Abstract
Origanum majorana L. commonly known as sweet marjoram has been used for variety of diseases in traditional and folklore medicines, including gastrointestinal, ocular, nasopharyngeal, respiratory, cardiac, rheumatologic, and neurological disorders. Essential oil containing monoterpene hydrocarbons and oxygenated monoterpenes as well as phenolic compounds are chemical constituents isolated and detected in O majorana. Wide range of pharmacological activities including antioxidant, hepatoprotective, cardioprotective, anti-platelet, gastroprotective, antibacterial and antifungal, antiprotozoal, antiatherosclerosis, anti-inflammatory, antimetastatic, antitumor, antiulcer, and anticholinesterase inhibitory activities have been reported from this plant in modern medicine. This article summarizes comprehensive information concerning traditional uses, phytochemistry, and pharmacological activities of sweet marjoram.
Keywords: Origanum majorana, traditional medicine, pharmacology, phytochemical constituent, Majorana hortensis
Origanum majorana L. from the family Lamiaceae (syn. Majorana hortensis Moench) is commonly known as sweet marjoram. This herb is native to Mediterranean region and cultivated in many countries of Asia, North Africa, and Europe, for example, Spain, Hungary, Portugal, Germany, Egypt, Poland, and France. Origanum majorana grows up to 30 to 60 cm. It is a perennial bushy plant. It has oblique rhizome, hairy shrub like stalks, opposite dark green oval leaves and white or red flowers in clustered bracts. The leaves are whole, larger ones being fragmented, oblate to broadly elliptical.1–3 This plant is widely used as a garnish and is used for different medicinal purposes in traditional and folklore medicine of different countries. Various compounds have been identified in sweet marjoram. Also, different pharmacological activities have been attributed to this plant. The present review summarizes comprehensive information concerning traditional uses, phytochemistry, and pharmacological activities of sweet marjoram. For this purpose, databases, including PubMed, Google Scholar, and Scopus were searched for studies focusing on the ethnomedicinal use, phytochemical compounds and pharmacological activities of sweet marjoram. Data were collected from 1980 to 2015 (up to July). The search terms were “sweet marjoram” or “Origanum majorana.”
Ethnomedicinal Uses
Ethnomedicinal uses of sweet marjoram in different countries are shown in Table 1. The parts of sweet marjoram that are used in folklore medicine are dried leaves, leaves extract, and essential oil. Origanum majorana leaves have been claimed to have antimicrobial and emmenagogue properties and be useful for treatment of respiratory and gastrointestinal problems.1, 4,5 It has been used in Morocco as an antihypertensive plant.8 The essential oil of the plant has been used for pains, gastrointestinal problems, and respiratory tract disorders.6,8–11
Table 1.
Ethnomedicinal Uses of Origanum Majorana.
| Region | Plant Part Used | Traditional Uses |
|---|---|---|
| Iran4,5 | Leaves | Antimicrobial, antiseptic, antidote, carminative, antitussive and used for gastrointestinal disorder, head cool, sniffle, vision performance, otitis, melancholia accompanied by flatulence, unilateral facial paralysis, headache, epilepsy, cataract, weakness of sight, ear pain, dyspnea, cardiac pain, dysrhythmia, cramp, obstruction of large intestine, emmenagogue, strangury, dropsy, spondilolysthesis, groin pain, back pain, fatigue, freckle, migraine |
| Azerbaijan6 | Essential oil | Flatulence, nervousness, diuretic, sedative |
| England1 | Leaves | Cold, bronchial coughs, asthmatic whooping |
| Egypt1 | Leaves | Cold, chill |
| India11 | Essential oil | Toothache, soothe joints, muscular pain |
| Austria7 | Leaves | Gastrointestinal tract diseases, infections |
| Turkey8 | Essential oil | Asthma, indigestion, headache, rheumatism |
| Morocco9 | Leaves | Hypertension |
Phytochemical Constituents
Table 2 shows the structure and phytochemical category of compounds isolated from different parts of sweet marjoram.
Table 2.
Phytochemical Constituents of Origanum majorana.
| Compound | Chemical Category | Part/Extract |
|---|---|---|
| α-Pinene | Monoterpene hydrocarbon | Essential oil2,13 |
| β-Pinene | Monoterpene hydrocarbon | Essential oil2,13 |
| ρ-Cymene | Monoterpene hydrocarbon | Essential oil2,14 |
| Camphene | Monoterpene hydrocarbon | Essential oil13 |
| α-Phellandrene | Monoterpene hydrocarbon | Essential oil2 |
| β-Phellandrene | Monoterpene hydrocarbon | Essential oil2 |
| γ-Terpinene | Monoterpene hydrocarbon | Essential oil14–16 |
| d-Limonene | Monoterpene hydrocarbon | Essential oil13 |
| α-Terpinene | Monoterpene hydrocarbon | Essential oil2,3,15,16 |
| Terpinolene | Monoterpene hydrocarbon | Essential oil2 |
| β-Myrcene | Monoterpene hydrocarbon | Essential oil2 |
| 2-Carene | Monoterpene hydrocarbon | Essential oil17 |
| β-Ocimene | Monoterpene hydrocarbon | Essential oil17 |
| Sabinene | Monoterpene hydrocarbon | Essential oil1,16,17 |
| α-Thujene | Monoterpene hydrocarbon | Essential oil2 |
| Carvone | Monoterpene hydrocarbon | Essential oil2,13 |
| Citronellol | Monoterpene hydrocarbon | Essential oil13 |
| Terpinen-4-ol | Oxygenated monoterpene | Essential oil14–16,19 / Leaf18 |
| cis-Sabinene hydrate | Oxygenated monoterpene | Essential oil10,14,15 |
| trans-Sabinene hydrate | Oxygenated monoterpene | Essential oil15,16 |
| Linalool | Oxygenated monoterpene | Leaf18 / Essential oil1,13 |
| Thymol | Oxygenated monoterpene | Essential oil10,13,19 |
| α-Terpineol | Oxygenated monoterpene | Essential oil1–3,13,15 |
| Linalyl acetate | Oxygenated monoterpene | Essential oil2,15 |
| Carvacrol | Oxygenated monoterpene | Essential oil10,13 |
| 1,8-Cineol | Oxygenated monoterpene | Essential oil17 |
| Fenchyl alcohol | Oxygenated monoterpene | Essential oil17 |
| Piperitol | Oxygenated monoterpene | Essential oil17 |
| trans-Carveol | Oxygenated monoterpene | Essential oil17 |
| cis-Carveol | Oxygenated monoterpene | Essential oil17 |
| Anethole | Oxygenated monoterpene | Essential oil17 |
| Geraniol | Oxygenated monoterpene | Essential oil13 |
| α-Terpinyl acetate | Oxygenated monoterpene | Essential oil2 |
| Geranyl acetate | Oxygenated monoterpene | Essential oil17 |
| α-Cubebene | Sesquiterpene hydrocarbon | Essential oil17 |
| Longicyclene | Sesquiterpene hydrocarbon | Essential oil17 |
| Copaene | Sesquiterpene hydrocarbon | Essential oil17 |
| β-Longipinene | Sesquiterpene hydrocarbon | Essential oil17 |
| β-Caryophyllene | Sesquiterpene hydrocarbon | Essential oil17 |
| Aromadendrene | Sesquiterpene hydrocarbon | Essential oil17 |
| α-Humulene | Sesquiterpene hydrocarbon | Essential oil17 |
| β-Farnesene | Sesquiterpene hydrocarbon | Essential oil17 |
| Alloaromadendrene | Sesquiterpene hydrocarbon | Essential oil17 |
| α-Selinene | Sesquiterpene hydrocarbon | Essential oil17 |
| ar-Curcumene | Sesquiterpene hydrocarbon | Essential oil17 |
| Germacrene D | Sesquiterpene hydrocarbon | Essential oil17 |
| Valencene | Sesquiterpene hydrocarbon | Essential oil17 |
| α-Muurolene | Sesquiterpene hydrocarbon | Essential oil17 |
| α-Farnesene | Sesquiterpene hydrocarbon | Essential oil17 |
| Spathulenol | Sesquiterpene alcohol | Essential oil2 |
| Caryophyllene oxide | Oxygenated sesquiterpene | Essential oil2,17 |
| Carnosic acid | Diterpenoid | Water extract20 |
| Carnosol | Diterpenoid | Water extract20 |
| Ursolic acid | Triterpenoid | Water extract20 |
| Sinapic acid | Phenolic acid | Essential oil1 |
| Vanillic acid | Phenolic acid | Hydroalcoholic extract21 / Essential oil1 |
| Ferulic acid | Phenolic acid | Hydroalcoholic extract21 / Essential oil1 |
| Caffeic acid | Phenolic acid | Hydroalcoholic extract21 / Essential oil1,22 |
| Syringic acid | Phenolic acid | Hydroalcoholic extract21 / Essential oil1 |
| ρ-Hydroxybenzoic acid | Phenolic acid | Hydroalcoholic extract21 / Essential oil1 |
| m-Hydroxybenzoic acid | Phenolic acid | Hydroalcoholic extract21 |
| Coumarinic acid | Phenolic acid | Essential oil1 |
| Gallic acid | Phenolic acid | Hydroalcoholic extract21 |
| Neochlorogenic acid | Phenolic acid | Hydroalcoholic extract21 |
| Protocatechuic acid | Phenolic acid | Hydroalcoholic extract21 |
| Caftaric acid | Phenolic acid | Hydroalcoholic extract21 |
| Rosmarinic acid | Phenolic acid | Ethyl acetate extract8 / Essential oil22 |
| Chlorogenic acid | Phenolic acid | Hydroalcoholic extract21 |
| Cryptochlorogenic acid | Phenolic acid | Hydroalcoholic extract21 |
| Coumaric acid | Phenolic acid | Hydroalcoholic extract21 |
| Lithospermic acid | Phenolic acid | Water extract23 |
| Methyl rosmarinate | Phenolic compound | Hydrophilic extract24 |
| Hydroquinone | Phenolic compound | Ethyl acetate extract8 / Essential oil10 |
| Arbutin | Phenolic glycosides | Ethyl acetate extract8 / Essential oil10,25 |
| Methyl arbutin | Phenolic glycoside | Essential oil10 |
| Vitexin | Phenolic glycoside | Essential oil10 |
| Orientinthymonin | Phenolic glycoside | Essential oil10 |
| Hesperetin | Flavonoid | Ethyl acetate extract8 |
| Catechin | Flavonoid | Hydroalcoholic extract21 |
| Quercetin | Flavonoid | Hydroalcoholic extract21 |
| Kaempferol | Flavonoid | Hydroalcoholic extract21 |
| Naringenine | Flavonoid | Hydroalcoholic extract21 |
| Eriodictyol | Flavonoid | Hydroalcoholic extract21 |
| Diosmetin | Flavonoid | Essential oil10 |
| Luteolin | Flavonoid | Essential oil10 |
| Apigenin | Flavonoid | Essential oil10 |
| 5,6,3′-Trihydroxy-7,8,4′-trimethoxyflavone | Flavonoid | Ethyl acetate extract8 |
| Kaempferol-3-O-glucoside | Flavonoid glycoside | Hydroalcoholic extract21 |
| Quercetin-3-O-glucoside | Flavonoid glycoside | Hydroalcoholic extract21 |
| Narigenin-O-hexoside | Flavonoid glycoside | Hydroalcoholic extract21 |
| Apigenin-glucuronide | Flavonoid glycoside | Water extract23 |
| Rutin | Flavonoid glycoside | Hydroalcoholic extract26 |
| Luteolin-7-O-β-glucuronide | Flavonoid glycoside | Hydrophilic extract24 |
| Eugenol | Phenyl propene | Essential oil13 |
| Ethyl cinnamate | Ester | Essential oil13 |
| Sitosterol | Phytosterol | Essential oil10 |
| Oleanolic acid | Fatty acid | Essential oil10 |
| Vitamin A | Vitamin | Essential oil1 |
| Vitamin C | Vitamin | Essential oil1 |
Essential Oil
Monoterpene hydrocarbons, including α and β-pinene, camphene, sabinene, α- and β- phellandrene, ρ-cymene, limonene, β-ocimene, γ-terpinene, terpinolene, α-terpinene, carvone, and citronellol have been detected in essential oil of O majorana.2,13,14 Terpinene 4-ol and cis-sabinene hydrate are 2 main oxygenated monoterpenes isolated from O majorana.14,15 Linalool, linalyl acetate, α-terpineol, trans- and cis-carveol, thymol, anethole, geraniol, and carvacrol are other oxygenated compounds identified in essential oil and leaves18 of O majorana.13,15,17
Phenolic Compounds
Vanillic acid, gallic acid, ferulic acid, caffeic acid, syringic acid, p- and m-Hydroxybenzoic acid, coumaric acid, neochlorogenic acid, protocatechuic acid, chlorogenic acid, cryptochlorogenic acid, caftaric acid are phenolic acids that have been detected in hydroalcoholic extract of leaves of sweet marjoram.21 Rosmarinic acid, sinapic acid, vanillic acid, ferulic acid, caffeic acid, syringic acid, p- and m-hydroxybenzoic acid, and coumarinic acid have been identified in essential oil of sweet marjoram.1,22 Arbutin, methyl arbutin, vitexin, and orientinthymonin have been reported to be the most predominant phenolic glycosides in essential oil of sweet marjoram.10 Hesperetin, catechin, quercetin, kaempferol, naringenine, eriodictyol, diosmetin, luteolin, and apigenin are the most abundant flavonoids detected in sweet marjoram10,21 and kaempferol-3-O-glucoside, quercetin-3-O-glucoside, narigenin-O-hexoside, and rutin are flavonoid glycosides identified in sweet marjoram.21,26,27
Pharmacological Activities
Table 3 shows pharmacological properties of O majorana in detail.
Table 3.
Pharmacological Properties of Origanum majorana in Detail.
| Pharmacological Activity | Plant part / Extract | Method | Result | Active Constituent |
|---|---|---|---|---|
| Antioxidant20 | Ethanol, n-hexane, supercritical CO2 and water extract of herb | DPPH method and chemiluminometric method | Antioxidant activities of all extracts | Ursolic acid, carnosic acid, carnosol |
| Antioxidant19 | Essential oil | DPPH reduction test | Low antioxidant activity with EC50 values >250μg/mL | — |
| Antioxidant17 | Essential oil | (1) DPPH assay (2) Percent inhibition in linoleic acid system (3) Bleaching of β-carotene | 1)IC50 of 89.2 µg/ml 2) 72.8% inhibition of linoleic acid oxidation 3)showed slow rate of color depletion | — |
| Antioxidant8 | Ethyl acetate extract and isolated compounds | DPPH | Significant antioxidant activities from extract and isolated compounds with IC50 of 2.77 and 1.92 µg/mL, respectively | Hydroquinone |
| Antioxidant22 | Essential oil / Water extract | ABTS + reducing power were examined for their effect against lipid oxidation in comparison to a tea water extract by measurement of the oil stability index | Remarkable capacity in retarding lipid oxidation with oil stability index 13.9 hours | Bound forms of phenolic compounds such as hydroxycinnamic acid and flavonoids |
| Antioxidant21 | Hydroalcoholic extract | ABTS + radical decolorization and DPPH assay | Significant antioxidant capacity with 0.84 and 0.33 mmol TE/g DW, respectively | Polyphenolic compounds |
| Antioxidant28 | Essential oil | Glutathione level and lipid peroxidation content as malondialdehyde in the testis, liver and brain in ethanol treatment male albino rat (ethanol induced reproductive disturbances and oxidative damage in different organs and lipid peroxidation due to the formation of free radicals) | Co-administration of the extract resulted in minimizing the hazard effects of ethanol toxicity on male fertility, liver and brain tissues | — |
| Antioxidant16 | Essential oil | DPPH, .OH, H2O2, reducing power and lipid peroxidation | IC50 values of 58.67, 67.11, 91.25, 78.67, and 68.75 µg/mL, respectively | — |
| Antioxidant29 | Water extract | DPPH | High antioxidant capacity | Phenolic compounds |
| Antioxidant30 | Isolated metabolite | Amyloid β–induced oxidative injury in PC12 nerve cells by MTT, LDH, and trypan blue assays | ↓ Amyloid β–induced neurotoxic effect | Ursolic acid |
| Antioxidant31 | Plant extract | DPPH and ferric ion reducing antioxidant power assays | A direct, positive, and linear relationship between antioxidant activity and total phenolic content of extract | Rosmarinic acid |
| Antimicrobial18 | Dried whole plant/oil/leaves aqueous extract | MIC | Better antimicrobial activity of essential oil rather than water extract; inhibition of yeast and lactic acid bacteria by essential oil at a concentration of 5 ppm | — |
| Antimicrobial32 | Essential oil | ND | The most susceptible organisms were Beneckea natriegens, Erwinia carotovora, and Moraxella sp. and Aspergillus niger | — |
| Antimicrobial26 | n-Hexane extract, aqueous ethanol, ethanolic ammonia extract | Disk-diffusion method for bacteria and serial dilution method for protozoa | n-Hexane extract showed the highest antibacterial activity and the ethanolic ammonia extract reduced the number of viable Pentatrichomonas hominis trophozoites by 50% at 160 µg/ml | — |
| Antimicrobial33 | Methanol extract | Filter paper disk diffusion method | Considerable activity against Aspergillus niger, Fusarium solani, and Bacillus subtilis with zone of inhibition 40, 28 and 42 mm, respectively | — |
| Antimicrobial17 | Essential oil | (1) Disk diffusion (2) Resazurin microtitre-plate | (1) Large zone of inhibition (16.5-27.0 mm) (2) Small MIC against Staphylococcus aureus, Bacillus cereus, B subtilis, Pseudomonas aeruginosa, Salmonella poona, Escherichia coli (40.9-1250.3 μg/mL) | — |
| Antimicrobial15 | Essential oil | Agar diffusion method | Active against Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, and Klebsiella pneumoniae with inhibition zone of 16, 12, 15, and 13 mm, respectively | cis-Sabinene hydrate |
| Antimicrobial19 | Essential oil | Microdilution | Inhibitory activity against Staphylococcus aureus and Streptococcus pyogenes with MICs of 125 and 250 μg/mL, respectively | — |
| Antimicrobial19 | Essential oil | Diffusion assay | Growth inhibitory activity against dermatophytes | — |
| Antimicrobial34 | Methanol extract of leaves | Zone of inhibition | Inhibitory activity against Escherichia coli with 16 mm diameter zone of inhibition | — |
| Anti-inflammatory35 | Essential oil | THP-1 human macrophage cells activated by LPS or human ox-LDL, and the cytokine secretion and gene expression, in vitro | Suppression of production of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6 and IL-10) and COX-2 and NFκB gene expression | Sabinene hydrate, terpineol |
| Anticancer17 | Essential oil | MTT assay | Cytotoxic effect against different cancer cell type, such as MCF-7, LNCaP, NIH-3T3 with IC50 s of 70.0, 85.3, 300.5 µg/ml respectively | — |
| Anticancer36 | Ethanol, methanol and water extract | MTT assay, trypan blue dye exclusion, AO/EB staining and fluorescence microscopical analysis and DNA fragmentation analysis | Significant cytotoxic activity of ethanolic extract on fibrosarcoma cancer cell line HT-1080 and least toxicity on normal human lymphocytes | — |
| Anticancer37 | Plant extract | Nonradioactive cytotoxicity assay on human lymphoblastic leukemia cell line Jurkat | ↓ Viability of cells with increase of concentration of plant extract. Induction of apoptosis through upregulation of p53 protein levels and downregulation of Bcl-2α. Strong radical scavenging activity | — |
| Anticancer38 | Ethanol extract | (1) Matrigel invasion assays (2) Gelatin zymography assay (3) Chick embryo tumor growth assay | (1) Significant inhibition of migration and invasion of the MDA-MB-231 cells. Induction of homotypic aggregation of cells associated with an up regulation of E-cadherin protein and decrease the adhesion of cells to HUVECs and inhibition of transendothelial migration of cells through TNF-α-activated HUVECs (2) Suppression of activities of MMP-2 and MMP-9 (3) Inhibition of tumor growth and metastasis | — |
| Anticancer8 | Ethyl acetate extract and isolated compounds | BrdU cell proliferation enzyme-linked immunosorbent assay and xCELLigence assay against C6 and HeLa cell lines | Strong antiproliferative activities against C6 and HeLa cells | Hesperetin, Hydroquinone |
| Antiplatelet12 | Methanol extract of leaves | Adhesion, aggregation and protein secretion of the activated platelet to laminin-coated plates | 40% inhibition of platelet adhesion to laminin-coated wells by ethanol extract at concentration of 200 µg/mL | — |
| Antiplatelet39 | Methanol extract | Platelet aggregation induced by collagen; ADP, arachidonic acid and thrombin | Strong inhibition of platelet aggregation induced by ADP, arachidonic acid and thrombin | Arbutin |
| Antiulcer27 | Ethanol extract | Hypothermic restraint stress-, indomethacin-, and necrotizing agents–induced ulcers and pylorus ligated Shay rat-model | ↓ Incidence of ulcers, basal gastric secretion and acid output. replenishment of the depleted gastric wall mucus and nonprotein sulfhydryls contents and ↓ malondialdehyde | — |
| Gastric secretory activity40 | Plant extract | Acid and pepsin secretions in normal Wistar rats | ↑ Basal acid and pepsin secretions | — |
| Cardioprotective activity41 | Leaves powder and aqueous extract | Isoproterenol-induced myocardial infarction in rats | Alleviation of erythrocytosis, granulocytosis, thrombocytosis, ↓ clotting time, ↑ relative heart weight, ↓ myocardial oxidative stress and the leakage of heart enzymes. inhibition of NO production and lipid peroxidation in heart tissues | — |
| Hepatoprotective activity10 | Essential oil | Pralletrin-induced oxidative stress in rats (prallethrin caused a significant decrease in the activity of SOD, CAT, and GST in liver of rats) | Depletion of serum marker enzymes and replenishment of antioxidative status | — |
| Antiacetylcholinesterase activities16 | Essential oil | ND | IC50 value was 36.40 µg/mL | — |
| Anticholinesterase activity42 | Ethanol extract | In vitro | The Ki value was 6 pM, and IC50 value was 7.5 nM | Ursolic acid |
| Hormonal activity and regulation of menstrual cycle43 | Water extract | 25 patients were received marjoram tea or a placebo tea twice daily for 1 month. Hormonal and metabolic parameters measured, including FSH, LH, progesterone, oestradiol, total testosterone, DHEA-S, fasting insulin and glucose | ↓ DHEA-S and fasting insulin levels | — |
Abbreviations: ABTS: 2, 2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid); ADP, adenosine diphosphate; CAT, catalase; COX, cyclooxygenase; DHEA-S, dehydroepiandrosterone-sulfate; DPPH, 1,1-diphenyl-2-picryl-hydrazyl; DW, dry weight; EC, effective concentration; FSH, follicle-stimulating hormone; GSH, glutathione S-transferase; IC, inhibitory concentration; IL, interleukin; LDH, lactate dehydrogenase; LH, luteinizing hormone; MIC, minimum inhibitory concentration; MMP, matrix metalloproteinase; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; ND, not determined; NO, nitric oxide; PCOS, polycystic ovary syndrome; SOD, superoxide dismutase; TE, trolox equivalent; TNF, tumor necrosis factor.
Antioxidant Activity
Water extract, essential oil, and ethyl acetate extract of aerial part of O majorana show significant antioxidant activity.8,16,17,22,29 Antioxidant properties were also reported from other extracts of sweet marjoram, including ethanolic, n-hexane, and hydroalcoholic extracta.20 Phenolic compounds such as hydroxycinnamic acid and flavonoids, ursolic acid, carnosic acid, carnosol, rosmarinic acid, and caffeic acid are responsible for antioxidant activity.20,22,30,31
Antimicrobial Activity
Dried whole plant and its essential oil and water extract of leaves have demonstrated antimicrobial effect and essential oil was more active against lactic acid bacteria and yeasts than water extract.18 Essential oil showed inhibitory activity against various pathogenic bacteria and fungi, including Beneckea natriegens, Erwinia carotovera, Moraxella, Aspergillus, Staphylococcus aureus, Streptococcus pyogenes, Bacillus cereus, B subtilis, Pseudomonas aeruginosa, Salmonella poona, Escherichia coli, and dermatophytes.15,17,19,32 Methanol extract of sweet marjoram exhibited antimicrobial activity against E, Aspergillus niger, Fusarium solani, and Bacillus subtilis.33,34 The ethanolic ammonia extract reduced the number of viable Pentatrichomonas hominis trophozoites.26 cis-Sabinene hydrate in essential oil of sweet marjoram have been claimed to be responsible for antibacterial effect.15
Anti-inflammatory Activity
Sabinene hydrate and terpineol in essential oil of sweet marjoram suppressed the production of Tumor necrosis factor-α (TNFα), interleukin 1β (IL-1β), IL-6, and IL-10 inhibited cyclooxygenase 2 (COX2) and NFκB gene expression.35
Anticancer and Antiproliferative Properties
Ethanol extract of plant have shown significant cytotoxicity against fibrosarcoma cancer cell line, promoting cell cycle arrest and apoptosis of the metastatic breast cell and inhibited the migration and invasion of the MDA-MB-231 cells.36,38 Ethyl acetate extract have strong antiproliferative activities against C6 and HeLa cells. Hesperetin and hydroquinone isolated from sweet marjoram extract have revealed strong antiproliferative activity.8
Antiplatelet Activity
Methanol extract of sweet marjoram leaves inhibit adhesion of platelet to laminin-coated plate12 and strongly inhibited platelet aggregation induced by adenosine diphosphate (ADP), arachidonic acid, and thrombin. Arbutin is responsible for this activity.39
Antiulcerogenetic Effect
Ethanol extract of sweet marjoram significantly decreased the incidence of ulcers, basal gastric secretion, and acid output and replenished the depleted gastric wall mucus.27
Cardioprotective and Hepatoprotective Activity
Leave powder and extract significantly alleviated erythrocytosis, granulocytosis, thrombocytosis, increase heart weight, and myocardial infarction oxidative stress in isoproterenol treated albino rats.41 Essential oil of sweet marjoram depleted serum marker enzymes and replenished antioxidant status in hepatic of rat.10
Anticholinesterase Inhibitory Activity
Essential oil and ethanol extract of sweet marjoram have exhibited anticholinesterase inhibitory activity.16 Ursolic acid is responsible for this effect.42
Regulation of Menstrual Cycle
Sweet marjoram tea significantly reduced dehydroepiandrosterone-S (DHEA-S) and was useful in treatment of polycystic ovary syndrome.43
Toxicity
Acute toxicity test has demonstrated a large margin of safety of O majorana extract in mice. Emmenagogue properties of sweet marjoram should be concerned during pregnancy.11 Its essential oil must not be used by lactating and pregnant women.44
Conclusion
Sweet marjoram is a medicinal plant with various proven pharmacological properties, including antioxidant, antibacterial, hepatoprotective, cardioprotective, antiulcer, anticoagulant, anti-inflammatory, antiproliferative, and antifungal activities. The flowering stems are the medicinal parts. Their constituents include 1% to 2% of an essential oil with a containing terpinenes and terpinols, plus tannins, bitter compounds, carotenes, and vitamin C. These substances give sweet marjoram stomachic, carminative, antispasmodic, and weak sedative properties.
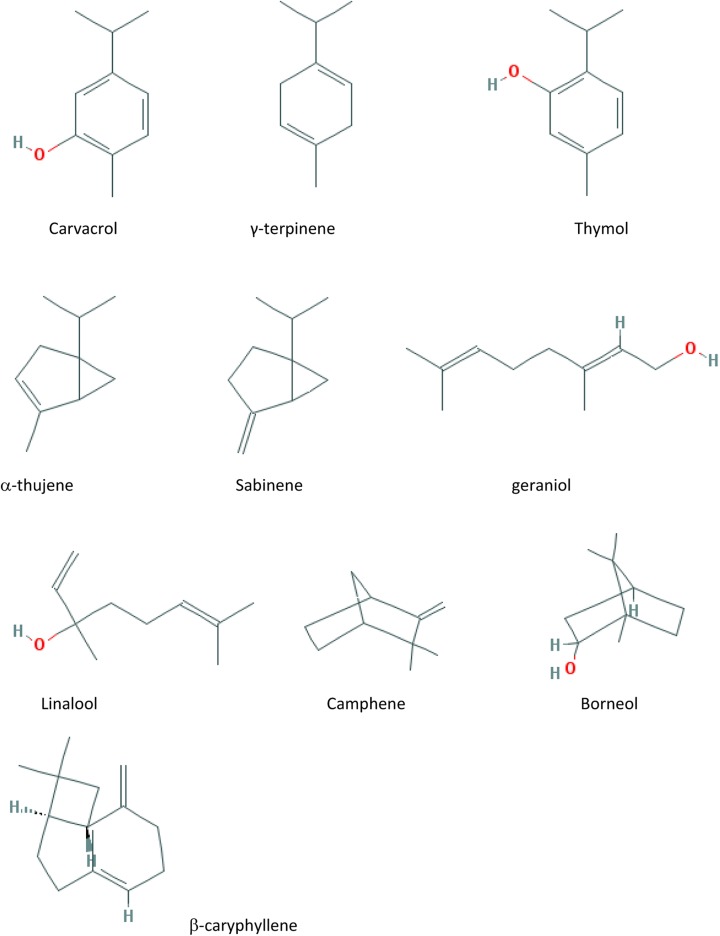
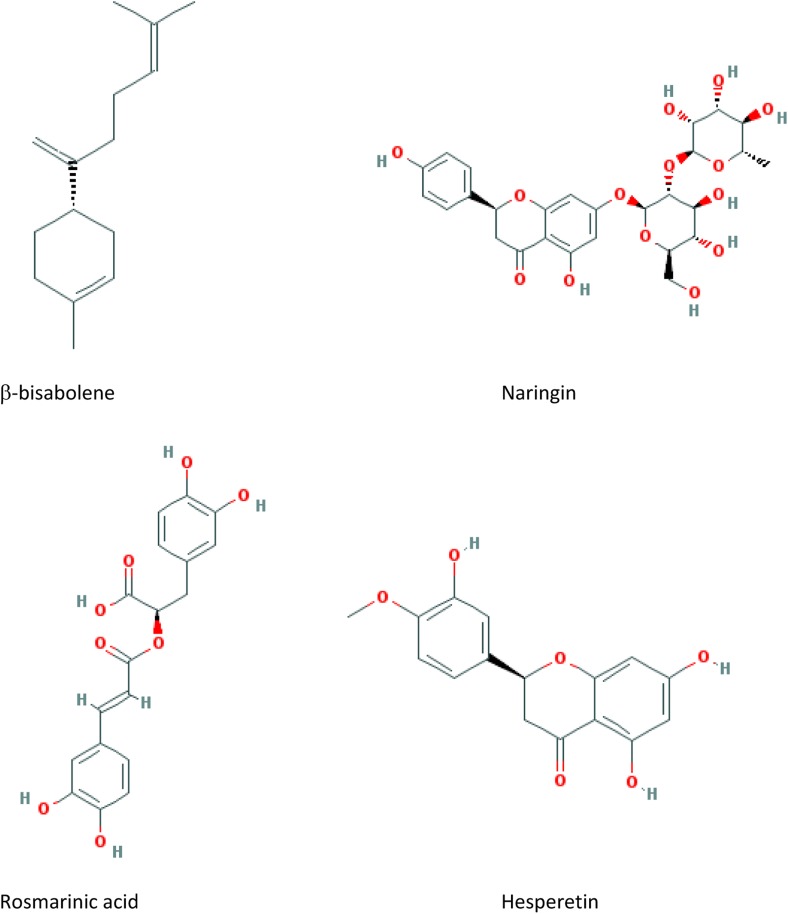
In herbalism, it is used mainly for various gastrointestinal disorders and to aid digestion. Novel investigations showed increase in acid and pepsin secretions by this plant. Also sweet marjoram showed antiulcer activity and mucus protecting effects in gastrointestinal tract. Ethnomedicinal use of O majorana on vaginitis and polycystic ovarian disease can be related to restoration of hormonal balance and reduction of DHEA-S by this plant. Efficacious uses of O majorana in cardiac disease and dysrhythmia were proved which may be related to its antiplatelet and cardioprotective activities through inhibition of production of nitric oxide and lipid peroxidation in heart tissues. Useful effect on head cool, sniffle, ear pain, and respiratory disorders may be related to its antimicrobial effect. Monoterpene hydrocarbons (such as α-pinene, β-pinene, camphene, and γ-terpinene), oxygenated monoterpenes particularly terpinene-4-ol, cis-sabinene hydrate and terpineol, phenolic compounds particularly flavonoids (such as apigenin, hesperetin, quercetin, kaempferol), and phenolic glycosides (such as arbutin) are the active components isolated and detected in O majorana. Figure 1 shows the structure of some main active compounds. Various bioactive compounds have been isolated and identified in O majorana, whereas many active compounds responsible for ethnomedicinal uses or proved pharmacological activities have not been completely evaluated. Therefore, new investigations are proposed to isolate, identify and obtain the O majorana active compounds in order to explore novel natural component for rectifying the stalemate on the way of modern medicine.
Figure 1.
The structure of some main active compounds of Origanum majorana.
Footnotes
Author Contributions: RR designed the study and edited the manuscript. FB collected data and wrote the manuscript.
Ethical Approval: This study did not need ethical approval as no animal or human subjects were involved.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Charles DJ. Marjoram sweet. In: Antioxidant Properties of Spices, Herbs and other Sources. New York, Springer; 2013:393–399. [Google Scholar]
- 2. Lis A, Piter S, Gora J. A comparative study on the content and chemical composition of essential oils in commercial aromatic seasonings. Herba Polonica. 2007;53:21–26. [Google Scholar]
- 3. Nahida J, Wissal D, Thouraya et al. Essential oil composition of Origanum majorana leaves. INIST-CNRS. 2007;1:190–193. [Google Scholar]
- 4. Aghili MH. In: Rahimi R, Shams Ardekani MR, Farjadmand F, eds. Makhzan-al-Advia. Tehran, Iran: Tehran University of Medical Sciences; 2009:735. [Google Scholar]
- 5. Tonkaboni MM. In: Rahimi R, Shams Ardekani MR, Farjadmand F, eds. Tohfeh-al-Momenin. Tehran, Iran: Shahid Beheshti University of Medical Sciences; 2007:290–291. [Google Scholar]
- 6. Alakbarov F. Aromatic herbal baths of the ancients. HerbalGram American Botanical Council. 2003;57:40–49. [Google Scholar]
- 7. Vogl S, Picker P, Mihaly-Bison J, et al. Ethnopharmacological in vitro studies on Austria’s folk medicine—an unexplored lore in vitro anti-inflammatory activities of 71 Austrian traditional herbal drugs. J Ethnopharmacol. 2013;149:750–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Erenler R, Sen O, Aksit H, et al. Isolation and identification of chemical constituents from Origanum majorana and investigation of antiproliferative and antioxidant activities. J Sci Food Agric. 2016;96:822–836. [DOI] [PubMed] [Google Scholar]
- 9. Tahraoui A, El-Hilaly J, Israili ZH, Lyoussi B. Ethnopharmacological survey of plants used in the traditional treatment of hypertension and diabetes in south-eastern Morocco (Errachidia province). J Ethnopharmacol. 2007;110:105–117. [DOI] [PubMed] [Google Scholar]
- 10. Mossa AT, Refaie AA, Ramadan A, Bouajila J. Amelioration of prallethrin-induced oxidative stress and hepatotoxicity in rat by the administration of Origanum majorana essential oil. Biomed Res Int. 2013;2013:859085 doi:10.1155/2013/859085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Always Ayurveda: A Sister Concern of Planet Ayurveda. Origanum majorana. http://www.alwaysayurveda.com/origanum%20majorana/. Accessed May 4, 2016.
- 12. Yazdanparast R, Shahriyary L. Comparative effects of Artemisia dracunculus, Satureja hortensis and Origanum majorana on inhibition of blood platelet adhesion, aggregation and secretion. Vascul Pharmacol. 2008;48:32–37. [DOI] [PubMed] [Google Scholar]
- 13. El-Moursi A, Talaat IM, Balbaa LK. Physiological effect of some antioxidant polyphenols on sweet marjoram (Majorana hortensis) plants. Nusantara Biosci. 2012;4:11–15. [Google Scholar]
- 14. Vera RR, Chane-Ming J. Chemical composition of the essential oil of marjoram (Origanum majorana L.) from Reunion Island. Food Chem. 1999;66:143–145. [Google Scholar]
- 15. Ramos S, Rojas LB, Lucena ME, et al. Chemical composition and antibacterial activity of Origanum majorana L. essential oil from the Venezuelan Andes. J Essential Oil Res. 2011;23(5):45–49. [Google Scholar]
- 16. Mossa AT, Nawwar GA. Free radical scavenging and antiacetylcholinesterase activities of Origanum majorana L. essential oil. Hum Exp Toxicol. 2011;30:1501–1513. [DOI] [PubMed] [Google Scholar]
- 17. Hussain AI, Anwar F, Rasheed Sh, et al. Composition, antioxidant and chemotherapeutic properties of the essential oils from two Origanum species growing in Pakistan. Rev Bras Farmacogn. 2011;21(6). doi:10.1590/S0102-695X2011005000165. [Google Scholar]
- 18. Charai M, Mosaddak M, Faid M. Chemical composition and antimicrobial activities of two aromatic plants: Origanum majorana L. and O. compactum Benth. J Essential Oil Res. 1996;8:657–664. [Google Scholar]
- 19. Guerra-Boone L, Alvarez-Roman R, Salazar-Aranda R, et al. Antimicrobial and antioxidant activities and chemical characterization of essential oils of Thymus vulgaris, Rosmarinus officinalis, and Origanum majorana from northeastern Mexico. Pak J Pharm Sci. 2015;28:363S–369S. [PubMed] [Google Scholar]
- 20. Vagi E, Rapavi E, Hadolin M, et al. Phenolic and triterpenoid antioxidants from Origanum majorana L. herb and extracts obtained with different solvents J Agric Food Chem. 2005;53:17–21. [DOI] [PubMed] [Google Scholar]
- 21. Vallverdu Queralt A, Regueiro J, Rinaldi Alvarenga JF, et al. Characterization of the phenolic and antioxidant profiles of selected culinary herbs and spices: caraway, turmeric, dill, marjoram and nutmeg. Food Sci Technol (Campinas). 2015;35(1).doi:10.1590/1678-457X.6580. [Google Scholar]
- 22. Triantaphyllou K, Blekas G, Boskoua D. Antioxidative properties of water extracts obtained from herbs of the species Lamiaceae. Int J Food Sci Nutr. 2001;52:313–317. [DOI] [PubMed] [Google Scholar]
- 23. Kaiser A, Carle R, Kammerer DR. Effects of blanching on polyphenol stability of innovative paste-like parsley (Petroselinum crispum (Mill.) Nym ex A. W. Hill) and marjoram (Origanum majorana L.) products. Food Chem. 2013;138:1648–1656. [DOI] [PubMed] [Google Scholar]
- 24. Fecka I, Turek S. Determination of polyphenolic compounds in commercial herbal drugs and spices from Lamiaceae: thyme, wild thyme and sweet marjoram by chromatographic techniques. Food Chem. 2008;108:1039–1053. [DOI] [PubMed] [Google Scholar]
- 25. Lukas B, Schmidere C, Mitteregger U, Novak J. Arbutin in marjoram and oregano. Food Chem. 2010;121:185–190. [Google Scholar]
- 26. Kozlowska M, Laudy AE, Starosciak BJ. Antimicrobial and antiprotozoal effect of sweet marjoram (Origanum majorana L.). Acta Sci Pol Hortorum Cultus. 2010;9:133–141. [Google Scholar]
- 27. Al-Howiriny T, Alsheikh A, Alqasoumi S, et al. Protective effect of Origanum majorana L. ‘Marjoram’ on various models of gastric mucosal injury in rats. Am J Chin Med. 2009;37:531–545. [DOI] [PubMed] [Google Scholar]
- 28. El-Ashmawy IM, Saleh A, Osama M. Effects of marjoram volatile oil and grape seed extract on ethanol toxicity in male rats. Basic Clin Pharmacol Toxicol. 2007;101:320–327. [DOI] [PubMed] [Google Scholar]
- 29. Chrpova D, Kourrimska L, Gordon MH, et al. Antioxidant activity of selected phenols and herbs used in diets for medical conditions. Czech J Food Sci. 2010;28:317–325. [Google Scholar]
- 30. Heo HJ, Cho HY, Hong B, et al. Ursolic acid of Origanum majorana L. reduces Abeta-induced oxidative injury. Mol Cells. 2002;13:5–11. [PubMed] [Google Scholar]
- 31. Hossain MB, Camphuis G, Aguilo-Aguayo I, et al. Antioxidant activity guided separation of major polyphenols of marjoram (Origanum majorana L.) using flash chromatography and their identification by liquid chromatography coupled with electrospray ionization tandem mass spectrometry. J Separation Sci. 2014;37:3205–3213. [DOI] [PubMed] [Google Scholar]
- 32. Deans SG, Svoboda KP. The antimicrobial properties of marjoram (Origanum majorana L.) volatile oil. Flavour Frag J. 1990;5:187–190. [Google Scholar]
- 33. Leeja L, Thoppil JE. Antimicrobial activity of methanol extract of Origanum majorana L. (sweet marjoram). J Environ Biol. 2007;28:145–146. [PubMed] [Google Scholar]
- 34. Bonjar GHS. Screening for antibacterial properties of some Iranian plants against Escherichia coli. Asian J Plant Sci. 2004;3:310–314. [Google Scholar]
- 35. Arranz E, Jaime L, Lopez MC, et al. Supercritical fluid extraction as an alternative process to obtain essential oils with anti-inflammatory properties from marjoram and sweet basil. Ind Crop Prod. 2015;67:121–129. [Google Scholar]
- 36. Rao Sh, Timsina B, Nadumane VK. Evaluation of the anticancer potentials of Origanum marjorana on fibrosarcoma (HT-1080) cell line. Asian Pac J Trop Dis. 2014;4:S389–S394. [Google Scholar]
- 37. Abdel-Massih RM, Fares R, Bazzi S, et al. The apoptotic and anti-proliferative activity of Origanum majorana extracts on human leukemic cell line. Leuk Res. 2010;34:1052–1056. [DOI] [PubMed] [Google Scholar]
- 38. Al Dhaheri Y, Attoub S, Arafat K, et al. Anti-metastatic and anti-tumor growth effects of Origanum majorana on highly metastatic human breast cancer cells: inhibition of NFκB signaling and reduction of nitric oxide production. PLoS One. 2013;8(7). doi:10.1371/journal.pone.0068808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Okazaki K, Nakayama Sh, Kawazoe K. Antiaggregant effects on human platelets of culinary herbs. Phytother Res. 1998;12:603–605. [Google Scholar]
- 40. Rafsanjani FN, Shahrani M, Ardakani ZV, Ardakani MV. Marjoram increases basal gastric acid and pepsin secretions in rats. Phytother Res. 2007;21:1036–1038. [DOI] [PubMed] [Google Scholar]
- 41. Ramadan G, El-Beih NM, Arafa NM, Zahra MM. Preventive effects of Egyptian sweet marjoram (Origanum majorana L.) leaves on hematological changes and cardiotoxicity in isoproterenol-treated albino rats. Cardiovasc Toxicol. 2013;13:100–109. [DOI] [PubMed] [Google Scholar]
- 42. Chung YK, Heo HJ, Kim EK, et al. Inhibitory effect of ursolic acid purified from Origanum majorana L on the acetyl cholinesterase. Mol Cells. 2001;11:137–143. [PubMed] [Google Scholar]
- 43. Haj-Husein I, Tukan S, Alkazaleh F. The effect of marjoram (Origanum majorana) tea on the hormonal profile of women with polycystic ovary syndrome: a randomized controlled pilot study. J Hum Nutr Diet. 2016;29:105–111. doi:10.1111/jhn.12290. [DOI] [PubMed] [Google Scholar]
- 44. Ernst E. Herbal medicinal products during pregnancy: are they safe? BJOG. 2002;109:227–235. [DOI] [PubMed] [Google Scholar]