Abstract
It was purposed to evaluate the biological potential of ethanol and isopropanol crude extracts of ripe Physalis peruviana fruits. Cytotoxic and immunomodulatory effects of the expression of interleukin-6, interleukin-8, and monocyte chemoattractant protein-1 (MCP-1) were evaluated on human cervical cancer (HeLa) and murine fibroblast (L929) cells. The composition was evaluated by high-performance liquid chromatography diode-array detection and high-performance liquid chromatography ultraviolet/visible detection. The presence of ursolic acid and rosmarinic acid was found in both solvents. However, gallic acid, quercetin, and epicatechin were higher in isopropanol extracts (P < .05). The results indicated a relationship among the total polyphenol content, antioxidant activity, and cytotoxic activity that was dependent on the solvent used. Isopropanol extracts presented a half-maximal inhibition concentration value (IC50) of 60.48 ± 3.8 μg/mL for HeLa cells and 66.62 ± 2.67 μg/mL for L929 fibroblasts. The extracts reduced the release of interleukin-6, interleukin-8, and MCP-1 in a dose-dependent manner. Extracts showed anticancer and immunomodulatory potential for new complementary pharmaceutical products development.
Keywords: cytotoxicity, immunomodulatory, antioxidants
Fruits and vegetables contain natural antioxidants that are associated with therapeutic effects, including several types of antioxidant compounds such as vitamin C, vitamin E, polyphenols, carotenoids, lutein, and lycopene.1 Among these compounds, polyphenols play an important role as antioxidants through several mechanisms associated with redox properties, reducing agents, hydrogen donors, and free radical capturing agents.2 Recent studies have shown that many polyphenols and carotenoids may contribute substantially to protective effects in vivo3 and in vitro,4 related to a broad spectrum of properties, including antimicrobial, antiallergy, anti-inflammatory, cardioprotective,5,6 and antineoplastic properties.7 The Physalis peruviana plant, known to have chemotherapeutic effects, is extensively used in folk medicine to treat malaria, asthma, hepatitis, dermatitis, diuresis, rheumatism,8 and visual acuity reduction9 with interesting polyphenols such as 28-hydroxywithanolide, withanolides, phygrine, kaempferol, and quercetin di- and triglycosides.10
The antioxidant and biological activities of Physalis peruviana have been reported in extracts of the calyxes, leaves, stems, and merely in fruits. These extracts have been shown to inhibit the growth of different cancer cell lines and exhibit protective effects in liver and other cells, as well as exhibit anti-inflammatory activity.11,12 Some studies have observed apoptosis activated by mitochondrial signaling in macrophages, hepatocytes, monocytes, T lymphocytes, and natural killer cells,13–15 which demonstrates the enormous potential of this Andean plant in food and herbal medicine applications. However, Physalis fruit studies have explored its traditional use as a juice directly instilled to reduce inflammatory ocular problems, like pterygium, due to its cytostatic effects on cultured rabbit fibroblasts.9 The L929 and HeLa cells are biological models useful to demonstrate the potential of natural extracts to function as tumor cell inhibitors as well as the impact of these natural extracts on epidermal cells and untapped potential with Physalis peruviana fruits extracts.
The aim of this study was to determine the antioxidant and biological activity of isopropanol and ethanol Physalis peruviana fruit extracts, grown in the same geographic location, on HeLa human cervical cancer cells and L929 murine fibroblasts to demonstrate the potential anticancer and anti-inflammatory effects, and the relationship of these effects with the polyphenol and carotenoid content.
Materials and Methods
Biological Materials and Extracts Preparation
Fruits, from 100% ripening of the Colombian ecotype of Physalis peruviana, native of the Cundinamarca region, were acquired-with the owners’ consent-from 2 commercial growers geographically located at 5°13″N 73°36″W and 2715 meters above sea level: Las Quebradas (C1) and Buenos Aires (C2). During the study period, the average relative humidity was 75%, with 990 mm of annual rainfall in the region, and a temperature of 13°C. The fruits were classified according to color using a color chart (NTC-4580).
Two kilograms of washed fruits from each location were independently macerated with ethanol (E) and isopropanol (I) (Merck; Darmstadt, Germany) at room temperature for 48 hours, which produced 4 extracts of the solvent-grower combinations: IC1 (I-C1), IC2 (I-C2), EC1 (E-C1), and EC2 (E-C2). All extracts were concentrated and stored in amber bottles at 4°C until use.
Quantification of Polyphenols
The Folin-Ciocalteu assay, based on a redox reaction, was used to quantify the total polyphenol content of the extracts by a modified Folin-Ciocalteu method.16 The Folin-Ciocalteu reagent (100 μL; Sigma Chemical Company; St Louis, MO) was diluted 1:10, and 80 μL of sodium carbonate solution (75 g/L) was added to 20 μL of each extract in a 96-well plate. After 2 hours of reaction at room temperature without light, the absorbance was measured at 750 nm using a microplate reader (Bio-Rad, iMark, Hercules, CA). Distilled water was used as a blank. Calibration curves were performed with 5 to 150 mg gallic acid/L as a standard, and methanol (80% v/v) was used as current negative control. The total phenolic content was expressed as mg gallic acid equivalents (GAE)/g extract.
Chromatographic Evaluation of Some Polyphenolic Compounds
Polyphenols qualitative presence were obtained by a solid-liquid extraction with 0.3% acetic acid and methanol 50:50 (v/v) and analyzed by high-performance liquid chromatography (HPLC) with a UV-Vis diode arrangement device (DAD), λ = 245 nm (Agilent Technologies, Palo Alto, CA). The following were used as patterns at 50 μg/μL concentration (Sigma-Aldrich, St Louis, MO): gallic acid (G27645), p-hydroxybenzoic acid (HB240141), vanillic acid (V94770), ferulic acid (128708), trans-cinnamic acid (T133770), xanthine caffeine (C8960), theobromine (T4500), teofilina (T1633), (±)-catechin (C1788), (±)-epicatechin (E1753), (−)-epigallocatechin gallate (EGCG 4143), (−)-epicatechin gallate (ECG, E3893), (−)-epigallocatechin (E3768), caffeic acid (C0625), p-cumaric acid (C9008), rosmarinic acid (R536954), quercetin (Q4951), naringenin (N5893), luteolin (L9283), kaempferol (K0133), ursolic acid (U6753), pinocembrin (P5239), carnosic acid (C0609), and apigenin (A3145). The separation was performed on a Kinetex (C18) column, 100 mm × 4.6 mm (id) × 2.6 μm (particle size) (Phenomenex; Torrance, CA) at a temperature of 35°C, a flow rate of 1.0 mL/min, and detection at 280 nm. The mobile phase consisted of A (0.3% acetic acid) and B (acetonitrile HPLC) according to the following gradient program (min): Time 0, 95.5:4.5; Time 13, 95.5:4.5; Time 14, 85:15; Time 17, 85:15; Time 20, 78:22; Time 28, 78:22; Time 30, 0:100; Time 33, 0:100; Time 34, 95.5:4.5; and Time 13, 95.5:4.5. Samples were analyzed in duplicate from 4 independent samples against a control extraction with methanol. Results were expressed by peak area in mAU s.
The quantification of gallic acid, catechin, epicatechin, and quercetin were completed by reverse-phase HPLC (RP-HPLC; LaChrom Chromatograph, Merck-Hitachi; Germany-Japan) with a C18 reverse-phase column (Phenomenex; Torrance, CA) at 25°C, a flow rate of 1.0 mL/min,17 and UV/VIS detector at 280 nm. The mobile phase consisted of A (2% acetic acid) and B (methanol) according to the following gradient program: Time: 0, 10, 60, and 70 minutes; % Phase A: 95%, 95%, 50%, and 95%; and % Phase B: 5%, 5%, 50%, and 5%, respectively. Reference standards of gallic acid (5-100 μg/mL), catechin (2-80 μg/mL), epicatechin (5-100 μg/mL), and quercetin (1-90 μg/mL) (Sigma-Aldrich; St Louis, MO)were prepared in a mixture of methanol and 2% acetic acid (6:4, v/v). Extracts were reconstituted in the same mixture, homogenized for 15 minutes by a 37-kHz Elmasonic S (Elma Hans Schmidbauer, Singen, Germany) and filtered through a 0.45 μm membrane to remove any remaining solid particles. Each extract (100 μL per sample) was analyzed by chromatography in triplicate.
Ferric Reducing Antioxidant Power (FRAP)
The FRAP assay measures the reducing potential of antioxidants by TPTZ (2,4,6-(tri-(2-pyridyl-s-triazine)); Sigma; St Louis, MO) reaction, yielding a blue ferrous complex. The results were expressed as μmol Trolox/g (TEAC/g).18 In accordance with López-Cobo et al,19 20 μL of extract was mixed with 30 μL of water in each well of a 96-well microplate. Then, 200 μL of the fresh FRAP reagent was added, acetate buffer (300 mM; pH 3.6), 10 mL of FeCl3 (20 mM), and 1 mL of TPTZ (10 mM) with HCl (40 mM). After 8 minutes without light, the absorbance was read at 595 nm (Bio-Rad Microplate, iMark, Hercules, CA) using water as a blank. The antioxidant activity was calculated using calibration curves of Trolox standard in a dose-response manner (5-150 mM).
DPPH Free Radical Scavenging Assay
The ability to inhibit free radicals was determined spectrophotometrically by monitoring the inhibition of the radical 2,2-diphenyl-1-picrylhydrazyl (DPPH).20 A series of 5 dilutions for each extract (dilution range: 150 mg/mL to 12 mg/mL) were prepared in 96-well plates, and 25 μL of the diluted extract was added to a 200 μL methanol solution of 150 mM DPPH with an absorbance of 1.1 at 515 nm. After 16-minute incubation at room temperature, the inhibition of the radicals was determined at 515 nm using a Varian Cary 100 UV-Vis spectrophotometer (GMI, Inc, Ramsey, MN). A calibration curve was prepared with Trolox (50-800 μM). Inhibition of the radicals was expressed as μmol TEAC/g extract, and also indicated the concentration of extract that caused a 50% decrease in the initial concentration of DPPH (EC50).21 The DPPH inhibition percentage (% IDPPH) was determined by using the Equation (1):
| 1 |
Carotene Content Determination
The carotenoids were quantified spectrophotometrically using a β-carotene standard (Sigma, St Louis, MO) according to AOAC Standard No. 938.04. Each extract (3.8 g) was macerated with acetone-hexane 1:9, (v/v) (5 mL) for 2 hours. Then, 20 mL of acetone-hexane (1:9) was added to the macerated extract, filtered, and diluted to 25 mL. Finally, 0.6 mL of this solution was added to a test tube with 4 mL of petroleum ether and stirred for 30 seconds. The absorbance was determined at a wavelength of 449 nm using a Cary Varian 100 UV-Vis spectrophotometer (GMI, Inc, Ramsey, MN). β-Carotene was used to generate a standard curve (0.1-0.8 mg/L) with petroleum ether as a blank, and the carotene content was expressed as μg equivalents of β-carotene/gram extract (EBC/g).
Cell Cultures
The human cervical cancer cells (HeLa) and murine fibroblasts cell line (L929), obtained from ATCC coding L-929 ATCCR CCL-1 and HeLa CCL-2, respectively, were cultured in RPMI 1640 medium supplemented with 5% fetal bovine serum (FBS; Microgen, Bogotá, Colombia). The cells were cultured separately at 37°C, 95% humidity, and 5% CO2. In all experiments, the cells were used in the exponential growth phase. The culture medium was changed twice per week.22 All experimental procedures included untreated viable cells as control. Cell lines selection was done taking into consideration low regulation consent and availability.
In Vitro Cytotoxicity by Resazurin Reduction
The cell suspension was at a density of 1 × 105 cells/mL in RPMI medium supplemented with 5% FBS. Cells were seeded in 192-well plates (100 μL/well) and incubated at 37°C, 95% humidity, and 5% CO2 for 24 hours. The cells were treated with each extract at 8 concentrations: 1000 μg/mL, 500 μg/mL, 250 μg/mL, 125 μg/mL, 62.5 μg/mL, 31.25 μg/mL, 15.6 μg/mL, and 7.8 μg/mL. The final volume was 200 μL. The extracts were diluted in RPMI 1640 medium and dimethyl sulfoxide (DMSO) at nontoxic concentrations. To rule out cytotoxicity unrelated to the compounds from Physalis peruviana, ethanol, isopropanol, and DMSO solvents were evaluated as controls at 6 concentrations between 0.3% and 10%. The tests were performed with paclitaxel (Taxol, conventional chemotherapy drug) at 7 concentrations between 0.67 and 42.9 μg/mL; these tests served as a positive control for the cytotoxic effect. The treated cells were incubated for 72 hours at 37°C, 95% humidity, and 5% CO2. The supernatant was removed, and some supernatant was cryopreserved at −20°C for later use in tests for immunomodulatory activity. The supernatant was added to 100 μL of 44 μM resazurin. After 4 hours of incubation at 37°C, 95% humidity, and 5% CO2, the test was read using a Tecan Fluorometer (Genius; Austria) with 535 nm filters for excitation and 595 nm filters for emission. The blue resazurin reduction method is an indicator of nontoxic viability and allows for the determination of the half-maximal inhibition concentration value (IC50) for each extract, as evaluated by measuring the fluorescence of nontreated cells as indicating 100% viability.23
Analysis of Immunomodulatory Activity by Quantifying Cytokines
Cytokines were quantified in the supernatants of cultured HeLa and L929 cells that had previously been cryopreserved with commercial kits for detecting cytokines and inflammation in human and murine cells using the Cytometric Bead Array system24 (CBA; BD Biosciences, San Diego, CA). The cytokines analyzed in murine cells included interleukin-6 (IL-6), IL-10, monocyte chemo-attractant protein (MCP-1), interferon-γ (IFN-γ), tumor necrosis factor (TNF), and interleukin-12p70 (IL-12p70). For human cells, the cytokines analyzed included IL-8, IL-1β, IL-6, IL-10, TNF, and IL-12p70. The tests were conducted according to the manufacturer’s instructions. The supernatants of treated cells were exposed to 3 concentrations of each extract, 1000 μg/mL, 9.5 μg/mL, and 3.13 μg/mL, and were incubated for 72 hours. For both tests, 50 μL of each secondary antibody was mixed with 50 μL of capture beads (a mixture containing specific beads for each of the cytokines and chemokines evaluated) and 50 μL of supernatant from the treated cultures. In addition, positive control incubations were performed using 10 mM Taxol (Paclitaxel). These controls were incubated for 2 hours at 18°C without light. In parallel, dilutions of recombinant proteins for each cytokine expressed by HeLa cells and L929 cells under baseline conditions were analyzed to perform the calibration curve for each kit. The samples were washed, and the acquisition of data for each sample was performed using a FACS Canto II flow cytometer (BD Bioscience). The results were analyzed using FCAP Array v2.0 software (Soft Flow Inc) by the average fluorescence intensity in pg/mL according to the calibration curve constructed for each cytokine.
Statistical Analysis
The experiment was conducted as a nonstructured arrangement of 4 treatments by triplicate. Values are expressed as the means ± standard deviations. Differences between the means were analyzed by ANOVA and compared with Tukey’s test (P < .05) and Duncan’s test using Minitab version 16 software (Minitab, State College, PA). In addition, correlations among variables were performed by Pearson correlation analysis with the same software. The IC50 values were calculated using GraphPad Prism 5 software (Graphpad Software, Las Jolla, CA). Dose-response curves were obtained by graphing the percentage of inhibition versus the concentration. Cytokine concentrations were calculated with FCAP Array v2.0 software.
Results and Discussion
Through result analysis, it was observed that the isopropanol (I) extract appeared to be superior to the ethanol fruit extract in terms of antioxidant activity. Additionally, there seemed to be a relationship between the antioxidant activities, polyphenols content, cytotoxic activity, and the suppression of pro-inflammatory cytokines and chemokines expressed for HeLa and L929 cell lines. The implication of this study was the potential use of this fruit in the development of functional products with possible anticancer and immunomodulatory properties.
Polyphenol Content and Preliminary Chromatographic Profile
The highest total polyphenol content was detected in the IC1 extract, followed by IC2, EC2, and EC1 extracts. This finding suggests better polyphenol extraction in isopropanol compared to ethanol, perhaps because isopropanol has more affinity to polyphenol than ethanol. Contents on polyphenol results of the 4 extracts from fresh Physalis peruviana are presented in Table 1.
Table 1.
Total Polyphenols, Antioxidant Activities, and Carotenoids Content in Physalis peruviana Fruit Extracts*.
| Sample | TP (mg GAE/g Extract) | FRAP (μmol TEAC/g Extract) | DPPH (μmol TEAC/g Extract) | EC50 DPPH (mg/mL Extract) | Total Carotenoids (μg EBC/g Extract) |
|---|---|---|---|---|---|
| EC1 | 2.03 ± 0.11b | 35.02 ± 7.61a | 11.3 ± 1.89a | 48.55 ± 8.12a | 3.65 ± 0.91b |
| EC2 | 2.37 ± 0.03a | 36.81 ± 4.33a | 10.3 ± 0.69a | 36.2 ± 2.42a | 4.91 ± 0.09a |
| IC1 | 2.97 ± 0.02c | 47.87 ± 5.71a | 14.6 ± 1.14b | 26.81 ± 2.09b | 3.17 ± 0.18b |
| IC2 | 2.94 ± 0.02c | 42.17 ± 5.45a | 12.18 ± 0.97ab | 34.17 ± 2.72a | 1.28 ± 0.15c |
Abbreviations: TP, total polyphenols; IC1, isopropanol extract from C1; IC2, isopropanol extract from C2; EC1, ethanol extract from C1; EC2, ethanol extract from C2.
*This data set corresponds to the mean (n = 3) ± standard deviation. Different letters indicate significant differences between the extracts (Tukey, P < .05).
According to findings from other studies, the extraction yield of phenolic compounds depends on the polarity and nature of the solvent.25 Polyphenol content in Physalis peruviana fruits had been evaluated at different harvesting time,2 showing a content range from 0.77 to 0.59 g GAE kg FW−1 and from 6.4 to 5.4 mg 100 g FW−1 depending on the ripening stage.26 Polyphenols are not uniformly distributed in plants, and the contents of a specific phenolic compound vary widely depending on the organ studied, growth stage when harvested, the geographic place of production and storage after harvesting, and the ripening stage of the fruit.16 There was a significant difference due to solvent, with a better performance of isopropanol (P < .05). Some of the phenolic compounds most represented in the extracts were preliminarily evaluated with RP-HPLC and identified by comparing the retention times of the signals in the samples with those obtained for the standards. Contents on polyphenol results of the 4 extracts from fresh Physalis peruviana are shown in Table 2 and original chromatograms appear on Supplementary Materials Figure 1 (available online). Mass spectrometry was performed as the next step to the final identification of those polyphenolic compounds, which have been related with antitumor and anti-inflammatory activities.26
Table 2.
Preliminary Content of Polyphenolic Compounds in Physalis peruviana Extracts*.
| Compound | Polyphenol Type | Retention Time (Minutes) | Content in Extract (μg/100 g Dry Weight) | |||
|---|---|---|---|---|---|---|
| IC1 | IC2 | EC1 | EC2 | |||
| Gallic acid | Phenolic acids | 8.6 | 65.2 ± 0.85a | 11.2 ± 1.93b | 11.2 ± 1.68b | 6.7 ± 1.35c |
| Catechin | Flavanol | 29.9 | 665.2 ± 0.8a | 509.8 ± 6.65b | 380.9 ± 6.71c | 422.8 ± 56.4c |
| Epicatechin | Flavanol | 38.5 | 21.2 ± 0.92b | 64.9 ± 8.75a | 55.4 ± 11.15a | 21.9 ± 1.53b |
| Quercetin | Flavanol | 65.2 | 1018.8 ± 1.3a | 9.9 ± 0.31bc | 5.6 ± 0.03c | 14.1 ± 0.93b |
Abbreviations: IC1, isopropanol extract from C1; IC2, isopropanol extract from C2; EC1, ethanol extract from C1; EC2, ethanol extract from C2.
*The data correspond to the mean (n = 3) ± standard deviation. Different letters indicate significant differences of each compound between the extracts (Tukey P < .05).
The evaluation of samples (from 4 independent replicates) by the time of retention compared with 24 patterns, including catechins, phenolic acids, xanthines, and flavonoids, indicated a trace presence of acid, and the outcomes showed differences among the samples due to the solvent extraction technique. Extracts obtained with isopropanol showed rosmarinic acid with an average of 23.97 ± 0.98 mAU s. Solvent extraction also showed difference at revealing ursolic acid, with an average of 11.56 ± 0.54 mAU s for ethanol extracts and 19.74 ± 2.79 mAU s for isopropanol extracts, with a significant higher extraction for isopropanol. Rosmarinic acid has been associated with anti-inflammatory, antioxidative, and, interestingly, anticancer activity against HepG2 cell proliferation when derived from natural extracts as Spica prunellae water extracts, or other Chinese traditional medicinal herbs.27 Ursolic acid has shown antitumor activity, inhibiting human breast cancer cell lines by ethanolic extracts of Wrightia tomentosa, an Indian traditional medicine used against several diseases.28 In sweet marjoram, a plant that exhibits anticancer activity, it has been reported that the presence of rosmarinic acid is associated with antioxidant activity, and also ursolic acid is associated with anticholinesterase inhibition activity.29 Epicatechin gallate was only found in isopropanol extracts, which in green tea has been associated with tumor viability reduction and apoptosis induction in human colon adenocarcinoma cells (HT29).30 Another study reported the presence of rutin, kaempferol, myricetin, and quercetin in a phytochemical screening (HPLC, Fourier transform infrared mass spectrometry) for Indian fruits of Physalis peruviana 31; none of these were found in this current study. The variation of polyphenolic composition depends on many factors, including among others, varietals, ripening stage, climate variations, and the progressive binding to the cell walls, as a defense mechanism against pathogen and stress factors.32
Carotene Content
The carotenoid substances in the extracts ranged between 1.28 ± 0.15 and 4.91 ± 0.09 μg EBC/g extract. These concentrations were highest for the C1 extract with ISO. In Physalis peruviana from Peru, the carotenoid content was estimated to be 2.64 mg carotene/100 g sample, which is equivalent to 4400 IU of vitamin A. In an extract of Physalis peruviana assisted by high hydrostatic pressure, an amount of 1074.67 ± 6.41 mg·100 g−1 was detected.33 Our findings indicate that a large proportion of the carotenoids present in the fruit were also found in the extracts. It was expected that a large content would be present in the extracts due to the high ripening stage, when the β-carotene accumulation is evident.
Antioxidant Activity
Antioxidant activity is vital to combating oxidative damage. An inverse association has been shown between the consumption of fruits and vegetables and morbidity and mortality due to degenerative diseases.34 The present study found values of total antioxidant capacity between 35.02 ± 7.61 and 47.87 ± 5.71 μmol TEAC/g extract. Although the data are slightly higher in the isopropanol extracts compared to the ethanol extracts, there was no significant difference between the extracts (P < .05).
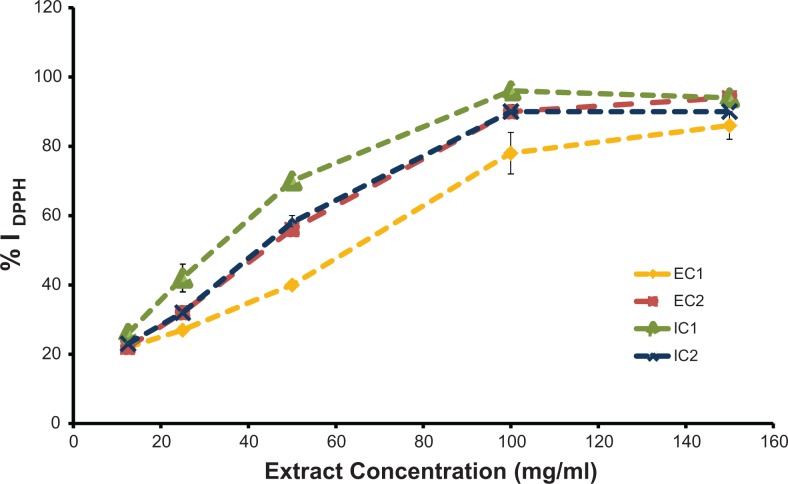
The results of the DPPH analysis for the 4 extracts ranged from 10.3 to 14.6 μmol TEAC/g extract. The values in the extracts with isopropanol were 23.98% significantly higher (P < .05) than those with ethanol (Table 2); however, there were no differences related to which crop was used. The percentage of DPPH inhibition was proportional to the concentration of antioxidants present in the Physalis peruviana extracts as shown in Figure 1. The best EC50 was from IC1 and was significantly higher than the other treatments (P < .05).
Figure 1.
Antioxidant activity dependence on extract concentration. Each value represents the mean of percentage of inhibition (% I DPPH) (n = 3 ± SD) obtained from the extracts of Physalis peruviana applied to L929 fibroblasts or HeLa cells. IC1, isopropanol extract from C1; IC2, isopropanol extract from C2; EC1, ethanol extract from C1; EC2, ethanol extract from C2. The letters indicate Tukey grouping (P < .05).
Cytotoxicity of Crude Extracts
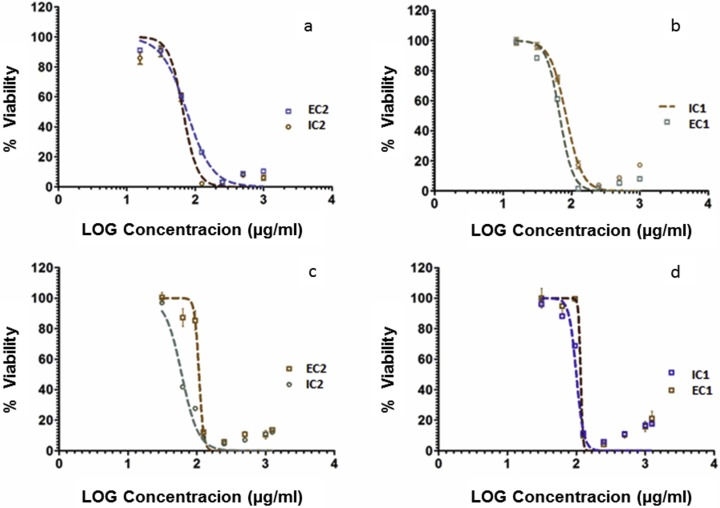
The effect of the Physalis peruviana extracts on HeLa cells and L929 murine fibroblast cells was evaluated using the blue resazurin reduction method as a colorimetric indicator of oxidation/reduction status and/or metabolic activities. The blue resazurin is reduced to a pink fluorescent compound called resorufin by metabolically active or viable cells.24 The cells were treated with 8 different concentrations (1000 μg/mL to 15.6 μg/mL) of the 4 extracts for 72 hours. Graphical representations of the viability and cytotoxic effect of the extracts on the 2 cell lines are presented in Figures 2 and 3.
Figure 2.
Cytotoxic effects of Physalis peruviana extracts on cell lines. The normalized concentration-response curves are shown for (a) EC2, IC2 on L929 fibroblasts; (b) IC1, EC1 on L929 fibroblasts; (c) EC2, IC2 on HeLa cells; (d) IC1, EC1 on HeLa cells. Values represent means ± SD at α = .05.
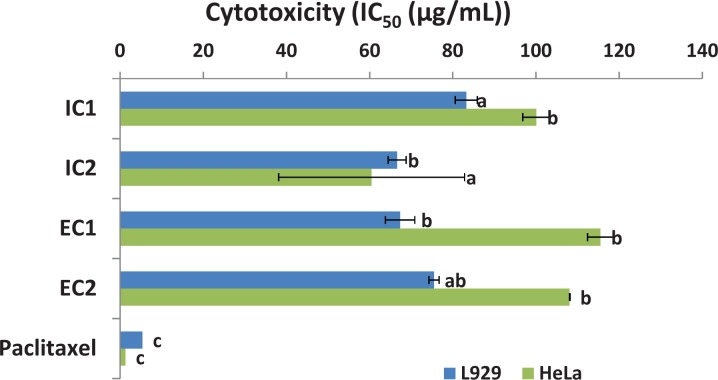
Figure 3.
In vitro cytotoxicity extracts testing compared to paclitaxel. Each value represents the mean IC50 (n = 3) ± SD obtained from the extracts of Physalis peruviana applied to L929 fibroblasts or HeLa cells. IC1, isopropanol extract from C1; IC2, isopropanol extract from C2; EC1, ethanol extract from C1; EC2, ethanol extract from C2. The letters indicate a Tukey grouping (P < .05).
After obtaining data on each cytotoxic profile, cellular inhibition was observed in a dose-dependent manner for both cell lines. In the cervical cancer line (HeLa), the best IC50 value, which corresponds to the minimum value (60.48 ± 3.28 μg/mL), was observed when using the IC2 extract, followed by the EC1, EC2, and IC1 extracts. In comparison, in L929 fibroblasts, the best IC50 value was 66.62 ± 2.67 μg/mL for the IC2 extract, followed by the IC1, EC2, and EC1 extracts.
The US National Cancer Institute suggests that IC50 values below 20 μg/mL correspond to good activity, although many authors have reported that IC50 values below 100 μg/mL show potential anticancer effects.35 Therefore, the IC50 values obtained support the suggestion that all of the extracts have a potential inhibitory effect against L929 cells, whereas only the IC2 extract showed significant inhibitory activity against HeLa cells. However, when comparing the data with values obtained with Taxol treatment (positive control), the extracts were between 12 and 16 times less effective in L929 fibroblasts, and between 50 and 80 times less effective for HeLa cells (Figure 3 and Supplementary Materials, Table 1 [available online]).
Studies on Physalis peruviana have not yet explored the biological activity of fruit extracts on L929 and HeLa cells. Studies on the human colon adenocarcinoma cell line (HT-29), human hepatoma cell line (Hep3B), human breast adenocarcinoma cell line (MCF-7), human neuroblastoma cell line (SH-SY5Y), human osteosarcoma cell line (SaOS-2), and human prostate adenocarcinoma cell line (LNCap) reported promising IC50 values of peruviana fruits (Antalya, Turkey) after 48 hours for SaOS-2, HT-29, Hep3B, and SH-SY5Y cells of 15.44, 40.79, 24.92, and 44.24 μg/mL.36 Studies performed on fruits from other species have been accomplished using murine fibroblasts (L929)37 and human cervical cancer cells (HeLa).38
The current results indicate that the extracts from Physalis fruit exhibited a stronger inhibition in L929 cells compared to HeLa cells; furthermore, the isopropanol extracts were less effective than the ethanol extracts on HeLa cells. The observation concerning the variability of each cell line in the level of response to treatment is supported biologically. Although both cell lines are eukaryotic and are transformed (a characteristic that allows for continuous in vitro culture), they have different biochemical characteristics that influence their susceptibility or resistance to treatment.39
Correlation Between Variables
Results indicate important relationships between these compounds and the antioxidant characteristics of the extracts when performing a Pearson correlation analysis (Supplementary Materials, Table 2 [available online]). The relation between the FRAP and DPPH showed a strong correlation coefficient of 0.925 (P < .01). Phenolic content and total FRAP antioxidant activity had a correlation coefficient of 0.918 (P < .05), while phenolic content and DPPH radical-capture activity correlation was 0.734 (P < .05). The correlation of polyphenols with EC50 was −0.895 (P < .05); hence, lower EC50 values, more effective antioxidant capacity. Similarly, high correlations were observed between catechin content and both FRAP 0.993 (P < .05) and DPPH 0.994 (P < .05), besides between quercetin content and both FRAP 0.851 (P < .05) and DPPH 0.91 (P < .05).
Cytotoxicity outcomes pointed out a high correlation between polyphenol content and the IC50 values in L929 fibroblasts: gallic acid 0.815 (P < .01), catechin 0.716 (P < .01), and quercetin 0.854 (P < .01). However, the IC50 values in HeLa cells exhibited a promising correlation of 0.88 (P < .01) with carotene content, suggesting that cytotoxicity can be dependent on carotene.
Cytokines Expression
The low cytotoxic activity of the extracts despite high antioxidant activity and a high content of polyphenols redirected the research to determine the expression of cytokines, which can provide resistance to the cytotoxic effect of the extracts.
In various types of cancer, a tumor microenvironment is generated in response to the expression or repression of different genes, such as Fas-ligand, cytokines, and chemokines. These genes are important in the immune response and in controlling tumor cells. Immunosuppressive cytokines are also produced at the tumor site, favoring the progression of the neoplastic process.40 It is possible that the cytokines promote tumor development by interacting with growth factors, inducing tumor activity and angiogenesis, and promoting metastasis by increasing cell adhesion.41 The development of anticancer drugs is an invaluable weapon. However, cancers may develop multiple mechanisms of drug resistance, including apoptosis inhibition, drug expulsion, and increased proliferation that reduce the effectiveness of the drug. The collective work of researchers has highlighted the role of cytokines in the mechanisms of cancer drug resistance, as well as in cancer cell progression. Recent studies have described how specific cytokines secreted by cancer stromal cells confer resistance to chemotherapeutic treatments.42
The anti-inflammatory activity of leaf and plant stem extracts from Physalis peruviana has been confirmed mainly in macrophages.11 Another study of Physalis peruviana ethanol extracts from leaves and shoots—not edible external and separated protection of the fruit also known as calyx—showed antibacterial activity, antioxidant activity, and protection to DNA damage by controlling the expression of Bcl-2 family genes, which regulate apoptosis in HeLa.37
Results on this current study showed that the only constitutively expressed cytokines in HeLa cells under baseline conditions were IL-6 and IL-8. A basal expression of MCP-1 was only found for L929 cells. IL-6 has been demonstrated to directly stimulate proliferation of tumor cells and promote angiogenesis.43 IL-6 levels correlate with disease progression and inversely correlate with response to treatment.44 IL-8 promotes tumor cell proliferation and metastasis,45 and MCP-1 also is able to stimulate migration of normal and malignant cells, as well as promote tumor angiogenesis.46 Overproduction of these factors by growing tumors has been shown to lead to resistance to therapy and overall poor prognosis.44,47
In our study, the residual expression of cytokines and chemokines exhibited a dose-dependent effect. The HeLa cells treated with the 4 extracts showed a dose-dependent decrease in IL-6 and IL-8 expression as observed in Table 3. The untreated viable cells’ basal expression was assumed as the maximum expected. Information in Table 3 allows comparison of the extracts’ performance at any evaluated concentration, pointing out the minimal concentration with similar behavior with paclitaxel.
Table 3.
Quantification of Cytokines and Chemokines Expression in HeLa and L929 Cells†.
| Sample | Extract Concentration (μg/mL) | HeLa | L929 | |
|---|---|---|---|---|
| IL-8 (pg/mL) | IL-6 (pg/mL) | MCP-1 (pg/mL) | ||
| Control* | 0 | 261.9 ± 12.8** | 1705.2 ± 123.7**a | 689.6 ± 3.3** |
| EC1 | 31.25 | 24.8 ± 10.7cd e | 23.6 ± 1.5c e | 200.6 ± 3.8b e |
| 95 | 1 ± 0.7cd f | 17.4 ± 2.3c f | 0.0b fg | |
| 1000 | 0.0cd f | 0.0c g | 0.0b g | |
| EC2 | 31.25 | 65.4 ± 1 9.4b e | 79.1 ± 18.7a e | 217.2 ± 4.3a e |
| 95 | 2.5 ± 1.9b f | 43.3 ± 4.7a f | 35.7 ± 2.5a fg | |
| 1000 | 0.0b f | 0.0a g | 0.0a g | |
| IC1 | 31.25 | 41.3 ± 0.9c e | 39.6 ± 5.3b e | 67.7 ± 3.9c e |
| 95 | 1.2 ± 0.3c f | 38.8 ± 0.2b f | 30.1 ± 4.3c fg | |
| 1000 | 0.0c f | 0.0b g | 0.0c g | |
| IC2 | 31.25 | 97.4 ± 10.2a e | 68.3 ± 0.4b e | 230.7 ± 22.7a e |
| 95 | 0.0a f | 18.9 ± 1.1b f | 28.1 ± 1.9a fg | |
| 1000 | 0.0a f | 0.0b g | 0.0a g | |
| Paclitaxel | 5.35 | 3.0 ± 0.6d f | 9.2 ± 0.8d g | 60.7 ± 0.6c f |
| 21.4 | 0.0d f | 0.0d g | 0.0c g | |
Abbreviations: IC1, isopropanol extract from C1; IC2, isopropanol extract from C2; EC1, ethanol extract from C1; EC2, ethanol extract from C2.
†The data set corresponds to the mean (n = 3) ± standard deviation.
*Refers to untreated viable cells’ basal expression.
**Significant difference with other treatments.
Letters a, b, c, and d indicate Duncan grouping for type of extract by column; letters e, f, and g indicate Duncan grouping for concentration by column (P < .05).
Paclitaxel is one of the most effective chemotherapy drug for several cancer types including HeLa. However, resistance is an adverse factor that reduce apoptosis effectivity and could induce autophagy.48 Hartman et al showed that IL-6 and IL-8 are produced for autocrine signaling in TNBCs (triple-negative breast cancers) cell lines in vitro. Additionally, it was reported that combined IL-6 and IL-8 inhibition in TNBCs enhanced paclitaxel-induced apoptosis, suggesting that concurrent IL-6 and IL-8 signaling plays a critical role in TNBC resistance to apoptosis.49 Additionally, human foreskin fibroblast immortalized treated with paclitaxel at a low concentration (100 nM) showed adverse inflammatory effects, associated with the increasing of luciferase activity, and promoting the emergence of a highly glycolytic, autophagic, and pro-inflammatory environment, transforming stromal fibroblast into cancer-associated fibroblast.50
Consistent with the findings in this article, a high expression of chemokine IL-8 in HeLa cells was previously reported.51 The chemokine IL-8 plays an important role in stimulating the regulation of integrins, elevating cytoplasmic calcium, and the adhesion of polymorphonuclear cells to the endothelium, as well as their transversion during the inflammatory process. When comparing the extracts applied with paclitaxel and control treatment, the results showed a significant effect on cytokine IL-8 and chemokine MCP-1M at a concentration of 95 μg/mL. There was no evidence of differential effects over IL-8 due to type of solvent (Duncan’s test, P < .05).
Treatment with extracts at 1000 μg/mL resulted in zero readings in all cases because this concentration induces total cell death. However, at 95 μg/mL, the extracts showed the same inhibition of IL-8 in HeLa cells as paclitaxel, at 5.35 and 21.4 μg/mL, respectively. The extracts inhibited IL-8 basal production by almost 18-fold with no significant difference (P < .05), and there was no evidence to confirm a solvent effect over its inhibition of IL-8.
When identifying potential molecular markers for uterine cervix cancer (UCCa), an overexpression of protein and gene transcripts of IL-6 in HeLa, SiHa, and CaSki cells, as well as in the biopsies of patients with UCCa, was observed.51 The levels of IL-6 were higher in the cervicovaginal secretions of patients with UCCa than in those from healthy controls, and its production increases with respect to the severity of the neoplasm.52 IL-6 is produced mainly in tumor cells and can act as a growth factor in cervical cell lines.53 The presence of IL-6 in 80.5% of the 36 samples tested on cervical tumor biopsies was reported.54 Current results of IL-6 in HeLa cells showed high inhibitory rates with Physalis fruit extracts (Table 3). The basal rate reduction of IL-6 levels, around 72-fold, was found similar after treatment with the extracts at 95 μg/mL, with no significant difference with paclitaxel treatment (P < .05), considering the common composition including rosmarinic acid and ursolic acid. A cytotoxicity assay performed with pure rosmarinic acid on MKN45 human gastric cancer cells reported an IC50 of 240.2 μM and the suppression of cytokines, including IL-6, IL-1β, TNF-α, and TNFsR-1.55 Ursolic acid is well recognized as a suppressor of angiogenesis by inhibiting IL-8 in hepatocellular carcinoma and IL-6 in prostate carcinoma.56
MCP-1 is a chemokine that induces monocyte infiltration at the inflammation site,57 presenting an important role in neoplasia by recruiting circulating monocytes to undergo differentiation in situ to macrophages associated with tumors.58 For MCP-1 in L929 murine fibroblasts, the highest level of product inhibition was observed with the IC1 extract at a concentration of 95 μg/mL, which resulted in a 23-fold reduction in basal expression. Reduced MCP-1 levels were also found after treatment with the EC1, EC2, and IC2 extracts at 95 μg/mL, with no significant difference with paclitaxel at 5.35 μg/mL.
Positive results of Physalis peruviana fruits extracts are indicators of the immunomodulatory effects of pro-inflammatory cytokines associated with neoplastic cells and tumor progress. Extracts from the leaves of Physalis peruviana obtained in supercritical CO2 showed significant protection against LPS-induced inflammation and inhibited the expression of iNOS and COX-2 in Raw 264.7 murine macrophages.8,59 There is also evidence of the anti-inflammatory activity of calyx extracts in a murine model of ear edema induced by 13-acetate and 12-tetradecanoilforbol.60 Similarly, J774 murine macrophages infected with Leishmania that were treated with ethanol extract from Physalis peruviana calyxes showed decreased expression in IL-6 and MCP-1.24
In general, a strong anti-inflammatory effect has been shown in analyzed extracts from Physalis peruviana fruits. The efficacy of those products depends on the tumor model and compound or compounds being analyzed, an aspect that requires further research.61 This finding should be confirmed through further studies that could involve a Physalis peruviana fruit source and crop effect assessment. Since Physalis peruviana fruits have lower amounts of active antioxidants compared to the other parts of the plant, it may be helpful to use other plant parts in combination with the fruit for future potential developments of functional products from this plant, as in the case of Psyllium with anti-inflammatory compounds that reduce the expression of IL-8 in gastric epithelial cells in response to Helicobacter pylori. 61, 62
Conclusions
Extracts from Physalis peruviana fruits showed an important role in L929 and HeLa cancer cell lines at the cellular level through cell inhibition and by blocking the release of pro-inflammatory cytokines in a dose-dependent manner. Antioxidant content, antioxidant activity, and biological activity seemed to be associated with the presence of catechin, gallic acid, rosmarinic acid, ursolic acid, and epicatechin gallate. The evidence also indicates that efficiency depended on the solvent used with better performance for isopropanol extracts.
The relevance of this study pointed out a possible alternative treatment of chronic inflammation as a determining factor in the onset, progression, and prognosis of some cancers such as HeLa, based on antioxidant capacity of Physalis extracts. Additionally, outcomes indicated a possible antineoplastic and immunomodulatory effect of interest for developing functional products derived from this exotic fruit that could be enhanced by using other parts of the plant. Although the negative modulation of cytokine production does not eliminate tumor cells or prevent carcinogenesis, fruits extracts could be useful in therapies that include tumoricidal drugs and applications in the field of anti-inflammatory drug innovation.
Supplementary Material
Acknowledgments
The authors thank the Grupo de Investigación en Inmunotoxicología, Departamento de Farmacia, Facultad de Ciencias, Universidad Nacional de Colombia, Bogotá, Colombia, for the technical support to perform biological tests. Special thanks are extended to Dr Diana Granados.
Footnotes
Author Contributions: Conceived and designed the experiments: LED, GCR. Performed the experiments: HM, GCR, LED. Analyzed the data: HM, LED, LGD, MV, GCR. Contributed reagents/materials/analysis tools: GCR, LGD, MV. All the authors contribute equally toward writing the first draft of the manuscript. GCR revised and edited the final version.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors received financial support to perform research from COLCIENCIAS (Colombian governmental agency of research), CCB (Bogotá Chamber of Trading), and Universidad de La Sabana (under Trust No. 04-2009 and ING-75-2008).
Ethical Approval: This study did not need ethical approval as no animal or human subjects were involved. It was carried out in accordance with the current ethical norms approved by Decree Law 1375-1376 (June 27, 2013) and the Resolution 1348 (August 14, 2014) from the Colombian Ministry of Environment and Development, which describes the accessibility to biological and genetic resources, its products and the intangible components, establishing no special permission to study commercial crops.
Supplemental Material: The supplemental materials for this article are available online.
References
- 1. Gülçin I. Antioxidant activity of food constituents: an overview. Arch Toxicol. 2012;86:345–391. [DOI] [PubMed] [Google Scholar]
- 2. Li X, Wang T, Zhou B, Gao W, Cao J, Huang L. Chemical composition and antioxidant and anti-inflammatory potential of peels and flesh from 10 different pear varieties (Pyrus spp.). Food Chem. 2014;152:531–538. [DOI] [PubMed] [Google Scholar]
- 3. Yeddes N, Chérif JK, Guyot S, Sotin H, Ayadi MT. Comparative study of antioxidant power, polyphenols, flavonoids and betacyanins of peel and pulp of three Tunisian opuntia forms. Antioxidants. 2013;2:37–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Niranjana R, Gayathri R, Mol SN, et al. Carotenoids modulate the hallmarks of cancer cells. J Funct Foods. 2015;18:968–985. [Google Scholar]
- 5. Diaz LE, Munoz DR, Prieto RE, et al. Antioxidant, antitubercular and cytotoxic activities of Piper imperiale . Molecules. 2012;17:4142–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oueslati S, Trabelsi N, Boulaaba M, Legault J, Abdelly C, Ksouri R. Evaluation of antioxidant activities of the edible and medicinal Suaeda species and related phenolic compounds. Ind Crops Prod. 2012;36:513–518. [Google Scholar]
- 7. Rabie MA, Soliman AZ, Diaconeasa ZS, Constantin B. Effect of pasteurization and shelf life on the physicochemical properties of Physalis (Physalis peruviana L.) juice. J Food Process Preserv. 2015;39:1051–1060. [Google Scholar]
- 8. Wu SJ, Tsai JY, Chang SP, et al. Supercritical carbon dioxide extract exhibits enhanced antioxidant and anti-inflammatory activities of Physalis peruviana . J Ethnopharmacol. 2006;108:407–413. [DOI] [PubMed] [Google Scholar]
- 9. Pardo JM, Fontanilla MR, Ospina LF, Espinosa L. Determining the pharmacological activity of Physalis peruviana fruit juice on rabbit eyes and fibroblast primary cultures. Invest Opthalmol Vis Sci. 2008;49:3074–3079. [DOI] [PubMed] [Google Scholar]
- 10. Arun M, Asha VV. Preliminary studies on antihepatotoxic effect of Physalis peruviana Linn. (Solanaceae) against carbon tetrachloride induced acute liver injury in rats. J Ethnopharmacol. 2007;111:110–114. [DOI] [PubMed] [Google Scholar]
- 11. Puente LA, Pinto-Muñoz CA, Castro ES, Cortés M. Physalis peruviana Linnaeus, the multiple properties of a highly functional fruit: a review. Food Res Int. 2011;44:1733–1740. [Google Scholar]
- 12. Al-Olayan EM, El-Khadragy MF, Aref AM, Othman MS, Kassab RB, Moneim AEA. The potential protective effect of Physalis peruviana L. against carbon tetrachloride-induced hepatotoxicity in rats is mediated by suppression of oxidative stress and downregulation of MMP-9 expression. Oxid Med Cell Longev. 2014;2014:381413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dkhil MA, Al-Quraishy S, Diab MM, Othman MS, Aref AM, Moneim AEA. The potential protective role of Physalis peruviana L. fruit in cadmium-induced hepatotoxicity and nephrotoxicity. Food Chem Toxicol. 2014;74:98–106. [DOI] [PubMed] [Google Scholar]
- 14. Ahmed LA. Renoprotective effect of Egyptian cape gooseberry fruit (Physalis peruviana L.) against acute renal injury in rats. ScientificWorldJournal. 2014;2014: 273870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moneim AEA. Prevention of carbon tetrachloride (CCl4)-induced toxicity in testes of rats treated with Physalis peruviana L. fruit. Toxicol Ind Health. 2016;32:1064–1073. [DOI] [PubMed] [Google Scholar]
- 16. Bravo K, Sepulveda-Ortega S, Lara-Guzman O, Navas-Arboleda AA, Osorio E. Influence of cultivar and ripening time on bioactive compounds and antioxidant properties in Cape gooseberry (Physalis peruviana L.). J Sci Food Agric. 2015;95:1562–1569. [DOI] [PubMed] [Google Scholar]
- 17. Ahmad N, Zuo Y, Lu X, Anwar F, Hameed S. Characterization of free and conjugated phenolic compounds in fruits of selected wild plants. Food Chem. 2016;190:80–89. [DOI] [PubMed] [Google Scholar]
- 18. Goyeneche R, Roura S, Ponce A, et al. Chemical characterization and antioxidant capacity of red radish (Raphanus sativus L.) leaves and roots. J Funct Foods. 2015;16:256–264. [Google Scholar]
- 19. López-Cobo A, Gómez-Caravaca AM, Cerretani L, Segura-Carretero A, Fernández-Gutiérrez A. Distribution of phenolic compounds and other polar compounds in the tuber of Solanum tuberosum L. by HPLC-DAD-q-TOF and study of their antioxidant activity. J Food Compos Anal. 2014;36:1–11. [Google Scholar]
- 20. İzli N, Yıldız G, Ünal H, Işık E, Uylaşer V. Effect of different drying methods on drying characteristics, colour, total phenolic content and antioxidant capacity of goldenberry (Physalis peruviana L.). Int J Food Sci Technol. 2014;49:9–17. [Google Scholar]
- 21. Vega-Gálvez A, Díaz R, López J, et al. Assessment of quality parameters and microbial characteristics of Cape gooseberry pulp (Physalis peruviana L.) subjected to high hydrostatic pressure treatment. Food Bioprod Process. 2016;97:30–40. [Google Scholar]
- 22. Franco LA, Ocampo YC, Gómez HA, De la Puerta R, Espartero JL, Ospina LF. Sucrose esters from Physalis peruviana calyces with anti-inflammatory activity. Planta Med. 2014;80:1605–1614. [DOI] [PubMed] [Google Scholar]
- 23. Ma L, Chen H, Dong P, Lu X. Anti-inflammatory and anticancer activities of extracts and compounds from the mushroom Inonotus obliquus . Food Chem. 2013;139:503–508. [DOI] [PubMed] [Google Scholar]
- 24. Martínez W, Ospina LF, Granados D, Delgado G. In vitro studies on the relationship between the anti-inflammatory activity of Physalis peruviana extracts and the phagocytic process. Immunopharmacol Immunotoxicol. 2010;32:63–73. [DOI] [PubMed] [Google Scholar]
- 25. Xu X, Li F, Zhang X, et al. In vitro synergistic antioxidant activity and identification of antioxidant components from Astragalus membranaceus and Paeonia lactiflora . PLoS One. 2014;9:e96780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Valdenegro M, Fuentes L, Herrera R, Moya-León MA. Changes in antioxidant capacity during development and ripening of goldenberry (Physalis peruviana L.) fruit and in response to 1-methylcyclopropene treatment. Postharvest Biol Technol. 2012;67:110–117. [Google Scholar]
- 27. Wu J, Zhu Y, Li F, et al. Spica prunellae and its marker compound rosmarinic acid induced the expression of efflux transporters through activation of Nrf2-mediated signaling pathway in HepG2 cells. J Ethnopharmacol. 2016;193:1–11. [DOI] [PubMed] [Google Scholar]
- 28. Chakravarti B, Maurya R, Siddiqui JA, et al. In vitro anti-breast cancer activity of ethanolic extract of Wrightia tomentosa: role of pro-apoptotic effects of oleanolic acid and urosolic acid. J Ethnopharmacol. 2012;142:72–79. [DOI] [PubMed] [Google Scholar]
- 29. Bina F, Rahimi R. Sweet marjoram: a review of ethnopharmacology, phytochemistry, and biological activities. J Evid Based Complementary Altern Med. 2017;22:175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sánchez-Tena S, Alcarraz-Vizán G, Marín S, Torres JL, Cascante M. Epicatechin gallate impairs colon cancer cell metabolic productivity. J Agric Food Chem. 2013;61:4310–4317. [DOI] [PubMed] [Google Scholar]
- 31. Sathyadevi M, Subramanian S. Extraction, isolation and characterization of bioactive flavonoids from the fruits of Physalis peruviana Linn extract. Asian J Pharm Clin Res. 2015;8:152–157. [Google Scholar]
- 32. Acosta-Estrada BA, Gutiérrez-Uribe JA, Serna-Saldívar SO. Bound phenolics in foods, a review. Food Chem. 2014;152:46–55. [DOI] [PubMed] [Google Scholar]
- 33. Briones-Labarca V, Giovagnoli-Vicuña C, Figueroa-Alvarez P, Quispe-Fuentes I, Pérez-Won M. Extraction of β-carotene, vitamin C and antioxidant compounds from Physalis peruviana (Cape gooseberry) assisted by high hydrostatic pressure. Food Nutr Sci. 2013;4:109–118. [Google Scholar]
- 34. Barreca D, Lagana G, Ficarra S, et al. Evaluation of the antioxidant and cytoprotective properties of the exotic fruit Annona cherimola Mill. (Annonaceae). Food Res Int. 2011;44:2302–2310. [Google Scholar]
- 35. Le Roux K, Hussein AA, Lall N. In vitro chemo-preventative activity of Crotalaria agatiflora subspecies agatiflora Schweinf. J Ethnopharmacol. 2011;138:748–755. [DOI] [PubMed] [Google Scholar]
- 36. Demir T, Özen MO, Hameş-Kocabaş E. Antioxidant and cytotoxic activity of Physalis peruviana . Med Plant Res. 2014;4:30–34. [Google Scholar]
- 37. Çakir Ö, Pekmez M, Çepni E, Candar B, Fidan K. Evaluation of biological activities of Physalis peruviana ethanol extracts and expression of Bcl-2 genes in HeLa cells. Food Sci Technol. 2014;34:422–430. [Google Scholar]
- 38. Amagase H, Farnsworth NR. A review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (Goji). Food Res Int. 2011;44:1702–1717. [Google Scholar]
- 39. Edison TJI, Sethuraman MG. Instant green synthesis of silver nanoparticles using Terminalia chebula fruit extract and evaluation of their catalytic activity on reduction of methylene blue. Process Biochem. 2012;47:1351–1357. [Google Scholar]
- 40. Siriwatanametanon N, Fiebich BL, Efferth T, Prieto JM, Heinrich M. Traditionally used Thai medicinal plants: in vitro anti-inflammatory, anticancer and antioxidant activities. J Ethnopharmacol. 2012;130:196–207. [DOI] [PubMed] [Google Scholar]
- 41. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jones VS, Huang RY, Chen LP, Chen ZS, Fu L, Huang RP. Cytokines in cancer drug resistance: cues to new therapeutic strategies. Biochim Biophys Acta. 2016;1865:255–265. [DOI] [PubMed] [Google Scholar]
- 43. Nilsson MB, Langley RR, Fidler IJ. Interleukin-6, secreted by human ovarian carcinoma cells, is a potent proangiogenic cytokine. Cancer Res. 2005;65:10794–10800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Salgado R, Junius S, Benoy I, et al. Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. Int J Cancer. 2003;103:642–646. [DOI] [PubMed] [Google Scholar]
- 45. Xu L, Fidler IJ. Interleukin 8: an autocrine growth factor for human ovarian cancer. Oncol Res. 2000;12:97–106. [DOI] [PubMed] [Google Scholar]
- 46. Ben-Baruch A. The multifaceted roles of chemokines in malignancy. Cancer Metastasis Rev. 2006;25:357–371. [DOI] [PubMed] [Google Scholar]
- 47. Benoy JH, Salgado R, Van Da P, et al. Increased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survival. Clin Cancer Res. 2004;10:7157–7162. [DOI] [PubMed] [Google Scholar]
- 48. Peng X, Gong F, Chen Y, et al. Autophagy promotes paclitaxel resistance of cervical cancer cells: involvement of Warburg effect activated hypoxia-induced factor 1-α-mediated signaling. Cell Death Dis. 2014;5(8):e1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hartman ZC, Poage GM, Hollander P, et al. Growth of triple-negative breast cancer cells relies upon coordinate autocrine expression of the proinflammatory cytokines IL-6 and IL-8. Cancer Res. 2013;73:3470–3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Peiris-Pagès M, Sotgia F, Lisanti MP. Chemotherapy induces the cancer-associated fibroblast phenotype, activating paracrine Hedgehog-GLI signalling in breast cancer cells. Oncotarget. 2015;6:10728–10745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269:199–225. [DOI] [PubMed] [Google Scholar]
- 52. Shukla S, Shishodia G, Mahata S, et al. Aberrant expression and constitutive activation of STAT3 in cervical carcinogenesis: implications in high-risk human papillomavirus infection. Mol Cancer. 2010;9:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ren C, Cheng X, Lu B, Yang G. Activation of interleukin-6/signal transducer and activator of transcription 3 by human papillomavirus early proteins 6 induces fibroblast senescence to promote cervical tumourigenesis through autocrine and paracrine pathways in tumour microenvironment. Eur J Cancer. 2013;49:3889–3899. [DOI] [PubMed] [Google Scholar]
- 54. Nagasaki T, Hara M, Nakanishi H, Takahashi H, Sato M, Takeyama H. Interleukin-6 released by colon cancer-associated fibroblasts is critical for tumour angiogenesis: anti-interleukin-6 receptor antibody suppressed angiogenesis and inhibited tumour-stroma interaction. Br J Cancer. 2014;110:469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bhairavabhotla RK, Verma V, Tongaonkar H, Shastri S, Dinshaw K, Chiplunkar S. Role of IL-10 in immune suppression in cervical cancer. Indian J Biochem Biophys. 2007;44:350–356. [PubMed] [Google Scholar]
- 56. Han S, Yang S, Cai Z, et al. Anti-Warburg effect of rosmarinic acid via miR-155 in gastric cancer cells. Drug Des Devel Ther. 2015;9:2695–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shanmugam MK, Dai X, Kumar AP, Tan BK, Sethi G, Bishayee A. Ursolic acid in cancer prevention and treatment: molecular targets, pharmacokinetics and clinical studies. Biochem Pharmacol. 2013;85:1579–1587. [DOI] [PubMed] [Google Scholar]
- 58. Van Linthout S, Miteva K, Tschöpe C. Crosstalk between fibroblasts and inflammatory cells. Cardiovasc Res. 2014;102:258–269. [DOI] [PubMed] [Google Scholar]
- 59. Richards DM, Hettinger J, Feuerer M. Monocytes and macrophages in cancer: development and functions. Cancer Microenviron. 2013;6:179–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. de Melo MMR, Silvestre AJD, Silva CM. Supercritical fluid extraction of vegetable matrices: applications, trends and future perspectives of a convincing green technology. J Supercrit Fluids. 2014;92:115–176. [Google Scholar]
- 61. Castro JP, Ocampo YC, Franco LA. In vivo and in vitro anti-inflammatory activity of Cryptostegia grandiflora Roxb. ex R. Br. leaves. Biol Res. 2014;47:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yakoob J, Jafri W, Mehmood MH, Abbas Z, Tariq K. Immunomodulatory effects of Psyllium extract on Helicobacter pylori interaction with gastric epithelial cells. J Evid Based Complementary Altern Med. 2016;21:NP18-NP24. doi:10.1177/2156587215611517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.