Abstract
In the present study, the aphrodisiac properties of the purple corn (Zea mays) in male rats were analyzed. The aqueous crude extract of purple corn (at 25, 50, and 75 mg/kg) was administered to (a) copulating male rats and (b) anesthetized and spinal cord transected male rats. Behavioral parameters of copulatory behavior and parameters of the genital motor pattern of ejaculation previous to its inhibition, under the influence of the purple corn extract, are described. Administration of the aqueous crude extract of purple corn significantly facilitates the arousal and execution of male rat sexual behavior without significant influences on the ambulatory behavior. In addition, purple corn extract elicit a significant increase in the number of discharges of the ejaculatory motor patterns and in the total number of genital motor patterns evoked in spinal rats. The present findings show that the aqueous crude extract of purple corn possesses aphrodisiac activity.
Keywords: aphrodisiac, medicinal plant, ejaculation, purple corn, copulatory behavior
Masculine sexual dysfunctions have a high prevalence in sexually active men and are considered as cause of sexual complains with a noteworthy impact on the quality of life. Studies on masculine sexual dysfunction have mainly focused on understanding erectile and ejaculatory disorders, which represent the most prevalent sexual problems.1 Sildenafil citrate is the first-line therapy for male sexual dysfunction including erectile and ejaculatory dysfunctions with excellent overall efficacy and satisfactory side effect profiles.2 Recently, it has been shown that men with comorbid erectile dysfunction and premature ejaculation may also be concomitantly prescribed the phosphodiesterase type 5 inhibitor and other compounds such as the antidepressant dapoxetine.2 In addition, the effects of new synthetic drugs derived from different sources to treat male sexual disorders has aroused a renewed interest in the study of medicinal plants with pro-sexual activity that favor sexual functions such as Montanoa tomentosa,3 Kaempferia parviflora,4 Boesenbergia rotunda,5 Bersama engleriana,6 and Dracaena arborea,7 among others. All these medicinal plants offer a significant potential for studying the male sexual response and its dysfunctions.
In the ancient Mexican traditional medicine some medicinal plants are reputed to possess aphrodisiac effects and currently are recommended for the treatment of sexual disorders. Ancient Mexican documents that include the Historia Natural de Nueva España by Francisco Hernández8 mentioned that the purple corn or Tlaolli prepared in a beverage known as atolli is employed to excite the venereal appetite by increasing the natural vigor. As a medicine, Tlaolli mitigates fever, provokes urine, and clean all paths.8 Aztecs prepared Tlaolli extracts to refresh untempered people, to temper the chest, to purge the belly by toning the stomach to support digestion, to eliminate adherent phlegms, and to clean the kidneys, and mixed with chili and honey, to increase the natural vigor and to excite the venereal appetite.8 In addition, Hernandez mentioned that purple corn is employed to provoke urination and to prevent irritation of the urinary ducts,8 and when it is mixed with pepper to prepare atolli, a remedy to mitigate fever and induce urination is obtained.8 In modern times, Tlaolli is still used by Mexicans to reduce cardiac pain, to increase milk ejection, during diarrheic states, to solve sterility problems, and as an antihypertensive and diuretic agent.9
Thus, though medicinal properties of purple corn were described in detail 5 centuries ago, scientific literature lacks experimental or clinical studies validating its aphrodisiac properties. Aphrodisiacs can be categorized according to their mode of action into 3 groups: substances that increase libido (ie, sexual desire, arousal), substances that increase sexual potency (ie, effectiveness of erection), and those that increase sexual pleasure.10
Purple corn is employed in México from ancient times, and studies of our laboratory exploring the pro-ejaculatory properties of Mexican plants revealed that the administration of purple corn extract elicits the activation of the spinal generator for ejaculation. This result prompted us to design experimental studies to describe the potential aphrodisiac properties of purple corn by hypothesizing that the purple corn aqueous crude extract could possess sexual stimulant properties improving the expression of male sexual behavior, by targeting the ejaculatory response. The neural commands that regulate ejaculation are organized both at brain and spinal levels. In the brain, the medial preoptic area and the paraventricular nucleus exert an excitatory influence on ejaculation and the nucleus paragigantocellularis an inhibitory one.11 At the spinal level, a central pattern generator regulates the ejaculatory response.12 This spinal generator for ejaculation receives sensory genital inputs and projects to supraspinal sites and also receives descending projections from higher centers. Finally, this generator connects to spinal autonomic and somatic centers.13
The aim of the present study was to describe the aphrodisiac properties of the purple corn aqueous crude extracts on the male rat copulatory behavior and on the fictive ejaculation model in spinal cord transected male rats. Copulatory behavior of male rats allows the evaluation of the 2 main physiological mechanisms of sexual behavior, the motivational and consummatory components.11 The fictive ejaculation model permits, in anesthetized and spinal cord transected rats, the quantitative recording and evaluation of the genital motor pattern of ejaculation (GMPE), which is a rhythmic motor pattern that includes a first ejaculatory motor train and an after-discharge component, obtained in the bulbospongiosus muscles in response to urethral stimulation.12–15 Fictive ejaculatory responses can be elicited at 3 min intervals, and previous reports have shown that repeated stimulation of the urethra induces an inhibitory effect on the GMPE, which is gradually evidenced in the parameters of the first ejaculatory motor trains and in its after-discharge component and by a progressive reduction in the number of motor discharges and its frequency.12–15 As a result, the fictive ejaculation model permits the analysis of ejaculatory potency and ejaculatory capacity.15 Fictive ejaculatory responses can be induced by physiological-like stimulation and by pharmacological means and is under the control of the spinal generator for ejaculation.12–15
Materials and Methods
Animals
Sexually vigorous male Wistar rats (300-350 g body weight) were used. Animals were housed in groups (4 rats per cage), under an inverted light-dark cycle 12:12 hours, at 22°C, and with free access to food and water. Prior to experimental testing all animals received 5 sexual behavior tests, and the sexually active males were selected and considered sexually experienced. The Local Committee of Ethics on Animal Experimentation approved all experimental procedures, which followed the regulations established in the Mexican Official Norm for the use and care of laboratory animals (NOM-062-ZOO-1999).
Sexual Behavior Testing Protocol
All sexual behavior tests were conducted 2 hours after the onset of darkness. Males were introduced into a cylindrical observation cage and a 5-minute adaptation period was allowed. Thereafter a receptive stimulus female was introduced and sexual behavior was recorded along 30 minutes in agreement to the original protocol proposed by Beach.11 Female receptivity was induced by the sequential subcutaneous administration of estradiol valerianate (4 μg/rat) followed 44 hours later by progesterone (2 mg/animal). Behavioral observations were conducted 4 hours after progesterone administration. Male rats displaying ejaculatory behavior in a period less than 15 minutes during at least 3 consecutive copulatory sessions were considered as sexually experienced male rats. The sexual behavior parameters analyzed were the following: mount latency, time from introduction of the female until the first mount with pelvic thrusting; intromission latency, time from introduction of the female until the first mount with pelvic thrusting and vaginal penetration (intromission); ejaculation latency, time from the first intromission until ejaculation; and the postejaculatory interval, time from ejaculation until the rat reassumes copulatory behavior with an intromission. In addition, the number of mounts and intromissions displayed in an ejaculatory series was recorded. Latency data were expressed in minutes as mean ± SEM, while the number of mounts and intromissions as median numbers.
General Surgical Procedures
Rats were anesthetized with urethane 0.7 g/kg. ip; adequacy of the anesthesia was assessed by the absence of a withdrawal reflex after noxious paw pinches. Once anesthetized, the body temperature of all animals was maintained at 37°C. After this, bulbospongiosus genital muscles were exposed by a surgical incision on the perineum. These muscles was selected from the genital striated muscles complex to monitor the ejaculatory response given its close relations with sexual structures such the penile crura and perineal fascia and based on the fact that this muscle is the most prominent genital muscle and easy to access. After exposing, the bulbospongiosus genital muscles were prepared for electromyographic recordings. Two platinum wires (Grass) were inserted into the muscles to record electromyographic activity, which was registered on a polygraph (Grass M7). For a better visualization of the motor genital activity, which includes penile erection and penile movements associated to the ejaculation, an additional surgery was performed to expose the bulbar portion of the penis and its anatomical connections with the striated bulbospongiosus muscles. The femoral vein was cannulated for treatment administration. At the end of the surgical approach, the spinal cord was blunt transected at T6 spinal cord segment level. This manipulation was carried out by exposing dorsally the spine after the removal of skin and dorsal muscles, and fixed on a stereotaxic apparatus. After exposing the spine, a laminectomy was done to uncover the spinal cord to be transected using bistouries, and once spinalized, the animals were prepared for physiological recording in a ventral position.
Activation and Recording of Fictive Ejaculation
Immediately after spinal cord transection, the fictive ejaculatory response can be expressed spontaneously and urethrally induced in all animals.12 Thus, in all spinal cord transected animals we permitted to express 2 spontaneous fictive ejaculatory responses only to verify the functioning of the spinal cord. After this procedure, the fictive ejaculatory responses were elicited by mechanical stimulation of the urethra and registered in the bulbospongiosus muscles as GMPEs. Mechanical stimulation of the urethra was achieved by injecting saline solution (200 μL/min) with a Harvard syringe pump, during 10 seconds, through a PE-50 catheter (0.965 mm OD) inserted into the pelvic urethra (via a bladder incision) simultaneous to the brief occlusion of the urethral meatus. In control animals and once 2 spontaneous responses were obtained, GMPEs were repeatedly evoked by urethral stimulation and the resulting motor activity of the selected muscles was registered until its inhibition. Rats receiving the purple corn extract were permitted to express 2 spontaneous fictive ejaculatory responses and then 1 fictive ejaculatory response was evoked by urethral stimulation. After this, the pharmacological treatments were intravenously injected and the GMPEs under its influence, if present, were registered. Finally, the fictive ejaculatory responses were evoked at 3-minute intervals by urethral stimulation until its inhibition. The pharmacologically induced responses were compared with GMPEs obtained after a first urethral stimulation in the control group, since vehicle administration has no effect on the GMPEs. After the inhibition of the fictive ejaculatory responses evoked in control conditions and under the presence of purple corn extracts, a 10-minute period was allowed to verify the total inhibition of the fictive ejaculatory response and then the experiment was ended. The parameters recorded for each motor train were the latency to the expression of ejaculatory motor patterns, the number and frequency of electromyographic bursts, and the total number of GMPEs expressed.
Preparation of Purple Corn Extracts
Purple corn was obtained from organic producers of Ixtenco Tlaxcala during December of 2013 and specimens were authenticated by Dr. José Luis Martínez y Pérez from Herbarium (TLXM) of the Universidad Autónoma de Tlaxcala; in this place voucher specimens are preserved (serial number of Zea mays TLXM MCarro04). Kernels were selected and prepared to be perfectly dried during 40 days. Once dried, kernels were weighted to prepare an infusion as indicated by the traditional medicine, which was obtained mixing 1 L of distilled water with 200 g of kernels and permitting the repose of 2 hours. After the repose, the mixture was warmed up approximately 15 minutes just before boiling and then allowed to cool at room temperature. The resulting extract was filtered using Whatman No. 1 filter paper and then lyophilized (Telstar freezer-dryer, −45°C) to obtain solid residues of the kernels that were quantified. Solid residuals were utilized to prepare dilutions for the administration of specific doses of the extract that were expressed as weight/volume of lyophilized powder. The doses of aqueous extract used in this study were expressed as milligram of dried extract/kilogram. Final dilutions were prepared to obtain the concentration used of 25, 50, or 75 mg/kg, which was administered per orale to behaving animals in a total volume of 1 mL or intravenously to spinal cord transected animals in 0.4 mL of saline solution. Doses of aqueous crude extracts of purple corn were selected on the basis of a previous pilot study. In brief, to select the convenient doses, we asked as to which quantity of purple corn employed by indigenous midwives in San Tadeo Huiloapan, Tlaxcala, México, improved sexual function of adult men.
Experimental Series
Experiment 1. Effect of purple corn extract administration on the expression of male rat sexual behavior
In order to establish the ability of the purple corn crude extract to influence masculine sexual behavior, rats of groups 2 to 4 (N = 8 each) received a single oral treatment with the aqueous crude extract of purple corn at 25, 50, or 75 mg/kg, via a catheter 1 hour after the onset of darkness, and after 30 minutes of the application of each dose were subjected to the evaluation of male sexual behavior. Sexual behavior data were compared with vehicle treated animals (G1).
Experiment 2. Effects of systemic injection of purple corn aqueous crude extract on the ejaculatory response in spinal male rats
In order to establish the responsive doses of purple corn extract on ejaculation in spinal male rats, animals from groups 6 to 8 (N = 3) received a single intravenous dose of 25, 50, or 75 mg/kg of the purple corn extract, and were subjected to the fictive ejaculation model. In these animals, the ejaculations obtained occurred within a 10-minute period and were graphed and registered. After purple corn extract injection, additional urethral stimulations were applied and its resulting genital responses, if present, were observed. When no response was obtained, the experiment was ended. Data were compared to vehicle treated animals (G5), which received vehicle solution (distilled water).
Experiment 3. Effect of purple corn extract administration on the expression of ambulatory behavior
In order to discard a possible purple corn extract–induced motor impairment that could influence the sexual behavior recordings, rats from groups 2 to 4 (N = 8 each) that received the doses of 25, 50, or 75 mg/kg of the purple corn aqueous crude extract were tested immediately after copulation in the open-field test during a 5-minute session. In brief, the apparatus consisted of an opaque Plexiglas box (40 × 30 × 20 cm) with the floor divided into 12 equal squares (10 × 10 cm2). As previously described,16 animals were placed in a corner of the apparatus, and an observer, always blind to the treatments, registered the number of times that the animal crossed squares during a 5-minute session. Results were compared to those obtained in the vehicle-treated animals (G1).
Drugs
All drugs were purchased from Sigma Chemical Co (St Louis, MO). Urethane was prepared and administered at 20%. Estradiol benzoate and progesterone were dissolved in sesame oil and subcutaneously injected to the females.
Data Analysis
All analyses were performed with the Sigma Stat program, version 3.1. The Kruskal-Wallis ANOVA on ranks followed by the Tukey’s test were used to analyze sexual behavior. Paired comparisons were conducted by using the t test. One-way ANOVA followed by the Holm-Sidak’s test were used to analyze GMPE parameters and to analyze motor behavior data obtained after purple corn treatments, respectively. Motor activity data were expressed as mean ± SEM of crosses and statistically evaluated by means of the Mann-Whitney U test. Bulbospongiosus electromyographic activity was recorded differentially, amplified, and filtered (1000×, 0.1-1 kHz bandpass; Poliview Data Acquisition System; Grass Astro-Med Inc, West Warwick, RI). Comparisons with P < .05 were considered to be statistically significant.
Results
Effect of Purple Corn Extract Administration on the Expression of Male Rat Sexual Behavior
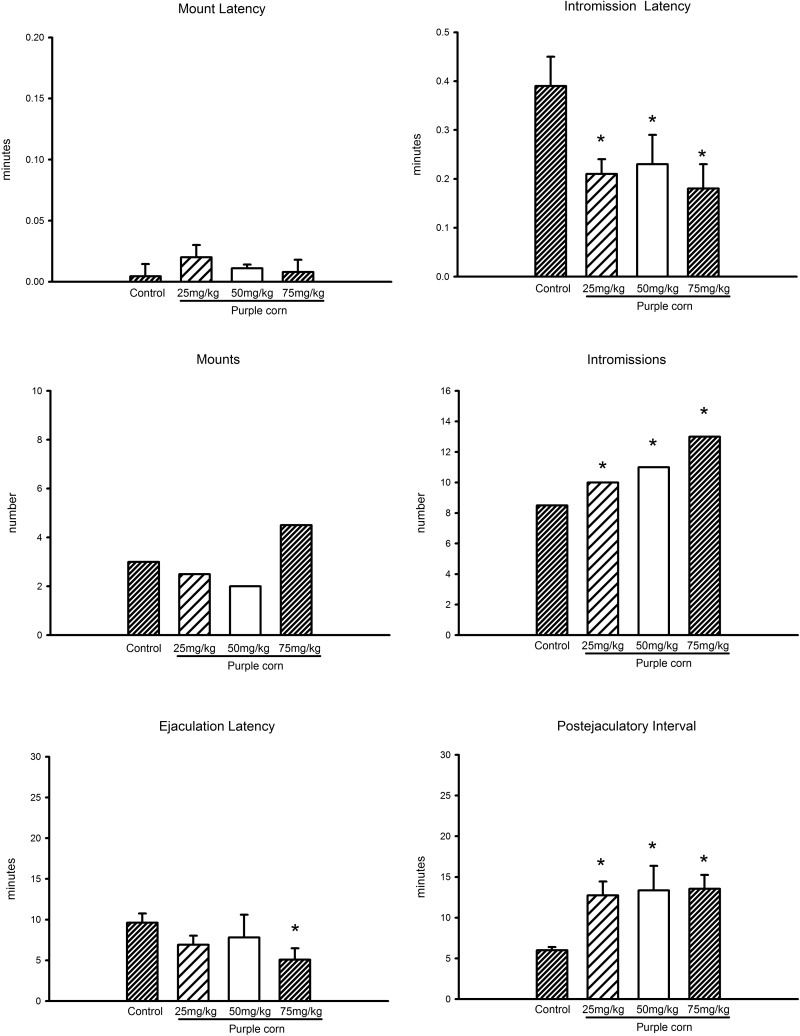
In the first part of the present study, we analyzed the copulatory behavior of male rats acutely treated with vehicle or with 25, 50, or 75 mg/kg of the crude extract of purple corn. Data show that purple corn extract at the doses tested lacked an effect on mount latency and the number of mounts (Figure 1). With regard to the intromission latency, we observed that all doses produced a statistically significant decrease of this parameter (P < .05; Figure 1) and a statistically significant and progressive augmented number of intromissions (P < .05). In contrast, the purple corn extract decreased the latency of ejaculation at the highest dose (P < .05; Figure 1). On the contrary, the postejaculatory interval exhibited a statistically significantly increase at all tested doses (P < .05). In Figure 1 are graphed the data obtained from specific sexual behavior parameters registered after the different doses of purple corn, with the dose of 75 mg/kg of the aqueous extract being the most effective to influence the male rat sexual behavior.
Figure 1.
Sexual behavior parameters of male rats treated with 25, 50, and 75 mg/kg of the purple corn extract. Latencies are expressed as mean ± SEM and numbers as medians. Kruskal-Wallis analysis of variance followed by Tukey’ test versus Control; *P < .05 for latency to intromission, intromissions, latency of ejaculation, and for postejaculatory interval.
Activation of Fictive Ejaculation by the Systemic Injection of Purple Corn Aqueous Crude Extract in Spinal Male Rats
In control animals, the first sensory elicited ejaculatory phase was the most potent and the last one prior to its inhibition the weakest. Once the ejaculatory capacity maximum level was accomplished, no further GMPEs including its associated penile movements or expulsion of seminal contents occurred. At this moment, the stimulation protocol was completed and the ejaculatory ability was considered as inhibited. The ejaculatory capacity of control animals consisted on the expression of a mean number of 6.8 ± 0.3 GMPEs.
Administration of purple corn extracts at all doses tested was able to activate the GMPE in spinal male rats that always consisted of the expression of highly rhythmic motor patterns registered in the bulbospongiosus muscles, very similar to those registered in control animals (Figure 2b-d). Purple corn extracts elicited ejaculatory motor responses without the after-discharge component. General visual observations of movements elicited by the purple corn extract at the dose of 75 mg/kg (Figure 2d) permitted us to notice that the expression of GMPEs was significantly more potent as compared to those elicited by urethral stimulation.
Figure 2.
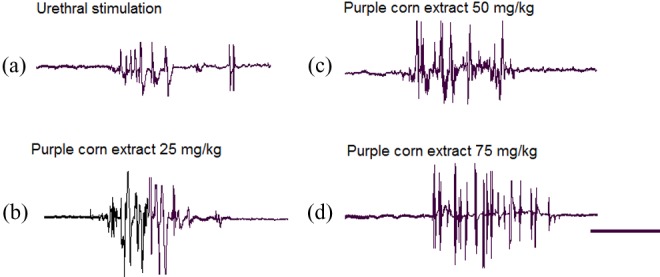
Facilitation of the genital motor pattern of ejaculation in rats under the influence of the purple corn extract, administered at different doses (25, 50, and 75 mg/kg). Electromyographic polygraphic traces obtained after urethral stimulation (a) and after the systemic injection of purple corn extract at the doses of 25 (b), 50 (c), and 75 mg/kg (d), respectively. Calibration bar 10 seconds.
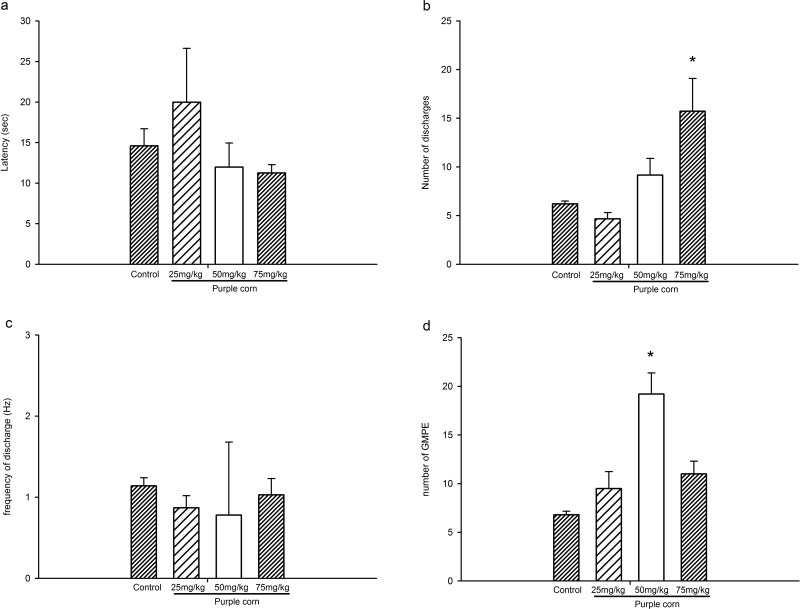
GMPEs elicited by purple corn extract consisted of a motor component that included a first motor train registered in bulbospongiosus muscles (Figure 2b-d), and the analysis of the specific parameters on the GMPE showed that the purple corn extract did not provoke any significant modification in the latency of ejaculation (Figure 3a), but a statistically significant increase in the number of discharges was observed in the animals treated with the highest dose of purple corn extract (P < .05), when compared to control animals (Figure 3b). We observed that the frequency of discharge in the genital motor patterns elicited by the purple corn extract was not significantly modified (Figure 3c). Finally, a trend to increase the number of GMPEs was observed at all tested doses but only the statistically significant increase in this parameter was observed with the 50 mg/kg doses (P < .05; Figure 3d).
Figure 3.
Specific parameters of genital motor patterns of ejaculation registered in the bulbospongiosus muscle under the influence of purple corn extract at different doses (25, 50, and 75 mg/kg). One-way analysis of variance followed by Holm-Sidak test versus Control. *P < .05.
Effects of Purple Corn Extract Administration on the Expression of Ambulatory Behavior
None of the 3 doses of the extract tested had significant effects on the locomotor activity of male rats (see Table 1).
Table 1.
Effects of PO Administration of Purple Corn Extract on the Open-Field Test in Male Ratsa.
| Treatment | Number of Crossed Squares in 5 Minutes |
|---|---|
| Saline solution | 50.12 ± 1.6 |
| Purple corn 25 mg/kg | 51.25 ± 2.2 |
| Purple corn 50 mg/kg | 51.37 ± 1.9 |
| Purple corn 75 mg/kg | 48.12 ± 1.8 |
aNo significant differences among groups were found.
Discussion
Purple corn has multiple uses including its outstanding use as nourishment, though experimental evidence on the medicinal uses of this kernel is incipient. Here we documented that the decoction of this kernel possesses aphrodisiac properties and present results are summarized as follows: (a) administration of the aqueous crude extract of purple corn significantly facilitates the arousal and execution of male rat sexual behavior without affecting the ambulatory activity and (b) this extract facilitates the ejaculatory potency and the ejaculatory capacity in spinal cord transected rats.
In the experimental analysis of male sexual behavior, the concept of the existence of 2 different physiological mechanisms responsible for sexual behavior expression was introduced in the late 1950s by Frank Beach (1956). This notion holds that one of these mechanisms is responsible for sexual arousal and the other for sexual performance.11,17 This concept has been fundamental for the neurobiology of sexual behavior. In the present study, we were interested in establishing, on the one hand, whether purple corn extract possesses sexual stimulant properties, and on the other, if this were the case, on which of the physiological mechanisms of the male rat sexual behavior were exerted. In addition, we were interested in establishing the possibility that the purple corn extract could influence the components of male rat copulatory behavior by acting on the ejaculatory response integrated at the spinal level.
As aforementioned in the introduction, pro-sexual effects of the aphrodisiacs might be exerted at different levels, that is, on sexual motivation (arousal) or performance (potency).10 Findings of the present study in sexually experienced rats treated with the purple corn extract show reductions in the intromission latency in copulating male rats. The experimental analysis of the male rat sexual behavior suggests that significant reductions in the intromission latency can be considered as an enhanced sexual arousal and also as an enhanced ability of the male to intromit.18,19 Accordingly, since this extract reduces the intromission latency, we propose that purple corn extract possesses aphrodisiac activity. Besides, we observed that the purple corn extract increases intromissions at all tested doses, reflecting facilitatory effects of the extract on the ability of the male to intromit. It has been described that as a result of sexual experience, male rats exhibit reductions in the amount of stimulation required to ejaculate.20 Thus, it could be thought that augmenting the intromissions would reveal the inhibitory influence of the purple corn extract on the copulatory efficiency of male rats and then an augmented ejaculatory latency would be expected. On the contrary, we observed that the purple corn extract augments the intromissions in a significant manner while significantly reduces the ejaculation latency (at the doses of 75 mg/kg), suggesting facilitatory influence of this extract on the sexual execution. Several authors have defined facilitation of male rat sexual behavior as a decrease in the number of intromissions preceding ejaculation.19 However, although a reduction in the intromission frequency may be viewed as a facilitation of ejaculation in copulating rats, it should be noted that such a reduction may actually diminish the vaginocervical stimulation necessary for implantation, and thus, may ultimately be viewed as a form of male sexual dysfunction.19 Conversely, we obtained profuse intromissions preceding ejaculation indicative of marked copulatory efficiency in addition to robust ejaculatory trains in spinal rats (vide infra), suggesting increased sexual potency. Accordingly, the present data show facilitatory effects of the purple extract on the copulatory efficiency, since it promotes profuse pre-ejaculatory intromissions.
Also, facilitatory effects of the purple corn extract could be extended to the ejaculatory behavior since we registered significant reductions in the ejaculatory latency (at the doses of 75 mg/kg), which reflect a positive influence of the extract on the ejaculatory mechanism, particularly on the ejaculatory threshold. As a result, we put forward that the aphrodisiac constituent of the purple corn extract can promote pro-ejaculatory effects.
Additionally, we observed that purple corn extract also impacts other components of sexual motivation such as the postejaculatory interval. The postejaculatory interval results from the expression of the ejaculatory response and during this period the male rat exhibits sexual quiescence, with erections and ejaculations inhibited.11 The fact that the postejaculatory interval was extended in all animals treated with the purple corn extract suggests that, after ejaculation, compounds contained in this extract promote a prolonged blockade of the mechanisms directing the resumption of copulation. Therefore, it could be suggested that the purple corn extract possesses aphrodisiac activity that significantly potentiate the expression of ejaculation and its physiological consequences, such as the male postejaculatory refractoriness.
A main effect of the purple corn extract on male rat sexual behavior reported here has to do with the reduction in the ejaculation threshold. We hypothesized that the purple corn extract is acting at the level of the spinal cord to exert its effects. Data of the present study support our hypothesis and establish the potential pro-ejaculatory properties of this extract at the higher tested doses in spinalized male rats. Thus, we observed that the purple corn extract when systemically injected can provoke significant increases in the number of discharges of the ejaculatory motor pattern at the higher doses and in the GMPEs evoked, at the doses of 50 mg/kg, suggesting the enhancement of the ejaculatory potency and capacity of spinal animals, respectively.
The number of discharges in the genital motor pattern of ejaculation is associated with the robustness of the rhythmic motor pattern and increases in this parameter have been paralleled to a facilitated ejaculatory response.21,22 Data reported here show that purple corn extract provokes a significantly increased number of ejaculatory rhythmic discharges after the extract administration (at the high doses), without significant changes in the frequency of discharge. The number of discharges and its frequency contribute to the evaluation of the ejaculatory potency, given that specific changes in these parameters of the motor pattern reflects its refinement. When physiological-like or pharmacological stimuli are applied, changes in the potency could be expected if changes in the number and frequency of discharge were obtained.22 In the present study, we only observed augmented numbers of discharges in the GMPE after purple corn extract administration at the higher doses. Thus, it is probable that these changes in the motor parameters of ejaculatory trains elicited by purple corn extract have to do with possible changes in the functioning of the specific modules of the spinal ejaculation generator controlling the rhythmic output.13 Accordingly, modules of the ejaculation generator driving the final ejaculatory motor output could be targeted by higher quantities of the compounds contained in the purple corn extract facilitating its activity and hence the ejaculatory potency.
Fictive ejaculation is a response that can be repeatedly elicited in the anesthetized and spinal cord transected rat,12–15 and the number of genital motor patterns of ejaculation evoked by sensorial or pharmacological stimulation is associated to the ejaculatory capacity of male rats.22 The ejaculatory capacity of male rats can be defined prior to the establishment of an inhibitory state induced after repeated urethral stimulation.12,13,23 Findings of the present study show that the purple corn extract increases the ejaculatory capacity of spinal male rats, by retarding the establishment of the inhibitory state that results from repeated sensorial stimulation. We do not have an explanation to this interesting result but may propose 2 potential explanations. One possibility is that in spinal rats the increased ejaculatory capacity seen after purple corn extracts administration follows sustained activation of the spinal generator for ejaculation’ pacemaker cells, promoted by compounds contained in extract at the doses of 50 mg/kg, which could retard the establishment of the inhibitory state that results from repeated elicitation of ejaculatory sequences. Additionally, the increased ejaculatory capacity promoted by the compounds of the purple corn extract could be due to the blockade of the mechanisms involved in the building of the inhibitory state resulting from continuous activation of GMPE.
Purple corn contain several compounds of medical importance such as anthocyanins that, utilized as a daily food, exert disease-preventive activities and is considered as functional food.24 Cyanidin-3-glucoside and its malonated derivative are the major anthocyanins present in the purple corn, though several dimalonylated monoglucosides of cyanidin, peonidin, and pelargonidin represent minor constituents.25 It has been shown that the physiological responses induced by purple corn extracts are mainly mediated by nitrergic activity.26 The exact mechanism through which the purple corn aqueous crude extract is exerting its aphrodisiac actions was not investigated in the present study, but it could proposed that its pro-sexual activity, centered on the sexual performance seen in copulating rats and those obtained in an increased ejaculation potency and capacity seen in the fictive ejaculation model, are due to its nitrergic activity. In line with notion, it has been demonstrated that nitric oxide participates in male sexual behavior of rats by increasing the indexes of sexual performance27 and by promoting penile erection in intact male rats.28 Moreover, a significant excitatory role for nitric oxide in the bursting pattern of synchronized discharge generated in autonomic and somatic outflows from the lumbosacral cord by neurons governing ejaculation has been reported.29
All in all, the present data support the notion that the aqueous crude extract of purple corn possesses aphrodisiac activity by acting at the 2 main levels of the central nervous system that control ejaculation, the brain and the spinal cord.
Acknowledgments
We thank Israel Montes for his skillful technical assistance.
Footnotes
Author Contributions: MCJ and MGRS designed the experimental protocol, the animal experiments, and data analyses and prepared the final version of the article. MAF conducted all animal experiments and contributed toward the preparation of the article. HSME participated in the experiments and data analyses and contributed toward the preparation of the article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: MCJ and MGRS received a grant from Universidad Autónoma de Tlaxcala to support the formation and consolidation of academic groups (Grant UATX-CA-215).
Ethical Approval: All animal experimental approaches were performed with ethical approval from the Local Committee of Ethics on Animal Experimentation of Escuela de Medicina Veterinaria y Zootecnia, Universidad Autónoma de Tlaxcala, in compliance with the regulations established in the Mexican Official Norm for the use and care of laboratory animals (NOM-062-ZOO-1999).
References
- 1. Akre C, Berchtold A, Gmel G, Suris JC. The evolution of sexual dysfunction in young men aged 18-25 years. J Adolesc Health. 2014;55:736–743. [DOI] [PubMed] [Google Scholar]
- 2. McMahon CG, Giuliano F, Dean J, et al. Efficacy and safety of dapoxetine in men with premature ejaculation and concomitant erectile dysfunction treated with a phosphodiesterase type 5 inhibitor: randomized, placebo-controlled, phase III study. J Sex Med. 2013;10:2312–2325. [DOI] [PubMed] [Google Scholar]
- 3. Carro-Juárez M, Cervantes E, Cervantes-Méndez M, Rodríguez-Manzo G. Aphrodisiac properties of Montanoa tomentosa aqueous crude extract in male rats. Pharmacol Biochem Behav. 2004;78:129–134. [DOI] [PubMed] [Google Scholar]
- 4. Chaturapanich G, Chaiyakul S, Verawatnapakul V, Pholpramool C. Effects of Kaempferia parviflora extracts on reproductive parameters and spermatic blood flow in male rats. Reproduction. 2008;136:515–522. [DOI] [PubMed] [Google Scholar]
- 5. Sudwan P, Saenphet K, Aritajat S, Sitasuwan N. Effects of Boesenbergia rotunda (L.) Mansf. on sexual behaviour of male rats. Asian J Androl. 2007;9:849–855. [DOI] [PubMed] [Google Scholar]
- 6. Watcho P, Wankeu-Nya M, Nguelefack TB, Tapondjou L, Teponno R, Kamanyi A. Pro-sexual effects of Dracaena arborea (wild) link (Dracaenaceae) in sexually experienced male rats. Pharmacologyonline. 2007;1:400–419. [Google Scholar]
- 7. Watcho P, Modeste WN, Albert K, Carro-Juárez M. Dracaena arborea extracts delay the pro-ejaculatory effect of dopamine and oxytocin in spinal male rats. Int J Impot Res. 2014;26:213–217. [DOI] [PubMed] [Google Scholar]
- 8. Hernandez F. Quatro libros de la naturaleza y virtudes de las plantas y animales que están recevidos en el uso de medicina en la Nueva España, y la método, y corrección y preperación que para administrarllas se requiere con lo que el doctor Francisco Hernández escrivio en lengua latina, Viuda de Diego López Davalos. Ciudad de México, México; 1615. [Google Scholar]
- 9. Biblioteca digital de la Medicina Tradicional Mexicana. www.medicinatradicionalmexicana.unam.mx. Accessed May 1, 2017.
- 10. Sandroni P. Aphrodisiacs past and present: a historical review. Clin Autonom Res. 2001;11:303–307. [DOI] [PubMed] [Google Scholar]
- 11. Hull EM, Rodríguez-Manzo G. Male Sexual Behavior: Hormones, Brain and Behavior. 2nd ed San Diego, CA: Academic Press/Elsevier; 2009. [Google Scholar]
- 12. Carro-Juárez M, Cruz SL, Rodríguez-Manzo G. Evidence for the involvement of a spinal pattern generator in the control of the genital motor pattern of ejaculation. Brain Res. 2003;975:222–228. [DOI] [PubMed] [Google Scholar]
- 13. Carro-Juarez M, Rodríguez-Manzo G. The spinal pattern generator for ejaculation. Brain Res Rev. 2008;58:106–120. [DOI] [PubMed] [Google Scholar]
- 14. Carro-Juárez M, Lobaton I, Benitez O, Espiritu A. Pro-ejaculatory effect of the aqueous crude extract of cihuapatli (Montanoa tomentosa) in spinal male rats. J Ethnopharmacol. 2006;106:111–116. [DOI] [PubMed] [Google Scholar]
- 15. Carro-Juárez M, Rodríguez-Manzo G. Participation of endogenous opioids in the inhibition of the spinal generator for ejaculation in rats. J Sex Med. 2009;6:3045–3055. [DOI] [PubMed] [Google Scholar]
- 16. Estrada-Camarena E, Fernández-Guasti A, López-Rubalcava C. Antidepressant-like effect of different estrogenic compounds in the forced swimming test. Neuropsychopharmacology. 2003;28:830–838. [DOI] [PubMed] [Google Scholar]
- 17. Beach FA. Characteristics of masculine “sex drive.” In: Jones MR, ed. The Nebraska Symposium of Motivation. Lincoln, NE: University of Nebraska Press; 1956. [Google Scholar]
- 18. Bitran D, Hull EM. Pharmacological analysis of male rat sexual behavior. Neurosci Biobehav Rev. 1987;11:365–389. [DOI] [PubMed] [Google Scholar]
- 19. Everitt BR. Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci Biobehav Rev. 1990;14:217–232. [DOI] [PubMed] [Google Scholar]
- 20. Toner JP, Adler NT. The pre-ejaculatory behavior of male and female rats affects the number of sperm in the vagina and uterus. Physiol Behav. 1986;36:363–367. [DOI] [PubMed] [Google Scholar]
- 21. Carro-Juárez M, Franco MÁ, Rodríguez-Peña ML. Increase of the ejaculatory potency by the systemic administration of aqueous crude extracts of cihuapatli (Montanoa genus) plants in spinal male rats. J Evid Based Complementary Altern Med. 2014;19:43–50. [DOI] [PubMed] [Google Scholar]
- 22. Birri MA, Franco MA, Vallejo MG, Carro-Juárez M, Agnese AM. Huperzia saururus facilitates ejaculation in spinal cord transected male rats. J Ethnopharmacol. 2014;157:38–44. [DOI] [PubMed] [Google Scholar]
- 23. Carro-Juárez M, Rodríguez-Manzo G. Sensory and motor aspects of the coital reflex in the spinal male rat. Behav Brain Res. 2000;108:97–103. [DOI] [PubMed] [Google Scholar]
- 24. Carhuapoma MY, López GS. Maíz morado. Moleculas bioactivas antioxidantes y anticancerígenas. Lima, Peru: Centro de Produccion editorial e imprenta de la Universidad Mayor de San Marcos; 2008:65–90. [Google Scholar]
- 25. Cuevas-Montilla E, Hillebrand S, Antezana A, Winterhalter P. Soluble and bound phenolic compounds in different Bolivian purple corn (Zea mays L.) cultivars. J Agric Food Chem. 2011;59:7068–7074. [DOI] [PubMed] [Google Scholar]
- 26. Moreno-Loaiza O, Paz-Aliaga A. Vasodilator effect mediated by nitric oxide of the Zea mays L (Andean purple corn) hydroalcoholic extract in aortic rings of rat [in Spanish]. Rev Peru Med Exp Salud Publica. 2010;27:527–531. [DOI] [PubMed] [Google Scholar]
- 27. Benelli A, Bertolini A, Poggioli R, et al. Nitric oxide is involved in male sexual behavior of rats. Eur J Pharmacol. 1995;294:505–510. [DOI] [PubMed] [Google Scholar]
- 28. Hull EM, Lumley LA, Matuszewich L, Dominguez J, Moses J, Lorrain DS. The roles of nitric oxide in sexual function of male rats. Neuropharmacology. 1994;33:499–1504. [DOI] [PubMed] [Google Scholar]
- 29. Brack KE, Watkins N, Pyner S, Coote JH. A physiological role for nitric oxide in the centrally mediated sympathetic and somatomotor ejaculatory response in anesthetized male Wistar rats. Neuroscience. 2007;150:487–497. [DOI] [PubMed] [Google Scholar]