Abstract
To evaluate the anticonvulsant activity of the aerial parts of Verbena officinalis used traditionally by local Iranians for the treatment of convulsion. The anticonvulsant activity of the extract was assessed in pentylenetetrazole (PTZ) and maximal electroshock (MES) induced seizures in mice. Diazepam was used as reference drug. In addition, for investigating the mechanism of V officinalis in PTZ model, flumazenil and naloxone were injected before V officinalis. The extract showed no toxicity and significantly increased the period taken before the onset and decreased the duration of the seizures induced by PTZ. In the MES test, V officinalis displayed significant reduction in hind limb tonic extension duration in a dose-dependent manner. The results propose that V officinalis ethanolic extract has anticonvulsant activity against seizure. It seems that these effects may be related to potentiating of GABAergic system. Moreover, this study supports the use of this plant by local Iranians in order to treat convulsion.
Keywords: Verbena officinalis, pentylenetetrazole, maximal electroshock, anticonvulsant, traditional Persian medicine
Epilepsy is one of the most serious neurological conditions. Although several anticonvulsant drugs are used to treat seizure attacks, about 30% of patients are medicated incompletely. The current therapeutic management of epilepsy with modern antiepileptic drugs is associated with side effects, dose-related chronic toxicity, and teratogenic effects. Also, it necessitates search for new therapeutic compounds for better treatment of epileptic disorders.1,2 Natural products from folk remedies have contributed significantly in the discovery of modern drugs because they have had potential different mechanisms and lower toxicity.3 Vervain, Verbena officinalis L (Verbenaceae) is a perennial flowering plant native to Iran and Europe, which has nearly a 200-year history of use in traditional folk medicine for the treatment of several diseases. It has been used as sedative, relaxant, nerve tonic, gastroprotective, anti-inflammatory, analgesic, and antidepressant.4–6 The aerial parts of V officinalis have been effectively used to alleviate conditions of anxiety, insomnia, and nervous irritability. Aqueous extract of V officinalis can significantly decrease sleep latency and increase the sleeping time of Sprague-Dawley rats that is reversed by flumazenil.7 Braga et al8 showed a dose-dependent antinociceptive activity of verbena genus that may be due to an agonistic effect of this plant on opioidergic system. Since there are some reports on the use of Vervain for the treatment of central nervous system diseases and its sedative and antinociceptive effects, the anticonvulsant activity and probable involvement of GABAergic and opioid systems of ethanolic extract of V officinalis were evaluated in this study.
Methods
Plant Material
The aerial parts of V officinalis were collected from the Golestan Province, Iran during April 2013. The aerial parts were identified and authenticated by Mis Farahmand, a plant taxonomist at the Iranian Biological Resource Center, where a voucher specimen with the number (1110) was deposited.
Preparation of Verbena officinalis Ethanol Extract (VOEE)
Dried aerial parts were milled into powder using a commercial grinder. The coarse powder (100 g) was extracted with 95% ethanol for 72 hours by refluxing extraction. The extract was then concentrated with a rotary evaporator apparatus at a temperature not exceeding 40°C. For animal experiments, VOEE was dissolved in carboxymethylcellulose 1%.
Animals
The experimental protocols used in this study were approved by the Ethics and Animal Care Committee of Tehran University of Medical Sciences and were performed in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals. Male NMRI mice weighing 26 ± 3 g (Razi Institute, Karaj, Iran) were used in this study. The animals were housed in standard polycarbonate cages in groups of 4 to 5 and kept in a temperature-controlled room (22°C) with a 12-hour light/12-hour dark cycle. Animals acclimated at least 2 days before experiments with free access to food and water. All procedures were carried out in accordance with institutional guidelines for animal care and use. The groups consisted of at least 6 animals, each animal used only once. Additionally, effort was made to reduce animal suffering and to use the number of animals necessary to produce reliable scientific data.
Drugs and Chemicals
Drugs used as follows: pentylenetetrazole (PTZ; Sigma, St Louis, MO, USA), flumazenil ampoule (2 mg/kg) (Roche, Switzerland), diazepam (Chemidaru, Iran), naloxone (Tolid Daru, Iran). PTZ, diazepam, and naloxone were dissolved in normal saline. All compounds were prepared freshly each time and administered intraperitoneally. Maximal electroshock (MES) apparatus (SATADIS-8810, Iran) was used in the present work.
Phytochemical Screening
Phytochemical screening of the ethanolic extract of V officinalis was performed using the following reagents and chemicals: alkaloids with Dragendorff, s reagent, flavonoids with the use of Mg and HCl, tannins with 1% gelatin and 10% NaCl solutions, glycoside with 10% lead acetate and saponins that can produce suds and induce hemolysis reaction.9
Acute Toxicity Study
A toxicity study of the extract was performed according to OECD guideline no. 425. A limit test was performed initially using NMRI mice 26 ± 3 g. Six mice were serially administered with a 2000 mg/kg dose of extract prepared in carboxymethylcellulose 1% as recommended in the guideline. After administration of the dose, each animal was observed every hour for signs of toxicity and abnormality in behavior up to the 48th hour. Subsequent daily observations were made for toxicity and mortality up to 14 days.
Anticonvulsant Activity
Maximal Electroshock Seizure Test
The mice were randomly divided into 5 groups of 6 animals each: (1) negative control group with normal saline (10 mL/kg), (2) positive control group with diazepam (1 mg/kg),3–5 and ethanolic extract–treated groups (100, 200, and 400 mg/kg, respectively). The mice were given ethanolic extract and controls, intraperitoneally, 30 minutes prior to the induction of MES. Tonic convulsions were induced in 99.99% of the untreated animals by alternative electrical current (50 mA, 50 Hz, 1-second duration) through ear-clip electrodes using a stimulator apparatus. A drop of 0.9% saline solution was applied on each ear of the animal prior to placing the electrode. The duration of hind limb tonic extension was recorded.10,11
PTZ-Induced Seizure
The mice were divided into groups of 6 animals each. In 3 groups, the mice were given VOEE at the doses (100, 200, 400 mg/kg) by oral gavage 30 minutes before the administration of PTZ (90 mg/kg, intraperitoneally), 2 groups were injected diazepam (1 mg/kg, intraperitoneally) and normal saline 30 minutes before the administration of PTZ (90 mg/kg, intraperitoneally).10 Each animal was placed into an individual plastic cage for observation lasting 30 minutes. The general clonus was characterized by forelimb clonus followed by full clonus of the body. The time taken before the onset of clonic convulsion, the duration of clonic convulsion, the percentage of seizure and mortality protection were recorded.12
The Effect of Flumazenil on the Anticonvulsant Activity of VOEE
We also studied the effects of a selective benzodiazepine receptor antagonist, flumazenil on the anticonvulsant of VOEE in order to investigate the probable involvement of benzodiazepine receptors.13 We selected 6 groups of 6 mice each. In the first group, mice were given flumazenil (2 mg/kg, intraperitoneally) 5 minutes before the administration of VOEE (400 mg/kg, oral gavage) and 35 minutes before the injection of PTZ. In the second group, the animals received flumazenil (2 mg/kg) 5 minutes before the administration of diazepam (1 mg/kg). Also, 3 groups were injected diazepam (1 mg/kg, intraperitoneally), flumazenil (2 mg/kg), and normal saline 30 minutes before the administration of PTZ (90 mg/kg, intraperitoneally), respectively.12–15 The anticonvulsant activity of VOEE and diazepam in mice pretreated with flumazenil assessed and compared with normal saline (10 mL/kg), flumazenil (2 mg/kg), diazepam (1 mg/kg), and VOEE (400 mg/kg) treated animals.
The Effect of Naloxone on the Anticonvulsant Activity of VOEE
We selected 4 groups of 6 mice each for further investigation of the probable modulatory activities of opioid receptors on the anticonvulsant activity of VOEE.16,17 naloxone was injected as an opioid receptor antagonist at a dose of (5 mg/kg) 5 minutes before the administration of VOEE (400 mg/kg) and 35 minutes before the injection of PTZ in group of 6 mice each.12–15 The anticonvulsant activity of VOEE in groups pretreated with naloxone was assessed and compared with animals pretreated only with VOEE (400 mg/kg), naloxone (5 mg/kg), and normal saline (10 mL/kg) groups.
Statistical Analysis
The results are reported as mean ± standard error of the mean. The statistical analyses were performed using 1-way analysis of variance by Graph Pad Prism 5.0 software. Group differences were calculated by post hoc analysis using Tukey test. For all tests, differences with values of P < .05 were considered significant.
Results
Acute Toxicity Study
In the acute toxicity study, neither death nor any observable neurobehavioral effects were observed in the limit test. Because of the lack of observable toxicity, no LD50 determination was carried out. This suggests that the plant extract is relatively safe or nontoxic in mice at the doses given.
Phytochemical Screening
Preliminary phytochemical screening of the extract indicated the presence of alkaloids and glycoside. However, flavonoids, tannins, and saponins were absent.
Anticonvulsant Activity
Maximal Electroshock Test
In the MES test, administration of the ethanolic extract at doses of 100, 200 mg/kg (P < .01) reduced the duration of hind limb tonic extension compared with saline control group. The VOEE at dose of 400 mg/kg (P < .001) was as effective as diazepam (1 mg/kg) in reduction of hind limb tonic extension time compared to saline control group (Figure 1).
Figure 1.
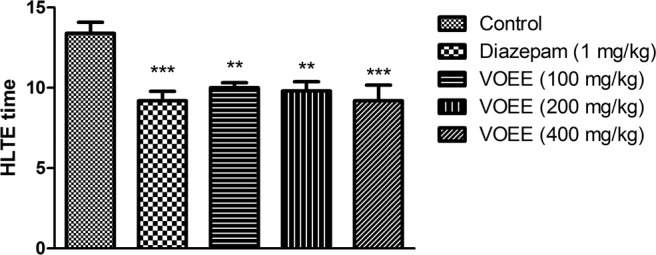
The effect of Verbena officinalis ethanol extract (VOEE) on maximal electroshock–induced seizure in mice. The ethanolic extracts (100, 200, 400 mg/kg, per os) and diazepam (1 mg/kg, intraperitoneally) administered 30 minutes prior to induction of maximal electroshock seizure. Values are mean ± standard error of the mean for 6 mice. **P < .01, ***P < .001 compared with the saline group. HLTE, hind limb tonic extension.
PTZ-Induced Seizure
VOEE only at the dose of 400 mg/kg prolonged the onset time of seizure (P < .05) and decreased the duration of seizures compared to saline group (P < .01) (Table 1). As shown in Table 1, VOEE displayed its protection against seizure in a dose-dependent manner. Moreover, diazepam prolonged the latency and shortened the duration of seizures compared with the saline group (Table 1).
Table 1.
The Effects of VOEE on PTZ-Induced Convulsion in Mice.a
| Treatment (Dose) | Onset (s) | Duration (s) | Seizure Protection (%) | Mortality Protection (%) |
|---|---|---|---|---|
| Normal saline (10 mL/kg) | 57.2 ± 9.45 | 19.20 ± 0.8 | 0 | 0 |
| Diazepam (1 mg/kg) | 405.6 ± 35.9** | 6 ± 0.45*** | 100 | 100 |
| VOEE (100 mg/kg) | 95.8 ± 15.42 | 17 ± 1.63 | 16 | 33 |
| VOEE (200 mg/kg) | 257.2 ± 102.4 | 14.8 ± 0.97* | 33 | 67 |
| VOEE (400 mg/kg) | 333 ± 77.40* | 10.4 ± 0.25*** | 83 | 100 |
Abbreviations: PTZ, pentylenetetrazole; VOEE, Verbena officinalis ethanol extract.
aNormal saline, diazepam, and VOEE were administered 30 minutes before the injection of PTZ (90 mg/kg, intraperitoneally). Values are mean ± standard error of the mean for 6 mice.
*P < .05, **P < .01, ***P < .001 compared with the saline group.
The Effect of Flumazenil on the Anticonvulsant Activity of VOEE
In the PTZ-induced seizure model, the administration of flumazenil (2 mg/kg) 5 minutes before VOEE (400 mg/kg) reversed the effect of VOEE in prolonging seizure latency and reducing the duration of seizures. There was no significant difference between the latency and duration of seizures in mice receiving VOEE (400 mg/kg) pretreated with flumazenil and the saline group. Therefore, flumazenil could reverse the anticonvulsant activity of diazepam (Table 2).
Table 2.
The Effect of Flumazenil on the Anticonvulsant Activity of VOEE and Diazepam in PTZ-Induced Convulsion in Mice.a
| Treatment (Dose) | Onset (s) | Duration (s) | Mortality Protection (%) |
|---|---|---|---|
| Normal saline (10 mL/kg) | 57.2 ± 9.45 | 19.20 ± 0.8 | 0 |
| Flumazenil (2 mg/kg) | 48 ± 1.9 | 17 ± 0.55 | 0 |
| Diazepam (1 mg/kg) | 405.6 ± 35.96*** | 6 ± 0.45*** | 100 |
| Diazepam + Flumazenil | 169.2 ± 1.16 | 17.8 ± 1.47 | 67 |
| VOEE (400 mg/kg) | 333 ± 77.40*** | 10.4 ± 0.25*** | 100 |
| VOEE + Flumazenil | 103.2 ± 22.55# | 18 ± 0.55## | 50 |
Abbreviations: PTZ, pentylenetetrazole; VOEE, Verbena officinalis ethanol extract.
aNormal saline, diazepam, and VOEE were administered 30 minutes before the injection of PTZ (90 mg/kg, intraperitoneally). Flumazenil was administered 35 minutes before the injection of PTZ (90 mg/kg, intraperitoneally). Values are mean ± standard error of the mean for 6 mice.
***P < .001 compared with the saline group. # P < .01, ## P < .001 compared with the VOEE (400 mg/kg) group.
The Effects of Naloxone on the Anticonvulsant Activity of VOEE
Pretreatment of mice with naloxone (5 mg/kg) 5 minutes before the administration of the VOEE (400 mg/kg) reversed the reduction in seizure duration and the prolongation in seizure latency (Table 3).
Table 3.
The Effect of Naloxone on the Anticonvulsant Activity of VOEE in PTZ-Induced Convulsion in Mice.
| Treatment (Dose) | Onset (s) | Duration (s) | Mortality Protection (%) |
|---|---|---|---|
| Normal saline (10 mL/kg) | 57.2 ± 9.45 | 19.20 ± 0.8 | 0 |
| Naloxone (5 mg/kg) | 83.6 ± 22.83 | 16.8 ± 2.04 | 0 |
| VOEE (400 mg/kg) | 333 ± 77.40* | 10.4 ± 0.25*** | 100 |
| VOEE + Naloxone | 235 ± 82.25 | 12 ± 0.45 | 50 |
Abbreviations: PTZ, pentylenetetrazole; VOEE, Verbena officinalis ethanol extract.
aNormal saline and VOEE were administered 30 minutes before the injection of PTZ (90 mg/kg, intraperitoneally). Naloxone was administered 35 minutes before the injection of PTZ (90 mg/kg, intraperitoneally). Values are mean ± standard error of the mean for 6 mice.
*P < .05, ***P < .001 compared with the saline group.
Discussion
The present study demonstrated the anticonvulsant effects of the ethanolic extract of aerial parts of V officinalis in chemically and electrically induced seizures in mice. The extract exhibited dose-dependent protection in both MES and PTZ tests. In the MES test, V officinalis extract showed significant effect in reducing hind limb tonic extension duration. Likewise, data showed that the ethanolic extract of V officinalis displayed anticonvulsant effect in the PTZ-induced seizure model. Thus, V officinalis has protective activity against both grand mal and petit mal epilepsy. To the best of our knowledge, our study is the first report about anticonvulsant effect of this plant in biomedical literature. The anticonvulsant activity of V officinalis may be due to the presence of glycoside and alkaloids, which have been found in its ethanolic extract by phytochemical screening. Some studies have shown the anticonvulsant effect of alkaloids such as piperine and berberine in animal model.18,19 Thus, unidentified alkaloids are speculated to account for the observed anticonvulsant effect of the plants extract. The findings of the study appear to suggest that VOEE could be used as a natural supplementary remedy in the management and/or control of partial and generalized seizures in order to reduce the toxic effects of synthetic anticonvulsant drugs. PTZ is the chemical substance inducing clonic seizure that blocks transmission of GABA.20 To investigate the possible interaction between GABAergic system and anticonvulsant activity of V officinalis, flumazenil, a benzodiazepine receptor antagonist was used.13 As shown in Table 2, flumazenil reversed the effects of V officinalis on seizure latency and duration of clonic seizure in the PTZ model. It is considerable that the anticonvulsant effect of V officinalis extract is antagonized by an antagonist of benzodiazepine receptor. Therefore, this effect may be related to benzodiazepine receptor activation. This result is similar to previous finding by Akanmu et al.4 They found that hypnotic effects of one verbena genus (Verbena hastate) were related to benzodiazepine receptor activation. Moreover, their study results showed the scientific validity for the use of verbena genus as a sedative substance.
We also found another mechanism for the anticonvulsant effects of V officinalis. As shown in Table 3, naloxone antagonized the effect of V officinalis on decreasing the duration and increasing the latency of clonic seizures in PTZ model compared with the saline group. The results of this study are in accordance with previous research about the activation of opioid receptor.14 The previous animal studies demonstrated the anticonvulsant activity of kappa opioid receptor (KOPr) against bicuculline, maximal electroshock and excitatory amino acid–induced convulsion. Moreover, KOPr agonist attenuated the kindling seizures produced by repeated administration of PTZ.21–24 On the other hand, some investigations showed that the anticonvulsant activity of KOPr agonist may be related to enhancement of GABAergic21 and attenuation of glutamatergic activity.25,26 Thus, there is one hypothesis that V officinalis could activate kappa opioid and benzodiazepine receptors and ultimately potentiate GABA transmission in central nervous system. However, there is limitation about our present information of the chemical constituent of V officinalis aerial parts. It is, therefore, impossible for us at this stage, to identify any other possible anticonvulsant constituent/s apart from the glycosides and alkaloids shown to be present in this plant. In addition, we imagine that the glycosides and alkaloids present in the plant aerial parts may account for the observed anticonvulsant effect of V officinalis, but there are no sufficient scientific data at present to verify this imagination. However, more research is needed to evaluate this speculation.
In conclusion, the present study provides scientific evidence for the use of aerial parts of V officinalis in the treatment of seizure disorders between local Iranians. It was further shown that the anticonvulsant activity of V officinalis may be due to the agonistic activity on BZDs and KOPr receptors that may lead to increase GABA transmission in the central nervous system. Therefore, this plant can be utilized as a therapeutic agent and/or supplement for treating epilepsy.
Footnotes
Author Contributions: The work presented in this article was carried out through collaboration between all authors. SMR and ARD made the initial hypothesis. All authors participated in defining the research theme and providing the proposal. AR, FM, and SM perform the experiments, collected the data, analyzed the data, and wrote the article. SMR, ARD, and SEM conceptualized the study, critically analyzed and discussed the data, and corrected and reviewed the article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was a part of a PharmD thesis by Fatemeh Kazemi, which was supported by Pharmaceutical Sciences Branch, Islamic Azad University (Grant Number: 94-169).
Ethical Approval: The experimental protocols used in this study were approved by the Ethics and Animal Care Committee of Tehran University of Medical Sciences and were performed in accordance with the US National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (NIH publication no. 85.23, revised 1985).
References
- 1. Poole K, Moran N, Bell G, et al. Patients’ perspectives on services for epilepsy: a survey of patient satisfaction, preferences and information provision in 2394 people with epilepsy. Seizure. 2000;9:551–558. [DOI] [PubMed] [Google Scholar]
- 2. Luszczki JJ, Czuczwar P, Cioczek-Czuczwar A, Czuczwar SJ. Arachidonyl-2′-chloroethylamide, a highly selective cannabinoid CB1 receptor agonist, enhances the anticonvulsant action of valproate in the mouse maximal electroshock-induced seizure model. Eur J Pharmacol. 2006;547:65–74. [DOI] [PubMed] [Google Scholar]
- 3. Raza M, Shaheen F, Choudhary MI, et al. Anticonvulsant effect of FS-1 subfraction isolated from roots of Delphinim denudatum on hippocampal pyramidal neurons. Phytother Res. 2003;17:38–43. [DOI] [PubMed] [Google Scholar]
- 4. Akanmu MA, Honda K, Inoue S. Hypnotic effects of total aqueous extracts of Vervain hastata (Verbenaceae) in rats. Psychiatry Clin Neurosci. 2002;56:309–310. [DOI] [PubMed] [Google Scholar]
- 5. Speroni E, Cervellati R, Costa S, et al. Effects of differential extraction of Verbena officinalis on rat models of inflammation, cicatrization and gastric damage. Planta Med. 2007;73:227–235. [DOI] [PubMed] [Google Scholar]
- 6. Zargari A. Medicinal Plants. Tehran, Iran: Tehrari University Publications; 1995. [Google Scholar]
- 7. Lai S-W, Yu M-S, Yuen W-H, Chang RC-C. Novel neuroprotective effects of the aqueous extracts from Verbena officinalis Linn. Neuropharmacology. 2006;50:641–650. [DOI] [PubMed] [Google Scholar]
- 8. Braga VF, Mendes GC, Oliveira RT, et al. Micropropagation, antinociceptive and antioxidant activities of extracts of Verbena litoralis Kunth (Verbenaceae). An Acad Bras Cienc. 2012;84:139–148. [DOI] [PubMed] [Google Scholar]
- 9. Trease G, Evans W. A Textbook of Pharmacognosy. London, England: Bailliare Tindall; 1983. [Google Scholar]
- 10. Hosseinzadeh H, Madanifard M. Anticonvulsant effects of Coriandrum sativum L. seed extracts in mice. Arch Iran Med. 2000;3:81–84. [Google Scholar]
- 11. Mandegary A, Arab-Nozari M, Ramiar H, Sharififar F. Anticonvulsant activity of the essential oil and methanolic extract of Bunium persicum (Boiss). B. Fedtsch. J Ethnopharmacol. 2012;140:447–451. [DOI] [PubMed] [Google Scholar]
- 12. Vogel H, Vogel W. Drug Discovery and Evaluation, Pharmacological Assays. Berlin, Germany: Springer-Verlag; 1997. [Google Scholar]
- 13. File SE, Pellow S. Intrinsic actions of the benzodiazepine receptor antagonist Ro 15-1788. Psychopharmacology. 1986;88:1–11. [DOI] [PubMed] [Google Scholar]
- 14. Nassiri-Asl M, Shariati-Rad S, Zamansoltani F. Anticonvulsant effects of aerial parts of Passiflora incarnata extract in mice: involvement of benzodiazepine and opioid receptors. BMC Complement Altern Med. 2007;7:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hosseinzadeh H, Parvardeh S. Anticonvulsant effects of thymoquinone, the major constituent of Nigella sativa seeds, in mice. Phytomedicine. 2004;11:56–64. [DOI] [PubMed] [Google Scholar]
- 16. Lauretti GR, Ahmad I, Pleuvry B. The activity of opioid analgesics in seizure models utilizing N-methyl-dl-aspartic acid, kainic acid, bicuculline and pentylenetetrazole. Neuropharmacology. 1994;33:155–160. [DOI] [PubMed] [Google Scholar]
- 17. Kaminski RM, Witkin JM, Shippenberg TS. Pharmacological and genetic manipulation of kappa opioid receptors: effects on cocaine-and pentylenetetrazol-induced convulsions and seizure kindling. Neuropharmacology. 2007;52:895–903. [DOI] [PubMed] [Google Scholar]
- 18. Bukhari IA, Pivac N, Alhumayyd MS, Mahesar AL, Gilani AH. The analgesic and anticonvulsant effects of piperine in mice. J Physiol Pharmacol. 2013;64:788–794. [PubMed] [Google Scholar]
- 19. Bhutada P, Mundhada Y, Bansod K, Dixit P, Umathe S, Mundhada D. Anticonvulsant activity of berberine, an isoquinoline alkaloid in mice. Epilepsy Behav. 2012;18:207–210. [DOI] [PubMed] [Google Scholar]
- 20. Riazi K, Honar H, Homayoun H, et al. Sex and estrus cycle differences in the modulatory effects of morphine on seizure susceptibility in mice. Epilepsia. 2004;45:1035–1042. [DOI] [PubMed] [Google Scholar]
- 21. Yajima Y, Narita M, Takahashi-Nakano Y, et al. Effects of differential modulation of μ-, δ-and κ-opioid systems on bicuculline-induced convulsions in the mouse. Brain Res. 2000;862:120–126. [DOI] [PubMed] [Google Scholar]
- 22. Manocha A, Mediratta PK, Sharma KK. Studies on the anticonvulsant effect of U50488 H on maximal electroshock seizure in mice. Pharmacol Biochem Behav. 2003;76:111–117. [DOI] [PubMed] [Google Scholar]
- 23. VonVoigtlander P, Hall E, Ochoa MC, Lewis RA, Triezenberg HJ. U-54494A: a unique anticonvulsant related to kappa opioid agonists. J Pharmacol Exp Ther. 1987;243:542–547. [PubMed] [Google Scholar]
- 24. Becker A, Braun H, Schröder H, Grecksch G, Höllt V. Effects of enadoline on the development of pentylenetetrazol kindling, learning performance, and hippocampal morphology. Brain Res. 1999;823:191–197. [DOI] [PubMed] [Google Scholar]
- 25. Rawls SM, McGinty JF. κ Receptor activation attenuates l-trans-pyrrolidine-2, 4-dicarboxylic acid-evoked glutamate levels in the striatum. J Neurochem. 1998;70:626–634. [DOI] [PubMed] [Google Scholar]
- 26. Wagner JJ, Caudle RM, Chavkin C. Kappa-opioids decrease excitatory transmission in the dentate gyrus of the guinea pig hippocampus. J Neurosci. 1992;12:132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]


