Abstract
We aimed to evaluate the antidiarrheal effect of black tea in pediatric patients with acute nonbacterial diarrhea. This single-blind randomized clinical trial study was performed on 2 to 12-year-old patients, with acceptable criteria for acute nonbacterial diarrhea in Shiraz, Iran. In total, 120 patients took part in this study. Blocked randomization method was used to allocate them into 2 groups of intervention (black tea tablet + standard treatment) and control group (standard treatment; 60 patients in each). Frequency of defecation, volume, and consistency of stool were registered on arrival and 24 hours later. We used χ2 test, t test, and Mann-Whitney U test. After a 24-hour follow-up, the proportion of patients with formed stool was higher in the intervention group when compared with the control group (P < .001). There was a significant difference between the mean number of defecations per 24 hours in both groups, after treatment (P < .001). We found a possible antidiarrheal effect of black tea.
Keywords: diarrhea, Camellia sinensis, pediatrics, outpatients, tea
Diarrhea is among the diseases that are major causes of mortality of under 5 children around the world, especially in developing countries, as 2195 children die of diarrhea every day, which is more than the deaths caused by other major killers such as AIDS, malaria, and measles.1 In Iran, diarrhea is the fifth leading infectious cause of mortality among children.2 Despite the application of effective prevention methods such as sanitation and hygiene improvement, vaccines, oral rehydration therapy, zinc supplement, and breastfeeding, it still disproportionately affects the children of poor developing countries, who may have limited access to these services.3
Complementary and alternative methods of treatment have increasingly gained attention in various diseases, including diarrhea. The effects of various traditional herbal remedies have been studied on acute diarrhea with some of them showing to be effective.4
The effects of savory on treatment of diarrhea, regressive effect of oregano on contractions of ileum and treatment of diarrhea, antisecretory effect of manna herb, and therapeutic effect of pennyroyal plant on diarrhea have been reported.5
Black tea is one of the most consumed beverages in the world, and especially in Iran.6 In Iranian traditional medicine, using black tea has been recommended to treat diarrhea. In this regard, the famous medical book Makhzan-ul-advia has pointed to the therapeutic and antidiarrheal effect of black tea.7 In addition, an Indian herbal medicine book has mentioned the antidiarrheal effect of black tea.8
Polyphenolic compounds in black tea undergo enzyme oxidation and convert into pigments called thearubigins and theaflavins. The beneficial effects of black tea in the treatment of peptic ulcer and the impacts of thearubigins pigment in the treatment of digestive disorders such as inflammatory bowel disease has been shown in a previous study.9 Another research conducted on the effects of black tea extract on bowel movement has shown that this extract can increase or decrease gastrointestinal motility in a dose-dependent way; hence, it can be used to treat gastrointestinal motility disorders such as diarrhea and constipation.10,11 It should be noted that all the studies mentioned above were animal studies, and before we conducted our clinical trial there was no human study about the antidiarrheal effect of black tea.
It is noteworthy to mention that no significant acute and subchronic toxicity of Camellia sinensis has been reported in rats.12 But, long time consumption of black tea can induce anorexia and anemia in humans.13
Regarding the popularity and accessibility of tea beverage around the world and lack of enough knowledge on its probable antidiarrheal benefits, we aimed to evaluate the effect of black tea (Camellia sinensis L Kuntze) on nonbacterial diarrhea including stool consistency, volume, and frequency of defecation per 24 hours in pediatrics patients.
Methodology
This was a single-blind clinical trial with parallel design conducted on pediatric patients aged 2 to 12 years with acute nonbacterial diarrhea referred to Dastgheib Hospital, Shiraz, in 2011. Ethical consideration of this study was confirmed by the Ethical Committee of Shiraz University of Medical Sciences (Approval No. 5617-90). This study is registered in Iranian Registry for Clinical Trials with code number IRCT201108272434N6.
The eligibility criteria were pediatric patients aged 2 to 12 years, who have had 3 or more loose stools per 24 hours for less than 14 days, had been referred to the hospital, and ought to have been managed as outpatients. According to the primary clinical judgment based on the patients’ symptoms, the majority of these patients had viral diarrhea. The exclusion criteria were those with underlying diseases including diabetes, immunodeficiency, and digestive diseases such as celiac, lactase deficiency, or malabsorption. Moreover, the patients with symptoms of severe vomit leading to oral treatment intolerance, fever >39°C, severe abdominal cramps, and bloody diarrhea were excluded. Needing hospitalization and not being within reaching distance for the next 24 hours were the other exclusion criteria. A trained research physician visited the patients for their eligibility to be included in the study. Written and informed consent from the parents or guardians of the children was obtained before their enrollment in the study. The research physician then collected the patient’s information including demographic data, disease history (duration of diarrhea and symptoms), and the characteristics of diarrhea (stool consistency, frequency, and volume) according to a checklist, on entering the trial, and then allocated the patient blindly to each treatment group.
We used blocked randomization method with blocks of size 4 with equal number of treatments A and B in each block, in order to have treatment arms with similar number of patients. Random number generator was used for generating a sequence of digits; each represented a block of successive number of 4 patients. A researcher from outside the hospital and not involved in the process of allocating the patients did the randomization. The treatment group was written on a card and sealed in numbered pockets, in order to assure the blinding process.
Accordingly, tablets containing 500 mg of dry black tea were prepared by pharmacists at the School of Pharmacy, Shiraz University of Medical Sciences. For tablets to be uniform and compressed as well as to be dissolved in time in the digestive tract, certain amounts of permitted additives including binding and disintegrating agents approved by British Pharmacopoeia, with no reported antidiarrhea effect, were used in the tablet. In this study, direct compressed tea tablets were used. The finishing weight of each tablet reached to 750 mg at the end. Beside dry black tea (500 mg of total weight), 3 other additives, avicel (15% of the black tea weight), magnesium stearate (5% of the black tea weight), and sodium starch monohydrate (20% of the black tea weight), were used in the studied tablet.
The 2 groups of treatment were group A (receiving black tea tablets in addition to the standard therapy) and group B (control; receiving just the standard treatment). We considered oral rehydration therapy by oral rehydration supplement and the administration of zinc syrup supplement (ages 2-6 [2.5 mL/day] and 6-12 [5 mL/day]) as standard treatment.14,15 Children aged 2 to 6 years received 1 black tea tablet, and the older children, aged 6 to 12 years, received 2 black tea tablets in a single dose in the presence of the researchers. Due to the difficulty of taking pills in young children, it was dissolved in water to form a suspension, as the tablet is soluble in water. We considered using compressed black tea tablets rather than regular steeped black tea because it was possible to control the consistency and similarity of dose of the active ingredient. Also, it was easier to assure similar use by each patient as the method of preparing tea and scaling the required amount can be different from person to person. Moreover, the concentration of steeped tea differs a few times after its preparation and it was not possible to refresh it for each patient at the hospital.
Another trained research physician who was unaware of the treatment group of each patient performed the follow-up of patients after 24 hours of receiving treatment and recorded the relevant information. The 2 examiners were trained in the beginning of the study and they used the same checklist to assure the consistency of data gathering.
Three characteristics of stool—volume (cup per defecation), stool consistency, and number of defecations per 24 hours reported by parents of the patients—were used to assess the stool form and were considered as the outcome measures. The duration of diarrhea was not an outcome measure, it was only took into account for exclusion of chronic (more than 14 days) of diarrhea.
The data were analyzed using SPSS software Version 20; χ2 test, independent and paired t test, and Mann-Whitney U test were used for data analysis, and P < .05 was considered to be statistically significant. We used per protocol analysis, and only patients who completed the treatment protocol were included in the statistical analysis.
Results
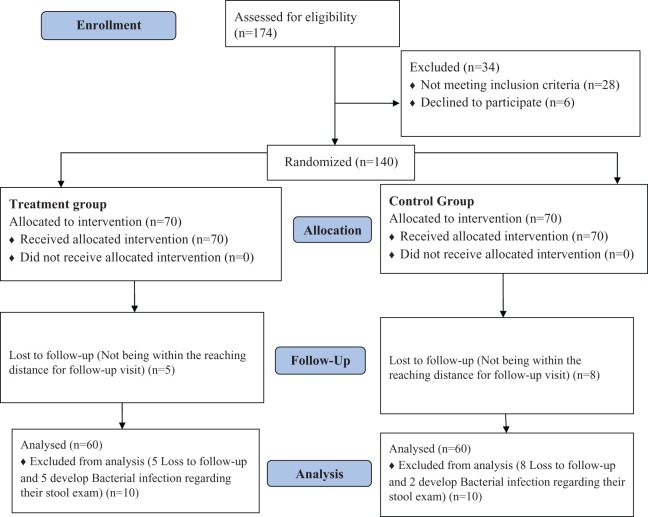
We assessed 174 patients for eligibility; 28 were excluded because of not meeting the eligibility criteria and 6 patients were declined to participate. Finally, 140 patients were selected to take part in the study. In the follow-up visit 20 patients were excluded after treatment, because of unavailability and worsening of their general condition and suspicion to bacterial infection, which was confirmed by presence of red blood cells and pus cells in their stool examinations. Sixty patients remained in each treatment group. Patients’ allocation process is shown in Figure 1. Mean age ± standard deviation of the patients was 4.35 ± 2.18 years. Seventy of them were male (58.3%) and 50 (41.7%) were female patients. The majority of the participants (115; 95.8%) were residences of Shiraz city and only 5 (4.2%) were from other counties in Fars province. The mean ± standard deviation duration of the participants’ symptoms from the onset to referral to the hospital was 2.92 ± 1.57 days. Patients’ demographic information with respect to treatment groups are shown in Table 1. There was no significant difference between demographic characteristics of group A (intervention) and group B (control) patients.
Figure 1.
Flow diagram of patients’ allocation process.
Table 1.
Patients’ Demographic Information With Regard to Treatment Groups A and B.
| Parameter | Treatment Group | P | |
|---|---|---|---|
| Group A | Group B | ||
| Gender | .711 | ||
| Female, n (%) | 24 (40%) | 26 (43.3%) | |
| Male, n (%) | 36 (60%) | 34 (56.7%) | |
| Age, mean ± SD (minimum, maximum) | 4.55 ± 1.83 (2-10) | 4.14 ± 2.48 (2-12) | .298 |
| Living place | .648 | ||
| Shiraz | 58 (96.7%) | 57 (95%) | |
| Other | 2 (3.3%) | 3 (5%) | |
| Symptom duration (days), mean ± SD (minimum, maximum) | 3.08 ± 1.55 (1-7) | 2.76 ± 1.59 (1-7) | .274 |
Thirty-two (53.3%) patients from intervention group and 36 patients (60%) from control group had fever of 38°C oral temperature. In group A, 29 (48.3%) and 33 (55%) patients, and in group B, 37 (61.7%) and 43 (71.1%) patients, had complaint of abdominal pain and vomiting, respectively. In addition, history of flatulence was noted in 32 (53.3%) patients in group A and in 36 patients (60%) in group B. No patient mentioned dysentery. There was no significant difference in the symptoms of patients on entering the hospital in the intervention and control groups. Patients’ symptoms with regard to intervention and control groups are shown in Table 2.
Table 2.
Patients’ Symptoms on Entering the Study With Regard to Intervention (A) and Control (B) Groups.
| Patients’ Symptoms | Group A, n (%) | Group B, n (%) | P |
|---|---|---|---|
| Fever | 32 (53.3%) | 36 (60%) | .58 |
| Abdominal pain | 29 (48.3%) | 37 (61.7%) | .19 |
| Vomiting | 33 (55%) | 43 (71.7%) | .08 |
| Flatulence | 23 (38.3%) | 31 (51.7%) | .19 |
| Rhinorrhea | 8 (13.3%) | 21 (35%) | .1 |
None of the patients from both intervention and control groups reported the history of medication use for their current situation. Among the patients, 2 cases from the intervention group and 1 case from the control group had background diseases including glucose-6-phosphate-dehydrogenase deficiency and anemia. Totally, 9 patients reported a history of travelling within the last 2 weeks (4 cases from the intervention group and 5 cases from the control group).
Three characteristics of stool, volume (cup per defecation), stool consistency, and number of defecations per 24 hours, were used to assess the stool form. Ten (16.7%) patients of the tea treatment group had loose stool, 27 (45%) had watery stools, and 23 (38.3%) had very watery stools. Eighteen (30%) patients from the standard treatment group had loose stool, 29 (48.3%) had watery stools, and 13 (21.7%) had very watery stool. There was no statistically significant difference in stool form between the 2 treatment groups in the beginning of the study (P = .07).
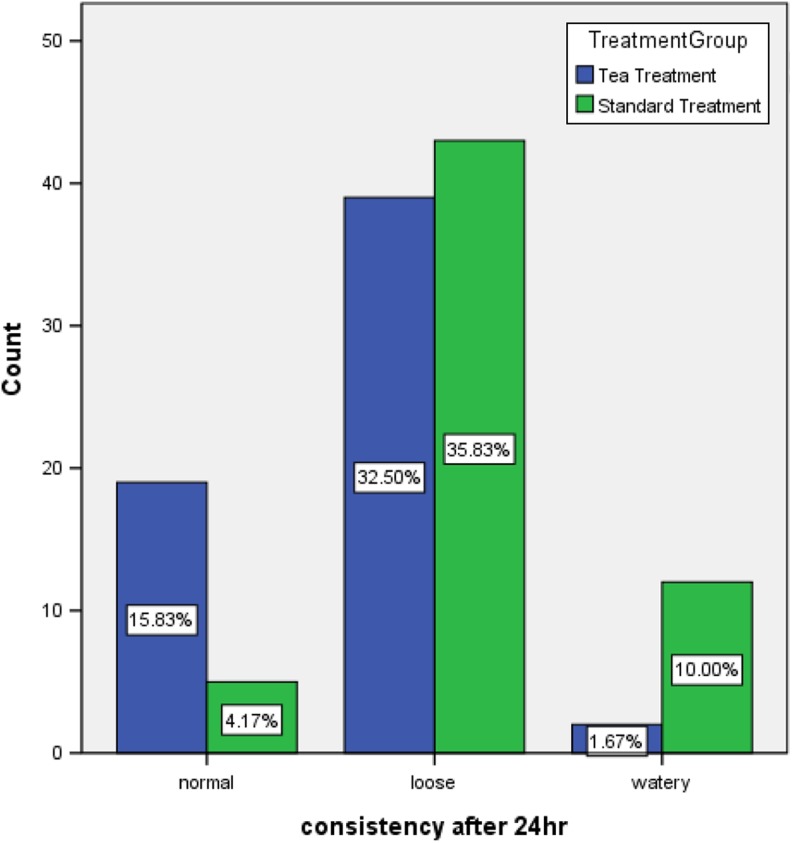
In the 24-hour follow-up visit, 19 patients (31.7%) had formed stool, 39 (65%) had loose stool, and 2 (3.3%) had watery stool in the intervention group. In addition, 5 (8.3%), 43 (71.7%), and 12 (20%) patients of the control group had normal, loose, and watery stools, respectively. Significant differences between pre- and posttreatment stool consistency were observed in both groups (P < .001; Figure 2).
Figure 2.
Comparison between stool form 24 hours after treatment, in the intervention and control groups.
Considering the incidence rate of formed stool in the treatment and control groups, 19/60 and 5/60, respectively, the number needed to treat is calculated, and it is approximately equal to 4.
At the beginning of the study, 5 (8.3%), 29 (48.3%), and 26 (43.3%) patients of the intervention group (black tea tablet group) had low (lower than half a cup), medium (half a cup to one cup), and high stool volume (more than a cup), respectively. In addition, 9 (15%), 41 (68.3%), and 10 (16.7%) patients of the control group had low, medium, and high stool volume, respectively. There was a significant difference between the stool volume of the intervention (black tea tablet group) and control groups prior to the treatment (P = .006). In the 24-hour follow-up visit, 45 (75%) and 15 (25%) patients of the intervention group had low and medium stool volume, and the number of patients with high stool volume decreased to zero. In addition, from the control group, the stool volume of 39 (65%) patients was low, 20 (33.3%) patients had medium, and 1 patient (1.7%) had high stool volume. There was no statistically significant differences between stool volumes after 24 hours of treatment between the intervention and control groups (P = .232).
The mean number of loose/watery defecation per 24 hours was 5.7 times pretreatment (minimum 3, maximum 10), and 6 times (maximum 10, minimum 3) in patients of the tea and standard treatment groups, respectively. In the 24-hour follow-up visit, these values changed to 2.26 (maximum 5, minimum 1) and 3.45 times (maximum 7, minimum 1) in the intervention and control groups, respectively. The mean number of loose/watery defecations was significantly reduced in both intervention and control groups in pre- and posttreatment time (P < .001). In addition, the mean number of loose/watery defecations were significantly lower in the intervention group than in the control group, after treatment (P < .001).
Discussion
Our study was the first to investigate the antidiarrheal effect of black tea in pediatric patients. In pharmaceutical evaluation, tea tablets were gently disintegrated in water and changed the color of water gradually. It is considerable that the main fraction of tea tablets that could act in this study is tannins. These components are highly soluble in water and possess astringent activity. We found that the patients who had used tea tablets in addition to the standard therapies had better results after 24 hours of follow-up. In the way, the proportion of the patients whose stool consistency had been transformed from loose or watery to formed stool was higher in the intervention group compared to the control group. Moreover, there was a decrease in defecation frequency and stool volume in the intervention group. We can see that there was an overall change to better situation in bowel habit after 24 hours in the intervention group compared to the control group. Although literature on the effect of Camellia sinensis (L) Kuntze on acute diarrhea is scarce, Besra et al showed the antidiarrheal concentration-dependent effect of black tea on Syrian mice.10
In addition, comparing the effect of black tea on diarrhea with that of loperamide and naloxone showed that the effect of black tea was similar to that of loperamide, and naloxone significantly inhibited the prokinetic activity of black tea extract as well as loperamide.9,10,16 In addition, it has been reported that black tea extract had an independent effect on lowering the diarrhea prevalence in piglets.17
There is no human clinical studies indicating the antidiarrheal effect of black tea.
We found that from every 3 patients treated by tea tablet plus standard therapy one proceed to have formed stool. However, standard treatment in 12 patients will lead to one of them having formed stool, although it is not clear whether black tea has synergistic or additive effect if taken simultaneously with other conventional medications. Increase in peristaltic activity of gastrointestinal tract and permeability changes in the intestinal mucosal membrane to electrolytes and water are seen in diarrhea conditions. In addition, these events are associated with prostaglandin release, which is the major cause of arachidonic acid–induced diarrhea. It seems that antidiarrheal activity of black tea may be due to inhibition of prostaglandin synthesis.10,16,18
This study had some limitations, the most important of which was the limited sample. Furthermore, since the present study was the first clinical trial to study the effect of black tea on human subjects, we choose just one dose according to indigenous medicine books, and no acute complications in patients occurred. Another limitation was the lack of blinding of the patients and not using placebo for the control group. Thus, future studies may be conducted on larger samples and with different doses and double-blind design, so that results that are more precise can be achieved regarding the use of these medications. Although there was a minimal difference between the 2 groups according to the sickness of patients at the first point, it was not statistically significant. Also, the sampling was done by randomization and this difference may not affect the validity of the results. Moreover, we have used per protocol analysis in this study. In per protocol analysis patients who deviated from the first assignment are excluded. It may lead to the groups of patients being compared no longer having similar characteristics. The results of this analysis usually provide a lower level of evidence. On the other hand, it better shows the effects of treatment when it is taken in an ideal mode. In our study, 10 patients were excluded for the analysis in each group due to loss to follow-up and developing bacterial diarrhea. As the excluded number was similar in both groups and there was no exchange of assigned treatment between them, we have assumed that per protocol analysis might not have a great impact on the results of the study.
Conclusion
Given the tendency of people toward traditional medicine and using herbal remedies, the findings of this study indicate that the use of the black tea tablet along with conventional drugs seems to be an effective, inexpensive, and safe treatment for nonbacterial acute diarrhea management. Further studies are needed to generalize these results.
Acknowledgment
The authors are thankful to Dr M. R. Dehghani, Dr Golzadeh, and Research Center for Traditional Medicine and History of Medicine of Shiraz University of Medical Science for providing necessary facilities for preparation of our article.
Footnotes
Author Contributions: Sareh Doustfatemeh contributed in the data collection, patient allocation and writing some parts of the article. Mohammad Hadi Imanieh, Abdolali Mohagheghzade and Gholamhossein Yousefi contributed in editing and proofing the final manuscript. Zahra Torkamani did parts of data gathering and final manuscript edit. Saman Farahangiz contributed in method design, data analysis, writing the article, edit, submission and proof reading of the final manuscript. Alireza Salehi is the corresponding author suggested the idea, took part in data anlysis, writing, editing and proofing the final manuscript.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: Ethical approval was provided by the Ethical Committee of Shiraz University of Medical Sciences (Approval No. 5617-90).
References
- 1. Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–2161. [DOI] [PubMed] [Google Scholar]
- 2. Naghavi M. Mortality Profile for 23 Provinces of Iran (2003) [in Farsi]. Tehran, Iran: Iranian Ministry of Health and Medical Education; 2006. [Google Scholar]
- 3. Young M, Wolfheim C, Marsh DR, Hammamy D. World Health Organization/United Nations Children’s Fund joint statement on integrated community case management: an equity-focused strategy to improve access to essential treatment services for children. Am J Trop Med Hyg. 2012;87(5 suppl):6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Asgari Z, Selwyn BJ, Vonville H, DuPont HL. A systematic review of the evidence for use of herbal medicine for the treatment of acute diarrhea. Natural Prod J. 2012;2(1):1–8. [Google Scholar]
- 5. Rezaei M, Ghafarian Shirazi HR, Karimi Z, Parsa M. The effect of Mentha longifolia on children: acute nonbacterial diarrhea in Pars. J Med Sci (Jahrom Med J). 2009;7(1):7–14. [Google Scholar]
- 6. Luczaj W, Skrzydlewska E. Antioxidative properties of black tea. Prev Med. 2005;40:910–918. [DOI] [PubMed] [Google Scholar]
- 7. Aghili Khorasani Shirazi MH. Makhzan al-Advieh. Tehran, Iran: Iran University of Medical Sciences; 2010. [Google Scholar]
- 8. Khare CP. Indian Medicinal Plants. An Illustrated Dictionary. Berlin, Germany: Springer; 2007. [Google Scholar]
- 9. Maity S, Vedasiromoni JR, Chaudhuri L, Ganguly DK. Role of reduced glutathione and nitric oxide in the black tea extract-mediated protection against ulcerogen-induced changes in motility and gastric emptying in rats. Jap J Pharmacol. 2001;85:358–364. [DOI] [PubMed] [Google Scholar]
- 10. Besra SE, Gomes A, Ganguly DK, Vedasiromoni JR. Antidiarrhoeal activity of hot water extract of black tea (Camellia sinensis). Phytother Res. 2003;17:380–384. [DOI] [PubMed] [Google Scholar]
- 11. Jafari K, Gharibzadeh S, Faghihi M, Karimian SM, Hamzehloo M, Keshavarz M. Effect of Iranian black tea extract and its isolated Thearubigins on intestinal transient time in mice. J Kerman Univ Med Sci. 2006;13(1):37–42. [Google Scholar]
- 12. Li B, Jin Y, Xu Y, Wu Y, Xu J, Tu Y. Safety evaluation of tea (Camellia sinensis (L.) O. Kuntze) flower extract: assessment of mutagenicity, and acute and subchronic toxicity in rats. J Ethnopharmacol. 2011;133:583–590. [DOI] [PubMed] [Google Scholar]
- 13. Fleming T. PDR for Herbal Medicine (Camellia Sinensis, pp. 256–268). Washington, DC: Thomson PDR; 2000. [Google Scholar]
- 14. Kosek M, Bern C, Guerrant RL. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull World Health Organ. 2003;81:197–204. [PMC free article] [PubMed] [Google Scholar]
- 15. Bhutta ZA. Acute gastroenteritis in children In: Kliegman RM, Stanton BF, St Geme JW, Schor NF, Behrman RE, eds. Nelson Textbook of Pediatrics. 19th ed Philadelphia, PA: Saunders; 2011:1323–1338. [Google Scholar]
- 16. Chaudhuri L, Basu S, Seth P, et al. Prokinetic effect of black tea on gastrointestinal motility. Life Sci. 2000;66:847–854. [DOI] [PubMed] [Google Scholar]
- 17. Bruins MJ, Vente-Spreeuwenberg MA, Smits CH, Frenken LG. Black tea reduces diarrhoea prevalence but decreases growth performance in enterotoxigenic Escherichia coli-infected post-weaning piglets. J Anim Physiol Anim Nutr (Berl). 2011;95:388–398. [DOI] [PubMed] [Google Scholar]
- 18. Szajewska H, Mrukowicz JZ. Use of probiotics in children with acute diarrhea. Paediatr Drugs. 2005;7:111–122. [DOI] [PubMed] [Google Scholar]