Abstract
The objective of this review is to identify, summarize, and evaluate clinical trials of berberine for the treatment of hyperlipidemia and other dyslipidemias. A literature search for randomized, controlled trials of berberine that assessed at least 2 lipid values as endpoints resulted in identification of 12 articles that met criteria. The majority of evaluated articles consistently suggest that berberine has a beneficial effect on low-density lipoprotein (reductions ranging from approximately 20 to 50 mg/dL) and triglycerides (reductions ranging from approximately 25 to 55 mg/dL). Common study limitations included lack of reporting of precision in their endpoints, description of blinding, transparency in flow of patients, and reporting of baseline concomitant medications. Berberine could serve as an alternative for patients who are intolerant to statins, patients resistant to starting statin therapy but who are open to alternative treatments, and for low-risk patients not indicated for statin therapy.
Keywords: berberine, hyperlipidemia, low-density lipoprotein, triglycerides
Hyperlipidemia is a major risk factor for cardiovascular disease that affects approximately 33.5% of the US population.1 When high lipid levels are present in the blood, lipids begin to deposit in the walls of the arteries, forming plaques. This leads to atherosclerosis and obstruction of blood flow.2 A 2010 meta-analysis found that every 38.67 mg/dL reduction in low-density lipoprotein (LDL) produces a 22% reduction in major vascular events.3 The most recent American College of Cardiology/American Heart Association guidelines (ATP IV, published in 2013) do not recommend treating to specific lipid targets due to lack of evidence from randomized controlled trials; however, many practitioners still monitor lipid values and use them to guide therapy for hyperlipidemia.4 Among patients with hyperlipidemia, only 48.1% are receiving treatment, and only 33.2% have LDL levels that would have been considered “controlled” under the previous ATP III guidelines.1 Some patients with hyperlipidemia may benefit from alternative lipid-lowering therapies, particularly those patients who cannot tolerate the recommended statin dose.
Several natural products have been studied for their effects on lipid values. Red yeast rice is the most well-studied among them, with one study demonstrating a significant reduction in cardiovascular events versus placebo (5.7% vs 10.4%).5 Red yeast rice inhibits HMG-CoA (3-hydroxy-3-methylglutaryl–coenzyme A) reductase, using a cholesterol-lowering mechanism similar to that of statins. In fact, the primary active ingredient (monacolin K) is currently marketed as lovastatin.5 Other natural products that have been studied for their lipid-lowering properties include policosanols, polyphenols, garlic, plant sterols, and berberine.5
Berberine is an isoquinolone alkaloid isolated from the bark, roots, rhizome, and stems of plants of the genus Berberis, as well as from plants such as Coptis chinensis (huanglian in traditional Chinese medicine) and Hydrastis canadensis (goldenseal).5,6 Berberine has been used in traditional Chinese medicine for thousands of years, and it has been studied for the treatment of many different conditions, including type 2 diabetes mellitus and hypertension. Berberine has been found to lower lipid levels by a different mechanism than that of statins and red yeast rice. It is thought to upregulate the expression of LDL receptors (LDLR) on hepatocytes by stabilizing LDLR mRNA, and suppress the expression of proprotein convertase substilisin/kexin type 9 (PCSK9) by accelerating degradation of hepatocyte nuclear factor 1α (HNF1α) and decreasing PCSK9 mRNA transcription.6–8 Its effects on PCSK9 are of particular interest, considering the recent Food and Drug Administration approval of PCSK9 inhibitors as a new class of highly effective lipid-lowering drugs. Berberine has the potential to interact with other drugs, as it is an inhibitor of cytochrome P450 (CYP) 2D6, 2C9, and 3A4.9 Its oral bioavailability is low due to poor absorption and significant first-pass metabolism.10 Potential side effects of berberine include constipation, diarrhea, abdominal distension, and bitter taste.11
Preliminary animal and pilot human studies have shown that berberine produces a positive effect on the lipid profile. In one study, 32 patients who were not receiving other lipid-lowering therapies were given berberine 500 mg twice daily for three months.12 In these patients, significant reductions in LDL, triglycerides, and total cholesterol were seen from baseline (25% reduction in LDL, 35% reduction in triglycerides, and 29% reduction in total cholesterol, P < .0001 for change from baseline).12 In a 2-month study of 63 patients, berberine 500 mg twice daily lowered LDL by an average of 23.8%, and a combination of simvastatin 20 mg daily and berberine lowered LDL by an average of 31.8%.13 These results need to be confirmed in larger, well-designed studies, and berberine needs to be evaluated both on its own and as an adjunct to other lipid-lowering agents.
The objective of this review is to identify, summarize, and evaluate clinical trials to determine the efficacy of berberine, both alone and in combination with other herbal products, for the treatment of hyperlipidemia and other dyslipidemias.
Data Sources, Selection, and Extraction
In March 2016, a literature search using a combination of the terms “berberine,” “hyperlipidemia,” “cholesterol,” and “dyslipidemia” was performed using PubMed, both with and without medical subject headings (MeSH) terminology. No publication date limits were applied to the search, but filters (“clinical trial,” “humans,” and “English”) were applied. Human clinical trials that were published in English were reviewed for inclusion. Titles and abstracts were examined by author LMK to identify citations related to berberine. References cited in identified studies were also examined for inclusion. In order to be included, studies had to be randomized, controlled trials of berberine that assessed at least 2 lipid values as endpoints. Included studies were reviewed and approved by the fourth author (RDB).
Excluding duplicates, a total of 21 articles were identified for possible inclusion. Figure 1 illustrates the primary reasons for exclusion of articles. The majority of studies were excluded because they did not assess efficacy of berberine against a separate control group. Ultimately, 11 articles from PubMed were selected for inclusion: one additional article was found through the examination of study references and included in the review. It was decided on initial review of the 12 articles to conduct the project as a narrative review. Study results were not pooled in a meta-analysis due to high anticipated heterogeneity among studies from differences in patients, intervention, control, duration, and blinding. The Consolidated Standards of Reporting Trials (CONSORT) Extension for Reporting Herbal Medicinal Interventions14 was used as the primary basis for evaluation of study quality; however, other author-identified limitations were also considered.
Figure 1.
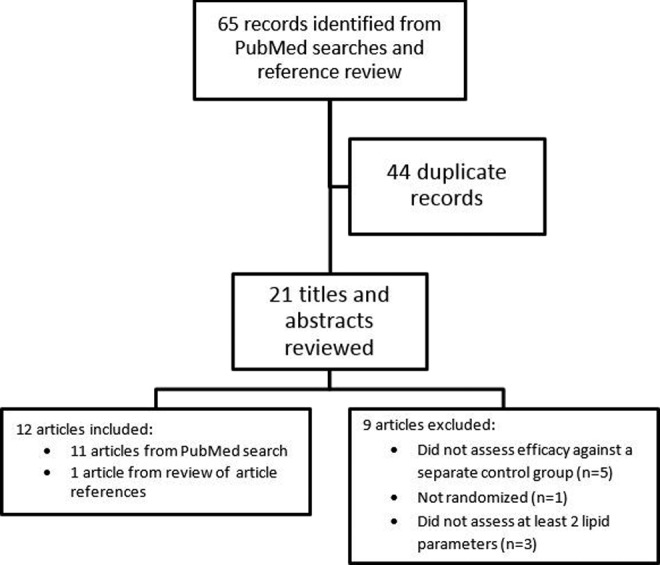
Studies that were identified through title and abstract review during the literature search, reasons for exclusion, and the ultimate number of studies included in the review.
Data Synthesis
See Table 1 for a side-by-side comparison of extracted study information and Table 2 for the study evaluations using the CONSORT Extension.
Table 1.
Summary of Clinical Trial Results.
| Citation | Author and Year | Design | Treatment | Control | Sample Size | Duration | LDL Changes | TC Changes |
|---|---|---|---|---|---|---|---|---|
| 15 | Wei, 2012 | R, SC | BBR 500 mg 3 times daily | Placebo twice daily | 100 | 3 months | Average baseline and study end values ± SD (mg/dL) | |
| T: 163 ± 22 to 140 ± 23 C: 166 ± 20 to 140 ± 23 P < .05a | T: 226 ± 15 to 179 ± 20 C: 220 ± 18 to 195 ± 15 P < .01a | |||||||
| 16 | Zhang, 2008 | R, DB, MC | BBR 500 mg twice daily | Placebo twice daily | 110 | 3 months | Average baseline and study end values ± SD (mg/dL) | |
| T: 125 ± 31 to 99 ± 30, P < .000b PC: 130 ± 28 to 125 ± 28 P = .138b; P < .000a | T: 205 ± 38 to 168 ± 37, P < .000b C: 208 ± 36 to 204 ± 30, P < .000b; P < .000a | |||||||
| 17 | Derosa et al, 2013 | R, DB | BBR 500 mg twice daily | Placebo | 144 | 14 months | 3 months postrandomization average values ± SD (mg/dL) | |
| T: 133±7 C: 147±8 P < .005a,b | T: 191±9 C: 201±9 P < .05a,b | |||||||
| 3 months after treatment restart, post-washout average values ± SD (mg/dL) | ||||||||
| T: 134±8 C: 147±8, P < .05a,b | T: 192±10 C: 202±10 P < .05a,b | |||||||
| 18 | Cicero et al, 2007 | R, SB | NC3 daily | BBR HCl 500 mg | 40 | 4 weeks | Absolute change from baseline ± SD (mg/dL) | |
| T: −44.4 ± 10.7 C: −35.6 ± 5.9 P < .000a,b | T: −52.9 ± 10 C: −42.0 ± 5.5 P < .000a,b | |||||||
| 19 | Affuso, 2008 | R, DB, SC | NC2 daily | Placebo daily | 50 | 6 weeks; followed by 4-week open-label | Absolute change from baseline ± SD (mg/dL) | |
| T: −41 ± 29 C: −2 ± 19 P < .001a | T: −44± 34 C: −1 ± 30 P < .001a | |||||||
| 20 | Marazzi, 2011 | R, SB, SC | NC1 (no frequency) | Placebo (no frequency) | 80 | 1 year | Average baseline and study end values ± SD (mg/dL) | |
| T: 172 ± 16 to 119 ± 21 C: 173 ± 10 to 175 ± 25 P < .05a | T: 252 ± 23 to 201 ± 26 C: 253 ± 19 to 175 ± 25 P < .05a | |||||||
| 21 | Sola, 2014 | R, DB, MC | NC1 daily | Placebo daily | 102 | 12 weeks | Least square means change between groups (95% CI) (mg/dL) | |
| −10.46 (−19.81 to −1.12) P = .029a | −12.12 (−21.28 to −2.95) P = .01a | |||||||
| 22 | Gonnelli et al, 2015 | R, SB | NC1 daily | Placebo | 60 | 24 weeks | Percent change from baseline ± SD | |
| T: 23.7% ± 32.6% C: Not reported P < .01a,b | T: 24.6% ± 32.1% C: Not reported P < .01a,b | |||||||
| 23 | Cianci et al, 2012 | R | NC4 daily | Calcium 240 mg + vitamin D3 5 µg | 120 | 12 weeks | Percent change from baseline ± SD | |
| T: −12.4% ± 1.5% C: 0.8% ± 0.7% P < .001a | T: −13.5% ± 0.7% C: −0.2% ± 0.5% P < .001a | |||||||
| 24 | Ruscica et al, 2014 | R, DB, C | NC1 daily | Placebo followed by pravastatin 10 mg daily | 30 | 16 weeks | Average baseline and study end values ± SD (mg/dL) | |
| T: 151 ± 23 to 119 ± 25 C: 150 ± 29 to 144 ± 33 P < .0001a,b | T: 239 ± 30 to 208 ± 27 C: 239 ± 38 to 243 ± 34 P < .0001a,b | |||||||
| 25 | Pisciotta, 2012 | R, DB | NC2 daily | EZE 10 mg daily | 135 | 6 months | Percent change from baseline ± SD | |
| T: −31.7 ± 7 C: −25.4 ± 6.4 P < .001a | T: −24.2 ± 5.2 C: −19.0 ± 4.6 P < .001a | |||||||
| 26 | Marazzi et al, 2015 | R, SB | NC1 (no frequency) | EZE 10 mg daily | 100 | Intervention × 12 weeks, 1 year follow-up | Average baseline and values after 12 weeks ± SD (mg/dL) | |
| T: 149 ± 16 to 109 ± 8 C: 150 ± 8 to 126 ± 11 P < .0001a | T: 218 ± 15 to 177 ± 12 C: 219 ± 14 to 194 ± 16 P < .0001a | |||||||
| Average values after 12 months ± SD (mg/dL) | ||||||||
| T: 95 ± 3 C: 95 ± 10 P < .0001a | T: 163 ± 7 C: 164 ± 13 P < .0001a | |||||||
Abbreviations: DB, double-blind; SB, single-blind; R, randomized; SC, single-center; MC, multicenter; C, crossover, BBR, berberine; EZE, ezetimibe; CI, confidence interval; SD, standard deviation; NC1, berberine 500 mg, red yeast rice extract 200 mg, policosanol 10 mg, folic acid 0.2 mg, coenzyme Q10 2 mg, and asthaxantin 0.5 mg; NC2, berberine 500 mg, policosanol 10 mg, and red yeast rice 200 mg; NC3, berberine 500 mg, policosanol 10 mg, red yeast extract 200 mg, folic acid 2 mg, coenzyme Q10 2 mg, and astaxanthin 0.5 mg; NC4, berberine 500 mg, soy isoflavones 60 mg, Lactobacillus sporogenes 1 × 109 spores, calcium phosphate 137 mg, vitamin D3 5 µg, and folic acid 0.2 mg; T, treatment; C, control.
a P value indicates between-group differences of study end results.
b P value indicates within-group difference (ie, from baseline).
Table 2.
Adapted CONSORT (Consolidated Standards of Reporting Trials) Checklist.14
| Citation | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | |
| 1. Title and abstract | ||||||||||||
| Description of how patients were allocated to interventions | × | × | × | × | × | × | × | × | × | × | × | |
| Latin binomial name | × | |||||||||||
| Part of the plant used | ||||||||||||
| 2. Introduction | ||||||||||||
| Statement of reasoning behind trial with reference to specific herbal product being tested | × | × | × | × | × | × | × | × | × | × | × | × |
| 3. Methods | ||||||||||||
| Eligibility criteria for participants | × | × | × | × | × | × | × | × | × | × | × | × |
| Setting and location where data were collected | × | × | × | × | × | × | × | |||||
| 4. Interventions | ||||||||||||
| Latin binomial name and common name | × | × | × | × | × | × | × | × | ||||
| Part of plant used | × | × | × | |||||||||
| Dosage and duration of administration | × | × | × | × | × | × | × | × | × | × | × | |
| Explanation of how the dose was determined | × | |||||||||||
| Content of all herbal products per dosage unit form | × | × | × | × | × | × | × | × | × | × | × | × |
| Rationale for type of control or placebo | × | × | × | × | × | × | × | × | ||||
| 5. Objectives | ||||||||||||
| Specific objectives and hypotheses | × | × | × | × | × | × | × | × | × | × | × | |
| 6. Outcomes | ||||||||||||
| Clearly defined primary and secondary outcomes | × | × | × | × | × | |||||||
| 7. Sample size | ||||||||||||
| How sample size was determined | × | × | × | × | × | |||||||
| 8-10. Randomization | ||||||||||||
| Methods used for randomization | × | × | × | × | × | × | × | × | × | × | ||
| 11. Blinding | ||||||||||||
| Description of who is blinded | × | × | × | |||||||||
| 12. Statistical methods | ||||||||||||
| Description of statistical methods used for analysis | × | × | × | × | × | × | × | × | × | × | × | × |
| 13. Results | ||||||||||||
| Flow of participants through each stage | × | × | × | × | × | × | × | |||||
| 14. Recruitment | ||||||||||||
| Dates of period of recruitment and follow-up | × | × | × | |||||||||
| 15. Baseline data | ||||||||||||
| Baseline demographic and clinical characteristics of each group, including concomitant medications | × | × | × | |||||||||
| 16. Numbers analyzed | ||||||||||||
| Number of subjects in each group included in the analysis | × | × | × | × | × | × | × | × | × | × | ||
| 17. Outcomes | ||||||||||||
| Summary of results for each group with precision | × | |||||||||||
| 18. Ancillary analyses | ||||||||||||
| Report of any other analyses performed distinguishing prespecified from exploratory | × | × | × | |||||||||
| 19. Adverse events | ||||||||||||
| All important adverse events or side effects in each group | × | × | × | × | × | × | × | × | × | × | ||
| 20. Discussion | ||||||||||||
| Interpretation of results taking study hypotheses into account as well as sources of potential bias or imprecision | × | × | × | × | × | × | × | × | ||||
| 21. Generalizability | ||||||||||||
| External validity of trial results | × | × | × | × | × | × | ||||||
| 22. Overall evidence | ||||||||||||
|
× | × | × | × | × | × | × | × | × | × | × | × |
|
× | × | × | × | × | × | × | × | × | × | × | |
Efficacy of Berberine Alone
A prospective, randomized trial evaluated the effects of berberine in polycystic ovary syndrome compared with placebo and metformin.15 A total of 89 Chinese females who had a diagnosis of polycystic ovary syndrome and were insulin resistant were included. Patients were excluded if they had an endocrine disorder such as Cushing’s syndrome, thyroid dysfunction, or diabetes mellitus; they were also excluded if they had coronary artery disease, if they were taking medications that affect insulin hemodynamics or ovulation, or if they had taken oral contraceptives within 3 months of the study. Patients were randomized to take berberine 500 mg 3 times daily, metformin 500 mg 3 times daily, or placebo twice daily; all patients were concomitantly taking a compound preparation of ethinyl estradiol 35 mg and cyproterone 2 mg, which was taken in a cyclic fashion, and all patients were provided with education on dietary changes. Endpoints reported in relation to the lipid profile were LDL, total cholesterol (TC), high-density lipoprotein (HDL), and triglycerides (TG). Patients had a mean age of 26 years and mean body mass index (BMI) of 25 kg/m2.15
The results comparing metformin with berberine are not reported here, and the 2 groups were not directly compared for lipid outcomes. Baseline TC levels in the berberine and placebo groups were 226 ± 15 and 220 ± 18 mg/dL respectively; at the end of 3 months, there was a significant difference in the TC levels between groups (179 ± 20 vs 195 ± 15 mg/dL in the berberine and placebo groups respectively, P < .01). The berberine group and placebo group baseline LDL values were 163 ± 22 and 166 ± 20 mg/dL, respectively. At the end of 3 months, the LDL levels were 140 ± 23 and 157 ± 20 mg/dL, respectively, in the berberine and placebo groups (P < .05). Both groups had baseline HDL levels of 43 ± 5 mg/dL; HDL levels increased to 48 ± 3 mg/dL in the berberine group and to 44 ± 4 mg/dL in the placebo group by the end of the study (P < .01). The TG levels in the berberine and placebo groups at baseline were 91 ± 10 and 89 ± 9 mg/dL, respectively. At the end of 3 months, a greater reduction was observed with the berberine group (75 ± 9 vs 81 ± 9 mg/dL in the placebo group, P < .05).15
A limitation of the study design was that the placebo group only received the product twice daily, whereas both intervention groups were taking the study medication 3 times daily. The difference in administration frequency and lack of blinding increases the risk of bias. Additionally, the patient population consisted entirely of females of Chinese descent with polycystic ovary syndrome, so application to other demographics may be limited.15
A randomized, double-blind, multicenter, placebo-controlled trial evaluated berberine use in diabetic patients with dyslipidemia.16 A total of 110 patients were included in the trial: These patients were aged between 25 and 70 years, had a new diagnosis of type 2 diabetes mellitus, and had a diagnosis of dyslipidemia. Patients were excluded if they had heart failure (New York Heart Association class III or IV), prior use of diabetic medications, or moderate to severe liver or renal dysfunction. Patients were randomized to receive either berberine 500 mg twice daily or placebo for a total of 3 months. Major endpoints collected for dyslipidemia monitoring were LDL, TC, HDL, and TG. At baseline, the participants had an average age of 51 years, an average BMI of 25 kg/m2, and an average blood pressure of 125/82 mm Hg.16
The berberine group had a change in TC from 205 ± 38 mg/dL at baseline to 168 ± 37 mg/dL at 3 months (P < .000), while the placebo group experienced a change from 208 ± 36 to 204 ± 30 mg/dL over the same 3-month time period (P < .001). The difference between 3-month TC values in the 2 groups was statistically significant (P < .001). The between-group comparisons observed for LDL and TG were also statistically significant (P < .001 for each endpoint). The change in LDL in the berberine group was 125 ± 31 to 99 ± 30 mg/dL (P < .000 for change from baseline), whereas in the placebo group, it was 130 ± 28 to 125 ± 28 mg/dL (P = .138 for change from baseline). The TG level in the berberine group decreased from 97 ± 79 mg/dL at baseline to 62 ± 43 mg/dL after 3 months (P < .000 for change from baseline); in the placebo group, TG increased from 76 ± 36 to 79 ± 49 mg/dL (P = .543 for change from baseline). No significant difference in the change in HDL levels was seen between the berberine group and the placebo group (51 ± 18 to 53 ± 31 mg/dL in the berberine group vs 50 ± 10 to 50 ± 9 mg/dL in the placebo group, P = .415).16
A limitation of this trial was the lack of statistical analysis: there was no discussion of effect size or confidence intervals. Including this information would have allowed for more meaningful interpretation of the intervention impact. The patient population was composed only of diabetic participants of Chinese descent; different outcomes may be seen in different patient populations.16
Another randomized, double-blind, placebo-controlled clinical trial evaluated the cholesterol-lowering effects of berberine in 144 Caucasian patients with low cardiovascular risk.17 Patients were eligible for the study if they were nonsmokers and had hypercholesterolemia (TC 200-240 mg/dL and TG <400 mg/dL), BMI 24.7-28.9 kg/m2, normal blood pressure (<140/90 mm Hg), and normal thyroid function. Of note, patients were excluded if they had diabetes, a history of cardiovascular disease, or a history of tobacco use. Authors specifically excluded patients taking some medications that can affect the lipid profile (ie, antidepressants, antiserotonergics, barbiturates, and oral corticosteroids, among others). However, statins and other cholesterol-lowering medications were not specifically excluded. Patients underwent a 6-month run-in period in which they were advised to follow a specific diet and exercise regimen. Patients were then randomized to receive placebo or berberine 500 mg twice daily for 3 months. After 3 months, a 2-month washout period occurred, during which both berberine and placebo agents were discontinued and patients were again instructed to follow diet and exercise regimens. At the end of the washout period, patients were restarted on the same medication, placebo, or berberine, for an additional 3 months. Changes in BMI and lipid profile were the primary efficacy measures: These were assessed at 3 and 6 months during the run-in phase, at randomization, before the washout period (at 1, 2, and 3 months), and after the washout period (1, 2, and 3 months). Safety data were also collected in this study by means of physical examination, vital sign assessment, weight, electrocardiogram, adverse events, and treatment tolerability.17
Of the 141 patients who completed the run-in phase, 71 were randomized to berberine and 70 to placebo. Attrition rate was low with 137 patients completing the study. Baseline characteristics were similar between groups. Average TC and LDL values at the point of randomization following the 6-month run-in period were 216 ± 13 and 159 ± 10 mg/dL, respectively. These values are slightly lower than the baseline values for TC and LDL, prior to the implementation of diet and exercise (225 ± 15 and 164 ± 12 mg/dL, respectively). Differences from baseline for TC and LDL after 6 months of the run-in period were not significant. Results are presented in reference to either change from baseline or change from the washout period if referring to the reintroduction phase. A significant decrease in TC was found in the berberine group 3 months postrandomization (191 ± 9 mg/dL, −11.6%, P < .05 compared with baseline and placebo) and as soon as 2 months after the reintroduction of berberine (204 ± 11 mg/dL, −12.9%, P < .05 compared with the washout period). Significant decreases in LDL were also evident in both time periods (191 ± 9 mg/dL, −16.4% after randomization, P < .05 compared with placebo; 143±10 mg/dL, −17.9% 2 months after reintroduction, P < .05 compared with the washout period). Decreases in TG were also observed (−21.2% [P < .05] 3 months after randomization and −25% [P < .05] 3 months after reintroduction). HDL increased 3 months after randomization (+9.1%) in the berberine group (P < .05), but a significant change was not found in the placebo group. For all other parameters (TC, LDL, TG), significant changes were not found in the placebo group (TC: 3 months postrandomization, 201 ± 9 mg/dL, 2 months post-reintroduction, 199 ± 10 mg/dL; LDL: 147 ± 8 mg/dL 3 months postrandomization, 141 ± 8 mg/dL 2 months post-reintroduction; TG: 81 ± 26 mg/dL 3 months postrandomization, 85 ± 24 mg/dL post-reintroduction). When the lipid profile values in the berberine group were compared with the placebo group, all differences were found to be statistically significant (P < .05). No serious adverse events were reported. One patient did report a headache for 1 day during the run-in phase, and 2 patients reported transient flatulence for 2 days.17
Patients included in this study were at low cardiovascular risk, which significantly limits the study applicability to populations who would typically receive a cholesterol-lowering drug (ie, diabetics, patients with cardiovascular disease). It is unknown if patients were allowed to take other cholesterol-lowering agents, since these were not specifically identified as exclusion criteria or addressed in the baseline characteristics. Authors neither discussed how sample size was determined, nor did they specify a particular lipid value as the primary efficacy endpoint. Additionally, effect sizes and confidence intervals were not reported for each endpoint in each group, which limits the interpretability of the results.17
Efficacy of Berberine Alone Versus Berberine in Combination With Other Natural Products
A randomized controlled trial evaluated berberine alone and in combination with other natural cholesterol-lowering agents in patients with moderate hyperlipidemia (TC 200-300 mg/dL, TG 200-300 mg/dL).18 Patients were eligible for inclusion if they had a 10-year atherosclerotic cardiovascular disease (ASCVD) risk between 10% and 20%, no previous cardiovascular events, no family history of severe dyslipidemia, no high cardiovascular risk independent of plasma lipids, no known liver and/or muscle disease, and no consumption of any chemical or natural agent affecting lipid metabolism. Patients were randomized to receive a food supplement (10 mg policosanol, 200 mg red yeast extract, 2 mg folic acid, 2 mg coenzyme Q10) plus berberine 500 mg once daily, collectively referred to as COMB, or berberine 500 mg alone (BERB) once daily. The authors analyzed the mean change in a variety of cholesterol-related outcomes from baseline to the end of the 4-week treatment period: These outcomes included TC, LDL, HDL, non-HDL cholesterol, TG, apolipoprotein B, apolipoprotein A, lipoprotein a, plasma glucose, glutamate oxaloacetate transaminase, glutamate pyruvate transaminase, and creatinine phosphokinase.18
Twenty patients were randomized to each group. The BERB group had 8 males (mean age: 55.4 ± 14.2 years) and 12 females (mean age: 63.8 ± 12.1 years); the COMB group also had 8 males (mean age: 57.8 ± 14.2 years) and 12 females (mean age: 63.1 ± 16.2 years). Baseline values for LDL, TC, and TG in the BERB group were 177.8 ± 13.8, 263.7 ± 14.2, and 191.8 ± 37.4 mg/dL, respectively. LDL, TC, and TG values for the COMB group were similar: 174.4 ± 21.9, 265.4 ± 20.1, and 202.4 ± 49.2 mg/dL, respectively. After 4 weeks of treatment, both the BERB and COMB groups showed statistically significant reductions in LDL, TC, and TG from baseline (LDL: −35.6 ± 5.9 and −44.4 ± 10.7 mg/dL, respectively, P < .000 for both; TC: −42 ± 5.5 and −52.9 ± 10 mg/dL, respectively, P < .000 for both; TG: −43 ± 17.2 and −52.1 ± 14.6 mg/dL, respectively, P < .000 for both). Increases in HDL were also statistically significant in both the BERB and COMB groups compared with baseline (+2.15 ± 5.06 and +1.95 ± 3.36 mg/dL for the BERB and COMB groups, respectively, P < .05 for both). The combination of Preparation plus berberine was found to be superior to berberine alone in terms of effect on LDL, TC, and TG (P < .000). The authors found no effect on their specified safety variables (β-glucuronidase, glutamate oxaloacetate transaminase, glutamate pyruvate transaminase, and creatinine phosphokinase) in either treatment group, and no adverse events were reported during the study.18
This study has several limitations. The study lacked a true placebo group, and the number of patients studied was small. The study failed to include specific objectives and hypotheses, and did not clearly differentiate between primary and secondary outcomes. The study also did not describe sample size calculations, methods used for randomization, who was blinded, or confidence intervals for their results, limiting applicability and reproducibility. The authors did not discuss how their results compared with similar trials. Finally, superiority of the preparation plus berberine compared with berberine was concluded; however, statistical tests reported differences in changes from baseline alone and failed to describe P values for between-group differences. While both groups exhibited statistically significant reductions in TC, LDL, and TG, a clinically significant effect size was not specified.18
Efficacy of Berberine in Combination With Other Natural Products
A single-center, double-blind, randomized, placebo-controlled 6-week trial evaluated the reduction in TC in patients given a nutraceutical combination (NC) pill containing berberine 500 mg, policosanol 10 mg, and red yeast rice 200 mg.19 Fifty patients were eligible for inclusion: These patients were between 18 and 70 years of age and had TC >220 mg/dL and LDL >130 mg/dL. Patients were excluded if they were pregnant, if they had used a lipid-lowering agent in the past 6 weeks, or if they had TG >500 mg/dL. The primary outcome was the reduction in TC at the end of 6 weeks; secondary endpoints included LDL, TG, and HDL. In both groups, age was approximately 55 years, and BMI was approximately 28 kg/m2. Baseline lipid values in the NC group and the placebo group were as follows: TC 255 ± 30 and 251 ± 31 mg/dL, respectively, and LDL cholesterol 176 ± 25 and 171 ± 22 mg/dL, respectively.19
At the end of 6 weeks, the NC treatment group had a mean reduction in TC of 44 ± 34 mg/dL, compared with a reduction of 1 ± 30 mg/dL in the placebo group (P < .001). There was also a significant reduction in LDL (41 ± 29 mg/dL in the NC group vs 2 ± 19 mg/dL in the placebo group, P < .001). No difference was detected in HDL or TG changes between the NC and placebo group (HDL: 11 ± 10 and 4 ± 6 mg/dL, respectively, P = not significant; TG: −7 ± 17 and 2 ± 25 mg/dL, respectively, P = .06).19
A limitation of the article was the effect size that was used to determine the sample size. The set effect size and standard deviation were 39 ± 46 mg/dL; variability was quite high considering the observed effect. Additionally, it is customary to check lipid panels 3 months after initiation of a lipid-lowering medication. Using this standard follow-up time period would have allowed for a better direct comparison between NC and different lipid-lowering medications.19
A prospective, single-center, single-blind, parallel group, placebo-controlled trial assessed the efficacy, safety, and tolerability of a different NC pill in elderly patients.20 Patients who were >75 years old, statin intolerant or refusing to take statins, and had TC >200 mg/dL were eligible for inclusion in the trial: patients were excluded if they were diabetic or if they had been on statin therapy in the previous 2 months. Eighty patients were randomized in a 1:1 ratio to receive a NC pill containing berberine 500 mg, policosanol 10 mg, red yeast rice 200 mg, folic acid 0.2 mg, coenzyme Q10 2 mg, and astaxanthin 0.5 mg or placebo; frequency of administration was not reported. No prespecified primary or secondary outcomes were stated; however, TC, LDL, HDL, and TG were all collected as endpoints. Approximately half of the participants were female, and the average age was 82 years. Approximately 60% of the patients had hypertension, and 45% had a history of ischemic heart disease.20
The baseline TC, LDL, and HDL levels for the NC group were 252 ± 23, 172 ± 16, and 44 ± 12 mg/dL, respectively: The baseline TC, LDL, and HDL levels for the placebo group were 253 ± 19, 173 ± 10, and 44 ± 8 mg/dL, respectively. The TC at the end of the study was 201 ± 26 mg/dL in the NC treatment group compared with 255 ± 28 mg/dL in the placebo group (P = .05). End-of-study LDL cholesterol values were also significantly reduced in the NC group versus the placebo group (119 ± 21 mg/dL in the NC group vs 175 ± 25 mg/dL in the placebo group, P = .05). No significant difference was observed in the HDL endpoint (49 ± 11 vs 45 ± 8 mg/dL) or TG levels (162 ± 33 vs 177 ± 49 mg/dL) between groups; no specific P values were stated. The investigators reported that no deaths were observed during the 12-month follow-up period. Four participants in the NC group and 7 in the placebo group reported adverse effects, including muscle pain (2 patients in each group) and muscle weakness (1 patient on NC vs 3 patients on placebo).20
One limitation of this study is that it does not specify a particular primary endpoint. No specific power calculations were described. There was also a lack of reported statistical analysis: there was no discussion of effect size or confidence intervals. Without this information, the readers’ ability to determine the impact of the intervention is limited. The study only assessed the use of the medication in elderly patients, so the results should only be applied to this patient population.20
A prospective, multicenter, double-blind, randomized trial assessed the effects of an NC pill versus placebo in participants who had mild to moderate hypercholesterolemia.21 The study included 102 patients who were >18 years of age, had LDL cholesterol between 130 and 189 mg/dL, and were not eligible for pharmacotherapy intervention but were eligible for lifestyle modifications. Patients were excluded from the trial if they were pregnant, or if they had diabetes mellitus, a history of cardiovascular disease, or TG >350 mg/dL. The identified patients were randomized in a 1:1 ratio to receive placebo or Armolipid Plus, a European NC product containing berberine 500 mg, policosanol 10 mg, red yeast rice 200 mg, folic acid 0.2 mg, coenzyme Q10 2 mg, and astaxanthin 0.5 mg, for a total of 12 weeks. The primary outcome of the study was serum LDL, and major secondary outcomes assessed were TC, HDL, and TG. Patients in the intervention and placebo groups had average ages of 49 and 52 years, respectively, and average BMIs of 25 and 28 kg/m2. The majority of patients had a low 10-year cardiovascular risk (78.4% and 82.4% of patients in the intervention and placebo groups, respectively). The average blood pressure in both groups was 122/76 mm Hg.21
The least square mean change in LDL from baseline to week 12 was −12 mg/dL (95% confidence interval [CI] −20.06 to −4.03 mg/dL) in the placebo group and −23.25 mg/dL (95% CI −27.08 to −15.34 mg/dL) in the NC group. The treatment difference between these groups was −10.46 mg/dL (95% CI −19.81 to −1.12 mg/dL, P = .029). The least square mean change in TC from baseline to 12 weeks was −13.36 mg/dL (95% CI −23.14 to −3.58 mg/dL) in the placebo group and -25.48 mg/dL (95% CI −35.98 to −14.99 mg/dL) in the NC group. The treatment difference for TC between groups was −12.12 mg/dL (95% CI −21.28 to −2.95 mg/dL, P = .010). The HDL and TG treatment differences between groups were 1.91 mg/dL (95% CI −1.50 to 5.32 mg/dL, P = .268) and −6.40 mg/dL (95% CI −36.26 to 23.47 mg/dL, P = .671), respectively.21
A limitation of the study was the effect size used to determine the sample size. The set effect size and standard deviation were 16 ± 28 mg/dL, which was high variability considering the observed effect. Using these factors in the sample size calculation resulted in an inappropriately small sample size. A larger sample size would have allowed for more precise results. The confidence intervals for the endpoints were all very wide, which decreases the likelihood that the results could be accurately reproduced.21
Gonnelli et al22 assessed the efficacy and safety of a combination nutraceutical containing berberine in 60 patients with low-to-moderate risk hypercholesterolemia. This was a 24-week, double-blind, randomized, placebo-controlled trial. Patients were eligible for inclusion in the study if they were between 18 and 60 years of age and had a BMI of 18.5 to 29.9 kg/m2, a serum LDL >150 mg/dL, and an estimated ASCVD risk of <20%. Patients were excluded from the trial if they had a history of cardiovascular disease or coronary risk equivalents, secondary hyperlipidemia caused by diabetes, renal, liver, or thyroid diseases, alcohol consumption >40 g/d, ASCVD risk >20%, muscular diseases, or abnormally elevated creatinine phosphokinase. They were also excluded from the trial if they were on medications with antiplatelet, anti-inflammatory, or hypolipidemic effects, or if they had received any type of hormone replacement therapy within the past 2 months. All patients were instructed to follow a hypolipidic diet (low cholesterol/low saturated fat). The intervention group received a combination pill (MBP-NC) containing 200 mg red yeast rice extract, 500 mg berberine, 10 mg policosanol, 0.2 mg folic acid, 2 mg coenzyme Q10, and 0.5 mg asthaxantin once daily. The MBP-NC pills were identical in taste and appearance to the placebo pills, which contained an inactive compound. Authors analyzed multiple efficacy and safety endpoints, including TC, LDL, HDL, and TG.22
Baseline characteristics were similar between groups, and the authors reported no statistically significant differences; however, baseline TC and TG levels varied somewhat between the MBP-NC and placebo groups (TC: 238.4 and 248.1 mg/dL, respectively; TG: 132.1 and 119.0 mg/dL, respectively). Twenty-eight patients in the MBP-NC group and 29 patients in the placebo group completed the study. In the MBP-NC group, statistically significant differences were found for percentage change in TC and LDL both from baseline and compared to placebo at week 24 (TC: −24.6% ± 32.1%, P < .01; LDL: −23.7% ± 32.6%, P < .01). Percentage change from baseline for TG was not significant in the MBP-NC or placebo group at 4 weeks. HDL decreased in both groups from baseline at all points of follow-up, although this difference was only significant for the placebo group at 4, 12, and 24 weeks (P < .01, P < .05, P < .01, respectively). Percent reductions in TC and LDL from baseline were decreased slightly at 24 weeks compared with the initial 4- and 12-week reductions (TC at 4 weeks: −30.3% ± 33.9%, 12 weeks: −26.7% ± 33.1%, 24 weeks: −24.6% ± 32.1%; LDL: −29.4% ± 35.3%, −25.6% ± 31.5%, and −23.7% ± 32.6%, respectively). However, reductions in TC and LDL observed after the first 4 weeks were largely maintained until the end of the study.22
This study had several significant limitations. The standard deviation found for the statistically significant endpoints (TC and LDL) was greater than the effect size (percentage change from baseline) at all points of the study. Wide standard deviations and the lack of confidence intervals allow for doubt about the efficacy of this intervention. The specific lipid values attained in each group were not reported—only percent reductions. Furthermore, the percent reductions were only shown graphically, without fully disclosing specific percentages. Reductions in TC and LDL were highest at 4 weeks but began to decline at each follow-up visit. It is unknown if further follow-up visits would have shown lipid values that suggest reduced efficacy of the intervention over time.22
A randomized controlled trial evaluated the percentage change in LDL cholesterol in 120 menopausal women taking a nutraceutical combination.23 Patients were eligible for inclusion if they had LDL cholesterol levels between 130 and 190 mg/dL and/or serum triglycerides between 150 and 400 mg/dL but were not taking any cholesterol- or TG-lowering medications. Women were excluded if they had familial hypertriglyceridemia or “acute forms of severe diseases,” or if they were receiving hormone replacement, statin, or fibrate therapy. Women were randomized to receive a NC formulation of soy isoflavones 60 mg, Lactobacillus sporogenes 1 × 109 spores, berberine 500 mg, calcium phosphate dehydrate 137 mg, vitamin D3 5 µg, and folic acid 0.2 mg (n = 60) or a combination of calcium 240 mg plus vitamin D3 5 µg (n = 60) at a dose of 1 tablet daily for 12 weeks. The tablets were similar in size and shape, and compliance was monitored at each visit. The primary endpoint was percentage change in LDL cholesterol at 12 weeks, although the authors also collected and reported data for changes in TC, HDL cholesterol, TG, menopause symptom severity, blood pressure, waist circumference, weight, transaminases, and creatinine phosphokinase.23
Baseline characteristics were similar between groups. Average LDL cholesterol levels at baseline were 148.2 ± 3.03 mg/dL for the NC group and 151.2 ± 3.02 mg/dL for the calcium + vitamin D group. Other cholesterol parameters measured at baseline included TC (239.4 ± 2.8 and 234.3 ± 2.9 mg/dL for the NC group and the calcium + vitamin D group, respectively), HDL cholesterol (53.9 ± 1.38 and 51.7 ± 1.26 mg/dL, respectively), and TG (180.9 ± 9.95 and 175.2 ± 7.51 mg/dL, respectively). The NC group had a significantly larger percent reduction in LDL cholesterol compared to placebo at 12 weeks (−12.4% ± 1.5% vs 0.8% ± 0.7%, P < .001). The percent change in TC was −13.5% ± 0.7% versus −0.2% ± 0.5% with placebo (P < .001), the percent change in HDL cholesterol was +4.7% ± 1.5% versus −1.2% ± 1.0% with placebo (P < .001), and the percent change in TG was −18.9% ± 2.5% versus −1.3% ± 1.2% with placebo (P < .001). The placebo group experienced slightly more adverse effects than the intervention group, including kidney stones, pruritus, dyspepsia, and constipation.23
A limitation of this study was that it did not use a validated menopause scale to assess severity of menopause symptoms. Additionally, it did not adequately define the exclusion criteria of “acute forms of severe diseases.” Concomitant disease states were not reported in the baseline characteristics, so it is unknown whether or not some of the women in the study had comorbid conditions that would have warranted treatment with a statin (ie, diabetes or conditions resulting in an ASCVD risk >7.5%). Data regarding concomitant medications were also absent. While the authors discussed the content of the placebo and the similarities in appearance to the active treatment, they did not provide a rationale for the choice of calcium and vitamin D over an inactive placebo. They also did not delineate specific primary and secondary outcomes. No effect sizes or confidence intervals were reported; these values would have strengthened the interpretability of their results.23
A randomized, double-blind, crossover study compared Armolipid Plus (200 mg red yeast rice, 500 mg berberine, 10 mg policosanol, 0.2 mg folic acid, 2 mg coenzyme Q10, and 0.5 mg astaxanthin) to placebo in 30 patients for 8 weeks.24 This initial treatment period was followed by a 4-week washout period, and then treatment with pravastatin 10 mg/d in all patients for an additional 8 weeks. Patients were eligible for inclusion if they were older than 18 years, diagnosed with moderate metabolic syndrome, and had LDL levels within the range of 130 to 170 mg/dL. The following patient populations were excluded: pregnant patients, patients with a history of cardiovascular disease, patients with chronic liver or renal disease, patients being treated with antidiabetic medications or insulin, patients with untreated hypertension, obese patients (BMI >30 kg/m2), patients taking treatments known to interfere with the study treatment, and patients who were enrolled in another research study in the past 90 days. The placebo medication was identical in taste and appearance to the Armolipid Plus product. The primary endpoint of the study was the reduction in LDL in the Armolipid Plus arm from baseline. Secondary endpoints included reduction in TC and changes in other cardiometabolic and inflammatory biomarkers related to cardiometabolic risk (TG, HDL, and glucose, among others). These results were also compared to those achieved with pravastatin. Authors did not specify that safety data would be collected; however, CPK and liver enzyme values were assessed.24
The number of patients placed in each group is unclear. Moreover, authors did not specify how many patients were retained in the study for analysis after 4 and 8 weeks. Thirty patients were initially included in the study, which the authors stated would provide 90% power with an alpha value of .05 to detect a reduction in LDL of 12% ± 20%. Baseline LDL was 148 ± 33 mg/dL for the 30 patients included in the study. Armolipid Plus reduced LDL from 151 ± 23 mg/dL at baseline to 119 ± 25 mg/dL at 8 weeks (P < .001). Armolipid Plus also significantly reduced TC from baseline to 8 weeks (239 ± 30 to 208 ± 27 mg/dL, P <0.001); however, reduction in TG from baseline after 8 weeks was not statistically significant (216 to 195 mg/dL, P = .726). Increase in HDL in the Armolipid Plus group was statistically significant from baseline after 8 weeks (40 ± 8 mg/dL to 42 ± 9 mg/dL, p=0.049). In the placebo group, change in LDL from baseline to 8 weeks was not significant (150 ± 29 mg/dL at baseline and 144 ± 33 mg/dL at 8 weeks, P = .617). When Armolipid Plus was compared with placebo for the primary endpoint, Armolipid Plus was significantly more effective in lowering LDL (119 ± 25 mg/dL at 8 weeks vs 144 ± 33 mg/dL, P < .0001). LDL changes were not significantly different in the pravastatin and Armolipid Plus groups after 8 weeks of treatment (118 ± 27 vs 119 ± 25 mg/dL, P = .974).24
Limitations of this study include its small sample size and lack of reporting on a number of different items. The number of patients in each group at each phase of the study was not reported. The study also did not describe how patients were allocated to each intervention, individuals blinded, or how adverse events were monitored (if at all). A lack of reported confidence intervals reduces the robustness of the results. Additionally, patients with diabetes and cardiovascular disease were excluded, limiting the applicability of this study to patient populations who are often indicated to receive lipid-lowering therapy.24
A prospective randomized controlled trial evaluated the lipid-lowering effect of an NC pill in 135 participants.25 The study randomized 270 patients who had primary polygenic hypercholesterolemia to receive either the NC pill (which contained berberine 500 mg, policosanol 10 mg, and red yeast rice 200 mg) or ezetimibe (EZE). The patients were on a lipid-lowering diet for 3 months prior to randomization and then were followed for another 3 months. No prespecified primary or secondary outcomes were stated: however, TC, LDL, HDL, and TG were evaluated as endpoints. Two additional analyses were conducted as part of this study. The first analysis assessed the impact of dual NC/EZE treatment on lipid values among 26 of the original study patients who had less than a 30% reduction in LDL with monotherapy. A completely separate analysis of 30 patients with familial hypercholesterolemia was also reported: These patients, who had been on stable doses of statin ± EZE for 1 year, had the NC intervention added to their therapy for 3 months. Their lipid values were assessed for change from baseline after 3 months of receiving the add-on NC pill.25
With the monotherapy interventions, the change in TC from baseline was −24.2% ± 5.2% in the NC group and −19.0% ± 4.6% in the EZE group (P < .001). Percent LDL reductions from baseline were also seen with the NC and ezetimibe groups (−31.7% ± 7% and −25.4% ± 6.4% respectively, P < .001). No difference was observed in terms of percent change in HDL (−0.64% ± 7.2% vs 1.24% ± 6.9% in the NC and ezetimibe groups, respectively) or percent change in TG (−19.5% ± 16.1% vs −14.9% ± 11.5% in the NC and EZE groups, respectively). Approximately 50% of the patients in the NC group were able to obtain an LDL reduction of >30% whereas only about 25% of patients in the EZE group were able to obtain a similar LDL reduction. When EZE was added to NC monotherapy, the changes in TC, LDL, and TG after 3 months of dual treatment were only −9.7%, −13.6%, and −4.5%, respectively; tests for statistical significance were not performed. Finally, in patients with familial hypercholesterolemia who were on a stable dose of statin ± EZE, the addition of the NC product resulted in a further reduction of TC and LDL of 8.2% and 10.6%, respectively; tests for statistical significance were not performed. An inverse correlation was found between the percentage decrease in LDL achieved with statin ± EZE and the additional percentage decrease seen with NC (r = −0.617, P < .001).25
The study provided an ample amount of data on three different groups of patients. However, the analysis of patients on a stable regimen of statin ± EZE should have been conducted as a separate study, as it included a completely different subset of patients. Including all these data makes it difficult to pinpoint the focus of this study. Additionally, the study only included patients with moderate cardiovascular risk and primary hypercholesteremia, so the results can only be applied to this subgroup of patients. This excludes a large number of patients who would normally benefit from lipid-lowering medication (ie, diabetics, individuals with elevated ASCVD risk, and individuals with coronary artery disease). Another limitation of the study was that 8 patients were excluded from the EZE group for poor compliance and adverse effects, but the severity of these side effects was not reported. These exclusions decrease the validity of trial results and decrease the applicability of the results to the general population. The flow of patients in the study was not clearly defined. The methods section indicated that 270 patients were randomized: however, baseline characteristics were only provided for 135 of the patients. The reason for leaving out these other patients was not specified, and the number of patients included in the final data analysis was not specified.25
A single-blind randomized controlled trial measured the efficacy and safety of an NC pill containing berberine in 100 patients with dyslipidemia and ischemic heart disease (stable or unstable angina) receiving percutaneous coronary interventions.26 Patients were randomized to receive either the NC pill (containing berberine 500 mg, policosanol 10 mg, red yeast rice 200 mg, folic acid 0.2 mg, coenzyme Q10 mg, and astaxanthin 0.5 mg) or EZE 10 mg once daily for 12 weeks. Eligible patients were required to have documented coronary heart disease treated with percutaneous coronary intervention, TC >200 mg/dL, LDL >160 mg/dL, and statin intolerance with refusal of other treatments for hypercholesterolemia. Statin intolerance was defined as myalgia, myositis, rhabdomyolysis, or gastrointestinal disorders (alanine transaminase or aspartate transaminase >2 times the upper limit of normal) while on statin therapy. Patients were ineligible for the study if their glomerular filtration rate was <30 mL/min or if they had used lipid-lowering therapy within 30 days of the initial study treatment. After 12 weeks of treatment, patients continued their assigned therapy only if they had achieved the primary endpoint goal of LDL <100 mg/dL. Patients who did not achieve this goal continued their assigned therapy with the therapy from the comparator group added on (ie, nutraceuticals were added on to the EZE group) for an additional 9 months. Secondary outcomes of the study included changes in TC, LDL, HDL, TG, and treatment tolerability.26
Fifty patients were randomized to each treatment group (NC pill or EZE). Baseline characteristics were similar between NC and EZE groups, with baseline LDL levels of 149 ± 16 and 150 ± 8 mg/dL in each group, respectively. After 3 months of therapy, 14 patients (28%) in the NC group and 0 patients in the EZE group achieved the primary outcome (P < .0001 for differences between groups). All 50 patients in the EZE group and the 36 patients in the NC group who did not reach LDL levels of <100 mg/dL after 3 months of treatment had the comparator therapy added on to their baseline therapy. At the 1-year visit, 58 patients (73%) in the EZE-nutraceutical combination therapy group had achieved LDL <100 mg/dL. The 14 patients in the NC monotherapy group maintained LDL <100 mg/dL at 1 year. In both the NC group and the combination therapy groups, LDL, TC, and TG significantly decreased from baseline over 12 months (NC values at baseline vs 12 months: 149 ± 16, 218 ± 15, and 166 ± 31 mg/dL, respectively, vs 95 ± 3, 163 ± 7, and 140 ± 21 mg/dL, respectively, P < .0001; combination group values at baseline vs 12 months: 150 ± 8, 219 ± 14, and 171 ± 25 mg/dL, respectively vs 95 ± 10, 164 ± 13, and 140 ± 21, respectively, P < .0001). HDL increased from baseline to 12 months in both groups (NC: 36 ± 8 vs 40 ± 7 mg/dL, P < .0001; combination: 34 ± 7 vs 41 ± 8 mg/dL, P < .0001). No patient discontinued study treatment or reported adverse effects.26
One caveat to this study is although baseline characteristics were similar between the NC and EZE groups at baseline, the average baseline LDL in both groups was below the prespecified inclusion criteria value of >160 mg/dL (mean LDL values were 149 ± 16 and 150 ± 8 mg/dL in the NC and EZE groups, respectively). Some patients may have had an LDL as low as 133 mg/dL. Patients with lower lipid values may not have been as difficult to control as other patients with more severe hyperlipidemia. It may have been beneficial to evaluate patients who required add-on therapy versus those who did not and observe whether there were any differences in baseline characteristics (such as LDL values) that were associated with the need for additional therapy. The authors neither specified which visit the primary endpoint was in relation to, nor did they state if the 2 intervention pills were similar in shape, size, and color. Finally, the primary endpoint involved achieving an LDL of <100 mg/dL, which is a surrogate endpoint rather than a clinical outcome.26
Discussion
Overall, the majority of evaluated articles consistently suggest that berberine has a beneficial effect on LDL (reductions ranging from approximately 20 to 50 mg/dL) and TG (reductions ranging from approximately 25 to 55 mg/dL).15,16,18–20,24–26 Favorable results have been demonstrated compared with both placebo and active controls, the latter of which included other herbal products, EZE, and low-dose pravastatin. It should be noted that most trials provided berberine in formulations that included other herbal products (see Table 2). Berberine has a theorized mechanism that is similar to PCSK9 inhibitors, and it has been postulated that combination therapy of berberine with red yeast rice is pharmacologically analogous to combination therapy of PCSK9 inhibitors with statins.
Included clinical trials demonstrated appropriate descriptions of the patient populations and the type of intervention provided. Additionally, berberine 500 mg once daily was almost consistently used in all trials either as a single agent or combined with other dietary supplements. These strengths improve both the applicability and reproducibility of the results.
There are a number of inherent limitations to most of the studies included in this review when assessed according to the CONSORT Extension for Herbal Medicine Interventions criteria.14 Many trials lacked reporting of precision in their endpoints (17), description of blinding (11), transparency in flow of patients (13), reporting of baseline concomitant medications (15), and prespecified primary and secondary outcomes (6). Additionally, most trials had very small sample sizes. Without pellucidity of these components, clinical reproducibility, potential for bias, and presence of confounding variables are of concern. There was also inconsistent presentation of the outcomes, especially in terms of adverse effects reporting, across the trials preventing direct comparison of the results among studies.
Strengths of this review include use of the CONSORT extension to guide a systematic evaluation of each study, increasing consistency and validity of the assessment. Objective, systematic methods were also used to determine which trials were included in the review in a way that selected for the strongest studies in terms of design. There were 2 key limitations. First is that the search was limited to PubMed. Second, only English language articles were included, and several trials of berberine have been published in Chinese. However, given the strict trial inclusion criteria, it is not expected that highly impactful additional studies would be found in other databases or published in other languages.
Berberine alone and in combination with other dietary supplements provides an average LDL percentage lowering capability of 20% to 30%.15–26 Moderate-intensity statin medications have been proven to lower LDL cholesterol by 30 to 50% and high-intensity statins lower LDL even further, upward of 50%.2 The American Heart Association and American College of Cardiology released clinical guidelines for cholesterol management that no longer recommend specific LDL goals, but suggest 4 statin benefit groups in which the use of statins is recommended not only for their benefits related to cholesterol lowering but also for their established efficacy in preventing nonfatal and fatal cardiovascular disease. Statins are recommended in individuals with clinical ASCVD, LDL >190 mg/dL, diabetes mellitus aged 40 to 75 years with LDL 70 to 189 mg/dL and without clinical ASCVD, or LDL 70 to 189 mg/dL and ASCVD risk at least 7.5%. Studies included in this review consistently assess effects of berberine and combination dietary supplements on surrogate lipid endpoints.15–26 While this information is necessary to gauge the lipid-lowering potential of berberine, future studies should focus on this product’s ability to reduce ASCVD in order to determine the relative clinical benefit compared to statins. Based on available studies, berberine alone or in combination with other dietary supplements could serve as an alternative for patients who are intolerant to statins, patients resistant to starting statin therapy but who are open to alternative treatments, and for low-risk patients not indicated for statin therapy (ie, do not fall into 1 of the 4 statin benefit groups).
Footnotes
Authors’ Note: This work was completed at the Manchester University Drug Information Center. No assistance was provided that did not merit authorship. At the time of writing, Dr Koppen was a PGY1 Pharmacy Resident at Parkview Regional Medical Center, Fort Wayne, Indiana. Dr Whitaker and Dr Rosene were PharmD candidates at Manchester University College of Pharmacy, Natural and Health Sciences.
Author Contributions: LMK is designated as first author who led the conduction of this work. LMK was responsible for developing the study criteria, conducting the literature search, and selecting studies based on criteria. AW and AR led the summary and evaluation of identified studies, and LMK led the editing and proofreading process. RDB contributed equally to this work as a mentor, and was responsible for developing the idea for the review, the literature search, and providing guidance through the summary and evaluation process. RDB reviewed and edited the article in its entirety.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: It was determined that this project was exempt from institutional review board review.
References
- 1. Centers for Disease Control and Prevention. Vital signs: prevalence, treatment, and control of high levels of low-density lipoprotein cholesterol—United States, 1999-2002 and 2005-2008. MMWR Morb Mortal Wkly Rep. 2011:60;109–114. [PubMed] [Google Scholar]
- 2. Dong H, Zhao Y, Zhao L, Lu F. The effects of berberine on blood lipids: a systemic review and meta-analysis of randomized controlled trials. Planta Med. 2013;79:437–446. [DOI] [PubMed] [Google Scholar]
- 3. Baigent C, Blackwell L, Emberson J, et al. ; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013. ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889–2934. [DOI] [PubMed] [Google Scholar]
- 5. Barbagallo CM, Cefalù AB, Noto D, Averna MR. Role of nutraceuticals in hypolipidemic therapy. Front Cardiovasc Med. 2015;2:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li Z, Jiang JD, Kong WJ. Berberine up-regulates hepatic low-density lipoprotein receptor through Ras-independent but AMP-activated protein kinase-dependent Raf-1 activation. Biol Pharm Bull. 2014;37:1766–1775. [DOI] [PubMed] [Google Scholar]
- 7. Abidi P, Zhou Y, Jiang JD, Liu J. Extracellular signal-regulated kinase-dependent stabilization of hepatic low-density lipoprotein receptor mRNA by herbal medicine berberine. Arterioscler Thromb Vasc Biol. 2005;25:2170–2176. [DOI] [PubMed] [Google Scholar]
- 8. Dong B, Li H, Singh AB, Cao A, Liu J. Inhibition of PCSK9 transcription by berberine involves down-regulation of hepatic HNF1α protein expression through the ubiquitin-proteasome degradation pathway. J Biol Chem. 2015;290:4047–4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guo Y, Chen Y, Tan ZR, Klaassen CD, Zhou HH. Repeated administration of berberine inhibits cytochromes P450 in humans. Eur J Clin Pharmacol. 2012;68:213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu CS, Zheng YR, Zhang YF, Long XY. Research progress on berberine with a special focus on its oral bioavailability. Fitoterapia. 2016;109:274–282. [DOI] [PubMed] [Google Scholar]
- 11. Mannarino MR, Ministrini S, Pirro M. Nutraceuticals for the treatment of hypercholesterolemia. Eur J Intern Med. 2014;25:592–599. [DOI] [PubMed] [Google Scholar]
- 12. Kong W, Wei J, Abidi P, et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10:1344–1351. [DOI] [PubMed] [Google Scholar]
- 13. Kong WJ, Wei J, Zuo ZY, et al. Combination of simvastatin with berberine improves the lipid-lowering efficacy. Metabolism. 2008;57:1029–1037. [DOI] [PubMed] [Google Scholar]
- 14. CONSORT. Extensions. Herbal medicinal interventions. http://www.consort-statement.org/extensions?ContentWidgetId=557. Accessed March 31, 2016.
- 15. Wei W, Zhao H, Wang A, et al. A clinical study on the short-term effect of berberine in comparison to metformin on the metabolic characteristics of women with polycystic ovary syndrome. Eur J Endocrinol. 2012;166:99–105. [DOI] [PubMed] [Google Scholar]
- 16. Zhang Y, Li X, Zou D, et al. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J Clin Endocrinol Metab. 2008;93:2559–2565. [DOI] [PubMed] [Google Scholar]
- 17. Derosa G, D’Angelo A, Bonaventura A, et al. Effects of berberine on lipid profile in subjects with low cardiovascular risk. Expert Opin Biol Ther. 2013;13:475–482. [DOI] [PubMed] [Google Scholar]
- 18. Cicero AF, Rovati LC, Setnikar I. Eulipidemic effects of berberine administered alone or in combination with other natural cholesterol-lowering agents. A single-blind clinical investigation. Arzneimittelforschung. 2007;57:26–30. [DOI] [PubMed] [Google Scholar]
- 19. Affuso F, Ruvolo A, Micillo F, et al. Effects of a nutraceutical combination (berberine, red yeast rice and policosanols) on lipid levels and endothelial function randomized, double-blind, placebo-controlled study. Nutr Metab Cardiovasc Dis. 2010;20:656–661. [DOI] [PubMed] [Google Scholar]
- 20. Marazzi G, Cacciotti L, Pelliccia F, et al. Long-term effects of nutraceuticals (berberine, red yeast rice, policosanol) in elderly hypercholesterolemic patients. Adv Ther. 2011;28:1105–1113. [DOI] [PubMed] [Google Scholar]
- 21. Solà R, Valls RM, Puzo J, et al. Effects of poly-bioactive compounds on lipid profile and body weight in a moderately hypercholesterolemic population with low cardiovascular disease risk: a multicenter randomized trial. PLoS One. 2014;9:e101978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gonnelli S, Caffarelli C, Stolakis K, et al. Efficacy and tolerability of a nutraceutical combination (red yeast rice, policosanols, and berberine) in patients with low-moderate risk hypercholesterolemia: a double-blind, placebo-controlled study. Curr Ther Res Clin Exp. 2014;77:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cianci A, Cicero AF, Colacurci N, et al. Activity of isoflavones and berberine on vasomotor symptoms and lipid profile in menopausal women. Gynecol Endocrinol. 2012;28:699–702. [DOI] [PubMed] [Google Scholar]
- 24. Ruscica M, Gomaraschi M, Mombelli G, et al. Nutraceutical approach to moderate cardiometabolic risk: results of a randomized, double-blind and crossover study with Armolipid Plus. J Clin Lipidol. 2014;8:61–68. [DOI] [PubMed] [Google Scholar]
- 25. Pisciotta L, Bellocchio A, Bertolini S. Nutraceutical pill containing berberine versus ezetimibe on plasma lipid pattern in hypercholesterolemic subjects and its additive effect in patients with familial hypercholesterolemia on stable cholesterol-lowering treatment. Lipids Health Dis. 2012;11:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marazzi G, Pelliccia F, Campolongo G, et al. Usefulness of nutraceuticals (Armolipid Plus) versus ezetimibe and combination in statin-intolerant patients with dyslipidemia with coronary heart disease. Am J Cardiol. 2015;116:1798–1801. [DOI] [PubMed] [Google Scholar]


