Abstract
Silybum marianum (L) Gaertn (milk thistle) seeds, Urtica dioica L (nettle) leaves, and Boswellia serrata (olibanum gum) resin are used traditionally by Iranian diabetic patients. The aim of this study was to evaluate the antihyperglycemic effects of these herbs in an herbal formulation in patients with type II diabetes mellitus. Sixty patients diagnosed as type II diabetes mellitus with fasting blood glucose level from 150 to 180 mg/dL, glycosylated hemoglobin level from 7.5% to 8.5%, and on oral antihyperglycemic drugs, were allocated to receive the mix herbal formulation or placebo for 90 days in a double-blind randomized placebo-controlled clinical trial. The mean serum fasting blood glucose, glycosylated hemoglobin, and triglyceride in the herbal drug group were significantly less than placebo group’s values after 3 months of the intervention. The study showed a potential antihyperglycemic and triglyceride lowering effect of the herbal formulation, while it did not have any significant cholesterol or blood pressure lowering effect.
Keywords: diabetes mellitus, Silybum marianum (L) Gaertn, olibanum gum, Urtica dioica L, traditional Persian medicine
Diabetes mellitus is one of the most important health issues worldwide. Almost 285 million people, 6.4% of the world population, suffered from diabetes in 2010.1 This prevalence is estimated to have increasing pattern to 7.7% of the world population (439 million patients) by 2030.1 This imposes an increasing economic burden on national health care systems worldwide estimated from 376 to 490 billion dollars in 2010 to 2030.2
Numerous herbs have been investigated for their use in patients with diabetes.3 Many of these herbs have been shown to possess hypoglycemic effects in animal and human studies.4–6 Milk thistle, nettle, and olibanum were among these herbs that were traditionally recommended for diabetes in Iran.7–9
Silybum marianum (L) Gaertn (milk thistle) has been evaluated for its antidiabetic properties in multiple studies with promising results.10–12 It is an annual plant of the Asteraceae family and native of southern Europe and Asia. Multiple flavonoids, including silibinin, silydianin, and silychristin, are isolated as its active components.13 It is reported to have regulatory and protective effect on pancreatic β-cell function14,15 and insulin secretagogues effect16 in animal models of diabetes.
Urtica dioica L (common nettle) has also shown antidiabetic effects in animal and human studies.9,17 It is a herbaceous perennial flowering plant, native to Europe, Asia, northern Africa, and North America. It is the best-known member of the nettle genus Urtica. Its most important active constituents are neophytadiene, phtaleic acid, dibutyl phthaleate, bis(2-ethyl hexyl) maleate, and 1,2-benzenocli carboxylic acid.18 It has demonstrated insulin secretagogue, insulin mimetics, PPAR (peroxisome proliferator-activated receptor)-γ agonistic, and α-glucosidase inhibitory effects.9,19–21
Olibanum (frankincense), is an aromatic resin obtained from Boswellia serrata trees in the family Burseraceae. The gum has demonstrated antihyperglycemic, antihyperlipidemic, and antioxidant effects in diabetic patients and animal models.7,22,23 Its antihyperlipidemic effect is known to be due to improving insulin sensitivity and protecting pancreas β-cells.7
The use of herbal combination has been popular in traditional Persian medicine. Multiple herbs were mixed to increase the efficacy, decreasing the side effects and target their drugs to specific organ.24 Among current investigations on herbal remedies for diabetes, multiple studies are focused on herbal combination.25,26 It is believed that using these formulations can target multiple glucose lowering pathways, which cause an enhanced antidiabetic activity.27
The aim of the study was to evaluate the antihyperglycemic effect of mixture of silymarin, nettle, and olibanum gum in a herbal formulation in patients with type II diabetes mellitus. The secondary goal of this study is to evaluate the effect of this formulation on the other co morbidities of diabetic patients along with its safety.
Methods
Preparation of the Herbal and Placebo Capsules
Silybum marianum (L) Gaertn seed extract (silymarin) and Urtica dioica L leaf extract (nettle) and olibanum gum (olibanum), all in powder form with certified botanical origin along with their specification were given by Institute of Medicinal Plants, Karaj, Iran. The herbal capsule was prepared by mixture of olibanum, 200 mg, silymarin 200 mg, and nettle 200 mg. Placebo capsules were filled with 600 mg of toasted powder.
The specification given by Institute of Medicinal Plants, Karaj, Iran, for each single herb in mix herbal formulation was as follows: Silibinin was the major constituent of Silybum marianum (L) Gaertn seed extract (38% w/w); α-pinene was the major constituent (41.5% w/w) of olibanum gum essential oil and total flavonoid content of urtica dioica L leaf extract was 293 mg of rutin equivalents per gram of the extract.
Trial Design
The study was designed as a 2-arm, double-blind randomized placebo-controlled clinical trial using a parallel design with a 1:1 allocation ratio. There was no change in methods after trial commencement.
Participants
Ninety-three patients attending the Diabetes Clinic of the Baghiatallah University of Medical Sciences between March 2014 and April 2015 with a clinical diagnosis of non–insulin-dependent diabetes mellitus were evaluated for inclusion in the study. Patients between 40 to 60 years old with fasting blood glucose level from 150 to 180 mg/dL and blood glycosylated hemoglobin level from 7.5% to 8.5%, under therapy of oral antihyperglycemic drugs maximum 10 mg glyburide and 1000 mg metformin daily, with no change in drug doses in previous 3 months were considered for inclusion in the study. Exclusion criteria were receiving insulin therapy and presence of any diagnosed diabetes-related or nonrelated cardiovascular, renal, hepatic, haematological, or neurologic diseases. Patients with a history of hypothyroidism, vertigo, seizure, gallstones, or gallbladder surgery and patients under estrogen, steroid, beta-blocker, and thiazide therapy were excluded from the study. Other exclusion criteria were current or planned pregnancy and breast-feeding.
The sample size of 25 patients in each group was calculated by statistician based on the formula n = (z1-α/2)2δ2/d2 considering standard deviation of 40 mg/dL, effect size of 30 mg/dL, a 1-sided significance level of .05, and a power of 0.80. Considering 20% probable drop in the sample at last 60 patients were allocated to intervention and control groups (30 in each group).
Randomization, Blinding, and Allocation Concealment
Sixty eligible patients were randomly allocated to 2 parallel groups (the drug and placebo groups) by the secretary of the clinic, who had been instructed to use a randomized list. The randomized list was generated using Microsoft Excel with a permuted balanced block randomization method, as previously described.28 The physicians, researchers, and statisticians were blind to the allocation of patients. Moreover, due to the same shape, colour, weight, and size of the drug and placebo capsules and containers the patients were blind to the drug allocation.
Interventions and Outcomes
Patients were instructed to take 1 capsule of herbal formulation or placebo 3 times per day before the meal. No change in the patients’ previous diet, physical exercise, and oral hypoglycemic medications were made. Patients were evaluated prior to and following three months of the intervention in terms of the fasting blood glucose and glycosylated haemoglobin as primary outcomes and 2-hour postprandial blood glucose (2hppG), lipid profile, complete blood count, liver, kidney function tests, and systolic and diastolic blood pressures as secondary outcomes. Fasting blood glucose (FBG) was measured at 0, 1, 2, 3, 5, 7, 14, 30, 45, 60, 75, and 90 days of the intervention for any hypoglycemic or hyperglycemic adverse effects.
Ethical Considerations
The study protocol was in compliance with the Declaration of Helsinki (1989 revision) and approved by the Local Medical Ethics Committee of Baghiatallah University of Medical Sciences. The trial was registered in the Iranian Registry of Clinical Trials (registration ID: IRCT201502011157N9).
Statistical Methods
The descriptive data were presented as numbers, frequencies, means and standard deviations. The paired and independent Student’s t test and chi-square test were used for statistical comparison of primary characteristics and outcomes between the drug and placebo groups. The intention to treat analysis protocol was used for analyzing the data of patients not fully adhered to the study protocol. A P value of less than .05 was considered significant. The SPSS software (version 17, IBM Corporation) was used for analysis of data.
Results
From March 2014 to April 2015, a total of 93 patients were assessed for eligibility and finally, 60 of them were eligible to be randomized to receive the trial drug or placebo. No patients were lost to follow-up and none of them dropped out. Two patients that had not full adherence to the study protocol were included in analysis according to intention to treat protocol of analysis. Figure 1 is a flowchart that reveals detailed descriptions of patients’ enrolment, randomization, and outcomes.
Figure 1.
Flowchart of study inclusion, allocation, and follow-up.
The baseline characteristics (demographic and clinical) of the patients in the 2 groups are shown in Table 1. There were no significant differences between the 2 arms in terms of patients’ mean age, gender, weight, duration of diabetes and baseline systolic and diastolic blood pressure.
Table 1.
Basic Characteristics of Patients in the Herbal Formulation and Placebo Groups.a
| Basic Characteristics | Groups | P | |
|---|---|---|---|
| Herbal Formulation (n = 30) | Placebo (n = 30) | ||
| Age (years) | 55.14 ± 10.60 | 57 ± 10.63 | .5b |
| Sex (female), n (%) | 13 (44.8) | 17 (54.8) | .3c |
| Weight (kg) | 75.48 ± 11.95 | 73.38 ± 9.87 | .46b |
| Blood pressure (mm Hg) | |||
| Systolic | 133.45 ± 15.76 | 132.74 ± 10.86 | .84b |
| Diastolic | 79.83 ± 9.77 | 81.61 ± 7.89 | .43b |
aValues are reported as mean ± standard deviation unless indicated otherwise.
bIndependent-samples t test.
cChi-square test.
The mean FBG level decreased significantly in the active drug group through the study (152.13 ± 44.63 vs 124.68 ± 21.94 mg/dL, P = .003). Mean FBG level in this group, after a temporary increase in the first day of the intervention, started a reducing trend from the second day through the end of the study resulted in about 28 mg/dL reduction (Figure 2). However, in the placebo group a fluctuating trend was seen in the mean FBG level resulted in nonsignificant reduction at the end of the study (155.41± 51.90 vs. 147.77 ± 43.34 mg/dL, P = .538) (Figure 2). The mean FBG in the active drug group was significantly less than placebo group’s value after 3 months of the intervention (P = .012).
Figure 2.
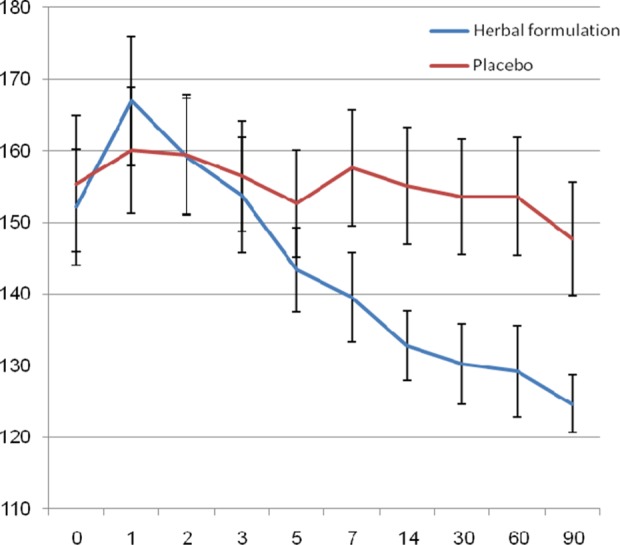
Changes in mean levels of fasting blood glucose in herbal formulation and placebo groups.
Despite the reduction in postprandial glucose in both the treated and placebo groups, there was no significant difference between serum 2hppG levels between the 2 groups after the intervention (P = .29). This indicates that it is likely due to increased compliance with diet and other medication.
A significant decrease in glycosylated hemoglobin (HbA1c) was observed in the 3-month score of both herbal formulation and placebo groups when comparing their base values. It fell by 19% with the herbal treatment but only by 7% in the placebo group (P < .001 and P = .004, respectively). The mean value of HbA1c was significantly lower in the active drug group than in the placebo group (P < .001) (Table 2).
Table 2.
Mean ± Standard Deviation Values for Fasting Blood Glucose, Postprandial Glucose, Glycosylated Hemoglobin (HbA1c), and Lipids Levels in Herbal Formulation and Placebo Groups Before and After the Intervention.
| Groups | P a | |||
|---|---|---|---|---|
| Herbal Formulation (n = 30) | Placebo (n = 30) | |||
| Fasting blood sugar (mg/dL) | Before | 152.13 ± 44.63 | 155.41 ± 51.90 | .59 |
| After | 124.68 ± 21.94 | 147.77 ± 43.34 | .01 | |
| P b | <.01 | .06 | ||
| Postprandial glucose (mg/dL) | Before | 245.13 ± 67.19 | 232.22 ± 70.57 | .47 |
| After | 192.10 ± 43.96 | 205.41 ± 51.99 | .29 | |
| P b | <.01 | <.01 | ||
| HbA1c (%) | Before | 8.05 ± 1.19 | 8.17 ± 1.00 | .66 |
| After | 6.56 ± 0.44 | 7.64 ± 0.83 | <.01 | |
| P b | <.01 | <.01 | ||
| Triglyceride (mg/dL) | Before | 171.79 ± 50.00 | 179.32 ± 58.12 | .59 |
| After | 148.89 ± 37.60 | 171.96 ± 47.90 | .04 | |
| P b | <.01 | .30 | ||
| Total cholesterol (mg/dL) | Before | 180.93 ± 57.84 | 182.48 ± 49.02 | .91 |
| After | 162.75 ± 37.65 | 171.09 ± 40.70 | .41 | |
| P b | .01 | .06 | ||
| Low-density lipoproteins (mg/dL) | Before | 142.66 ± 40.51 | 143.63 ± 40.40 | .99 |
| After | 141.54 ± 39.39 | 141.60 ± 38.54 | .99 | |
| P b | .74 | .64 | ||
| High-density lipoproteins (mg/dL) | Before | 42.48 ± 7.17 | 43.67 ± 5.74 | .47 |
| After | 41.20 ± 7.01 | 44.09 ± 5.43 | .07 | |
| P b | .37 | .47 | ||
aIndependent-samples t test.
bPaired-samples t test.
There was a significant reduction in the serum triglyceride and total cholesterol values in the herbal formulation group, but these reductions were not significantly different from those of placebo group. There was also no significant change in mean values of low- and high-density lipoprotein, systolic and diastolic blood pressure in the active drug and placebo groups (Tables 2 and 3). The mean values for the kidney and liver function tests showed no significant changes during the study period (Table 3). No patient in the study groups reported any adverse events.
Table 3.
Mean ± Standard Deviation Values for Blood Pressure and Liver and Kidney Function Tests in Herbal Formulation and Placebo Groups Before and After the Intervention.
| Groups | P a | |||
|---|---|---|---|---|
| Herbal Formulation (n = 30) | Placebo (n = 30) | |||
| Systolic blood pressure (mm Hg) | Before | 133.45 ± 15.76 | 132.74 ± 10.86 | .84 |
| After | 129.65 ± 12.02 | 129.35 ± 8.13 | .91 | |
| P | ||||
| Diastolic blood pressure (mm Hg) | Before | 79.83 ± 9.77 | 81.61 ± 7.89 | .43 |
| After | 76.86 ± 6.47 | 77.58 ± 6.30 | .66 | |
| P | ||||
| Aspartate transaminase (U/L) | Before | 28.65 ± 9.47 | 25.35 ± 8.03 | .13 |
| After | 29.03 ± 9.89 | 24.64 ± 8.03 | .06 | |
| P b | .77 | .17 | ||
| Alanine transaminase (U/L) | Before | 29.48 ± 12.10 | 25.25 ± 9.16 | .15 |
| After | 30.55 ± 10.26 | 26.90 ± 8.47 | .13 | |
| P b | .38 | .24 | ||
| Alkaline phosphatase (U/L) | Before | 164.37 ± 54.19 | 184.93 ± 65.08 | .19 |
| After | 170.75 ± 54.56 | 170.61 ± 42.51 | .99 | |
| P b | .47 | .08 | ||
| Blood urea nitrogen (mg/dL) | Before | 22.58 ± 7.87 | 20.54 ± 7.05 | .26 |
| After | 24.31 ± 6.11 | 23.22 ± 6.06 | .49 | |
| P b | .19 | .21 | ||
| Creatinine (mg/dL) | Before | 1.08 ± 0.19 | 1.12 ± 0.19 | .51 |
| After | 1.06 ± 0.15 | 1.12 ± 0.18 | .21 | |
| P b | .29 | .83 | ||
aIndependent-samples t test.
bPaired-samples t test.
Discussion
This study showed that the combination therapy with silymarin, olibanum, and nettle can cause hypoglycemic effect in the diabetic patients which was demonstrated as a significant reduction in the level of serum FBG and HbA1c in these patients. It has also a small size reducing effect on the level of serum triglyceride in diabetic patients. However, this combination seems to have no significant effect on the level of total cholesterol, low- and high-density lipoproteins, or on systolic and diastolic blood pressure in these patients.
The observed result was compatible with previously shown antidiabetic effects of silymarin, olibanum and nettle in the form of single herbal therapy. Silymarin at the dose of 600 mg per day caused about 20 mg/dL reduction in FBG and 1-unit reduction in HbA1c of diabetic patients.10 Gum olibanum and nettle also showed about 0.4- and 0.7-unit reduction in HbA1c of these patients, respectively.7,23 The combination of these herbs could cause a greater reduction in HbA1c (1.5 units) in this study. According the results of previous studies different parts of this combination can target of different glucose lowering pathways including induction of insulin secretion (by silymarin), insulin mimetics effect (by olibanum gum) and improving insulin sensitivity.7,10,23 This may justify the observed enhanced effect.
Combination therapy with the medications affecting multiple parts of glucose metabolisms is an area of increasing research interest in the field of diabetes.29 It is believed that combination therapy may provide multiple benefits including increase in the glucose control efficacy and decrease the risk of adverse events such as hypoglycemia and weight gain.30 Using polyherbal formulations in the treatment of diabetes is also a popular traditional approach.31 These traditional formulations also were supposed to increase the efficacy and safety of treatment.32 Multiple studies have reported significant hypoglycemic benefit of these traditional herbal formulations.33
The selection of the study herbs for formulating a combination were based on traditional Persian medicine view on treatment of diabetes.34 This traditional medical system is originated mainly from the manuscripts of Persian scholars such as Avicenna (980-1037 ce), Rhazes (865-925 ce), Haly Abbas (949-982 ce), and Jorjani (1040-1136 ce).35–38 Diabetes (known as Ziabites in Persian medicine) was remarked as excessive thirst and urination due to inability of kidneys to save water.39
Recently, several medicinal herbs were tested for their efficacy and safety in a wide range of chronic diseases. This is a necessity for integration of trusted herbal remedies into the standard health care system.40–42
Strict eligibility criteria of the study such as including the patients with a specific narrow range of FBG (150-180 mg/dL) and HbA1c (7.5%-8.5%) and excluding the patients with any diagnosed micro- and macrovascular complications of diabetes, limits the generalizability of results. Another limitation of the study is that it only demonstrates the effect of adjuvant use of the herbal formulation with the patients’ routine oral hypoglycemic agents. On the other hand, the use of this new herbal formula with discontinuing the patients’ standard therapies was not possible due to major ethical issues. Small size improvement of FBG and HbA1C in placebo group is also important point to be noticed. It may be due to increase patient compliance to his/her previous medication and/or lifestyle as a result of being involved in the study with regular follow-up. Multiple measurements of fasting blood sugar besides considering the evaluation of the effect of the intervention on other cardiovascular risk factors such as lipid profile and blood pressure were the major strengths of this study.
To summarize, this study showed a potential antihyperglycemic and triglyceride-lowering effect of the mixture of silymarin, olibanum, and nettle extracts, which caused a significant reduction of HbA1c, FBG, and triglyceride after 3 months of intervention. The formulation did not show any significant cholesterol or blood pressure lowering effect in this study. Further studies with larger sample size and without adjuvant use of other hypoglycemic agents should be done to confirm the observed effects.
Footnotes
Author Contributions: The work presented in this article was carried out through collaboration between all authors. HFH and NK made the initial hypothesis. All authors participated in defining the research theme and provided the proposal. SM, MH, RF, and RM performed the experiments, collected the data, analyzed the data, and wrote the article. HFH, NK, SM, and MH conceptualized the study, critically analyzed and discussed the data, and corrected and reviewed the article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a grant from the Baqiyatallah University of Medical Sciences and Iranian Academic Center for Education, Culture and Research (ACECR).
Ethical Approval: The study protocol was in compliance with the Declaration of Helsinki (1989 revision) and approved by the Local Medical Ethics Committee of Baghiatallah University of Medical Sciences. The trial was registered in the Iranian Registry of Clinical Trials (registration ID: IRCT201502011157N9).
References
- 1. Shaw JE, Sicree RA, Zimmet PZ, Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. [DOI] [PubMed] [Google Scholar]
- 2. Zhang P, Zhang X, Brown J, et al. Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:293–301. [DOI] [PubMed] [Google Scholar]
- 3. Hashempur MH, Heydari M, Mosavat SH, Heydari ST, Shams M. Complementary and alternative medicine use in Iranian patients with diabetes mellitus. J Integr Med. 2015;13:319–325. [DOI] [PubMed] [Google Scholar]
- 4. Yeh GY, Eisenberg DM, Kaptchuk TJ, Phillips RS. Systematic review of herbs and dietary supplements for glycemic control in diabetes. Diabetes Care. 2003;26:1277–1294. [DOI] [PubMed] [Google Scholar]
- 5. Hosseini S, Jamshidi L, Mehrzadi S, et al. Effects of Juglans regia L. leaf extract on hyperglycemia and lipid profiles in type two diabetic patients: a randomized double-blind, placebo-controlled clinical trial. J Ethnopharmacol. 2014;152:451–456. [DOI] [PubMed] [Google Scholar]
- 6. Mehrzadi S, Ghaznavi H, Tajallizadehkhoob Y, Fakhrzadeh H. Effects of Pinus eldarica Medw. nut extract on blood glucose and cholesterol levels in hypercholesterolemic alloxan-induced diabetic rats. J Med Plants. 2013;1:68–74. [Google Scholar]
- 7. Azadmehr A, Ziaee A, Ghanei L, et al. A randomized clinical trial study: anti-oxidant, anti-hyperglycemic and anti-hyperlipidemic effects of olibanum gum in type 2 diabetic patients. Iran J Pharm Res. 2014;13:1003–1009. [PMC free article] [PubMed] [Google Scholar]
- 8. Hasani-Ranjbar S, Zahedi HS, Abdollahi M, Larijani B. Trends in publication of evidence-based traditional Iranian medicine in endocrinology and metabolic disorders. J Diabetes Metab Disord. 2013;12:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kianbakht S, Khalighi-Sigaroodi F, Dabaghian FH. Improved glycemic control in patients with advanced type 2 diabetes mellitus taking Urtica dioica leaf extract: a randomized double-blind placebo-controlled clinical trial. Clin Lab. 2013;59:1071–1076. [DOI] [PubMed] [Google Scholar]
- 10. Huseini HF, Larijani B, Heshmat R, et al. The efficacy of Silybum marianum (L.) Gaertn. (silymarin) in the treatment of type II diabetes: a randomized, double-blind, placebo-controlled, clinical trial. Phytother Res. 2006;20:1036–1039. [DOI] [PubMed] [Google Scholar]
- 11. Fallahzadeh MK, Dormanesh B, Sagheb MM, et al. Effect of addition of silymarin to renin-angiotensin system inhibitors on proteinuria in type 2 diabetic patients with overt nephropathy: a randomized, double-blind, placebo-controlled trial. Am J Kidney Dis. 2012;60:896–903. [DOI] [PubMed] [Google Scholar]
- 12. Vessal G, Akmali M, Najafi P, Moein MR, Sagheb MM. Silymarin and milk thistle extract may prevent the progression of diabetic nephropathy in streptozotocin-induced diabetic rats. Renal Fail. 2010;32:733–739. [DOI] [PubMed] [Google Scholar]
- 13. Rui YC. Advances in pharmacological studies of silymarin. Mem Inst Oswaldo Cruz. 1991;86(suppl 2):79–85. [DOI] [PubMed] [Google Scholar]
- 14. Chang CL, Lin Y, Bartolome AP, Chen YC, Chiu SC, Yang WC. Herbal therapies for type 2 diabetes mellitus: chemistry, biology, and potential application of selected plants and compounds. Evid Based Complement Alternat Med. 2013;2013:378657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Soto C, Mena R, Luna J, et al. Silymarin induces recovery of pancreatic function after alloxan damage in rats. Life Sci. 2004;75:2167–2180. [DOI] [PubMed] [Google Scholar]
- 16. El-Far M, Negm A, Wahdan M. Promising biopharmaceutical use of silymarin and silibinin as antidiabetic natural agents in streptozotocin-induced diabetic rats: first comparative assessment. World J Pharm Pharm Sci. 2014;4:7–28. [Google Scholar]
- 17. Qujeq D, Tatar M, Feizi F, Parsian H, Sohan Faraji A, Halalkhor S. Effect of Urtica dioica leaf alcoholic and aqueous extracts on the number and the diameter of the islets in diabetic rats. Int J Mol Cell Med. 2013;2:21–26. [PMC free article] [PubMed] [Google Scholar]
- 18. Habibi Lahigi S, Amini K, Moradi P, Asaadi K. Investigating the chemical composition of different parts extracts of bipod nettle Urtica dioica L. in Tonekabon region. Iran J Plant Physiol. 2011;2:337–340. [Google Scholar]
- 19. Namazi N, Esfanjani AT, Heshmati J, Bahrami A. The effect of hydro alcoholic nettle (Urtica dioica) extracts on insulin sensitivity and some inflammatory indicators in patients with type 2 diabetes: a randomized double-blind control trial. Pak J Biol Sci. 2011;14:775–779. [DOI] [PubMed] [Google Scholar]
- 20. Domola MS, Vu V, Robson-Doucette CA, Sweeney G, Wheeler MB. Insulin mimetics in Urtica dioica: structural and computational analyses of Urtica dioica extracts. Phytother Res. 2010;24(suppl 2):S175–S182. [DOI] [PubMed] [Google Scholar]
- 21. Onal S, Timur S, Okutucu B, Zihnioglu F. Inhibition of alpha-glucosidase by aqueous extracts of some potent antidiabetic medicinal herbs. Prep Biochem Biotechnol. 2005;35:29–36. [DOI] [PubMed] [Google Scholar]
- 22. Shehata AM, Quintanilla-Fend L, Bettio S, Singh CB, Ammon HP. Prevention of multiple low-dose streptozotocin (MLD-STZ) diabetes in mice by an extract from gum resin of Boswellia serrata (BE). Phytomedicine. 2011;18:1037–1044. [DOI] [PubMed] [Google Scholar]
- 23. Ahangarpour A, Heidari H, Fatemeh RA, et al. Effect of Boswellia serrata supplementation on blood lipid, hepatic enzymes and fructosamine levels in type2 diabetic patients. J Diabetes Metab Disord. 2014;13:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. IbneSina H. Al-Qanoon fi al-Tibb (The Canon of Medicine), Beirut, Lebanon: Dare Ehia Attorath Al Arabi; 2005:423–424. [Google Scholar]
- 25. Srivastava S, Lal VK, Pant KK. Polyherbal formulations based on Indian medicinal plants as antidiabetic phytotherapeutics. Phytopharmacology. 2012;2:1–15. [Google Scholar]
- 26. Dwivedi C, Daspaul S. Antidiabetic herbal drugs and polyherbal formulation used for diabetes: a review. J Phytopharmacol. 2013;2:44–51. [Google Scholar]
- 27. Ghorbani A. Clinical and experimental studies on polyherbal formulations for diabetes: current status and future prospective. J Integr Med. 2014;12:336–345. [DOI] [PubMed] [Google Scholar]
- 28. Matts JP, Lachin JM. Properties of permuted-block randomization in clinical trials. Control Clin Trials. 1988;9:327–344. [DOI] [PubMed] [Google Scholar]
- 29. Phung OJ, Sobieraj DM, Engel SS, Rajpathak SN. Early combination therapy for the treatment of type 2 diabetes mellitus: systematic review and meta-analysis. Diabetes Obes Metab. 2014;16:410–417. [DOI] [PubMed] [Google Scholar]
- 30. Bailey T. Options for combination therapy in type 2 diabetes: comparison of the ADA/EASD position statement and AACE/ACE algorithm. Am J Med. 2013;126(9 suppl 1):S10–S20. [DOI] [PubMed] [Google Scholar]
- 31. Petchi RR, Vijaya C, Parasuraman S. Antidiabetic activity of polyherbal formulation in streptozotocin-nicotinamide induced diabetic Wistar rats. J Tradit Complement Med. 2014;4:108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Parasuraman S, Thing GS, Dhanaraj SA. Polyherbal formulation: concept of ayurveda. Pharmacogn Rev. 2014;8:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu JP, Zhang M, Wang WY, Grimsgaard S. Chinese herbal medicines for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2004;(3):CD003642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rashidi AA, Mirhashemi SM, Taghizadeh M, Sarkhail P. Iranian medicinal plants for diabetes mellitus: a systematic review. Pak J Biol Sci. 2013;16:401–411. [DOI] [PubMed] [Google Scholar]
- 35. Mosavat SH, Ghahramani L, Rahmanian Haghighi E, Rostami Chaijan M, Hashempur MH, Heydari M. Anorectal diseases in Avicenna’s “Canon of Medicine”. Acta Med Hist Adriat. 2015;13:103–114. [PubMed] [Google Scholar]
- 36. Dalfardi B, Heydari M, Golzari SE, Mahmoudi Nezhad GS, Hashempur MH. Al-Baghdadi’s description of venous blood circulation. Int J Cardiol. 2014;174:209–210. [DOI] [PubMed] [Google Scholar]
- 37. Heydari M, Shams M, Hashempur MH, Zargaran A, Dalfardi B, Borhani Haghighi A. The origin of the concept of neuropathic pain in early medieval Persia (9th-12th century CE). Acta Med Hist Adriat. 2015;13:9–22. [PubMed] [Google Scholar]
- 38. Hashempur MH, Hashempour MM, Mosavat SH, Heydari M. Rhazes—his life and contributions to the field of dermatology. JAMA Dermatol. 2017;153:70 doi:10.1001/jamadermatol.2016.0144. [DOI] [PubMed] [Google Scholar]
- 39. Zarshenas MM, Khademian S, Moein M. Diabetes and related remedies in medieval Persian medicine. Indian J Endocrinol Metab. 2014;18:142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mosavat SH, Ghahramani L, Sobhani Z, Haghighi ER, Heydari M. Topical Allium ampeloprasum Subsp Iranicum (Leek) extract cream in patients with symptomatic hemorrhoids: a pilot randomized and controlled clinical trial. J Evid Based Complementary Altern Med. 2015;20:132–136. [DOI] [PubMed] [Google Scholar]
- 41. Jabbari M, Hashempur MH, Razavi SZ, Shahraki HR, Kamalinejad M, Emtiazy M. Efficacy and short-term safety of topical dwarf elder (Sambucus ebulus L.) versus diclofenac for knee osteoarthritis: a randomized, double-blind, active-controlled trial. J Ethnopharmacol. 2016;188:80–86. [DOI] [PubMed] [Google Scholar]
- 42. Hashempur MH, Ghasemi MS, Daneshfard B, et al. Efficacy of topical chamomile oil for mild and moderate carpal tunnel syndrome: a randomized double-blind placebo-controlled clinical trial. Complement Ther Clin Pract. 2016;26:61–67. [DOI] [PubMed] [Google Scholar]