Abstract
BACKGROUND
Autologous hematopoietic cell transplant (autoHCT) is a standard therapy for relapsed Hodgkin lymphoma (cHL) and diffuse large B-cell lymphoma (DLBCL); however, long-term outcomes are not well described.
METHODS
We analyzed survival, non-relapse mortality, late effects and subsequent malignant neoplasms (SMN) in 1617 patients who survived progression-free for ≥2 years after autoHCT for cHL or DLBCL, between 1990 and 2008. The median age at autoHCT was 40 years; median follow-up was 10.6 years.
RESULTS
Five-year overall survival was 90% (95%CI, 87–92) for cHL and 89% (95%CI, 87–91) for DLBCL. The risk of late mortality compared with the general population was 9.6-fold higher for cHL patients (standardized mortality ratio [SMR] = 9.6) and 3.4-fold (SMR = 3.4) higher for DLBCL patients. Relapse accounted for 44% of late deaths. At least one late effect was reported in 9% of patients. A total of 105 SMN were confirmed, 44 in the cHL and 61 in the DLBCL group. By multivariate analysis, older age, male sex, Karnofsky score <90, total body irradiation (TBI) exposure, and higher numbers of lines of chemotherapy prior to autoHCT were risk factors for overall mortality in cHL. Risk factors in DLBCL were older age and TBI-exposure. A sub-analysis of 798 adolescent and young adult patients mirrored outcomes of the overall study population.
CONCLUSION
Despite generally favorable outcomes, two-year survivors of autoHCT for cHL or DLBCL have an excess late mortality risk when compared to the general population and experience an assortment of late complications.
Keywords: late effects, survival, non-relapse mortality, autologous hematopoietic cell transplant, Hodgkin lymphoma, diffuse large B-cell lymphoma
Introduction
High-dose chemotherapy followed by autologous hematopoietic cell transplant (autoHCT) remains the standard treatment for medically-fit patients with relapsed or refractory aggressive lymphomas.1,2 Reported survival rates 3–5 years after autoHCT for classical Hodgkin lymphoma (cHL) and diffuse large B-cell lymphoma (DLBCL) range between 40 and 70%.2–8 Treatment failure is most commonly due to relapse or progression of the underlying disease, which primarily occurs within the first two years after autoHCT.1 For those patients who survive the initial post-autoHCT period, long-term outcomes are not well described.
Prior reports of long-term outcomes after autoHCT were limited by short follow-up times, inclusion of patients who received older therapies, or small, non-representative patient cohorts. The first major study of late mortality described outcomes of 854 patients who survived at least two years after autoHCT for leukemia or lymphoma, between 1981–1998.9 With a median follow-up of 7.6 years and 68% having received total-body irradiation (TBI) based preparative regimens, the cohort had a 13-fold increased risk of late death compared with the general population. Subsequent reports have confirmed an increased risk of late mortality.10–13 Additional, mostly single center studies have reported high rates of organ impairments, functional limitations, and subsequent malignant neoplasms (SMN) in autoHCT survivors.13–15
To address the gaps in the literature, we selected a representative, multicenter cohort of two-year disease-free survivors of autoHCT for cHL or DLBCL who were reported to the Center for International Blood and Marrow Transplant Research (CIBMTR). We sought to evaluate: (1) long-term survival and mortality risk as compared to the general population, (2) SMN, (3) non-malignant late effects and (4) predictive factors for worse long-term outcomes. Since cHL is the most common cancer in adolescents and young adults (AYA), we also conducted a sub-analysis of AYA survivors.16
Methods
Data Source
The CIBMTR is a voluntary working group comprised of over 450 transplantation centers worldwide that contribute detailed hematopoietic cell transplant data to a statistical center at the Medical College of Wisconsin in Milwaukee and the National Marrow Donor Program in Minneapolis. Participating centers are required to report all transplants consecutively; compliance is monitored by on-site audits. Patients are followed longitudinally. Computerized checks for discrepancies, physician’s review of submitted data and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants and are under the guidance of the institutional review board of the National Marrow Donor Program.
The CIBMTR collects transplant-essential data for all patients, which includes demographic, disease type and stage, pre-transplant chemotherapy, graft type, preparative regimen, development of a new malignancy, survival, relapse and cause of death data. A subset of patients, selected by a weighted randomization scheme, also have comprehensive research level data collected. Late effects data are obtained from this subset and therefore, only this group of patients was included in the current study. The presence of the following specific late effects are reported: avascular necrosis, bronchiolitis obliterans, pulmonary hemorrhage, cataracts, congestive heart failure, myocardial infarction, diabetes/hyperglycemia, gonadal dysfunction/infertility requiring hormone replacement, growth hormone deficiency/growth disturbance, hypothyroidism, hemorrhagic cystitis/hematuria requiring medical intervention, non-infectious liver toxicity, pancreatitis, post-transplant microangiopathy-thrombotic thrombocytopenic purpura, hemolytic uremic syndrome, renal failure or stroke/seizure. The data are collected pre-transplant, 100 days and six months post-transplant and then annually.
To confirm diagnoses of SMN, pathology reports were obtained from reporting transplant centers. Any new malignancy diagnosis that could not be confirmed on central review was not included in the analysis.
Study population
The study population consisted of AYA and older adults (age > 39 years) who had survived progression-free for ≥2 years following autoHCT for cHL or DLBCL between January 1, 1990 and December 31, 2008. Patients ages 15–39 years were characterized as AYA, based on the National Cancer Institute’s definition.17 The study was restricted to patients treated in the United States and Canada.
Of 5171 patients undergoing their first autoHCT for cHL or DLBCL at age ≥15 years, 1023 were excluded based on their transplant center’s location or completeness of follow-up. Another 2250 patients were excluded because of death (n=1594), relapse (n=584), or loss to follow-up (n=72) within two years of autoHCT. Other exclusion criteria were nodular lymphocyte predominant HL (n=5), low-grade lymphomas or chronic lymphocytic leukemia transforming to DLBCL (n=102), known HIV positive status (n=53), subsequent transplant within two years of the first transplant (n=52), a pre-autoHCT history of another malignancy (other than non-melanomatous skin cancers, n=56), and missing CIBMTR forms (n=13). The final study cohort consisted of 1617 patients from 134 centers.
Outcomes
Outcomes studied included overall survival (OS), progression-free survival (PFS), relapse and non-relapse mortality (NRM). OS was defined as time to death from any cause and was censored at last point of contact. For PFS, a patient was considered a treatment failure at the time of progression/relapse or death from any cause. Relapse was defined as progressive disease after autoHCT or disease recurrence after a complete remission; death in remission was considered a competing risk. NRM was defined as death without evidence of progression/relapse; relapse was considered a competing risk.
Additional outcomes included the development of SMN and non-malignant organ impairments ≥2 years after autoHCT. Late effects were censored at the time of second transplant or relapse. Only the specific organ impairments collected on the CIBMTR forms described above were analyzed.
Statistical analysis
Descriptive statistics were calculated for patient demographic, disease and treatment-related variables. The Kaplan-Meier method was used to estimate the probability of overall survival, which was calculated from the two-year landmark after autoHCT to the date of death or last follow-up. The cumulative incidence function was used to estimate relapse, relapse-related death, and NRM. The frequencies of individual late effects (censored at relapse or second transplant) were calculated. In addition, the cumulative incidence of developing a SMN, ≥1 non-malignant late effect, or ≥2 late effects was estimated. Standardized mortality ratios (SMR) analyzed the ratio of observed deaths in the study population relative to expected deaths in country, age, race, and sex-matched controls from the general population in the United States and Canada. General population data was obtained from the National Center for Health Statistics.
Cox proportional hazards analysis was used to identify multivariate risk factors for overall mortality, NRM, SMN, and ≥1 late effect. The stepwise selection method with a significance level of 0.05 was used to identify multivariate risk factors. The proportional hazard assumption was checked. If violated, it was included as a time-dependent covariate. Pairwise interaction between significant variables was examined. Separate models were created for patients with HL and those with DLBCL.
The AYA sub-analysis used the same methodology at the main analysis. Analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC).
RESULTS
Patient and Transplant Characteristics
Characteristics of the study population are presented in Table 1. Of the 1617 patients eligible for analysis, 836 had cHL 781 had DLBCL. The median duration of follow-up was 127 (range 24–292) months after autoHCT. For cHL patients, the median age at autoHCT was 33 years; 72% (n=606) were included in the AYA group. Forty-four percent (n=371) received radiation therapy prior to autoHCT conditioning and 5% (n=39) received a TBI-based preparative regimen.
Table 1.
Patient Characteristics
| Variable | cHL N (%) |
DLBCL N (%) |
|---|---|---|
| Number of patients | 836 | 781 |
| Median age at autoHCT (range), years | 33 (15–77) | 51 (15–77) |
| 15–19 | 79 (9) | 9 (1) |
| 20–29 | 274 (33) | 66 (8) |
| 30–39 | 253 (30) | 117 (15) |
| 40–49 | 132 (16) | 169 (22) |
| 50–59 | 66 (8) | 213 (27) |
| 60–69 | 27 (3) | 180 (23) |
| 70+ | 5 (<1) | 27 (3) |
| Sex | ||
| Male | 504 (60) | 441 (56) |
| Female | 332 (40) | 340 (44) |
| Karnofsky/Lansky score at autoHCT | ||
| <90% | 240 (29) | 238 (30) |
| ≥90% | 575 (69) | 509 (65) |
| Missing | 21 (3) | 34 (4) |
| Race/Ethnicity | ||
| Caucasian/White | 691 (83) | 686 (88) |
| Other | 139 (17) | 91 (12) |
| Unknown/Declined | 6 (<1) | 4 (<1) |
| Disease remission status at autoHCT | ||
| Complete response | 240 (29) | 338 (43) |
| Partial response | 417 (50) | 353 (45) |
| Chemorefractory | 77 (9) | 57 (7) |
| Untreated | 41 (5) | 6 (<1) |
| Unknown | 61 (7) | 27 (3) |
| Extranodal involvement at autoHCT | ||
| No | 648 (78) | 556 (71) |
| Yes | 158 (19) | 429 (55) |
| Missing | 30 (4) | 60 (8) |
| Median (range) interval from diagnosis to autoHCT, months | 25 (2–374) | 13 (<1–184) |
| Number of lines of chemotherapy prior to autoHCT conditioning | ||
| 1 | 95 (11) | 134 (17) |
| 2 | 462 (55) | 356 (46) |
| 3 | 196 (23) | 196 (25) |
| 4+ | 65 (8) | 60 (8) |
| Missing | 18 (2) | 35 (4) |
| Lines of anthracyclines prior to autoHCT conditioning | ||
| 0 | 37 (4) | 28 (4) |
| 1 | 621 (74) | 599 (77) |
| ≥2 | 163 (19) | 111 (14) |
| Missing | 15 (2) | 43 (6) |
| Lines of bleomycin prior to autoHCT conditioning | ||
| 0 | 116 (14) | 652 (83) |
| 1 | 628 (75) | 80 (10) |
| ≥2 | 81 (10) | 8 (1) |
| Missing | 11 (1) | 41 (5) |
| Auto-HCT conditioning regimen | ||
| TBI-based | 39 (5) | 115 (15) |
| BuMel/BuCy/Bu+Other | 82 (10) | 88 (11) |
| CBV or similar | 403 (48) | 200 (26) |
| BEAM or similar | 193 (23) | 304 (39) |
| Other | 119 (14) | 74 (9) |
| Radiation therapy prior to autoHCT conditioning | ||
| No | 299 (36) | 373 (48) |
| Yes | 371 (44) | 219 (28) |
| Missing | 166 (20) | 189 (24) |
| Stem cell source | ||
| Bone marrow | 193 (23) | 99 (13) |
| Peripheral blood | 643 (77) | 682 (87) |
| Median follow-up of survivors (range), months | 127 (24–292) | 121 (24–289) |
For DLBCL patients, the median age at autoHCT was 51 years; 25% (n=192) were included in the AYA group. Twenty-eight percent (n=218) received radiation therapy prior to conditioning and 15% (n=115) received a TBI-based preparative regimen.
Survival and Relapse Outcomes
Figure 1 displays the Kaplan Meier estimates and cumulative incidences of survival outcomes. Among two-year survivors of autoHCT for cHL, OS at 3- and 5-years after autoHCT was 96% (95%CI, 95–98%) and 90% (95%CI, 87–92%). PFS at 3- and 5-years was 93% (95%CI, 91–95%) and 84% (95%CI, 81–86%). The cumulative incidence of relapse was 4% (95%CI, 3–6%) at 3-years and 9% (95%CI, 8–12%) at 5-years after autoHCT. The incidence of NRM was 3% (95%CI, 2–4%) at 3-years and 7% (95%CI, 5–9%) at 5-years.
Figure 1.
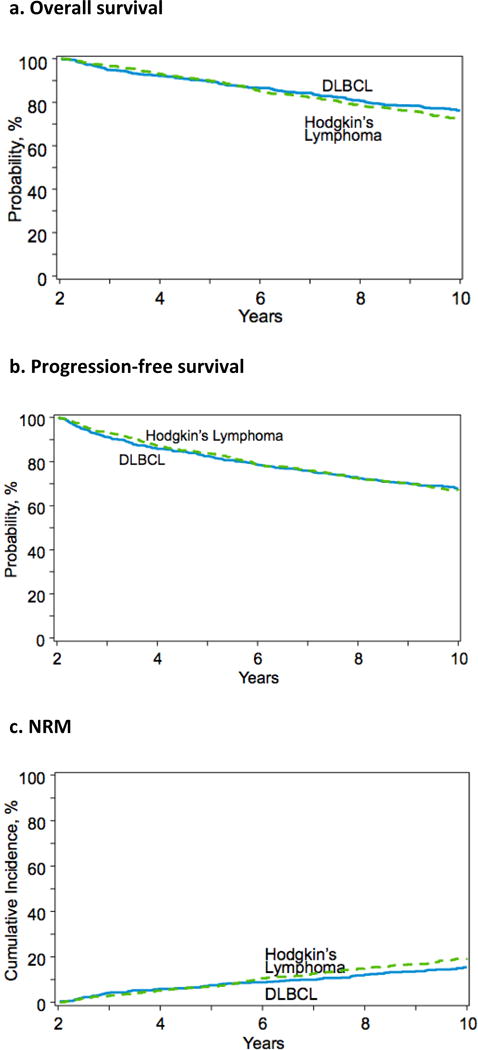
Survival outcomes among two-year survivors of autoHCT for cHL and DLBCL
Of the 256 patients with cHL who died >2 years after autoHCT, relapse accounted for 44% (n=113) of deaths and NRM accounted for 56% (n=143) of deaths. Causes of NRM are presented in Table 2. Of the 173 patients who died >5 years after autoHCT, relapse accounted for 28% (n=49) and NRM accounted for 72% (n=124) of deaths. The SMR was 9.6 (95%CI, 8.1–11.2) for two-year cHL survivors compared to the general population.
Table 2.
Cause of death for deceased patients who survived progression-free for ≥2 years
| Cause of death | cHL (n=256) N (%) |
DLBCL (n=223) N (%) |
|---|---|---|
| Relapse | 113 (44) | 96 (43) |
| SMN | 23 (9) | 24 (11) |
| Organ failure | 17 (7) | 15 (7) |
| Infection | 7 (3) | 13 (6) |
| Graft rejection (post-allogeneic HCT) | 1 (<1) | 0 |
| GVHD (post-allogeneic HCT) | 2 (1) | 0 |
| Other | 37 (14) | 36 (16) |
| Unknown | 56 (22) | 39 (17) |
Among two-year survivors of autoHCT for DLBCL, OS at 3- and 5-years after autoHCT was 95% (95%CI, 93–96%) and 89% (95%CI, 87–91%). PFS at 3- and 5-years was 91% (95%CI, 89–93%) and 82% (95%CI, 79–85%). The cumulative incidence of relapse was 4% (95%CI, 3–6%) at 3-years and 7% (95%CI, 6–9%) at 5-years after autoHCT. The incidence of NRM was 4% (95%CI, 3–6%) at 3-years and 7% (95%CI, 6–9%) at 5-years.
Of the 223 patients with DLBCL who died >2 years after autoHCT, relapse accounted for 43% (n=96) of deaths and NRM accounted for 57% (n=127) of deaths. Causes of NRM are presented in Table 2. Of the 142 patients who died >5 years after autoHCT, relapse accounted for 29% (n=41) and NRM accounted for 71% (n=101) deaths. The SMR was 3.4 (95% CI, 2.9–4.1) for two-year DLBCL survivors compared to the general population.
Predictors of Mortality
Table 3 displays the multivariate models of overall mortality and NRM in cHL patients. Risk factors for overall mortality included older age (p <.001), male sex (p =0.039), Karnofsky score <90% at the time of autoHCT (p=0.011), TBI-exposure (p <.001), and higher numbers of lines of chemotherapy prior to autoHCT (p=0.015). Predictors of NRM included older age (p <.0001), Karnofsky score <90% at the time of autoHCT (p=0.011) and TBI-exposure (p <.0001).
Table 3.
Risk factors for overall mortality and NRM in cHL patients
| Overall mortality | NRM | ||||
|---|---|---|---|---|---|
| N | RR (95% CI) | P-value | RR (95% CI) | P-value | |
| Age at transplant, years | <.001 | <.001 | |||
| 15–39 | 606 | 1.00 | 1.00 | ||
| 40–54 | 169 | 1.31 (0.97–1.76) | 0.07 | 1.27 (0.89–1.81) | 0.18 |
| 55+ | 61 | 2.66 (1.82–3.90) | <.01 | 2.67 (1.69–4.21) | <.01 |
| Sex1 | 0.039 | ||||
| Male | 504 | 1.00 | |||
| Female | 332 | 0.76 (0.58–0.99) | |||
| Karnofsky score at autoHCT | 0.011 | 0.011 | |||
| ≥90 | 575 | 1.00 | 1.00 | ||
| <90 | 240 | 1.49 (1.14–1.94) | <.01 | 1.49 (1.09–2.04) | 0.01 |
| Missing | 21 | 1.55 (0.72–3.35) | 0.26 | 2.36 (1.02–5.45) | 0.05 |
| Number of lines of chemotherapy | <.001 | Not significant | |||
| 1 | 95 | 1.00 | |||
| 2 | 462 | 0.91 (0.60–1.39) | 0.66 | ||
| 3 | 196 | 1.23 (0.78–1.94) | 0.37 | ||
| 4+ | 65 | 1.71 (1.01–2.87) | 0.04 | ||
| Missing | 18 | 2.87 (1.44–5.70) | <.01 | ||
| TBI as part of conditioning | <.001 | <.001 | |||
| No | 797 | 1.00 | 1.00 | ||
| Yes | 39 | 2.65 (1.73–4.05) | <.001 | 2.62 (1.54–4.45) | <.001 |
Table 4 displays the multivariate models of overall mortality and NRM in DLBCL patients. Risk factors for overall mortality included older age (p <.001) and TBI-exposure (p =0.013). Predictors of NRM were also older age (p <.0001) and TBI-exposure (p=0.043).
Table 4.
Risk factors for overall mortality and NRM in DLBCL patients
| Overall mortality | NRM | ||||
|---|---|---|---|---|---|
| N | RR (95% CI) | P-value | RR (95% CI) | P-value | |
| Age at transplant, years | <.001 | <.001 | |||
| 15–39 | 192 | 1.00 | 1.00 | ||
| 40–54 | 277 | 2.02 (1.32–3.10) | <.01 | 1.79 (1.08–2.96) | 0.02 |
| 55+ | 312 | 4.23 (2.80–6.39) | <.01 | 4.22 (2.60–6.85) | <.01 |
| TBI as part of conditioning | 0.013 | 0.043 | |||
| No | 666 | 1.00 | 1.00 | ||
| Yes | 115 | 1.51 (1.09–2.08) | 0.013 | 1.51 (1.01–2.25) | 0.043 |
Non-Malignant Late Effects
At least one non-malignant late effect was reported in 9% (n=148) of patients. Two or more late effects were reported in 2% (n=30) of patients.
The incidences of specific late effects are described in Table 5. The most frequently reported non-malignant late effects were endocrine impairments, including hypothyroidism (n=50), diabetes (n=23) and gonadal dysfunction (n=16). Cataracts (n=16) and cardiac impairments, including congestive heart failure (n=14) and myocardial infarction (n=11), were the next most commonly reported late effects. Other complications that were reported in ≥10 patients included interstitial pneumonitis/idiopathic pneumonia syndrome (n=12) and stroke/seizures (n=10).
Table 5.
Incidence of non-malignant late effects occurring ≥2 years after autoHCT
| Organ Impairment | Incidence, N (%) |
|---|---|
| Hypothyroidism | 50 (3) |
| Diabetes | 23 (1) |
| Gonadal dysfunction | 16 (<1) |
| Cataracts | 16 (<1) |
| Congestive heart failure | 14 (<1) |
| Interstitial pneumonitis / idiopathic pneumonia syndrome | 12 (<1) |
| Myocardial infarction | 11 (<1) |
| Stroke/seizures | 10 (<1) |
| Non-infectious liver toxicity | 8 (<1) |
| Renal failure (severe) warranting dialysis | 7 (<1) |
| Avascular necrosis | 6 (<1) |
| Hemorrhagic cystitis | 4 (<1) |
| Thrombotic thrombocytopenic purpura / hemolytic uremic syndrome | 4 (<1) |
| Bronchiolitis obliterans | 2 (<1) |
| Pulmonary hemorrhage | 2 (<1) |
| Pancreatitis | 1 (<1) |
SMN
A total of 105 confirmed SMN occurred ≥2 years post-autoHCT, 44 in cHL and 61 in DLBCL patients. Among cHL patients, biopsy-documented SMN included myelodysplastic/myeloproliferative syndrome (n=12), genitourinary tract cancers (n=6), acute myeloid leukemia (n=5), gastrointestinal cancer (n=5) and breast cancer (n=5). Predictors of SMN included older age (p <.001) and higher number of lines of chemotherapy (p=0.015, Table 6).
Table 6.
Risk factors for the development of SMN in cHL and DLBCL survivors
| cHL patients | DLBCL patients | |||||
|---|---|---|---|---|---|---|
| N | RR (95% CI) | P-value | N | RR (95% CI) | P-value | |
| Age at transplant, years | <.001 | <.001 | ||||
| 15–39 | 570 | 1.00 | 182 | 1.00 | ||
| 40–54 | 145 | 1.54 (0.73–3.25) | 0.25 | 252 | 3.42 (1.14–10.23) | 0.03 |
| 55+ | 52 | 5.20 (2.38–11.36) | <.01 | 271 | 13.56 (4.76–38.63) | <.01 |
| Number of lines of chemotherapy | 0.015 | Not significant | ||||
| 1 | 88 | 1.00 | ||||
| 2 | 428 | 0.95 (0.32–2.84) | 0.92 | |||
| 3 | 177 | 2.31 (0.77–6.94) | 0.14 | |||
| 4+ | 57 | 2.57 (0.72–9.15) | 0.14 | |||
| Missing | 17 | 6.36 (1.12–36.01) | 0.04 | |||
Among DLBCL patients, biopsy-documented SMN included myelodysplastic/myeloproliferative syndrome (n=24), gastrointestinal (n=7), genitourinary tract cancers (n=7), acute myeloid leukemia (n=5), and breast cancer (n=5). Older age (p <.001) was the only risk factor identified for SMN in DLBCL patients (Table 6).
AYA Sub-analysis
Outcomes of the AYA subpopulation largely mirrored outcomes of the overall population. For cHL, OS was 91% (95%CI, 88–93%), NRM was 7% (95%CI, 5–9%) and relapse was 8% (95%CI, 6–10%) at 5-years after autoHCT. For DLBCL, OS was 97% (95%CI, 94–99%), NRM was 2% (95%CI, 1–5%), and relapse was 5% (95%CI, 2–8%) at 5-years after autoHCT.
Relapse accounted for 47% of deaths in cHL and 31% of deaths in DLBCL. At least one non-malignant late effect was reported in 8% of patients; ≥2 late effects were reported in 1% of the patients. A total of 30 SMN were reported, 25 in cHL and 5 in DLBCL.
For cHL patients, predictors of overall mortality included Karnofsky score <90 (p=0.0004), higher numbers of lines of chemotherapy (p <.0001), and TBI-exposure (p <.0001). Risk factors for NRM included Karnofsky score <90 (p <.0001) and TBI-exposure (p=0.0003). For DLBCL patients, TBI-exposure (p=0.011) was predictive of late mortality. No significant risk factors were identified for NRM.
Discussion
We present major long-term outcomes, including survival, SMN and late effects, of a multicenter cohort of 1617 two-year autoHCT survivors for cHL or DLBCL treated in a modern era of therapies and reported to the CIBMTR. We found that patients who survived progression-free for ≥2 years after autoHCT had favorable long-term survival. Five years after autoHCT, OS was 90% among cHL and 89% among DLBCL patients. Despite favorable survival outcomes, however, cHL patients had a 9.6-fold increased risk of late mortality and DLBCL patients had a 3.4-fold increased risk of late mortality when compared to an age- and gender-matched general population. Relapse of the primary disease continued to be a major cause of late mortality, accounting for 44% of deaths in cHL patients and 43% of deaths in DLBCL patients. We report a myriad of SMN and non-malignant late effects experienced by survivors. By adjusted analyses, we found that the most consistent risk factors for worse long-term outcomes were TBI-exposure and older age at the time of autoHCT.
The strengths of the study are the representative patient cohort and long follow-up time. The 1617 patients were reported to the CIBMTR from 134 centers in the United States and Canada, representing a range of community and tertiary-care centers from both rural and urban regions. The median follow-up time was 10.6 years, which was longer than comparable studies and allowed for more thorough assessment of mortality and late effects.9,10,14,18
In order to focus on treatment-related toxicities of autoHCT, the study population was chosen to represent patients most likely to be cured by transplant – namely the two most common aggressive lymphomas, cHL and de novo DLBCL. Despite selecting for patients who survived progression-free for ≥2 years after transplant, relapse remained a major cause of late mortality. Relapse accounted for 44% of deaths among two-year survivors and 29% of deaths among five-year survivors. Consistent with our findings, previous reports have also found relapse to be a leading cause of late deaths among patients who survive the initial post-autoHCT period.9,10,13,18 This observation highlights the importance of post-transplantation relapse prevention. In this regard, brentuximab vedotin was recently approved as post-transplant consolidation therapy in certain high-risk cHL patients.19 For DLBCL, while rituximab maintenance was not shown to improve post-transplant disease control, the recently activated BMT CTN IronCLAD trial will examine the role of ibrutinib in ABC type DLBCL.20 Other agents that warrant investigation as post-transplant relapse prevention strategies include checkpoint inhibitors, immunomodulators, and PI3K inhibitors. Nevertheless, the majority of two- and five-year survivors in the current study did not die due to relapse of their underlying lymphoma. For these patients, the patterns and risk factors for treatment-related morbidity and mortality are most informative.
Similarly to the other large series of long-term outcomes after autoHCT for lymphoma, our study found relatively high rates of SMN.9,18 This was true despite the lower use of TBI-containing preparative regimens than was reported in the other, less contemporary studies. The development of SMN is an unfortunate yet inherent late effect of administration of myeloablative therapy. When questioning ways to minimize this risk, perhaps the most important modifiable risk factor identified in this study for impaired overall survival and SMN is the use of TBI. Other reports of SMN after autoHCT for lymphoma did not identify risk factors for the development of SMN.9,13,15,18 There are, however, conflicting reports about the association of TBI and long-term survival after autoHCT. Worse PFS in patients has been described in patients who received TBI.18 In contrast, Bhatia, et al’s large report found that TBI provided a protective effect such that the risk of late death was two-fold higher among patients who did not receive TBI compared with those who did.9 It should be noted the study captured patients with a range of hematologic malignancies between 1981–1998, thus making the applicability of the TBI findings difficult to compare to the current study.
Our study reported a low incidence of non-malignant late effects; 9% of the cohort had at least one late effect that occurred at least two years after autoHCT and 2% had multiple late effects. Data on late effects after autoHCT for lymphoma are limited, but the few available studies found late effects in the majority of survivors. The largest series reported outcomes of 458 patients who were part of the Bone Marrow Transplant Survivor Study (BMTSS).21 Of the survivors, 60.7% reported at least one nonmalignant late effect. Notably, data was collected via self-report and the study included patients with different types of hematologic malignancies. A separate analysis that only included patients from the BMTSS cohort who had underwent autoHCT for lymphoma (n=276) also reported high incidences of late effects.14 For instance, 33%, 23%, and 22% of the study population had neurosensory, endocrine and cardiopulmonary impairments, respectively. The differences in outcomes between the BMTSS analysis and our study would not be explained by differences in follow-up time, as the BMTSS analysis had a median follow-up time of 6 years, compared to 10 years in our study. As the patients in the BMTSS analysis were treated between 1974 and 1998, the majority (69%) received a TBI-based conditioning regimen. This is in contrast to the few patients in the current study treated with TBI-based regimens and may, in part, account for the differences in late effects findings. It is also important to acknowledge that CIBMTR data forms capture a limited number of late effects and screening practices for late effects are not consistent among centers. Therefore, the low incidence of late morbidities in our sample was likely an underrepresentation of the patients’ true symptoms burden. Our results, in combination with prior data, support the need for continued long-term follow-up of patients after autoHCT to screen for late effects.22
The current study has some limitations that should be considered in interpreting the results. It utilized a retrospective cohort design and specific drug and radiation dosing data were not available. As explained above, only selected late effects data were collected. Furthermore, autoHCT patients are often only followed by their transplant centers for a short time after transplant. Therefore, it can be challenging for centers to ascertain long-term follow up information, which leads to missing data. In addition, we required original biopsy reports to confirm SMN, but were unable to obtain reports from several centers. Hence, we may have underestimated the true incidence of SMN. Though we had a median follow-up time of 10.6 years, it is possible that a longer follow-up time would reveal a larger burden of late effects.
In summary, we found that patients who survived progression-free for at least two years after autoHCT for cHL or DLBCL had favorable long-term outcomes. Five years after autoHCT, 90% of the two-year cHL survivors were alive and 89% of the two-year DLBCL survivors were alive. Survivors, however, continue to be at excess risk for late mortality compared to the general population due to relapse, SMN, and other late effects. Our results affirm the need to build therapies to augment the efficacy of autoHCT and treat relapse after autoHCT. And as outcomes continue to improve, our study highlights the importance of close, systematic follow-up of autoHCT patients to screen for and treat late effects.
Acknowledgments
We would like to thank the University of Nebraska Medical Center for contributing a significant proportion of patients to this study.
Funding details: CIBMTR is supported by 5U24-CA076518 from NCI, NHLBI, NIAID and 5U10HL069294 from NHLBI, NCI.
CIBMTR Support List
The CIBMTR is supported primarily by Public Health Service Grant/Cooperative Agreement 5U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-15-1-0848 and N00014-16-1-2020 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals, Inc.; Alexion; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; AstraZeneca; Atara Biotherapeutics, Inc.; Be the Match Foundation; *Bluebird Bio, Inc.; *Bristol Myers Squibb Oncology; *Celgene Corporation; Cellular Dynamics International, Inc.; Cerus Corporation; *Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Genentech, Inc.; Genzyme Corporation; Gilead Sciences, Inc.; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Janssen Scientific Affairs, LLC; *Jazz Pharmaceuticals, Inc.; Jeff Gordon Children’s Foundation; The Leukemia & Lymphoma Society; Medac, GmbH; MedImmune; The Medical College of Wisconsin; *Merck & Co, Inc.; *Mesoblast; MesoScale Diagnostics, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Otsuka America Pharmaceutical, Inc.; Otsuka Pharmaceutical Co, Ltd. – Japan; PCORI; Perkin Elmer, Inc.; Pfizer, Inc; *Sanofi US; *Seattle Genetics; *Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; *Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum, Inc.; Takeda Oncology; Telomere Diagnostics, Inc.; University of Minnesota; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
*Corporate Members
Footnotes
None of the authors have any relevant conflict of interest to disclose.
Author contributions: The study was designed and interpreted by RM, BTH, BES, SK, HRM, MB, BNS, MEDF, MH, and PS. Data analysis was performed by SK and HM. All other authors participated in writing and reviewing the manuscript.
Discipline: Hematopoietic cell transplantation
References
- 1.Hamadani M, Hari PN, Zhang Y, et al. Early failure of frontline rituximab-containing chemo-immunotherapy in diffuse large B cell lymphoma does not predict futility of autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014;20:1729–36. doi: 10.1016/j.bbmt.2014.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333:1540–5. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 3.Hazar V, Kesik V, Aksoylar S, et al. Outcome of autologous hematopoietic stem cell transplantation in children and adolescents with relapsed or refractory Hodgkin’s lymphoma. Pediatr Transplant. 2015;19:745–52. doi: 10.1111/petr.12573. [DOI] [PubMed] [Google Scholar]
- 4.Garfin PM, Link MP, Donaldson SS, et al. Improved outcomes after autologous bone marrow transplantation for children with relapsed or refractory Hodgkin lymphoma: twenty years experience at a single institution. Biol Blood Marrow Transplant. 2015;21:326–34. doi: 10.1016/j.bbmt.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 5.Lazarus HM, Loberiza FR, Jr, Zhang MJ, et al. Autotransplants for Hodgkin’s disease in first relapse or second remission: a report from the autologous blood and marrow transplant registry (ABMTR) Bone Marrow Transplant. 2001;27:387–96. doi: 10.1038/sj.bmt.1702796. [DOI] [PubMed] [Google Scholar]
- 6.Robinson SP, Boumendil A, Finel H, et al. Autologous stem cell transplantation for relapsed/refractory diffuse large B-cell lymphoma: efficacy in the rituximab era and comparison to first allogeneic transplants. A report from the EBMT Lymphoma Working Party . Bone Marrow Transplant. 2016;51:365–71. doi: 10.1038/bmt.2015.286. [DOI] [PubMed] [Google Scholar]
- 7.Satwani P, Ahn KW, Carreras J, et al. A prognostic model predicting autologous transplantation outcomes in children, adolescents and young adults with Hodgkin lymphoma. Bone Marrow Transplant. 2015;50:1416–23. doi: 10.1038/bmt.2015.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vanderwalde AM, Sun CL, Laddaran L, et al. Conditional survival and cause-specific mortality after autologous hematopoietic cell transplantation for hematological malignancies. Leukemia. 2013;27:1139–45. doi: 10.1038/leu.2012.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhatia S, Robison LL, Francisco L, et al. Late mortality in survivors of autologous hematopoietic-cell transplantation: report from the Bone Marrow Transplant Survivor Study. Blood. 2005;105:4215–22. doi: 10.1182/blood-2005-01-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majhail NS, Weisdorf DJ, Defor TE, et al. Long-term results of autologous stem cell transplantation for primary refractory or relapsed Hodgkin’s lymphoma. Biol Blood Marrow Transplant. 2006;12:1065–72. doi: 10.1016/j.bbmt.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Hill BT, Rybicki L, Bolwell BJ, et al. The non-relapse mortality rate for patients with diffuse large B-cell lymphoma is greater than relapse mortality 8 years after autologous stem cell transplantation and is significantly higher than mortality rates of population controls. Br J Haematol. 2011;152:561–9. doi: 10.1111/j.1365-2141.2010.08549.x. [DOI] [PubMed] [Google Scholar]
- 12.Czyz A, Lojko-Dankowska A, Dytfeld D, et al. Prognostic factors and long-term outcome of autologous haematopoietic stem cell transplantation following a uniform-modified BEAM-conditioning regimen for patients with refractory or relapsed Hodgkin lymphoma: a single-center experience. Med Oncol. 2013;30:611. doi: 10.1007/s12032-013-0611-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodman KA, Riedel E, Serrano V, et al. Long-term effects of high-dose chemotherapy and radiation for relapsed and refractory Hodgkin’s lymphoma. J Clin Oncol. 2008;26:5240–7. doi: 10.1200/JCO.2007.15.5507. [DOI] [PubMed] [Google Scholar]
- 14.Majhail NS, Ness KK, Burns LJ, et al. Late effects in survivors of Hodgkin and non-Hodgkin lymphoma treated with autologous hematopoietic cell transplantation: a report from the bone marrow transplant survivor study. Biol Blood Marrow Transplant. 2007;13:1153–9. doi: 10.1016/j.bbmt.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lavoie JC, Connors JM, Phillips GL, et al. High-dose chemotherapy and autologous stem cell transplantation for primary refractory or relapsed Hodgkin lymphoma: long-term outcome in the first 100 patients treated in Vancouver. Blood. 2005;106:1473–8. doi: 10.1182/blood-2004-12-4689. [DOI] [PubMed] [Google Scholar]
- 16.Hochberg J, Waxman IM, Kelly KM, et al. Adolescent non-Hodgkin lymphoma and Hodgkin lymphoma: state of the science. Br J Haematol. 2009;144:24–40. doi: 10.1111/j.1365-2141.2008.07393.x. [DOI] [PubMed] [Google Scholar]
- 17.Institute NC. Adolesent and Young Adult Cancer by Site: Incidence, Survival and Mortality. SEER Cancer Statistics Review 1975–2011. 2014 [Google Scholar]
- 18.Majhail NS, Bajorunaite R, Lazarus HM, et al. Long-term survival and late relapse in 2-year survivors of autologous haematopoietic cell transplantation for Hodgkin and non-Hodgkin lymphoma. Br J Haematol. 2009;147:129–39. doi: 10.1111/j.1365-2141.2009.07798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moskowitz CH, Nademanee A, Masszi T, et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;385:1853–62. doi: 10.1016/S0140-6736(15)60165-9. [DOI] [PubMed] [Google Scholar]
- 20.Gisselbrecht C, Schmitz N, Mounier N, et al. Rituximab maintenance therapy after autologous stem-cell transplantation in patients with relapsed CD20(+) diffuse large B-cell lymphoma: final analysis of the collaborative trial in relapsed aggressive lymphoma. J Clin Oncol. 2012;30:4462–9. doi: 10.1200/JCO.2012.41.9416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun CL, Francisco L, Kawashima T, et al. Prevalence and predictors of chronic health conditions after hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study. Blood. 2010;116:3129–39. doi: 10.1182/blood-2009-06-229369. quiz 3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashmi SK, Bredeson C, Duarte RF, et al. National Institutes of Health Blood and Marrow Transplant Late Effects Initiative: The Healthcare Delivery Working Group Report. Biol Blood Marrow Transplant. 2017;23:717–725. doi: 10.1016/j.bbmt.2016.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]


