Abstract
The present study was designed to investigate the protective effect of the aqueous extract of Portulaca oleracea against hyperglycemic, oxidative damage and inflammation in the serum of streptozotocin (STZ)-induced diabetic rats. In the present study, the rats were divided into the following groups of 8 animals each: control, untreated diabetic, 3 Portulaca oleracea (100, 200, 400 mg/kg/d)–treated diabetic groups. At the end of the 4-week period, glucose, interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), malondialdehyde (MDA), glutathione (GSH), and total antioxidant status (TAS) levels were measured. STZ caused an elevation in the serum levels of glucose, MDA, IL-6, and TNF-α with reduction in the levels of GSH and TAS (P < .01). Portulaca oleracea ameliorated glucose, MDA, IL-6, TNF-α, GSH, and TAS levels in diabetic groups versus to the untreated groups (P < .05). Taken together, Portulaca oleracea prevented hyperglycemia by preventing the oxidative stress and inflammation.
Keywords: Portulaca oleracea, diabetes, rat, streptozotocin, oxidative indices, inflammation
The metabolic syndrome is characterized by insulin resistance, dyslipidemia, and hypertension and is associated with type 2 diabetes and cardiovascular disease.1
Diabetes is a complex metabolic disorder that consists of progressive hyperglycemia in the context of insulin resistance, which precedes insulin deficiency as a result of β-cell failure.1 Accumulating evidence shows that β-cell loss in diabetes due to the combination of oxidative stress and inflammation. Oxidative stress is caused by imbalance between oxidant-antioxidant systems, which could be because of elevated free radical generation and decreased activity of antioxidants.2 Involvement of oxidative stress in the pathogenesis of diabetes is suggested not only by the generation of free radicals especially reactive oxygen species (ROS) but also because of nonenzymatic protein glycosylation, auto-oxidation of glucose, impaired glutathione metabolism, modification in antioxidant enzymes and lipid peroxides formation.3 On the other hand, oxidative stress induces overproduction of ROS, which activates several inflammatory signaling cascades that will contribute to inflammation.4 Expression of pro-inflammatory molecules might attract local inflammatory cells, which may further exacerbate the local inflammation, causing β-cell apoptosis and type 2 diabetes.5 Therapeutic approaches were designed to improve oxidative stress and inflammation and prove new drugs for the prevention of diabetes.6 In the past decade, there is a growing interest to evaluate biological and medical properties of herbal remedies against diabetes.6 Portulaca oleracea L (Portulaceae, common name purslane) is a warm-climate annual, used traditionally for alleviating pain and swelling.7 It has been shown a wide range of pharmacological effects including antibacterial,8 antioxidant,9,10 anti-inflammatory,11 skeletal muscle relaxant,12,13 and wound healing14 activities. It is also serves as a vegetable, widely sold in shops in China, United Arab Emirates, and Oman15 and has been reported to be rich in α-linolenic acid and β-carotene.16 In addition, purslane consists of flavonoids17, coumarins18 and monoterpene glycoside, in particular, contain dopamine, N-trans-feruloyltyramine,19 high concentration of noradrenaline,20 adenosine,21 and ferulic acid.22 The protective effect of purslane against type 2 diabetes remains uncertain. Hence, the present study was conducted to find the effect of purslane as antidiabetic agent in streptozotocin (STZ)-induced diabetic rats by measuring glucose, interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), malondialdehyde (MDA), glutathione (GSH), and total antioxidant status (TAS) levels in the serum.
Materials and Methods
Reagents
All purified enzymes, coenzymes, substrates, standards, buffers, kits, and other chemicals were purchased from Sigma-Aldrich Chemical (St Louis, MO, USA). Glucose kit was purchased from Pars Azmoon kit (Iran).
Animals
Wistar albino rats (2 months; 200 ± 13 g) were bred at the university experimental animal care center. Animals were maintained under standard environmental conditions and had free access to standard rodent feed and water.
Study Design
Forty male Wistar albino rats were randomly allotted to 5 experimental groups (n = 8 per group) as follows: group 1, control (C); group 2, diabetic (D); group 3, diabetic and Portulaca oleracea–treated (100 mg/kg/d) (D + PO1); group 4, diabetic and Portulaca oleracea–treated (200 mg/kg/d) (D + PO2); and group 5, diabetic with Portulaca oleracea–treated (400 mg/kg/d) (D + PO3). Rats were kept in their own cages at constant room temperature (21°C ± 2°C) under a normal 12-hour light/dark cycle with free access to food and water. The animals were housed according to regulations for the welfare of experimented animals. The study was conducted in Mashhad Medical University Experimental Animal Research Laboratory. Protocols were approved by the Ethical Research Committee of Mashhad University of Medical Sciences. On the first day of the study, the diabetic groups were given STZ in a single intraperitoneal injection at a dose of 60 mg/kg for induction of diabetes. Blood was extracted from the tail vein for glucose analysis 72 hours after STZ injection. Rats with blood glucose levels higher than 250 mg/dL were accepted as being diabetic. In the control groups (C), physiological saline (intraperitoneally) was injected as vehicle. Portulaca oleracea was injected (intraperitoneally) to the treatment groups from 3 days after STZ administration for 4 weeks. Blood glucose level was recorded at weekly intervals. At the end of the 4-week period, animals were anesthetized by ether and blood was subsequently collected from the retro orbital sinus. Blood and sera were separated by centrifugation at 3000 rpm for 10 minutes for glucose, IL-6, TNF-α, MDA, GSH, and TAS.
Biochemical Analysis
During the experiment, the serum glucose concentration was measured with the Ames One Touch glucometer (One-Touch Basic; Lifescan, Johnson & Johnson, New Brunswick, NJ, USA) in rat tail vein blood. At the end of experiment, blood glucose was estimated using the diagnostic kits (Pars Azmoon kit, Iran) on an automatic analyzer (Abbott, model Alcyon 300, Dallas, TX, USA). TNF-α and IL-6 was measured by enzyme-linked immunosorbent assay method according to the manufacturer’s instructions. The serum MDA was estimated by TBARS (thiobarbituric acid reactive substances) method.23,24 The whole blood reduced GSH was measured using Ellman’s reagent (5,5′-dithio-bis-2-nitrobenzoic acid,) as described by Beutle et al.25 The TAS was measured by FRAP (ferric reducing antioxidant power) method.26,27
Statistical Analysis
All experiments were carried out at least in duplicate. Each group consisted of 8 rats. One-way analysis of variance was performed and Tukey’s post hoc test was used for multiple comparisons. Statistical analyses were performed using the InStat 3.0 program. The results are expressed as mean ± standard error of the mean. The results originated from analysis of serum. Linear correlation tests were also performed. Differences of P < .05 were considered significant.
Results
After the experimental period (4-week), STZ-diabetic rats exhibited significant (P < .001) hyperglycemia compared with the control rats (Figure 1). Purslane decreased blood glucose level in the diabetic rats compared to the untreated diabetic rats in a dose-dependent manner (P < .001) (Figure 1). Purslane (200 and 400 mg/kg/d) significantly decreased glucose in STZ diabetic rats only at the fourth week of the study (P < .05 and P < .01, respectively), while the highest dose of purslane (80 mg/kg/d) was significantly reduced blood glucose in diabetic rats in the beginning of the first week of treatment (P < .05 and P < .01, respectively) (Figure 1).
Figure 1.
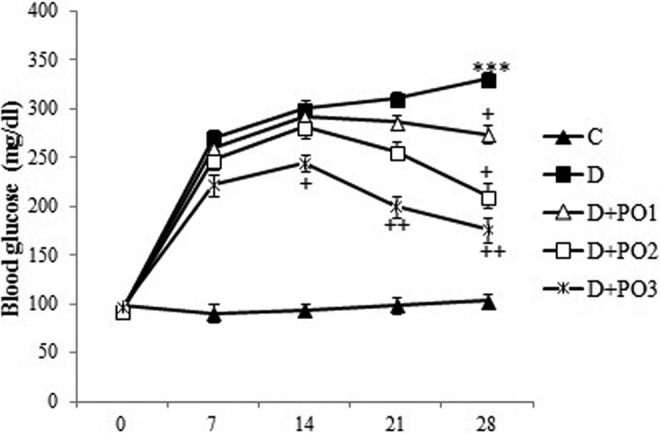
Effect of purslane on blood glucose level (mg/dL). Control (C), untreated diabetic rats (D), Portulaca oleracea (PO)–treated (100 mg/kg/d) diabetic rats (D + PO1), PO-treated (200 mg/kg/d) diabetic rats (D + PO2), and PO-treated (400 mg/kg/d) diabetic rats (D + PO3) during 4 weeks of study (n = 8, for each group). Values are the means ± standard error of the mean (SEM) for 8 rats in each group. Significantly different from normal control (group C) rats (***P < .001). Significantly different from streptozotocin (STZ)-treated (group D) rats (+ P < .05, ++ P < .01).
STZ injection produced significant changes in oxidative stress and inflammatory parameters in the serum of diabetic rats 4 weeks after diabetes induction, as shown by increased lipid peroxidation product (MDA) (P < .001), TNF-α (P < .01), IL-6 (P < .001) and decreased GSH (P < .001) and TAS (P < .01) compared with the control group (Table 1). The highest dose of purslane (400 mg/kg/d) decreased the serum MDA (P < .01), IL-6 (P < .05), TNF-α (P < .01) and increased GSH (P < .001), and TAS (P < .05) content versus the untreated diabetic groups (Table 1). Dosage of 200 mg/kg/d of purslane decreased the serum levels of TNF-α (P < .05) and increased GSH (P < .01) versus the untreated diabetic groups (Table 1). A significant difference was observed between MDA and GSH levels in rats that received the lowest dose (100 mg/kg) and the highest dose (200 mg/kg) (P < .05) (Table 2).
Table 1.
Effect of PO on Serum MDA, GSH, TAS, IL-6, and TNF-α in Control, Untreated Diabetic Rats, and PO-Treated Diabetic Rats During 4 Weeks of Study (n = 8 for Each Group).a
| C | D | D + PO1 | D + PO2 | D + PO3 | |
|---|---|---|---|---|---|
| MDA (nmol/mL) | 1.66 ± 0.26 | 4.45 ± 0.40*** | 4.02 ± 0.34*** | 3.21 ± 0.35*### | 2.47 ± 0.29++# |
| GSH (nmol/mL) | 2.78 ± 0.19 | 1.06 ± 0.11*** | 1.56 ± 0.18*** | 1.97 ± 0.22*++ | 2.36 ± 0.15+++# |
| TAS (nmol/mL) | 4.55 ± 0.70 | 1.78 ± 0.35** | 2.37 ± 0.38* | 3.11 ± 0.52 | 1.87 ± 0.35+ |
| IL-6 (pg/mL) | 2.12 ± 0.13 | 5.34 ± 0.54*** | 4.56 ± 0.45*** | 4.00 ± 0.32* | 3.45 ± 0.36+ |
| TNF-α (pg/mL) | 9.82 ± 0.60 | 15.11 ± 1.13** | 13.21 ± 0.90 | 11.54 ± 0.49+ | 10.26 ± 1.05++ |
Abbreviations: PO, Portulaca oleracea; MDA, malondialdehyde; GSH, glutathione; TAS, total antioxidant status; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α.
aC denotes controls; D, denotes untreated diabetic rats; D + PO1 denotes PO-treated (100 mg/kg/d) diabetic rats; D + PO2 denotes PO-treated (200 mg/kg/day) diabetic rats; and D + PO3 denotes PO-treated (400 mg/kg/day) diabetic rats. Significantly different from normal control (group C) rats (*P < .05, **P < .01; ***P < .001). Significantly different from streptozotocin-treated (group D) rats (+ P < .05, ++ P < .05, +++ P < .001). Significant difference between D + PO1 group versus D + PO3 group (# P < .05).
Table 2.
Effect of PO on Serum MDA, GSH, and Total Protein in in Control, Untreated Diabetic Rats, and PO-Treated Diabetic Rats During 4 Weeks of Study (n = 8 for Each Group).a
| C | D | D + PO1 | D + PO2 | D + PO3 | |
|---|---|---|---|---|---|
| MDA (nmol/mg protein) | 1.66 ± 0.26 | 4.45 ± 0.40*** | 4.02 ± 0.34*** | 3.21 ± 0.35*### | 2.47 ± 0.29++# |
| GSH (nmol/mg protein) | 2.78 ± 0.19 | 1.06 ± 0.11*** | 1.56 ± 0.18*** | 1.97 ± 0.22*++ | 2.36 ± 0.15+++# |
| Total protein (mg/g) | 4.55 ± 0.70 | 1.78 ± 0.35** | 2.37 ± 0.38* | 3.11 ± 0.52 | 1.87 ± 0.35+ |
Abbreviations: PO, Portulaca oleracea; MDA, malondialdehyde; GSH, glutathione.
aC denotes controls; D, denotes untreated diabetic rats; D + PO1 denotes PO-treated (100 mg/kg/d) diabetic rats; D + PO2 denotes PO-treated (200 mg/kg/day) diabetic rats; and D + PO3 denotes PO-treated (400 mg/kg/day) diabetic rats. Significantly different from normal control (group C) rats (*P < .05, **P < .01, ***P < .001). Significantly different from streptozotocin-treated (group D) rats (+ P < .05, ++ P < .05, +++ P < .001). Significant difference between D + PO1 group versus D + PO3 group (# P < .05).
Discussion
The results of the present study indicate that intraperitoneal injection of purslane significantly ameliorated the adverse metabolic effects in the serum of rats treated with STZ. Purslane injection after STZ treatment resulted in lower serum glucose level when compared with rats treated with STZ alone. In addition, purslane treatment of diabetic rats recovered decreased GSH and TAS levels as well as increased MDA, TNF-α, and IL-6 levels. The results confirmed the previous findings showed by others using STZ to induce diabetes in rats and enhance the susceptibility to lipid peroxidation.28,29 Oxidative stress pays an essential role in the complications and pathogenesis of diabetes. Hyperglycemia induces extra production of oxygen free radicals, which is involved in the development of diabetes and its complications.28 Several investigations have indicated that STZ disrupts balance between plasma oxidant and antioxidant system, causes the progression of diabetes mellitus and its complications.29,30 STZ enters the pancreatic β cell by the low-affinity glucose protein-2 transporter, making the selective damage of the insulin-producing islet β cells and, in turn, a significant decrease in insulin secretion.29 Therefore, diabetes progressed in the STZ-treated rats via damage to the pancreas due to induction of oxidative stress both locally and systemically.29 These results may be the important mechanism in STZ-induced hyperglycemia complications.30
Oxidative stress is associated with the activation of a cascade of inflammatory signaling pathways and characterized by abnormal cytokine production (TNF-α and IL-6).31 TNF-α has been shown to enhance adipocyte lipolysis, which further increases free fatty acids and also elicits its own direct negative effects on the insulin signaling pathway by altering the tyrosine/serine phosphorylation of insulin receptor substrate.32
In our experimental model of diabetes mellitus, it was seen that STZ administration led to a significant decrease in plasma GSH and TAS accompanied by a significant increase in MDA, TNF-α and IL-6 in serum. MDA is an aldehydic product of lipid peroxidation that combine quickly with biomolecules and contributes to cellular disturbance, including β-pancreatic cell, thus, deregulating glucose metabolism.33,34 The amelioration of variable measurements in STZ-diabetic rats after purslane treatment might indicate a protective effect of purslane against STZ function that could be mediated via reduction of oxygen free radicals. Purslane treatment of the diabetic rats restored oxidative stress indices. This may be due to a reduction in free radical production and also elevation in antioxidant content. In the current study, GSH and TAS levels were increased in purslane-treated diabetic rats versus the untreated diabetic rats. Purslane may also decrease lipid peroxidation by increasing the antioxidant content.
It is also presumed that the reduction of pro-inflammatory mediators by purslane treatment might have improved insulin signaling pathways and glucose uptake process. This observation perfectly agrees with those of Lee et al35 who demonstrated hypoglycemic and anti-inflammatory activity of purslane in diabetic mice. Their work showed that purslane treatment to diabetic mice could make helpful effects by reducing blood glucose, ameliorating renal function via decreasing inflammation in diabetes. Similarly, El-Sayed36 observed that purslane seed may effectively control hypoglycemic, hypolipidemic, and insulin resistance in diabetic patient; possibly due to its contents of polyunsaturated fatty acids, flavonoids, and polysaccharides. In addition, it was demonstrated that aqueous extract of purslane could markedly alleviate high fat diet-induced oxidative injury by enhancing blood and liver antioxidant enzyme activities.37 Another study indicated that purslane leaves had a protective effect against oxidative stress caused by vitamin A deficiency.38 It was shown that betacyanins from purslane ameliorated cognition deficits and attenuate oxidative damage induced by d-galactose in the brains of senescent mice.38
In our study, prooxidant-antioxidant balance was evaluated by measuring MDA, GSH, and TAS levels in the serum of the diabetic rats. Elevated MDA and reduced GSH and TAS levels pointed out that the balance changed toward pro-oxidation in STZ-induced diabetic rats. Purslane treatment of diabetic rats improved GSH and TAS levels due to reduction in free radical production and enhancement antioxidant defenses. Purslane consists of ingredients with its antioxidant activity such as flavonoids, amino acids, proteins, and enzymes. Flavonoids are a group of phenolic compounds with its hydrogen donating activity39 and a scavenger of free radicals. In biological systems, Purslane showed its antioxidant impact via hydrogen donating antioxidant activity39 and a scavenger of free radicals.
Purslane modulates oxygen radical production, which may be responsible at least in part for the amelioration hyperglycemia, inflammation, and oxidative stress seen in the present study in STZ-diabetic rats. Moreover, elevated GSH and TAS levels after Purslane treatment play an additional role in decreasing oxidative stress. In summary, the present study showed that the association between hypoglycemic effect of purslane with antioxidant and anti-inflammatory activities in STZ-diabetic rats. However, detailed studies are required for the evaluation of the exact protective mechanism of purslane against diabetes and its complications in human and animal models.
Acknowledgments
The authors would like to thank Research Affairs of Neyshabur University of Medical Sciences for financially supporting this work.
Author Contributions: SS was involved with supervising this work, writing the manuscript, study conception and design, analysis and interpretation of data, and critical revision. AB performed the experiment, and was involved in analysis and interpretation of data. TF was involved with writing and editing the manuscript, helped design the manuscript, performed the experiment, and was involved with acquisition of data.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was financially supported by the Research Affairs of Neyshabur University of Medical Sciences.
Ethical Approval: Ethical approval for this study was granted by the Ethical Research Committee of Mashhad University of Medical Sciences.
References
- 1. Chaiyasut C, Kusirisin W, Lailerd N, Lerttrakarnnon P, Suttajit M, Srichairatanakool S. Effects of phenolic compounds of fermented Thai indigenous plants on oxidative stress in streptozotocin-induced diabetic rats. Evid Based Complement Alternat Med. 2011;2011:749307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Samarghandian S, Farkhondeh T, Samini F, Borji A. Protective effects of carvacrol against oxidative stress induced by chronic stress in rat’s brain, liver, and kidney. Biochem Res Int. 2016;2016:2645237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moghaddam HS, Samarghandian S, Farkhondeh T. Effect of bisphenol A on blood glucose, lipid profile and oxidative stress indices in adult male mice. Toxicol Mech Methods. 2015;25:507–513. [DOI] [PubMed] [Google Scholar]
- 4. Samarghandian S, Azimi-Nezhad M, Afshari R, Farkhondeh T, Karimnezhad F. Effects of buprenorphine on balance of oxidant/antioxidant system in the different ages of male rat liver. J Biochem Mol Toxicol. 2015;29:249–253. [DOI] [PubMed] [Google Scholar]
- 5. Samarghandian S, Azimi-Nezhad M, Samini F, Farkhondeh T. Chrysin treatment improves diabetes and its complications in streptozotocin-induced diabetic rats. Can J Physiol Pharmacol. 2016;94:388–393. [DOI] [PubMed] [Google Scholar]
- 6. Samarghandian S, Borji A, Delkhosh MB, Samini F. Safranal treatment improves hyperglycemia, hyperlipidemia and oxidative stress in streptozotocin-induced diabetic rats. J Pharm Pharm Sci. 2013;16:352–362. [DOI] [PubMed] [Google Scholar]
- 7. Mohanapriya S, Senthilkumar P, Sivakumar S, Dineshkumar M, Subbhuraam CV. Effects of copper sulfate and copper nitrate in aquatic medium on the restoration potential and accumulation of copper in stem cuttings of the terrestrial medicinal plant, Portulaca oleracea Linn. Environ Monit Assess. 2006;121:233–244. [DOI] [PubMed] [Google Scholar]
- 8. Zhang XJ, Ji YB, Qu ZY, Xia JC, Wang L. Experimental studies on antibiotic functions of Portulaca oleracea L. in vitro. Chin J Microecol. 2002;14:277–280. [Google Scholar]
- 9. Xiang L, Xing D, Wang W, Wang R, Ding Y, Du L. Alkaloids from Portulaca oleracea L. Phytochemistry. 2005;66:2595–2601. [DOI] [PubMed] [Google Scholar]
- 10. Yang Z, Liu C, Xiang L, Zheng Y. Phenolic alkaloids as a new class of antioxidants in Portulaca oleracea . Phytother Res. 2009;23:1032–1035. [DOI] [PubMed] [Google Scholar]
- 11. Chan K, Islam MW, Kamil M, et al. The analgesic and anti-inflammatory effects of Portulaca oleracea L. subsp. sativa (Haw.) Celak. J Ethnopharmacol. 2000;73:445–451. [DOI] [PubMed] [Google Scholar]
- 12. Parry O, Okwuasaba F, Ejike C. Skeletal muscle relaxant action of an aqueous extract of Portulaca oleracea in the rat. J Ethnopharmacol. 1987;19:247–253. [DOI] [PubMed] [Google Scholar]
- 13. Parry O, Marks JA, Okwuasaba FK. The skeletal muscle relaxant action of Protulaca oleracea . J Ethnopharmacol. 1993;40:187–194. [DOI] [PubMed] [Google Scholar]
- 14. Rashed AN, Afifi FU, Disi AM. Simple evaluation of the wound healing activity of a crude extract of Portulaca oleracea L. (growing in Jordan) in Mus musculus JVI-1. J Ethnopharmacol. 2003;88:131–136. [DOI] [PubMed] [Google Scholar]
- 15. Miller AG, Morris M. Plants of Dhofar, the Southern Region of Oman: Traditional, Economic, and Medicinal Uses. Office of the Adviser for Conservation of the Environment, Diwan of Royal Court, Sultanate of Oman; 1988. [Google Scholar]
- 16. Liu L, Howe P, Zhou YF, Xu ZQ, Hocart C, Zhan R. Fatty acids and β-carotene in Australian purslane (Portulaca oleracea) varieties. J Chromatogr A. 2000;893:207–213. [DOI] [PubMed] [Google Scholar]
- 17. Awad NE. Lipid content and antimicrobial activity of phenolic constituents of cultivated Portulaca oleracea L. Bull Fac Pharm Cairo Univ. 1994;32:137–142. [Google Scholar]
- 18. Mizutani M, Hashidoko Y, Tahara S. Factors responsible for inhibiting the motility of zoospores of the phytopathogenic fungus Aphanomyces cochlioides isolated from the non-host plant Portulaca oleracea . FEBS Lett. 1998;438:236–240. [DOI] [PubMed] [Google Scholar]
- 19. Feng PC, Haynes LJ, Magnus KE. High concentration of (−)- noradrenaline in Portulaca oleracea L. Nature. 1961;191:2–4. [DOI] [PubMed] [Google Scholar]
- 20. Meng XZh, Ni SF, Suo HX. Studies on Ixeris sonchifolia Hance used for coronary heart disease 6. Isolation and identification of the active constituents for dilating coronary blood vessel. J Shenyang Coll Pharm. 1981;32–35. [Google Scholar]
- 21. Chen D. Manual of Standard Compounds of Traditional Chinese Medicine. Beijing, China: China Medico-Pharmaceutical Science and Technology Press; 2000. [Google Scholar]
- 22. Yagi K. Assay for blood plasma or serum lipid peroxides. Methods Enzymol. 1984;105:28–31. [DOI] [PubMed] [Google Scholar]
- 23. Biswas D, Banerjee M, Sen G, et al. Mechanism of erythrocyte death in human population exposed to arsenic through drinking water. Toxicol Appl Pharmacol. 2008;230:57–66. [DOI] [PubMed] [Google Scholar]
- 24. Drabkin DL, Austin JN. Spectrophotometric studies. I. Spectrophotometric constants for common hemoglobin derivatives in human, dog, and rabbit. J Biol Chem. 1932;98:719–733. [Google Scholar]
- 25. Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–888. [PubMed] [Google Scholar]
- 26. Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. [DOI] [PubMed] [Google Scholar]
- 27. Budin SB, Othman F, Louis SR, Bakar MA, Das S, Mohamed J. The effects of palm oil to cotrienol rich fraction supplementation on biochemical parameters, oxidative stress and the vascular wall of streptozotocin-induced diabetic rats. Clinics (Sao Paulo). 2009;64:235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yazdanparast R, Ardestani A, Jamshidi S. Experimental diabetes treated with Achillea santolina: effect on pancreatic oxidative parameters. J Ethnopharmacol. 2007;112:13–18. [DOI] [PubMed] [Google Scholar]
- 29. Samarghandian S, Borji A, Tabasi SH. Effects of Cichorium intybus Linn on blood glucose, lipid constituents and selected oxidative stress parameters in streptozotocin-induced diabetic rats. Cardiovasc Hematol Disord Drug Targets. 2013;13:231–236. [DOI] [PubMed] [Google Scholar]
- 30. Samarghandian S, Azimi-Nezhad M, Samini F. Ameliorative effect of saffron aqueous extract on hyperglycemia, hyperlipidemia, and oxidative stress on diabetic encephalopathy in streptozotocin induced experimental diabetes mellitus. Biomed Res Int. 2014;2014:920857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shahady E. Hyperlipidemia in diabetes—etiology, consequences and treatment. Circulation. 1995;91:2844–2850.7758192 [Google Scholar]
- 32. El-Abhar HS, Schaalan MF. Topiramate-induced modulation of hepatic molecular mechanisms: an aspect for its anti-insulin resistant effect. PLoS One. 2012;7:e37757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sivaraman K, SenthilKumar GP, Sankar P, Bobby Z. Attenuation of oxidative stress, inflammation and insulin resistance by Allium sativum in fructose-fed male rats. J Clin Diagn Res. 2013;7:1860–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grune T, Siems WG, Petras T. Identification of metabolic pathways of the lipid peroxidation product 4-hydroxynonenal in situ perfused rat kidney. J Lipid Res. 1997;38:1660–1665. [PubMed] [Google Scholar]
- 35. Lee AS, Lee YJ, Lee SM, et al. An aqueous extract of Portulaca oleracea ameliorates diabetic nephropathy through suppression of renal fibrosis and inflammation in diabetic db/db mice. Am J Chin Med. 2012;40:495–510. [DOI] [PubMed] [Google Scholar]
- 36. El-Sayed MI. Effects of Portulaca oleracea L. seeds in treatment of type-2 diabetes mellitus patients as adjunctive and alternative therapy. J Ethnopharmacol. 2011;137:643–651. [DOI] [PubMed] [Google Scholar]
- 37. Chen B, Zhou H, Zhao W, Zhou W, Yuan Q, Yang G. Effects of aqueous extract of Portulaca oleracea L. on oxidative stress and liver, spleen leptin, PARα and FAS mRNA expression in high-fat diet induced mice. Mol Biol Rep. 2012;39:7981–7988. [DOI] [PubMed] [Google Scholar]
- 38. Arruda SF, Siqueira EM, Souza EM. Malanga (Xanthosoma sagittifolium) and purslane (Portulaca oleracea) leaves reduce oxidative stress in vitamin A-deficient rats. Ann Nutr Metab. 2004;48:288–295. [DOI] [PubMed] [Google Scholar]
- 39. Wang CQ, Yang GQ. Betacyanins from Portulaca oleracea L. ameliorate cognition deficits and attenuate oxidative damage induced by d-galactose in the brains of senescent mice. Phytomedicine. 2010;17:527–532. [DOI] [PubMed] [Google Scholar]


