Abstract
Linum usitatissimum L is traditionally used for relief of pain and inflammation. In this study, the analgesic and anti-inflammatory effects of this plant were evaluated. Xylene test was used for anti-inflammatory evaluation in which 48 mice were randomly designated into 6 groups of 8 each including: control, dexamethasone as positive control (15 mg/kg), and experimental groups (42, 85, 170, and 340 mg/kg, respectively). For analgesic evaluation, 192 mice were randomly designated into 4 sets of 6 groups of 8 mice, including control, morphine as positive control, morphine plus naloxone, experimental groups (200 and 500 mg/kg extract), and extract along with naloxone group, which received 500 mg/kg. The analgesic activities were evaluated at 5, 15, 30, and 60 minutes, respectively, in each set. Both doses showed analgesic activity, the 200 mg/kg possessed higher effects (P < .05). Naloxone reduced a section of its effect (P < .001). The 170 mg/kg dose of the extract showed anti-inflammatory activity (P < .05). The extract had phenolic, flavonoid, and flavonol compounds with antioxidant activity. Linum usitatissimum L dose dependently had analgesic activity partially like morphine and might be used as analgesic and anti-inflammatory agent.
Keywords: Linum usitatissimum L, pain, inflammation, mice
Pain and inflammation are parts of the complex responses of the body to harmful stimuli. The inflammation can help eliminate the initial cause of cell injury and initiate tissue repair; however, if the inflammation persists, it may become destructive and cause tissue damage.1 Inflammation is a type of potential immunity that is caused by setting up arachidonic acid pathway flow and prostaglandins synthesis, which cause nerves irritation and increase of arterial permeability.2 Prostaglandins are produced by the help of cyclooxygenase-1 and -2 from arachidonic acid, which have a crucial role in creating pain and inflammation. Nowadays, common chemical drugs such as steroids and nonsteroids are used in the relief of pain and inflammation. However, most of them have side effects. New researches recommend the use of medicinal plants for the treatment of various diseases.3–7 A lot of these plants have shown promising results in prevention and treatment of diseases and complications.8–12 These plants generally have low cost and minimum side effects.13 Linum usitatissimum L with the local name of “Gole Katan” is an oily, annual and herbaceous plant from the Linaceae family that can grow in dry and hot climate of Iran.14 Linseed is a rich source of useful fatty acids and unsaturated fatty acids, a 0.3 to 1 ratio of omega-6/omega-3, α-linoleic, lignans, dietary and protein fiber, minerals, and vitamins such as vitamin E. Linoleic acid available in fatty acids of linseed has antioxidant activity and protective effect against some diseases such as cardiac diseases and atherosclerosis.14 Some waxes are extracted from processing of L usitatissimum fibers. Compounds of this are traditionally used for treating skin problems, relief of pain, especially headache, and for its sedative properties.15 Lignans from phytoestrogens have a structure similar to human estrogen and protects body against some diseases like diabetes, cardiovascular diseases, chest cancer, large intestine disorder, prostate, digestive diseases, migraine, and osteoporosis. Other compounds available in plant include phenols, flavons, flavonoids, tannins, antocianins, carotenoids, lignans, vitamin E, vitamin C, and amino acids like tryptophan, tyrosine, and proline, and phenolic compounds. These compounds also have different ecologic and physiologic roles like defense and antioxidant activities.15 Some of these compounds such as flavonoids have previously shown to have analgesic and anti-inflammatory activities.16
In result of increase in inflammatory factors and damages resulted from them in different tissues, the level of enzymatic antioxidants (superoxide dismutase, catalase, glutathione peroxidase) and nonenzymatic antioxidants (vitamins C and E) in blood circulation and tissues decrease. Hence, antioxidants have been shown to relieve pain and inflammation. Some of these compounds, especially phenols, flavons, flavonoids, have also reported in the leaves of L usitatissimum.15 Generally, with regard to prevalence of inflammation and pain complications and the active role of antioxidants and flavonoids in prevention of pain and inflammation and antioxidant activity of flavonoids, which are present in the leaves, as well as traditional uses of this plant, this study was performed with the aim of investigating the anti-inflammatory and analgesic effects of linseed leaves and evaluating its antioxidant, flavonoid, flavonol, and phenolic compounds.
Materials and Methods
Extracting
The leaves of linseed plant, provided from a grocery in Shahrekord city, was used in this study. The plant was approved in the herbarium center of Medical Plants Research Center of Shahrekord University of medical Sciences and a herbarium specimen was deposited there (Herbarium No. 458). Dried plant leaves were milled to 40 and 80 mesh powder. One hundred grams of the powder was macerated with 200 mL of 80% ethanol for 72 hours.16,17 In the next stage, its extract was dried by the help of a rotary evaporator device at 35°C to 40°C.18
Animals
In this research, 240 male Balb/c mice in the weight range of 20 to 25 g were bought from Pasteur Institute (Iran) and maintained in 12-hour dark-light condition at 20°C to 24°C with pellet food of livestock food factory of Pasteur Institute, Iran. Mice were transferred to animal house, where the experiments were done, 7 days before experiment and weighted 1 hour before performing experiment. Then they were maintained in cages individually without having access to water and food during experiment.
Method of Studying Inflammation Effect
To study the anti-inflammatory effect, the xylene method was used. To conduct this test, 48 male mice were randomly designated into 6 groups of 8 each, including control, dexamethasone as positive control (15 mg/kg), and experimental groups (42, 85, 170, and 340 mg/kg, respectively). For analgesic evaluation, 192 male Balb/c mice were randomly designated into 4 sets of 6 groups (8 mice in each group). The groups consisted of control group, which received 10 mL/kg normal saline, morphine (2.5 mg/kg) as positive control, morphine (2.5 mg/kg) plus naloxone, experimental groups (200 and 500 mg/kg extract), and extract (500 mg/kg) along with naloxone (4 mg/kg) groups. The analgesic activities were evaluated at 5, 15, 30, and 60 minutes, respectively, in each set.
To create inflammation in mice’s ear, 30 µL of xylene (purchased from Romil Company, Germany) was injected in anterior and posterior surfaces of the earlobe of animals’ right ears 15 minutes after the mentioned intraperitoneal injections and then the animals were killed and both ears of each animal were cut and 7-mm slices from both ears were prepared and weighed and results of groups were compared with each other.19
Method of Investigating Pain Relief
To study the analgesic effect, the hot plate test was used. The analgesic activities were evaluated at 5, 15, 30, and 60 minutes, respectively, in each set. At first 4 mg/kg naloxone was injected subcutaneously in the back area of animal and 5 minutes later, 500 mg/kg of plant extract was injected intraperitoneally. Delay in animal’s reaction in the hot plate device was measured, respectively, in 5, 15, 30, and 60 minutes after the injection. One set of animals was used for each time to prevent the effect of pain experience of animals in one time to the other time. Hot plate test was considered with constant temperature of 52°C ± 2°C. The cutoff time of the test was considered as 25 seconds, to prevent animal injury. Animal response criterion was licking forelimbs or jumping.19 The dosage selection was based on a pilot study.
Studying the Amounts of Antioxidant Activity
To measure antioxidant activity of the extract, at first a stock solution of DPPH (2,2-diphenyl-1-picrylhydrazyl) was prepared by adding 0.01 mg powder to 50 mL of methanol. Then, in the next stage 10 mg of the extract was dissolved in 1 mL of methanol and was diluted 10 times more. Half a milliliter (0.5 mL) of the dissolved extract was poured into 4.5 mL of methanol and the desired concentrations were prepared. Colorimetric method was conducted by spectrophotometer. First, the device was set to zero by ethanol and then the absorption of the samples was read at wavelength of 517 nm during 1 hour.20
Measuring Phenolic Compounds
To determine total phenolic compounds, 10 mg of extract was dissolved in 10 mL methanol (60%) and total phenolic compounds were determined by the Folin-Ciocalteu method.21 A volume of 0.1 mL of extract solution was added to 0.5 mL of Folin-Ciocalteu reagent and was maintained in room temperature for about 8 minutes. Then, 0.4 mL of 7.5% sodium carbonate was added to it and then the absorption was measured at wavelength of 765 nm by spectrophotometer device after 30 minutes.
Measuring Flavonoids
To determine flavonoids of total extracts, 0.01 g of dried extract powder was dissolved in 60% methanol and was increased to 10 mL volume. Then total flavonoid was determined based on the aluminum chloride colorimetric method. Then, 1 mL of this solution was transferred to experiment tube and 1 mL of 2% aluminum chloride solution was added to it. Then, 6 mL of 5% potassium acetate solution was added it and absorption at wavelength of 415 nm was measured after 40 minutes and amount of total flavonoid content in mg/g was calculated.22
Measuring Flavonols
Total flavonol compounds was estimated by the aluminum chloride colorimetric method in accordance with the Rutin method with the difference that absorbance was read at wavelength of 440 nm after 2.5 hours.23
Statistical Methods
Information obtained from data was statistically analyzed by SPSS software. Changes of contractile force resulted from extract and antagonist compared to extract were calculated as mean ± SEM. The analysis of variance test was used to compare different concentrations and Student’s t test was used to compare 2 groups. P < .05 was considered as significant difference.
Results
Comparing the Analgesic Tolerance in Different Doses of the Extract
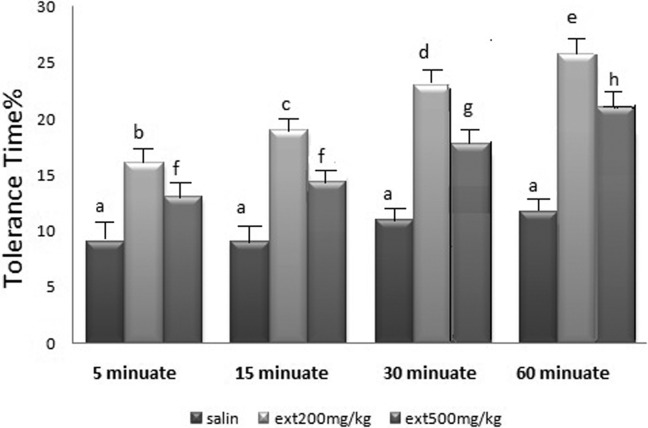
The 200 and 500 mg/kg of leaves alcoholic extract in each of the 4 time periods (5, 15, 30, and 60 minutes) used in the test indicated a significant difference in the pain tolerance time duration in mice in comparison with the normal saline and increased the pain tolerance time in mice, respectively (P < .0001, n = 8).
The pain tolerance time duration in the 500 mg/kg of the leaves alcoholic extract was significantly less than the 200 mg/kg one in each of the mentioned stages (Figure 1).
Figure 1.
The analgesic effect of different doses of the extract in different time periods. a: P < .05: in relation to the control group and each other in all groups. b, c, d, f: P < .05: in relation to the control group and each other in all groups and in each case with regard to 200 mg/kg dosage.
Comparing the Pain Tolerance Time Duration of the Leaves Toadflax Extract With Morphine and Naloxone Drugs
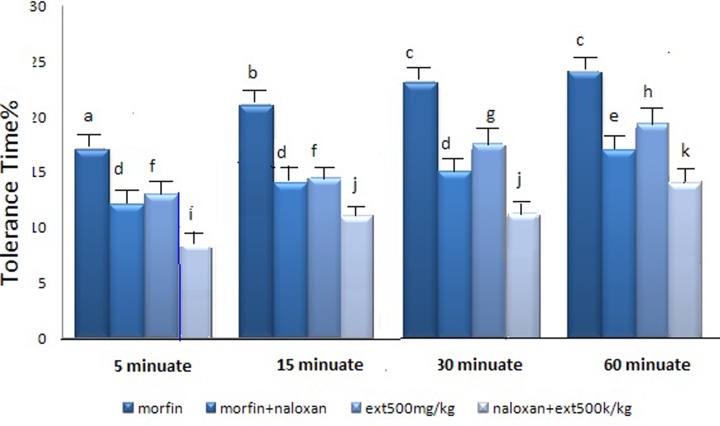
In order to study the possible mechanism of the extract through opioid receptors and to compare the pain tolerance time resulting from the extract and morphine, the mice received 500 mg/kg of extract or extract plus naloxone.
T test statistical analysis of the data showed a significant difference between groups before and after receiving naloxone. Following occupation of the opioid receptors by naloxone, the extract had analgesic ability. However, the effect was less than the time when opioid receptors had not been occupied with naloxone (P < .001). The same happened in the morphine plus naloxone receiving group but morphine effect was returned completely (P < .001, n = 8) (Figure 2).
Figure 2.
Comparison of time of pain tolerance of the leaves of linseed extract in comparison with morphine drugs. d, e: P < .001: in relation to morphine + naloxone group in each case. f, g, h: P < .001: in relation to group compared with the extract group. i, j, k: P < .001: in relation to group compared with the extract + naloxone group. a, b, c: P < .05 compared with each other.
Effect of Plant Extract on Inflammation
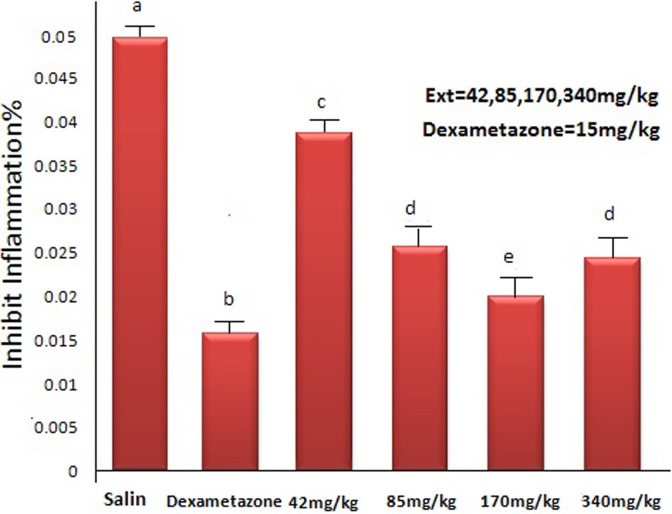
Figure 3 shows that 42, 85, 170, and 340 mg/kg extract of linseed could significantly and in a dose-dependent manner decrease the inflammatory effect induced by xylene (P < .05). Also, there was a significant difference between control group and the groups receiving 42, 85, 170, and 340 mg/kg extract P < .0001, n = 8) (analysis of variance). By increasing the dose, the anti-inflammatory effect of the extract was increased in the ear of mice affected against inflammation resulted from xylene.
Figure 3.
Effect of the plant leaves extract on inflammation resulted from xylene injection in mice ear. a: P < .0001 compared with saline group. b, c, d, e: P < .05 compared with saline group. d: P < .05: compared with e, c, b.
Amount of antioxidant, phenol, flavonol, and flavonoid compounds in linseed extract in mg/g were measured that values are presented as follows: The antioxidant activity of the extract was 27.4% of the DPPH. The flavonol and flavonoid compounds were, respectively, 14.9 and 20.7 mg/g of dried extract, and the total phenolic compounds was 23.9 mg/g dried extract.
Discussion
The present study indicates increase of pain tolerance in groups receiving morphine or extract in comparison with control group and also suggests that 200 mg/kg dosage of L usitatissimum extract is more effective than 500 mg/kg dosage of the same extract. So that there was no significant statistical relationship between amount of receiving 200 mg/kg dose of extract and morphine but significant difference was seen between 2 dosages of 200 and 500 mg/kg. Naloxone is an inhibitor of opioid receptors and results showed that the analgesic effect of the extract plus naloxone was decreased significantly compared with extract group, indicating that analgesic effect of the extract was, at least in part, through the opioid system.
Inflammation level in groups receiving the leaves extract was significantly decreased in comparison with saline group. Also, in the groups receiving dexamethasone, significant difference was seen compared with control group. Data suggested more relief effect in groups receiving dexamethasone than groups receiving extract. The same as analgesic activity, this effect was more pronounced in the group that received 170 mg/kg of extract than other groups receiving the extract. More analgesic and anti-inflammatory activities of the extract with lower doses are somewhat surprising and the mechanisms involved are not clear. Hence, more researches are needed to elucidate the possible mechanisms.
Prostaglandins also have crucial role in providing pain and inflammation in the body. These compounds are produced from acid arachidonic by cyclooxygenase-1 and -2 enzymes.24 Most of drugs with analgesic and anti-inflammatory effects reduce pain and inflammation by inhibiting these enzymes. Release of internal glucocorticoids also is of other important factors in decreasing pain and inflammation.25 So, the compounds available in this plant, like steroid drugs or the compounds with opioid activity, should apply analgesic effects through one of the mentioned mechanisms. Lack of complete inhibition of analgesic effect of the extract by naloxone, and having anti-inflammatory effect confirm that the effect of the extract is exerted also through nonopioid pathways. In previous researches, analgesic and anti-inflammatory effects of substances effective in pain have been attributed to some compounds like alkaloids, tannins, flavonoids, anthocyanins, glycosides, saponins, organic acids, carotenoids, vitamin C, vitamin E, and caffeic acid derivatives.26 However, which of these or other substances in the extract are responsible for the effects of the extract is not clear and should be elucidated.
With regard to clinical effects of compounds containing flavonoids, the effect of the extract can possibly be partly based on central and peripheral inhibition of prostaglandins production by flavonoid compounds. Regarding that, flavonoids can prevent metabolism of arachidonic acid2 and with regard to the fact that prostaglandins’ effect on creation of inflammation and intensity of pain and originate from arachidonic acids, so it can be said that possibly flavonoids—which are natural polyphenol compounds in this plant—play a role in creation of analgesic and anti-inflammatory effects. Presence of flavonoids and steroids and their analgesic effects have been reported in Euphorbia decipiens species.27 Also, anti-inflammatory effects of flavonoids and steroids compounds have been proved. It seems that steroids available in the plant extract inhibit production of prostaglandins reducing inflammation by inhibiting cyclooxygenase.28 In studies conducted on aqueous extract of Teucrium hyrcanicum in male mice and rats, analgesic and anti-inflammation effects of extract have been attributed to alkaloid, flavonoid, and triterpenoids compounds and central and peripheral mechanisms have been stated as responsible for these effects.29
Possible decrease of prostaglandins and interference in release of internal glucocorticoids are considered in peripheral mechanisms of extract performance.24 These findings are in agreement with researches conducted on most of medicinal compounds bearing anti-inflammatory and analgesic properties and confirm their therapeutic effects in decreasing production of prostaglandins, mediators, and enzymes that create pain and inflammation as important peripheral factors in decreasing pain inflammation process.30 Furthermore, these effects have also been, in part, attributed to antioxidant properties of these plants. There are a lot of other plants with flavonoid and antioxidant activities (30-35). Therefore, these plant which possess antioxidant activities may have analgesic and anti-inflammatory activities, too.
Conclusion
Linum usitatissimum L has analgesic activity partially like morphine. Regarding the safety and possessing antioxidant and various effects of plants with antioxidant activities,31–35 L usitatissimum might be used as analgesic and anti-inflammatory agent, as well as treatment of for other diseases.
Acknowledgments
The authors thank the stuff of Medical Plants Research Center of this university for their cooperation.
Footnotes
Author Contributions: All the authors contributed equally toward writing the first draft of the manuscript. MRK revised and edited the final version. All authors read and confirmed its publication.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was financially supported by research deputy of Shahrekord University of Medical Sciences.
Ethical Approval: The study was approved by Ethical Committee of Shahrekord University of Medical Sciences, Shahrekord, Iran.
References
- 1. Patton HD, Fuchs AF, Hills AF, Scher AM, Steiner R. Textbook of Physiology. 21st ed Philadelphia, PA: W. B. Saunders; 2003. [Google Scholar]
- 2. Koo KL, Ammit AJ, Tran VH, Duke CC, Roufogalis BD. Gingerols and related analogues inhibit arachidonic acid induced human platelet serotonin release and aggregation. Thromb Res. 2001;103:387–397. [DOI] [PubMed] [Google Scholar]
- 3. Asadi-Samani M, Kooti W, Aslani E, Shirzad H. A systematic review of Iran’s medicinal plants with anticancer effects. J Evid Based Complementary Altern Med. 2016;21:143–153. [DOI] [PubMed] [Google Scholar]
- 4. Fasihzadeh S, Lorigooini Z, Jivad N. Chemical constituents of Allium stipitatum regel (Persian shallot) essential oil. Der Pharmacia Lettre. 2016;8:175–180. [Google Scholar]
- 5. Ghasemi S, Lorigooini Z. A review of significant molecular mechanisms of flavonoids in prevention of prostate cancer. J Chem Pharm Sci. 2016;9:3388–3394. [Google Scholar]
- 6. Rabiei Z, Bigdeli M, Lorigooini Z. A review of medicinal herbs with antioxidant properties in the treatment of cerebral ischemia and reperfusion. J Babol Univ Med Sci. 2015;17:45–76. [Google Scholar]
- 7. Hajian S. Positive effect of antioxidants on immune system. Immunopathol Persa. 2015;1(1):e02. [Google Scholar]
- 8. Nasri H. Herbal drugs and new concepts on its use. J Prev Epidemiol. 2016;1(1):e01. [Google Scholar]
- 9. Nasri H. Impact of garlic extract on platelet function and structure. Ann Res Platelets. 2016;1(1):e01. [Google Scholar]
- 10. Dehghan Shahreza F. Hibiscus esculentus and diabetes mellitus. J Nephropharmacol. 2016;5:104–105. [PMC free article] [PubMed] [Google Scholar]
- 11. Kafeshani M. Ginger, micro-inflammation and kidney disease. J Renal Endocrinol. 2015;1:e04. [Google Scholar]
- 12. Amiri M, Hosseini SM. Diabetes mellitus type 1: is it a global challenge? Acta Epidemioendocrinol. 2016;1(1):e02. [Google Scholar]
- 13. Nasri H. Improving the nephrotoxicity of cyclosporine; the role of herbal drugs. Toxicol Persa. 2016;1(1):e05. [Google Scholar]
- 14. Oomah BD. Flaxseed as functional source. J Sci Food Agric. 2001;81:889–894. [Google Scholar]
- 15. Nakhlawy FS. Inheritance of oil content, unsaturated fatty acid composition and iodine number of oil in flax. Menofiya J Agric Res Egypt. 1996;20:483–492. [Google Scholar]
- 16. Samani BH, Khoshtaghaza MH, Lorigooini Z, Minaei S, Zareiforoush H. Analysis of the combinative effect of ultrasound and microwave power on Saccharomyces cerevisiae in orange juice processing. Innov Food Sci Emerging Technol. 2015;32:110–115. [Google Scholar]
- 17. Zomorodian K, Moein M, Lori ZG, et al. Chemical composition and antimicrobial activities of the essential oil from Myrtus communis leaves. J Essent Oil Bearing Plants. 2013;16:76–84. [Google Scholar]
- 18. Mohammadparast V. Antioxidant efficacy of Hibiscus esculentus . Front Biomed. 2016;1(1):e04. [Google Scholar]
- 19. Haj Hashemi V, Ghanadi AR, Mousavi D. Analgesic and anti-inflammatory effects to total extract flavonoid fraction and volatile oil of Salvia hydrangea . J Res Med Sci. 2000;5(2):10–14. [Google Scholar]
- 20. Samani BH, Zareiforoush H, Lorigooini Z, Ghobadian B, Rostami S, Fayyazi E. Ultrasonic-assisted production of biodiesel from Pistacia atlantica Desf. oil. Fuel. 2016;168:22–26. [Google Scholar]
- 21. Lorigooini Z, Ayatollahi SA, Amidi S, Kobarfard F. Evaluation of anti-platelet aggregation effect of some Allium species. Iranian J Pharm Res. 2015;14:1225. [PMC free article] [PubMed] [Google Scholar]
- 22. Lorigooini Z, Kobarfard F, Ayatollahi SA. Anti-platelet aggregation assay and chemical composition of essential oil from Allium atroviolaceum Boiss growing in Iran. Int J Biosci. 2014;5:151–156. [Google Scholar]
- 23. Asadi SY, Parsaei P, Karimi M, et al. Effect of green tea (Camellia sinensis) extract on healing process of surgical wounds in rat. Int J Surg. 2013;11:332–337. [DOI] [PubMed] [Google Scholar]
- 24. Cheraghi J, Valadi A. Effects of anti-nociceptive and anti-inflammatory component of limonenein herbal drugs. Iranian J Med Arom Plants Fall. 2010;26:415–422. [Google Scholar]
- 25. Ahmadiani A, Fereidoni M, Semnanian S, Kamalinejad M, Saremi S. Antinociceptive and anti-inflammatory effect of Sambucus ebulus rhizome extract in rats. J Ethnopharmacol. 1998;61:229–235. [DOI] [PubMed] [Google Scholar]
- 26. Ghannadi AR, Ghassemi-Dehkordi N. Pharmacognostical investigations on Sambucus ebulus L. and Sambucus nigra L. Daru. 1997;7:55–65. [Google Scholar]
- 27. Ahmad VU, Ahmad H, Hussain I, Bukhari A. Antinociceptive diterpene from Euphorbia decipiens . Fitoterapia. 2005;76:230–232. [DOI] [PubMed] [Google Scholar]
- 28. Recio MC, Giner RM, Manes S, et al. Anti-inflammatory activity of flavonol glycosides from Erythrospermum monticolum depending on single or repeated local TPA administration. Planta Med. 1995;61:502–504. [DOI] [PubMed] [Google Scholar]
- 29. Farshchi A, Ghiasi G, Abdollahuasl A. Antinociceptive and antiinflammatory effects of Teucrium hyrcanicum aqueous extract in male mice and rats. Physiol Pharmacol. 2010;14:78–84. [Google Scholar]
- 30. Lantz RC, Chen GJ, Sarihan M, Sólyom AM, Jolad SD, Timmermann BN. The effect of extracts from ginger rhizome on inflammatory mediator production. Phytomedicine. 2007;14:123–128. [DOI] [PubMed] [Google Scholar]
- 31. Bahmani M, Asadi-Samani M. Native medicinal plants of Iran effective on peptic ulcer. J Inj Inflamm. 2016;1(1):e05. [Google Scholar]
- 32. Nasri P. Cancers and herbal antioxidants. Front Biomark. 2017;2(1):e01. [Google Scholar]
- 33. Seddighi S, Bahmani M, Asgary S, et al. A review of plant-based compounds and medicinal plants effective on atherosclerosis. J Res Med Sci. 2017;22:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sedighi M, Rafieian-kopaei M, Noori-Ahmadabadi M. Effect of Allium ampeloprasum on ileum function: involopersum of beta-adrenergic receptors and voltage depend calcium channels. Life Science journal. 2012;9(3):1660–7. [Google Scholar]
- 35. Baradaran A. Administration of herbal drugs in geriatric individuals: trends on its helps and hazards. Geriatr Persia. 2017;1(1):e01. [Google Scholar]