Abstract
This study was conducted to evaluate the prophylactic effect of ginger extract on ethanol-induced reproductive toxicity in male rats. Twenty-eight adult male Sprague-Dawley rats were randomly divided into 4 groups and treated daily for 28 days as follows: control, control-ginger (1 g/kg of body weight [BW]/day by gavage), ethanol group (ethanol 4 g/kg of BW/day by gavage), and ginger-ethanol group. At the end of the experiment, all the rats were sacrificed and their testes were removed and used for measurement of the total homocysteine (tHcy), trace elements, antioxidant enzymes activity, and malondialdehyde (MDA). The results in the ethanol group indicate that ethanol decreased antioxidant enzymes activity and increased MDA and tHcy compared with the control groups (P < .05). In ginger-ethanol group, ginger improved antioxidant enzymes activity and reduced tHcy and MDA compared to ethanol group (P < .05). It can be concluded that ginger protects the ethanol-induced testicular damage and improves the hormonal levels, trace elements, antioxidant enzymes activity, and decreases tHcy and MDA.
Keywords: Zingiber officinale Roscoe, ginger, oxidative stress, ethanol, testis, traditional medicine
Infertility is a major clinical problem in the realm of medicine and psychiatry. About 5% to 15% of couples are infertile, and the male factor is responsible for 50% of these causes.1–3 Many factors in men have been considered as potential causes, such as trauma, obstructive lesions, varicocele, cryptorchidism, cystic fibrosis, infections, and tumors; and an important cause has been identified—oxidative stress.3 Oxidative stress is a result of a discrepancy between the production of reactive oxygen species (ROS) and antioxidant defense mechanism. Oxidative stress affects the male fertility in many ways such as lipid peroxidation,4 crosslinking and inactivation of proteins,3 fragmentation of DNA,5 and impairment of sperm motility.6 Lifestyle stressors such as casting, welding, bakery, driving and lifestyle factors, such as cigarette smoking and excessive alcohol consumption, are known to affect the levels of free radicals and oxidative stress.1 Oxidative stress and ROS-mediated toxicity are considered as the main etiological origins of alcohol-induced ultrastructural changes in the reproductive system. The role of alcohol in the etiology of reproductive pathology has been debated for several decades.6,7 The effect of alcohol consumption on the male fertility has been of great concern, because its abuse impairs the reproductive function and sexual behavior.8 Consumption of alcohol has been popular in many societies and it seems to continue to be so in the future. According to the World Health Organization,9 alcohol consumption represents the third largest risk factor for disease burden in high-income countries, just after smoking and hypertension. Studies showed that both chronic and acute consumption of alcohol cause fertility disturbances such as decreased sperm count, low sperm motility, reduced serum testosterone level, atrophy, and irregularity in the diameter of the seminiferous tubules of the testes in human and animal models.6,7,10 There are several studies showing that excessive alcohol consumption is associated with a decrease in the percentage of normal sperm, semen production, and sperm quality6–11 while better sperm morphology in those who drink moderately has been observed.12
Today people tend to use complementary and alternative medicine,13 especially herbal drugs, because of fewer side effects and in some cases lower cost.14,15 Plants are very useful in curing many diseases and for hygiene via producing secondary metabolites like proteins, alkaloids, steroids, flavonoids, and phenolic compounds.16,17
Medicinal plants have attracted the attentions due to the inherent antioxidant potential of the phytochemicals that reduce the free-radical induced oxidative damage. Consumption of plants parts that have high amounts of total phenolic and antioxidant compounds is important in protecting against oxidative damage.
Ginger (Zingiber officinale Roscoe) contains flavonoids and polyphenolic constituents that have antioxidant,18 anti-inflammatory,19 antidiabetic and hypolipidemic properties,20 and anti-carcinogenic activity.21,22 The antioxidant activity of ginger has been attributed to its major ingredients, namely zingerone, gingerdiol, zingiberene, gingerols, and shogoals (23). Ginger-free phenolic and also ginger hydrolyzed phenolic fractions of ginger extract exhibited free radical scavenging properties and also demonstrated inhibition of lipid peroxidation, protection of DNA and reduction of power abilities, indicating strong antioxidant properties.23,24 Also, ginger rhizome can inhibit nuclear factor-κB (NF-κB) activation induced by a variety of agents,25,26 and has been shown to downregulate NF-κB regulated gene products involved in cellular proliferation and angiogenesis, including interleukin-8 (IL-8)27 and angiogenesis28 Ginger extract has a potential remedy against cardiovascular disease29 and also improves the testicular function.30,31 It is reported that oral administration of ginger extract significantly increased the number, viability, and motility of the sperms and also serum total testosterones in rats. Ginger rhizome was found to overcome reproductive toxicity of cyclophosphamide32 and gentamicin.33
Ginger has been traditionally used as a male reproductive organs enhancer through the world for a long time.34,35 As a hot remedy, ginger has been mentioned in traditional Persian medicine resources such as Avicenna’s (980-1037 AD)36 Canon of Medicine and The Storehouse of Medicaments (written in 1772 AD)37 by Aghili Shirazi38–40 to increase sexual energy, semen volume, and ejections.35,41 It is also popular among western Ugandans to take ginger in tea for impotence.42 Its use as moxibustion is known in traditional Chinese medicine for the same indication.43 In contrast to ginger, excessive alcohol consumption is known to decrease the semen production and sexual desire according to traditional Persian medicine.44 The present study was designed to evaluate the protective effects of Zingiber officinale Roscoe extract against ethanol-induced reproductive toxicity in male rats.
Materials and Methods
Plant Material and Extraction
The fresh ginger rhizome was purchased from herbal market, Shiraz, Iran. Identification was made by the herbalist of Shiraz University of Medical Sciences and a voucher specimen was deposited in the university herbarium (voucher number: PM-948). The ginger was dried and powdered in the air temperature. Two hundred and fifty grams of dried powder of ginger was moved to a sterile Erlenmeyer flask and ethyl alcohol was added until the mix turned into a suspension. It was shaken by a shaker for 48 hours and then the solution was filtered using filter paper. The obtained liquid was heated in oven at 40°C to evaporate the alcohol. The residual was extracted. By adding definite amounts of corn oil, the dose of the extract was prepared for oral administration.
Phytochemical Analysis
Determination of Total Phenolic Content
The total phenolic content (TPC) of the plant extract was evaluated according to the modified Folin-Ciocalteu spectrophotometric method described previously by Waterhouse.45,46 The method involves the reduction of Folin-Ciocalteau reagent by phenolic compounds. An aliquot of 40 μL solution of the extract was added to 3.16 mL water and 200 μL Folin-Ciocalteure agents. The mixture was mixed well for 8 minutes. To this solution, 600 μL of a 0.25% sodium carbonate solution was added. The above solution was further incubated at room temperature for 2 hours in the dark and its absorbance was measured at 765 nm against the blank using an ultraviolet-visible spectrophotometer. The same procedure was repeated for the standard solution of gallic acid and the calibration standard curve was construed. The measurement was compared to a standard curve prepared with gallic acid solution. The concentration of the total phenols was expressed as milligrams of gallic acid equivalents per gram of dry extract (mg of GAE/g of dE).47
The TPC in the 80% hydroethanolic extract was calculated from equation of calibration curve and it was 89.84 as mg GAE/g dE.
Total Antioxidant Activity Assay
2,2-Diphenyl-1-picrylhydrazyl (DPPH) is a stable free radical that accepts an electron or hydrogen radical and converts into yellow-colored diphenylpicyl hydrazine. The reduction capacity of DPPH is estimated by the decrease in the absorbance at 517 nm induced by antioxidants. DPPH scavenging activity was measured by the spectrophotometric method. A solution of DPPH (0.3 mM) in methanol was prepared freshly. Three-milliliter aliquot of this solution was mixed with 0.1 mL of the samples. The solution in the test tubes was shaken well and incubated in the dark for 30 minutes at room temperature. The absorbance was measured at 517 nm against methanol as blank. Control tube containing 1.0 mL methanol and 3.0 mL DPPH reagent was also measured for absorbance.48
Animal Ethics
This experiment was performed under the approval of the state committee on animal ethics, Shiraz University, Shiraz, Iran. Also, the recommendations of European Council Directive (86/609/EC) of November 24, 1986, regarding the standards in the protection of animals used for experimental purposes were followed.
Animals
Twenty-four adult male Sprague-Dawley rats (220 ± 15 g) colony-bred in the Animal House Center, Iran, were housed (6 rats per cage) in the animal room under controlled lighting (12-hour light:12-hour darkness) and temperature (20°C ± 2°C) conditions and had free access to laboratory food and tap water. All the experimental procedures were carried out between 09:00 am and 09:15 am.
Experimental Design and Protocol
The effect of ethanol on the oxidative stress biomarkers, trace elements, and hormonal levels in the testes of male rat and the role of ginger extract were studied by dividing the animals into four groups, each cage included 6 animals and were treated orally as follows:
Group I: Control rats or vehicle group, which was assigned to receive 2 mL/d of corn oil as vehicle by oral administration.
Group II: Ethanol group was assigned to receive ethanol (4 g/kg of body weight [BW]/d) by oral administration.
Group III: Ginger group was given ginger rhizome extract (1 g/kg of BW/d) by oral administration.
Group IV: As the ginger-ethanol group, this group received ginger (1 g/kg of BW/d) by oral administration before administration of ethanol (4 g/kg of BW/d).
The extract and the vehicle were orally administered between the 09:00 am and 09:15 am daily for 28 days. Alcohol (ethanol 96%) was purchased from Razi Chemical Company (Tehran, Iran).
Sampling and Tissue and Blood Preparation for Biochemical Assay
On the final day of experiment, the animals of all groups were sacrificed under ether anesthesia, by whole blood collection through heart puncture. Blood samples were drawn into blood-collecting tubes. After blood clotting, the sera were collected and stored at −20°C until analysis. The left testis was quickly removed and carefully dissected from the surrounding fat and tissue and immediately rinsed in ice saline. It was rapidly homogenized manually in cold phosphate buffer (pH 7.4), and the debris was removed by centrifugation at 3000 × g for 10 minutes. The upper clear supernatants were then recovered and stored at −70°C for later enzymes activity and protein assays.
Biochemical Analysis
Measurement of Superoxide Dismutase Activity
Total superoxide dismutase (SOD) activity was evaluated with SOD detection kit (RANSOD kit produced by RANDOX Company, Northern Ireland Antrim, UK) according to the manufacturer’s instructions. The role of SOD is to accelerate the dismutation of the toxic superoxide produced during oxidative energy processes to hydrogen peroxide and molecular oxygen. This method employs xanthine and xanthine oxidase to generate superoxide radicals which react with 2-(4-iodophenyl)-3-(4- nitrophenol)-5-phenyltetrazolium chloride (INT) to form a red formazan dye. The SOD activity is then measured by the degree of inhibition of this reaction. One unit of SOD causes 50% inhibition in the rate of reduction of INT under the conditions of the assay. SOD levels were recorded at 505 nm and through a standard curve, and expressed as unit per milligram of tissue protein (U/mg protein).
Measurement of Glutathione Peroxidase (GPx) Activity
The activity of glutathione peroxidase (GPx) was evaluated with GPx detection kit (RANSEL kit; RANDOX Company) according to the manufacturer’s instructions. GPx catalyzes the oxidation of glutathione (GSH) by cumene hydroperoxide. In the presence of glutathione reductase and NADPH, the oxidized glutathione is immediately converted into the reduced form with aconcomitant oxidation of NADPH to NADP+. The decrease in absorbance at 340 nm against blank was measured spectrophotometrically. One unit (U) of GPx activity was defined as the amount of enzyme that converts 1 mmol of NADPH into NADP+ per minute. The GPx activity was expressed as the unit per milligram of tissue protein (U/mg protein).
Measurement of Catalase Activity
Tissue catalase (CAT) activity was assayed spectrophotometrically by monitoring the decomposition of H2O2 using the procedure proposed by Aebi.49 Briefly, 0.5 mL of 30 mmol/L H2O2 solution in 50 mmol/L phosphate buffer (pH 7.0), 1 mL of 1:10 diluted testis supernatant, was added and the consumption of H2O2 was followed spectrophotometrically at 240 nm for 2 minutes at 25°C. The molar extinction coefficient was 43.6 L/mol per cm for H2O2. CAT activity was expressed as the unit that is defined as mmol H2O2 consumed/min per mg tissue protein.
Measurement of Lipid Peroxidation
To evaluate lipid peroxidation in the testis, we used a modified high-performance liquid chromatography (HPLC) method, which is based on the reaction of malondialdehyde (MDA) with thiobarbituric acid (TBA) to form a colored MDA-TBA adduct.50 Briefly, 0.5 mL testis supernatant was added to 2 mL TBA reagent containing 0.375% TBA, 15% trichloroacetic acid, and 0.25 mol/L HCl; the mixture was immediately heated (60 minutes at 958°C) and cooled with running water and thereafter butanol-pyridine (15:1, v/v) (1 mL) was added and the final volume was adjusted to 2 mL with distilled water. After vigorous mixing, the organic layer was separated by centrifugation (16 000 × g, 3 minutes, at room temperature). The supernatant was analyzed on an ultraviolet-visible spectrophotometer fitted with an 80-mL flow cell.51 The absorbance was measured at 532 nm (the mobile phase consisted of 300 mL/L methanol in 50 mM KH2PO4, pH 7.0). 1,1,3,3-Tetraethoxypropane was used as the standard, and MDA-TBA reactive substances values were expressed as nanomoles per milligram of tissue protein (nmol/mg protein). The HPLC system consisted of a solvent delivery pump (980-PU, Jasco, Tokyo, Japan), a reversed-phase column (Luna C18, 250 mm × 4.6 mm; Phenomenex, Torrance, CA, USA), and an ultraviolet-visible detector (UV-975, Jasco) operating at 532 nm.
Protein Content
The total protein concentration of tissue homogenates was determined according to Lowry et al.52
Measurement of Total Homocysteine Concentration
Total homocysteine (tHcy) of the testis serum, which refers to the sum of protein-bound, free-oxidized and reduced species of homocysteine, was determined by the Axis Homocysteine EIA kit. The sample volume used was 25 μL. Absorbance was measured at a wavelength of 450 nm using an enzyme-linked immunosorbent assay (ELISA) reader (STAT FAX 2100, USA). All estimations were performed in duplicate and the intra-assay coefficient of variation was <10%.
Measurement of Trace Elements
Serum trace elements were measured by atomic absorption spectrophotometry (ShimadzuAA-670, Kyoto, Japan). One milliliter of the serum was mixed with the same volume of a solution of hydrochloric acid and nitric acid and digested at 80°C in a water bath for 16 hours. The samples were then diluted with deionized water to 1 mL. Working standard solutions of Zn, Mg, Cu, and Fe were prepared by dilution of 1000 ppm certified standard solutions (Merck; Darmstadt, Germany).
Hormonal Measurements
The serum levels of total testosterone, sex hormone–binding globulin (SHBG), and dehydroepiandrosterone sulfate (DHEAs) were measured using commercially available ELISA kits according to the manufacturer’s instructions.
Statistical Analysis
The results were expressed as means ± standard error of the mean (mean ± SEM). All data were recorded with the Statistical Package for Social Sciences (SPSS 16.0). The variables for between groups comparison were analyzed using 1-way analysis of variance (ANOVA). Where a significant difference was found with ANOVA, the source of difference was located followed by post hoc multiple comparisons Tukey test for comparison. Statistical significance was set at P < .05.
Results
In this study, the antioxidant capacity of hydroalcoholic extract of Zingiber officinale Roscoe (ginger) was evaluated. The DPPH inhibition of the sample was expressed as the percentage of inhibition. Hydroethanolic extract of ginger roscoe possessed the reasonable DPPH scavenging activity (IC50 = 75.0% ± 1.41% mg/mL inhibition of the DPPH radical). The antioxidant activity of the hydroethanolic extract of ginger roscoe could be due to the presence of a wide variety of bioactive compounds, such as phenolics, flavonoids, carotenoids, and tannins in this plant. The potential medicinal use of ginger extract is supported by the presence of the aforementioned antioxidants and polyphenolic compounds. This study indicates that ginger is one of the most effective plants in terms of antioxidant properties and can serve as natural sources to the free radical scavengers and antioxidant agents.
The mean values (±SEM) of body weight and testicular weight are presented in Tables 1 and 2. Administration of ethanol significantly decreased the body weight in the ethanol group compared with the control group, while administrated ginger raised its weight to the normal level in the ginger-ethanol group (P < .05) (Table 1). Administration of ethanol also significantly decreased the testicular weight in the ethanol group compared with other groups, while administration of ginger in the ginger-ethanol group could significantly increase the testicular weight and bring it to the normal level with no significant difference compared with the control group (P > .05) (Table 2).
Table 1.
The Mean ± Standard Error of the Mean for Body Weight (g) in Different Groups.*
| Day | Group | |||
|---|---|---|---|---|
| Control | Ginger | Ethanol | Ginger + Ethanol | |
| Day 0 | 260.57 ± 7.14aA | 265.77 ± 4.60aA | 262.17 ± 3.16aA | 274.17 ± 4.36aA |
| Day 7 | 278.73 ± 7.28aA | 273.48 ± 4.67aA | 248.34 ± 3.37aA | 254.33 ± 4.42aA |
| Day 14 | 286.47 ± 7.59abA | 283.23 ± 4.58abA | 232.73 ± 3.16abB | 264.33 ± 4.2aA |
| Day 21 | 296.80 ± 7.79bcA | 299.91 ± 6.80bcA | 216.87 ± 3.11bcB | 271.89 ± 4.21aA |
| Day 28 | 321.17 ± 7.61cA | 326.27 ± 6.74cA | 199.79 ± 2.99cdB | 279.25 ± 3.90aC |
*Different uppercase letters show significant differences between groups. Different lowercase letters show significant differences between days of evaluation (P < .05).
Table 2.
The Mean ± Standard Error of the Mean for Testes Weight (g) in Different Groups at the End of Treated Period.*
| Group | ||||
|---|---|---|---|---|
| Control | Ginger | Ethanol | Ginger + Ethanol | |
| Day 28 | 1.37 ± 0.06a | 1.42 ± 0.07a | 0.78 ± 0.04b | 1.32 ± 0.09a |
*Different lowercase letters show significant difference between groups (P < .05).
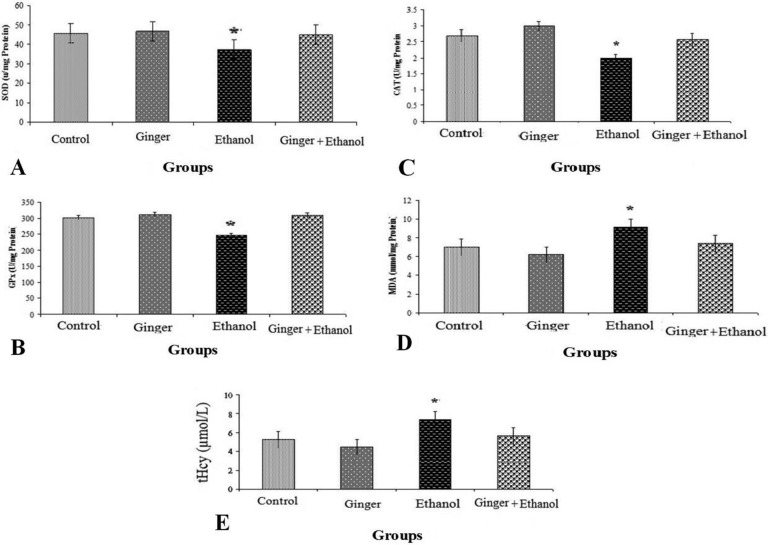
The mean values (±SEM) of GPx, SOD, CAT, MDA activity, and tHcy level in the rat testes are presented in Figure 1. Administration of ethanol significantly decreased the activity of SOD in ethanol group compared with other groups, while administration of ginger in the ginger-ethanol group could significantly increase the activity of this enzyme and bring it to the normal level with no significant difference compared with the control group (P > .05) (Figure 1A). Consumption of ethanol also in the ethanol group significantly decreased the activity of GPx compared with control group and administrated ginger raised its activity to the normal level in the ginger-ethanol group (Figure 1B). CAT activity was significantly lower in the ethanol group compared with the other groups (Figure 1C) and pretreatment by ginger in the ginger-ethanol group could have prevented this effect. Moreover, consumption of ethanol in the ethanol group significantly increased lipid peroxidation products (MDA) and tHcy concentration as compared with the control group, while pretreatment of rats with ginger suppressed the MDA and tHcy concentrations significantly in the ginger-ethanol group (Figure 1D and E).
Figure 1.
Comparison of superoxide dismutase (SOD) (A), glutathione peroxidase (GPx) (B), catalase (CAT) (C), and malondialdehyde (MDA) (D) activity and total homocysteine (tHcy) (E) among the control and treated rats (n = 6). Values represent mean ± standard error of the mean of enzyme activity (U/mg protein of testes tissue). Asterisk show significant difference with other groups (P < .05).
The mean values (±SEM) of trace elements (Zn, Mg, Fe, and Cu) and hormonal levels (total testosterone, SHBG, and DHEAs) are presented in Tables 3 and 4, respectively. Administration of ethanol significantly decreased the total testosterone, SHBG, and DHEAs level in the ethanol group compared with the other group, while administration of ginger in the ginger-ethanol group could significantly increase the level of their values (P < .05) (Table 4).
Table 3.
The Mean ± Standard Error of the Mean for Zinc, Copper, Iron, and Magnesium (μg/g) in Different Groups.*
| Element | Group | |||
|---|---|---|---|---|
| Control | Ginger | Ethanol | Ginger + Ethanol | |
| Zn | 131.54 ± 20.26a | 71.94 ± 18.21b | 90.45 ± 18.46c | 77.647 ± 17.31b |
| Mg | 2.866 ± 0.13a | 2.40 ± 0.27b | 2.52 ± 0.15b | 2.75 ± 0.25a |
| Fe | 640.86 ± 20.37a | 860.87 ± 20.6b | 584 ± 21.61c | 973 ± 16.41d |
| Cu | 16.54 ± 0.92a | 15.53 ± 0.4b | 13.09 ± 0.31c | 14.69 ± 0.53c |
*Different lowercase letters show significant difference between groups (P < .05).
Table 4.
The Mean ± Standard Error of the Mean for Total Testosterone, SHBG, and DHEAs (nmol/L) in Different Groups.*
| Group | ||||
|---|---|---|---|---|
| Control | Ginger | Ethanol | Ginger + Ethanol | |
| Total testosterone | 3.43 ± 0.22a | 2.242 ± 0.11b | 0.822 ± 0.10c | 2.0874 ± 0.38d |
| SHBG | 1.51 ± 0.12a | 1.445 ± 0.08a | 1.184 ± 0.10b | 1.44 ± 0.21a |
| DHEAs | 0.17 ± 0.012a | 0.169 ± 0.018a | 0.162 ± 0.009b | 0.167 ± 0.016a |
Abbreviations: SHBG, sex hormone–binding globulin; DHEAs, dehydroepiandrosterone sulfate.
*Different lowercase letters show significant difference between groups (P < .05).
Discussion
The findings of the present study show the significant changes in the testicular weight of the test group following ethanol consumption, which is in agreement with other previous reports.10,53 In the treated group, administration of ginger could significantly increase the testicular weight and bring it to normal level.
Both in vivo and in vitro studies indicated that ethanol consumption causes changes of morphology and testicular atrophy.10,53 Reduction of testicular weight and atrophy of seminiferous tubules leads to reduction in seminiferous testicular diameter.53,54 One of the effects of ethanol consumption on the testes is a change in the structure of the mitochondria.55 These alterations within the mitochondria may promote both apoptotic and necrotic cell death, which causes degeneration of the germinal epithelium and interstitial cells. In addition, ROS promotes the inappropriate activation of the mitochondrial permeability transition, leading the cells to pro-apoptotic pathways.56 Hence, ethanol-induced elevation of germ cell apoptosis together with necrosis and suppression of cell proliferation may contribute to testicular atrophy57 and decrease the testicular weight.
In our study, oral administration of ginger could significantly increase the testicular weight and bring it to normal level, which is in agreement with Morakinyo et al58 and Mohammadi et al.32 Morakinyo et al59 reported that ginger in sodium arsenite–induced reproductive toxicity protects against the adverse change reproductive organ weight and the decrease in sperm function. Khaki et al44 reported that administration of ginger significantly increased sperm percentage, viability, and motility and serum total testosterones in rats. The results of Mohammadi et al32 indicated that ginger extract could cause a significant increase testosterone and the number of germ cells and sertoli cells in seminiferous tubules. The increase sertoli cells and testosterone might be reasons for the significant improvement of testicular weight and spermatogenesis by ginger extract.
The results of this study also indicated that indices of oxidative stress and trace elements exchanged in test group compared with the control group, which agrees with previous reports.6,10,32,60,61 Many processes and factors are involved in causing alcohol-induced oxidative stress. Alcohol abuse associated with mitochondrial damage and increased microsomal proliferation.62 Alcohol metabolism results in the formation of NADH, which enhances activity of the respiratory chain, including heightened O2 use and ROS formation.63 One of the byproducts of alcohol metabolism, acetaldehyde, interacts with proteins and lipids to form ROS. When acetaldehyde is oxidized, it produces superoxide free radicals that are able to react with hydrogen peroxide to form other types of free radicals, such as hydroxyl radicals.64 Alcohol also induces hypoxia, which is as a cause ROS production.3 It is well known that ROS reacts with major cell macromolecules, such as lipids, proteins, and nucleic acids, and causes tissue injury. ROS are scavenged by antioxidant enzymes (SOD, CAT, and GPx), vitamins, and sulfhydryl group donors, including glutathione.3
SOD, CAT, and some enzymes contain metal ions as an integral part of their active sites to battle against toxic effects of metal-induced free radicals.65 The biochemistry and molecular structure of 3 SOD isoforms found in different body compartments have been characterized in humans. Cu/Zn-SOD or SOD1 is found in the cytoplasm, whereas EC-SOD or SOD3 is extracellular. The 2 isoforms use copper and zinc as cofactors. Manganese and Fe2+ are the cofactor for SOD2 and CAT, respectively, which are found in mitochondria and cytosol.65,66 O2−. formed within the mitochondrion rapidly converted in the matrix compartment to H2O2 by Mn-SOD, or in the intermembrane space and the cytosol space by ZnCu-SOD.66 In turn, H2O2 is decomposed by catalase to H2O and O2 or reduced to H2O by glutathione peroxidases and by glutathione.65 Because trace elements are cofactors of antioxidant enzymes; their deficiency may be associated with increased oxidative stress and increased lipid peroxidation. In agreement with our study, Dreosti and Pitman67 reported that cellular metabolism of ethanol could lead to the generation of damaging superoxide and hydroxyl radicals; trace elements, notably zinc, manganese, selenium, and copper, may function protectively as free radical scavengers and as antioxidants.
Ginger contains high level of total phenolic and flavonoid, responsible for its high antioxidant activities.18 Also, the antioxidant activity of ginger was attributed to its major ingredients namely zingerone, gingerdiol, zingiberene, gingerols, and shogoals.23,68,69 Siddaraju and Dharmesh24 elucidated that ginger-free phenolic and ginger-hydrolyzed phenolic fractions of ginger exhibited free radical scavenging, inhibited lipid peroxidation, DNA protection and reduced power abilities indicating strong antioxidant properties.
In our study, ethanol consumption caused significant increase in MDA and tHcy concentration in the test group, and oral administration of ginger restored them to normal level in treated group. Many studies have evaluated the association between homocysteine and alcohol consumption.70,71 Previous studies showed that short-term and chronic ethanol consumption of alcohol impairs methionine synthetase activity and decreases S-adenosylmethionine (SAM) levels and increases S-adenosyl Hcy (SAH) levels, which indicate interference with homocysteine remethylation.71,72
It is assumed that hyperhomocysteinemia and oxidative stress may play a role in development of alcoholic patients.70 Previous studies suggest that chronic ethanol consumption increases homocysteine accumulation in hepatocyte and high level of homocysteine induces oxidative stress via thiolactone formation and disrupts endothelium integrity.73,74 However, lack of folate causes methylation cycle impairment. Bleich et al75 showed that ethanol decreases liver ability in folate maintenance and storage.75 Bree et al76 also explained an inverse relation between folate and Hcy level.
MDA is the byproduct of the major chain reactions leading to the oxidation of polyunsaturated fatty acids, and thus serves as a marker of oxidative stress–mediated lipid peroxidation. Increased MDA level in spermatozoa leads to impaired sperm function and decreased sperm motility.3 Testis is more vulnerable to oxidative damage than other organs due to its low antioxidant capacity and it also has a high content of polyunsaturated fatty acids, which are easy targets to oxidative damage by free radicals due to the unsaturated bonds. Use of ginger was reported to improve antioxidant enzymes activity and lipid proxidation in testis.32,58,59,77 Morakinyo et al58 suggested that ginger extract (500 and 1000 mg/kg) doses have a beneficial effect on male reproductive functions in rats. The authors observed an increased epididymal sperm count, motility, viability, and testosterone and a decreased MDA level after ginger intake.58 Morakinyo et al59 showed that ginger enhances plasma reproductive hormones level, antioxidants activities and lipid peroxidation in sodium arsenite–induced reproductive toxicity in male rats. Mohammadi et al32 indicated that ginger treatment increased the level of antioxidants in serum. Because a higher level of testosterone has been recorded after ginger extract administration in several studies, including Morakinyo et al,32 Khaki et al,44 and Mohammadi et al,58 positive effects of ginger on reproductive functions might be a consequence of both its potent antioxidant properties and androgenic activities.
Conclusion
The findings of this study indicated that oral administration of ginger can improve the body weight, trace element, tHcy and indices of oxidative stress in ethanol-induced reproductive toxicity in the rat testis. This finding is consistent with other traditional use of ginger for controlling antioxidant enzymes activity, although further investigation with longer period or higher dose may show clearer feature of this finding. In this study, ethanol-induced toxicity was conducted by gavage; it is suggested that future studies use self-administration method.
Acknowledgments
We are grateful to the Shiraz University of Medical Sciences, Shiraz, Iran for sponsoring this study.
Footnotes
Author Contributions: The work presented in this article was carried out through collaboration between all authors. AA made the initial hypothesis. All authors participate in defining the research theme and providing the proposal. AA and KA performed the experiments on animal models. AA, MH, AI did the phytochemical analysis and SHM performed the laboratory measurements. AA and MH interpreted the data, wrote and edited the article. MH supervised the work. All authors have contributed to, edited, and approved the article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Shiraz University of Medical Sciences.
Ethical Approval: This study is approved by Committee of Ethics of Shiraz University of Medical Sciences (reference number 94-01-64-10080).
References
- 1. Akbari A, Jelodar GA. The effect of oxidative stress and antioxidants on men fertility. Zahedan J Res Med Sci. 2013;15(7):1–7. [Google Scholar]
- 2. Gaur DS, Talekar M, Pathak VP. Effect of cigarette smoking on semen quality of infertile men. Singapore Med J. 2007;48:119–123. [PubMed] [Google Scholar]
- 3. Agarwal A, Prabakaran SA. Mechanism, measurement, and prevention of oxidative stress in male reproductive physiology. Indian J Exp Biol. 2005;43:963–974. [PubMed] [Google Scholar]
- 4. Suleiman SA, Elamin Ali M, Zaki Z, El-Malik E, Nasr M. Lipid peroxidation and human sperm motility: protective role of vitamin E. J Androl. 1996;17:530–537. [PubMed] [Google Scholar]
- 5. Moustafa MH, Sharma RK, Thornton J, et al. Relationship between ROS production, apoptosis and DNA denaturation in spermatozoa from patients examined for infertility. Hum Reprod. 2004;19:129–138. [DOI] [PubMed] [Google Scholar]
- 6. Alirezaei M, Jelodar G, Ghayemi Z. Antioxidant defense of betaine against oxidative stress induced by ethanol in the rat testes. Int J Peptide Res Ther. 2012;18:239–247. [Google Scholar]
- 7. Dosumu O, Duru F, Osinubi A, Oremosu A, Noronha C. Influence of virgin coconut oil (VCNO) on oxidative stress, serum testosterone and gonadotropic hormones (FSH, LH) in chronic ethanol ingestion. Agric Biol J North Am. 2010;6:1126–1132. [Google Scholar]
- 8. Van Thiel D, Gavaler J, Eagon P, Chiao Y-B, Cobb C, Lester R. Alcohol and sexual function. Pharmacol Biochem Behav. 1980;13:125–129. [DOI] [PubMed] [Google Scholar]
- 9. World Health Organization. Global Status Report on Alcohol 2004 Geneva, Switzerland: World Health Organization; 2004. [Google Scholar]
- 10. Dosumu O, Osinubi A, Duru F. Alcohol induced testicular damage: can abstinence equal recovery? Middle East Fertil Soc J. 2014;19:221–228. [Google Scholar]
- 11. Goverde H, Dekker HS, Janssen H, Bastiaans BA, Rolland R, Zielhuis GA. Semen quality and frequency of smoking and alcohol consumption—an explorative study. Int J Fertil Menopausal Stud. 1994;40:135–138. [PubMed] [Google Scholar]
- 12. Marinelli D, Gaspari L, Pedotti P, Taioli E. Mini-review of studies on the effect of smoking and drinking habits on semen parameters. Int J Hyg Environ Health. 2004;207:185–192. [DOI] [PubMed] [Google Scholar]
- 13. Jabbari M, Hashempur MH, Razavi SZE, Shahraki HR, Kamalinejad M, Emtiazy M. Efficacy and short-term safety of topical Dwarf Elder (Sambucus ebulus L.) versus diclofenac for knee osteoarthritis: a randomized, double-blind, active-controlled trial. J Ethnopharmacol. 2016;188:80–86. [DOI] [PubMed] [Google Scholar]
- 14. Mosavat SH, Ghahramani L, Haghighi ER, Chaijan MR, Hashempur MH, Heydari M. Anorectal Diseases in Avicenna’s “Canon of Medicine”. Acta Med Hist Adriat. 2015;13(Suppl 2):103–14. [PubMed] [Google Scholar]
- 15. Roozbeh J, Hashempur MH, Heydari M. Use of herbal remedies among patients undergoing hemodialysis. Iran J Kidney Dis. 2013;7:492–495. [PubMed] [Google Scholar]
- 16. Heydari M, Homayouni K, Hashempur MH, Shams M. Topical Citrullus colocynthis (bitter apple) extract oil in painful diabetic neuropathy: a double-blind randomized placebo-controlled clinical trial. J Diabetes. 2016;8:246–252. [DOI] [PubMed] [Google Scholar]
- 17. More SK, Lande AA, Jagdale PG, Adkar PP, Ambavade SD. Evaluation of anti-inflammatory activity of Solanum xanthocarpum Schrad and Wendl (Kaṇṭakāri) extract in laboratory animals. Anc Sci Life. 2013;32:222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ghasemzadeh A, Jaafar HZ, Rahmat A. Antioxidant activities, total phenolics and flavonoids content in two varieties of Malaysia young ginger (Zingiber officinale Roscoe). Molecules. 2010;15:4324–4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lantz RC, Chen G, Sarihan M, Solyom A, Jolad S, Timmermann B. The effect of extracts from ginger rhizome on inflammatory mediator production. Phytomedicine. 2007;14:123–128. [DOI] [PubMed] [Google Scholar]
- 20. Al-Amin ZM, Thomson M, Al-Qattan KK, Peltonen-Shalaby R, Ali M. Anti-diabetic and hypolipidaemic properties of ginger (Zingiber officinale) in streptozotocin-induced diabetic rats. Br J Nutr. 2006;96:660–666. [DOI] [PubMed] [Google Scholar]
- 21. Ramachandran C, Lollett IV, Escalon E, Quirin K-W, Melnick SJ. Anticancer potential and mechanism of action of mango ginger (Curcuma amada Roxb.) supercritical CO2 extract in human glioblastoma cells. J Evid Based Complementary Altern Med. 2015;20:109–119. [DOI] [PubMed] [Google Scholar]
- 22. Shukla Y, Singh M. Cancer preventive properties of ginger: a brief review. Food Chem Toxicol. 2007;45:683–690. [DOI] [PubMed] [Google Scholar]
- 23. Zancan KC, Marques MO, Petenate AJ, Meireles MAA. Extraction of ginger (Zingiber officinale Roscoe) oleoresin with CO2 and co-solvents: a study of the antioxidant action of the extracts. J Supercrit Fluids. 2002;24:57–76. [Google Scholar]
- 24. Siddaraju MN, Dharmesh SM. Inhibition of gastric H+, K+-ATPase and Helicobacter pylori growth by phenolic antioxidants of Zingiber officinale . Mol Nutr Food Res. 2007;51:324–332. [DOI] [PubMed] [Google Scholar]
- 25. Aktan F, Henness S, Tran VH, Duke CC, Roufogalis BD, Ammit AJ. Gingerol metabolite and a synthetic analogue Capsarol™ inhibit macrophage NF-κB-mediated iNOS gene expression and enzyme activity. Planta Med. 2006;72:727–734. [DOI] [PubMed] [Google Scholar]
- 26. Kim SO, Chun K-S, Kundu JK, Surh Y-J. Inhibitory effects of [6]-gingerol on PMA-induced COX-2 expression and activation of NF-κB and p38 MAPK in mouse skin. Biofactors. 2004;21:27–31. [DOI] [PubMed] [Google Scholar]
- 27. Nonn L, Duong D, Peehl DM. Chemopreventive anti-inflammatory activities of curcumin and other phytochemicals mediated by MAP kinase phosphatase-5 in prostate cells. Carcinogenesis. 2007;28:1188–1196. [DOI] [PubMed] [Google Scholar]
- 28. Kim E-C, Min J-K, Kim T-Y, et al. [6]-Gingerol, a pungent ingredient of ginger, inhibits angiogenesis in vitro and in vivo. Biochem Biophys Res Commun. 2005;335:300–308. [DOI] [PubMed] [Google Scholar]
- 29. Nicoll R, Henein MY. Ginger (Zingiber officinale Roscoe): a hot remedy for cardiovascular disease? Int J Cardiol. 2009;131:408–409. [DOI] [PubMed] [Google Scholar]
- 30. Afolabi A, Alagbonsi I, Oyebanji T. Beneficial effects of ethanol extract of Zingiber officinale (ginger) rhizome on epididymal sperm and plasma oxidative stress parameters in experimentally cryptorchid rats. Annu Res Rev Biol. 2014;4:1448–1460. [Google Scholar]
- 31. Bordbar H, Esmaeilpour T, Dehghani F, Panjehshahin MR. Stereological study of the effect of ginger’s alcoholic extract on the testis in busulfan-induced infertility in rats. Iran J Reprod Med. 2013;11:467–472. [PMC free article] [PubMed] [Google Scholar]
- 32. Mohammadi F, Nikzad H, Taghizadeh M, et al. Protective effect of Zingiber officinale extract on rat testis after cyclophosphamide treatment. Andrologia. 2014;46:680–686. [DOI] [PubMed] [Google Scholar]
- 33. Zahedi A, Khaki A, Ahmadi-Ashtiani H, Rastegar H, Rezazadeh S. Zingiber officinale protective effects on gentamicin’s toxicity on sperm in rats. J Med Plants. 2010;3(35):93–98. [Google Scholar]
- 34. Heyadri M, Hashempur MH, Ayati MH, Quintern D, Nimrouzi M, Mosavat SH. The use of Chinese herbal drugs in Islamic medicine. J Integr Med. 2015;13:363–367. [DOI] [PubMed] [Google Scholar]
- 35. Khodaie L, Sadeghpoor O. Ginger from ancient times to the new outlook. Jundishapur J Nat Pharm Prod. 2015;10(1):e18402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shoara R, Hashempur MH, Ashraf A, Salehi A, Dehshahri S, Habibagahi Z. Efficacy and safety of topical Matricaria chamomilla L.(chamomile) oil for knee osteoarthritis: a randomized controlled clinical trial. Complement Ther Clin Pract. 2015;21:181–187. [DOI] [PubMed] [Google Scholar]
- 37. Hashempur MH, Lari ZN, Ghoreishi PS, et al. A pilot randomized double-blind placebo-controlled trial on topical chamomile (Matricaria chamomilla L.) oil for severe carpal tunnel syndrome. Complement Ther Clin Pract. 2015;21:223–228. [DOI] [PubMed] [Google Scholar]
- 38. Mosavat SH, Ghahramani L, Sobhani Z, Haghighi ER, Heydari M. Topical Allium ampeloprasum subsp Iranicum (Leek) extract cream in patients with symptomatic hemorrhoids: a pilot randomized and controlled clinical trial. J Evid Based Complementary Altern Med. 2015;20:132–136. [DOI] [PubMed] [Google Scholar]
- 39. Qasemzadeh MJ, Sharifi H, Hamedanian M, et al. The effect of Viola odorata flower syrup on the cough of children with asthma a double-blind, randomized controlled trial. J Evid Based Complementary Altern Med. 2015;20:287–291. [DOI] [PubMed] [Google Scholar]
- 40. Sharifi H, Minaie MB, Qasemzadeh MJ, Ataei N, Gharehbeglou M, Heydari M. Topical use of Matricaria recutita L (Chamomile) oil in the treatment of monosymptomatic enuresis in children: a double-blind randomized controlled trial. J Evid Based Complementary Altern Med. 2017;22:12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ghadiri MK, Gorji A. Natural remedies for impotence in medieval Persia. Int J Impotence Res. 2004;16:80–83. [DOI] [PubMed] [Google Scholar]
- 42. Kamatenesi-Mugisha M, Oryem-Origa H. Traditional herbal remedies used in the management of sexual impotence and erectile dysfunction in western Uganda. Afr Health Sci. 2005;5:40–49. [PMC free article] [PubMed] [Google Scholar]
- 43. Liang X. Clinical observation on impotence treated by ginger-partition moxibustion [in Chinese]. Zhen Ci Yan Jiu. 1992;17:263–265. [PubMed] [Google Scholar]
- 44. Khaki A, Fathiazad F, Nouri M, et al. The effects of ginger on spermatogenesis and sperm parameters of rat. Int J Reprod Biomed. 2009;7:7–12. [Google Scholar]
- 45. Blainski A, Lopes GC, De Mello JCP. Application and analysis of the Folin Ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules. 2013;18:6852–6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Waterhouse AL. Determination of total phenolics In: Wrolstad RE, ed. Current Protocols in Food Analytical Chemistry. New York, NY: Wiley; 2003:1.1.1–1.1.8. doi:10.1002/0471142913.faa0101s06. [Google Scholar]
- 47. Noorafshan A, Dabbaghmanesh M, Tanideh N, et al. Stereological study of the effect of black olive hydroalcoholic extract on osteoporosis in vertebra and tibia in ovariectomized rats. Osteoporos Int. 2015;26:2299–2307. [DOI] [PubMed] [Google Scholar]
- 48. Leong L, Shui G. An investigation of antioxidant capacity of fruits in Singapore markets. Food Chem. 2002;76:69–75. [Google Scholar]
- 49. Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. [DOI] [PubMed] [Google Scholar]
- 50. Lykkesfeldt J. Determination of malondialdehyde as dithiobarbituric acid adduct in biological samples by HPLC with fluorescence detection: comparison with ultraviolet-visible spectrophotometry. Clin Chem. 2001;47:1725–1727. [PubMed] [Google Scholar]
- 51. Hagar HH, El Etter E, Arafa M. Taurine attenuates hypertension and renal dysfunction induced by cyclosporine A in rats. Clin Exp Pharmacol Physiol. 2006;33:189–196. [DOI] [PubMed] [Google Scholar]
- 52. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 53. Weinberg J, Vogl AW. Effects of ethanol consumption on the morphology of the rat seminiferous epithelium. J Androl. 1988;9:261–269. [DOI] [PubMed] [Google Scholar]
- 54. Martinez M, Macera S, De Assis G, et al. Structural evaluation of the effects of chronic ethanol ingestion on the testis of Calomys callosus . Tissue Cell. 2009;41:199–205. [DOI] [PubMed] [Google Scholar]
- 55. Kiessling K, Tobé U. Degeneration of liver mitochondria in rats after prolonged alcohol consumption. Exp Cell Res. 1964;33:350–354. [DOI] [PubMed] [Google Scholar]
- 56. Sastre J, Serviddio G, Pereda J, et al. Mitochondrial function in liver disease. Front Biosci. 2007;12:1200–1209. [DOI] [PubMed] [Google Scholar]
- 57. Zhu Q, Meisinger J, Emanuele NV, Emanuele MA, LaPaglia N, Thiel DH. Ethanol exposure enhances apoptosis within the testes. Alcohol Clin Exp Res. 2000;24:1550–1556. [PubMed] [Google Scholar]
- 58. Morakinyo A, Adeniyi O, Arikawe A. Effects of Zingiber officinale on reproductive functions in the male rat. Afr J Biomed Res. 2008;11:329–334. [Google Scholar]
- 59. Morakinyo A, Achema P, Adegoke O. Effect of Zingiber officinale (ginger) on sodium arsenite-induced reproductive toxicity in male rats. Afr J Biomed Res. 2010;13:39–45. [Google Scholar]
- 60. Guthauser B, Boitrelle F, Plat A, Thiercelin N, Vialard F. Chronic excessive alcohol consumption and male fertility: a case report on reversible azoospermia and a literature review. Alcohol Alcohol. 2014;49:42–44. [DOI] [PubMed] [Google Scholar]
- 61. Villalta J, Ballesca J, Nicolas J, Martinez de Osaba M, Antunez E, Pimentel C. Testicular function in asymptomatic chronic alcoholics: relation to ethanol intake. Alcohol Clin Exp Res. 1997;21:128–134. [PubMed] [Google Scholar]
- 62. Castro GD, Costantini MH, Delgado de Layño AM, Castro JA. Rat liver microsomal and nuclear activation of methanol to hydroxymethyl free radicals. Toxicol Lett. 2002;129:227–236. [DOI] [PubMed] [Google Scholar]
- 63. Liesivuori J. Methanol and formic acid toxicity: biochemical mechanisms. Pharmacol Toxicol. 1991;69:157–163. [DOI] [PubMed] [Google Scholar]
- 64. Smith AM, Zeve DR, Grisel JJ, Chen W-JA. Neonatal alcohol exposure increases malondialdehyde (MDA) and glutathione (GSH) levels in the developing cerebellum. Dev Brain Res. 2005;160:231–238. [DOI] [PubMed] [Google Scholar]
- 65. Afonso V, Champy R, Mitrovic D, Collin P, Lomri A. Reactive oxygen species and superoxide dismutases: role in joint diseases. Joint Bone Spine. 2007;74:324–329. [DOI] [PubMed] [Google Scholar]
- 66. Schönfeld P, Wojtczak L. Fatty acids as modulators of the cellular production of reactive oxygen species. Free Radic Biol Med. 2008;45:231–241. [DOI] [PubMed] [Google Scholar]
- 67. Dreosti IE, Pitman L. Interactions between trace elements and alcohol in rats. Mechanisms of alcohol damage in utero. Ciba Foundation Symposium; 2009. [DOI] [PubMed]
- 68. Souri E, Amin G, Farsam H, Jalalizadeh H, Barezi S. Screening of thirteen medicinal plant extracts for antioxidant activity. Iran J Pharm Res. 2010;7:149–154. [Google Scholar]
- 69. Stoilova I, Krastanov A, Stoyanova A, Denev P, Gargova S. Antioxidant activity of a ginger extract (Zingiber officinale). Food Chem. 2007;102:764–770. [Google Scholar]
- 70. Looker H, Fagot-Campagna A, Gunter E, et al. Homocysteine as a risk factor for nephropathy and retinopathy in type 2 diabetes. Diabetologia. 2003;46:766–772. [DOI] [PubMed] [Google Scholar]
- 71. Berlin KN, Cameron LM, Gatt M, Miller RR. Reduced de novo synthesis of 5-methyltetrahydrofolate and reduced taurine levels in ethanol-treated chick brains. Comp Biochem Physiol C Toxicol Pharmacol. 2010;152:353–359. [DOI] [PubMed] [Google Scholar]
- 72. Carrasco M, Jimenez-Lopez J, Segovia J, Marco C. Comparative study of the effects of short- and long-term ethanol treatment and alcohol withdrawal on phospholipid biosynthesis in rat hepatocytes. Comp Biochem Physiol B Biochem Mol Biol. 2002;131:491–497. [DOI] [PubMed] [Google Scholar]
- 73. Tyagi N, Sedoris KC, Steed M, Ovechkin AV, Moshal KS, Tyagi SC. Mechanisms of homocysteine-induced oxidative stress. Am J Physiol Heart Circ Physiol. 2005;289:H2649–H2656. [DOI] [PubMed] [Google Scholar]
- 74. Barak AJ, Beckenhauer HC, Kharbanda KK, Tuma DJ. Chronic ethanol consumption increases homocysteine accumulation in hepatocytes. Alcohol. 2001;25:77–81. [DOI] [PubMed] [Google Scholar]
- 75. Bleich S, Bandelow B, Javaheripour K, et al. Hyperhomocysteinemia as a new risk factor for brain shrinkage in patients with alcoholism. Neurosci Lett. 2003;335:179–182. [DOI] [PubMed] [Google Scholar]
- 76. de Bree A, Verschuren WM, Blom HJ, Kromhout D. Association between B vitamin intake and plasma homocysteine concentration in the general Dutch population aged 20-65 y. Am J Clin Nutr. 2001;73:1027–1033. [DOI] [PubMed] [Google Scholar]
- 77. Zahedi A, Fathiazad F, Khaki A, Ahmadnejad B. Protective effect of ginger on gentamicin-induced apoptosis in testis of rats. Adv Pharm Bull. 2012;2:197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]