Abstract
Polysaccharide extracted from the Maitake mushroom (MP) is considered as a potential anticancer agent. The present study was performed to investigate the cytotoxic effects of MP and vitamin C (VC) alone and in combination on the viability of human neuroglioma M059 K cells in vitro. A combination of MP (1.0 mg/mL) and VC (0.4 mmol/L) led to a 53.10% reduction in cell viability and this treatment induced cell cycle arrest at the G2/M phase, and apoptosis occurred in 38.54% of the cells. Results of Hoechst 33258 staining and Western blot showed apoptotic cells appeared and changes in the expression of apoptosis-related proteins (upregulation of Bax and caspase-3, downregulation of Bcl-2, and activation of poly-(ADP-ribose)-polymerase). Moreover, the activities of caspase-3, caspase-8, and caspase-9 were enhanced in M059 K cells. The inhibiting effect of combined treatment with MP and VC on M059 K cells indicates the mechanism of anticancer activity involved induction of cell apoptosis.
Keywords: antitumor, apoptosis, Maitake, vitamin C, neuroglioma
Malignant gliomas and glioblastomas (GBM) are considered to be the most common primary brain tumors in adults, which are closely related to poor quality of life and lamentable prognosis.1,2 Virtually, the current standard of therapy for all patients with high-grade glioma is surgical resection with concurrent radiation and chemotherapy.3 Despite aggressive diagnosis and treatment methods and recent clinical advances, the median survival with gliomas patients is only 15 months and the survival statistics for them have not improved significantly over the past decades.4,5 This highlights the need to establish more effective therapeutic strategies to GBM.
Maitake, usually called Grifola frondosa, is a basidiomycete fungus belonging to the polyporaceae family. Investigation of mechanism of how the biological behavior of tumor cells is affected by this polysaccharide has attracted a lot of research interests in recent years. The study of Martin and Brophy6 showed that Grifola frondosa polysaccharide emerged its anticancer activity by inducing cell apoptosis in breast cancer MCF-7 cells. The same research result was demonstrated by Shomori et al7 where water extract from Maitake induced gastric cancer cell apoptosis. A recent study showed that a sulfate synthesized from Maitake polysaccharide induced liver cancer cell HepG2 apoptosis8 in order to play a role of anticancer effect. Vitamin C (VC), a water-soluble enzyme, usually is regarded as a powerful antioxidant in plants and animals. The effect of it is participation in many processes biological intracellular and extracellular reactions to effectively scavenge free radicals.9 In recent years, researches suggested that VC can reduce the adverse reactions induced by chemotherapy in clinical cancer treatment.10 Leon et al11 showed that VC could add the level of reactive oxygen species (ROS) scavengers and thus underlie the main mechanisms of action of osteosarcoma cell apoptosis. In their study, the performances of cell apoptosis such as disruption of the mitochondria membrane potential (MMP), increased levels of caspase-3 and DNA fragmentation were all observed. Moreover, VC appears to induce tumor cell apoptosis by decreasing the expression of the anti-apoptotic protein p34SEI-1.12
Over the years 2 studies have suggested that Maitake polysaccharide (MP) and VC have a synergistic effect of inducing tumor cell apoptosis. Alexander et al13 have shown that a combination of MP and VC can induce kidney cancer cell apoptosis. Our previous research also has proved this anticancer effect, a kind of MP (D-fraction from Grifola frondosa) and VC have synergistic apoptotic effect on hepatocellular carcinoma SMMC-7721 cells.14 While the combined application of MP and VC can induce apoptosis in some tumor cell lines, further studies examining the anticancer effects of their combination are needed. In this study, the mechanism of antitumor activity of the combination of MP and VC was investigated in human neuroglioma M059 K cells.
Materials and Methods
Reagents
Dulbecco’s modified Eagle medium: Nutrient mixture F-12 (DMEM/F12) was bought from Gibco (Grand Island, NY, USA). Fetal bovine serum (FBS) was purchased from Sijiqing Biological Co (Hangzhou, China). Sodium dodecyl sulfate (SDS), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reagent was bought from Amresco (Cleveland, OH, USA). Annexin V-FITC/PI (propidium iodide) apoptosis detection kit, Cell Cycle detection kit, the fluorescent dye Hoechst 33258 were purchased from BeyGen Biotech Co (Nanjing, China). Anti-Bax (#5023), anti-Bcl-2 (#3041), anti-PRAP(#9542), anti-cleaved-caspase-3 (#5049) were from Cell Signaling Technology (Danvers, MA, USA). Anti-rabbit IgG secondary antibody was from ErWan Biotechnology Co (Shanghai, China). The polysaccharide of Maitake (Grifola frondosa) was purchased from Zelang Biotech Co (Nanjing, China), with a purity of over 90% as proved by high-performance liquid chromatography. VC was bought from Seebio Biotech Co (Shanghai, China).
Cell Line and Culture
The human neuroglioma cell line M059 K was a gift from Dr. Guo in Lanzhou University. Cells were maintained in DMEM/F12 supplemented with 10% heat-inactivated FBS, penicillin (100 U/mL), and streptomycin (100 μg/mL) at 37°C in a 5% CO2 incubator. Cells were seeded at an initial cell density of 6 × 105 cells/mL in 60-mm dishes or T-25 flasks. When cells were treated with varying drug concentrations, the serum concentration of the medium was reduced to 8%.
Cell Viability Assay
The viability of M059 K cells was assessed by the MTT assay. The cells were seeded in 96-well microtiter plates at an initial cell density of 5 × 103 cells/well. After culturing overnight, cells were treated with varying concentrations of MP (0.1, 0.2, 0.4, 0.6, 0.8, and 1.0 mg/mL) or VC (0.1, 0.2, 0.3, 0.4, 0.5 and mmol/L) for 48 hours. Control group and zero adjustment wells were included. MTT was added to each well at a final concentration of 0.5 mg/mL. Dimethyl sulfoxide was added to each well after 4 hours to dissolve the formazan crystals. The absorbance value per well at 490 nm was read using a microplate reader (Bio-Rad, Hercules, CA, USA). The percentage inhibition for proliferation of M059 K cells was calculated according to the formula: (1 − experimental absorbance value/control absorbance value) × 100%. All experiments were performed in triplicate. The IC50 (50% inhibition concentration) of MP and VC was calculated using the data generated from the MTT experiment. Then detections of cell inhibition rate with different concentration of the combination of MP and VC were carried out by using MTT method in order to determine the best combination concentration.
Cell Cycle Analysis and Apoptosis Detection
M059 K cells were treated with medium (without drugs), MP (1.0 mg/mL), VC (0.4 mmol/L), and their combination at the same concentrations for 48 hours. To determine cell cycle distribution, approximately 1 × 106 floating and attached cells were collected, washed twice with cold phosphate- buffered saline (PBS), and fixed in 70% ethanol at 4°C overnight. Then, cells were resuspended in 400 μL propidium iodide solution (containing 0.2 mg/mL RNase and 1% Triton X-100) and incubated in the dark at room temperature for 60 minutes. Cell cycle analysis was performed using a NovoCyte flow cytometer (ACEA Biosciences, San Diego, CA, USA). The Annexin V-FITC/propidium iodide double staining assay was performed on 1 × 105 M059 K cells that were harvested and resuspended in 500 μL binding buffer containing 5 μL Annexin V-FITC and 10 μL propidium iodide. The cell suspension was incubated for 20 minutes at room temperature in the dark. Flow cytometry was used to count cells stained for apoptosis. All experiments were performed in triplicate and the results were analyzed statistically.
Hoechst 33258 Staining
Hoechst 33258 staining was performed to assess apoptosis as reported previously.15 M059 K cells were seeded in 24-well plates (8 × 103 cells/well) and treated with medium alone or medium containing MP (1.0 mg/mL), VC (0.4 mmol/L), or a combination of MP and VC at the same concentrations for 48 hours. The cells were then washed twice with PBS and the nuclei stained with 0.1 mM Hoechst 33258 in the dark for 10 minutes. The cells were washed with flowing water and immediately analyzed under an IX50 fluorescence microscope.
Western Blot Analysis of Apoptosis-Related Proteins
Approximately 2 × 106 cells were harvested and lysed in 200 μL of RIPA lysis buffer to obtain total protein extracts after incubation with control treatment, MP, VC, and MP/VC for 48 hours. Equal amounts of cell lysate (20 µg) were first subjected to 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to a nitrocellulose membrane. The blot membrane was incubated with the primary antibodies against Bcl-2 (1:1500 dilution), Bax (1:1000 dilution), PARP (1:1500 dilution) or caspase-3 (1:1000 dilution) at 4°C overnight, followed by a 30-minute incubation with the secondary antibody conjugates. The Electro Chemi Luminescence (ECL) reagent was used to develop the membrane, which was exposed under X-ray for observing results.
Assay of the Activity of Caspase-3, -8, and -9
After cells were treated with MP (1.0 mg/mL), VC (0.4 mmol/L), and MP/VC for 48 hours, the activities of caspase-3, caspase-8, and caspase-9 in cells were examined. In brief, M059 K Cells were seeded in 10-mm-diameter culture dishes at a density of 6 × 105 cells/mL, 150 μL cell lysis buffer was used to lyse cells on ice for 15 to 30 minutes, followed by centrifugation (4°C, 10 000 rpm, 15 minutes). The resultant supernatants were then used for further evaluation. The activity of caspase enzymes was measured using the caspase activity assay kits according to the manufacturer’s instructions after the total protein concentration was determined using the BCA protein assay kit (Erwan Biotechnology Co, Shanghai, China). The assay was based on the absorbance value of the chromophore p-nitroanilide (pNA) at 405 nm, which is cleaved from DEVD-pNA for activated caspase-3, IETD-pNA for activated caspase-8, and LEHD-pNA for activated caspase-9.
Statistical Analysis
All experiments were performed in triplicate. Data are presented as means ± standard deviation (SD). Statistical analyses were performed with GraphPad Prism software (GraphPad, San Diego, CA, USA) and significant differences between or within the groups were assessed with either one-way analysis of variance or the unpaired Student’s t test. P values less than .05 were regarded as statistically significant. IC50 values were determined by linear regression analyses.
Results
Effects of MP and VC on M059 K Cell Proliferation
M059 K cells were treated separately with different concentration gradient of MP or VC for 48 hours. The results of MTT assay showed that both MP and VC alone had dose-dependent inhibition of cell proliferation. MP produced a 4.66% to 17.28% reduction in cell viability in the dose range of 0.6 to 2.0 mg/mL at 48 hours. And when MP <0.6 mg/mL, it has no inhibitory effect to M059 K cells (Figure 1A). In contrast, VC produced an 8.15% to 57.51% reduction in cellular viability over the dose range of 0.1 to 1.0 mmol/L at 48 hours (Figure 1B). Differences in cell viability rates between the MP or VC treatment group compared with the control group were statistically significant (*P < .05). The IC50 values of MP and VC at 48 hours were 4.73 ± 0.18 mg/mL and 0.98 ± 0.27 mmol/L, respectively.
Figure 1.
Effects of Maitake polysaccharide (MP) or vitamin C (VC) on M059 K cell viability. Cells were treated with different concentrations of MP (0.1-2.0 mg/mL) (A) or VC (0.1-1.0 mmol/L) (B) for 48 hours. Cell inhibition (absorbance at 490 nm) was determined by a MTT assay and compared with control.
Synergistic Cytotoxic Effect of MP and VC
According to previous research, VC has been supposed to play a role of the modulator of bioactivity of β-glucan in MP.16 In this study, various concentrations of combinations of MP and VC were tested in M059 K cells. The results of the MTT assay showed that a synergistic effect was observed when cells were treated with both MP and VC for 48 hours. As shown in Figure 2A, when using 1.0 mg/mL MP (approximate 1/5 IC50) in combination with 0.4 mmol/L VC (2/5 IC50), or 2.5 mg/mL MP (about 1/2 IC50) with 0.4 mmol/L VC (2/5 IC50) inhibited the proliferation of M059 K cells by more than 50%. In addition, as showed by Figure 2B, the 1.0 mg/mL MP and 0.4 mmol/L VC combination produced 55% inhibition while using same concentration of MP or VC alone both had little inhibitory effect (MP: 7.56% ± 1.80% and VC: 14.43% ± 1.02%). So 1.0 mg/mL MP and 0.4 mmol/L VC doses were chosen as the best concentration for following test ultimately.
Figure 2.
Synergy effect of the combinations of Maitake polysaccharide (MP) and vitamin C (VC) to inhibit cell proliferation in M059 K cells. (A) MTT assay of combined application with different concentrations MP and VC. (B) Cell inhibition rate for without drugs, 1.0 mg/mL MP, 0.4 mmol/L VC, and MP/VC. Data are expressed as mean ± SD of 3 separate experiments (*P < .5; **P < .01 compared with control).
MP and VC Promote Apoptosis of M059 K Cells
The Annexin-V-FITC/propidium iodide double staining assay showed that there were 0.294% ± 0.15% apoptotic cells in the control group, increasing to 38.54% ± 2.2% in cells treated with the optimal combination of MP (1.0 mg/mL) and VC (0.4 mM) for 48 hours. In contrast, only 3.153% ± 1.32% and 6.581% ± 0.67% of M059 K cells were apoptotic when treated for 48 hours with MP or VC alone and the apoptosis rate of M059 K cells after MP/VC treatment increased by 12- and 5.86-fold, respectively, compared with using MP or VC alone (Figure 3A).
Figure 3.
Effect of Maitake polysaccharide (MP) or/and vitamin C (VC) on M059 K cell apoptosis. (A) Flow cytometry (FCM) analysis: cell apoptosis was induced by control (without treatment), MP (1.0 mg/mL), VC (0.4 mmol/L), and MP/VC after 48 hours using Annexin V-FITC/propidium iodide staining. The cells are characterized as early apoptosis (bottom right quadrant), late apoptosis (top right quadrant), necrotic (top left quadrant), and healthy cells (bottom left quadrant) based on the FCM results, and the total apoptosis rate is summarized in a bar chart on the right. (B) Histogram of corresponding apoptotic ratios. Data are expressed as mean ± SD of 3 separate experiments. *P < .05; **P < .01 compared with control.
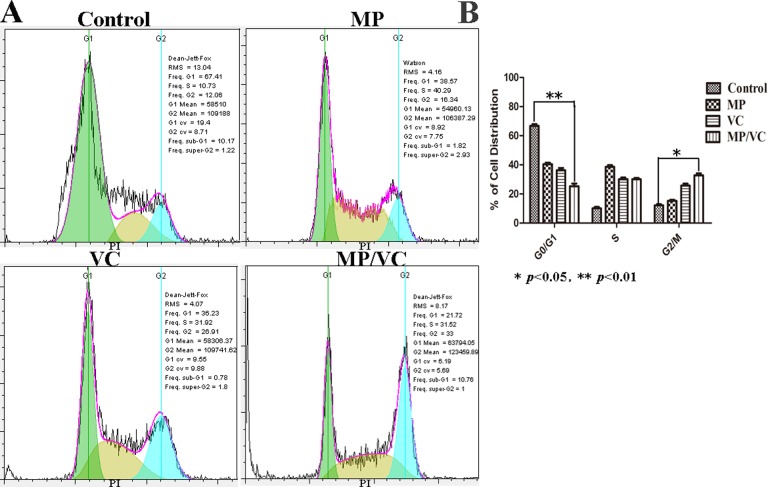
MP/VC Induced Cell Cycle Arrest in the G2/M Phase
Detection of percentages of cells in each phase of the cell cycle reveals how cell proliferation is progressing and, when inhibited, which phase is most affected. As shown in Figure 4A, the cycling of cells in the MP group was mainly unchanged after drug treatment for 48 hours, compared with cells in the control group (the number of cells in G2 phase from 12.06% ± 2.62% to 16.34% ± 2.57%). In contrast, the proportion of cells in the G2 phase increased significantly in cells treated with VC and MP/VC, growth as 26.91% ± 2.62% and 33.00% ± 2.57%, respectively. The cycling of cells treated with the combination MP, VC, and MP/VC also changed significantly, decreasing the number of cells in the G1 phase (from 67.41% to 38.57%, 36.23%, and 21.72%) and increasing the number of cells in the S phase (from 10.73% to 40.29%, 31.92%, and 31.52%). The differences between control, VC, and MP/VC treated cells were statistically significant (*P < .05; **P < .01).
Figure 4.
Effect of Maitake polysaccharide (MP) or/and vitamin C (VC) on cell cycle in M059 K cells. (A) Diagram of cell cycle analyzed by flow cytometry (FCM). Cells were treated with control, Maitake polysaccharide (MP) (1.0 mg/mL), VC (0.4 mmol/L), and MP/VC for 48 hours. (B) Histogram of cell cycle. The results shown are mean ± SD of 3 separate experiments, *P < .05; **P < .01 compared with control.
Changes in Apoptotic Cell Nuclei
Changes of the cell nucleus typical of apoptosis could be observed under a fluorescence microscope after staining cells with Hoechst 33258.17 After M059 K cells were treated with MP, VC, or MP/VC, the nuclei of cells in the control group were intact, the chromatin distributed uniformly, and the cells showed only weak light blue fluorescence (Figure 5A). There was also no obvious change in the nuclei of cells treated with MP alone (Figure 5B). However, some cells treated with VC exhibited migration of the nuclear chromatin toward one side, forming a crescent with strong fluorescence (as indicated by the arrows, Figure 5C). Treatment with the combination of MP and VC resulted in the presence of a large number of cells with typical apoptotic nuclear changes such as nuclear tear, nuclear fragmentation, enhanced fluorescence staining, nuclear chromatin condensation, and the marginalization of the nucleus (Figure 5D-F).
Figure 5.
Detection of apoptosis in M059 K cells after Maitake polysaccharide (MP) or/and vitamin C (VC) treated by fluorescent staining. After cells were treated with control, MP (1.0 mg/mL), VC (0.4 mmol/L), and MP/VC for 48 hours. Hoechst 33258 staining was performed, the stained M059 K cells were immediately examined by fluorescence microscope. (A) Control group. (B) MP treatment group. (C) VC treatment group. (D-F) Apoptotic cells were stained by Hoechst 33258 dye, the morphological changes of nucleus (nuclear tear, nuclear fragmentation, nuclear hyperchromatism, and nuclear enrichment) were indicated by arrows.
Effects of MP, VC, and their Combination on Apoptosis-Related Proteins
To determine the effect of treating M059 K cells with MP or VC on the expression of apoptosis marker proteins, Western blot analyses of the anti-apoptotic protein, Bcl-2, and the pro-apoptotic proteins, Bax, cleaved caspase-3, and PARP were performed. After treatment with MP, VC, or MP/VC for 48 hours, the expression of Bax, cleaved caspase-3, and PARP all increased, while the expression of Bcl-2 decreased (Figure 6). Compared to treatment with MP or VC alone, the changes of protein expression of cells in combination of MP and VC group significantly. These findings are consistent with previous research showing that the upregulation of BAX and PARP, combined with the downregulation of Bcl-2, is indicative of apoptosis.18,19 Thus, it is plausible that combination treatment with MP and VC induces M059 K cell death through apoptosis.
Figure 6.
Effect of Maitake polysaccharide (MP) or/and vitamin C (VC) on expression of apoptosis-related proteins in M059 K cells. Cells were treated with control, MP (1.0 mg/mL), VC (0.4 mmol/L), and MP/VC for 48 hours. (A) Expressions of Bax, Bcl-2, cleaved caspase-3, and PARP were analyzed with Western blotting. In addition, β-actin is shown as a protein loading control. (B) Histogram of Western blotting results. Data are expressed as mean ± SD of 3 separate experiments. # P < .05; ## P < .01 compared with control.
Activity Assay of Caspase-3, Caspase-8, and Caspase-9
As shown in Figure 7, the activity of caspase-3 increased by 1.1-, 1.3-, and 2.1-fold after M059 K cells were treated for 48 hours with MP, VC, and MP/VC, respectively, and these differences were statistically significant compared with controls (# P < .05). The activities of caspase-8 and caspase-9 in M059 K cells both enhanced in our study. However, there was no significant difference in caspase-8 activity between MP-treated or VC-treated and control cells. The change of caspase-9 activity also revealed that caspase-9 activity was not significantly increased in cells compared with controls following treatment with MP alone. Interestingly, the activities of caspase-8 and caspase-9 in cells that were treated with the combination of MP and VC significantly increased compared with cells that were treated in control group after 48 hours (approximately 1.52- and 1.75-fold for caspase-8 and caspase-9, respectively) (# P < .05).
Figure 7.

Activation of caspase-3, caspase-8, and caspase-9 in M059 K cells treated with Maitake polysaccharide (MP), vitamin C (VC), and MP/VC after 48 hours. Caspase-3 activity significantly increased in cells treated with VC and MP/VC. Caspase-8 and caspase-9 activity significantly increased in cells treated with MP/VC. * P < .05 compared with control.
Discussion
With its increasing incidence worldwide, glioma has become a major challenge for the neurosurgeon, because of its complicated pathogenesis mechanisms, unfavorable prognosis, and higher recurrence rate.20 The current clinical treatment efficacy is still unfavorable and the main reason is that glioma cells has intrinsic features of migration and invasion. These features are both correlated with anti-apoptosis and enhanced cell adhesion of tumor cells.21,22 Therefore, the establishment of effective apoptosis-inducing drugs can improve the treatment efficacy of glioma.
The inhibition effect of the combination of the MP and VC on the growth of human neuroglioma M059 K cells were investigated in our study. When MP or VC was applied separately, cell viability was not obviously decreased and the IC50 values of MP was 4.73 mg/mL, which showed that the cell toxicity of MP to M059 K is very small. In addition, the analytical results of MTT indicated that MP (1.0 mg/mL) and VC (0.4 mmol/L) at their respective optimal concentrations have synergistic cytotoxic effects in M059 K cells (55%). As the same as our previous study’s results,14 MP or VC at low concentrations has no direct inhibitory effect on M059 K cells, but MP may influence the basis of cell metabolism, which sensitized the cells to VC. VC can effectively clear reactive oxygen, and regulate oxidation/reduction homeostasis via anti-oxidation pathway. So that the VC play a more important antitumor effect in the process.
In addition, the flow cytometry results revealed that more cell apoptosis (38.54%) was induced by the combination than that by the individual agents and that the number of cells in the G2/M phase of the cell cycle increased (from 12.06% to 33%) compared with controls. The morphological changes associated with apoptosis in cells were observed by fluorescence staining. The overexpression of Bcl-2 and inhibition of Bax expression are closely correlated with anti-apoptosis/apoptosis imbalance of glioma cells.23 This study demonstrated that after MP/VC treatment, the proliferation of glioma cells was remarkably inhibited, enhancing tumor cell apoptosis, decreasing mRNA/protein expression of Bcl-2 while increasing Bax mRNA or protein expression. Meanwhile, the expression of caspase-3 and its endogenous substrate, cleavage of PARP, were enhanced in our study. Furthermore, we also evaluated the activity of caspase-3, caspase-8, and caspase-9 in M059 K cells. The activation levels of these key mediators in the apoptosis pathway were all significantly increased after MP/VC treatment. All these results suggested that the combination of MP and VC induces M059 K cell apoptosis. As shown in Figure 7, treatment of M059 K cells for 48 hours with MP or VC resulted neither increase in caspase-8 nor increase in caspase-9 activity, but they both increased by treating with MP/VC. This result demonstrates that the apoptosis of M059 K cells is induced ultimately by the activation of caspase-3 through the activation of either caspase-8 or caspase-9.
Conclusion
As one of the effective natural compounds for antitumor treatment currently studied in recent years, MP can induce the apoptosis of some human tumor cells. When used in combination with VC, the effect of inducing tumor cell apoptosis is more apparent.24 However, the mechanism has not been illustrated yet. In summary, the combined use of MP and VC has significant antitumor effect and additional studies needed to verify the therapeutic potential of this strategy for treatment of glioblastoma patients.
Acknowledgments
Our sincere appreciation goes to the Evidence-Based Medicine Center, School of Basic Medical Sciences, Lanzhou University, Lanzhou, China.
Authors’ Note: Authors Lei Duan and Xiao-Lu Wu contributed equally to this work.
Author Contributions: LD: Study design, Literature research, experimental studies, Data acquisition and analysis, Statistical analysis, Manuscript writing and editing, Manuscript revision/review.
XLW: Study design, experimental studies, Data interpretation, Manuscript preparation, Manuscript revision/review.
FZ: Literature research, experimental studies, Statistical analysis and Manuscript editing.
RZ: Experimental studies and Data acquisition and analysis.
KHY: Guarantor of integrity of entire study and Manuscript final version approval.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Natural Science Foundation of Gansu Province of China (No. 1308RJZA192) and the Science and Technology Development Project of Lanzhou City (No.2016-3-37).
Ethical Approval: The project received approval from the School of Basic Medical Sciences, Lanzhou University, Lanzhou, China.
References
- 1. Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310:1842–1850. [DOI] [PubMed] [Google Scholar]
- 2. Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro Oncol. 2012;14(suppl 5):v1–v49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnson DR, Galanis E. Medical management of high-grade astrocytoma: current and merging therapies. Semin Oncol. 2014;41:511–522. [DOI] [PubMed] [Google Scholar]
- 4. Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009; 10:459–466. [DOI] [PubMed] [Google Scholar]
- 5. Mitteer RA, Wang Y, Shah J, et al. Proton beam radiation induces DNA damage and cell apoptosis in glioma stem cells through reactive oxygen species. Sci Rep. 2015;5:13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martin KR, Brophy SK. Commonly consumed and specialty dietary mushrooms reduce cellular proliferation in MCF-7 human breast cancer cells. Exp Biol Med (Maywood). 2010;235:1306–1314. [DOI] [PubMed] [Google Scholar]
- 7. Shomori K, Yamamoto M, Arifuku I, Teramachi K, Ito H. Antitumor effects of a water-soluble extract from Maitake (Grifola frondosa) on human gastric cancer cell lines. Oncol Rep. 2009;22(3): 615–620. [DOI] [PubMed] [Google Scholar]
- 8. Wang CL, Meng M, Liu SB, Wang LR, Hou LH, Cao XH. A chemically sulfated polysaccharide from Grifola frondosa induces HepG2 cell apoptosis by notch1-NF-κB pathway. Carbohydr Polym. 2013;95:282–287. [DOI] [PubMed] [Google Scholar]
- 9. Sorice A, Guerriero E, Capone F, Colonna G, Castello G, Costantini S. Ascorbic acid: its role in immune system and chronic inflammation diseases. Mini Rev Med Chem. 2014;14:444–452. [DOI] [PubMed] [Google Scholar]
- 10. Guerriero E, Sorice A, Capone F, et al. Vitamin C effect on mitoxantrone-induced cytotoxicity in human breast cancer cell lines. PLoS One. 2014;9:e115287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leon IE, Di Virgilio AL, Porro V, et al. Antitumor properties of a vanadyl (IV) complex with the flavonoid chrysin [VO(chrysin)2EtOH]2 in a human osteosarcoma model: the role of oxidative stress and apoptosis. Dalton Trans. 2013;42:11868–11880. [DOI] [PubMed] [Google Scholar]
- 12. Lee S, Kim J, Jung S, et al. SIAH1-induced p34SEI-1 polyubiquitination/degradation mediates p53 preferential vitamin C cytotoxicity. Int J Oncol. 2015;46:1377–1384. [DOI] [PubMed] [Google Scholar]
- 13. Alexander B, Fishman AI, Eshghi M, Choudhury M, Konno S. Induction of cell death in renal cell carcinoma with combination of D-fraction and vitamin C. Integr Cancer Ther. 2013;12:442–448. [DOI] [PubMed] [Google Scholar]
- 14. Zhao F, Wang YF, Song L, et al. Synergistic apoptotic effect of D-fraction from Grifola frondosa and vitamin C on hepatocellular carcinoma SMMC-7721 cells [published online May 5, 2016]. Integr Cancer Ther. doi:10.1177/1534735416644674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fu H, Wang QS, Luo Q, et al. Simvastatin inhibits apoptosis of endothelial cells induced by sepsis through upregulating the expression of Bcl-2 and downregulating Bax. World J Emerg Med. 2014;5:291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Briolant S, Garin D, Scaramozzino N, Jouan A, Crance JM. In vitro inhibition of Chikungunya and Semliki Forest viruses replication by antiviral compounds: synergistic effect of interferon-alpha and ribavirin combination. Antiviral Res. 2004;61:111–117. [DOI] [PubMed] [Google Scholar]
- 17. Shi S, Wang Q, Xu J, et al. Synergistic anticancer effect of cisplatin and Chal-24 combination through IAP and c-FLIPL degradation, ripoptosome formation and autophagy-mediated apoptosis. Oncotarget. 2015;6:1640–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yip KW, Reed JC. Bcl-2 family proteins and cancer. Oncogene. 2008;27:6398–6406. [DOI] [PubMed] [Google Scholar]
- 19. Soldani C, Scovassi AI. Poly(ADP-ribose) polymerase-1 cleavage during apoptosis: an update. Apoptosis. 2002;7:321–328. [DOI] [PubMed] [Google Scholar]
- 20. Waghmare I, Roebke A, Minata M, Kango-Singh M, Nakano I. Intercellular cooperation and competition in brain cancers: lessons from Drosophila and human studies. Stem Cells Transl Med. 2014;3:1262–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brower JV, Clark PA, Lyon W, Kuo JS. MicroRNAs in cancer: glioblastoma and glioblastoma cancer stem cells. Neurochem Int. 2014;77:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang L, Shi ZM, Jiang CF, et al. MiR-143 acts as a tumor suppressor by targeting N-RAS and enhances temozolomide-induced apoptosis in glioma. Oncotarget. 2014;5:5416–5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bi D, Yang M, Zhao X, Huang S. Effect of cnidium lactone on serum mutant P53 and BCL-2/BAX expression in human prostate cancer cells PC-3 tumor-bearing BALB/C Nude Mouse Model. Med Sci Monit. 2015;21:2421–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Konno S. Synergistic potentiation of D-fraction with vitamin C as possible alternative approach for cancer therapy. Int J Gen Med. 2009;30:91–108. [DOI] [PMC free article] [PubMed] [Google Scholar]