Abstract
Chronic stress has been associated with a number of illnesses, including obesity. Ashwagandha is a well-known adaptogen and known for reducing stress and anxiety in humans. The objective of this study was to evaluate the safety and efficacy of a standardized root extract of Ashwagandha through a double-blind, randomized, placebo-controlled trial. A total of 52 subjects under chronic stress received either Ashwagandha (300 mg) or placebo twice daily. Primary efficacy measures were Perceived Stress Scale and Food Cravings Questionnaire. Secondary efficacy measures were Oxford Happiness Questionnaire, Three-Factor Eating Questionnaire, serum cortisol, body weight, and body mass index. Each subject was assessed at the start and at 4 and 8 weeks. The treatment with Ashwagandha resulted in significant improvements in primary and secondary measures. Also, the extract was found to be safe and tolerable. The outcome of this study suggests that Ashwagandha root extract can be used for body weight management in adults under chronic stress.
Keywords: stress, food craving, Withania somnifera, weight gain, serum cortisol
Chronic psychological stress is a major health concern worldwide and has been associated with numerous serious illnesses, including depression, cardiac disease, diabetes, hypertension, and possibly even cancer.1 Excess stress is also associated with symptoms such as muscle tension, gastrointestinal disturbances, sleep disturbances, cognitive dysfunction, headaches, and fatigue.
Psychological stress has also been linked to weight-gain and obesity.2,3 Stress causes systemic elevation of stress hormones such as cortisol, and chronic elevation of these hormones leads to increased visceral adiposity and other metabolic syndrome.4
Chronic stress may also lead to changes in eating behavior.5,6 The exacerbation of negative mood in response to external stress elements is highly correlated with increased food intake,2 and chronic stress is also associated with reduced physical activity.5 Both these behaviors may have a significant impact on body weight.2 Increased cortisol production has been shown to potentiate hunger.7–9 Thus, there may be a physiological component to the tendency to overeat during times of stress. In addition, stress tends to elicit cravings for sweet and fried foods, soft drinks, and alcoholic beverages2,10; these cravings are linked to increased caloric consumption and resultant higher body mass index.10,11 This may be due to the fact that chronic stress increases activation of the hypothalamic-pituitary-adrenal axis, which has been found to increase sweet cravings in persons prone to binge-eating sweet and fried foods, soft drinks, and alcoholic beverages.2
In double-blind studies of various herbal extracts traditionally associated with calming and adaptogenic properties, links were found between anxiety, stress, and body weight, and patients who were treated showed marked reduction in body weight and serum cortisol levels compared with placebo.12,13
The root of the Ashwagandha plant (also known as Withania somnifera), has a long history of use as an adaptogen in the ayurvedic system of complementary medicine, and is used to counteract the negative effects of stress. Modern research has begun to identify a number of active components in Ashwagandha that may have useful therapeutic applications.14 The plant contains a range of bioactive constituents, including withanolides, glycowithanolides, sitoindosides, withaferin A, and other therapeutically active phytochemicals.15 Other studies have identified anticancer, antidepressant, anxiolytic, cardioprotective, antioxidant, thyroid modulating, immunomodulating, antibacterial, neuroprotective, antifungal, anti-inflammatory, and hematopoietic activities.16
Several preclinical studies have indicated that Ashwagandha does indeed have adaptogenic and antistress activities. Jain et al17 observed that Ashwagandha extract reduced damage to hippocampal neurons in the CA2 and CA3 region by 80%. In another study, rodents pretreated with Ashwagandha extract showed significant attenuation of hypercortisolemia and other physiological indictors of stress. Also, the Ashwagandha component withanolide A was found to reverse the memory deficits and induce regeneration of dendritic spines and axons in mice.18 In another study with rodents, the antistress potential of the Ashwagandha components sitoindoside VII and VIII was established.19
Additional studies have determined that extracts of Ashwagandha root have significant anxiolytic properties in humans, as measured both by patient-reported instruments and by quantitative analysis of serum biomarkers.3,20,21 For example, because of its GABAergic activity on ionotropic GABA-A and GABA-ρ receptors, it has shown efficacy in the treatment of insomnia.14 Chandrasekhar et al1 evaluated the efficacy of a standardized extract versus placebo in a 60-day clinical trial. Significant differences were found for all the outcome measures, including scores on the Perceived Stress Scale (P < .0001), the General Health Questionnaire (P < .0001), and levels of cortisol in the bloodstream (P = .0006).12,13
Based on previous works linking stress to anxiety and weight gain, the aim of the present randomized, double-blind, placebo-controlled clinical study was to assess the efficacy of a root extract of Ashwagandha root in improving general well-being and reducing physiological markers of stress that have been associated with obesity in adults under chronic stress. We hypothesized that treatment with this extract would yield anxiolytic and antistress effects, thus improving patient-reported measures of psychological and physical well-being, and normalizing serum cortisol levels, thereby reducing hunger and stress-eating behaviors and reducing weight gain.
Materials and Methods
Patient Enrollment
Study subjects were selected from several outpatient clinics in the city of Pune, India, who were intended for the treatment of stress and overweight. Subjects were invited to the study center at Chaitanya Hospital & Nursing Home, Pune, India for the study. All the subjects received interventions at the study center only. Inclusion criteria included the following: symptoms of chronic, routine work stress; age between 18 and 60 years; ability to provide written informed consent; a Perceived Stress Scale (PSS)22 score ≥20, and a body mass index between 25 and 39.9 kg/m2. Exclusion criteria included the following: a diagnosable eating disorder; participation in a weight-loss program in the past 3 months; predisposition to weight gain due to genetic or endocrine conditions; diagnosed neurologic disorder, unstable medical condition, or known allergy/side effects to Ashwagandha root extract; pregnancy or lactation; taking medications known to affect weight (eg, corticosteroids, antidepressants, antipsychotics, mood stabilizers, and antiepileptic drugs); participation in other clinical trials during the previous 3 months; history of alcohol abuse or smoking; and clinically significant acute unstable hepatic, renal, cardiovascular, or respiratory disease.
The study was conducted in accordance with the Declaration of Helsinki (1989) and “Guidelines for Clinical Trials on Pharmaceutical Products in India”—GCP Guidelines issued by the Central Drugs Standard Control Organization, Ministry of Health, and Government of India. Institutional review board approval was obtained from the study center at Chaitanya Hospital & Nursing Home, Pune, India. Ethics committee notifications as per Good Clinical Practice Guidelines, issued by Central Drugs Standard Control Organization and Ethical Guidelines for Biomedical Research on Human Subjects, issued by Indian Council of Medical Research, were followed.
Data Collection
This 8-week prospective clinical trial was conducted using a random-assignment, parallel-group, single-centre, double-blind, placebo-controlled design to evaluate the efficacy of Ashwagandha root extract compared with placebo in reducing markers of stress, and in controlling weight gain and improving general well-being in adults under chronic stress.
The study comprised a screening visit followed by an 8-week treatment period. At the screening visit, medical history was obtained from each subject and symptoms of chronic stress were assessed. A general physical examination was conducted and vital parameters, baseline body weight, body mass index, and baseline serum cortisol levels were recorded. Each subject was then assessed using the PSS.22 A qualified psychiatrist performed a clinical psychiatric examination on each subject to check for primary psychiatric disorders that would warrant exclusion from the study.
Randomization and Blinding
Following screening, eligible subjects were randomized through a computer-based predetermined randomization (Rando version 1.0) in a 1:1 ratio to receive either Ashwagandha root extract or placebo. The randomization list had blocks of the same length and was nonstratified. The study was a double blind one, that is, doctors and subjects were unaware about the study groups. Both the drug and placebo capsules were prepared as hard gelatin capsules having identical size, shape, color, texture, and weight. Also, the investigational products were packaged in such a way that the extract and placebo medication packs were identical in appearance. The packs were coded to conceal their contents, and the label contained the subject serial number (ID of the study). After the subject was enrolled, he or she was provided with the medication pack having the corresponding serial number. During data collection, neither the researchers nor the physicians had access to the randomization codes and were blinded to the allocations. The unbinding was allowed only after completion of entire data collection process or in case of serious adverse events. The data analysts and the persons in charge of reporting the study results were unaware of the identity of the study groups. The data were double entered and blinded to the statisticians.
The study group received 300 mg of a standardized (containing 5% withanolides) Ashwagandha root extract (KSM-66 Ashwagandha, Ixoreal Biomed, Los Angeles, CA) in capsule form, twice daily with water for 8 weeks. The control group received identical placebo capsules containing inert filler for the same period. Placebo capsules were kept with a cloth-covered envelope that contained Ashwagandha root extract for few days, so that the smell of Ashwagandha is permeated to the placebo capsules and the smell of those capsules became similar to Ashwagandha capsules. At the beginning of the study and at the end of 4 and 8 weeks, subjects were assessed using the outcome measures described below. In addition, body weight, body mass index, serum cortisol levels, and vital parameters were also recorded. Data on safety and adverse effects of the investigational drug were collected at the end of 8 weeks. Patients Global Assessment of Tolerability to Therapy (PGATT) was assessed on a 5-point Likert-type scale at the end of therapy.
Clinical safety was assessed based on the adverse events reported by the subjects during the follow-up or during clinical evaluation of subjects. Adverse events were recorded, along with their severity, duration and relationship to study drug. Assessment of the tolerability of the Ashwagandha root extract was done through PGATT on a 5-point scale of “worst,” “poor,” “moderate,” “good,” and “excellent” tolerability at the end of therapy.
Outcome Measures
The primary outcome measures were the PSS22 and the Food Cravings Questionnaire–Trait (FCQ-T).23 Secondary outcome measures included the Oxford Happiness Questionnaire (OHQ),24,25 the Three-Factor Eating Questionnaire (TFEQ),26 serum cortisol levels, initial and final body weight, and body mass index.
The PSS instrument is used to measure psychological stress. This 14-item scale determines general stress experienced in the previous month, with higher scores representing higher stress and possible values ranging from 0 to 56. PSS evaluates physical and mental depressive symptoms, requirement of health services, social anxiety, and correlates with life-event scores.22 Perceived stress was used as a continuous variable in the present analysis to determine any effect of treatment.
The FCQ-T is a 39-item, self-reported questionnaire that is used to measure stable dimensions of food cravings, with answers based on a 6-point Likert-type scale ranging from 1(never/not applicable) to 6 (always). FCQ-T records 9 domains of food cravings: (1) planning to eat food, (2) positive reinforcement from eating, (3) relief from negative mood by eating, (4) lack of control on overeating of food, (5) thoughts about food, (6) physiological state, (7) emotions that are involved during food cravings or eating, (8) environmental cues that may trigger food cravings, and (9) guilt experienced due to food craving.23
The OHQ24,25 consists of 29 questions that are answered on a 6-point Likert-type scale (1 = strongly disagree, 6 = strongly agree). The OHQ is an effective tool to measure happiness, well-being, and optimism. In general, happiness and stress are believed to be inversely proportional.27 Therefore, reduction of the effects of stress may be expected to improve general well-being in study subjects.
As noted above, serum cortisol levels are an indicator of stress4 and have been shown to affect appetite.7–9 Therefore, cortisol represents an effective parameter for measuring the antistress effect of Ashwagandha in subjects under chronic stress and its impact on weight gain.7–9
The TFEQ used in this study was the Revised-TFEQ as explained and revised by Cappelleri et al28 and Karlsson et al26 from the original version of TFEQ by Stunkard and Messick.29 This questionnaire is used to determine eating behavior. It is a 4-point Likert-type response format with a 3-factor structure containing 18 items. The 3-factor scales are “cognitive restraint,” “uncontrolled eating,” and “emotional eating.”
Statistical Analyses
Baseline scores were compared with posttreatment scores using a Friedman test followed by post hoc individual comparisons using a Wilcoxon test. The 2 groups were compared for changes from baseline in the scores using a Wilcoxon test.
All data were expressed as means with standard deviation. Categorical data and discrete data were expressed as numbers with percentages. Changes in the scores from baseline were calculated and expressed as mean change and percent change from baseline. Differences at the level of P < .05 were regarded as statistically significant.
Results
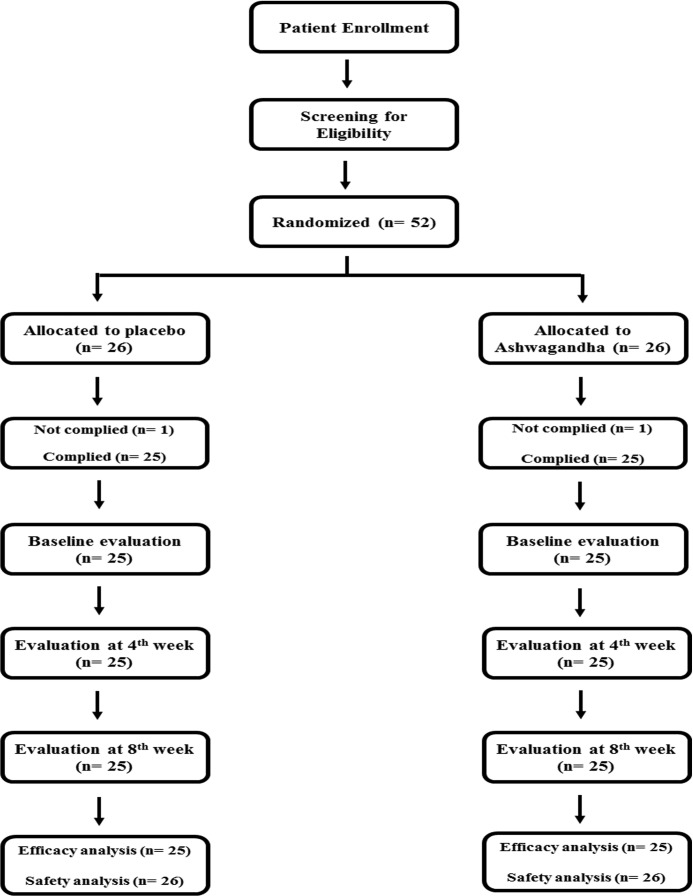
A total of 52 adults (38 men and 14 women) between the ages of 18 and 60 years were enrolled in the present study, and randomized to receive either treatment or placebo (Figure 1).
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) flow diagram. Patient distribution and study design.
Of the 52 enrolled subjects, 2 (1 each in the placebo and treatment group) were not compliant with the study protocol. The data for the remaining 50 subjects were used for efficacy analysis as per-protocol (PP) datasets. For safety analysis, intent-to-treat (ITT) datasets were used. The ITT dataset included all 52 subjects recruited for the study irrespective of their study completion status.
The efficacy of the Ashwagandha root extract with regard to weight management was evaluated using FCQ scores, body weight, body mass index, and TFEQ scores, while the efficacy in stress management was evaluated through PSS and OHQ scores.
Baseline Occupational and Illness Characteristics
Occupational and baseline characteristics were comparable across treatment groups in the trial (Table 1). The majority of subjects (72% in Ashwagandha group and 68% in placebo group) were found to be employed. The remaining subjects were either students or housewives. All subjects in the trial had chronic stress symptoms. The majority were troubled with difficulties in concentration (60% in Ashwagandha group and 44% in placebo group) and insomnia (60% in Ashwagandha group and 44% in placebo group). About 44% of the subjects in Ashwagandha group and 52% in placebo group had problems with anxiety and restlessness. Other major symptoms included physical exhaustion, mental fatigue, and headaches.
Table 1.
Baseline Occupational and Illness Characteristics (Intent-to-Treat Population).a
| Ashwagandha (n = 25) | Placebo (n = 25) | |
|---|---|---|
| Employment status | ||
| Housewife | 3 (12) | 4 (16) |
| Employed | 18 (72) | 17 (68) |
| Student | 4 (16) | 4 (16) |
| Stress symptoms | ||
| Difficulty in concentration | 15 (60) | 11 (44) |
| Physical exhaustion | 7 (28) | 1 (4) |
| Anxiety, restlessness | 11 (44) | 13 (52) |
| Insomnia | 15 (60) | 11 (44) |
| Headache | 5 (20) | 9 (36) |
| Fatigue | 4 (16) | 5 (20) |
| Loss of appetite | 1 (4) | 0 (0) |
| Mental confusion | 0 (0) | 1 (4) |
a Data are presented as number (percentage).
Outcome Measures
The primary and secondary outcomes of the trial are shown in Table 2. The primary outcome of the study was obtained from the PSS score. The treatment (Ashwagandha) and placebo groups were similar with respect to baseline PSS scores (P = .759). At both subsequent time points, however, the mean PSS score of the treatment group decreased significantly (Table 2). This was a superior response compared with the placebo group (P = .05 at 4 weeks and P = .0015 at 8 weeks). A reduction in PSS scores was observed at the end of fourth and eighth weeks for both the treatment and placebo groups. However, the treatment group experienced a significantly greater degree of reduction than the placebo group at the end of the fourth week (22.1%, P = .0025) and the eighth week (32.7% reduction at 8 weeks, P < .0001).
Table 2.
Efficacy Analysis: Primary and Secondary Outcomes (Per-Protocol Population).a
| Ashwagandha (n = 25) | Placebo (n = 25) | P Value | |
|---|---|---|---|
| Primary outcomes | |||
| Mean Perceived Stress Scale score | |||
| Baseline | 20.31 (4.04) | 19.96 (3.99) | .759 |
| Week 4 | 15.73 (4.38) | 18.50 (5.33) | .0519 |
| Week 8 | 13.65 (3.14) | 17.83 (5.16) | .0015 |
| Mean change from baseline: | |||
| At 4 weeks | −4.48 (4.16) | −1.46 (2.57) | .0025 |
| At 8 weeks | −6.65 (4.80) | −2.12 (2.68) | <.0001 |
| Mean Food Cravings Questionnaire scores—Component 1: Planning | |||
| Baseline | 11.54 (4.55) | 12.62 (3.87) | .366 |
| Week 4 | 10.12 (4.14) | 11.92 (3.74) | .1127 |
| Week 8 | 9.35 (4.18) | 11.67 (4.01) | .0406 |
| Mean change from baseline | |||
| At 4 weeks | −1.42 (1.17) | −0.71 (1.04) | .0269 |
| At 8 weeks | −2.19 (1.70) | −0.96 (1.49) | .0087 |
| Mean Food Cravings Questionnaire scores—Component 2: Positive Reinforcement | |||
| Baseline | 19.12 (6.45) | 20.54 (5.74) | .4121 |
| Week 4 | 16.65 (5.82) | 19.92 (6.07) | .0589 |
| Week 8 | 15.92 (6.16) | 19.62 (5.44) | .0287 |
| Mean change from baseline: | |||
| At 4 weeks | −2.46 (1.88) | −0.62 (2.58) | .0067 |
| At 8 weeks | −3.19 (1.96) | −0.92 (2.10) | <.0001 |
| Mean Food Cravings Questionnaire scores—Component 3: Negative Reinforcement | |||
| Baseline | 10.73 (3.67) | 10.46 (4.05) | .805 |
| Week 4 | 9.46 (4.23) | 10.42 (4.11) | .4218 |
| Week 8 | 8.85 (4.08) | 10.08 (4.17) | .2947 |
| Mean change from baseline | |||
| At 4 weeks | −1.27 (1.78) | −0.04 (1.33) | .008 |
| At 8 weeks | −1.88 (2.32) | −0.38 (1.47) | .0083 |
| Mean Food Cravings Questionnaire scores—Component 4: Lack of Control | |||
| Baseline | 15.96 (4.32) | 14.50 (4.49) | .2476 |
| Week 4 | 14.04 (3.49) | 14.08 (4.20) | .9676 |
| Week 8 | 13.00 (3.64) | 13.83 (4.31) | .466 |
| Mean change from baseline | |||
| At 4 weeks | −1.92 (2.83) | −0.42 (2.32) | .0443 |
| At 8 weeks | −2.96 (3.49) | −0.67 (2.46) | .0097 |
| Mean Food Cravings Questionnaire scores—Component 5: Thoughts About Food | |||
| Baseline | 19.88 (6.87) | 21.62 (5.48) | .3253 |
| Week 4 | 18.31 (6.69) | 21.21 (6.06) | .1142 |
| Week 8 | 17.58 (7.27) | 20.88 (5.97) | .0851 |
| Mean change from baseline | |||
| At 4 weeks | −1.58 (2.94) | −0.42 (2.95) | .1704 |
| At 8 weeks | −2.31 (3.43) | −0.75 (2.63) | .0764 |
| Mean Food Cravings Questionnaire scores—Component 6: Physiological | |||
| Baseline | 11.73 (5.41) | 12.33 (5.07) | .6861 |
| Week 4 | 10.96 (5.57) | 12.08 (5.08) | .46 |
| Week 8 | 10.77 (5.64) | 12.08 (4.94) | .3843 |
| Mean change from baseline | |||
| At 4 weeks | −0.77 (1.82) | −0.25 (1.94) | .3347 |
| At 8 weeks | −0.96 (1.89) | −0.25 (1.67) | .1642 |
| Mean Food Cravings Questionnaire scores—Component 7: Emotion | |||
| Baseline | 14.46 (5.49) | 13.21 (6.39) | .4625 |
| Week 4 | 12.85 (4.89) | 12.83 (6.49) | .9938 |
| Week 8 | 12.15 (4.65) | 12.67 (5.87) | .7352 |
| Mean change from baseline | |||
| At 4 weeks | −1.62 (2.26) | −0.38 (1.76) | .0352 |
| At 8 weeks | −2.31 (2.65) | −0.54 (1.67) | .0068 |
| Mean Food Cravings Questionnaire scores—Component 8: Environment | |||
| Baseline | 15.46 (4.69) | 15.67 (4.86) | .8802 |
| Week 4 | 13.62 (5.35) | 14.79 (5.32) | .4401 |
| Week 8 | 12.77 (5.16) | 14.29 (5.36) | .3124 |
| Mean change from baseline | |||
| At 4 weeks | −1.85 (2.44) | −0.88 (2.35) | .1583 |
| At 8 weeks | −2.69 (2.33) | −1.38 (2.06) | .039 |
| Mean Food Cravings Questionnaire Score—Component 9: Guilt | |||
| Baseline | 8.69 (3.28) | 8.04 (3.64) | .5115 |
| Week 4 | 8.12 (3.96) | 7.79 (4.05) | .7768 |
| Week 8 | 7.81 (3.67) | 7.58 (3.97) | .8368 |
| Mean change from baseline | |||
| At 4 weeks | −0.58 (1.65) | −0.25 (1.36) | .4475 |
| At 8 weeks | −0.88 (1.42) | −0.46 (1.44) | .299 |
| Secondary outcomes | |||
| Mean Oxford Happiness Questionnaire score | |||
| Baseline | 28.88 (5.26) | 29.17 (5.83) | .8586 |
| Week 4 | 32.58 (4.62) | 30.21 (7.59) | .1949 |
| Week 8 | 34.42 (4.69) | 30.21 (6.01) | .0087 |
| Mean change from baseline | |||
| At 4 weeks | 3.69 (4.43) | 1.04 (4.16) | .0342 |
| At 8 weeks | 5.54 (4.64) | 1.04 (3.06) | <.0001 |
| Mean serum cortisol level (μg/dL) | |||
| Baseline | 17.25 (4.41) | 16.76 (3.95) | .6835 |
| Week 4 | 14.47 (2.94) | 15.63 (3.07) | .1798 |
| Week 8 | 13.41 (2.17) | 15.44 (3.20) | .0132 |
| Mean change from baseline | |||
| At 4 weeks | −2.77 (3.09) | −1.13 (2.14) | .0328 |
| At 8 weeks | −3.83 (3.18) | −1.32 (2.10) | .0019 |
| Mean body weight (kg) | |||
| Baseline | 76.35 (8.71) | 77.16 (8.46) | .7383 |
| Week 4 | 74.70 (7.81) | 76.32 (7.99) | .4733 |
| Week 8 | 74.03 (7.29) | 76.03 (7.72) | .3523 |
| Mean change from baseline | |||
| At 4 weeks | −1.64 (1.57) | −0.84 (1.24) | .0503 |
| At 8 weeks | −2.32 (1.99) | −1.13 (1.24) | .0148 |
| Mean body mass index (kg/m2) | |||
| Baseline | 26.88 (1.62) | 27.17 (1.32) | .4793 |
| Week 4 | 26.32 (1.51) | 26.89 (1.30) | .1579 |
| Week 8 | 26.09 (1.34) | 26.80 (1.35) | .0696 |
| Mean change from baseline | |||
| At 4 weeks | −0.56 (0.51) | −0.28 (0.42) | .0429 |
| At 8 weeks | −0.79 (0.65) | −0.38 (0.40) | .0096 |
| Mean Three-Factor Eating Questionnaire score—Component 1: Cognitive Restraint | |||
| Baseline | 13.19 (4.78) | 13.50 (6.15) | .8452 |
| Week 4 | 12.08 (4.96) | 13.00 (6.19) | .5656 |
| Week 8 | 11.54 (4.82) | 12.38 (5.33) | .5644 |
| Mean change from baseline | |||
| At 4 weeks | −1.12 (2.37) | −0.50 (1.98) | .3228 |
| At 8 weeks | −1.65 (2.17) | −1.12 (2.33) | .4114 |
| Mean Three-Factor Eating Questionnaire score—Component 2: Uncontrolled Eating | |||
| Baseline | 23.69 (5.36) | 22.75 (5.42) | .5399 |
| Week 4 | 19.92 (6.10) | 20.83 (5.66) | .5867 |
| Week 8 | 18.85 (4.67) | 20.17 (6.13) | .399 |
| Mean change from baseline | |||
| At 4 weeks | −3.77 (3.28) | −1.92 (3.35) | .0542 |
| At 8 weeks | −4.85 (3.78) | −2.58 (3.11) | .0247 |
| Mean Three-Factor Eating Questionnaire score—Component 3: Emotional Eating | |||
| Baseline | 9.46 (2.28) | 9.46 (2.38) | .9961 |
| Week 4 | 8.27 (2.49) | 9.21 (2.19) | .1623 |
| Week 8 | 7.96 (2.65) | 9.08 (2.08) | .1011 |
| Mean change from baseline | |||
| At 4 weeks | −1.19 (1.55) | −0.25 (1.22) | .0207 |
| At 8 weeks | −1.50 (1.86) | −0.38 (1.17) | .0135 |
| Vital parameters | |||
| Mean systolic blood pressure (mm Hg) | |||
| Baseline | 120.88 (8.04) | 122.80 (7.42) | .385 |
| Change at 4 weeks | −0.96 (6.83) | −2.08 (7.99) | .597 |
| Change at 8 weeks | −2.24 (13.62) | −4.56 (6.42) | .445 |
| Mean diastolic blood pressure (mm Hg) | |||
| Baseline | 74.72 (4.96) | 75.60 (4.83) | .528 |
| Change at 4 weeks | 2.00 (6.63) | −0.16 (5.51) | .216 |
| Change at 8 weeks | 0.08 (5.90) | −0.96 (5.45) | .521 |
| Mean pulse rate (per minute) | |||
| Baseline | 76.16 (4.20) | 76.64 (3.82) | .674 |
| Change at 4 weeks | −0.80 (4.65) | 0.08 (4.14) | .484 |
| Change at 8 weeks | −1.76 (4.70) | −2.08 (4.67) | .810 |
| Mean respiratory rate (breaths per minute) | |||
| Baseline | 15.92 (0.64) | 16.00 (0.29) | .572 |
| Change at 4 weeks | 0.08 (0.64) | 0.04 (0.35) | .785 |
| Change at 8 weeks | 0.24 (0.66) | 0.16 (0.37) | .602 |
| Mean body temperature (°F) | |||
| Baseline | 98.08 (0.09) | 98.09 (0.07) | .481 |
| Change at 4 weeks | 0.03 (0.11) | 0.00 (0.08) | .390 |
| Change at 8 weeks | −0.04 (0.11) | −0.04 (0.10) | 1.000 |
aData are presented as mean (standard deviation).
The scores of FCQ components are also shown in Table 2. The FCQ component “Planning” scores for the treatment group were compared with the placebo group at baseline and at the end of the fourth and eighth weeks. No significant differences between the 2 groups were observed at baseline (P = .366) or fourth week (P = .113) score. However, at the end of the eighth week, the mean FCQ “Planning” score of the treatment group was significantly lower than that of the placebo group (P = .0406). A reduction of the mean FCQ “Planning” score from baseline to 4 and 8 weeks was observed for both the treatment and placebo groups. However, the reductions of FCQ “Planning” scores for the treatment group at the end of fourth week (P = .0269) and eighth week (P = .0087) were statistically significant compared with the placebo group. The mean FCQ “Positive Reinforcement” score of the treatment group at the eighth week was found to be significantly lower than that of the placebo group (P = .0287). The mean difference from the baseline FCQ “Positive Reinforcement” score at the end of the fourth week (P = .0067) and eighth week (P < .0001) for the treatment group were found to be significant compared with the placebo group.
The mean FCQ “Negative Reinforcement” scores of the treatment group at the fourth and eighth weeks did not show any significant difference compared with the placebo group. However, the mean reduction from the baseline FCQ “Negative Reinforcement” score for the treatment group showed a significant difference from the placebo group at the fourth week (P = .008) and the eight week (P = .0083). The mean FCQ scores of the treatment group showed a significant reduction from baseline compared with the placebo group for the following components: “Lack of Control” (fourth week, P = .0443; eighth week, P = .0097), “Emotion” (fourth week, P = .0352; eighth week, P = .0068), and “Environment” (eighth week, P = .039), during the study. However, the mean FCQ component scores of the treatment group for the “Thoughts about Food,” “Physiological,” and “Guilt” components did not show any significant differences compared with the placebo group.
With regard to the secondary outcomes of this study, mean OHQ scores were found to improve in both the placebo and treatmentgroups over the 8-week period of study. However, at the end of the eighth week, the mean OHQ score of the treatment group improved significantly compared with the placebo group (P = .0087). At the end of the fourth week (P = .0342) and the eighth week (P < .0001), the mean increase of the OHQ score from baseline for treatment group was significantly better than for the placebo group, with an overall improvement of 19.18%.
Table 2 summarizes the changes in serum cortisol levels, measured in μg/dL. Both the treatment and placebo groups had similar serum cortisol levels at baseline (P = .6835). However, by the end of the study (eighth week), mean serum cortisol levels of the treatmentgroup were significantly lower compared with the placebo group (P = .0132). After 4 and 8 weeks of treatment, a reduction from baseline of 16.05% and 22.2%, respectively, was observed in the treatment group. The difference between the mean reductions of serum cortisol levels in the 2 groups after the fourth week (P = .0328) and eighth week (P = .0019) of treatment were statistically significant.
Mean changes in body weight are shown in Table 2. The body weight for both the treatment and placebo groups was found to be reduced during the 8-week period of the study. After 4 weeks of treatment, a mean reduction of 2.14% and 1.09%, from baseline was observed in the treatment and placebo groups, respectively, but the difference in reduction in the 2 groups was not statistically significant after 4 weeks (P = .0503). However, at the end of 8 weeks, the reduction of body weight for the treatmentand placebo groups was 3.03% and 1.46%, respectively. The data collected after 8 weeks of treatment suggests a significant difference in mean reduction of body weight for both groups (P = .0148).
The mean body mass index for both groups was reduced during the study (Table 2). After 4 and 8 weeks of treatment, a mean reduction from baseline of 2.08% (P = .0429) and 2.93% (P = .0096), respectively, was observed in the treatment group, which was statistically significant compared with the placebo group (fourth week, 1.03%; eighth week, 1.4%).
Mean scores for the TFEQ components are shown in Table 2. During the study, the TFEQ-“Cognitive Restraint” scores of the treatment group did not show significant differences when compared with the placebo group. However, the mean TFEQ “Uncontrolled Eating” and “Emotional Eating” scores showed marked reduction from baseline scores for the treatment group, which were statistically significant compared with the placebo group at the end of the fourth week (“Emotional Eating,” P = .0207) and the eighth week (“Uncontrolled Eating,” P = .0247; “Emotional Eating,” P = .0135) of the study.
Vital parameters such as systolic blood pressure, diastolic blood pressure, pulse rate, respiratory rate and body temperature are summarized in Table 2. Mean systolic blood pressure, diastolic blood pressure, and pulse rate were observed to change to a similar extent in both groups. Respiratory rate and body temperatures of the subjects of both groups were found to be unaltered during the 8 weeks of the trial. Therefore, it can be concluded that the treatment with Ashwagandha root extract did not produce any significant changes in vital parameters when compared with placebo.
Safety Analysis
At the end of 8 weeks of treatment, subjects were evaluated with the PGATT test, on a 5-point scale, which is done based on the ITT population. The majority of the subjects in both the treatment (96%) and placebo group (96%) reported “excellent” tolerability.
Adverse Events
Data on adverse events were collected and analyzed for the ITT population, considering all 52 subjects. Only 2 subjects (4%) out of 52 reported effects such as giddiness, heaviness of head, blurring of vision, and/or hyperacidity. The severity of these adverse events was mild and temporary. The treatment was tolerable to most of the subjects in both groups.
Discussion
This prospective, randomized, double-blind clinical study evaluated the safety and efficacy of a standardized Ashwagandha root extract in 52 subjects suffering from chronic stress and related disorders. The aim of the study was to analyze the impact of the extract on food cravings and body weight management compared with a placebo. The results indicate that the treatment with Ashwagandha root extract was more effective than that with placebo.
Treatment with Ashwagandha root extract resulted in a marked reduction of mean scores on the PSS compared with baseline values at both 4 and 8 weeks. The treatment group exhibited significantly greater improvement than the placebo group. This result is in accordance with the findings of Chandrasekhar et al,1 who observed a 44% reduction of PSS score from baseline was observed at the end of a 60-day study with 64 subjects. The other measures of efficacy used in this study also showed significantly greater improvement in the treatment group than the placebo group. These included measures of well-being and happiness, food cravings, reactive eating, serum cortisol levels, and body weight.
Chronic stress is a common problem in modern life. Individuals experiencing prolonged stress are prone to overeating and improper diet maintenance.2 Food cravings can be linked with higher consumption of palatable foods, thereby leading to increased body mass index. These cravings are known to mediate stress-related weight gain.11 In the present study, mean FCQ scores for “Planning,” “Positive Reinforcement,” “Negative Reinforcement,” “Lack of Control,” “Emotion,” and “Environment” were reduced significantly (P < .05) after 8 weeks of treatment with Ashwagandha root extract, when compared with placebo. However, mean FCQ component scores of the treatment group for “Thoughts about Food,” “Physiological,” and “Guilt” did not show significant changes during the study. These results support the conclusion that because of the anxiolytic and antistress properties of Ashwagandha, subjects rejected the use of food as a method for coping with stress. Cravings for food due to stress can lead to unconscious eating. Consequently, we can see that “Thoughts about Food,” “Physiological,” and “Guilt” parameters were not affected due to the treatment. Similar results were obtained from the TFEQ study. Scores for “Uncontrolled Eating” and “Emotional Eating” were reduced significantly (P < .05) after 8 weeks of Ashwagandha treatment but were not reduced for “Cognitive Restraint.” It has been observed previously that higher scores on the “Uncontrolled Eating” and “Emotional Eating” subscales are related to a higher preference of energy-dense foods.30
The reduction of body weight and body mass index observed in the present study further supports hypothesis that Ashwagandha root extract exerts antistress activity, resulting in reduced food cravings and better eating behaviors (as reflected in improved FCQ and TFEQ scores).
Stress-induced increase in serum cortisol level leads to increased visceral fat deposition in humans. Prolonged stress also increases circulating glucocorticoid concentrations, which eventually promotes the ingestion of carbohydrates and fat and decreased energy expenditure by suppressing corticotropin-releasing hormone and stimulating neuropeptide hypothalamic secretion.4 Low leptin levels have also been associated with increased symptoms of depression.30 Leptin is a hormone that regulates energy balance by suppressing food intake, and thereby induces weight loss. Stress reduction restores normal leptin levels and helps control obesity.31 These results further support the hypothesis that treatment with Ashwagandha can be helpful in limiting stress-associated weight gain in humans.
The potential of Ashwagandha as a natural antistress and antianxiety therapeutic has been strongly supported by previous researchers.1,3,20,21 The results of the present study have taken this analysis a step further and demonstrated that Ashwagandha may provide a potential additional benefit of supporting the maintenance of normal weight (or even weight loss) in people living with chronic stress.
Ashwagandha is generally considered a harmless and easily tolerated medication with few adverse effects or withdrawal symptoms. Long-term administration of Ashwagandha root extract has been found to be safe in various studies.1,32–34 The results of the present study are consistent with those of previous studies, with Ashwagandha exhibiting a good safety profile and negligible adverse events.
Limitations
The major limitation of the present study design is the relatively small sample size. A study with a larger population involving a wider cross-section of the subjects with regard to age groups, occupation, and socioeconomic background would provide more conclusive results. Study duration should also be increased in future research to evaluate the long-term effects of Ashwagandha root extract. Also, it would be useful to measure additional parameters, such as serum leptin and ghrelin levels involved in appetite regulation. However, this preliminary study offers a useful guide for future studies with Ashwagandha root extract or other herbal medicines.
Conclusion
The results of this study suggest that Ashwagandha root extract reduces psychological and physiological markers of stress, improves mental well-being, and reduces serum cortisol level and food cravings and improves eating behaviors. A statistically significant reduction in body weight and body mass index were observed in patients treated with Ashwagandha root extract compared to placebo. Therefore, we conclude that Ashwagandha root extract can be useful for body-weight management in patients experiencing chronic stress. However, further studies are required to bolster the potential of Ashwagandha to prevent weight gain caused by long-term chronic stress.
Acknowledgments
The authors thank Ixoreal BioMed of Los Angeles, California, USA, for supplying the KSM-66 high-concentration root extract used in the study treatment.
Footnotes
Author Contributions: DC, principal investigator, was involved int designing clinical trial and protocol, data collection, and data analysis. SB contributed toward data analysis, writing and drafting the manuscript, presentation, bibliography, and managed correspondence. KJ was responsible for data collection, data treatment, and data analysis. All authors were involved in the writing and drafting, and all read and approved the final manuscript.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The parent organization of the principal investigator has provided the support for the research and publication of this article.
Ethical Approval: The study was conducted in accordance with the Declaration of Helsinki (1989) and ‘Guidelines for Clinical Trials on Pharmaceutical Products in India—GCP Guidelines issued by the Central Drugs Standard Control Organization, Ministry of Health, and Government of India. Institutional Review Board approval was obtained from the study center at Chaitanya Hospital & Nursing Home, Pune, India. Reference number: ECR/66/Inst/MH/2013. Ethics Committee notifications as per Good Clinical Practice Guidelines, issued by Central Drugs Standard Control Organization and Ethical Guidelines for Biomedical Research on Human Subjects, issued by Indian Council of Medical Research, were followed.
References
- 1. Chandrasekhar K, Kapoor J, Anishetty S. A prospective, randomized double-blind, placebo-controlled study of safety and efficacy of a high-concentration full-spectrum extract of ashwagandha root in reducing stress and anxiety in adults. Indian J Psychol Med. 2012;34:255–262. doi:10.4103/0253-7176.106022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nevanperä NJ, Hopsu L, Kuosma E, Ukkola O, Uitti J, Laitinen JH. Occupational burnout, eating behavior, and weight among working women. Am J Clin Nutr. 2012;95:934–943. doi:10.3945/ajcn.111.014191. [DOI] [PubMed] [Google Scholar]
- 3. Cooley K, Szczurko O, Perri D, et al. Naturopathic care for anxiety: A randomized controlled trial ISRCTN78958974. PLoS One. 2009;4(8):e6628 doi:10.1371/journal.pone.0006628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kyrou I, Tsigos C. Chronic stress, visceral obesity and gonadal dysfunction. Hormones. 2008;7:287–293. [DOI] [PubMed] [Google Scholar]
- 5. Tsutsumi A, Kayaba K, Yoshimura M, et al. ; Jichi Medical School Cohort Study Group. Association between job characteristics and health behaviors in Japanese rural workers. Int J Behav Med. 2003;10:125–142. doi:10.1207/S15327558IJBM1002_03. [DOI] [PubMed] [Google Scholar]
- 6. Sulkowski ML, Dempsey J, Dempsey AG. Effects of stress and coping on binge eating in female college students. Eat Behav. 2011;12:188–191. doi:10.1016/j.eatbeh.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 7. Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women: A laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26:37–49. doi:10.1016/S0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- 8. Willox JC, Corr J, Shaw J, Richardson M, Calman KC, Drennan M. Prednisolone as an appetite stimulant in patients with cancer. BMJ (Clin Res Ed). 1984;288(6410):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tataranni PA, Larson DE, Snitker S, Young JB, Flatt JP, Ravussin E. Effects of glucocorticoids on energy metabolism and food intake in humans. Am J Physiol. 1996271(2 pt 1):E317–E325. [DOI] [PubMed] [Google Scholar]
- 10. Moore CJ, Cunningham SA. Social position, psychological stress, and obesity: a systematic review. J Acad Nutr Diet. 2012;112:518–526. doi:10.1016/j.jand.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 11. Chao A, Grilo CM, White MA, Sinha R. Food cravings mediate the relationship between chronic stress and body mass index. J Health Psychol. 2015;20:721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kalman DS, Feldman S, Feldman R, Schwartz HI, Krieger DR, Garrison R. Effect of a proprietary magnolia and phellodendron extract on stress levels in healthy women: a pilot, double-blind, placebo-controlled clinical trial. Nutr J. 2008;7:11 doi:10.1186/1475-2891-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garrison R, Chambliss WG. Effect of a proprietary magnolia and phellodendron extract on weight management: a pilot, double-blind, placebo-controlled clinical trial. Altern Ther. 2006;12:50–53. [PubMed] [Google Scholar]
- 14. Candelario M, Cuellar E, Reyes-Ruiz JM, et al. Direct evidence for GABAergic activity of Withania somnifera on mammalian ionotropic GABA A and GABAρ receptors. J Ethnopharmacol. 2015;171:264–272. [DOI] [PubMed] [Google Scholar]
- 15. Singh N, Bhalla M, de Jager P, Gilca M. An overview on Ashwagandha: a rasayana (rejuvenator) of ayurveda. Afr J Tradit Complement Altern Med. 2011;8:208–213. doi:10.4314/ajtcam.v8i5S.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Verma SK, Kumar A. Therapeutic uses of Withania somnifera (Ashwagandha) with a note on withanolides and its pharmacological actions. Asian J Pharm Clin Res. 2011;4(suppl 1):1–4. [Google Scholar]
- 17. Jain S, Shukla SD, Sharma K, Bhatnagar M. Neuroprotective effects of Withania somnifera Dunn. in hippocampal sub-regions of female albino rat. Phyther Res. 2001;15:544–548. doi:10.1002/ptr.802. [DOI] [PubMed] [Google Scholar]
- 18. Kuboyama T, Tohda C, Komatsu K. Neuritic regeneration and synaptic reconstruction induced by withanolide A. Br J Pharmacol. 2005;144:961–971. doi:10.1038/sj.bjp.0706122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bhattacharya S, Goel R, Kaur R, Ghosal S. Anti- stress activity of sitoindosides VII and VIII, new acylsterylglucosides from Withania somnifera. Phyther Res. 1987;1:32–37. [Google Scholar]
- 20. Andrade C, Aswath A, Chaturvedi SK, Srinivasa M, Raguram R. A double-blind, placebo-controlled evaluation of the anxiolytic efficacy of an ethanolic extract of Withania somnifera. Indian J Psychiatry. 2000;42:295–301. [PMC free article] [PubMed] [Google Scholar]
- 21. Auddy B, Hazra J, Mitra A, et al. A standardized Withania somnifera extract significantly reduces stress-related parameters in chronically stressed humans. J Am Nutraceut Assoc. 2008;11:51–57. [Google Scholar]
- 22. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 23. Cepeda-Benito A, Gleaves DH, Williams TL, Erath SA. The development and validation of the State and Trait Food-Cravings Questionnaires. Behav Ther. 2000;31:151–173. doi:10.1016/S0005-7894(00)80009-X. [DOI] [PubMed] [Google Scholar]
- 24. Argyle M, Martin M, Crossland J. Happiness as a function of personality and social encounters In: Forgas JP, Innes JM, eds. Recent Advances in Social Psychology: An International Perspective. New York, NY: Elsevier; 1989:189–203. [Google Scholar]
- 25. Hills P, Argyle M. The Oxford Happiness Questionnaire: a compact scale for the measurement of psychological well-being. Pers Individ Diff. 2002;33:1073–1082. doi:10.1016/S0191-8869(01)00213-6. [Google Scholar]
- 26. Karlsson J, Persson L-O, Sjöström L, Sullivan M. Psychometric properties and factor structure of the Three-Factor Eating Questionnaire (TFEQ) in obese men and women. Results from the Swedish Obese Subjects (SOS) study. Int J Obes Relat Metab Disord. 2000;24:1715–1725. [DOI] [PubMed] [Google Scholar]
- 27. Torrubia R, Avila C, Molto J, Grande I. Testing for stress and happiness: the role of the behavioral inhibition system. Stress Emot Anxiety Anger Curiosity. 1995;15:189–211. [Google Scholar]
- 28. Cappelleri JC, Bushmakin AG, Gerber RA, et al. Psychometric analysis of the Three-Factor Eating Questionnaire-R21: results from a large diverse sample of obese and non-obese participants. Int J Obes (Lond). 2009;33:611–620. doi:10.1038/ijo.2009.74. [DOI] [PubMed] [Google Scholar]
- 29. Stunkard AJ, Messick S. The Three-Factor Eating Questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi:10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 30. Schembre SM. Weight-related eating behavior questionnaires: applying theory to measurement In: Preedy VR, Watson RR, Martin CR, eds. Handbook of Behavior, Food and Nutrition. New York, NY: Springer; 2011:3487–3506. [Google Scholar]
- 31. Lawson EA, Miller KK, Blum JI, et al. Leptin levels are associated with decreased depressive symptoms in women across the weight spectrum, independent of body fat. Clin Endocrinol (Oxf). 2012;76:520–525. doi:10.1111/j.1365-2265.2011.04182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ambiye VR, Langade D, Dongre S, Aptikar P, Kulkarni M, Dongre A. Clinical evaluation of the spermatogenic activity of the root extract of Ashwagandha (Withania somnifera) in oligospermic males: a pilot study. Evid Based Complement Altern Med. 2013;2013:571420 doi:10.1155/2013/571420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mahdi AA, Shukla KK, Ahmad MK, et al. Withania somnifera improves semen quality in stress-related male fertility. Evid Based Complement Altern Med. 2011;2011:576962 doi:10.1093/ecam/nep138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sandhu JS, Shah B, Shenoy S, Chauhan S, Lavekar GS, Padhi MM. Effects of Withania somnifera (Ashwagandha) and Terminalia arjuna (Arjuna) on physical performance and cardiorespiratory endurance in healthy young adults. Int J Ayurveda Res. 2010;1:144–149. doi:10.4103/0974-7788.72485. [DOI] [PMC free article] [PubMed] [Google Scholar]