Abstract
Plants are an important source of natural active products that are different, based on mechanism and biological properties. Celery (Apium graveolens L) is a plant from the apiaceae family and phenolic and antioxidant compounds of this plant have been studied by several scientists. The aim of this study was to review systematically the antioxidant activity of celery. Required articles were searched from databases such as Science Direct, PubMed, Scopus, and Springer. Keywords used in this study were Apium graveolens L, celery, antioxidant, free radical, leaf, and seed. Out of 980 collected articles (published in the period 1997-2015), 9 studies finally met the inclusion criteria and were considered. Celery, because of compounds such as caffeic acid, p-coumaric acid, ferulic acid, apigenin, luteolin, tannin, saponin, and kaempferol, has powerful antioxidant characteristics, to remove free radicals. It is clear that celery, with different compounds and diverse concentration can have varied healing effects. It is suggested that the next studies concentrate on other therapeutic and industrial attributes of celery.
Keywords: Apium graveolens L, celery, antioxidant, free radical, leaf
Use of medicinal plants to treat common ailments has been prevalent since ancient times and different parts of the plants were used for public health. The use of natural treatments is cost-effective.1,2 Since ancient times, plants have been important in reducing pain and today the focus is on their role and ability in healing and their treatment properties for various diseases.3–5 Many studies have shown the positive effects of various herbs and medicinal plants on infertility,6 hormone disorders,7 liver disorders,8 anemia,9 renal diseases,10 and neurologic and mental disorders.11
Flavonoids and other phenolic compounds spread widely in plants, and their diverse biological activities such as antioxidant effects have been investigated in many studies such as coronary heart diseases,7,12 diabetes,13 and cancer.14 Medicinal herbs have fewer side effects than chemical drugs and their antioxidant attributes decrease the toxicity of these drugs.7 Today herbal drugs are used as an alternative to chemical drugs and the main reason is their low level of side effects compared with chemical drugs.15
Celery (Apium graveolens L) is a plant from the apiaceae family, and is one of the annual or perennial plants that grow throughout Europe and the tropical and subtropical regions of Africa and Asia16 (Figure 1).
Figure 1.
Apium graveolens L.
In India, approximately 40 000 tons of celery are produced and 29 250 tons are exported each year. Celery for its growth needs high levels of moisture, but requires lower temperature. Therefore, the highest quality celery is found growing in cold and mild environments.17 The parts that are used in this plant include seeds, leaves, and essential oils.18 Among the phytochemical compounds of celery, one can mention carbohydrates, phenols such as flavonoids, alkaloids, and steroids.19
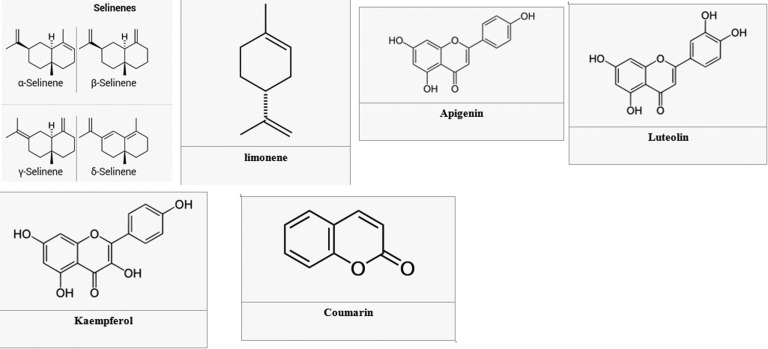
Presence of compounds such as limonene, selinene, frocoumarin glycosides, flavonoids, and vitamins A and C are the reason that celery is the most widely used plant in traditional medicine.20 Some main constituents of celery with chemical structures are shown in Figure 2.
Figure 2.
Some of the main constituents of celery.
Celery can prevent cardiovascular diseases,21 jaundice, liver and lien diseases,22 urinary tract obstruction,18 gout,22 and rheumatic disorders.23 Research on rats shows that ethanol extracts of celery leaves increases spermatogenesis24 and also improves their fertility.15,25,26 Celery reduces glucose, blood lipids,7,27 and blood pressure, which can strengthen the heart.28 Experimental studies show that celery has antifungal29 and anti-inflammatory properties.30 Moreover, its essential oils have antibacterial effects.31 Its seeds are useful in the treatment of bronchitis,22 asthenopia, asthma, chronic skin disorders, including psoriasis,32 vomiting, fever, and tumors.33 The root of the celery is diuretic and it is used for the treatment of colic.33 Plants are an important source of natural active products, which vary, based on mechanism and biological properties. Various phytochemical compounds, especially polyphenols (such as flavonoids, phenolic acids, and tansipropanoids) are responsible for collecting free radicals and antioxidant activities of plants.34 Polyphenols have biological effects. These effects, especially the antioxidant activities, are inductors for restraining free radicals and peroxidation. Polyphenols generally exhibit similar chemical properties, which means that one or more number of the phenolic groups can react with hydrogen donors and neutralize free radicals.35 Many studies examine the effects of celery antioxidants. Phenolic and antioxidant compounds of celery have been studied by several scientists.36 Celery root and its leaves have the property to remove OH and DPPH (2,2-diphenyl-1-picrylhydrazyl) radicals and the plant also reduces the intensity of liposomal peroxidation that represents the plant’s protection.37 The aim of this study was to review systematically the antioxidant activity of celery.
Method
Search Strategy
In order to collect data, the following keywords were searched separately or in combination in domestic databases such as Magiran, Sid, and Iranmedex, and the international databases of Science Direct, PubMed, Scopus, and Springer: Apium graveolens L, celery, antioxidant, free radical, leaf, and seed. The search was limited to articles in Persian and English, and the time period was the year 1997 to December 2015.
Study Selection and Data Extraction
Investigation and information gathering was conducted by 2 experts. Experimental studies and clinical trials met the inclusion criteria. In the first stage, 980 articles were collected. After reviewing titles and abstracts, the unrelated articles were deleted. In addition, the studies lacking full information, and the abstracts of conference proceedings were also eliminated. Finally, 9 studies met the inclusion criteria. According to the research type, the findings of studies were divided into 2 parts: in vitro and in vivo. Table 1 summarizes the findings of the reviewed studies.
Table 1.
Summary of Antioxidant Activity of Celery.
| Type of Extract | Used Part(s) | Model | Dose | Results | Reference |
|---|---|---|---|---|---|
| Aqueous extract | Root and leaves | In vivo | 1.5 mg/kg |
|
38 |
| Aqueous extract | Seed | In vivo | 60 mg/kg |
|
39 |
| Ethanolic extract | Leaves | In vivo and in vitro | 50-600 μg/mL |
|
40 |
| Methanolic and ethanolic extracts | Leaves | In vitro | 70% and 50% |
|
36 |
| Ethanolic extract | All of the parts | In vitro | — |
|
41 |
| — | Leaves | In vitro | 0, 25, 50, 100, 200, and 500 μg/0.05 mL |
|
42 |
| Methanolic, diethyl ether, and aqueous extracts | Seeds | In vitro | 20, 40, 60 and 80 μg/mL |
|
43 |
| Methanolic, ethanol, and hexane extracts | — | In vitro | — |
|
44 |
| Aqueous extract | Seeds | In vitro | 50, 100, and 150 ppm |
|
45 |
Abbreviations: CAT, catalase; FRAP, ferric reducing antioxidant power; GSH, reduced glutathione; GSH-PX, glutathione peroxidase; LPF, lipofuscin; MDA, malondialdehyde; SOD, superoxide dismutase; TAOC, total antioxidant capacity.
Results and Discussion
In Vivo Studies
The study by Kolarovic et al38 of the antioxidant activity of celery in treated rats with doxorubicin, was investigated and furthermore, the effect of the celery itself, and its combination with doxorubicin on the statue of antioxidant was studied. In this study, reduced glutathione (GSH) and ferric reducing antioxidant power (FRAP) in liver homogenate and blood hemolysate, as well as the contents of cytochrome P450 in liver homogenate were investigated. Results showed that doxorubicin significantly reduced glutathione content and total antioxidant status (FRAP) in liver homogenate and blood, but it does not have any effect on the cytochrome p540 content, while these items after treatment with celery juice and celery roots, had significantly increased. Celery root water increases antioxidant capacity and content of GSH and total antioxidant capacity (FRAP) in liver homogenate. Water of celery leaves increases GSH content but it does not have any effect on FRAP in liver homogenate. Water of the roots and leaves of celery, in combination with doxorubicin, have protective effects, which increase FRAP in the liver homogenate compared to animals treated with doxorubicin. Generally, the results of this study indicated that celery can have antioxidant effects.38 In the study by Al Sa’aidi et al,39 the potential of n-butanol extract from celery seed (Apium graveolens) in ameliorating of lipid peroxidation and its antioxidant properties in diabetic rats by streptozotocin was investigated. In this study, there were 32 male rats divided into 4 groups, including a control group and 3 diabetic groups. An amount of 60 mg/kg of aqueous extract of n-butanol was administered to the diabetic group, and 4 IU/animal insulin injected respectively for 21 days. On day 22, weight gain was recorded and male rats were killed. Liver blood and intracellular liquid were used for investigating blood glucose level and subcellular activity of alanine aminotransferase (ALT), aspartate aminotransferase (AST), catalase (CAT), superoxide dismutase (SOD), glutathione (GSH) transferase and reductase were assessed, along with the density of malondialdehyde (MDA) and glutathione.
Results were that these diabetic rats had hyperglycemia, weight loss, increased activity of ALT, SOD, CAT, GSH, reduced GSH reductase, and normal AST. Treatment with n-butanol extract from celery seed modified the glycemic and insulin level and increased body weight in the normal range and normal activity of all antioxidant enzymes. This study concluded that n-butanol extracts from celery seed has significant potential for the treatment of diabetic rats and also improving antioxidant enzymes in diabetic rats.39 Another study, conducted in 2014 by Li et al40 investigated the in vivo and in vitro effect of antioxidant flavonoids isolated from celery. In this study, flavonoid from leaves was extracted and purified and then its ethanol extract of celery, by column chromatography and crystallization were produced. This product was identified as apiin by liquid chromatography/electrospray ionizations/mass spectrometry and its antioxidant activities in in vitro inhibition of DPPH˙, superoxide mg/mL for O2 −˙ and hydroxyl radical (OH˙) were activated. The IC50 value was 68.0 μg/mL for DPPH, 0.39 mg/mL for O2 −˙ and 48.0 μg/mL for the OH˙. Also, the antioxidant activity was evaluated in murine models in vivo. All the data, including the contents of MDA, lipofuscin (LPF) activities of SOD, glutathione peroxidase, CAT, and total antioxidant capacity (TAOC) in serum, brain, and heart were assessed. Results showed that apiin has significant inhibitory activity of MDA and LPF, to strengthen TAOC, and it dramatically enhances the activity of SOD, GSH peroxidase, and CAT.40
In Vitro Studies
In a study conducted in 2008 by Yildiz et al,36 essential antioxidant compounds of celery leaves were evaluated. In this study, the TAOC by the method of CUPRAC (cupric ion reducing antioxidant capacity) and ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate)) spectrophotometry and TAOC relationship with high-performance liquid chromatography (HPLC) were measured. In the subsequent levels, CUPRAC spectrophotometric methods of TAOC assay and copper (II)-neocuproine (2,9-dimethyl-1,10-phenanthroline) were used as the chromogenic oxidant in the laboratory. Antioxidant compounds in celery plant extract by HPLC were analyzed on one column of C18 and then it was measured. TAC was measured by HPLC in the 41% to 57% of celery leaves compounds (in different levels of hydrolyses). According to data from HPLC it was shown that the celery leaves have antioxidant properties.36 Yao et al,40 in 2010, analyzed the structure of phenolic compounds and phenolic activities of 11 antioxidant celery cultivars. The major phenolic acids in extracts included caffeic acid, p-coumaric acid, and ferulic acid, while the identified flavonoids included apigenin, luteolin, and kaempferol. The total phenol content was measured by using the Folin-Ciocalteu method, and the TAOC with the help of DPPH and ABTS radicals were investigated.
Apigenin was the main flavonoid in this sample, and the most abundant phenolic acid was p-coumaric acid. Studied plants had high levels of phenolic compounds and antioxidant capacity. Generally, there was a positive correlation between total flavonoid contents and antioxidant activity, total phenolic acids or total phenolic were observed in this study.41 In another study conducted in 2012 by Nagella et al,42 chemical compounds and antioxidant activity of essential oils isolated from celery leaves were investigated. First, the celery leaves were distillated and they were analyzed by gas chromatography/mass spectrometry and essential oils of leaves were removed. A total of 73.72% of leaf content were essential oils. In the next step, the essential oils, isolated from the leaves of celery to inhibit the DPPH radical, were studied and the results showed that the isolated oil from celery has natural antioxidant capacity. The above information shows that the major compounds of celery can have a key role as a natural antioxidant.42 A study was carried out by Shanmugapriya and Ushadevi43 in 2014 on Apium graveolens seed extract antioxidant activity. First, celery seed extracts with solvents such as ethanol, ether, and water were prepared. Then the activity of celery seeds was analyzed using the DPPH assay method. Among the various extracts under assessment, methanol extract had the highest antioxidant activity. In the density of 80 μg/mL of extracts, methanol extract had the maximum antioxidant activity, 63.28% ± 0.86%, while this activity for diethyl ether extract was 54.04% ± 0.21%, and for the aqueous extract was 52.97% ± 0.64%. Similarly in the entire concentration of methanol extract of the plant, the seed had highest antioxidant activity. Other extracted luteolin and flavonoids reduced reactive oxygen, but on the other hand increased SOD enzymes that had protective effects against damages. So it can be concluded that these compounds may be responsible for antioxidant activity of celery seeds.43 In 2015, Uddin et al44 investigated the activities of celery antioxidant phytochemical compounds by photochemical screening in presence of flavonoid, tannins, saponins, and showed the absence of terpenoids. Total methanol content (63.46 ± 12.00 mg gallic acid equivalent [GAE]/g) was slightly higher. Fraction for ethanol was (36.60 ± 12.28 mg GAE/g) and for hexane fraction (34.86 mg ± 6.96 GAE/g). Flavonoid content in methanol extract was 56.95 ± 7.14 mg quorcetin/g and lowest of this content in methyl extract was 29.2 ± 3.15 mg quercitin/g. The activity of FRAP was analyzed as higher in comparison with other extracts. Generally, it was concluded that the celery plant has antioxidant characteristics.44 Naglaa et al,45 in 2015, investigated the Apium graveolens seeds, and the objective of this study was to evaluate the antioxidant activity of essential oil of the celery seed. First the chemical compounds of essential oils were obtained by hydrodistillation and were analyzed by gas chromatography/mass spectrometry. The most prevalent essential oil was limonene. The antioxidant activities of volatile oils extracted from the celery and seeds powders were assessed by the Rancimat apparatus and DPPH. Results from this test explained that all essential oils having different concentrations had antioxidant activity and this means that the entire added essential oils, whether added individually or mixed, possessed an antioxidant effect. Overall, this research showed that essential oils and natural antioxidant can be used in industrial food and drugs.45
Conclusion
This study investigated further the properties of celery leaves and seeds. Celery, because of compounds such as caffeic acid, p-coumaric acid, ferulic acid, apigenin, luteolin, tannin, saponin, and kaempferol, has powerful antioxidant characteristics, while different compounds of this plant with diverse concentration can have different healing effects. It is suggested that the next studies concentrate on other therapeutic and industrial attributes of celery.
Acknowledgements
The authors of the present article would like to express their sincere gratitude to all those who have supported them during the research.
Footnotes
Author Contributions: WK and ZA were involved with study concept, design, and critical revision of the manuscript for important intellectual content.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: Ethical approval was not required for this article as no human subjects were involved.
References
- 1. Ghasemi Pirbalouti A. Iranian Medicinal and Aromatic Plants. Shahrekord, Iran: Islamic Azad University; 2009. [Google Scholar]
- 2. Tang SY, Halliwell B. Medicinal plants and antioxidants: what do we learn from cell culture and Caenorhabditis elegans studies? Biochem Biophys Res Commun. 2010;394:1–5. [DOI] [PubMed] [Google Scholar]
- 3. Ghasemiboron M, Mansori E, Asadi-Samani M, et al. Effect of ointment with cabbage, pomegranate peel, and common plantain on wound healing in male rat. J Shahrekord Univ Med Sci. 2014;15(6):92–100. [Google Scholar]
- 4. Kooti W, Ghasemiboroon M, Ahangarpoor A, et al. The effect of hydro-alcoholic extract of celery on male rats in fertility control and sex ratio of rat offspring. J Babol Univ Med Sci. 2014;16(4):43–49. [Google Scholar]
- 5. Noori Ahmad Abadi M, Mortazavi M, Kalani N, Zare Marzouni H, Kooti W, Ali-Akbari S. Effect of hydroalcoholic extract of Rosmarinus officinalis L. leaf on anxiety in mice. J Evid Based Complementary Altern Med. 2016;21:NP85–NP90. doi:10.1177/2156587216642101. [DOI] [PubMed] [Google Scholar]
- 6. Kooti W, Ghasemiboroon M, Asadi-Samani M, et al. The effect of alcoholic extract of celery leaves on the delivery rate (fertilization and stillbirths), the number, weight and sex ratio of rat off spring. Adv Environ Biol. 2014;8:824–830. [Google Scholar]
- 7. Kooti W, Ghasemiboroon M, Asadi-Samani M, et al. The effects of hydro-alcoholic extract of celery on lipid profile of rats fed a high fat diet. Adv Environ Biol. 2014;8:325–330. [Google Scholar]
- 8. Lone ZA LY, Khan SS, Wani AA, Reshi MI. Hepatoprotective medicinal plants used by the Gond and Bhill tribals of District Raisen, Madhya Pradesh, India. J Med Plants Res. 2015;9:400–406. [Google Scholar]
- 9. Mansouri E, Kooti W, Bazvand M. The effect of hydro-alcoholic extract of Foeniculum vulgare Mill on leukocytes and hematological tests in male rats. Jundishapur J Nat Pharm Prod. 2015;10:e18396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu S-Y, Shen J-L, Man K-M, et al. An emerging translational model to screen potential medicinal plants for nephrolithiasis, an independent risk factor for chronic kidney disease. Evid Based Complement Alternat Med. 2014;2014:972958 doi:10.1155/2014/972958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saki K, Bahmani M, Rafieian-Kopaei M. The effect of most important medicinal plants on two importnt psychiatric disorders (anxiety and depression)—a review. Asian Pac J Trop Med. 2014;7:34–42. [DOI] [PubMed] [Google Scholar]
- 12. Kooti W, Moradi M, Ali-Akbari S, Sharafi-Ahvazi N, Asadi-Samani M, Ashtary-Larky D. Therapeutic and pharmacological potential of Foeniculum vulgare Mill: a review. J Herb Med Pharmacol. 2015;4:1–9. [Google Scholar]
- 13. Kooti W, Farokhipour M, Asadzadeh Z, Ashtary-Larky D, Asadi-Samani M.The role of medicinal plants in the treatment of diabetes: a systematic review. Electronic Physician. 2016;8:1832–1842. doi:10.19082/1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Asadi-Samani M, Kooti W, AE, Shirzad H. A systematic review of Iran’s medicinal plants with anticancer effects. J Evid Based Complementary Altern Med. 2015;21:145–153. [DOI] [PubMed] [Google Scholar]
- 15. Grzanna R, Lindmark L, Frondoza C. Ginger—an herbal medicinal product with broad anti-inflammatory actions. J Med Food. 2005;8:125–132. [DOI] [PubMed] [Google Scholar]
- 16. Gauri M, Javed Ali S, Shahid Khan M. A review of Apium graveolens (Karafs) with special reference to Unani medicine. Int Arch Integr Med. 2015;2:131–136. [Google Scholar]
- 17. Kolarovic J, Popovic M, Mikov M, Mitic R, Gvozdenovic L. Protective effects of celery juice in treatments with doxorubicin. Molecules. 2009;14:1627–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bhattacharjee SK. Handbook of Medicinal Plants. 4th ed Jaipur, India: Pointer; 2004. [Google Scholar]
- 19. Khare CP. Indian Medicinal Plants. London, England: Springer Science; 2008. [Google Scholar]
- 20. Kooti W, Ali-Akbari S, Asadi-Samani M, Ghadery H, Ashtary-Larky D. A review on medicinal plant of Apium graveolens . Adv Herb Med. 2014;1:48–59. [Google Scholar]
- 21. Sowbhagya HB, Srinivas P, Krishnamurthy N. Effect of enzymes on extraction of volatiles from celery seeds. Food Chem. 2010;120:230–234. [Google Scholar]
- 22. Nadkarni KM. Indian Materia Medica. 2nd ed Mumbai, India: Popular Prakashan; 2010. [Google Scholar]
- 23. Karnick CR. Pharmacopoeial Standards of Herbal Plants. New Delhi, India: Sri Satguru Publications; 1994. [Google Scholar]
- 24. Kooti W, Mansouri E, Ghasemiboroon M, Harizi M, Ashtary-Larky D, Afrisham R. The effects of hydroalcoholic extract of Apium graveolens leaf on the number of sexual cells and testicular structure in rat. Jundishapur J Nat Pharm Prod. 2014;9(4):e17532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zare Marzouni H, Daraei N, Sharafi-Ahvazi N, Kalani N, Kooti W. The effects of aqueous extract of celery leaves (Apium graveolens) on fertility in female rats. World J Pharm Pharm Sci. 2016;5:1710–1714. [Google Scholar]
- 26. Kooti W, Mansori E, Ghasemiboroon M, Harizi M, Amirzarga A. Protective effects of celery (Apium graveolens) on testis and cauda epididymal spermatozoa in rat. Iranian J Reprod Med. 2014;12:365–366. [PMC free article] [PubMed] [Google Scholar]
- 27. Gelodar G, Nazify H, Abadi S. Effect of celery, apple tart and carrots on some biochemical parameters in diabetic rats. J Kerman Univ Med Sci. 1997;3:114–119. [Google Scholar]
- 28. Lans CA. Ethnomedicines used in Trinidad and Tobago for urinary problems and diabetes mellitus. J Ethnobiol Ethnomed. 2006;2:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Momin RA, Nair MG. Mosquitocidal, nematicidal, and antifungal compounds from Apium graveolens L. seeds. J Agric Food Chem. 2001;49:142–145. [DOI] [PubMed] [Google Scholar]
- 30. Mencherini T, Cau A, Bianco G, Della Loggia R, Aquino RP, Autore G. An extract of Apium graveolens var. dulce leaves: structure of the major constituent, apiin, and its antiinflammatory properties. J Pharm Pharmacol. 2007;59:891–897. [DOI] [PubMed] [Google Scholar]
- 31. Atta A. Anti-nociceptive and anti-inflammatory effects of some Jordanian medicinal plant extracts. J Ethnopharmacol. 1998;60:117–124. [DOI] [PubMed] [Google Scholar]
- 32. Khare CP. Indian Medicinal Plants. New Delhi, India: Springer; 2007. [Google Scholar]
- 33. Kritikar KR, Basu BD. Indian Medicinal Plants. 2nd ed Vols 1 and 2 Dehradun, India: International Book Distributors; 2008. [Google Scholar]
- 34. Nickavar B, Kamalinejad M, Izadpanah H. In vitro free radical scavenging activity of five Salvia species. Pak J Pharma Sci. 2007;20:291–294. [PubMed] [Google Scholar]
- 35. Melo EA, Filho JM, Guerra NB. Characterization of antioxidant compounds in aqueous coriander extracts (Coriander sativum L.). LWT-Food Sci Technol. 2005;38:15–19. [Google Scholar]
- 36. Yildiz L, Başkan KS, Tütem E, Apak R. Combined HPLC-CUPRAC (cupric ion reducing antioxidant capacity) assay of parsley, celery leaves, and nettle. Talanta. 2008;77:304–313. [DOI] [PubMed] [Google Scholar]
- 37. Zidorn C, Johrer K, Ganzera M, et al. Polyacetylenesfrom the apiaceae vegetables carrot, celery, fennel, parsley, and parsnip and their cytotoxic activities. J Agric Food Chem. 2005;53:2518–2523. [DOI] [PubMed] [Google Scholar]
- 38. Kolarovic J, Popovic M, Zlinska J, Trivic S, Vojnovic M. Antioxidant activities of celery and parsley juices in rats treated with doxorubicin. Molecules. 2010;15:6193–6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Al Sa’aidi JAA, Alrodhan MNA, Ismael AK. Antioxidant activity of n-butanol extract of celery (Apium graveolens) seed in streptozotocin-induced diabetic male rats. Res Pharm Biotechnol. 2012;4:24–29. [Google Scholar]
- 40. Li P, Jia J, Zhang D, Xie J, Xu X, Wei D. In vitro and in vivo antioxidant activities of a flavonoid isolated from celery (Apium graveolens L. var. dulce). Food Funct. 2014;5:50–60. [DOI] [PubMed] [Google Scholar]
- 41. Yao Y, Sang W, Zhou M, Ren G. Phenolic composition and antioxidant activities of 11 celery cultivars. J Food Sci. 2010;75:C9–C13. [DOI] [PubMed] [Google Scholar]
- 42. Nagella P, Ahmad A, Kim SJ, Chung IM. Chemical composition, antioxidant activity and larvicidal effects of essential oil from leaves of Apium graveolens . Immunopharmacol Immunotoxicol. 2012;34:205–209. [DOI] [PubMed] [Google Scholar]
- 43. Shanmugapriya R, Ushadevi T. In vitro antibacterial and antioxidant activities of Apium graveolens L. seed extracts. Int J Drug Dev Res. 2014;6:165–170. [Google Scholar]
- 44. Uddin Z, Shad AA, Bakht J, Ullah I, Jan S. In vitro antimicrobial, antioxidant activity and phytochemical screening of Apium graveolens . Pak J Pharm Sci. 2015;28:1699–1704. [PubMed] [Google Scholar]
- 45. Naglaa HM, Hassanen A, Eissa MF, et al. Antioxidant and antimicrobial activity of celery (Apium graveolens) and coriander (Coriandrum sativum) herb and seed essential oils. Int J Curr Microbiol App Sci. 2015;4:284–296. [Google Scholar]