Abstract
The objective of present investigation was to determine antimicrobial activity of Thymus vulgaris oil on some oral pathogens. Thymus vulgaris oil was prepared by hydrodistillation and tested against 30 clinical isolates of each of Streptococcus pyogenes, Streptococcus mutans, Candida albicans, Porphyromonas gingivalis, and Aggregatibacter actinomycetemcomitans, prepared from related oral infections using agar disk diffusion and broth microdilution methods. Thymus vulgaris oil at concentrations of 16 to 256 μg/mL exhibited strong inhibitory activity on all clinical isolates producing inhibition zones of 7.5 to 42 mm as measured by agar disk diffusion method. Streptococcus pyogenes and Streptococcus mutans were the most sensitive isolates with minimum inhibitory concentrations of 1.9 and 3.6 μg/mL, respectively. The minimum inhibitory concentration values for C albicans, A actinomycetemcomitans, and P gingivalis were 16.3, 32, and 32 μg/mL, respectively.
Keywords: Streptococcus pyogenes, Candida albicans, Thymus vulgaris
Thymus vulgaris is a species of ever green plant in the Lamiaceae family originated from Mediterranean regions and has been adapted to many different climates around the world. It is a bushy, woody based shrub, 10 to 40 cm high with small and highly aromatic gray-green oval leaves containing numerous small glands with clusters of pink or purple flowers. The genus Thymus comprises approximately 400 species, several of which are widely used in traditional medicine.1,2 Thymus vulgaris is the most important species and traditionally has been administered for whooping cough, bronchitis, laryngitis gastritis, upper respiratory congestion, and diarrhea. Thymus vulgaris leaves oil or extract has also been used in the treatment of sore throat, tonsillitis, gum diseases, rheumatism, and arthritis.3–5 This essential oil has been considered as an antiseptic, antimicrobial, antispasmodic, antioxidant, and antitussive agent. There have been a number of reports validating the in vitro antibacterial and antifungal activities of this essential oil on some human pathogens, including Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Candida albicans, Mycobacterium smegmatis, Proteus mirabilis, Propionebacterium acnes, and Salmonella species.6–11 The main constituents of Thymus vulgaris leaves essential oil are 2 phenolic compounds, thymol (2-isopropyl-5-methylphenol) and its conformational isomer, carvacrol (5-isopropyl-2-methylphenol). Further components in the essential oil are thymol methyl ether, cineol, cymene, α-pinene, and borneol.10,12 The antimicrobial activities of Thymus vulgaris oil is mostly believed to be related to the thymol and carvacrol contents of the oil.
Dental caries, periodontal diseases, and streptococcal pharyngitis are the most common oral infectious diseases of man. Dental caries is a multifactorial condition in which diet, nutrition, resident microbial oral flora, and the host responses interact to determine whether infection occurs. Streptococcus mutans and Streptococcus sobrinus are known as the main etiological agents of dental caries. These endogenous cariogenic bacteria adhere and colonize the tooth surface and produce a sticky glycocalyx film composed of glucan resulting from the action of Streptococcal glucosyl transferase on dietary carbohydrates (mainly sucrose). Accumulation of bacteria on the enamel causes dental plaques formation within which there is continuing acid production by bacterial plaques, which causes demineralization of enamel and consequently leads to caries formation.13
Periodontitis is a chronic, slowly progressive polymicrobial infectious disease that affects the entire tooth and supporting tissues. In this infection, the gingival crevice enlarges to become a “pocket” with local inflammation. Periodontal disease is characterized by destruction of periodontal ligaments, alveolar bone, and gingival pocket formation, which consequently leads to tooth loss. This infection is known to be caused by Aggregatibacter actinomycetemcomitans, Prevotella intermedia, Porphyromonas gingivalis, and Tannerlla forythus, which are frequently isolated from gingival pocket and subgingival plaques of patients with periodontitis.14
Streptococcal pharyngitis is a bacterial infection of oropharynx that affects tonsils and possibly larynx and characterized by fever, sore throat, cervical lymphadenopathy, and tonsillar exudates. This infection is caused by Group A, β-hemolytic Streptococci or Streptococcus pyogenes. Although untreated Streptococcal pharyngitis usually resolves within a few days, antibiotic treatment will shorten the acute illness by about 16 hours, and hence reduce the risk of post–streptococcal pharyngitis complications such as rheumatic fever and glomerulonephritis. Although penicillin has long been regarded as the treatment of choice in tonsillopharyngitis caused by S pyogenes, since late 1970, bacteriological and clinical failure rate with penicillin therapy begun to increase, ranging from 2% to 30% among these patients and asymptomatic carriers.15–18 Erythromycin and related macrolide antibiotics are considered as an alternative among patients with allergy to penicillin.19 However, increasing incidence of erythromycin resistance has also been reported in several parts of the world.20 It is therefore essential to discover new antibacterial agents to combat strains expressing resistance to available antibiotics.
Although the in vitro antimicrobial activity of Thymus vulgaris leaves essential oil on some human pathogens are widely documented, the effects of this oil on oral pathogens such as periodontopathic and cariogenic microorganisms are not fully understood. In the present study, we are reporting in vitro inhibitory activity of Thymus vulgaris oil on some clinical isolates of oral pathogens, including Streptococcus pyogenes, Streptococcus mutans, Candida albicans, Aggregatibacter actinomycetemcomitans, and Porphyromonas gingivalis.
Materials and Methods
Preparation of Thymus vulgaris Oil
Fresh Thymus vulgaris were purchased from the local market and were kept in dark at room temperature. One hundred grams of dried leaves was crashed and extracted by conventional steam distillation using a Clevenger apparatus for 3 hours, and condensation took place continuously at 4°C in cold water. The essential oil was then dried over sodium sulfate (Sigma-Aldrich, St Quentin-Fallaveier, France) and stored at 4°C in dark vials until used. The aforementioned experiment was repeated 3 times and the mean of the yield ± standard deviation was recorded. A 1 mg/mL solution of Thymus vulgaris oil was prepared in 10% aqueous dimethyl sulfoxide containing 0.5% Tween 80 (for easy diffusion) and used as stock solution for determination of antimicrobial activities of this oil.
Isolation of Streptococcus pyogenes (β-Hemolytic Streptococci Group A)
Suspected patients with pharyngitis, mostly children below 10 years of age, were examined, and exudates were obtained from the posterior part of the pharynx using sterile cotton swabs. The swabs were then cultured on sheep blood agar (SBA) plates and kept at 37°C, 5% CO2, for 48 hours. The suspicious colonies with β-hemolysis were subjected to the bacitracin sensitivity test by using a 0.04 mg disk for identification of S pyogenes. Pure cultures of each strain isolated from the patients were obtained on sheep blood agar plates and kept at 4°C until used.21
Isolation of Streptococcus mutans From Carious Teeth
Streptococcus mutans was isolated from carious teeth as described previously.21–23 Briefly, the extracted carious teeth were incubated in 10 mL Todd-Hewitt Broth (THB) (Merck, Germany) at 37°C, 5% CO2, for 48 hours. A Mitis-Salivarious-Bacitracin-Agar (MSBA) was subcultured from THB and incubated at 37°C, 5% CO2, for 72 hours. S mutans was identified by standard bacteriological and biochemical procedures, including colony morphology (greenish hemolysis), catalase, Voges-Proskauer, arginine dihydrolase, hippurate hydrolysis, and fermentation of glucose, manitol, raffinose, melobiose, and sorbitol.23–25 Pure culture of each clinical isolate of S mutans was obtained on MSBA medium and kept at 4°C until used.
Isolation of Periodontopathic Bacteria
Patients with either aggressive or localized aggressive periodontitis were examined and sampled for isolation of A actinomycetemcomitans and P gingivalis. 21,22,26 Subgingival pocket samples were taken from the deepest part of periodontal pocket (probing depth ≥6 mm) by insertion of sterile paper point (Iso 35, Bocht, Offenburg, Germany). Each sample was inoculated into 4 mL Trypticase Soy Broth (TSB) containing 5 μg/mL of hemin and menadione (Becton Dickinson Microbiology System) and kept under anaerobic condition at 37°C, 5% CO2, for 48 hours. Bacteria from TSB were subcultured on Trypticase Soy-Blood Agar (TSBA) plates (composed of 40 g/L Trypticase soy agar, 5 mg/L hemin,10 mg/L N-acetylmuramic, acid and 50 mL/L defibrinated sheep blood) and kept under anaerobic condition at 37°C, 5% CO2, for 72 hours. A actinomycetemcomitans and P gingivalis were identified according to our previous publications.21,22,26 Pure culture of each clinical isolates was prepared on TSBA and kept at 4°C until used.
Isolation of Candida albicans
Patients with denture stomatitis, oral candidiasis, and infected root canal were sampled and cultured on Sabouraud dextrose agar (SDA) and kept at 37°C for 72 hours. C albicans was diagnosed on the basis of colonial morphology and other conventional mycological procedures.10,21 Pure cultures of C albicans were prepared on SDA and kept at 4°C until used. In the present study, 30 strains of each of S mutans, A actinomycetemcomitans, P gingivalis, C albicans, and S pyogenes isolated from patients with various oral infections were used for Thymus vulgaris oil antimicrobial determination by standard assays.
Agar Disk Diffusion
The antibacterial activities of Thymus vulgaris oil were determined by the standard disk diffusion susceptibility test on solid media. MSBA plates were used for S mutans, SBA plates for S pyogenes, SDA for C albicans, and TSBA for A actinomycetemcomitans and P gingivalis. S mutans ATCC 25175, A actinomycetemcomitans ATCC 29523, strains which were maintained anaerobically on TSBA supplemented with 10% defibrinated horse blood and hemin (5 μg/mL; Wako Pure Chemical Industries, Osaka, Japan), and C albicans ATCC10231 were used as control.
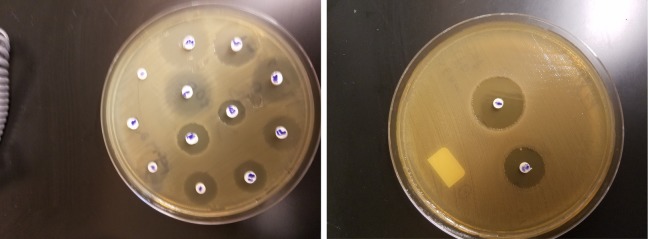
Pure microbial cell suspensions of each clinical isolates were obtained in 5 mL THB for S mutans and S pyogenes, TSB for A actinomycetemcomitans and P gingivalis, and Sabouraud dextrose broth (SDB) for C albicans. The suspension turbidity of these microorganisms was adjusted to 1.5 × 108 colony forming unit/mL (# 0.5 McFarland) and 100 μL of this suspension was seeded on appropriate solid culture media. A 6-mm-diameter sterile Whatman filter paper No. 5 (round filter Machery-Nagel, Doren, Germany) was impregnated with 50 μL of various concentrations of Thymus vulgaris oil and placed on the aforementioned culture media, followed by incubation at 37°C for 72 hours. Sabouraud dextrose agar containing C albicans was incubated at 37°C for 72 hours. The growth inhibition zones around the filter paper were measured in millimeters; means and standard deviations were calculated and recorded. Those disks containing Thymus vulgaris oil that did not produced inhibition zones were considered negative results. Sterile filter paper soaked in 50 μL of 10% dimethyl sulfoxide and antibiotic disks of vancomycin (30 μg), amikacin (30 μg), and nystatin (25 μg) were also used as control.
Determination of Minimum Inhibitory Concentration of Thymus vulgaris Oil
The minimum inhibitory concentration of Thymus vulgaris oil against bacterial and fungal (C albicans) isolates from oral infections was carried out by broth microdilution method using 96-well cell culture plates27 (Greiner Bio-One, Bergamo Italy). Todd-Hewitt Broth was used for S mutans and S pyogenes, SDB broth for C albicans, and TSB containing hemin and menadione (5 μg/mL) for A actinomycetemcomitans and P gingivalis. Cell suspensions of the clinical isolates were prepared in the appropriate liquid culture media and their concentrations were adjusted to 107 colony forming units/mL. Two-fold dilutions of Thymus vulgaris oil were prepared from the stock solution. Aliquots (100 μL) of each dilution of Thymus vulgaris oil were dispensed in 96-well cell culture plates. One hundred microliters of each bacterial suspension was added to each well and incubated under anaerobic conditions at 37°C, 5% CO2, for 48 hours. Microplates containing C albicans were incubated under aerobic condition at 37°C for 48 hours. The absorbance was then measured at 595 nm and the highest dilution at which no growth (OD ≤ 0.05) observed was defined as the minimum inhibitory concentration. All experiments were done in triplicates, and means ± standard deviations were recorded.
Statistical Analysis
Statistical analysis was performed by the χ2 and Fisher’s exact tests using the SPSS software package, version 11.5.
Results
The average yield of Thymus vulgaris essential oil on the basis of 3 successive extractions by hydrodistillation was 1.6 ± 0.34 g oil/100 g dried leaves. The oil was clear light yellow with pleasant odor. The results of agar disk diffusion assay regarding the growth inhibition zones (mean ± standard deviation) of the tested isolates against various concentrations (256 to 2 μg/mL) of Thymus vulgaris oil are summarized in Table 1. In this test, inhibition zones above 6 mm in diameter were taken as positive results. At the concentrations of 64 to 256 μg/mL, all (100%) the microbial isolates were found sensitive and produced inhibition zones ranging from 7.5 ± 0 to 42 ± 0.8 mm in diameter. S pyogenes was the most sensitive isolate since all (100%) of these clinical isolates produced the widest inhibition zones against all Thymus vulgaris oil concentrations (4-256 μg/mL). On the other hand, all strains (100%) of S pyogenes (n = 30), S mutans (n = 30), and C albicans (n = 30) were sensitive to the 32 μg/mL dose of Thymus vulgaris oil, producing inhibition zones ranging from 10.5 to 12.7 mm, while 40% (12/30) of P gingivalis and 60% (18/30) of A actinomycetemcomitans were sensitive to this oil concentration. None of the clinical isolates in this study showed inhibition zones against 2 μg/mL or lower concentration of Thymus vulgaris oil. Agar disk diffusion test carried out on 10% dimethyl sulfoxide showed no inhibitory activity on oral pathogens used in this study (Figure 1). The results of minimum inhibitory concentrations of Thymus vulgaris oil as measured by broth microdilution method are summarized in Table 2. S pyogenes with the minimum inhibitory concentration 1.9 ± 0.2 μg/mL followed by S mutans with minimum inhibitory concentration 3.6 ± 0.9 μg/mL were the most sensitive microorganism tested. The minimum inhibitory concentration of Thymus vulgaris oil on C albicans, P gingivalis, and A actinomycetemcomitans isolates were 16.3 ± 4, 32 ± 0, and 32 ± 0 μg/mL, respectively. Thymus vulgaris oil minimum inhibitory concentration on standard strains of S mutans (ATCC 25175), C albicans (ATCC 10231), and A actinomycetemcomitans (ATCC 29523) were 16 ± 0, 32 ± 0, and 32 ± 0 μg/mL, respectively.
Table 1.
Antimicrobial Activity of Thymus vulgaris Oil on Some Clinically Isolated Oral Pathogens by Agar Disk Diffusion Testsa.
| Thymus vulgaris Oil (μg/mL) | Streptococcus pyogenes (n = 30) | Streptococcus mutans (n = 30) | Candida albicans (n = 30) | Porphyromonas gingivalis (n = 30) | Aggregatibacter actinomycetemcomitans (n = 30) |
|---|---|---|---|---|---|
| 256 | 42 ± 0.8 (100%) | 38.1 ± 1 (100%) | 37.4 ± 1 (100%) | 29.9 ± 0.8 (100%) | 32.7 ± 0.7 (100%) |
| 128 | 29.4 ± 0.8 (100%) | 29.2 ± 1 (100%) | 29.8 ± 0.7 (100%) | 16.9 ± 0.8 (100%) | 24.4 ± 0.7 (100%) |
| 64 | 21.1 ± 0.8 (100%) | 18.9 ± 1 (100%) | 18.3 ± 0.7 (100%) | 9.5 ± 0.5 (100%) | 16.7 ± 1 (100%) |
| 32 | 12.7 ± 1.3 (100%) | 11.7 ± 1 (100%) | 10.5 ± 0.7 (100%) | 8.2 ± 0.4 (40%) | 10.9 ± 0.9 (60%) |
| 16 | 9.6 ± 0.8 (100%) | 9.1 ± 0.6 (76.6%) | 8.7 ± 0.6 (36.6%) | 7.5 ± 0 (16.6%) | 8 ± 0.7 (26.6%) |
| 8 | 8.8 ± 0.8 (80%) | 8 ± 0.2 (50%) | 0 ± 0 (0%) | 0 ± 0 (0%) | 0 ± 0 (0%) |
| 4 | 8.1 ± 0.3 (26.6%) | 0 ± 0 (0%) | 0 ± 0 (0%) | 0 ± 0 (0%) | 0 ± 0 (0%) |
| 2 | R | R | R | R | R |
| Vancomycin | 21.5 ± 0.8 | 21.2 ± 0.9 | R | R | R |
| Amikacin | R | R | R | 9.8 ± 0.7 | 9.1 ± 0.2 |
| Nystatin | R | R | 18. ± 0.8 | R | R |
| 10% DMSO | R | R | R | R | R |
Abbreviations: R, resistance (no inhibition zone); DMSO, dimethyl sulfoxide. Vancomycin disk (30 μg), amikacin disk (30 μg), nystatin disk (25 μg).
aData presented are inhibition zone diameter in mm (Mean ± SD). The values in parentheses are sensitivity percentages.
Figure 1.
Agar disk diffusion tests showing inhibition zone around disk containing various concentrations of Thymus vulgaris extract.
Table 2.
Minimum Inhibitory Concentration of Thymus vulgaris Oil on Some Clinically Isolated Oral Pathogens by Broth Microdilution Methoda.
| Antimicrobials | Streptococcus pyogenes (n = 30) | Streptococcus mutans (n = 30) | Candida albicans (n = 30) | Porphyromonas gingivalis (n = 30) | Aggregatibacter actinomycetemcomitans (n = 30) |
|---|---|---|---|---|---|
| TVO | 3.6 ± 0.9 | 1.9 ± 0.2 | 16.3 ± 4 | 32 ± 0 | 32 ± 0 |
| Vancomycin | 0.95 ± 0.5 | 0.66 ± 0.2 | R | R | R |
| Amikacin | R | R | R | 29.1 ± 1.9 | 24.1 ± 1.8 |
| Nystatin | R | R | 15 ± 1.7 | R | R |
| 10% DMSO | R | R | R | R | R |
Abbreviations: TVO, Thymus vulgaris oil; R, resistance; DMSO, dimethyl sulfoxide.
aData presented are minimum inhibitory concentration in μg/mL (mean ± SD).
Discussion
Data presented in this study revealed strong inhibitory activity of Thymus vulgaris oil on some oral pathogens, including S pyogenes, S mutans, C albicans, A actinomycetemcomitans, and P gingivalis as measured by agar disk diffusion and broth microdilution methods. S pyogenes isolated from patients with pharyngitis were the most sensitive strains to Thymus vulgaris oil as they produced the widest growth inhibition zones (42 ± 0.8 mm) and lowest minimum inhibitory concentration (1.9 ± 0.2 μg/mL). Sfeir et al28 using the same techniques found S pyogenes highly sensitive to Thymus vulgaris oil with growth inhibition zone of 38 mm and minimum inhibitory concentration as low as 0.87 μg/mL. The antimicrobial activity of various thymus species essential oils on oral Streptococci were documented by Nikolic et al.10 Thymus serpyllum oil showed strongest activity against S pyogenes clinical isolates with minimum inhibitory concentration of 2.5 ± 0.23 μg/mL, while Thymus vulgaris oil exhibited lower activity against S pyogenes with minimum inhibitory concentration of 80 μg/mL.10 Moreover, these investigators have reported stronger activity of T serpyllum oil than streptomycin and ampicillin against S pyogenes as measured by minimum inhibitory concentration determinations. Solano et al29 reported that S pyogenes isolates produced wider growth inhibition zones (34 mm) against Thymus vulgaris oil versus 6-unit penicillin disk, which produced 24 mm inhibition zones. In vitro antibacterial activity of Thymus vulgaris vapor against S pyogenes is also documented.30 Considering the strong inhibitory activity of Thymus vulgaris oil against S pyogenes as presented in our study and others,10,28–30 it seems reasonable to use this essential oil in aromatherapy, particularly among patients with respiratory tract infections caused by S pyogenes such as tonsillitis, pharyngitis, sinusitis, and bronchitis.
Ghorab et al2 reported that incubation of S mutans, the main etiologic agent of dental caries, with 20% Thymus vulgaris oil resulted in 96% growth inhibition of this bacteria after 48 hours. Moreover, significant reduction of S mutans adherence to buccal epithelial cells after mouth washing with 20% Thymus vulgaris oil has been observed.31 Nikolic et al10 found Thymus vulgaris oil less effective against S mutans than Thymus serpyllum, as this oil revealed higher minimum inhibitory concentration (160 ± 4.61 μg/mL). Data presented in our study exhibit strong inhibitory activity of Thymus vulgaris oil with minimum inhibitory concentration of 3.6 ± 0.9 μg/mL on S mutans clinical isolates. On the contrary, Babpour et al32 have detected no inhibitory effects of methanolic and aqueous Thymus vulgaris extract on S mutans even at concentrations over 500 μg/mL. Very weak inhibitory activity of Thymus vulgaris oil with minimum inhibitory concentration of 2670 μg/mL on oral Streptococci were also reported by Imelouane et al.9 Thymus vulgaris oil analysis by gas chromatography-mass spectrometry carried out by these investigators exhibited no carvacrol and presence of very low amount of thymol (0.24%). These 2 phenolic compounds play major roles in bacterial growth inhibition, and therefore, these findings may be an explanation for the weak (high value of minimum inhibitory concentration) or no inhibitory activity of Thymus vulgaris oil on oral Streptococci as reported by the investigators.9 Denture-related stomatitis is a very common form of oral candidiasis and is referred to as mild inflammation and erythema of mucosa beneath a denture. In our study C albicans isolated from patients with denture stomatitis (21/30) and infected root canal from patients with advanced periodontitis (9/30) were all sensitive to Thymus vulgaris oil with mean minimum inhibitory concentration of 16.3 ± 4 μg/mL. Minimum inhibitory concentrations values of the oil as low as 1.62 ± 0 μg/mL3 to as high as 3300 μg/mL5 for clinical isolates of C albicans are documented by other investigators. Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis are the 2 anaerobic gram negative rods that are the most prevalent etiological agents of periodontal diseases. Data on the inhibitory activity of Thymus vulgaris oil on these periodontopathic bacteria are very limited in the literature. In our study, A actinomycetemcomitans and P gingivalis clinical isolates were sensitive to Thymus vulgaris oil with mean minimum inhibitory concentration 32 μg/mL; however, higher value of Thymus vulgaris oil minimum inhibitory concentration (62.5 mg/mL) on these periodontopathic bacteria are reported by other investigators.33 These discrepancies in minimum inhibitory concentration values reported by different investigators from various regions are mainly attributed to the fact that Thymus vulgaris oil chemical composition and active ingredients’ (thymol, carvacrol, P-cymene, etc) concentrations are greatly determined by the plant genotype and influence of environmental factors including geographical conditions, nature of soil, temperature, season of collection and harvesting plant, and more important, the oil extraction procedure.4,9,34–37 Much of the antimicrobial activities of Thymus vulgaris oil appear to be associated with the phenolic compounds thymol and carvacrol.38 Although the mode of action of these compounds are not clearly understood, it is mostly believed that the hydroxyl group on these 2 compounds interacts with the cytoplasmic membrane, changes its permeability, and affects the lipid ordering and stability of its bilayer, resulting in an increase of proton passive flux across the membrane, leading to disruption of cytoplasmic membrane and leakage of cellular contents.38–42 The antifungal activity of the oil is mostly associated with the direct interaction of thymol, carvacrol, and P-cymene with cytoplasmic membrane ergostrol, which consequently leads to fungal cell membrane disruption and release of the cellular contents.8,12 Most studies reporting the antimicrobial activity of plant essential oils against foodborne and human pathogens agree that essential oils are relatively more active against gram positive than gram negative bacteria.43 Results obtained in our study showed gram positive bacteria were more susceptible to Thymus vulgaris oil than the gram negatives as measured by agar disk diffusion and minimum inhibitory concentration determinations. Zaika et al44 proposed that gram positive bacteria were more resistant to the plant volatile oils than to the gram negatives. This is in contrast to the hypothesis proposed by Dean et al,45 who observed little or no differences between gram positive and gram negative bacteria regarding the inhibitory effects of plant essential oils. However, greater susceptibility of gram negatives against Thymus vulgaris oil than the gram positive bacteria is documented.9 The greater resistance of gram negatives might be associated with the presence of an outer membrane hydrophilic lipopolysaccharide, which inhibits accumulation of hydrophobic plant essential oil on the cell membrane.46 Consumption of Thymus vulgaris flowers and leaves are safe; however, caution is warranted with the use of thyme oil, which should not be taken orally and should be diluted with a suitable oil (olive or almond oil) before use. Side effects of thyme oil if taken orally may include headache, dizziness, low blood pressure, gastrointestinal irritation, nausea, vomiting, and diarrhea.47
Data presented in this study revealed strong in vitro antimicrobial activity of Thymus vulgaris oil on clinical isolates of S pyogenes, S mutans, C albicans, A actinomycetemcomitans, and P gingivalis and therefore might be used in mouth rinse, toothpaste, or aromatherapy for prevention and treatment of related oral infections.
Acknowledgments
The authors would like to thank the Vice Chancellor of Research, Shiraz University of Medical Sciences, Shiraz, Iran. The editorial assistance of Miss Azadeh Kohanteb is greatly appreciated.
Footnotes
Author Contributions: Both authors have equally contributed to the research and preparation of this article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was financially supported by the Vice Chancellor of Research, Shiraz University of Medical Sciences (Grant No. 5638).
Ethical Approval: Written consents were obtained from the School of Dentistry Ethics Committee and patients prior to sample collection.
References
- 1. Javadi H, Hesamzadeh Hejazi SM, Babayev MSH. Chromosome reports on two species of Thymus (Lamiaceae). Iran J Bot. 2011;18:108–111. [Google Scholar]
- 2. Ghorab H, Kabouche A, Kabouche Z. Comparative composition of essential oil of Thymus growing in various soil and climate of North Africa. J Mater Environ Sci. 2014;5:298–303. [Google Scholar]
- 3. Tsai ML, Lin CC, Lin WC, Yang CH. Antimicrobial, antioxidant, and anti-inflammatory activities of essential oils from five selected herbs. Biosci Biotechnol Biochem. 2011;75:1977–1983. [DOI] [PubMed] [Google Scholar]
- 4. Ali Ahmad Al Maqtari M, Alghalibi SM, Alhamzy EH. Chemical composition and antimicrobial activity of essential oil of Thymus vulgaris from Yemen. Turk J Biochem. 2011;36:342–349. [Google Scholar]
- 5. Van Vuuren SF, Suliman S, Viljoen AM, et al. The antimicrobial activity of four commercial essential oils in combination with conventional antimicrobials. Lett Appl Microbiol. 2009;48:440–446. [DOI] [PubMed] [Google Scholar]
- 6. Azza S, Lyoussi B, Megias G, et al. Antioxidant, anti-inflammatory and antiproliferative activities of Moroccan commercial essential oils. Nat Prod Commun. 2014;9:587–594. [PubMed] [Google Scholar]
- 7. Azza S, Lyoussi B, Miguel MG. Antioxidant and antiacetylcholinesterase activities of some commercial oils and their major compounds. Molecules. 2011;16:7672–7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lira Mota KS, Oliveira Pereia F, Oliveira WA, et al. Antifungal activity of Thymus vulgaris L. essential oil and its constituent phytochemicals against Rhizopus oryzae: interaction with ergosterol. Molecules. 2012;17:14418–14433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Imelouane B, Amhamdi H, Wathelet JP, et al. Chemical composition and antimicrobial activity of essential oil of thyme (Thymus vulgaris) from Eastern Morocco. Int J Agric Biol. 2009;11:205–208. [Google Scholar]
- 10. Nikolic M, Glamoclija J, Ferreira ICFR, et al. Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum, Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. essential oils. Ind Crop Prod. 2014;52:183–190. [Google Scholar]
- 11. Sokovic M, Glamoclija J, Marin PD, Brkic D. Antibacterial effects of the essential oil of commonly consumed medicinal herbs using in vitro model. Molecules. 2010;15:7532–7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pina-Vaz C, Goncalves Rodrigues A, Pinto E, et al. Antifungal activity of Thymus oil and their major compounds. J Eur Acad Dermatol Venereol. 2004;18:73–78. [DOI] [PubMed] [Google Scholar]
- 13. Goncalves GMS, Bottaro M, Nilson AC. Effects of Thymus vulgaris essential oil on the growth of Streptococcus mutans . J Basic Appl Pharm Sci. 2011;32:375–380. [Google Scholar]
- 14. Kononen E, Paju S, Hyvonen M, et al. Population-based study of salivary carriage of periodontal pathogens in adults. J Clin Microbiol. 2007;45:2446–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Neeman R, Keller N, Barzilai A, et al. Prevalence of internalization-associated gene prtF1, among persisting group A Streptococcus strains isolated from asymptomatic carriers. Lancet. 1998;352:1974–1977. [DOI] [PubMed] [Google Scholar]
- 16. Gillespie SH. Failure of penicillin in Streptococcus pyogenes pharyngeal infection. Lancet. 1998;352:1954–1956. [DOI] [PubMed] [Google Scholar]
- 17. Pichichero ME, Casey JR, Mayes T, et al. Penicillin failure in Streptoccocal tonsillo pharyngitis: cases and remedies. Pediatr Infect Dis J. 2000;19:917–923. [DOI] [PubMed] [Google Scholar]
- 18. Ibrahim SB, Rehab H, El-Sokkary, et al. Emerging resistance to erythromycin and penicillin among Streptococcus pyogenes isolates in Zagazing, Egypt. Int J Curr Microbiol Appl Sci. 2014;3:750–756. [Google Scholar]
- 19. Malli E, Tatsidov E, Damani A. Macrolides-resistant Streptococcus pyogenes in central Greece; prevalence, mechanism and molecular identification. Int J Antimicrob Agents. 2010;35:614. [DOI] [PubMed] [Google Scholar]
- 20. Ying-Huang C, Fen-Lai J, Wen-Huang I, Chen P. Epidemiology and molecular characterization of macrolide-resistant Streptococcus pyogenes in Taiwan. J Clin Microbiol. 2014;52:508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fani MM, Kohanteb J, Araghizadeh A. Inhibitory activity of Myrthus communis oil on some clinically isolated oral pathogens. Med Princ Pract. 2014;23:363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fani MM, Kohanteb J. Inhibitory activity of Aloe vera gel on some clinically isolated cariogenic and periodontopathic bacteria. J Oral Sci. 2012;54:15–21. [DOI] [PubMed] [Google Scholar]
- 23. Fani MM, Kohanteb J, Dayaghi M. Inhibitory activity of garlic (Allium sativum) extract on multidrug-resistant Streptococcus mutans . J Indian Soc Pedodont Prev Dent. 2007;25:164–168. [DOI] [PubMed] [Google Scholar]
- 24. Toivianen A, Jalasvuori H, Lahti E, et al. Impact of orally administered lozenges with Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp. Lactis BB12 on the number of salivary mutan streptococci, amount of plaque, gingival inflammation and oral microbiome in healthy adults. Clin Oral Investig. 2015;19:77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Beighton D, Russel RR, Whilly RA. A simple biochemical scheme for differentiation of Streptococcus mutans and Streptococcus sobrinus . Caries Res. 1991;25:174–178. [DOI] [PubMed] [Google Scholar]
- 26. Araghizadeh A, Kohanteb J, Fani MM. Inhibitory activity of green tea (Camelia sinensis) extract on some clinically isolated cariogenic and periodontopathic bacteria. Med Princ Pract. 2013;22:368–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kohanteb J, Sadeghi E. Penicillin-resistant Streptococcus pneumoniae in Iran. Med Princ Pract. 2007;16:29–33. [DOI] [PubMed] [Google Scholar]
- 28. Sfeir J, Lefrancois C, Baudoux D, et al. In vitro antibacterial activity of essential oils against Streptococcus pyogenes . Evid Based Complement Alternat Med. 2013;2013:269161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Solano E, Cruz CC, Estrada LA, et al. Effect of the essential oil, infusion and ethanol extract of Thymus vulgaris L., on the growth in vitro of group A β-hemolytic Streptococcus pyogenes . TIP Rev Esp Cienc Quim Biol. 2006;9:73–77. [Google Scholar]
- 30. Inouye S, Takizawa T, Yamagouchi H. Antibacterial activity of essential oil and their major constituents against respiratory tract pathogens by gaseous contact. J Antimicrob Chemother. 2001;47:565–573. [DOI] [PubMed] [Google Scholar]
- 31. Hammad M, Sallal AK, Darmani H. Inhibition of Streptococcus mutans adhesion to buccal epithelial cells by an aqueous extract of Thymus vulgaris . Int J Dent Hyg. 2007;4:232–235. [DOI] [PubMed] [Google Scholar]
- 32. Babpour E, Angaji S, Angaji SM. Antimicrobial effects of four medicinal plants on dental plaque. J Med Plants Res. 2009;3:123–137. [Google Scholar]
- 33. Rodriguez-Garcia A, Galan-Wong LJ, Alevalo-Nino K. Development and in vitro evaluation of biopolymers as a delivery system against periodontopathogen microorganism. Acta Odontol Latinoam. 2010;23:158–163. [PubMed] [Google Scholar]
- 34. Sokovic M, Vukojevic J, Marin P, et al. Chemical composition of essential oil of Thymus and menthe species and their antifungal activities. Molecules. 2009;14:238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kabouche A, Ghannadi A, Kabouche Z. Thymus cilitus—the highest thymol containing essential oil of the genus. Nat Prod Commun. 2009;4:1251–1252. [PubMed] [Google Scholar]
- 36. Verma R, Rahman L, Chanotiya C, et al. Essential oil composition of Thymus serpyllum cultivated in the Kumaon region of Western Himalaya, India. Nat Prod Commun. 2009;4:987–988. [PubMed] [Google Scholar]
- 37. Hernandez T, Canales M, Duran A, et al. variation of the hexanic extract composition of Lippia graveolans in an arid zone from Mexico: environmental influence or true chemotypes? Open Plant Sci J. 2009;3:29–34. [Google Scholar]
- 38. Rota MC, Herrera RM, Martinez JA, et al. Antimicrobial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis essential oils. Food Control. 2007;19:681–687. [Google Scholar]
- 39. Dorman HJD, Deans SG. Antimicrobial agents from plants: antimicrobial activity of plant volatile oils. J Appl Microbiol. 2000;88:308–316. [DOI] [PubMed] [Google Scholar]
- 40. Xu J, Zhou F, Ji BP, et al. The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett Appl Microbiol. 2008;47:174–179. [DOI] [PubMed] [Google Scholar]
- 41. Ahmad A, Vuuren SV, Viljoen A. Unraveling the complex antimicrobial interactions of essential oils—the case of Thymus vulgaris (thyme). Molecules. 2014;19:2896–2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shapiro S, Guggenheim B. The action of thymol on oral bacteria. Oral Microbiol Immunol. 1995;10:241–246. [DOI] [PubMed] [Google Scholar]
- 43. Lambert RJW, Skandamis PN, Coote PJ, Nychas JE. A study of minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J Appl Microbiol. 2001;91:453–462. [DOI] [PubMed] [Google Scholar]
- 44. Zaika LL. Spices and herbs: their antimicrobial activity and its determination. J Food Saf. 1988;9:97–118. [Google Scholar]
- 45. Dean SG, Noble RC, Hiltunen R, Wuryani W. Antimicrobial and antioxidant properties of Syzygium aromatica Merr Perry: impact upon bacteria, fungi and fatty acid levels in aging mice. Flav Frag J. 1995;10:323–328. [Google Scholar]
- 46. Bezic N, Venskutonis M, Dunkic V, Darbrauskiene E. Composition and antimicrobial activity of Achillea clavennae essential oil. Phytother Res. 2003;17:1037–1040. [DOI] [PubMed] [Google Scholar]
- 47. Fachini-Queiroz FC, Kummer R, Estevao-Silva CF, Carvalho MD. Effects of thymol and carvacrol constituents of Thymus vulgaris essential oil on the inflammatory response. Evid Based Complement Alternat Med. 2012;2012:657026. [DOI] [PMC free article] [PubMed] [Google Scholar]