Abstract
The present study was conducted to explore the efficacy and safety of a herbal combination in the treatment of women with hyperlipidemic type 2 diabetes. The herbal combination capsule (600 mg) contained Terminalia chebula fruit extract (200 mg), Commiphora mukul (200 mg), and Commiphora myrrha oleo-gum-resin (200 mg), and the placebo capsule contained 600 mg toast powder. The patients in one group took the herbal combination and those in the other group took placebo capsules 3 times a day for 3 months. In the herbal combination–treated patients, the fasting blood glucose, total cholesterol, and low-density lipoprotein cholesterol levels were decreased and hidh-density lipoprotein cholesterol levels was increased significantly at the endpoint compared with the placebo and baseline. Other blood parameters such as glycosylated hemoglobin, triglyceride, blood urea nitrogen, creatinine, SGOT, and SGPT levels were not significantly changed after 3 months in both groups. In conclusion, the herbal combination improves glycemic control and lipid profile in women with hyperlipidemic type 2 diabetes without any adverse events.
Keywords: Commiphora mukul, Commiphora myrrha, Terminalia chebula, diabetes mellitus, hyperlipidemia
Diabetes mellitus type 2 is one of the most prevalent and fast-growing diseases in most countries with life-threatening complications leading to heavy personal and national economic burdens.1 It is estimated that of the total 80 million Iranian population 7.7% of adults aged 25 to 64 years, or 2 million, have diabetes, among whom one half are undiagnosed. An additional 16.8% or 4.4 million of Iranian adults have impaired fasting glucose.2
Dyslipidemia is believed to be a one of the major risk factors for coronary heart disease in diabetic patients.3 One study reported that 73.2% of type 2 diabetic patients in Tehran city in Iran had some form of hyperlipidemia.4 Although considerable progress has been made in the pharmacotherapy of type 2 diabetes, new remedies are still in great demand because of limited efficacy and undesirable side effects of the present antidiabetic drugs.5,6
Several herbal medicines are used for the treatment of diabetes mellitus.7 In Iranian traditional medicine, a combination of 3 herbal medicines—Commiphora mukul oleo-gum-resin, Commiphora myrrha oleo-gum-resin, and Terminalia chebula fruit—is used for the treatment of diabetes.8 C mukul is a medicinal plant native to Africa. In Indian traditional medicine, C mukul oleo-gum-resin has been used for the treatment of several diseases including hyperlipidemia.9 In 2 clinical studies, the resin improved blood glucose and lipid profile in hyperlipidemic patients.10,11 C myrrha is indigenous to Somalia. In an experimental study, an aqueous extract of the C myrrha oleo-gum-resin reduced the blood glucose levels in diabetic rats.12 Oral administration of 2 furano-sesquiterpenes isolated from the resin significantly reduced the blood glucose levels in the genetically altered obese diabetic mice.13 Hypocholesterolemic effect of the resin has also been reported in experimentally induced hypercholesterolemia in Albino rats.14 In Indian traditional medicine, T chebula has been used for treatment of several diseases including hyperglycemia.15,16 In experimental studies, the hypoglycemic effect of the T chebula extract has been reported in diabetic rats.17,18 In our previous studies, we demonstrated that some herbal product can improve glycemic control in diabetes,19,20 but in this study we have worked on the herbal mixture for the first time.
Considering the traditional use of T chebula fruit, C mukul, and C myrrha oleo-gum-resin in the treatment of diabetes and hyperlipidemia and their favorable effects on glucose and lipid metabolism reported in experimental studies, the present study was conducted to investigate the efficacy and safety of the combination of these agents in the treatment of women with hyperlipidemic type 2 diabetes.
Methods
Preparation of Herbal Combination and Placebo Capsules
The plant materials were purchased from Himalayan Herbaria Inc, India. The herbal capsule was filled with C mukul (200 mg), C myrrh (200 mg), and T chebula (200 mg) powder and placebo with toast powder. The herbal and placebo capsules were prepared with the same appearance and packed in identical boxes with different code numbers by the Institute of Medicinal Plants (Karaj, Iran). The herbal and placebo capsules were packed in boxes with the same shape and color but with different code numbers.
Standardized Herbal Materials
The C mukul oleo-gum resin was standardized for 2.5% guggulsterones E and Z; C myrrha oleo-gum-resin was standardized for 5% organic acid; and T chebula fruit was standardized for 10% to 40% tannins by Himalayan Herbaria Inc, India.
Identification of the Volatile Oil Constituents of Commiphora mukul and Commiphora myrrh
The volatile constituents were analyzed using an Agilent 6890 GC equipped with BPX5 capillary columns (30 m × 0.25 mm i.d. 0.25 μm film thicknesses), and a mass spectrometer column temperature was gradually increased from 50°C to 240°C at a rate of 3°C/min. For gas chromatography-mass spectrometry detection, an electron ionization system was used with an ionization energy of 70 eV. The injector and ion source temperatures were set at 290°C and 220°C, respectively. One microliter of the sample was injected manually in split less mode. C9-C20 n-alkanes were used as reference points in the calculation of the Kovats indices. Tentative identification of the compounds was based on the comparison of their relative retention time and mass spectra with those of the NIST and Wiley library data of the gas chromatography-mass spectrometry system and literature data.21,22
Total Phenols Determination in Terminalia chebula
The dried seeds of T chebula were extracted with 80% hydroalcoholic solvent by a percolator apparatus at room temperature. The plant materials were concentrated by rotary evaporator and dried in freeze dryer. Total phenols of dry extracts were determined by the Folin-Ciocalteu reagent method.20,23 A dilute extract of the plant (0.5 mL of 1:10 g/mL methanol) or gallic acid (standard phenolic compound) was mixed with Folin-Ciocalteu reagent (5 mL, 1:10 diluted with distilled water) and aqueous Na2CO3 (4 mL, 1 M). The mixtures were allowed to stand for 15 minutes and total phenols were determined by colorimeters at 765 nm. The standard curve was prepared using 0, 50, 100, 200, and 250 mg/L solution of gallic acid in methanol. Total phenol values are expressed in terms of gallic acid equivalent (mg/g of dry extract), which is a common reference compound.
Study Protocol
Participants
Eighty-six hyperlipidemic type 2 diabetic women patients who visited the the Diabetes Research Clinic of the Afshar Hospital (Yazd, Iran) from 2013 to 2014 were enrolled in the study. This study was performed on women regularly attending a women’s swimming pool.
The inclusion criteria for enrolment were Iranian female type 2 diabetic patients; aged 40 to 60 years; fasting serum glucose levels between 150 and 180 mg/dL; blood glycosylated hemoglobin levels between 7.5% and 8.5%; low-density lipoprotein cholesterol >100 mg/dL; and daily oral intake of not more than 10 mg glyburide and 1000 mg metformin at maximum. The exclusion criteria for enrolment were patients receiving insulin therapy; patients that changed food, drug, or other complementary medicine regimen 2 months prior; patients with hypothyroidism, vertigo, seizure, and cardiac, renal, hepatic, and hematological diseases; patients with a history of gallstones or gall bladder surgery; and patients using estrogens, steroids, beta-blockers, and thiazides drugs.
Sample Size
Eighty-six patients in 2 groups was the sample size required to estimate 30 mg/dL and 25 mg/dL difference of fasting glucose and total cholesterol, respectively, between the groups, considering type I error of 0.05 and 80% power.24
Randomization
The enrolled patients were randomly assigned into 2 groups with a randomized block design. Each block contained 2 × 2 treatments (AABB, ABAB, etc) and were selected in a random order. The patients in each block were not significantly different in terms of glycosylated hemoglobin and fasting serum glucose levels. Three different persons generated the random allocation sequence, enrolled the participants, and assigned them to interventions. The care providers and participants were blinded to interventions.
The principal investigator of the research project arranged identification code numbers (1 or 2) on herbal and placebo capsules boxes. After giving the herbal or placebo capsules to the patient, the care providers wrote down the boxes identification code numbers on the patient’s records file.
Interventions
The enrolled participants were randomly assigned to either herbal combination or placebo groups. All patients were instructed to take 1 herbal combination capsule or 1 placebo capsule 3 times daily immediately before starting breakfast, lunch, and dinner for 3 months. The dose of the herbal combination, 1800 mg daily, was used based on Iranian traditional medicine.8 To monitor consumption of allocated treatments, the patients were asked to return any capsules left, on their monthly visit. Patients’ compliance was assessed by pill count method, and those who used more than 80% of the interventions were included in the analysis. All patients took conventional oral antihyperglycemic drugs during the trial as before and without dose changes. The patients were advised not to change their food or other nutritional intake during the study. The physical activity (swimming) was same for all. All participants were requested to report any adverse effects. The medical ethics committee of the Ebne-Sina Research Institute (Iran, Tehran) approved the protocol. All patients signed written informed consent forms before participation in the study. The trial was registered in the Iranian Registry of Clinical Trials with the identification number IRCT138706161157N3.
Outcomes
Fasting blood samples were collected after 10-hour fasting for measurement of the glucose, glycosylated hemoglobin, low-density lipoprotein cholesterol, total cholesterol, high-density lipoprotein cholesterol, and triglyceride as primary outcomes and blood urea nitrogen, creatinine, SGOT, and SGPT levels as secondary outcomes, at the baseline and after 3 months in both groups. Blood biochemical parameters were determined by an auto-analyzer (Hitachi 902) using standard commercial kits provided by Pars Azmoon Company (Iran, Tehran).
Statistical Analysis
Intention-to-treat analysis was used for analysis of data. Statistical analysis was done by SPSS software (version 17). Quantitative variables were described by mean and standard deviation. Data were checked for normal distribution by Kolmogorov-Smirnov test. Student’s t test was used for analyzing the differences of quantitative variables between groups. Within-group analysis was conducted by paired test. A P value of less than .05 was considered significant.
Results
Standardized Herbal Materials
The total phenolic contents of the ethanol extract of T chebula was equal to 18 mg/g of dry extract.
Phytochemical analysis results of C myrrha and C mukul are summarized in Tables 1 and 2, respectively. Only phytochemical contents above 1% are shown in the tables. α-Pinene and 3Z-cembrene A were the major compounds in the volatile components of the C mukul oleo-gum resin; and curzerene, furanoeudesma-1,3-diene, and lindestrene were the major compounds in the volatile components of the C myrrha oleo-gum resin.
Table 1.
Chemical Compounds in Commiphora myrrha by Gas Chromatography–Mass Spectrometry.
| No. | Components | Percentage |
|---|---|---|
| 1 | β-Elemene | 3.04 |
| 2 | Curzerene | 16.87 |
| 3 | Curzerenone | 1.32 |
| 4 | Furanoeudesma-1,3-diene | 47.22 |
| 5 | Lindestrene | 11.74 |
| 6 | Elemol acetate | 1.79 |
Table 2.
Chemical Compounds in Commiphora mukul by Gas Chromatography–Mass Spectrometry.
| No. | Components | Percentage |
|---|---|---|
| 1 | α-Pinene | 9.72 |
| 2 | Myrcene | 6.02 |
| 3 | p-Methyl anisole | 1.71 |
| 4 | Limonene | 1.80 |
| 5 | β-Phellandrene | 1.62 |
| 6 | β-Elemene | 2.40 |
| 7 | E-Caryophyllene | 4.01 |
| 8 | Caryophyllene oxide | 1.75 |
| 9 | Cembrene | 2.62 |
| 10 | 3Z-Cembrene A | 36.96 |
| 11 | Phyllocladene | 4.06 |
| 12 | Abietadiene | 9.19 |
Baseline Characteristics Data
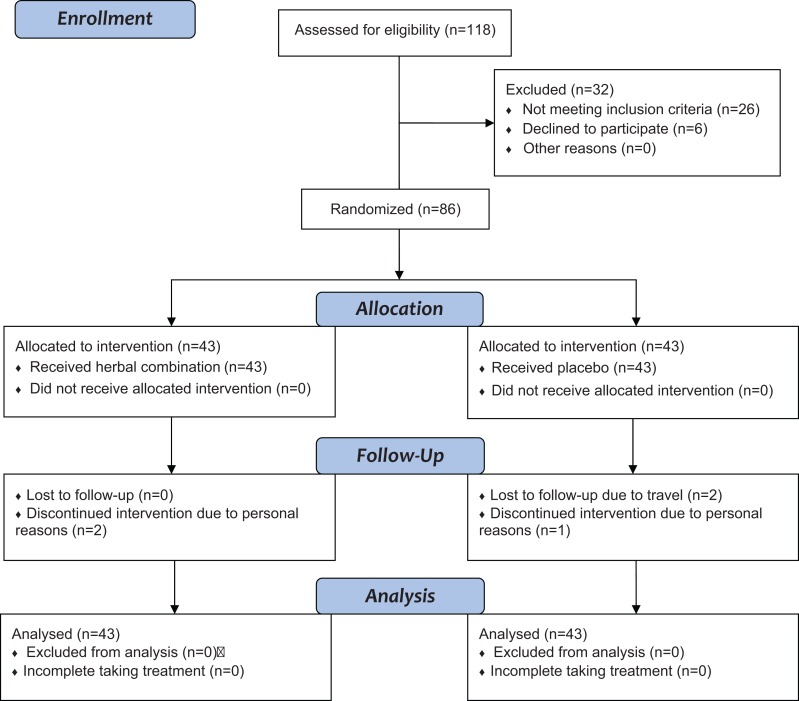
Out of 118 type 2 diabetic outpatients screened, according to the inclusion and exclusion criteria, 86 eligible patients participated in the study. Five patients discontinued intervention, 3 due to personal reasons and 2 due to travel. Finally, data of 86 patients, 43 in the herbal combination and 43 in the placebo group, were included in the statistical analysis (Figure 1). Both groups were matched with regard to demographic data such as age, gender, and duration of diabetes (Table 3).
Figure 1.
Flowchart of the study’s inclusion, allocation, and follow-up.
Table 3.
The Demographical Data of the Patients in the Herbal Combination and Placebo Groupsa,b.
| Group | |||
|---|---|---|---|
| Herbal Combination | Placebo | P Valuec | |
| Age (years) | 48.7 ± 7.3 | 50.7 ± 5.6 | P = 0.167 |
| Duration of diabetes (years) | 5.9 ± 2.4 | 6.5 ± 3.1 | P = 0.142 |
| Weight (kg) | 69.7 ± 4.6 | 70.5 ± 5.1 | P = 0.316 |
aThe data are presented as mean ± standard deviation.
bPaired t test was used to compare the groups.
c P values less than .05 were considered as significant.
Blood Parameters Levels
The blood parameter levels at baseline and after 3 months of the study are summarized in Table 4. The baseline blood levels of all parameters were not significantly different between the 2 groups. In the herbal combination group, fasting blood glucose, cholesterol, and low-density lipoprotein cholesterol decreased significantly and high-density lipoprotein cholesterol increased significantly at the endpoint compared with the placebo group and baseline. The HbA1c and other blood parameters levels were not significantly changed in the herbal combination group at the endpoint compared with the placebo group and baseline.
Table 4.
Changes in Fasting Blood Parameter Levels During the 3-Month Study in the Herbal Combination and Placebo Groups (Groups 1 and 2, Respectively)a,b.
| Blood Parameter | Group | Baseline | Baseline Group 1 vs 2 P Value | Endpoint | Endpoint Group 1 vs 2 P Valuec | Endpoint vs Baseline P Valued | % Change Endpoint vs Baseline |
|---|---|---|---|---|---|---|---|
| Fasting blood glucose (mg/dL) | 1 | 190.2 ± 45.6 | .98 | 160.0 ± 44.3 | .043 | .040 | 26.3 ↓ |
| 2 | 189.4 ± 48.3 | 184.2 ± 49.0 | .082 | 5.7 ↓ | |||
| HbA1c (%) | 1 | 8.1 ± 1.5 | .13 | 7.7 ± 1.2 | .90 | .22 | 4.5 ↓ |
| 2 | 7.9 ± 1.1 | 7.8 ± 1.4 | .94 | 1.3 ↓ | |||
| Total cholesterol (mg/dL) | 1 | 210.6 ± 30.1 | .83 | 183.9 ± 29.4 | .040 | .031 | 15.7 ↓ |
| 2 | 208.4 ± 39.4 | 209.5 ± 39.7 | .95 | 0.5 ↑ | |||
| LDL-C (mg/dL) | 1 | 139.6 ± 28.0 | .89 | 104.8 ± 38.0 | .048 | .001 | 25.2 ↓ |
| 2 | 127.2 ± 27.3 | 122.0 ± 32.9 | .100 | 6.9 ↓ | |||
| HDL-C (mg/dL) | 1 | 40.3 ± 12.1 | .29 | 49.1 ± 12.3 | .01 | .001 | 21.8 ↑ |
| 2 | 45.5 ± 12.0 | 43.9 ± 9.5 | .870 | 4.4 ↓ | |||
| Triglyceride (mg/dL) | 1 | 178.7 ± 78.0 | .68 | 165.0 ± 54.7 | .07 | .22 | 7.3 ↓ |
| 2 | 201.2 ± 25.8 | 212.9 ± 45.6 | .75 | 5.5 ↑ | |||
| BUN (mg/dL) | 1 | 13.9 ± 2.8 | .69 | 10.4 ± 3.8 | .60 | .10 | 2.5 ↓ |
| 2 | 12.7 ± 2.7 | 12.3 ± 3.2 | .91 | 0.7 ↓ | |||
| Creatinine (mg/dL) | 1 | 0.91 ± 0.2 | .24 | 0.93 ± 0.3 | .76 | .79 | 2.1 ↑ |
| 2 | 0.88 ± 0.2 | 0.91 ± 0.3 | .21 | 3.4 ↑ | |||
| SGOT (U/L) | 1 | 16.6 ± 4.2 | .59 | 15.7 ± 4.1 | .71 | .20 | 6.5 ↓ |
| 2 | 16.1 ± 3.4 | 15.4 ± 4.6 | .30 | 4.4 ↓ | |||
| SGPT (U/L) | 1 | 16.4 ± 2.2 | .12 | 17.2 ± 3.7 | .91 | .42 | 4.8 ↑ |
| 2 | 18.6 ± 2.4 | 17.1 ± 4.0 | .23 | 8.1 ↓ |
Abbreviations: LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; BUN, blood urea nitrogen.
aThe data are presented as mean ± standard deviation.
b P values below .05 are significant.
cStudent’s t test.
dPaired t test.
Adverse events
All patients took the medication completely during the study and no adverse effect was reported.
Discussion
The results suggest that the herbal combination improves glycemic control, lowers total cholesterol and low-density lipoprotein cholesterol, and increases high-density lipoprotein cholesterol levels in hyperlipidemic type 2 diabetic patients. There was a small but nonsignificant fall in HbA1c compared with baseline, but treatment does not affect triglyceride levels. The herbal combination is well tolerated and does not cause any hepatic, renal, or other adverse effects. However, the main shortcoming of the present study is the short duration of the study. Another limitation is lack of identifying the actual mechanism of drug action.
The hypoglycemic effect of the herbal combination is in line with its traditional use by the physician Dr A. Ahmadiah.8 He pointed out that several of his diabetic patients were treated with the mixture of C mukul, C myrrha, and T chebula. But limitation was lack of a placebo group, duration of study was not same for all patients, and HbA1c was determined. Several studies reported beneficial effects of each component in the herbal combination on glucose and lipid metabolism. C mukul is one of the components in the polyherbal formulation used in the treatment of diabetic patients in clinical trials.25 The blood glucose lowering effects observed in the present study is in accordance with previous studies in which antihyperglycemic and antioxidant effects have been demonstrated for C mukul in streptozotocin-induced diabetic rats.26,27 The hypoglycemic effect of T chebula, another component in the herbal combination, has also been reported in in vivo and in vitro studies.17,18 T chebula is one of the components in some polyherbal formulations with antihyperglycemic properties in alloxan-induced diabetic rats.25 The antidiabetic effects C myrrha have been reported in streptozotocin-induced diabetic rats.28
The hypoglycemic effects of furano-sesquiterpenes, a chemical constituent of C myrrha resin, another component of the herbal combination, have been reported in diabetic rat.13 The improved antihyperlipidemic effect observed in the present study is in accordance with previous clinical trial in which C mukul, one of the components in the herbal combination, lowered total cholesterol and increased high-density lipoprotein cholesterol in healthy adults with moderately increased total cholesterol level.29 In another clinical trial, C mukul decreased total cholesterol, low-density lipoprotein cholesterol, and triglycerides levels in hypercholesterolemic patients.11 The antilipid peroxidation effects have been demonstrated for C mukul in diabetic rats.27,30 Also the hypolipidemic effects of C myrrha resin, another component of the herbal combination, have been reported in hypercholesterolemic rats.14 In another study, hydroalcoholic extract of C myrrha resin decreased body weight gain and normalized high blood lipids level in obese hyperlipidemic rats.31
The mechanisms and bioactive(s) mediating the beneficial effects of the herbal combination on glucose and lipid levels are not yet characterized. Different hypotheses may explain the effects. The favorable effects of dietary free fatty acid and antioxidant intake on lipid and glucose metabolism have been reported in several studies.32–35 In the favor of this claim, the antioxidant properties of C mukul,26,27,30 C myrrha,36 and T chebula 37,38 have been reported in previous studies. In addition, the chemical constituents of C myrrha such as curzerene, furanoeudesma-1,3-diene, and lindestrene determined in present study (Table 1) significantly reduced blood glucose levels reported in a previous study.13 The blood glucose lowering effects of C myrrha may be due to reduction in the rate of gluconeogenesis in hepatocytes.31 Furthermore, the inhibitory effect of T chebula on α-glucosidase prevents the digestion of carbohydrates, resulting in inhibiting the intestinal absorption of glucose, and it may be another mechanism for the herbal combination to improve glycemic control.39 The hypolipidemic effect observed in the present study in part may be due to the phenolic compounds in T chebula.40
Furthermore, it has been reported that tannic acid, a component in T chebula, induced phosphorylation of insulin receptors, stimulates insulin-mediated glucose transport, and inhibits expression of key genes for adipogenesis in favor of the glucose metabolism.41,42 In addition, the herbal combination contains several components (Tables 1 and 2) with diverse mechanisms of action that may influence directly or indirectly lipid and glucose metabolism in the diabetic patients.43
However, the lack of significant effects of the herbal combination on HbA1c level in the present study may be attributed to small sample size, short duration of the study, or its low dosage. Whereas the lack of significant effects of the herbal combination on the levels of SGOT, SGPT, and creatinine may indicate its safety as it does not have hepatic and renal toxicities. However, the present favorable effects on hyperglycemia and lipid profile and safety of the herbal combination may not justify the use of this herbal combination in diabetic patients, unless its safety and beneficial therapeutic effect is approved by larger multicenter clinical trials.
In conclusion, it seems that the herbal combination containing C mukul, C myrrha, and T chebula is safe and may have a beneficial effect on lipid profile in hyperlipidemic type 2 diabetic patients. Further larger clinical trials concerning the efficacy and safety of this herbal combination in the treatment of patients with type 2 diabetes mellitus and/or hyperlipidemia as well as more studies addressing the bioactives and mechanisms involved in the effects of the herbal combination on the blood glucose and lipid levels seem necessary.
Footnotes
Authors’ Note: The trial was registered in the Iranian Registry of Clinical Trials (Registration ID: IRCT138706161157N3).
Author Contributions: The work presented in this article was carried out through collaboration between all authors. HFH and RS made the initial hypothesis. All authors participated in defining the research theme and laid out the research project proposal. SM, SK, MF, MR, and FN performed the experiments, collected the data, analyzed the data, and wrote the article. HFH, RS, and SK conceptualized the study, critically analyzed and discussed the data, and corrected and reviewed the article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article. This study was supported by a grant from the Iranian Academic Center for Education, Culture and Research (ACECR).
Ethical Approval: The study protocol was in compliance with the Declaration of Helsinki (1989 revision) and approved by the Local Medical Ethics Committee of Baghiatallah University of Medical Sciences (ET-BMUS-92- 15-650).
References
- 1. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. [DOI] [PubMed] [Google Scholar]
- 2. Esteghamati A, Gouya MM, Abbasi M, et al. Prevalence of diabetes and impaired fasting glucose in the adult population of Iran: National Survey of Risk Factors for Non-Communicable Diseases of Iran. Diabetes Care. 2008;31:96–98. [DOI] [PubMed] [Google Scholar]
- 3. Javadi E, Yarahmadi S, Larijani B, Mohammadi S, Shafaei AR, Jalili RB. Prevalence of dyslipidemia in diabetic patients attending the diabetes clinic at Doctor Shariati University Hospital between October 1998 and September 2000. Iran J Diabetes Metab. 2003;1:133–137. [Google Scholar]
- 4. Mooradian AD. Dyslipidemia in type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab. 2009;5:150–159. [DOI] [PubMed] [Google Scholar]
- 5. Krentz AJ, Bailey CJ. Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs. 2005;65:385–411. [DOI] [PubMed] [Google Scholar]
- 6. Howlett HC, Bailey CJ. A risk-benefit assessment of metformin in type 2 diabetes mellitus. Drug Saf. 1999;20:489–503. [DOI] [PubMed] [Google Scholar]
- 7. Dey L, Attele AS, Yuan CS. Alternative therapies for type 2 diabetes. Altern Med Rev. 2002;7:45–58. [PubMed] [Google Scholar]
- 8. Ahmadiah A. Raz-e-darman. Vol. 2. Tehran, Iran: Eghbal Press; 2002:34–36. [Google Scholar]
- 9. Shen T, Li GH, Wang XN, Lou HX. The genus Commiphora: a review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol. 2012;142:319–330. [DOI] [PubMed] [Google Scholar]
- 10. Nityanand S, Srivastava JS, Asthana OP. Clinical trials with gugulipid. A new hypolipidaemic agent. J Assoc Physicians India. 1989;37:323–328. [PubMed] [Google Scholar]
- 11. Singh RB, Niaz MA, Ghosh S. Hypolipidemic and antioxidant effects of Commiphora mukul as an adjunct to dietary therapy in patients with hypercholesterolemia. Cardiovasc Drugs Ther. 1994;8:659–664. [DOI] [PubMed] [Google Scholar]
- 12. Al-Awadi FM, Gumaa KA. Studies on the activity of individual plants of an antidiabetic plant mixture. Acta Diabetol Lat. 1987;24:37–41. [DOI] [PubMed] [Google Scholar]
- 13. Ubillas RP, Mendez CD, Jolad SD, et al. Antihyperglycemic furanosesquiterpenes from Commiphora myrrha . Planta Med. 1999;65:778–779. [DOI] [PubMed] [Google Scholar]
- 14. Al-Amoudi NS. Hypocholesterolemic effect of some plants and their blend as studied on albino rats. Int J Food Saf Nutr Publ Health. 2009;2:176–188. [Google Scholar]
- 15. Ainslie W. Terminalia chebula and Terminalia citrina. Vol 1 London; 1826:236–242. [Google Scholar]
- 16. Bag A, Bhattacharyya SK, Chattopadhyay RR. Therapeutic potential of Terminalia chebula Retz. (Combretaceae): the ayurvedic wonder. Asian Pac J Trop Biomed. 2013;3:244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Senthilkumar GP, Subramanian SP. Biochemical studies on the effect of Terminalia chebula on the levels of glycoproteins in streptozotocin-induced experimental diabetes in rats. J Appl Biomed. 2008;6:105–115. [Google Scholar]
- 18. Murali YK, Chandra R, Murthy PS. Antihyperglycemic effect of water extract of dry fruits of Terminalia chebula in experimental diabetes mellitus. Indian J Clin Biochem. 2004;19:202–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mehrzadi S, Ghaznavi H, Huseini HF, et al. Effects of Pinus eldarica medw: nut extract on blood glucose and cholesterol levels in hypercholesterolemic alloxan-induced diabetic rats. J Med Plant Res. 2013;1:68–74. [Google Scholar]
- 20. Hosseini S, Jamshidi L, Mehrzadi S, et al. Effects of Juglans regia L. leaf extract on hyperglycemia and lipid profiles in type two diabetic patients: a randomized double-blind, placebo-controlled clinical trial. J Ethnopharmacol. 2014;152:451–456. [DOI] [PubMed] [Google Scholar]
- 21. Duchateau G, Van Oosten H., Vasconcellos M. Analysis of cis- and trans-fatty acid isomers in hydrogenated and refined vegetable oils by capillary gas-liquid chromatography. J Am Oil Chem Soc. 1996;73:275–282. [Google Scholar]
- 22. Masada Y. Analysis of Essential Oils by Gas Chromatography and Mass Spectrometry. New York, NY: John Wiley; 1976:214–218. [Google Scholar]
- 23. Gutfinger T. Polyphenols in olive oils. J Am Oil Chem Soc. 1981;58:966–968. [Google Scholar]
- 24. Kobayashi K, Pillai SK. A Handbook of Applied Statistics in Pharmacology. Boca Florida, FL: CRC Press; 2013:183–185. [Google Scholar]
- 25. Ghorbani A. Clinical and experimental studies on polyherbal formulations for diabetes: current status and future prospective. J Integr Med. 2014;12:336–345. [DOI] [PubMed] [Google Scholar]
- 26. Bellamkonda R, Rasineni K, Singareddy SR, et al. Antihyperglycemic and antioxidant activities of alcoholic extract of Commiphora mukul gum resin in streptozotocin induced diabetic rats. Pathophysiology. 2011;18:255–261. [DOI] [PubMed] [Google Scholar]
- 27. Ramesh B, Saralakumari D. Antihyperglycemic, hypolipidemic and antioxidant activities of ethanolic extract of Commiphora mukul gum resin in fructose-fed male Wistar rats. J Physiol Biochem. 2012;68:573–582. [DOI] [PubMed] [Google Scholar]
- 28. al-Awadi F, Fatania H, Shamte U. The effect of a plants mixture extract on liver gluconeogenesis in streptozotocin induced diabetic rats. Diabetes Res. 1991;18:163–168. [PubMed] [Google Scholar]
- 29. Nohr LA, Rasmussen LB, Straand J. Resin from the mukul myrrh tree, guggul, can it be used for treating hypercholesterolemia? A randomized, controlled study. Complement Ther Med. 2009;17:16–22. [DOI] [PubMed] [Google Scholar]
- 30. Ramesh B, Karuna R, Sreenivasa RS, et al. Effect of Commiphora mukul gum resin on hepatic marker enzymes, lipid peroxidation and antioxidants status in pancreas and heart of streptozotocin induced diabetic rats. Asian Pac J Trop Biomed. 2012;2:895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shalaby MA, Hammouda AA. Analgesic, anti-inflammatory and anti-hyperlipidemic activities of Commiphora molmol extract (Myrrh). J Intercult Ethnopharmacol. 2014;3:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Montonen J, Knekt P, Järvinen R, Reunanen A. Dietary antioxidant intake and risk of type 2 diabetes. Diabetes Care. 2004;27:362–366. [DOI] [PubMed] [Google Scholar]
- 33. Huseini HF, Larijani B, Heshmat R, et al. The efficacy of Silybum marianum (L.) Gaertn. (silymarin) in the treatment of type II diabetes: a randomized, double-blind, placebo-controlled, clinical trial. Phytother Res. 2006;20:1036–1039. [DOI] [PubMed] [Google Scholar]
- 34. Ruhe RC, McDonald RB. Use of antioxidant nutrients in the prevention and treatment of type 2 diabetes. J Am Coll Nutr. 2001;20(5 suppl):363S–369S. [DOI] [PubMed] [Google Scholar]
- 35. Boden G. Free fatty acids, insulin resistance, and type 2 diabetes mellitus. Proc Assoc Am Physicians. 1999;111:241–248. [DOI] [PubMed] [Google Scholar]
- 36. Ashry KM, El-Sayed YS, Khamiss RM, El-Ashmawy IM. Oxidative stress and immunotoxic effects of lead and their amelioration with myrrh (Commiphora molmol) emulsion. Food Chem Toxicol. 2010;48:236–241. [DOI] [PubMed] [Google Scholar]
- 37. Chen X, Sun F, Ma L, Wang J, Qin H, Du G. In vitro evaluation on the antioxidant capacity of triethylchebulate, an aglycone from Terminalia chebula Retz fruit. Indian J Pharmacol. 2011;43:320–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cheng HY, Lin TC, Yu KH, Yang CM, Lin CC. Antioxidant and free radical scavenging activities of Terminalia chebula . Biol Pharm Bull. 2003;26:1331–1335. [DOI] [PubMed] [Google Scholar]
- 39. Gao H, Huang YN, Xu PY, Kawabata J. Inhibitory effect on α-glucosidase by the fruits of Terminalia chebula Retz. Food Chem. 2007;105:628–634. [Google Scholar]
- 40. Balaji K, Ni L, Rajindran B, et al. Determination of total phenolic, flavonoid content and antioxidant activity of Terminalia chebula (fruit). Res J Pharm Biol Chem Sci. 2015;6:413–417. [Google Scholar]
- 41. Juang LJ, Sheu SJ, Lin TC. Determination of hydrolyzable tannins in the fruit of Terminalia chebula Retz. by high-performance liquid chromatography and capillary electrophoresis. J Sep Sci. 2004;27:718–724. [DOI] [PubMed] [Google Scholar]
- 42. Liu X, Kim JK, Li Y, Li J, Liu F, Chen X. Tannic acid stimulates glucose transport and inhibits adipocyte differentiation in 3T3-L1 cells. J Nutr. 2005;135:165–171. [DOI] [PubMed] [Google Scholar]
- 43. Triggiani V, Resta F, Guastamacchia E, et al. Role of antioxidants, essential fatty acids, carnitine, vitamins, phytochemicals and trace elements in the treatment of diabetes mellitus and its chronic complications. Endocr Metab Immune Disord Drug Targets. 2006;6:77–93. [DOI] [PubMed] [Google Scholar]