Abstract
The aim of the study was to evaluate the biological activities and safety of commercial herbal concoctions manufactured in Ga Maja (Limpopo province). Microbial contamination was evaluated by spread-plating the concoctions on agar plates. The VITEK 2 instrument was used for identification of the pure cultures. Nutritional content of the concoctions was determined. Thin layer chromatography was used to analyze the chemical constituents of the extracts. The microdilution assay and bioautography were used to evaluate antimicrobial activity against selected microorganisms. Sodium, potassium, and zinc were elements most abundant in the concoctions. Phytochemical screening revealed the presence of various phytoconstituents. Acetone extracts of Hypoxis hemerocallidea and Kirkia wilmsii extracts had antioxidant activity. The minimum inhibitory concentrations values against test bacteria ranged between 0.02 and 0.63 mg/mL. Further studies are required to isolate bioactive compounds and evaluate their cytotoxicity. Caution in the consumption of the herbal mixtures should be adhered to.
Keywords: alternative medicine, antimicrobial activity, antioxidants
The majority of people in developing countries, estimated to be about 80% of the world’s population, depend on medicinal plants for their principal health care needs.1 In South Africa, an estimated 65% to 80% of the citizens depend on medicinal plants as the primary health care system to treat various ailments.2 The Limpopo province of South Africa is predominantly rural and has high levels of unemployment among its locals, subsequently poverty is prevalent. It is within these factors that the locals are heavily reliant on medicinal plants and use herbal medicines in combination with Western medicines or alone.3
Herbal concoctions have been described as a mixture of different plant species or plant parts to treat various health ailments. The formulas to these concoctions range from simple home remedies to more complex formulas administered by traditional healers to manage life-threatening diseases. Moreover, the latter ranges from simple preparations to more elaborate methods involving organic solvents and alcohols for more detailed extraction of crude plant chemicals.4 However, boiling of fresh herbal portions with water as means of extraction, thus a decoction, was a more favored method used traditionally to prepare herbal concoctions.5 Various plant parts such as leaves, flowers, stems, and/or roots from different or the same plant species are used as ingredients for the medicines.6
The increase in commercialization of herbal mixtures in South Africa has been attributed to increased globalization and urbanization. Many traditional healers and herbalists have integrated into the system of using media outlets such as radio, television, and newspapers as a marketing platform for their herbal products and these include the Internet whereby social networks are of popular use.7
The characteristic(s) of the recipes and the manner of preparation are based on traditional theories and beliefs; however, the packaging and presentation of these medicines rather adopt a Western influence. They are packaged in bottles provided by various distributors and some herbalists use recycled bottles and attach hand-written labels.8 The increase of commercialization of herbal products is observed in South Africa as there is an increase in herbal shops, hawkers, wholesalers, and plant gatherers.2
Traders from Ga Maja (24.1631° S, 29.5543° E) have set up market places where they sell herbal concoctions and the plants used to prepare them. The concoctions are sold in recycled 2-L and 500-mL plastic bottles. They boil the leaves of Monsonia angustifolia, the corms of Drimia elata, Hypoxis hemerocallidea, Kirkia wilmsii, and 2 kinds of powders locally known as Tšhikwana (white powder), Tšhikwana/moroto wa tšhwene (black powder). The contents of the powders have not been specified. These concoctions are claimed to relieve back pains, coughs, and stomach aches. They are also prescribed for hypertension and as aphrodisiacs.
The extent of the use of K wilsmii had been investigated among Bapedi traditional healers9 and in such surveys, it was reported that the dominant ethnic group, Bapedi (Northern Sotho), which is 57% of the Limpopo population10 refer to K wilmsii as Legaba or Modumela in their vernacular and use it to treat hypertension. Diverse African ethnic groups use the genus Hypoxis as a drug to cure and manage a range of health conditions and disorders. It is for this reason that it became an integral ingredient in a number of herbal concoctions.11 Indigenously, health ailments such as cancer, prostatitis, headaches, testicular tumors, impotency, cardiac diseases, and intestinal parasites were managed by a dosage of a decoction of the plant.12 However, urinary tract infections, dizziness, and mental disorders have been alleviated by the infusion of the corm of the plant.11
D elata belongs to the family Hyacinthaceae and is commonly known as sekanama in vernacular. The bulb of the plant is used in combination with Elaeodendron transvaalense, Elephantorrhiza elephantina (roots), Sclerocarya birrea (bark), Zanthoxylum capense (root) and Sarcostemma viminale (twigs) by Bapedi traditional healers for the treatment of sexually transmitted infections.13 The cardiotoxic bufadienolides present in D elata species have also been implicated in human poisoning. These plants are used by traditional healers as “blood purifiers” and for treating a variety of conditions such as headaches, edema, infertility, and bladder complaints.14
Monsonia is a genera classified under the Geraniaceae family. The family is distributed from Australia to Asia, Europe, America, and widely distributed in Africa.15 M angustifolia is a traditional herb most commonly used for the treatment of erectile dysfunction, premature ejaculatory disorders, and for libido enhancement.16
Tšhikwana and “Tšhikwana/moroto wa tšhwene are a combination of roots from different plants being ground together to form a powder (vendors refused to give the exact plants being used). Tšhikwana/moroto wa tšhwene contains extended types of roots more than Tšhikwana alone. Both plant mixtures are claimed to have potentials in the treatment of erectile dysfunction and for the enhancement of libido. Tšhikwana /moroto wa tšhwene is black in color due to addition of more plant roots and normally takes 2 hours before being active in the body. There is insufficient documentation of these powders and the traders claim secrecy regarding the components used to prepare them.
Phytochemicals are naturally occurring plant-synthesized secondary metabolites, which have associated biological activities but are not regarded as part of dietary nutrition. These compounds have a potential on health effects and their biological significance includes antibacterial, antifungal, antioxidant, anti-inflammatory, anticancer, antiaging, and antiatherosclerotic activities. However, these phytochemicals need to be researched in order to confirm and determine their therapeutic value(s) and activities.17
In spite of the knowledge of phytochemicals, a high percentage of them are yet to be identified.18,19 Various environmental factors in growing locations such as temperature, moisture, and illumination are associated with synthesis and accumulation of secondary metabolites. This suggests that production of secondary metabolites depends on growing environment for many plant species.20
Free radicals, commonly referred to as reactive oxygen species are compounds that have one or more unpaired electrons. They are produced in vivo from normal cell metabolism and a number of biochemical reactions such as the ones involved in respiration.21
These reactive oxygen species are involved in the degenerative or pathological reactions such as neurodegenerative disorders, atherosclerosis, inflammation, cataracts,22 cancer, coronary heart disease, and Alzheimer’s disease.23,24 Therefore, therapeutic utilization of substances that can eliminate these reactive oxygen species may aid in the treatment of diseases associated with the accumulation of the compounds.25 Plants have natural antioxidants that have been shown to play a role in scavenging and inhibiting the production of free radicals thus making plants an abundant source of such chemicals.26
Bacteria can develop antimicrobial resistance due to membrane translocases that remove toxins from the cell and multidrug resistance pumps. The latter confer protection from both synthetic and natural antimicrobial compounds.27 However, the synergistic interaction of plant extracts possessing antimicrobial activity and other antibiotics can provide a pathway to curbing the rise of antimicrobial resistant microbes and lead to the development of sanative drugs.28
The increasing cost of medicine(s), the low number, and unavailability of professional health practitioners and public primary health care establishments contribute to the disadvantages of the sole reliance on Western medicine. Consequently, this creates a huge gap of inaccessibility of therapeutic drugs to aid in alleviation of various ailments. This serves as motivation to investigate, screen, identify, and eventually isolate bioactive compounds from plants which exhibit biological activity properties. The benefit of such research is the distribution of more effective drugs than those currently on the market and would provide rural areas with an alternative to inaccessible Western-orientated primary health care.29 In this study, the biological activities of the selected plant species and herbal concoctions were evaluated to validate their use as potent therapeutics.
Methods and Materials
Herbal Concoctions and Plant Collection
Five different herbal concoctions were purchased in late summer of 2016 from 5 independent traders who had set up a market place alongside the main road leading into Ga Maja (Limpopo province). The medicines from all the traditional healers were left exposed to environmental factors such as alternating daily temperatures and exposure to sunlight rays. Collected alongside were parts of the plant species, which were used as ingredients in the recipe for these medicines. Of these were the corms of K wilmsii, D elata, and H hemerocallidea, powdered plant material locally known as Tšhikwana and Tšhikwana/moroto wa tšhwene and the leaves of M angustifolia. The traders prepare these concoctions by mixing and boiling all the mentioned plant material with water in large pots. The decoctions were then cooled and dispensed into recycled bottles. The herbal concoctions were cultured on Sabouraud dextrose agar plates immediately on the day of collection using the spread-plate technique. The corms and leaves of the plant species were cut into smaller pieces and dried at room temperature. A commercial blender was used to grind the dried plant material. The leaves of M angustifolia and the corm of D elata and were ground to fine powder; however, the corm of H hemerocallidea was ground to minute granules and that of K wilmsii was fibrous to be ground to fine powder, hence ground fibrous material of the plant was used in subsequent chemical tests and bioassays.
Extraction of Phytoconstituents From the Ground Plant Materials
The ground plant material (1 g) from each plant species was weighed in 50-mL polyester centrifuge tubes and extracted with 10-mL organic solvents of varying polarity, namely, acetone and n-hexane. In order to completely deplete the extracts from the plant material, the centrifuge tubes were shaken for 30, 20, and 10 minutes in a shaker set at 200 rpm. The resulting extracts from each extraction time were filtered using Whatman No. 1 filter paper and all the 3 filtrates were added together into preweighed glass vials according to the solvent used. To enable quantification of the extracts, the glass vials were placed under a stream of air from a bench-top fan to evaporate the solvents. The mass of the extracts was obtained as the difference between the mass of the glass vial containing the dried extracts and the mass of the same glass vial, which was determined before adding the filtrate plant extracts. All the extracts were reconstituted with acetone to a concentration of 10 mg/mL.
Microorganisms Used in This Study
Bacteria Species
Two Gram-positive bacteria (Staphylococcus aureus ATCC 29213 and Enterococcus faecalis ATCC 29212) and 2 Gram-negative bacteria (Escherichia coli ATCC 28922 and Pseudomonas aeruginosa ATCC 27853) were used. They are major causes of nosocomial infections in hospitals30 and are mainly the strains recommended for use by the National Committee for Clinical Laboratory Standards.31 The bacterial species were maintained on nutrient agar at 4°C. The cells were inoculated and incubated at 37°C in nutrient broth for 12 hours prior to screening tests.
Fungal Species
Candida albicans isolates were used. The fungal culture was maintained at 4°C on Sabouraud dextrose agar plates. The culture was inoculated in 100 mL of yeast extract–peptone–dextrose medium and incubated at 35°C for 24 hours. This served as a stock solution for bioassays that follow.
Evaluation of the Nutritional Content of the Collected Concoctions
The composition of nutrients and the metals present in the concoctions were determined with a plasma atomic emission spectrophotometer (Model ICPE-900, Shimadzu).
Bacterial Identification
The VITEK 2 instrument was used for identification of the pure cultures of the bacterial isolates. The instrument is housed at the Limpopo Agro-food Technology Station at the University of Limpopo. The manufacturer’s protocol was followed for analysis. The VITEK 2 system is a fully automated microbiology system utilizing growth-based technology system and operates in vitro. A suspension of a pure culture was prepared by suspending well isolated colonies in 3.0 mL of sterile saline (aqueous 0.45%-0.50% NaCl, pH 4.5-7.0) in a 12 × 75 mm clear plastic (polystyrene) test tubes using a sterile swab. The test kit card with the transferred suspensions were placed in the VITEK incubator. The VITEK system analyses the card as growth of the organism that occurs and gives an identity of the organism.32
Phytochemical Analysis (Fingerprint Profile)
Extracted chemical components were analyzed by separation with thin layer chromatography (TLC) using aluminum-backed TLC plates (Fluka, silica gel F254). TLC plates were developed in saturated chambers using mobile phases of different polarities, namely benzene/ethanol/ammonia hydroxide (BEA) (nonpolar/basic) (18:2:0.2), chloroform/ethyl acetate/formic acid (CEF) (intermediate polarity/acidic) (10:8:2), and ethyl acetate/methanol/water (EMW) (polar/neutral) (10:5.4:4).33 Separated compounds on the TLC plates were examined under ultraviolet light (254 and 365 nm) then sprayed with vanillin-sulfuric acid reagent (0.1 g vanillin [Sigma]: 28 mL methanol:1 mL concentrated sulfuric acid) and heated at 110°C for optimal color development.
Phytoconstituents Screening
The following photochemical analyses were performed, terpenoids (Salkowski test),34 flavonoids,34 cardiac glycosides (Keller-Killiani test),34 phlobatannin,34 tannins,34 saponins (froth test),35 and alkaloids (Drangendoff’s reagent test).35
Qualitative Antioxidant Activity (DPPH assay) on TLC Plates
Thin layer chromatography was used to separate compounds present in the extracts and the TLC plates were prepared and developed as described above. The chromatograms were air dried and sprayed with 0.2% 2,2-diphenyl-2-picryl-hyrazyl (DPPH) (Sigma) to detect any antioxidant compounds present in the separated plant extracts. The presence of antioxidant activity was detected by yellow spots against a purple background on TLC plates sprayed with 0.2% DPPH in methanol.36
Microdilution Assay by Minimum Inhibitory Concentration
The antibacterial activity was evaluated using the broth microdilution.37 The antifungal activity of the plant extracts was evaluated by determining the minimal inhibitory concentration against Candida albicans with slight modifications38 to suit fungal growth requirements. Ampicillin and amphotericin B were used as positive control(s) for bacterial and fungal strains, respectively, while acetone was used as the negative control. Total activity of the extracts was calculated by dividing the minimum inhibitory concentration (MIC) values with the mass extracted from 1 g of the plant material.
Antimicrobial Activity by Bioautography on TLC
Qualitative antibacterial activity of the extracts was evaluated using the bioautography method39 coupled with modifications for antifungal activity.38 The different plant extracts were redissolved in acetone to a concentration of 10 mg/mL and 20 µL of this resuspension was loaded onto the TLC plates. The plates were prepared and developed under saturated conditions using 3 different solvent systems.
Results and Discussion
The current study was conducted to evaluate the safety and efficacy of 5 different herbal concoctions commercially available at Ga Maja and the biological activities of the plants used to prepare the concoctions. With the lack of regulation and standardization, the consumption of the concoctions is anecdotal in that the claims made by the traditional healers about the efficacy and safety of their products have not been substantiated by scientific evidence. Also, issues of safety arise due to no apparent quality control of the manufacture and packaging of the products.
Generally, the contamination of the herbal concoctions by commonly occurring fungal species (Figure 1) presents a health hazard to consumers. Fungi produce low-molecular-weight secondary metabolites known as mycotoxins and exposure to these toxic metabolites could lead to the onset of diseases in animals and humans generally known as mycotoxicoses.
Figure 1.
Different herbal concoctions (A-D) (100 µL) were cultured on Sabouraud dextrose agar using the spread-plate technique. Growth appeared after 24 hours of incubation at 25°C.
The predominant microbial organism contaminating the concoction was Klebsiella pneumoniae (91% identified) (Table 1). The possible reason for the presence of K pneumoniae is that it occupies habitats such as sewage, soil, drinking water (surface of water), and vegetation; thus, the concoction bottles might have been collected from those areas or the bottle was not properly washed. In sample B, Gram-negative, rod-shaped, and cocci-structured were identified as Pseudomonas fluorescens, Burkholderia mallei, and Staphylococcus lentus, with 34%, 33%, and 93% identification, respectively. This indicates that the containers of 5 vendors used were hypothetically not collected from the same area or they were not disinfected the same way or could have not been disinfected at all. In sample C, rod-shaped Gram-negative cells and a huge clump of cells near one another was observed (Figure 2C). P fluorescens and Burkholderia cepacia were identified as 94% and 87%, respectively (Table 1). This suggests that these plant materials could have been collected from different areas because mostly these microorganisms are found in the soil and water. Thus P fluorescens has been reported as an indicator of water quality.40 In sample D, Klebsiella oxytoca was 99% identified and in sample E, distinctive red/pink elongated rod shaped bacterial cells were observed and identified as Pantoea spp (95%). We concluded that these herbal concoctions contain microorganisms that can affect human health. The most prominent bacterial strains identified were K pneumoniae and P fluorescens. This suggest(s) that prolonged use of these concoctions may expose people to infections such urinary tract infections and hemodynamic shock.41
Table 1.
The Bacterial Identification of All 5 Herbal Concoctions Including Characteristics and Infections.
| Samples (Bottle Labels) | Identified Species | Characteristics | Infections | Percentage Identification Score (%) |
|---|---|---|---|---|
| A | Klebsiella pneumoniae | Gram-negative Rod-shaped | Surgical wound Urinary tract | 91 |
| B | Pseudomonas fluorescens | Gram-negative Rod-shaped | Hemodynamic shock | 34 |
| Burkholderia mallei | Gram-negative Rod-shaped | Glanders infection | 33 | |
| Staphylococcus lentus | Gram-positive Cocci-shaped | Mastitis | 93 | |
| C | Pseudomonas fluorescens | Gram-negative Rod-shaped | Hemodynamic shock | 94 |
| Burkholderia cepacia | Gram-negative Rod-shaped | Pneumonia | 87 | |
| D | Klebsiella oxytoca | Gram-negative Rod-shaped | Nosocomial infection | 99 |
| E | Pantoea spp | Gram-negative Rod-shaped | 95 |
Figure 2.

Gram staining shows the presence of Gram-positive (purple) and negative (pink) diplococci and bacilli (A, B, and C) and Gram-negative (pink) bacilli (D).
The hawkers claim that these remedies can cure rash, flu, urine burns, cleans blood, stomach pains, waist problems, and kidney problems involving numerous microorganisms that are potentially pathogenic.42 The vendors recommend different consumption of each herbal concoction, that is, first vendor suggests that the herbal concoction must be taken 3 times daily, second vendor recommends that the 2-L bottle be finished in 2 days and others suggest that these bottles should be used every day (500 mL). Spoilage of these bottles are said to be after of 3 to 7 days and storage is anywhere but most preferably in the fridge.
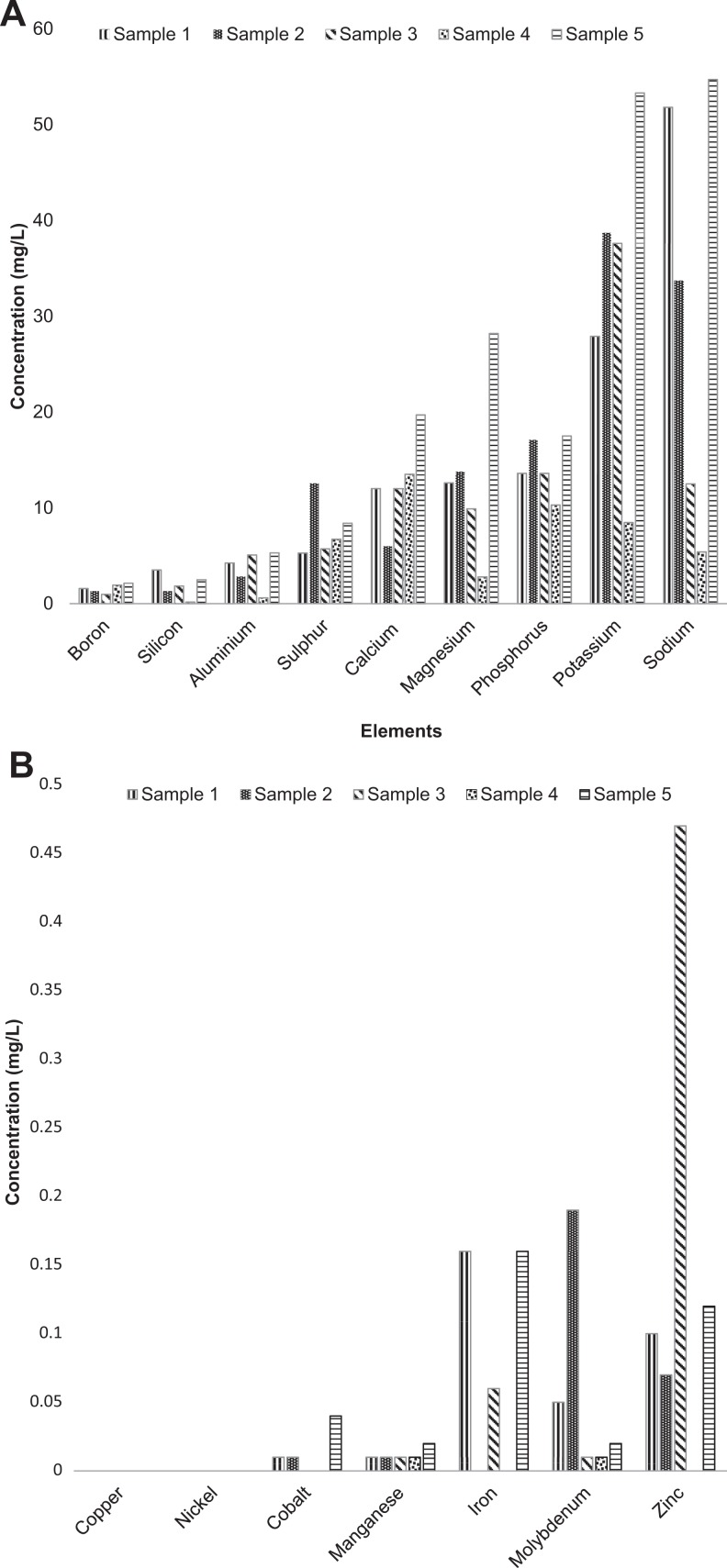
Heavy metals were also checked in the concoctions. They are native constituents of the Earth’s crust and thus cannot be completely removed. Food and beverages contain a variety of trace metals such as sodium (Na), potassium (K), iron (Fe), calcium (Ca), boron (B), magnesium (Mg), aluminum (Al), copper (Cu), zinc (Zn), molybdenum (Mo), cobalt (Co), manganese (Mn), silicon (Si), and nickel (Ni) (Figure 3A and B).43–45 They are present in consumables due to their natural occurrence in the environment and the product, or as a result of human activities, which include agricultural and industrial processes. These metals are known as essential micronutrients because of their functionality in biological systems and are required only in trace amounts.46 However, these essential nutrients are toxic at excessive levels and can be damaging to cells and their related processes. Therefore, the knowledge of their concentrations in consumables is essential to designate recommended dietary intakes of such products.47
Figure 3.
(A) The concentrations of elements present in the herbal concoctions. (B) Concentrations of various transition metals present in the different herbal concoction samples.
The concentrations of the elements in the 5 herbal concoctions were inconsistent and varied significantly. The difference in the concentrations could be due to the heterogeneity of the samples as a result of being collected from different traditional healers. The consequence of the latter is that the traditional healers may have dissimilar sources of raw materials and different methods of preparing and packaging of their products. Of the raw materials, tap water is the main solvent of choice for the preparation of the concoctions and as such the concentrations of the elements could be related to the purity of the water used.48 These variations pose problems for the patients and other consumers in that they would be unable to regulate the amount of elements they consume in order to conform to recommended adequate daily intakes and avoid toxicities.
The parts of the plant species used in this study were chosen because they are used by the Bapedi traditional healers to manufacture herbal concoctions that are ultimately commercialized (personal communication, Ga Maja traders). The choice of an appropriate solvent for the extraction of compounds present in the plant material is an important step in order to accurately evaluate and ascertain the biological activities associated with the particular plant species. Solvents that are preferred by researchers have low toxicity, they are easily removable from the plant extracts at low temperatures and do not induce the extract to form complexes, precipitate, and/or disintegrate into smaller subunits. Moreover, the solvents used have the properties of being able to extract a high yield of compounds of different chemical properties therefore increasing the opportunity of obtaining bioactive compounds in the extract.37,49 It was noted that to prepare the decoctions of the plant material, the traditional healers used water. Water is not only nontoxic but easily accessible and inexpensive for the traditional healers; however, it does not extract nonpolar compounds.50
Acetone and hexane were used to extract the plant material. Acetone was chosen because of its ability to extract compounds of varying polarities and was found to be harmless to fungi.51 In order to extract nonpolar compounds, hexane was selected as an appropriate solvent.52 The extraction yields obtained using the 2 solvents (Figure 4) indicate that acetone was a superior extractant because it was able to extract more plant material compared with hexane.
Figure 4.
The mass of plant extracts obtained from different plant species using acetone and hexane.
Thin layer chromatography was used to investigate the properties of the phytochemicals extracted using acetone and hexane. The colored bands represent the separated compounds and therefore the number of bands on a chromatogram is indicative of the number of compounds present and their relative polarities (Figure 5). A larger number of bands were visible on the CEF developed chromatogram followed by the EMW and BEA developed chromatograms. This demonstrates that overall, the polarity of the compounds present in the extracts are intermediate in nature. In addition, more compounds were observed in the acetone extracts than those obtained using hexane. This shows that the mass yielded with acetone was indicative of the amount of compounds extracted from the plant material.
Figure 5.

Thin layer chromatography chromatograms showing the diversity of compounds present in the hexane (H) and acetone (A) extracts of Kirkia wilmsii (P1), Hypoxis hemerocallidea (P2), Tšhikwana (P3), Tšhikwana/Morotwa tšhwene (P4), Drimia elata (P5), and Monsonia angustifolia (P6).
Chemicals such as antioxidants that can eliminate these free radicals are important in dietary intakes and can thus reduce the risk of the development of diseases associated with high concentrations of free radicals. This study presents the antioxidant activity of the acetone extracts of the corms of K wilmsii and H hemerocallidea and the hexane extracts of corm of D elata and the leaves of M angustifolia (Figure 6). Neither the acetone nor the hexane extracts of Tšhikwana and Tšhikwana/Morotwa tšhwene had antioxidant activity.
Figure 6.

Chromatograms of separated phytochemicals present in the hexane (H) and acetone (A) extracts developed in different solvent systems. Kirkia wilmsii (P1), Hypoxis hemerocallidea (P2), Tšhikwana (P3), Tšhikwana/Morotwa tšhwene (P4), Drimia elata (P5), and Monsonia angustifolia (P6). Chromatograms were sprayed with 0.2% DPPH (2,2-diphenyl-2-picryl-hyrazyl) dissolved in methanol. Yellow zones indicate the free radical scavenging activity of compounds.
The antioxidant compounds of the corms of K wilmsii and H hemerocallidea had high activity and were separated well on the EMW developed chromatogram and to a lesser extent on the CEF-developed chromatogram. The observed high activity on the CEF chromatogram of the acetone extract of H hemerocallidea was in agreement to that observed by other researchers.53 The BEA solvent system was unable to separate the antioxidant compounds of the 2 plant species. The polarity of the compounds might have been so high that they could not be separated with a nonpolar solvent system. Polar solvents such as methanol, acetone, and ethyl acetate are commonly used to extract polyphenols from plant material.54 Therefore, the acetone plant extracts of H hemerocallidea and K wilmsii showing the antioxidant activity could be a reflection of the presence of highly polar polyphenols.
Table 2 indicates the presence of all tested phytoconstituents except alkaloids in H hemerocallidea and D elata. The difference between Tšhikwana and Tšhikwana/Morotwa tšhwene was demonstrated by presence of flavonoids only in Tshikwana and presence of tannins, phlobatannins, and cardiac glycosides.
Table 2.
Demonstration of Various Phytochemicals Present in the Plant Species Used to Brew Herbal Concoctions From Ga Maja.a
| Phytochemicals | P1 | P2 | P3 | P4 | P5 | P6 |
|---|---|---|---|---|---|---|
| Tannins | + | + | − | + | + | + |
| Phlobatannins | + | + | − | + | + | + |
| Flavonoids | − | + | + | − | + | − |
| Saponins | + | + | − | − | + | − |
| Alkaloids | − | − | − | − | − | − |
| Terpenoids | + | + | − | − | + | + |
| Cardiac glycosides | − | + | − | + | + | + |
a(+), presence; (−) absence; P1, Kirkia wilmsii; P2, Hypoxis hemerocallidea; P3, Tšhikwana; P4, Tšhikwana/morotwa tšhwene; P5, Drimia elata; P6, Monsonia angustifolia.
The microdilution and bioautography on TLC assays were used to evaluate the antimicrobial activity of the plant extracts. Solvents can be toxic to microorganisms, therefore inhibiting growth and consequently this interferes with bioassays. Thus, prior to conducting the bioassays, acetone was used to reconstitute the air-dried extracts of both acetone and hexane because acetone was reported to be nontoxic to fungi.51
The microdilution assay was used to determine the lowest amount of the extracts (mg/mL) that is able to inhibit the growth of microorganisms. The study demonstrated that the acetone and hexane extracts had no antifungal activity against C albicans at ≤2.5 mg/mL. (results not shown). MIC values <1 mg/mL are foundations for further pharmacological testing. These results are comparable with those obtained by Katerere and Eloff (2008)53 whereby it was reported that the petroleum ether, dichloromethane, ethanol, and water extracts of the corm of H hemerocallidea had high MIC values (3.13-6.25 mg/mL) against C albicans. The extract of the leaf of K wilmsii was reported to have good antifungal activity against C albicans.55 Escherichia coli and S aureus were the least inhibited by the crude extracts (Table 3). Therefore, in applying medicinal plants for their antimicrobial activities, the choice of the part of the plant to be used is important. Total activity of plants is defined as the amounts of material extracted from a single gram of plant dried material divided by the MIC value (mL/g). Total activity (Table 4) indicates largest volume to which biologically active compounds extracted from 1 g of plant material can be diluted and still inhibits growth of tested organisms. Total activity is important when evaluating potential use of plant extracts for treating fungal and bacterial infections.56
Table 3.
The Minimum Concentrations of the Extracts Capable of Inhibiting the Bacterial Growth.a
| Plant | Extraction Solvent | Mass Plant Extract in (mg/mL) | Minimum Inhibitory Concentration Value (mg/mL) | ||||
|---|---|---|---|---|---|---|---|
| Escherichia coli | Pseudomonas aeruginosa | Enterococcus faecalis | Staphylococcus aureus | Average | |||
| P1 | Acetone | 16.9 | 0.63 | Not applicable | 0.31 | 2.50 | 1.15 |
| Hexane | 9 | − | 0.63 | 2.50 | − | 1.56 | |
| P2 | Acetone | 14.6 | − | 2.50 | 2.50 | − | 2.50 |
| Hexane | 8.3 | − | 0.63 | 2.50 | − | 1.56 | |
| P3 | Acetone | 11.1 | − | 0.02 | 0.16 | − | 0.09 |
| Hexane | 75.2 | − | 0.16 | 0.63 | − | 0.39 | |
| P4 | Acetone | 65.2 | − | 0.63 | 0.63 | − | 0.63 |
| Hexane | 5.6 | − | 0.63 | 0.63 | − | 0.63 | |
| P5 | Acetone | 30.9 | − | 0.32 | 0.20 | − | 0.26 |
| Hexane | 14.2 | − | 2.50 | 0.20 | − | 1.35 | |
| P6 | Acetone | 27.9 | − | 2.50 | 0.03 | 0.63 | 1.05 |
| Hexane | 15.3 | − | 2.50 | 0.03 | 2.50 | 1.68 | |
a(−), no activity; P1, Kirkia willmsi; P2, Drimia elata; P3, Tšhikwana; P4, Tšhikwana/moroto watšhwene; P5, Hypoxis hemerocallidea; P6, Monsonia angustifolia.
Table 4.
The Total Activity Was Calculated by Dividing Quantity of the Extract From 1 g of Crude Extract by Minimum Inhibitory Concentration Value Recorded.a
| Total Activity | |||||
|---|---|---|---|---|---|
| Plant | Extraction Solvent | Escherichia coli | Pseudomonas aeruginosa | Enterococcus faecalis | Staphylococcus aureus |
| P1 | Acetone | 27.04 | Not applicable | 54 | 6.67 |
| Hexane | − | 14.4 | 3.6 | − | |
| P2 | Acetone | − | 5.84 | 5.84 | − |
| Hexane | − | 13.28 | 3.32 | − | |
| P3 | Acetone | − | 555 | 71.5 | − |
| Hexane | − | 482.05 | 120.32 | − | |
| P4 | Acetone | − | 104.32 | 104.32 | − |
| Hexane | − | 9.28 | 9.28 | − | |
| P5 | Acetone | − | 97.17 | 158.46 | − |
| Hexane | − | 5.68 | 728.21 | − | |
| P6 | Acetone | − | 11.6 | 930 | 44.64 |
| Hexane | − | 6.12 | 510 | 6.12 | |
a(−), no activity; P1, Kirkia willmsi; P2, Drimia elata; P3, Tšhikwana; P4, Tšhikwana/moroto watšhwene; P5, Hypoxis hemerocalledea; P6, Monsonia angustifolia.
The lack of activity observed from the different parts of the plant species could be due to the seasonal variation of the compounds responsible for antimicrobial activity. The presence of phytochemicals in plants is due to outside stimuli such as the amount of light, soil humidity, and temperatures.57 Therefore, depending on the time of the year, different compounds can be produced or repressed. Another reason for the observed inactivity could be that in summer the compounds responsible for antifungal activity were insufficient in the plant matrices to inhibit microbial growth.58 The plants could also be strictly lacking in antifungal compounds, which lead to the failure to inhibit growth.
In order to gain more information about the possible antifungal properties of the phytochemicals in both the acetone and hexane the plant species, bioautography on TLC was used. There were no clear bands against the purple background on the bioautograms (results not shown). This indicated that the separated compounds were unable to inhibit the fungal growth. This observation could be due to the low concentration of the phytochemicals in the corms and leaves of the plant species. The fungicidal and/or bactericidal phytochemicals could be volatile compounds that evaporated during the prolonged drying process of the chromatograms. Moreover, the separation of the compounds on the TLC plates has the consequence of diminishing the antimicrobial activity that results from compounds that utilize synergy to either gain or enhance the property.
Conclusion
The results from this study demonstrated the presence of various compounds. The inconsistent concentrations of the elements in the concoctions demonstrate a lack of standardization of the micronutrients. This deprives the consumers the knowledge of how much elements they take in order to conform to recommended dietary allowances. It is also vital to educate people about these herbal concoctions as well as the practices of sterility of products. Bioassays provide fundamental information about the biological activities of the medicinal plants. Although the biological activities reported in this study do not necessarily indicate the effectiveness of the herbal concoctions, they provide substantial data that can be the basis for a more detailed evaluation of the activity of the plant species. Therefore, anti-inflammatory and cytotoxicity tests on mammalian cells are needed to elucidate the extent of the effect of biological activities associated with the use of the plants used to brew herbal concoctions.
Considering herbal mixtures, the degree of fungal contamination in the concoctions is overwhelming and thus highlights a lack of quality control over the manufacture and packaging of the products. Moreover, this contamination poses a threat to the health of patients that are dependent on the medicines. An additional question to be answered is the effect of the synergistic or antagonistic interaction of different phytoconstituents present in the crude extracts. Moreover, further studies are required to isolate bioactive compounds and evaluate their cytotoxicity on mammalian cells. Caution in the consumption of the herbal mixtures should be adhered to.
Acknowledgments
We would like to thank University of Limpopo and National Research Foundation for financial assistance. We would like to thank Prof Abotsi for proofreading.
Footnotes
Author Contributions: PM was involved conception and design of the study. MMM carried out the experiments and analyzed the data. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Financial assistance was provided by the University of Limpopo and National Research Foundation.
Ethical Approval: The research was approved by University of Limpopo Ethics Committee, approval number TREC/248/2017:IR.
References
- 1. World Health Organization. Traditional Medicine: Growing Needs and Potential. WHO Policy Perspectives on Medicines. Geneva, Switzerland: World Health Organization; 2002. [Google Scholar]
- 2. Dausdardt R. The changing geography of traditional medicine: urban herbalism on the Witwatersrand, South Africa. Geogr J. 1990;22:275–283. [Google Scholar]
- 3. Semenya SS, Potgieters MJ, Erasmus L. Ethnobotanical survey of medicinal plants used by Bapedi healers to treat diabetes mellitus in the Limpopo Province, South Africa. J Ethnopharmacol. 2012;141:440–445. [DOI] [PubMed] [Google Scholar]
- 4. Pujol J. The Herbalist Handbook: African Flora, Medicinal Plants. Durban, South Africa: Nature Africa; 1990. [Google Scholar]
- 5. Afanas IB, Dorozhko AI, Brodskii AV, Kostyuk VA, Potapovitch AI. Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochem Pharmacol. 1989;38:1763–1769. [DOI] [PubMed] [Google Scholar]
- 6. Rates SM. Plants as source of drugs. Toxicon. 2001;39:603–613. [DOI] [PubMed] [Google Scholar]
- 7. Bonora F. The Modernity/Traditional Interface Amongst Urban Black South Africans: An Investigation of the Current themes [master’s thesis]. Johannesburg, South Africa: University of South Africa. [Google Scholar]
- 8. Ndhlala AR, Stafford GI, Finnie JF, Van Staden J. In vitro pharmacological effects of manufactured herbal concoctions used in KwaZulu-Natal South Africa. J Ethnopharmacol. 2009;122:117–122. [DOI] [PubMed] [Google Scholar]
- 9. Semenya SS, Potgieters MJ. Kirkia wilmsii: a Bapedi treatment for hypertension. South Afr J Botany. 2015;100:228–232. [Google Scholar]
- 10. Limpopo Provincial Government. The ethnic groups, languages and races. 2013. http://www.limpopo.gov.za/index.php?option=com_content&view=article&id=8&Itemid=13. Accessed June 16, 2016.
- 11. Hutchings A, Scott AH, Lewis G, Cunningham AB. Zulu Medicinal Plants: An Inventory. Pietermaritzburg, South Africa: Natal University Press; 1996. [Google Scholar]
- 12. Drewes SE, Elliot E, Khan F, Dhlamini JTB, Gcumisa MSS. Hypoxis hemerocallidea not merely a cure for benign prostate hyperplasia. J Ethnopharmacol. 2008;119:593–598. [DOI] [PubMed] [Google Scholar]
- 13. Semenya SS, Potgieters MJ, Erasmus L. Bapedi phytomedicine and their use in the treatment of sexually transmitted infections in Limpopo Province, South Africa. Afr J Pharm Pharmacol. 2013;7:250–262. [Google Scholar]
- 14. Van Wyk BE, Van Oudtshoorn B, Gericke N. Medicinal Plants of South Africa. 2nd ed Pretoria, South Africa: Briza; 2000. [Google Scholar]
- 15. Hutchinson J. Evolution and Phylogeny of Flowering Plants. London, England: Academic Press; 1969. [Google Scholar]
- 16. Fouche G, Afolayan AJ, Wintola OA, Khorombi TE, Senabe J. Effect of the aqueous extract of the aerial parts of Monsonia angustifolia E. Mey. Ex A. Rich., on the sexual behaviour of male Wistar rats. BMC Complement Alternat Med. 2015;15:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mulaudzi RB, Ndhlala AR, Kulkarni MG, Van Staden J. Pharmacological properties and protein binding capacity of phenolic extracts of some Venda medicinal plants used against cough and fever. J Ethnopharmacology. 2012;143:185–193. [DOI] [PubMed] [Google Scholar]
- 18. Tomás-Barberán FA, Andrés-Lacueva C. Polyphenols and health: current state and progress. J Agric Food Chem. 2012;60:8773–8775. [DOI] [PubMed] [Google Scholar]
- 19. Wang H, Khor TO, Shu L, Su ZY, Fuentes F, Lee JH. Plants vs. cancer: a review on natural phytochemicals in preventing and treating cancers and their druggability. Anticancer Agents Med Chem. 2012;12:1281–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Quideau S, Deffieux D, Douat-Casassus C, Pouységu L. Plant polyphenols: chemical properties, biological activities, and synthesis. Angew Chem Int Ed. 2011;50:586–621. [DOI] [PubMed] [Google Scholar]
- 21. Gilham B, Papachristodoulou K, Thomas JH. Wills Biochemical Basis of Medicine. Oxford, England: Butterworth-Heinemann; 1997. [Google Scholar]
- 22. Aruoma OI. Free radicals, oxidative stress and antioxidants in human health and disease. J Am Oil Chem. 1998;75:199-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Diaz MN, Frei B, Vita JA, Keaney JF., Jr Antioxidants and atherosclerotic heart disease. N Engl J Med. 1997;337:408-416. [DOI] [PubMed] [Google Scholar]
- 24. Smith MA, Perry G, Richey PL, Sayre LM, Anderson VE, Beal MF. Oxidative damage in Alzheimer’s. Nature. 1996;382:120-121. [DOI] [PubMed] [Google Scholar]
- 25. Selkoe DJ. Defining molecular targets to prevent Alzheimer’s disease. Arch Neurol. 2005;62:192–195. [DOI] [PubMed] [Google Scholar]
- 26. Fusco D, Colloca G, LoMonaco MR, Cesari M. Effects of antioxidant supplementation on the aging process. Clin Intervent Aging. 2007;2:377–387. [PMC free article] [PubMed] [Google Scholar]
- 27. Stermitz FR, Lorenz P, Tawara JN, Zenewicz LA, Lewis K. Synergy in a medicinal plant: antimicrobial action of berberine potentiated by 5′-methoxyhydnocarpin, a multidrug pump inhibitor. Proc Natl Acad Sci U S A. 2000;97:1433–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gibbons S. An overview of plant extracts as potential therapeutics. Expert Opin Ther Patents. 2003;13:489–497. [Google Scholar]
- 29. Shai LJ, McGaw LJ, Masoko P, Eloff JN. Antifungal and antibacterial activity of seven traditionally used South African plant species active against Candida albicans . South Afr J Botany. 2008;74:677–684. [Google Scholar]
- 30. Sacho H, Schoub BD. Current Properties on Nosocomial Infections. (Glaxo Wellcome sponsored pamphlet). Natal, South Africa: The Natal Witness Printing and Publishing Company; 1993. [Google Scholar]
- 31. National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk Susceptibility Tests. 4th ed Approved Standard NCCLS Document M2-A4, Villanora; 1992. [Google Scholar]
- 32. Pincus DH. Microbial identification using the bioMérieux Vitek 2 system In: Encyclopedia of Rapid Microbiological Methods. Bethesda, MD: Parenteral Drug Association; 2006. [Google Scholar]
- 33. Kotze M, Eloff JN, Houghton PJ. Extraction of antibacterial compounds from Combretum microphyllum (Combretaceae). South Afr J Botany. 2002;68:62–67. [Google Scholar]
- 34. Borokini TI, Omotayo TO. Phytochemical and ethnobotanical study of some selected medicinal plants from Nigeria. J Med Plants Res. 2012;6:1106–1118. [Google Scholar]
- 35. Odebiyi OO, Sofowara EA. Phytochemical screening of Nigerian medicinal plants II. Llodydia. 1978;41:234–246. [PubMed] [Google Scholar]
- 36. Deby C, Margotteaux G. Relationship between essential fatty acids and tissue antioxidant levels in mice [in French]. C R Seances Soc Biol Fil. 1970;165:2675–2681. [PubMed] [Google Scholar]
- 37. Eloff JN. A sensitive and quick method to determine the minimum inhibitory concentration of plant extracts for bacteria. Planta Med. 1998;60:1–8. [DOI] [PubMed] [Google Scholar]
- 38. Masoko P, Picard J, Eloff JN. Antifungal activities of six South African Terminalia species (Combretaceae). J Ethnopharmacol. 2005;99:301–308. [DOI] [PubMed] [Google Scholar]
- 39. Begue WJ, Kline RM. The use of tetrazolium salts in bioautographic procedure. J Chromatogr. 1972;88:182–184. [DOI] [PubMed] [Google Scholar]
- 40. Wolf HW. The coliform count as a measure of water quality In: Mitchell R, ed. Water Pollution Microbiology. New York, NY: Wiley-Interscience; 1972:333–345. [Google Scholar]
- 41. Gupta A, Ampofo K, Rubenstein D, Saiman L. Extended spectrum beta lactamase-producing Klebsiella pneumoniae infections: a review of the literature. J Perinatol. 2003;23:439–443. [DOI] [PubMed] [Google Scholar]
- 42. Luseba D, Elgorashi EE, Ntloedibe DT, Van Staden J. Antibacterial, anti-inflammatory and mutagenic effects of some medicinal plants used in South Africa for treatment of wounds and retained placenta in livestock. South Afr J Botany. 2007;73:378–383. [Google Scholar]
- 43. Alka SE. A Textbook of Medical Biochemistry. New Delhi, India: Brothers Medical; 2000. [Google Scholar]
- 44. Garcia-Rico L, Leyva-Perez J, Jara-Marini ME. Content and daily intake of copper, zinc, lead, cadmium and mercury from dietary supplements in Mexico. Food Chem Toxicol. 2007;45:1599–1605. [DOI] [PubMed] [Google Scholar]
- 45. Hecht H, Kumpulainen J. Essential and toxic elements in meat and eggs. Mitteilungen Klosterneuburg. 1995;34:46–52. [Google Scholar]
- 46. Iwuoha GN, Uporo VB, Onwuachu UI. Variation of heavy metals in canned Geisha and Founty mackerel fish brands obtained from Choba Market Port Harcourt, Nigeria. J Appl Sci Environ Manage. 2013;17:577–580. [Google Scholar]
- 47. Soliman K, Zikovsky L. Determination of Br, Cd, U, Co, Cu, J, K, Mg, Mn, Na, Rb, S, Ti, V in cereals, oil, sweeteners and vegetables sold in Canada by neutron activation analysis. J Food Compos Anal. 1999;12:85–89. [Google Scholar]
- 48. Iwegbue CMA. Composition and daily intakes of some trace metals from canned beers in Nigeria. J Inst Brew. 2010;116:312–315. [Google Scholar]
- 49. Ncube NS, Afolayan AJ, Okoh AI. Assessment techniques of antimicrobial properties of natural compounds of plant origin: current methods and future trends. Afr J Biotechnol. 2008;7:1797–1806. [Google Scholar]
- 50. Masoko P, Mmushi TJ, Mogashoa MM, Mokgotho MP, Mampuru LJ, Howard RL. In vitro evaluation of the antifungal activity of Sclerocarya birrea extracts against pathogenic yeasts. Afr J Biotechnol. 2008;7:3521–3526. [Google Scholar]
- 51. Masoko P, Eloff JN. Screening of twenty-four South African Combretum and six Terminalia species (Combretaceae) for antioxidant activities. Afr J Tradit Complement Alternat Med. 2007;4:231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Masoko P, Makgapeetja DM. Antibacterial, antifungal and antioxidant activity of Olea africana against pathogenic yeast and nosocomial pathogens. BMC Complement Altern Med. 2015;15:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Katerere DR, Eloff JN. Antibacterial and anti-oxidant activity of Hypoxis hemerocallidea (Hypoxidaceae): can leaves be substituted for corms as a conservation strategy? South Afr J Botany. 2008;74:613–616. [Google Scholar]
- 54. Dai J, Mumper RJ. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15:7313–7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Eloff JN, Suleiman MM, McGaw LJ, Naidoo V. Evaluation of several tree species for activity against the animal fungal pathogen Aspergillus fumigatus . South Afr J Botany. 2010;76:64–71. [Google Scholar]
- 56. Eloff JN. Quantification the bioactivity of plant extracts during screening and bioassay guided fractionation. Phytomedicine. 2004;11:370–371. [DOI] [PubMed] [Google Scholar]
- 57. Figueiredo AC, Barroso JG, Pedro LG, Scheffer JJC. Factors affecting secondary metabolite production in plants: volatile components and essential oils. Flavour Fragr J. 2008;23:213–226. [Google Scholar]
- 58. Ncube B, Finnie JF, Van Staden J. Seasonal variation in antimicrobial and phytochemical properties of frequently used medicinal bulbous plants from South Africa. S Afr J Botany. 2011;77:387–396. [Google Scholar]