Abstract
Hydrosol beverages in Persian nutrition culture and ethnomedicine are the side products of essential oil industry that are used as delicious drinks or safe remedies. To investigate indications and chemical composition of hydrosol beverages for hyperlipidemia and cardiovascular conditions, Fars province was selected as the field of study. Ethnomedical data were gathered by questionnaires. The constituents of hydrosols were extracted with liquid/liquid extraction and analyzed by gas chromatography–mass spectrometry. Statistical analysis were used to cluster their constituents and find the relevance of their composition. A literature survey was also performed on plants used to prepare them. Thymol was the major or second major component of these beverages, except for wormwood and olive leaf hydrosols. Based on clustering methods, although some similarities could be found, composition of barberry, will fumitory, dill, and aloe hydrosols have more differences than others. These studies may help in developing some functional beverages or new therapeutics.
Keywords: essential oil, cardiovascular, hydrosol
Cardiovascular disease is a class of diseases that involve the heart or blood vessels and includes coronary artery diseases such as angina, myocardial infarction, stroke, hypertensive heart disease, cardiomyopathy, congenital heart disease, rheumatic heart disease, aortic aneurysms, peripheral artery disease, and venous thrombosis.
Coronary artery disease, stroke, and peripheral artery disease involve atherosclerosis. This also may be caused by high blood pressure, diabetes, smoking, lack of exercise, obesity, hypercholesterolemia, poor diet, and excessive alcohol consumption. According to the World Health Organization estimate, about 31% of all deaths worldwide are due to cardiovascular disease.1,2
Functional beverages are nonalcoholic drinks that contains ingredients such as herbs, vitamins, minerals, raw fruit, or vegetable, which are consumed to provide specific health benefits beyond those of general nutrition. Most of the well-known functional beverages are used to boost energy, enhance the immune system, or increasing sense of well-being. These are marketed as sports drinks, energy drinks, enhanced fruit drinks, and enhanced water.
Aromatic waters, also known as floral water, distillate water, or hydrosols, are the side products of the essential oil and natural perfumery industry.3 They are prepared by dispersion of the plant materials via industrial hydrodistillation. This water is evaporated simultaneously with the essential oil of the plants as the container is heated. These vapors are condensed and liquefied together in a collecting vessel to give 2 phases. An essential oil phase and aromatic water enriched with different amounts of the volatile constituents of the plant that are partly or completely soluble in water.4,5 These 2 phases are then separated; the essential oil goes to the pharmaceutical or cosmetic industry while the aromatic water depending on its unique properties is diluted 1:8 or 1:12 with water. They might go directly for marketing in big (250-1000 liters) containers without any further processing or be subjected to pasteurization in the factory. Subsequently, these preparations are kept in small (1-5 liters) plastic or glass containers for retail or wholesale marketing. In Iranian nutrition culture, they are used with sweeteners such as sugar or honey and served as natural delicious drinks. In Persian nutrition culture and folk medicine, aromatic waters are considered as very safe beverages used for medicinal purposes depending on the plants used for their production. Most aromatic waters are monoherbal but some have polyherbal constituents.6,7 Depending on the plants used for preparation of each aromatic water, an overall nature is considered including, hot, cold, wet, dry, or moderate. They are also used as remedies to treat several conditions in oral and/or topical applications. Some adverse effects have been reported in folk medicine due to their improper application or ingestion. But, in general, they are considered as a safe and effective way of consuming essential oils and vital essence of medicinal plants or vegetables. In contrast to the pure essential oils, which are usually very potent or even harsh in terms of their biological activities, aromatic waters are moderate and balanced by the water and its water soluble volatile components.8,9 Any of the aromatic waters has its own individual smell and composition, which is considerably different from the pure essential oil with which it was codistilled. The aromatic water has therefore additional properties not possessed by the essential oil alone.10 The moderate activity of these waters makes facilitates their use as daily soft drinks keeping their therapeutic features.
More than 50 different types of aromatic waters are produced and marketed in Iran, but as far as we know, the chemical constituents and biological activities of most of them have not been evaluated. Also, to the best of our knowledge no commercial products of them has been presented to the world markets. The aim of this study was to investigate constituents of aromatic waters and hydrosols used in Persian nutrition culture and folk medicine for hyperlipidemia and cardiovascular conditions as well as presenting them as potential functional soft drinks. Their nature and therapeutic indications have been also introduced in this study.
Materials and Methods
Information and Sample Collection
Fars Province, which is located in the south of Iran, was selected as the field of study. To gather information about different aromatic waters that are produced and used in Persian nutrition culture and folk medicine, the field study was conducted from March 2013 to March 2014 under the supervision of one local person as a native guide in all visits (84 manufactories). A suitable questionnaire was also prepared for this study, which was filled according to the information gathered in visits of the local manufactories or their shops. The frequency of each therapeutic effects for these aromatic waters from all questionnaires were calculated. The manufactories were also asked to rank these aromatic waters from 1 to 14 according to their mean of annual production over the past 3 years. The aromatic water with the lowest level of production was ranked 1. The ranking values from different manufactories are presented as mean ± standard deviation.
On the other hand, different aromatic waters that are used in Persian folk medicine as cardiovascular tonic or therapeutic beverages were purchased for further analysis. They are listed in Table 1 and coded as 1 to 14.
Table 1.
Plants’ Names and Their Medicinal Parts That Are Used to Prepare Aromatic Waters for Cardiovascular Diseases.
| Aromatic Water Beverage Name | Aromatic Water Name in Persian | Scientific Name | Family | Plant Parts |
|---|---|---|---|---|
| Aloe | Aragh-e-Sabre zard; Aragh-e-Aloe | Aloe spp. | Xanthorrhoeaceae | Leaf |
| Azarole hawthorn | Aragh-e-Keyalak | Crataegus azarolus L. | Rosaceae | Leaf and fruits |
| Barberry | Aragh-e-Zereshk | Berberis vulgaris L. | Berberidaceae | Fruits |
| Dill | Aragh-e-Shevid | Anethum graveolens L. | Apiaceae | Leaf |
| Fenugreek | Aragh-e-Shanbaleile | Trigonella foenum-graecum L | Fabaceae | Leaf |
| Garlic | Aragh-e-Sir | Allium sativum L. | Amaryllidaceae | Bulb |
| Olive | Aragh-e-Zeytoon | Olea europaea L. | Oleaceae | Leaf |
| Oriental plane | Aragh-e-Chenar | Platanus orientalis L. | Platanaceae | Leaf |
| Parsley | Aragh-e-Jafari | Petroselinum crispum Mill. | Apiaceae | Leaf |
| Poleygermander | Aragh-e-Kalpooreh | Teucrium polium L. | Lamiaceae | Aerial parts |
| Turnip | Aragh-e-Shalgham | Brassica rapa L. | Brassicaceae | Root |
| Wormwood | Aragh-e-Dermaneh | Artemisia sieberi Besser | Asteraceae | Aerial parts |
| Will fumitory | Aragh-e- Shatareh | Fumaria parviflora Lam. | Papaveraceae | Aerial parts |
| A mixture of nettle, walnut, saatar (Shirazi thyme), olive, and celery leaves | Aragh-e-Taadol | A mixture of the following: Urtica dioica L. | Urticaceae | Leaf |
| Juglans regia L. | Juglandaceae | Leaf | ||
| Zataria multiflora Boiss. | Lamiaceae | Leaf | ||
| Olea europaea L. | Oleaceae | Leaf | ||
| Apium graveolens var. dulce | Apiaceae | Aerial parts |
Phytochemical Analysis
Essential oils in each sample were extracted using a glass liquid extractor system. Five hundred milliliters of each sample was extracted with 500 mL of petroleum-ether as solvent. Petroleum-ether was heated to evaporation during 150 minutes. The solvent vapor was then transferred to the bottom of the beverage container. The vapor was liquefied in the beverage and due to the lower density it passed through the beverage toward the upper side of the container. At the same time, the essential oil of the sample was transferred from the aqueous phase to the petroleum-ether phase. In order to increase the essential oil concentration in the organic phase, after 150 minutes the used beverage was replaced with fresh beverage and the extraction procedure was continued for another 150 minutes. The extract of each sample was concentrated to approximately 10 mL at 40°C and 60 rpm using a basic rotary evaporator (IKA RV10), equipped with a Heidolph Rotavac vacuum pump.11
Gas Chromatography–Mass Spectrometry
The concentrated extract of each aromatic water beverage was dehydrated and subjected to gas chromatography–mass spectrometry for the analysis of the respective essential oils. Agilent Technologies 7890 Gas Chromatograph with a mass detector (Model 5975C) was used in the present study. The gas chromatograph was equipped with a HP-5MS capillary column (phenyl-methylsiloxan, 30 m, 0.25 mm i.d.; Agilent Technologies; model 19091S-433 [60°C to 325/350°C]) and a mass spectrometer (Agilent Technologies; model 5975C), which was operating in EI mode at 70 eV. The interface temperature was 280°C, and the mass range was 30 to 600 m/z. The oven was heated (5°C/min) from 60°C to 220°C and then it was held for 10 minutes at 220°C. Helium was the carrier gas, and the flow rate was set to 1 mL/min. The components were identified by comparing the mass spectra and retention times with those of reference compounds, or with mass spectra in NIST or Willey libraries or in literature.12–14
Statistical Analysis
Principal Component Analysis
In order to cluster the aromatic water samples based on their constituents resulting from gas chromatography–mass spectrometry analyses, principal component analysis was used as an unsupervised clustering analysis technique. Briefly, all aromatic samples together with their corresponding vectors of constituents generated a matrix in MATLAB (Mathworks Inc, Natick, MA). Principal components of the resulted matrix were thereafter extracted using singular value decomposition algorithm as implemented in MATLAB software. Principal component analysis theory is based on a ranking approach where principal components are sorted according to their eigenvalues in such a way that the first one contains the most variation inside the data set. Consequently, the next principal component is extracted to be orthogonal with respect to the previous one. The plot of the first 2 principal components is therefore representative of the whole data in a 2-dimensional space. The orthogonal feature of the first 2 principal components makes a representation of the data set in a 2-dimensional space.
Hierarchical Cluster Analysis
To perform hierarchical cluster analysis, the resultant matrix as prepared in the previous experiment was subjected once again to MATLAB software. Cluster definitions were done by means of Euclidean distance as a way to measure similarities using unweighted pair group method (UPGMA). The plot of the distances versus samples was used to represent the data based on their similarities. The final dendrogram could represent the similarities between the samples via its connectivity patterns.
K-Means Analysis
K-means separates the points of an N-by-P data matrix into K clusters. These partitions are designed in such a way to minimize the sum of the within-cluster sums of point-to-cluster-centroid distances. K-means returns an N-by-1 vector representing the cluster index for each sample. Euclidean distances were used for clustering purposes in this experiment.15
Results and Discussion
Fars province is located in the south of Iran. It has an area of 122 400 km2 and a population of 4.59 million people. Fars, or known in Old Persian as Pârsâ, is the original homeland of the ancient Persians. More than 84 manufactories are producing different medicinal aroma waters with traditional (65 manufactories) or full industrial techniques and equipment (about 19 manufactories). Most of these manufactories are located in Meymand and Darab cities, and their products are distributed all over the country.
Hydrosols and Their Phytochemicals
A list of aromatic waters that are used for hyperlipidemia and cardiovascular conditions was prepared according to indications on package labels or brochures written by their manufacturers or according to the information gathered via questionnaires (Tables 1 and 2).
Table 2.
Aromatic Waters’ Indications in Cardiovascular and Other Diseases.
| Aromatic Water Beverage Name | Nature | Cardiovascular Indication | Other Indications | Dosing |
|---|---|---|---|---|
| Aloe | Cold nature | Anti-anemia Antidiabetic Antihypertension Blood cleansing | Antidandruff and skin lightening Gastrointestinal tonic To treat peptic ulcers To treat insomnia | 100 mL BID or TID; before meal |
| Azarole hawthorn | Cold nature | Antiarrhythmic Anti-atherosclerosis Antipalpitation Cardio tonic | Antidiarrhea Antiepileptic Gastrointestinal tonic | 100 mL TID; before meal |
| Barberry | Cold nature | Antiatherosclerosis Antidiabetic Antihypertension Cholesterol lowering | Antidysentery Choleretic and chologue Liver tonic To treat kidney stones To treat intestinal cancers | 150 mL TID; after meal |
| Dill | Warm nature | Antihypertension Cholesterol lowering | Gastrointestinal tonic Galactogogue, menstruation inducer To treat urinary tract pain | 150 mL TID; after meal |
| Fenugreek | Warm nature | Anti-anemia Antidiabetic Antihypertension | Anti-rickets For weight gain Hair tonic | 150 mL TID; before meal |
| Garlic | Warm nature | Antihypertension Blood thinning Cholesterol lowering | Antibacterial Anthelmintic Hair tonic | 100 mL TID; after meal |
| Olive | Cold nature | Antidiabetic Antihypertension Diuretic | Liver tonic To improve memory To treat headache and toothache | 100-250 mL TID; before meal |
| Oriental plane | Cold nature | Antihypertension | Antipyretic For weight gain Nerve tonic; relief of pain | 250 mL TID; before meal |
| Parsley | Cold nature | Anti-anemia Antihypertension Blood cleansing Diuretic | Anti-arthritis Antipyretic Galactogogue Gastrointestinal tonic | 100 mL TID; before meal |
| Poleygermander | Warm nature | Antihypertension Antidiabetic Blood cleansing | Antiasthma Antibacterial Antiemetic Appetizer and liver tonic | 100 mL TID; before meal |
| Turnip | Warm nature | Antihypertension Antidiabetic | Appetizer Antitussive Eye tonic | 100 mL TID; before meal |
| Wormwood | Antihypertension Diuretic Perspirant | Antidiarrhea Appetizer Vermicide | 100 mL TID; before meal | |
| Will fumitory | Moderate nature | Antihypertension Blood cleansing Diuretic | Anti-scurvy Digestant | 100 mL TID; before meal |
| Taadol (a mixture of nettle, walnut, saatar, olive and celery leaves) | Warm nature | Anti-atherosclerosis Antihypertension Antidiabetic Blood thinning Lipid lowering | 100 mL TID; before meal |
The aim of this study was to investigate the aromatic waters that are used in Persian nutrition culture and folk medicine, but some aromatic waters listed in Tables 1 and 2 have been mentioned also in some traditional manuscript such as Qarabadin-e-salehi 16 and Qarabadin-e-kabir.17 Most current ethnopharmacological knowledge in Iran has been derived from historical manuscripts.18
Traditional knowledge of aromatic waters recorded in historical manuscripts can help unravel the ethnopharmacological roots of traditional Iranian concepts and herbal classifications.
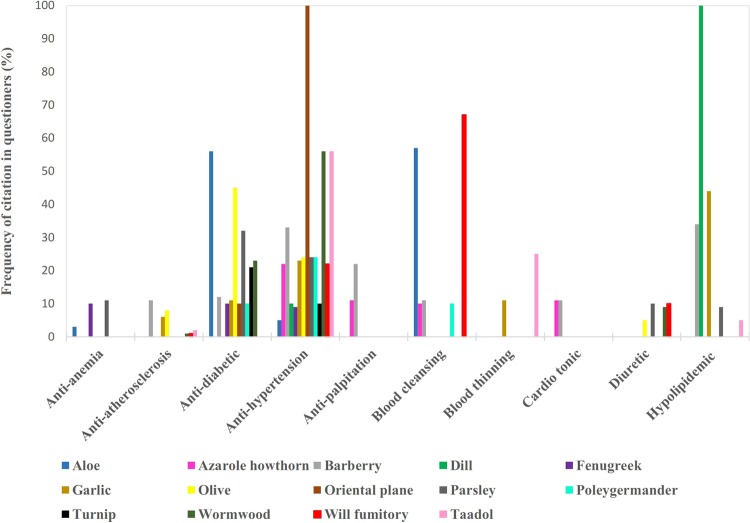
As seen in Table 1, the plants that are used to prepare these beverages belong to 11 different plants families. Apiaceae, Lamiaceae, and Asteraceae had a greater proportion than other families. The percentage of frequency of each cardiovascular application for these aromatic waters in all gathered questionnaires is shown in Figure 1. The higher percentage of frequency can show the higher importance of an application for a beverage. For example, in all questionnaires (100%), oriental plane aromatic water was suggested as a hypotensive and dill aromatic water as a hypolipidemic agent. while only a few informants believed that aloe aromatic water has anti-anemia properties. In ethnomedical surveys, cultural importance of species can reflect more accurate and more informants’ data obtained from questionnaires.19
Figure 1.
Frequency of citations in questionnaires for aromatic waters with cardiovascular effects.
As seen in Figure 1, most of these beverages were believed to show antihypertension properties. The second frequently cited application was antidiabetic effects.
In order to roughly evaluate the popularity of these aromatic waters in folk medicine, manufactories were also asked to rank these aromatic waters from 1 to 14 according to their mean of annual production over the past 3 years. Since these data were confidential for these manufactories, we used a ranking system. The aromatic water with the lowest level of production was ranked 1. The obtained ranking data from different manufactories are presented as mean ± standard deviation in Figure 2. Dill, will fumitory, Taadol, and oriental plane aromatic waters had higher annual production levels during the past 3 years. This popularity might be due to their efficacy, differences in prevalence of cardiovascular conditions in the region, or even the aromatic waters’ taste, aroma, or possible side effects during longer period of consumption.
Figure 2.
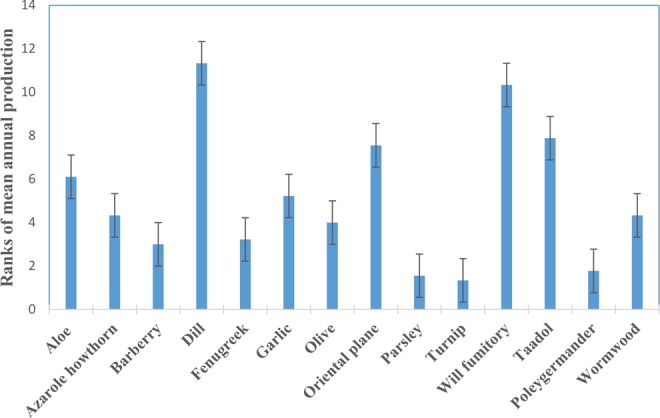
Ranking (1-14) of annual production level of aromatic waters in different manufactories over the past 3 years. Data are presented as mean ± standard deviation.
Most of these beverages are prepared from aerial parts (leaf and fruits) of the plants except in case of turnip (roots) and garlic (bulb). Different indications for cardiovascular conditions including cardiotonic, antihypertension, anti-arrhythmic, antipalpitation, blood cleansing, blood thinning, anti-anemia, anti-atherosclerosis, lipid lowering, antidiabetic, and diuretic were mentioned for these aromatic waters. It should be also mentioned that some of these beverages were believed to have cold nature while others had warm features. Other indications apart from cardiovascular specifications were also mentioned for these beverages, as summarized in Table 2.
As discussed earlier, aromatic waters have their own individual smell and compositions that are considerably irrelevant to the pure essential oils they were codistilled with. Therefore, it was necessary to elucidate chemical constituents of these aromatic waters by gas chromatography–mass spectrometry analysis after liquid-liquid extraction. The results are summarized in Table 3. In most of these aromatic waters, thymol is major or second major component except for wormwood and olive leaf aromatic waters. Carvacrol was also detected in all of these aromatic waters except for azarole hawthorn, wormwood, and olive leaf.
Table 3.
Aromatic Water Constituents Resulting From Gas Chromatography–Mass Spectrometry Analysis.
| Aloe | Azarole hawthorn | Barberry | Dill | Fenugreek | Garlic | Olive | Oriental plane | Parsley | Poley-germander | Taadol | Turnip | Will -fumitory | Worm-wood | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2,3-Dimethoxytoluene | — | — | — | — | — | — | 2.56 | — | — | — | — | — | — | |
| Acetophenone | — | — | — | — | — | — | — | 4.41 | — | — | — | — | ||
| Anethole (E) | — | — | — | 0.53 | — | — | — | — | — | 0.98 | — | — | — | — |
| Anethole (Z) | — | — | — | — | — | — | — | — | — | 1.52 | — | — | — | — |
| Apiole | — | — | — | — | — | — | — | — | 1.28 | — | — | — | — | — |
| Artemisia alcohol | — | — | — | — | — | — | — | — | — | — | — | — | — | 2.99 |
| Beta-fenchyl alcohol | — | — | — | — | — | — | 2.14 | — | — | — | — | — | — | — |
| A bisabolol oxide derivative | — | — | — | — | — | — | — | — | — | 4.28 | — | — | — | — |
| Bisabolol oxide A (α-) | — | — | 39.98 | — | — | — | — | — | — | — | — | — | — | — |
| Bisabolone oxide | — | — | 16.54 | — | — | — | — | — | — | — | — | — | — | — |
| Borneol | — | — | — | — | — | — | — | — | — | — | — | — | — | 1.84 |
| Camphor | — | — | — | — | — | — | — | — | — | — | — | — | — | 23.15 |
| Carvacrol | 6.17 | 6.69 | 12.14 | 5.31 | 24.07 | — | — | 2.74 | 36.90 | 13.80 | 22.22 | — | 1.30 | |
| Carvone | 3.89 | — | — | 9.90 | 12.88 | 2.37 | 1.93 | 23.22 | — | — | 15.84 | 5.18 | — | |
| 1,8-Cineole | 3.94 | — | — | — | — | 1.54 | 1.24 | 0.88 | 0.85 | 1.29 | — | 16.80 | ||
| m-Cumenol | — | — | — | — | — | — | — | — | — | — | — | — | — | 0.27 |
| p-Cymen-7-ol | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Damascenone (E-β) | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Davanone | — | — | — | — | — | — | — | — | — | — | — | 0.35 | ||
| Dihydro carveol | — | — | — | — | — | — | 5.96 | — | — | 8.93 | — | — | ||
| Dihydro carveol (iso) | — | — | — | — | — | — | — | — | — | — | — | — | — | |
| Dihydro carveol (neo) | 1.87 | — | — | — | — | 1.83 | — | — | — | — | 2.35 | — | — | |
| Dihydro carvone (cis) | 1.80 | — | — | 1.32 | 5.31 | 5.06 | — | — | — | — | 5.76 | 1.09 | — | — |
| Dihydro carvone (trans) | — | — | — | 0.66 | 2.74 | 1.79 | — | — | — | — | — | — | — | — |
| Dihydroactinidiolide | — | — | — | — | — | — | 6.43 | — | — | — | — | 6.70 | — | |
| Dill apiole | — | — | — | 5.96 | — | 6.15 | 1.34 | 8.02 | — | — | — | 0.67 | 20.29 | — |
| Dill ether | — | — | — | 40.91 | — | 4.32 | — | — | 1.56 | — | — | — | — | — |
| Ethylbenzene | — | — | — | — | — | — | — | 1.26 | — | — | — | — | — | — |
| Ethanone, 1-[2-(1,1-dimethylethyl)-1H-imidazol-4-yl]) | — | — | — | — | — | — | — | — | — | — | 1.08 | — | — | — |
| Eugenol | — | — | 0.91 | — | — | — | — | — | 5.09 | — | — | |||
| Fenchone | — | — | — | — | 0.36 | — | — | — | — | — | 0.58 | — | — | |
| Guaiacol (ρ-vinyl) | — | — | — | — | — | 0.70 | — | — | — | — | — | — | — | |
| Hexadecanoic acid | — | 7.71 | — | — | — | — | — | — | — | — | — | — | — | |
| Intermedeol (neo) | — | — | — | — | — | — | — | — | — | — | — | — | 0.37 | |
| Methyleugenol | — | — | — | — | — | — | 0.68 | — | — | — | — | — | — | |
| Jasmine (Z) | — | — | — | — | — | — | — | — | — | — | — | — | — | 0.25 |
| Linalool | — | — | — | — | — | 0.48 | — | — | — | — | — | 0.57 | — | 1.10 |
| Menth-2-en-1-ol (cis-ρ-) | — | — | — | — | — | — | — | — | — | — | — | — | 0.35 | |
| Menthol | 37.48 | — | — | 3.80 | — | 5.20 | — | — | — | 1.01 | — | — | — | — |
| Menthone (trans) | 5.46 | — | — | 2.41 | — | 1.13 | — | — | — | — | — | 0.53 | — | — |
| Menthone (cis) | 2.94 | — | — | 0.82 | — | 1.28 | — | — | — | — | — | — | — | — |
| Methyl hexadecanoate | — | — | 8.47 | — | 1.08 | — | — | — | 7.61 | 2.34 | 38.40 | 0.62 | ||
| Methyl jasmonate (Z) | — | — | — | — | — | — | — | — | — | — | — | — | — | 0.35 |
| Methyl octadecanoate | — | — | 1.16 | — | — | — | — | — | — | — | — | — | 5.82 | — |
| Methyl 5-vinylnicotinate | — | — | — | — | — | — | 29.75 | — | — | — | — | — | — | — |
| Muurolol (α) | — | — | — | — | — | — | — | — | — | 1.47 | — | — | — | — |
| m-Xylene | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Myristicin | — | — | — | — | — | 0.33 | — | 34.00 | 2.03 | — | — | — | 0.42 | |
| Myrtenol | — | — | — | — | — | — | — | — | — | — | — | — | — | 0.83 |
| Nerol | — | — | — | — | — | — | — | — | — | — | — | — | — | 0.26 |
| o-Xylene | 0.57 | — | — | — | — | — | — | 2.36 | — | — | — | — | — | — |
| Phenol-4,ethyl-2-methoxy | — | — | — | — | — | — | — | — | — | — | — | 7.53 | — | |
| Phenyl ethyl alcohol | — | — | — | — | — | — | 0.79 | — | — | — | — | — | — | — |
| Piperitenone | 2.77 | — | — | — | — | 2.45 | — | — | — | 1.52 | 0.76 | 2.66 | — | — |
| Piperitone | 2.02 | — | — | — | — | 0.43 | — | — | — | — | — | — | — | — |
| Pulegone | 5.38 | — | — | 0.57 | — | 3.50 | — | 5.04 | 0.99 | 1.67 | 6.13 | 6.07 | — | |
| Pulegone ethanoate | — | — | — | — | 3.01 | — | — | — | — | — | — | — | — | — |
| p-Xylene | 2.74 | 20.12 | — | — | — | — | 1.99 | 12.53 | — | — | — | — | — | — |
| Spathulenol | — | — | — | — | — | — | — | — | — | — | — | — | 0.75 | |
| Terpinen-4-ol | 3.07 | — | — | 0.56 | — | 1.07 | — | — | — | — | 0.49 | 0.67 | — | 6.08 |
| Terpineol (α) | 1.68 | — | — | — | 0.46 | — | — | — | — | — | 1.83 | |||
| Thujone (cis) | — | — | — | — | — | — | — | — | — | — | — | — | — | 0.74 |
| Thujone (trans) | — | — | — | — | — | — | — | — | — | — | — | — | — | 5.63 |
| Thymol | 11.09 | 28.71 | 23.82 | 19.49 | 20.04 | 32.00 | 4.34 | 6.25 | 56.61 | 26.19 | 44.98 | 49.20 | 6.75 | 2.93 |
| Thymol ethanoate | — | 2.34 | — | — | — | 0.35 | 6.49 | 1.24 | — | — | — | — | — | — |
| Yomogi alcohol | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
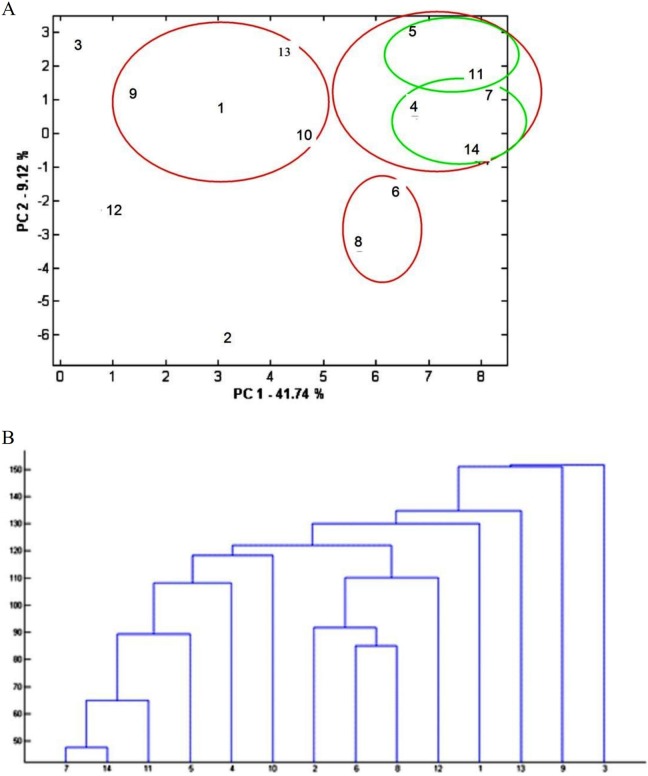
According to both hierarchical cluster analysis and K-means, oriental plane, fenugreek, and azarole hawthorn aromatic waters make a distinct cluster (Figure 3). The certain similarity of azarole hawthorn and fenugreek was also seen by means of principal component analysis. The reason for the observed similarities between these samples based on clustering analysis was the presence of comparable amounts of thymol (6.2% to 28.7%) in all 3 aromatic waters. In addition, carvone (23.22%) was the main component of oriental plane aromatic water, which was not detected in azarole hawthorn. According to hierarchical cluster analysis, fenugreek and azarole hawthorn made a subcluster that could be pertained to their similar thymol content.
Figure 3.
Cluster analysis of aromatic waters constituents based on principal component analysis (A) and hierarchical cluster analysis (B). The aromatic waters are as follows: 1 = aloe, 2 = oriental plane, 3 = wormwood, 4 = parsley, 5 = poleygermander, 6 = azarole hawthorn, 7 = turnip, 8 = fenugreek, 9 = will fumitory, 10 = dill, 11 = garlic, 12 = olive, 13 = barberry, and 14 = taadol.
Turnip, parsley, taadol, garlic, and poleygermander aromatic waters were classified as one cluster based on clustering analysis. According to K-means, there are 2 subclusters: one for turnip, parsley, taadol due to thymol (44.97% to 56.61%) as their main constituents and another for garlic and poleygermander, which contained 26% to 32% thymol. These aromatic waters (except for parsley) also contained comparable amount of carvacrol, 1,8-cineol, piperitenone, and pulegone. Parsley contained a considerable amount of myristicin (34%), which was not detected in other aromatic waters (Table 4).
Table 4.
Analysis of the Aromatic Waters’ Constituents Based on K-Means (sqEuclidean, 10 Epochs of Training).
| Aromatic Waters’ Name | Class |
|---|---|
| Barberry | I |
| Dill | II |
| Parsley, turnip, and taadol | III |
| Will fumitory | IV |
| Aloe | V |
| Garlic and poleygermander | VI |
| Olive and wormwood | VII |
| Azarole hawthorn, fenugreek, and oriental plane | VIII |
In contrast to other aromatic waters, wormwood and olive leaf had low thymol content (2%-6%). The main component of wormwood was camphor (23%), while in the case of olive leaf methyl 5-vinylnicotinate composed 29.76% of the aromatic water. Since these components were not detected in others they were clustered at distinct groups.
Based on clustering methods applied in this study, although some similarities could be found, composition of barberry, will fumitory, dill, and aloe aromatic waters revealed more differences than others. The main components of these aromatic waters were menthol (37%, aloe), methyl hexadecanoate (38.40%, will fumitory), bisabolol oxide A (39.98%, barberry), and dill ether (40.91%, dill).
Literature Survey
We could not find any reports on chemical composition of aromatic waters of the plants mentioned in Table 1. Thus, it was not possible to compare the results, but the major components of the reported essential oils are summarized in Table 5.
Table 5.
Profile of Essential Oils Reported in the Literature for the Plants Being Used to Prepare Cardiovascular Aromatic Waters and Hydrosols.
| Plant Name | Profile of Essential Oils Reported in the Literature | |
|---|---|---|
| Aloe | Profile of volatile components was not found in literature24,25 | |
| Azarole hawthorn | Fruits: Limonene, 2-furaldehyde, 3-cyclohexane-2-methyl-1-propenyl, γ-terpinene26 | |
| Leaves and flowers: n-Hexadecanoic acid, α-farnesene, alkanes27 | ||
| Barberry | Fruit: Benzaldehyde, benzyl alcohol, 1-hexanol, and (E)-2-hexenal | |
| Leaves and flowers: p-Cymene, limonene, ocimene28 | ||
| Dill | Limonene, Phellandrene, dihydrocarvone, and carvone29 | |
| α-Phellandrene, myristicin, dill ther, β-phellandrene22 | ||
| Phellandrene, limonene, dill ether23 | ||
| Fenugreek | Aerial parts: ω-Cadinene, α-cadinol, γ-eudesmol, and α-bisabolol30 | |
| Garlic | Leaves: Diallyl trisulfide, diallyl disulfide, methyl allyl trisulfide21 | |
| Bulb: Diallyl disulfide, diallyl trisulfide, methyl allyl trisulfide20 | ||
| Olive | Leaf: 2-Hexenal, α-farnesene, linalool31 | |
| Oriental plane | Leaf: Profile of volatile components was not found in literature32 | |
| Parsley | Myristicin, apiol, α-pinene, β-pinene | |
| β-Phellandrene, 1,3,8-p-menthatriene, α-,p-dimethylstyrene, myristicin, and β-myrcene33 | ||
| Myristicin, β-phellandrene, p-1,3,8-menthatriene22 | ||
| Poleygermander | α-Pinene, β-pinene, and p-cymene34 | |
| α-Cadinol, 3-β-hydroxy-a-muurolene, a-pinene, and β-pinene35 | ||
| Caryophyllene, torreyol, α-cadinol, and α-humulene36 | ||
| α-Pinene, linalool, caryophyllene oxide, β-pinene, caryophyllene37 | ||
| Turnip | 3-Butenylisothiocyanate, 4-pentenyl isothiocyanate, 2-methyl-5-hexenenitrile38 | |
| 2-Butylisothiocyanate, 3-butenylisothiocyanate, ionone, menthol39 | ||
| Wormwood | Camphor, 1,8-cineole, and bornyl acetate40 | |
| Artemisia ketone, 1, 8-cineole, selin-11-en-4-a-ol, and lavandulon41 | ||
| Camphor, camphene, 1,8-cineol, β-thujone, and α-pinene42 | ||
| β-thujone, camphor and α-thujone43 | ||
| Will fumitory | Profile of volatile components was not found in literature44 | |
| Taadol | Celery | Leaf: 4-Chloro-4,4-dimethyl-3-(1-imidazolyl)-valerophenone, 1-dodecanol45 |
| Leaf, stalk and roots: (Z)-3-butylidenephthalide, 3-butyl-4,5-dihydrophthalide, and α-thujene46 | ||
| Leaf: Limonene, β-caryophyllene, and 3-butyl-4,5-dihydrophthalide47 | ||
| Leaf: α-Pinene, β-pinene, myrcene, limonene, γ-terpinene, β-elemene, β-caryophyllene44 | ||
| Nettle | Profile of volatile components was not found in literature | |
| Saatar | Thymol, carvacrol, linalool48 | |
| Walnut | Husks: (E)-4,8-Dimethyl-1,3,7-nonatriene, pinocarvone, pinocarveol, myrtenal, myrtenol49 | |
| (E,E)-4,8,12-Trimethyl-1,3,7,11-tridecatetraene, caryophyllene epoxide, verbenol, verbenone | ||
| Leaf: Germacrene D, methyl salicylate50 | ||
For aloe leaf, oriental plane leaf, and will fumitory, we could not find any reports and our article seems to be the first report on their volatile components. For some of these aromatic waters, such as barberry and poleygermander, garlic, and turnip, the major components in the aromatic waters and essential oils are completely different. Different allyl sulfides were reported as the major components of the garlic essential oils20,21 and isothiocyanate derivatives as the major components of the turnip essential oil but none of these components were detected in the aromatic waters in the present study. In the case of dill essential oil, the major components were reported to be phellandrene, limonene, and myristicin, followed by dill ether.22,23 In the present study, the major components of dill aromatic water was dill ether (40.9%), followed by thymol and carvacrol. On the other hand, the major components of parsley leaf (myristicin) and wormwood (camphor) were similar in aromatic waters and reported essentials but their percentage as well as nonmajor components are different (Tables 3 and 5). As it was expected, comparing the results of this study on components of the aromatic waters (Table 3) with the reports on essential oils (Table 5) shows that there is a remarkable difference between aromatic waters and essential oil components. This might be due to different water solubility of the volatile components; thus, some of these volatile components did not enter in the water phase while preparing aromatic waters. It seems that it is essential to consider different biological activities for aromatic waters due to different chemical compositions compared with pure essential oils.
Different cardiovascular effects of the plants used to prepare identified aromatic waters were investigated from the literature and are summarized in Table 6. We could not find any report on cardiovascular activity for any of the aromatic waters. But for some of these plants including fenugreek, wormwood, and celery there are some reports on extracted essential oil. Although it is not possible to compare the observed effects of the essential oils with aromatic waters due to differences in constituents as well as constituent’s concentrations, these reports strengthen the hypothesis of cardiovascular tonic effects for these aromatic waters.
Table 6.
Literature Review on Plants Used in Preparing Aromatic Waters With Cardiovascular Indications.
| Aloe spp (Aloe vera, Aloe babadensis) | Antidiabetic and obesity | Phytosterol | In vivo51 | |
| Antihypertensive | Leaf extracts and constituents (Aloe-emodin. Aloin A, etc) | In vivo52 | ||
| Cardioprotective | Leaf gel | In vivo53,54 | ||
| Hypoglycemic and hypolipidemic | Leaf gel | Clinical trial55–58 | ||
| Gel extracts | In vivo59,60 | |||
| Azarole howthorn (Crataegus azarolus L.) | Cardioprotective | Aqueous extract of aerial part | In vivo61 | |
| Antiarrhythmic | Aqueous extract of aerial part | In vivo62 | ||
| Anti-atherosclerosis | Aqueous extract of aerial part | In vitro63 | ||
| In vivo64,65 | ||||
| Antipalpitation | Aqueous extract of aerial part | Clinical trial66,67 | ||
| Hypotensive | Aqueous extract of aerial part | In vivo68 | ||
| Positive inotropic and negative chronotropic | Aqueous extract of aerial part | In vivo69 | ||
| Positive inotropic, diuretic and natriuretic | Procyanidine of the fruit | In vivo70 | ||
| Vasorelaxant | Aqueous extract of aerial part | In vivo71 | ||
| Barberry (Berberis vulgaris L.) | Antihypertension | Fruits in apple vinegar | Clinical trial72 | |
| Fruits aqueous extract | In vivo73,74 | |||
| Methanolic extract of root and bark | In vivo75 | |||
| Effects on non-alcoholic fatty liver | Fruits aqueous extract | Clinical trial76 | ||
| Hypoglycemic | Berberine | In vivo77 | ||
| Fruits aqueous extract | In vivo78,79 | |||
| Hypolipidemic | Fruits aqueous extract | In vivo80 | ||
| Ethanolic extracts of roots | In vivo81 | |||
| Dill (Anethum graveolens L.) | Antihypertension | Hydroalcoholic extract of aerial part | Clinical trial82 | |
| Hypolipidemic | Hydroalcoholic extract of aerial part | Clinical trial82–85 | ||
| Different fractions of leaves | In vivo86 | |||
| Fenugreek (Trigonella foenum-graecum L.) | Anti-anemia (increase hemoglobin and WBC level) | Seed extracts | Clinical trial87 | |
| In vivo88–91 | ||||
| Antidiabetic | Seed extracts | Clinical trial92–95 | ||
| In vivo96–98 | ||||
| Antihypertension | Essential oil | In vivo99 | ||
| Hypolipidemic | Seed extract | Clinical trial100 | ||
| Seed extract | In vivo101 | |||
| Leaf extract | In vivo102,103 | |||
| Garlic (Allium sativum L.) | Anti-atherosclerosis | Aged garlic extract supplement | Clinical trial104,105 | |
| Antihypertension | Aqueous extract or powder | Clinical trial106–109 | ||
| Aqueous extract or powder | In vivo110–112 | |||
| Hypoglycemic effects | Aqueous extracts or powder | Clinical trial113,114 | ||
| Bulb extracts or powder | In vivo115–118 | |||
| Garlic oil | In vivo117,119 | |||
| Hypolipidemic | Aqueous extracts or powder | Clinical trial and In vivo114,120–126 | ||
| Effects on thrombocyte aggregation | Aqueous extract | In vivo127,128 | ||
| Olive (Olea europaea L.) | Antihypertension | Leaf extracts | Clinical trial129–134 | |
| Triterpenoids of the leaf | In vivo135–138 | |||
| Leaf extracts | In vivo135 | |||
| Cardiovascular protection | Olive oil | Clinical trial131,137–141 | ||
| Diuretic | Leaf extracts | In vivo142 | ||
| Hypoglycemic effects | Leaf extracts | Clinical trial and In vivo143–145 | ||
| Parsley (Petroselinum crispum Mill.) | Antidiabetic | Extracts of aerial part | In vivo146,147 | |
| Antihypertension | Extracts of aerial part | In vivo146,148 | ||
| Antiplatelet | Aqueous extracts | In vitro148–150 | ||
| Cardiovascular protection | Extracts of aerial part | In vivo151,152 | ||
| Diuretic | Extracts of aerial part | In vivo and in vitro153,154 | ||
| Poleygermander (Teucrium polium L.) | Antidiabetic | Extracts of aerial part | Clinical trial155 | |
| In vivo156–159 | ||||
| Antihypertension | Extracts of aerial part | In vivo160,161 | ||
| Hypolipidemic | Aqueous extract of aerial parts | In vivo159,162–164 | ||
| Turnip (Brassica rapa L.) | Antidiabetic | Root extracts | In vivo165–168 | |
| Hypolipidemic | Seed oil | Clinical trial169 | ||
| Root extracts | In vivo170,171 | |||
| Wormwood (Artemisia sieberi Besser) | Antidiabetic | Essential oil from aerial parts | In vivo172–174 | |
| Antihypertension | Essential oil | Hypothesis175 | ||
| Cardiovascular protection | Essential oil from aerial parts | In vivo176 | ||
| Hypolipidemic | Hydroethanolic extract | In vivo177 | ||
| Will fumitory (Fumaria parviflora Lam.) | Hypoglycemic | Extracts of aerial parts | In vivo178,179 | |
| Hypolipidemic | Aerial parts | In vivo180,181 | ||
| Taadol | Celery (Apium graveolens) | Antihypertension | Extracts of aerial parts | Clinical trial182,183 |
| Extracts of aerial parts | In vivo184–187 | |||
| Hypoglycemic | Extracts of aerial parts | In vivo188–190 | ||
| Hypolipidemic | Seed extract | In vivo191–194 | ||
| Essential oil | In vivo187 | |||
| Nettle (Urtica dioica L.) | Antihypertension | Root extracts | Clinical trial195 | |
| Root extracts | In vivo196 | |||
| Extracts of aerial parts | In vivo195–199 | |||
| Hypoglycemic | Extracts of aerial parts | Clinical trial200 | ||
| Extracts of aerial parts | In vivo195,197,199,201–205 | |||
| Seed extracts | In vivo206 | |||
| Hypolipidemic | Extracts of aerial parts | In vivo207–209 | ||
| Antihypertension | Extracts of aerial parts | In vivo210 | ||
| Saatar (Zataria multiflora Boiss.) | Antidiabetic | Essential oil | In vivo208,211,212 | |
| Extracts of aerial parts | In vivo210,213 | |||
| Walnut (Juglans regia L.) | Antihypertension | Leaf extracts | In vivo214 | |
| Antidiabetic | Leaf extracts | Clinical trial214 | ||
| Seed extract | Clinical trial215–218 | |||
| Leaf extracts | In vivo219 | |||
| Septum extract | In vivo219 | |||
| Flower extract | In vivo219 | |||
| Hypolipidemic | Seed extract | Clinical trial214,219–223 | ||
| In vivo224 | ||||
For other plants, different aqueous, ethanol, methanol extracts or plants powders were investigated and it is not clear if the volatile components had a role in observed effects. On the other hand, for many of the plants listed in the Table 6, the medicinal parts that were investigated are different from those that are used to prepare the aromatic waters in Persian ethnomedicine. For oriental plane we could not find any related report. This study was not intended to investigate the efficacy of these aromatic waters, but high production level and consumption of these aromatic waters in Persian nutrition culture and folk medicine might be related to their efficacy.
Overall, this article introduced some aromatic waters that are used for hyperlipidemia and cardiovascular conditions in Persian nutrition culture and folk medicine with different popularity and sales values. As was expected, their chemical composition was different from the essential oils of the plants used for their production. But cluster analysis showed that despite the differences in the plant family and medicinal parts used to prepare them, some similarity can be found in their chemical compositions. In most cases thymol was the major or second major component of these beverages.
Investigating aromatic waters scientifically may lead to the development of some functional beverages and soft drinks as a safe way of administration of essential oils or even new therapeutic components.
Acknowledgments
The authors also want to thank Nahal Shamaeezadeh (PharmD student at Shiraz University of Medical Sciences) for helping in extraction procedures.
Authors’ Note: This study was part of the PharmD thesis project of Seyed Mahmoud Moheimani.
Author Contributions: AH wrote the draft and contributed in guidance and data collection. AS contributed in the guidance and revisions of the final version of the article. SM, MM, and HE contributed in data collection and analyzing data.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Shiraz University of Medical Sciences (Grant # 92-01-70-7065).
Ethical Approval: This study was an experimental and laboratorial work and did not require ethical approval.
References
- 1. Laslett LJ, Alagona P, Clark BA, et al. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol. 2012;60(25 suppl):S1–S49. [DOI] [PubMed] [Google Scholar]
- 2. Wilson P. Overview of the risk equivalents and established risk factors for cardiovascular disease. http://www.uptodate.com/contents/overview-of-the-risk-equivalents-and-established-risk-factors-for-cardiovascular-disease. Accessed January28,2016.
- 3. Sağdιç O. Sensitivity of four pathogenic bacteria to Turkish thyme and oregano hydrosols. LWT—Food Sci Technol. 2003;36:467–473. [Google Scholar]
- 4. Aazza S, Lyoussi B, Miguel MG. Antioxidant activity of eight hydrosols from Morocco. Asian J Plant Sci. 2012;11(3):137–142. [Google Scholar]
- 5. Schorr S. Bioresonance and Phytotherapeutic Hydrosols in Healing. Kihei, HI: Bioponic Phytoceuticals; 2004. [Google Scholar]
- 6. Ghaieni Hravi M. The Book of Gharabadin Salehi (Lithograph in Persian; );1766. [Google Scholar]
- 7. Aghili Shirazi S. Qarabadin-e-Kabir. Tehran, Iran: Ostad Allah Qoli Khan Qajar (Lithograph in Persian); 1772/1855. [Google Scholar]
- 8. Rose J. 375 Essential Oils and Hydrosols. Berkeley, CA: Frog Books; 1999. [Google Scholar]
- 9. Catty S. Hydrosols: The Next Aromatherapy. Tumwater, WA: Capital City Press; 2001. [Google Scholar]
- 10. Price L, Price S. Understanding Hydrolats: The Specific Hydrosols for Aromatherapy: A Guide for Health Professionals. London, England: Churchill Livingstone; 2004. [Google Scholar]
- 11. Kurkcuoglu M, Baser KH. Studies on Turkish rose concrete, absolute, and hydrosol. Chemistry of natural compounds. Chem Nat Comp. 2003;39:457–464. [Google Scholar]
- 12. Hamedi A, Mohagheghzadeh A, Rivaz S. Preliminary pharmacognostic evaluation and volatile constituent analysis of spathe of Phoenix dactylifera L. (Tarooneh). Phcog J. 2013;5(2):83–86. [Google Scholar]
- 13. Hamedi A, Mohagheghzadeh A, Rivaz S. Hydrodistilled volatile constituents obtained from the roots of Operculina turpethum . Phcog J. 2014;6(2):36. [Google Scholar]
- 14. Mojab F, Hamedi A, Nickavar B, Javidnia K. Hydrodistilled volatile constituents of the leaves of Daucus carota L. subsp. sativus (Hoffman.) Arcang. (Apiaceae) from Iran. J Essential Oil Bearing Plants. 2008;11:271–277. [Google Scholar]
- 15. Torras-Claveria L, Berkov S, Codina C, Viladomat F, Bastida J. Metabolomic analysis of bioactive Amaryllidaceae alkaloids of ornamental varieties of Narcissus by GC-MS combined with k-means cluster analysis. Ind Crop Prod. 2014;56:211–222. [Google Scholar]
- 16. Heravi MG. Qarabadin-e-Salehi. Tehran, Iran: Dar-ol-khalafeh (Lithograph in Persian); 1765. [Google Scholar]
- 17. Aghili Shirazi S. Qarabadin-e-Kabir. Tehran, Iran: Ostad Allah Qoli Khan Qajar (Lithograph in Persian); 1772. [Google Scholar]
- 18. Hamedi A, Zarshenas MM, Sohrabpour M, Zargaran A. Herbal medicinal oils in traditional Persian medicine. Pharm Biol. 2013;51:1208–1218. [DOI] [PubMed] [Google Scholar]
- 19. Heinrich M, Edwards S, Moerman DE, Leonti M. Ethnopharmacological field studies: a critical assessment of their conceptual basis and methods. J Ethnopharmacol. 2009;124:1–17. [DOI] [PubMed] [Google Scholar]
- 20. Kimbaris AC, Siatis NG, Daferera DJ, Tarantilis PA, Pappas CS, Polissiou MG. Comparison of distillation and ultrasound-assisted extraction methods for the isolation of sensitive aroma compounds from garlic (Allium sativum). Ultrason Sonochem. 2006;13:54–60. [DOI] [PubMed] [Google Scholar]
- 21. Edris AE, Fadel HM. Investigation of the volatile aroma components of garlic leaves essential oil. Possibility of utilization to enrich garlic bulb oil. Eur Food Res Technol. 2002;214:105–107. [Google Scholar]
- 22. Vokk R, Lõugas T, Mets K, Kravets M. Dill (Anethum graveolens L.) and parsley (Petroselinum crispum (Mill.) Fuss) from Estonia: seasonal differences in essential oil composition. Agron Res. 2011;9:515–520. [Google Scholar]
- 23. Orhan IE, Senol FS, Ozturk N, Celik SA, Pulur A, Kan Y. Phytochemical contents and enzyme inhibitory and antioxidant properties of Anethum graveolens L. (dill) samples cultivated under organic and conventional agricultural conditions. Food Chem Toxicol. 2013;59:96–103. [DOI] [PubMed] [Google Scholar]
- 24. Hadjimitsi E, Zabetakis I. The aroma of jam prepared from fruits of mosphilla (Crataegus azarolus L.). Flavour Fragr J. 2005;20:507–511. [Google Scholar]
- 25. Edwards JE, Brown PN, Talent N, Dickinson TA, Shipley PR. A review of the chemistry of the genus Crataegus . Phytochemistry. 2012;79:5–26. [DOI] [PubMed] [Google Scholar]
- 26. Lakache Z, Tigrine-Kordjani N, Tigrine C, Kameli A, Meklati BY. Volatile constituents, phenolic compounds, and antioxidant activity of Crataegus azarolus leaves and flowers growing in Algeria. Chem Nat Comp. 2014;50:1132–1135. [Google Scholar]
- 27. Dolezal M, Velíšek J, Famfulíková P, et al. Chemical composition of less-known wild fruits. Biologically-active phytochemicals in food: analysis, metabolism, bioavailability and function. Paper presented at: Proceedings of the Eurofood Chem XI Meeting; Norwich, UK; 2001. [Google Scholar]
- 28. Naef A, Roy BA, Kaiser R, Honegger R. Insect-mediated reproduction of systemic infections by Puccinia arrhenatheri on Berberis vulgaris . New Phytol. 2002;154:717–730. [DOI] [PubMed] [Google Scholar]
- 29. Porter NG, Shaw ML, Shaw GJ, Ellingham PJ. Content and composition of dill herb oil in the whole plant and the different plant parts during crop development. N Z J Agric Res. 1983; 26:119–127. [Google Scholar]
- 30. Ahmadiani A, Rustaiyan A, Karimian M, Kamalinejad M. Volatile constituents from the oil of Trigonella foenum-graecum L. J Essent Oil Res. 2004;16:356–357. [Google Scholar]
- 31. Flamini G, Cioni PL, Morelli I. Volatiles from leaves, fruits, and virgin oil from Olea europaea Cv. Olivastra Seggianese from Italy. J Agric Food Chem. 2003;51:1382–1386. [DOI] [PubMed] [Google Scholar]
- 32. Zhang H, Chen F, Wang X, Yaoa HY. Evaluation of antioxidant activity of parsley (Petroselinum crispum) essential oil and identification of its antioxidant constituents. Food Res Int. 2006;39:833–839. [Google Scholar]
- 33. Petropoulos SA, Daferera D, Polissiou MG, Passam HC. The effect of water deficit stress on the growth, yield and composition of essential oils of parsley. Sci Hort. 2008;115:393–397. [Google Scholar]
- 34. Cozzani S, Muselli A, Desjobert JM, Casanova J. Chemical composition of essential oil of Teucrium polium subsp. capitatum (L.) from Corsica. Flavour Fragr J. 2005;20:436–441. [Google Scholar]
- 35. Kabouche A, Kabouche Z, Ghannadi A, Sajjadi S E. Analysis of the essential oil of Teucrium polium ssp. aurasiacum from Algeria. J Essent Oil Res. 2007;19:44–46. [Google Scholar]
- 36. Menichini F, Conforti F, Rigano D, et al. Phytochemical composition, anti-inflammatory and antitumour activities of four Teucrium essential oils from Greece. Food Chem. 2009;115:679–686. [Google Scholar]
- 37. Moghtader M. Chemical composition of the essential oil of Teucrium polium L. from Iran. Am Eurasian J Agric Environ Sci. 2009;5:843–846. [Google Scholar]
- 38. Miyazawa M, Nishiguchi T, Yamafuji C. Volatile components of the leaves of Brassica rapa L. var. perviridis bailey. Flavour Fragr J. 2005;20:158–160. [Google Scholar]
- 39. Taveira M, Fernandes F, de Pinho PG, et al. Evolution of Brassica rapa var. rapa L. volatile composition by HS-SPME and GC/IT-MS. Microchem J. 2009;93:140–146. [Google Scholar]
- 40. Sefidkon F, Jalili A, Mirhaji T. Essential oil composition of three Artemisia spp. from Iran. Flavour Fragr J. 2002;17:150–152. [Google Scholar]
- 41. Behmanesh B, Heshmati G, Mazandarani M, et al. Chemical composition and antibacterial activity from essential oil of Artemisia sieberi Besser subsp. Sieberi in North of Iran. Asian J Plant Sci. 2007;6:562–564. [Google Scholar]
- 42. Ghasemi E, Yamini Y, Bahramifar N, Sefidkon F. Comparative analysis of the oil and supercritical CO2 extract of Artemisia sieberi . J Food Eng. 2007;79:306–311. [Google Scholar]
- 43. Khosravi AR, Shokri H, Kermani S, Parsa S. Antifungal properties of Artemisia sieberi and Origanum vulgare essential oils against Candida glabrata isolates obtained from patients with vulvovaginal candidiasis. J Mycol Med. 2011;21:93–99. [Google Scholar]
- 44. Wilson C. Terpene and sesquiterpene hydrocarbons in the essential oil from fresh celery. J Food Sci. 1969;34:521–523. [Google Scholar]
- 45. Nagella P, Ahmad A, Kim SJ, Chung IM. Chemical composition, antioxidant activity and larvicidal effects of essential oil from leaves of Apium graveolens . Immunopharmacol Immunotoxicol. 2012;34:205–209. [DOI] [PubMed] [Google Scholar]
- 46. Sellami IH, Bettaieb I, Bourgou S, et al. Essential oil and aroma composition of leaves, stalks and roots of celery (Apium graveolens var. dulce) from Tunisia. J Essent Oil Res. 2012;24:513–521. [Google Scholar]
- 47. Pino JA, Rosado A, Fuentes V. Leaf oil of celery (Apium graveolens L.) from Cuba. J Essent Oil Res. 1997;9:719–720. [Google Scholar]
- 48. Saei-Dehkordi SS, Tajik H, Moradi M, Khaliqhi-Sigaroodi F. Chemical composition of essential oils in Zataria multiflora Boiss. from different parts of Iran and their radical scavenging and antimicrobial activity. Food Chem Toxicol. 2010;48:1562–1567. [DOI] [PubMed] [Google Scholar]
- 49. Buttery RG, Light DM, Nam Y, Merrill GB, Roitman JN. Volatile components of green walnut husks. J Agric Food Chem. 2000;48:2858–2861. [DOI] [PubMed] [Google Scholar]
- 50. Farag MA. Headspace analysis of volatile compounds in leaves from the Juglandaceae (walnut) family. J Essent Oil Res. 2008;20:323–327. [Google Scholar]
- 51. Misawa E, Tanaka M, Nomaguchi K, Iwatsuki K. Oral ingestion of Aloe vera phytosterols alters hepatic gene expression profiles and ameliorates obesity-associated metabolic disorders in Zucker diabetic fatty rats. J Agric Food Chem. 2012;60:2799–2806. [DOI] [PubMed] [Google Scholar]
- 52. Saleem R, Faizi S, Siddiqui BS, et al. Hypotensive effect of chemical constituents from Aloe barbadensis . Planta Med. 2001;67:757–760. [DOI] [PubMed] [Google Scholar]
- 53. Jain N, Vijayaraghavan R, Pant SC, Lomash V, Ali M. Aloe vera gel alleviates cardiotoxicity in streptozocin-induced diabetes in rats. J Pharm Pharmacol. 2010;62:115–123. [DOI] [PubMed] [Google Scholar]
- 54. Sakai T, Repko B, Griffith B, Waters JH, Kameneva MV. IV infusion of a drag-reducing polymer extracted from aloe vera prolonged survival time in a rat model of acute myocardial ischaemia. Br J Anaesth. 2007;98:23–28. [DOI] [PubMed] [Google Scholar]
- 55. Vogler B, Ernst E. Aloe vera: a systematic review of its clinical effectiveness. Br J Gen Pract. 1999;49:823–828. [PMC free article] [PubMed] [Google Scholar]
- 56. Huseini HF, Kianbakht S, Hajiaghaee R, Dabaghian FH. Anti-hyperglycemic and anti-hypercholesterolemic effects of Aloe vera leaf gel in hyperlipidemic type 2 diabetic patients: a randomized double-blind placebo-controlled clinical trial. Planta Med. 2012;78:311–316. [DOI] [PubMed] [Google Scholar]
- 57. Ulbricht C, Armstrong J, Basch E, Weissner W. An evidence-based systematic review of Aloe vera by the Natural Standard Research Collaboration. J Herb Pharmacother. 2008;7:279–323. [DOI] [PubMed] [Google Scholar]
- 58. Yagi A, Hegazy S, Kabbash A, Wahab EA. Possible hypoglycemic effect of Aloe vera L. high molecular weight fractions on type 2 diabetic patients. Saudi Pharm J. 2009;17:209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rajasekaran S, Sivagnanam K, Ravi K, Subramanian S. Hypoglycemic effect of Aloe vera gel on streptozotocin-induced diabetes in experimental rats. J Med Food. 2004;7:61–66. [DOI] [PubMed] [Google Scholar]
- 60. Surjushe A, Vasani R, Saple D. Aloe vera: a short review. Indian J Dermatol. 2008;53:163–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shatoor AS, Ahmed MAAS. Cardioprotective effect of Crataegus aronia syn. azarolus (L) aqueous extract against doxorubicin-induced cardiotoxicity and heart failure in Wistar rats. J Basic Appl Sci Res. 2014;4:102–114. [Google Scholar]
- 62. Garjani A, Nazemiyeh H, Maleki N, Valizadeh H. Effects of extracts from flowering tops of Crataegus meyeri A. Pojark. on ischaemic arrhythmias in anaesthetized rats. Phytother Res. 2000;14:428–431. [DOI] [PubMed] [Google Scholar]
- 63. Belkhir M, Rebai O, Dhaouadi K, et al. Antioxidant and antimicrobial activities of Tunisian azarole (Crataegus azarolus L.) leaves and fruit pulp/peel polyphenolic extracts. Int J Food Prop. 2013;16:1380–1393. [Google Scholar]
- 64. Xu H, Xu HE, Ryan D. A study of the comparative effects of hawthorn fruit compound and simvastatin on lowering blood lipid levels. Am J Chin Med. 2009;37:903–908. [DOI] [PubMed] [Google Scholar]
- 65. Rajendran S, Deepalakshmi PD, Parasakthy K, Devaraj H, Devaraj SN. Effect of tincture of Crataegus on the LDL-receptor activity of hepatic plasma membrane of rats fed an atherogenic diet. Atherosclerosis. 1996;123:235–241. [DOI] [PubMed] [Google Scholar]
- 66. Wang J, Xiong X, Feng B. Effect of Crataegus usage in cardiovascular disease prevention: an evidence-based approach. J Evid Based Complementary Altern Med. 2013;2013:149363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Eggeling T, Regitz-Zagrosek V, Zimmermann A, Burkart M. Baseline severity but not gender modulates quantified Crataegus extract effects in early heart failure—a pooled analysis of clinical trials. Phytomedicine. 2011;18:1214–1229. [DOI] [PubMed] [Google Scholar]
- 68. Shatoor AS. In vivo hemodynamic and electrocardiographic changes following Crataegus aronia syn. azarolus (L) administration to normotensive Wistar rats. Saudi Med J. 2013;34:123–134. [PubMed] [Google Scholar]
- 69. Shatoor AS. Cardio-tonic effect of the aqueous extract of whole plant of Crataegus aronia syn: azarolus (L) on isolated rabbits heart. Afr J Pharm Pharmacol. 2012;6:1901–1909. [Google Scholar]
- 70. Dizaya K, AL-Jeboory A, AL-Jaff H. The pharmacological studies of procyanidine isolated from Crataegus azarolus (Iraqi endigenous). Pak J Pharmacol. 2005;22:57–79. [Google Scholar]
- 71. Al-Habib O, Shekha M. Vasorelaxant effect of aqueous extract of Crataegus azarolus aronia and quercetin on isolated albino rat’s thoracic aorta. J Duhok Univ. 2010;13:1–9. [Google Scholar]
- 72. Golzarand M, Ebrahimi-Mamaghani M, Arefhosseini S, Asgarzadeh AA. Effect of processed Berberis vulgaris in apple vinegar on blood pressure and inflammatory markers in type 2 diabetic patients. J Diabetes Metab Disord. 2008;7(3):3. [Google Scholar]
- 73. Fatehi-Hassanabad Z, Jafarzadeh M, Tarhini A, Fatehi M. The antihypertensive and vasodilator effects of aqueous extract from Berberis vulgaris fruit on hypertensive rats. Phytother Res. 2005;19:222–225. [DOI] [PubMed] [Google Scholar]
- 74. Fatehi M, Saleh TM, Fatehi-Hassanabad Z, et al. A pharmacological study on Berberis vulgaris fruit extract. J Ethpharmacol. 2005;102:46–52. [DOI] [PubMed] [Google Scholar]
- 75. Azmat A, Ahmed M, Zafar NU, Ahmad ASI. Hypotensive activity of methanolic extract of Berberis vulgaris (root pulp and bark). Pak J Pharmacol. 2009;26(2):41–47. [Google Scholar]
- 76. Iloon Kashkooli R, Najafi SS, Sharif F, et al. The effect of Berberis vulgaris extract on transaminase activities in non-alcoholic fatty liver disease. Hepat Mon. 2015;15(2):e25067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ding Y, Ye X, Zhu J, et al. Structural modification of berberine alkaloid and their hypoglycemic activity. J Funct Foods. 2014;7:229–237. [Google Scholar]
- 78. Hajzadeh M, Rajaei Z, Shafiee S, et al. Effect of barberry fruit (Berberis vulgaris) on serum glucose and lipids in istreptozotocin-diabetic rats. Pharmacology. 2011;1:809–817. [Google Scholar]
- 79. Meliani N, Dib MEA, Allali H, Tabti B. Hypoglycaemic effect of Berberis vulgaris L. in normal and streptozotocin-induced diabetic rats. Asian Pac J Trop Biomed. 2011;1:468–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Shidfar F, Seyyed Ebrahimi S, Hosseini S, Heydari I, Shidfar S, Hajhassani G. The effects of Berberis vulgaris fruit extract on serum lipoproteins, apoB, apoA-I, homocysteine, glycemic control and total antioxidant capacity in type 2 diabetic patients. Iran J Pharm Res. 2012;11:643–652. [PMC free article] [PubMed] [Google Scholar]
- 81. Changizi Ashtiyani S, Zarei A, Taheri S, et al. A comparative study of hypolipidemic activities of the extracts of Melissa officinalis and Berberis vulgaris in rats. J Med Plants. 2013;3(47):38–47. [Google Scholar]
- 82. Mansouri M, Nayebi N, Hasani-Ranjbar S, et al. The effect of 12 weeks Anethum graveolens (dill) on metabolic markers in patients with metabolic syndrome; a randomized double blind controlled trial. Daru. 2012;20(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mirhosseini M, Baradaran A, Rafieian-Kopaei M. Anethum graveolens and hyperlipidemia: a randomized clinical trial. J Res Med Sci. 2014;19:758–761. [PMC free article] [PubMed] [Google Scholar]
- 84. Kojuri J, Vosoughi AR, Akrami M. Effects of Anethum graveolens and garlic on lipid profile in hyperlipidemic patients. Lipids Health Dis. 2007;6:1476–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rashid Lamir A, Hashemi Javaheri SAA. The effect of 4-weeks aerobic training according with the usage of Anethum graveolens on blood sugar and lipoproteins profile of diabetic women. Ann Biol Res. 2012;3:4313–4319. [Google Scholar]
- 86. Bahramikia S, Yazdanparast R. Efficacy of different fractions of Anethum graveolens leaves on serum lipoproteins and serum and liver oxidative status in experimentally induced hypercholesterolaemic rat models. Am J Chin Med. 2009;37:685–699. [DOI] [PubMed] [Google Scholar]
- 87. Doshi M, Mirza A, Umarji B, Karambelkar R. Effect of Trigonella foenum-graecum (fenugreek/methi) on hemoglobin levels in females of child bearing age. Biomed Res. 2012;23:47–50. [Google Scholar]
- 88. Balaraman R, Dangwal S, Mohan M. Antihypertensive effect of Trigonella foenum-greacum seeds in experimentally induced hypertension in rats. Pharm Biol. 2006;44:568–575. [Google Scholar]
- 89. Ramesh H, Yamaki K, Tsushida T. Effect of fenugreek (Trigonella foenum-graecum L.) galactomannan fractions on phagocytosis in rat macrophages and on proliferation and IgM secretion in HB4C5 cells. Carbohydr Polym. 2002;50:79–83. [Google Scholar]
- 90. Abdel-Daim MM, Abd Eldaim MA, Mahmoud MM. Trigonella foenum-graecum protection against deltamethrin-induced toxic effects on haematological, biochemical, and oxidative stress parameters in rats. Can J Physiol Pharmacol. 2014;92:679–685. [DOI] [PubMed] [Google Scholar]
- 91. Chaudhary R, Jahan S, Gupta U, Goyal PK. Radioprotective potential of Trigonella foenum graecum seeds extract. Pharmacologyonline. 2008;2:14–26. [Google Scholar]
- 92. Phadnis M, Malhosia A, Singh SM, Malhosia A. Therapeutic effect of fenugreek seed on the patients suffering from diabetes mellitus type II. J Biol Agric Healthc. 2011;1(2):50–55. [Google Scholar]
- 93. Sharma R. Effect of fenugreek seeds and leaves on blood glucose and serum insulin responses in human subjects. Nutr Res. 1986;6:1353–1364. [Google Scholar]
- 94. Gupta A, Gupta R, Lal B. Effect of Trigonella foenum-graecum (fenugreek) seeds on glycaemic control and insulin resistance in type 2 diabetes mellitus: a double blind placebo controlled study. J Assoc Physicians India. 2001;49:1057–1061. [PubMed] [Google Scholar]
- 95. Srinivasan K. Fenugreek (Trigonella foenum-graecum): a review of health beneficial physiological effects. Food Rev Int. 2006;22:203–224. [Google Scholar]
- 96. Kumar P, Bhandari U, Jamadagni S. Fenugreek seed extract inhibit fat accumulation and ameliorates dyslipidemia in high fat diet-induced obese rats. Biomed Res Int. 2014;2014:606021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Jelodar GA, Maleki M, Motadayen M, Sirus S. Effect of fenugreek, onion and garlic on blood glucose and histopathology of pancreas of alloxan-induced diabetic rats. Indian J Med Sci. 2005;59:64–69. [PubMed] [Google Scholar]
- 98. Xue WL, Li XS, Zhang J, et al. Effect of Trigonella foenum-graecum (fenugreek) extract on blood glucose, blood lipid and hemorheological properties in streptozotocin-induced diabetic rats. Asia Pac J Clin Nutr. 2007;16:422–426. [PubMed] [Google Scholar]
- 99. Hamden K, Keskes H, Belhaj S, et al. Inhibitory potential of omega-3 fatty and fenugreek essential oil on key enzymes of carbohydrate-digestion and hypertension in diabetes rats. Lipids Health Dis. 2011;10:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kassaian N, Azadbakht L, Forghani B, Amini M. Effect of fenugreek seeds on blood glucose and lipid profiles in type 2 diabetic patients. Int J Vitam Nutr Res. 2009;79:34–39. [DOI] [PubMed] [Google Scholar]
- 101. Handa T, Yamaguchi K, Sono Y, Yazawa K. Effects of fenugreek seed extract in obese mice fed a high-fat diet. Biosci Biotechnol Biochem. 2005;69:1186–1188. [DOI] [PubMed] [Google Scholar]
- 102. Annida B, Stanely Mainzen P. Supplementation of fenugreek leaves lower lipid profile in streptozotocin-induced diabetic rats. J Med Food. 2004;7:153–156. [DOI] [PubMed] [Google Scholar]
- 103. Roberts KT. The potential of fenugreek (Trigonella foenum-graecum) as a functional food and nutraceutical and its effects on glycemia and lipidemia. J Med Food. 2011;14:1485–1489. [DOI] [PubMed] [Google Scholar]
- 104. Budoff MJ, Ahmadi N, Gul KM, et al. Aged garlic extract supplemented with B vitamins, folic acid and L-arginine retards the progression of subclinical atherosclerosis: a randomized clinical trial. Prev Med. 2009;49:101–107. [DOI] [PubMed] [Google Scholar]
- 105. Ahmadi N, Zeb I, Rezaeian P, et al. Aged garlic extract supplemented with B vitamins, folic acid and L-arginine reduce the progression of adipose tissue and coronary artery calcium. Circulation. 2010;122:A20824. [Google Scholar]
- 106. Suetsuna K. Isolation and characterization of angiotensin I-converting enzyme inhibitor dipeptides derived from Allium sativum L (garlic). J Nutr Biochem. 1998;9:415–419. [Google Scholar]
- 107. Ried K, Frank OR, Stocks NP, Fakler P, Sullivan T. Effect of garlic on blood pressure: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2008;8:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Rahman K, Lowe GM. Garlic and cardiovascular disease: a critical review. J Nutr. 2006;136:736–740. [DOI] [PubMed] [Google Scholar]
- 109. Simons S, Wollersheim H, Thien T. A systematic review on the influence of trial quality on the effect of garlic on blood pressure. Neth J Med. 2009;67:212–219. [PubMed] [Google Scholar]
- 110. Pantoja CV, Chiang LC, Norris BC, Concha JB. Diuretic, natriuretic and hypotensive effects produced by Allium sativum (garlic) in anaesthetized dogs. J Ethnopharmacol. 1991;31:325–331. [DOI] [PubMed] [Google Scholar]
- 111. Bayan L, Koulivand PH, Gorji A. Garlic: a review of potential therapeutic effects. Avicenna J Phytomed. 2014;4(1):1–14. [PMC free article] [PubMed] [Google Scholar]
- 112. Cavagnaro PF, Camargo A, Galmarini CR, Simon PW. Effect of cooking on garlic (Allium sativum L.) antiplatelet activity and thiosulfinates content. J Agric Food Chem. 2007;55:1280–1288. [DOI] [PubMed] [Google Scholar]
- 113. Sitprija S, Plengvidhya C, Kangkaya V, Bhuvapanich S, Tunkayoon M. Garlic and diabetes mellitus phase II clinical trial. Chot Mai Het Thang Phaet. 1987;70:223–227. [PubMed] [Google Scholar]
- 114. Stevinson C, Pittler MH, Ernst E. Garlic for treating hypercholesterolemia: a meta-analysis of randomized clinical trials. Ann Intern Med. 2000;133:420–429. [DOI] [PubMed] [Google Scholar]
- 115. Jalal R, Bagheri SM, Moghimi A, Rasuli MB. Hypoglycemic effect of aqueous shallot and garlic extracts in rats with fructose-induced insulin resistance. J Clin Biochem Nutr. 2007;41:218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. El-Demerdash F, Yousef M, El-Naga NA. Biochemical study on the hypoglycemic effects of onion and garlic in alloxan-induced diabetic rats. Food Chem Toxicol. 2005;43:57–63. [DOI] [PubMed] [Google Scholar]
- 117. Ahmad MS, Ahmed N. Antiglycation properties of aged garlic extract: possible role in prevention of diabetic complications. J Nutr. 2006;136:796S–799S. [DOI] [PubMed] [Google Scholar]
- 118. Eidi A, Eidi M, Esmaeili E. Antidiabetic effect of garlic (Allium sativum L.) in normal and streptozotocin-induced diabetic rats. Phytomedicine. 2006;13:624–629. [DOI] [PubMed] [Google Scholar]
- 119. Edris AE. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review. Phytother Res. 2007;21:308–323. [DOI] [PubMed] [Google Scholar]
- 120. Thomson M, Al-Amin ZM, Al-Qattan KK, Shaban LH, Ali M. Anti-diabetic and hypolipidaemic properties of garlic (Allium sativum) in streptozotocin-induced diabetic rats. Int J Diabetes Metab. 2007;15:108–115. [Google Scholar]
- 121. Banerjee SK, Maulik SK. Effect of garlic on cardiovascular disorders: a review. Nutr J. 2002;1(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Yeh YY, Liu L. Cholesterol-lowering effect of garlic extracts and organosulfur compounds: human and animal studies. J Nutr. 2001;131:989S–993S. [DOI] [PubMed] [Google Scholar]
- 123. Alder R, Lookinland S, Berry JA, Williams M. A systematic review of the effectiveness of garlic as an anti-hyperlipidemic agent. J Am Acad Nurs Pract. 2003;15:120–129. [DOI] [PubMed] [Google Scholar]
- 124. Pittler MH, Ernst E. Clinical effectiveness of garlic (Allium sativum). Mol Nutr Food Res. 2007;51:1382–1385. [DOI] [PubMed] [Google Scholar]
- 125. Gardner CD, Lawson LD, Block E, et al. Effect of raw garlic vs commercial garlic supplements on plasma lipid concentrations in adults with moderate hypercholesterolemia: a randomized clinical trial. Arch Intern Med. 2007;167:346–353. [DOI] [PubMed] [Google Scholar]
- 126. Bongiorno PB, Fratellone PM, LoGiudice P. Potential health benefits of garlic (Allium sativum): a narrative review. J Complement Integr Med. 2008;5:1553–3840. [Google Scholar]
- 127. Kiesewetter H, Jung F, Pindur G, et al. Effect of garlic on thrombocyte aggregation, microcirculation, and other risk factors. J Clin Pharmacol Ther Toxicol. 1991;29:151–155. [PubMed] [Google Scholar]
- 128. Rahman K, Billington D. Dietary supplementation with aged garlic extract inhibits ADP-induced platelet aggregation in humans. J Nutr. 2000;130:2662–2665. [DOI] [PubMed] [Google Scholar]
- 129. Susalit E, Agus N, Effendi I, et al. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: comparison with captopril. Phytomedicine. 2011;18:251–258. [DOI] [PubMed] [Google Scholar]
- 130. Cherif S, Rahal N, Haouala M, et al. A clinical trial of a titrated Olea extract in the treatment of essential arterial hypertension. J Pharm Belg. 1995;51:69–71. [PubMed] [Google Scholar]
- 131. El Sedef N, Karakaya S. Olive tree (Olea europaea) leaves: potential beneficial effects on human health. Nutr Rev. 2009;67:632–638. [DOI] [PubMed] [Google Scholar]
- 132. Simon A. Pharmacological research and clinical trials on Olea europea . AJMH. 2005;17:61–62. [Google Scholar]
- 133. Ghanbari R, Anwar F, Alkharfy KM, Gilani AH, Saari N. Valuable nutrients and functional bioactives in different parts of olive (Olea europaea L.)—a review. Int J Mol Sci. 2012;13:3291–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Somova L, Shode F, Mipando M. Cardiotonic and antidysrhythmic effects of oleanolic and ursolic acids, methyl maslinate and uvaol. Phytomedicine. 2004;11:121–129. [DOI] [PubMed] [Google Scholar]
- 135. Somova L, Shode F, Ramnanan P, Nadar A. Antihypertensive, antiatherosclerotic and antioxidant activity of triterpenoids isolated from Olea europaea, subspecies africana leaves. J Ethnopharmacol. 2003;84:299–305. [DOI] [PubMed] [Google Scholar]
- 136. Khan Y, Panchal S, Vyas N, Butani A, Kumar V. Olea europaea: a phyto-pharmacological review. Pharmacogn Rev. 2007;1:114–118. [Google Scholar]
- 137. Khayyal MT, El-Ghazaly MA, Abdallah DM, et al. Blood pressure lowering effect of an olive leaf extract (Olea europaea) induced hypertension in rats. Arzneimittelforschung. 2002;52:797–802. [DOI] [PubMed] [Google Scholar]
- 138. Perrinjaquet-Moccetti T, Busjahn A, Schmidlin C, et al. Food supplementation with an olive (Olea europaea L.) leaf extract reduces blood pressure in borderline hypertensive monozygotic twins. Phytother Res. 2008;22:1239–1242. [DOI] [PubMed] [Google Scholar]
- 139. Covas MI. Olive oil and the cardiovascular system. Pharmacol Res. 2007;55:175–186. [DOI] [PubMed] [Google Scholar]
- 140. Lou-Bonafonte JM, Arnal C, Navarro MA, Osada J. Efficacy of bioactive compounds from extra virgin olive oil to modulate atherosclerosis development. Mol Nutr Food Res. 2012;56:1043–1057. [DOI] [PubMed] [Google Scholar]
- 141. Widmer R, Freund M, Flammer A, et al. Beneficial effects of polyphenol-rich olive oil in patients with early atherosclerosis. Eur J Nutr. 2013;52:1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Ghibu S, Morgovan C, Vostinaru O, et al. Diuretic, antihypertensive and antioxidant effect of Olea europaea leaves extract, in rats. Arch Cardiovasc Dis Suppl. 2015;7:184. [Google Scholar]
- 143. Wainstein J, Ganz T, Boaz M, Madar Z. Olive leaf extract as a hypoglycemic agent in both human diabetic subjects and in rats. J Med Food. 2012;15:605–610. [DOI] [PubMed] [Google Scholar]
- 144. Omar SH. Oleuropein in olive and its pharmacological effects. Sci Pharm. 2010;78:133–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Boaz M, Leibovitz E, Dayan YB, Wainstein J. Functional foods in the treatment of type 2 diabetes: olive leaf extract, turmeric and fenugreek, a qualitative review. Funct Foods Health Dis. 2011;1:472–481. [Google Scholar]
- 146. Branković S, Radenković M, Veljković S, et al. Acute effects of the Petroselinum crispum extracts on the mean arterial blood pressure in rats. Iugoslav Physiol Pharmacol Acta. 2002;38:33–40. [Google Scholar]
- 147. Yanardağ R, Bolkent Ş, Tabakoğlu-Oğuz A, Sacan O. Effects of Petroselinum crispum extract on pancreatic B cells and blood glucose of streptozotocin-induced diabetic rats. Biol Pharm Bull. 2003;26:1206–1210. [DOI] [PubMed] [Google Scholar]
- 148. Gadi D, Bnouham M, Aziz M, et al. Parsley extract inhibits in vitro and ex vivo platelet aggregation and prolongs bleeding time in rats. J Ethnopharmacol. 2009;125:170–174. [DOI] [PubMed] [Google Scholar]
- 149. Farzaei MH, Abbasabadi Z, Ardekani MRS, Rahimi R, Farzaei F. Parsley: a review of ethnopharmacology, phytochemistry and biological activities. J Tradit Chin Med. 2013;33:815–826. [DOI] [PubMed] [Google Scholar]
- 150. Mekhfi H, Haouari ME, Legssyer A, et al. Platelet anti-aggregant property of some Moroccan medicinal plants. J Ethnopharmacol. 2004;94:317–322. [DOI] [PubMed] [Google Scholar]
- 151. Sener G, Saçan Ö, Yanardag R, Ayanoglu-Dülger G. Effects of parsley (Petroselinum crispum) on the aorta and heart of STZ induced diabetic rats. Plant Foods Hum Nutr. 2003;58(3):1–7.12859008 [Google Scholar]
- 152. Kolarovic J, Popovic M, Zlinská J, Trivic S, Vojnovic M. Antioxidant activities of celery and parsley juices in rats treated with doxorubicin. Molecules. 2010;15:6193–6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Gbolade A, Lockwood G. Petroselinum crispum (Mill.) Nyman (parsley): in vitro culture, production and metabolism of volatile constituents In: Medicinal and Aromatic Plants XI. Berlin, Germany: Springer; 1999. [Google Scholar]
- 154. Kreydiyyeh SI, Usta J. Diuretic effect and mechanism of action of parsley. J Ethnopharmacol. 2002;79:353–357. [DOI] [PubMed] [Google Scholar]
- 155. Asl AA, Soveid M, Azadbakht M, Omrani GhR, Mohammadi SS. The effect of extract of Teucrium polium on blood sugar and insulin levels of type 2 diabetic patients. Shiraj E-Med J. 2003;4(4). [Google Scholar]
- 156. Baluchnejadmojarad T, Roghani M, Roghani-Dehkordi F. Antinociceptive effect of Teucrium polium leaf extract in the diabetic rat formalin test. J Ethnopharmacol. 2005;97:207–210. [DOI] [PubMed] [Google Scholar]
- 157. Gharaibeh MN, Elayan HH, Salhab AS. Hypoglycemic effects of Teucrium polium . J Ethnopharmacol. 1988;24:93–99. [DOI] [PubMed] [Google Scholar]
- 158. Ardestani A, Yazdanparast R, Jamshidi S. Therapeutic effects of Teucrium polium extract on oxidative stress in pancreas of streptozotocin-induced diabetic rats. J Med Food. 2008;11:525–532. [DOI] [PubMed] [Google Scholar]
- 159. Hasani-Ranjbar S, Nayebi N, Larijani B, Abdollahi. A systematic review of the efficacy and safety of Teucrium species; from anti-oxidant to anti-diabetic effects. Int J Pharmacol. 2010;6:315–325. [Google Scholar]
- 160. Niazmand S, Esparham M, Hassannia T, Derakhshan. Cardiovascular effects of Teucrium polium L. extract in rabbit. Pharmacogn Mag. 2011;7(27):260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Suleiman MS, Abdul-Ghani AS, Al-Khalil S, Amin R. Effect of Teucrium polium boiled leaf extract on intestinal motility and blood pressure. J Ethnopharmacol. 1988;22:111–116. [DOI] [PubMed] [Google Scholar]
- 162. Rasekh H, Khoshnood-Mansourkhani M, Kamalinejad M. Hypolipidemic effects of Teucrium polium in rats. Fitoterapia. 2001;72:937–939. [DOI] [PubMed] [Google Scholar]
- 163. Shahraki MR, Arab MR, Mirimokaddam E, Palan MJ. The effect of Teucrium polium (Calpoureh) on liver function, serum lipids and glucose in diabetic male rats. Iran Biomed J. 2007;11:65–68. [PubMed] [Google Scholar]
- 164. Khleifat K, Shakhanbeh J, Tarawneh K. The chronic effects of Teucrium polium on some blood parameters and histopathology of liver and kidney in the rat. Turk J Biol. 2002;26:65–71. [Google Scholar]
- 165. Jung UJ, Baek NI, Chung HG, et al. Effects of the ethanol extract of the roots of Brassica rapa on glucose and lipid metabolism in C57BL/KsJ-db/db mice. Clin Nutr. 2008;27:158–167. [DOI] [PubMed] [Google Scholar]
- 166. Akbari F, Karimi A, Shahinfard N. Effect of turnip on glucose and lipid profiles of alloxan-induced diabetic rats. Iran J Endocrinol Metab. 2013;14:492–497. [Google Scholar]
- 167. Liu H, Jiang SP, Yang LL, et al. Hypoglycemic effect of crude saponins of turnip (Brassica rapa L.) on diabetic mice. J Northwest Agric Forestry Univ. 2012;6:4. [Google Scholar]
- 168. Fard MH, Naseh G, Lotfi N, Hosseini SM, Hosseini M. Effects of aqueous extract of turnip leaf (Brassica rapa) in alloxan-induced diabetic rats. Avicenna J Phytomed. 2015;5:148–156. [PMC free article] [PubMed] [Google Scholar]
- 169. Palomäki A, Pohjantähti-Maaroos H, Wallenius M, et al. Effects of dietary cold-pressed turnip rapeseed oil and butter on serum lipids, oxidized LDL and arterial elasticity in men with metabolic syndrome. Lipids Health Dis. 2010;9:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. An S, Han JI, Kim MJ, et al. Ethanolic extracts of Brassica campestris spp. rapa roots prevent high-fat diet-induced obesity via β3-adrenergic regulation of white adipocyte lipolytic activity. J Med Food. 2010;13:406–414. [DOI] [PubMed] [Google Scholar]
- 171. Birjand I. Hypolipidemic activity of aqueous extract of turnip (Brassica rapa) root in hyperlipidemic rats. Ofogh-E-Danesh. 2015;21:45–51. [Google Scholar]
- 172. Irshaid F, Mansi K, Aburjai T. Antidiabetic effect of essential oil from Artemisia sieberi growing in Jordan in normal and alloxan induced diabetic rats. Pak J Biol Sci. 2010;13:423–430. [DOI] [PubMed] [Google Scholar]
- 173. Khayoon HA, Ali AH, Kadhim TA, Abdulhadi HA. Antidiabetic effect of Artemisia sieberi in rabbits that induced diabetic by alloxan. Health Perspect. 2001;109:69. [Google Scholar]
- 174. Hamza N, Berke B, Cheze C, et al. Treatment of high fat diet induced type 2 diabetes in C57BL/6J mice by two medicinal plants used in traditional treatment of diabetes in the east of Algeria. J Ethnopharmacol. 2011;133:931–933. [DOI] [PubMed] [Google Scholar]
- 175. Ben-Nasr H, Abderrahim MAB, Salama M, Ksouda K, Zeghal KM. Potential phytotherapy use of Artemisia plants: insight for anti-hypertension. J Appl Pharm Sci. 2013;3(5):120–125. [Google Scholar]
- 176. Irshaid F, Mansi K, Bani-Khaled A, Aburjia T. Hepatoprotetive, cardioprotective and nephroprotective actions of essential oil extract of Artemisia sieberi in alloxan induced diabetic rats. Iran J Pharm Res. 2012;11:1227–1234. [PMC free article] [PubMed] [Google Scholar]
- 177. Al-Dulaimi OAA. Evaluation of the antihyperlipidemic effect of herbal preparation prepared from Artemisia herba alba, and Nigella sativa . J US China Med Sci. 2011:419. [Google Scholar]
- 178. Fathiazad F, Hamedeyazdan S, Khosropanah MK, Khaki A. Hypoglycemic activity of Fumaria parviflora in streptozotocin-induced diabetic rats. Adv Pharm Bull. 2013;3:207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Jelodar G, Maleki M, Sirus S. Effect of fumitory, celery and lemon on blood glucose and histopathology of pancreas of alloxan diabetic rats. J Appl Anim Res. 2007;31:101–104. [Google Scholar]
- 180. Roghani M. Fumaria parviflora Lam. effect on serum levels of glucose and lipids in streptozocin-induced diabetic rats. J Basic Clin Pathophysiol. 2014;2(2):35–42. [Google Scholar]
- 181. Mandal U, Nandi DK, Chatterjee K, et al. Effect of different solvent extracts of Fumaria vaillantii L. on experimental hypochlorhydria in rat. Asian J Pharm Clin Res. 2011;4:136–141. [Google Scholar]
- 182. Madhavi D, Kagan D, Rao V. A pilot study to evaluate the antihypertensive effect of a celery extract in mild to moderate hypertensive patients. Age. 2013;57:10. [Google Scholar]
- 183. Sowbhagya H. Chemistry, technology, and nutraceutical functions of celery (Apium graveolens L.): an overview. Crit Rev Food Sci Nutr. 2014;54:389–398. [DOI] [PubMed] [Google Scholar]
- 184. Tang FF, Guo JX, Zhang J, Li J, Su M. Study on hypotensive and vasodilatory effects of celery juice. Food Sci. 2007;28:322–325. [Google Scholar]
- 185. Branković S, Kitić D, Radenković M, et al. Hypotensive and cardioinhibotory effects of the aqueous and ethanol extracts of celery (Apium graveolens, apiaceae). Acta Med Med. 2010;49:13–16. [Google Scholar]
- 186. Moghadam MH, Imenshahidi M, Mohajeri SA. Antihypertensive effect of celery seed on rat blood pressure in chronic administration. J Med Food. 2013;16:558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Cheng MC, Ker YB, Yu TH, et al. Chemical synthesis of 9(Z)-octadecenamide and its hypolipidemic effect: a bioactive agent found in the essential oil of mountain celery seeds. J Agric Food Chem. 2010;58:1502–1508. [DOI] [PubMed] [Google Scholar]
- 188. Survay NS, Ko E, Upadhyay CP, et al. Hypoglycemic effects of fruits and vegetables in hyperglycemic rats for prevention of type-2 diabetes. Kor J Hort Sci Technol. 2010;28:850–856. [Google Scholar]
- 189. Al-Sa’aidi JA, Alrodhan MN, Ismael AK. Antioxidant activity of n-butanol extract of celery (Apium graveolens) seed in streptozotocin-induced diabetic male rats. Res Pharm Biotech. 2012;4(2):24–29. [Google Scholar]
- 190. Fazal SS, Singla RK. Review on the pharmacognostical & pharmacological characterization of Apium graveolens Linn. Indo Glob J Pharm Sci. 2012;2:36–42. [Google Scholar]
- 191. Mansi K, Abushoffa AM, Disi A, Aburjai T. Hypolipidemic effects of seed extract of celery (Apium graveolens) in rats. Pharmacogn Mag. 2009;5(20):301. [Google Scholar]
- 192. Cheng MC, Lin LY, Yu TH, Peng RY. Hypolipidemic and antioxidant activity of mountain celery (Cryptotaenia japonica Hassk) seed essential oils. J Agric Food Chem. 2008;56:3997–4003. [DOI] [PubMed] [Google Scholar]
- 193. Ahmed Q, Sayedda K. Effect of celery (Apium graveolens) seeds extract on protease inhibitor (ritonavir) induced dyslipidemia. Natl J Integr Res Med. 2012;3:52–56. [Google Scholar]
- 194. Wang WB, Li J, Liu YL, et al. Effects of celery seed powder on blood lipid and liver function in high fat-diet rats. JJMC. 2012;3:9. [Google Scholar]
- 195. Zhang H, Yan X, Jiang Y, Han Y, Zhou Y. The extraction, identification and quantification of hypoglycemic active ingredients from stinging nettle (Urtica angustifolia). Afr J Biotechnol. 2013;10:9428–9437. [Google Scholar]
- 196. Testai L, Chericoni S, Calderone V, et al. Cardiovascular effects of Urtica dioica L. (Urticaceae) roots extracts: in vitro and in vivo pharmacological studies. J Ethnopharmacol. 2002;81:105–109. [DOI] [PubMed] [Google Scholar]
- 197. Irshaid F, Mansi K. The effects of methanol extract derived from Urtica pilulifera leaves on some hematological and biochemical parameters of diabetic rats. Res J Biol Sci. 2009;4:675–681. [Google Scholar]
- 198. Tahri A, Yamani S, Legssyer A, et al. Acute diuretic, natriuretic and hypotensive effects of a continuous perfusion of aqueous extract of Urtica dioica in the rat. J Ethnopharmacol. 2000;73:95–100. [DOI] [PubMed] [Google Scholar]
- 199. Chrubasik JE, Roufogalis BD, Wagner H, Chrubasik SA. A comprehensive review on nettle effect and efficacy profiles, Part I: herba urticae. Phytomedicine. 2007;14:423–435. [DOI] [PubMed] [Google Scholar]
- 200. Namazi N, Tarighat A, Bahrami A. The effect of hydro alcoholic nettle (Urtica dioica) extract on oxidative stress in patients with type 2 diabetes: a randomized double-blind clinical trial. Pak J Biol Sci. 2012;15:98–102. [DOI] [PubMed] [Google Scholar]
- 201. Ahangarpour A, Mohammadian M, Dianat M. Antidiabetic effect of hydroalcholic Urtica dioica leaf extract in male rats with fructose-induced insulin resistance. Iran J Med Sci. 2012;37:181–186. [PMC free article] [PubMed] [Google Scholar]
- 202. Das M, Sarma BP, Rokeya B, Das M. Antihyperglycemic and antihyperlipidemic activity of Urtica dioica on type 2 diabetic model rats. J Diabetol. 2011;2(2):1–6. [Google Scholar]
- 203. Ghafari S, Balajadeh BK, Golalipour M. Effect of Urtica dioica L. (Urticaceae) on testicular tissue in STZ-induced diabetic rats. Pak J Biol Sci. 2011;14:798–804. [DOI] [PubMed] [Google Scholar]
- 204. Patel SS, Udayabanu M. Effect of Urtica dioica on memory dysfunction and hypoalgesia in an experimental model of diabetic neuropathy. Neurosci Lett. 2013;552:114–119. [DOI] [PubMed] [Google Scholar]
- 205. Domola MS, Vu V, Robson-Doucette CA, Sweeney G, Wheeler MB. Insulin mimetics in Urtica dioica: structural and computational analyses of Urtica dioica extracts. Phytother Res. 2010;24:175–182. [DOI] [PubMed] [Google Scholar]
- 206. Yener Z, Celik I, Ilhan F, Bal R. Effects of Urtica dioica L. seed on lipid peroxidation, antioxidants and liver pathology in aflatoxin-induced tissue injury in rats. Food Chem Toxicol. 2009;47:418–424. [DOI] [PubMed] [Google Scholar]
- 207. Safamehr A, Mirahmadi M, Nobakht A. Effect of nettle (Urtica dioica) medicinal plant on growth performance, immune responses, and serum biochemical parameters of broiler chickens. Int Res J Appl Basic Sci. 2012;3:721–728. [Google Scholar]
- 208. Mansoub H, Nezhad M. The effects of using thyme, garlic and nettle on performance, carcass quality and blood parameters. Ann Biol Res. 2011;2:315–320. [Google Scholar]
- 209. Mahjoub S, Davari S, Moazezi Z, Qujeq D. Hypolipidemic effects of ethanolic and aueous extracts of Urtica dioica in Rats. World Appl Sci J. 2012;17:1345–1348. [Google Scholar]
- 210. Sharifi N, Souri E, Ziai SA, Amin G, Amanlou M. Discovery of new angiotensin converting enzyme (ACE) inhibitors from medicinal plants to treat hypertension using an in vitro assay. Daru. 2013;21(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Sepehri G, Khaksari M, Najar AG. The effect of water extract of Zataria multiflora on microvascular permeability in streptozocin induced diabetic rats. Annu Res Rev Biol. 2014;4:3119–3127. [Google Scholar]
- 212. Kavoosi G. Zataria multiflora essential oil reduces diabetic damages in streptozotocin-induced diabetic rats. Afr J Biotechnol. 2013;10:17632–1769. [Google Scholar]
- 213. Mohammadi A, Gholamhoseinian A, Fallah H. Zataria multiflora increases insulin sensitivity and PPARγ gene expression in high fructose fed insulin resistant rats. Iran J Basic Med Sci. 2014;17:263–270. [PMC free article] [PubMed] [Google Scholar]
- 214. Ma Y, Njike VY, Millet J, et al. Effects of walnut consumption on endothelial function in type 2 diabetic subjects: a randomized controlled crossover trial. Diabetes Care. 2010;33:227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Asgary S, Parkhideh S, Solhpour A, Rahimi P. Effect of ethanolic extract of Juglans regia L. on blood sugar in diabetes-induced rats. J Med Food. 2008;11:533–538. [DOI] [PubMed] [Google Scholar]
- 216. Mohammadi J, Delaviz H, Malekzadeh JM, Roozbehi A. The effect of hydroalcoholic extract of Juglans regia leaves in streptozotocin-nicotinamide induced diabetic rats. Pak J Pharm Sci. 2012;25:407–411. [PubMed] [Google Scholar]
- 217. Mohammadi J, Saadipour K, Delaviz H, Mohammadi B. Anti-diabetic effects of an alcoholic extract of Juglans regia in an animal model. Turk J Med Sci. 2011;41:685–691. [Google Scholar]
- 218. Hassanshahi MR, Eghbali H, Hosseini Zijoud SM, et al. Study of the effects of walnut leaf on some blood biochemical parameters in hypercholesterolemic rats. Biochem Anal Biochem. 2011;1(103):1–2. [Google Scholar]
- 219. Dehghani F, Mashhoody T, Panjehshahin M. Effect of aqueous extract of walnut septum on blood glucose and pancreatic structure in streptozotocin-induced diabetic mouse. Iran J Pharmacol Ther. 2012;11:10–14. [Google Scholar]
- 220. Almario RU, Vonghavaravat V, Wong R, Kasim-Karakas SE. Effects of walnut consumption on plasma fatty acids and lipoproteins in combined hyperlipidemia. Am J Clin Nutr. 2001;74:72–79. [DOI] [PubMed] [Google Scholar]
- 221. Srinath S. Effects of walnuts on serum cholesterol levels in people with normo- or hyperlipidemia. Nutrition Bytes. 2003;9(2). http://escholarship.org/uc/item/39p4j7mj. Accessed December 19, 2016. [Google Scholar]
- 222. Banel DK, Hu FB. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: a meta-analysis and systematic review. Am J Clin Nutr. 2009;90:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Zibaeenezhad M, Shamsnia S, Khorasani M. Walnut consumption in hyperlipidemic patients. Angiology. 2005;56:581–583. [DOI] [PubMed] [Google Scholar]
- 224. Nergiz-Ünal R, Kuijpers MJ, de Witt SM, et al. Atheroprotective effect of dietary walnut intake in ApoE-deficient mice: involvement of lipids and coagulation factors. Thromb Res. 2013;131:411–417. [DOI] [PubMed] [Google Scholar]