Abstract
Aim.
This systematic review is aimed at evaluating the literature on the efficacy of naturally available extracts that inhibit cancer.
Methods.
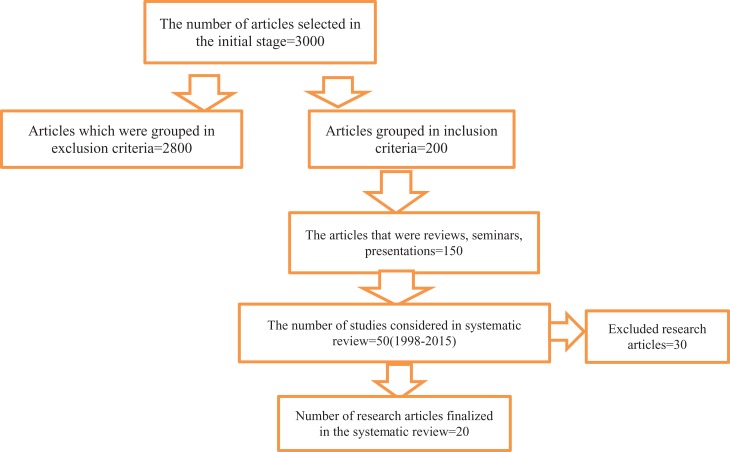
A literature search was performed to strengthening the reporting of observational studies in epidemiology analysis. Approximately 3000 research articles were initially selected. Of these articles, 200 were included, and 2800 were excluded. On further scrutiny, 150 of the 200 studies were reviews, seminars, and presentations, and 50 were original study articles. Among these articles, 20 studies were selected for the systematic review.
Results.
The predominant molecular pathways followed by natural extracts were nuclear factor kappa B ligand, suppression of the protein kinase B-Akt/P13K pathway (an intracellular signaling pathway important in regulating cell cycle), vascular endothelial growth factor downregulation, and tumor protein-P53 tumor suppressor upregulation.
Conclusions.
It is evident that natural extracts have the ability to inhibit cancer progression. Continued research in this field could facilitate the use of natural extracts with currently available anticancer agents.
Keywords: antineoplastic agents, cell cycle, downregulation, nuclear factor-kappa B, neoplasms, neoplastic processes, proto oncogene protein c-AKT, tumor suppressor protein p53, upregulation, vascular endothelial growth factor A
Naturally available extracts obtained from plants, animal sources, and marine sources have the ability to destroy abnormally proliferating cells. Traditional medicines and therapies have been produced from different types of natural extracts (Table 1). Many of the current studies have demonstrated the ability of natural extracts to inhibit the growth and proliferation of cancer cells.1–34
Table 1.
An Overview on Awareness of Naturally Available Extracts in Ancient Times.
| Researcher | Year | Source | Finding |
|---|---|---|---|
| Castiglioni35 | 1941 | A History of Medicine—Hippocrates (460-377 BC) | The extract of veratrum album as emetic olive oil aided in wound healing |
| Zhong and Wan31 | 1999 | Egyptian Ebbers Papyrus (1500 BC) | 800 prescriptions, 700 natural agents with medicinal properties, such as Aloe vera, Boswellia carteri, Frankincense, oil of Ricinus communis |
| Dev36 | 1999 | Charaka Samhita 900 BC, Susrutha Samhitha 600 BC | Concepts and practice of Indian ayurveda priority to surgical aspects mentioned the use of 395 medicinal plants and 57 animal products |
| Newman et al37 | 2000 | Archaeological investigations | Medical texts on clay tablets in 2600 BC |
| Wermuth38 | 2003 | Pedanius Dioscorides—40-90 AD | Material medica described the efficiency and dosage of 600 medicines |
| Zhen39 | 2004 | Literature by Galen 129-200 AD | Plants have beneficial and harmful effects |
| Newman40 | 2008 | Literature by Wilhelm Sertuner in 1805 | Isolation of morphine from opium |
| Koehn and Carter41 | 2005 | Pharmaceutical companies | Attempts were made to synthesize naturally available drugs |
| Newman and Cragg42 | 2012 | Influence of natural products | Advent of anticancer drugs from 1940 to 2010, including paclitaxel and vincristine |
Naturally available extracts exhibit high antioxidant abilities that favor the prevention and treatment of several cancers, including oral cancer and carcinomas of stomach, breast, prostrate, pancreas, and liver. Naturally available extracts target several pathways that aid tumor progression, such as the induction of cytotoxicity, autophagia, apoptosis, and necrosis of abnormally proliferating cells in the body. Naturally available extracts selectively inhibit abnormal cell proliferation without interrupting normally functioning cells. As a result, toxicity is rarely reported following their ingestion.43 Extracts from different sources may be combined to be more effective. Studies have demonstrated that naturally available extracts combined with the regularly used chemotherapeutics would minimize the adverse effects of chemotherapy by refining their pharmacodynamics and pharmacokinetics.6,10,12,17
Few studies23,30 have assessed the role of naturally available extracts against oral squamous cell carcinoma and its proliferative metastatic potential. The concentration and duration at which naturally available extracts portray their potential for treating oral squamous cell carcinoma are yet to be identified. Limited studies have been performed to determine the ability of naturally available extracts to induce cell cycle arrest, apoptosis, and cytotoxicity in oral squamous cell carcinoma. The precise chemical composition of the components within naturally available extracts that exhibits anticancer potential is still undetermined.
Although studies have demonstrated the anticancer properties of natural extracts, limited studies have been performed on pathways and the concentration and dosage to be administered to an individual. Therefore, the aim of this review was to evaluate the efficacy and ability of naturally available extracts to inhibit cancer.
Methodology
A systematic review was conducted according to the strengthening reporting of observational studies in epidemiology checklist (Table 2).
Table 2.
Objectives and Methodologies With Resources Utilized.
| Statement of the Objective | Methodology | Resources Utilized |
|---|---|---|
| It is essential to determine which of the molecular pathways were commonly followed by the natural extracts to inhibit cancer and the various investigative methods to explore cell cytotoxicity and cell viability of cancer cells in the reviewed literature. Analysis was performed to explore as to which were more common phases that exhibited cell cycle arrest by natural extract. | Numerous studies were reviewed where different concentrations of various natural extracts can accomplish lethality of cancer cells. Extensive search was done on research articles related to natural extracts with potential to kill cancer cells. Different analytical articles were probed that furnished data on ingesting different natural extracts at intervals or as a single dose to cause an effective mortality of cancer cells. Analysis was done as per STROBE criteria. | PLoS One, Scientific Reports, Oncology Reports, Cancer Letters, Cancer Journal, Molecular Cancer and Therapeutics, Carcinogenesis, Planta Medicine |
Abbreviation: STROBE, Strengthening the Reporting of Observational Studies in Epidemiology.
Focus Question
Following the preferred reporting items for systematic reviews and meta-analyses guidelines,44 a focused question was formulated based on the participants, interventions, control, and outcome principle.45 The focused question for this review was, “Do naturally available extracts have the ability to inhibit cancer progression?”
Eligibility Criteria
Original articles, experimental studies, clinical studies, and articles published in English that focused on naturally available extract anticancer properties in various types of human tissues, cell lines, animal models, and xenografts were included. Studies that reported upregulation/downregulation of different types of proteins responsible for neoplastic growth and natural extracts that regulated those protein levels were also considered.
Studies that examined anticancer drugs with their refined properties compared with different groups of anticancer drugs and advanced modes to induce different types of anticancer drugs were excluded from the study. Unpublished data, letters to the editor, reviews, and studies that lacked proper validation of their results were also excluded.
Search Strategy
Databases such as PubMed, Google Scholar, Scopus, and Science Direct were electronically searched from 1998 up to and including March 2016 using various combinations of the following keywords: “cancer,” “carcinoma,” “NAE,” “pomegranate,” “curcumin,” and “Aloe vera.” References of the selected articles were cross-checked to include those not identified by electronic search.
The titles and abstracts of studies that fit the eligibility criteria were screened by 2 researchers (AD, RSR) and reviewed for agreement. In case of any disagreement, a third reviewer (SVS) was consulted. Quality of the included studies was assessed according to the consolidated standards of reporting trials statement.46
Data Collection Process
The data collection was performed in 2 phases. In the first phase, all articles pertaining to naturally available extracts, combinations with currently available anticancer drugs, comparative studies of different naturally available extracts, effects on various diseases, and cross references were analyzed. In the second stage, the selection was narrowed to original research from 2009 to 2015 that concentrated on naturally available extracts’ potential to cause cytotoxicity and kill cancer cells.
Results
The initial search yielded 200 studies of which 180 did not conform to our eligibility criteria and were excluded from the study. In total, 20 studies were included. Figure 1 presents an overview of the study selection process.
Figure 1.
A flow chart illustration of the study selection.
Most studies were performed in 2015 and on cell lines, and only few studies utilized animal models. The most commonly used cell lines included MDA MB231, MDA MB137, and MDA MB157. Nine studies2,4,5,8,10,19,20,25,29 were performed on the anticancer properties of natural extracts on breast cancer cell lines, whereas only one study each was performed using ovarian cancer cell lines11 and cervical cancer cell lines.34 Three studies were conducted on pancreatic cell lines.1,6,28 Of the 20 research articles reviewed, the anticancer efficacy of the naturally available extract was determined “in vitro” in 80% of the research articles, and in vivo research was conducted in 6 studies. In 4 studies6,22,28,29 both in vitro and in vivo evaluation of the naturally available extract were performed. The animal models used in the study were Sprague Dawley rats and nude mice.
The commonly affected pathways included nuclear factor kappa B (NFkB), AKt, and vascular endothelial growth factor (VEGF) downregulation. Cell cytotoxicity (Table 3) was analyzed by annexin V propidium iodide assays, flow cytometry assays, trypan blue exclusion assays, TUNEL assays, Hoechst assays, bromodeoxyuridine (Brdu) assays, acridine orange ethidium bromide assays, and tube formation assay. In these assays, most of the cancer cells were destroyed by 24 to 72 hours. Most of the cancer cells were arrested at the G2M phase.2,4–8,10,11,14,15,19,23,25,28,29,34
Table 3.
The Extracts Employed and the Types of Assays Conducted to Determine Cell Viability and Cytotoxicity.
| Author, Year, and Extracts | Journal | Type of Cell Viability Assay/Proliferation | Type of Cell Cytotoxicity Assay |
|---|---|---|---|
| Giménez et al (2010)8 on mitraphylline | Planta Medicine | MTS | Trypan blue luciferase assay |
| Lee et al (2011)14 on alpha tomatine | PLoS One | MTT | Annexin V propidium iodide assay |
| Kim et al (2012)11 on cranberry proanthrocyanidin | International Journal of Oncology | Brdu assay, MMP assay | Annexin V propidium iodide assay |
| Meng et al (2011)19 on ECMS | Asian Pacific Journal of Cancer Prevention | MTT assay | Flow cytometry |
| Torres et al (2012)28 on graviola | Cancer Letters | MTT assay, western blot analysis | Flow cytometry analysis, confocal analysis |
| Elias et al (2013)20 on pouteria torta | Journal of Cancer Research and Therapeutics | MTT assay | DNA fragmentation assay |
| Fan et al (2014)7 on curcumin | Target Oncology | MTT assay | Annexin V propidium assay |
| Edderkaoui et al (2013)6 on ellagic acid and embellin | Nutrition and Cancer | MTT assay | Dual luciferase reporter assay, flow cytometry |
| Ling et al (2014)15 on diallyldisulfide | Oncology Reports | MTT assay | |
| Zhu et al (2013)33 on phyllanthus embellica | European Journal of Medicine and Research | MTT assay | Immunofluorescence assay |
| Pan et al (2013)22 on Aloin | Cancer Cell International | MTT assay, matrigel tube formation assay | Scratch assay, transwell invasion assay |
| Yen et al (2014)30 Soleria Robusta | Molecules | MTS assay, MMP analysis assay | Flow cytometry assay |
| Xiao et al (2014)29 on dialyl disulfide | PLoS One | Trypsininzation wound healing assay | Transwell assay |
| Arumugam et al (2014)2 on neem | Cancer Biology and Therapeutics | Western blot analysis | Immunofluorescence analysis |
| Sodde et al (2015)25 on Macrosolen parasiticus | Pharmacogn Magazine | MTT assay, brine shrimp lethality assay | SRB assay, acridine orange/ethidium bromide staining analysis |
| Samal et al (2015)23 on ketorolac | Scientific Reports | MTT scratch ATPase activity assay | Acridine orange/ethidium bromide staining analysis, RT-PCR analysis |
| Akimoto et al (2015)1 on ginger | PLoS One | MTT analysis, western blot analysis TEM analysis | Flow cytometry analysis, annexin V propidium iodide staining |
| Bassa et al (2016)4 on Rhidiola crenulata | Phytomedicine | RT-PCR analysis, western blot analysis, IHC | Trypan blue dye exclusion assay, dual reporter luciferase assay |
| Kala et al (2015)10 on resveratrol and pterostilbene | BMC Cancer | MTT analysis, cell colony forming analysis | Apoptosis analysis, cell cycle analysis, RT-PCR analysis, TRAP assay, western blot analysis, sirtuin DNMT activity analysis |
| Bishayee et al (2016)5 on pomegranate | Nutrition and Cancer | Carcinogenesis bioassay, RT-PCR | Immunofluorescence |
Abbreviations: RT-PCR, reverse transcription polymerase chain reaction; TEM, transmission electron microscopy; IHC, immunohistochemistry; SRB, sulforhodamine B.
The preferred analysis for cell viability studies was the MTT assay. Cell viability was analyzed by one-step MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay and XTT (2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) assays and was adequately validated.
Additional Findings
The cell lines treated with natural extracts were cultured in Dulbecco modified Eagle medium (DMEM), fetal bovine serum (FBS, 10%), and Roswell Park Memorial Institute medium (RPMI1640).
Naturally available extracts have the potential to kill the cancer cells at a lesser dose than the synthetically prepared chemotherapeutics
In vitro studies outnumbered the in vivo studies.
Discussion
Traditionally, natural compounds have been a main source of medicines to treat various ailments. Although rapid recoveries occurred following the administration of chemotherapy for cancer, adverse effects persisted. Our data concluded that naturally available extracts have the potential to prevent cancer progression with minimal adverse effects. The in vitro studies conducted (Table 4) have demonstrated that the natural extracts have the ability to kill cancer cells at doses less than synthetic chemotherapeutics.2,4–8,10,11,14,15,19,23,25,28,29,34
Table 4.
The Time Duration for Cell Cycle Arrest, the Phase at Which the Cell Cycle Was Arrested, Culture Media, and Cell Line Used.
| Author and Year | Culture Media | Cell Line In Vitro, Animal Model In Vivo | Time Duration for Cell Cycle Arrest | Cell Cycle Phase Arrest |
|---|---|---|---|---|
| Giménez et al (2010)8 | RPMI 1640 | In vitro on MHH ES1, MT3 | The IC50 for MHH-ES1 = 17.15 ± 0.82 µM in 30 hours | G2M |
| The IC50 for MT3 = 11.8 ± 1.03 µM in30 hours | ||||
| Lee et al (2011)14 | DMEM | In vitro on RWPE1s | At 24 hours the IC50 with a-tomatine for PC3 (prostrate cancer cells) was 1.67 ± 0.3 µM | G2M |
| Kim et al (2012)11 | DMEM | In vitro on SKOV-3 | The SKOV-3 cells incubated with PAC1 at 75 µg/mL in 24 hours, reduced its proliferation by 64.1% | G2M |
| Meng et al (2011)19 | DMEM | In vitro on MDA MB 231 | Treating MDA MB-231 cells with 0.1, 0.2, 0.4 mg/mL ECMS for 48 hours increased the proportion for apoptotic cells from 3.1% to 33% | G2M |
| Torres et al (2012)28 | DMEM | In vitro on FG/COL357 and CD18/HPAF and in vivo on athymic nude mice | At 48 hours the IC50 of graviola on FG/COLO357 was 200 µg/mL and CD18/HPAF was 73 µg/mL | G2M |
| Elias et al (2013)20 | DMEM | In vitro on MCF7, OSCC3 | The MCF7 cells seeded at 1.5 × 104 cells/well density on 24 well plates were treated with 500 mg/mL of P tortua extract at 24 and 48 hours caused death of 60% to 67% of cells | G1 |
| Fan et al (2014)7 | DMEM | In vitro on HepG2 | At 24 hours 30 µg/mL curcumin suppressed HepG2 cell growth by 93% | G2M |
| Edderkaoui et al (2013)6 | DMEM | In vitro on PaCa-2, HPAF-II cell lines and in vivo on athymic nude mice | At 72 hours, ellagic acid and embellin caused decreased cell proliferation at 0.5 µM in MIA PaCa-2, HPAF-II at 1 µM | G2M |
| Zhu et al (2013)33 | DMEM | In vitro on SW629 | At 48 hours, 150 mg/mL PEEP caused increase in apoptosis from 4.8% to 45% | G2M |
| Pan et al (2013)22 | FBS | In vitro on HUVEC, SW-620, HCT-116 cell lines and nude mice in vivo | Aloin at 200-240 µmol/L showed an IC50, the apoptotic activity increased by 33% in 72 hours | G1 |
| Yen et al (2014)30 | DMEM | In vitro on Ca 922 | After 24 hours of treatment, the cell viability drastically dropped from 100 ± 6.3 to 3.7 ± 1.3 at concentrations ranging from 0.05 to 1 mg/mL | G1 |
| Xiao et al (2014)29 | RPMI1640 | In vitro on MDA-MB 231 and in vivo on nude mice | The cancer cells show growth inhibition of 34.2% with 5 mg/L at 96 hours, the IC50 of DADS was 15 mg/L at 96 hours | G2M |
| Arumugam et al (2014)2 | In vivo study on Sprague-Dawley rats | At 48-72 hours apoptotic changes increased significantly than that at 24 hours in a dose-dependent manner | G2M | |
| Ling et al (2014)15 | RPMI1640 | In vitro on MGC803 | 30 mg/mL DADS enhanced phospho-Chk1 protein levels in a time dependent pattern in 12 hours | G2M |
| Sodde et al (2015)25 | DMEM | In vitro on MCF7 | In 48 hours M parasiticus showed IC50 59.33 ± 3.3 μg/mL | G2M |
| Samal et al (2015)23 | DMEM | In vitro on H357 | In 48 hours, the cell growth was declined by 1 μM; the IC50 was 2.6 μM | G2M |
| Akimoto et al (2015)1 | DMEM, FBS | In vitro on Panc1 | When Panc1 cells were treated with ginger extract 200 μg/mL for 20 hours causes cell cycle arrest | G0-G1 |
| Bassa et al (2016)4 | DMEM | In vitro on MCF7 | Following a 24 hour treatment, the proliferation of cancer cells was reduced by 50% | G2M |
| Kala et al (2015)10 | RPMI1640 | In vitro on MDA MB 157, HCC1806 | In 24 hours, there was an ER transcriptional activation, greater duration suppressed the activity | G2M, S |
| Bishayee et al (2016)5 | DMEM | In vitro on HCC1806, MDA MB 157 cancer cell Sprague-Dawley rat | In 72 hours, HCC1806 cancer cells were arrested at G2M phase, MDA MB 157 cancer cells with 15 μM combination treatment | G2M |
| At 5 g/kg tumor incidence was reduced by 54% |
Abbreviations: DMEM, Dulbecco modified Eagles medium; FBS, fetal bovine serum; PEEP, polyphenol extract of Phyllanthus emblica; RPMI1640, Roswell Park Memorial Institute Medium.
Mode Employed by Natural Extracts to Inhibit Cancer Cell Proliferation
In our review, naturally available extracts exhibited their potential to arrest the abnormal proliferation of cells. In majority of the studies (18 out of 20 studies), the anticancer properties of a single natural extract were evaluated.1,2,4,5,8,11,14,15,19,22,23,25,28–30,34 All these studies demonstrated that naturally available extracts have a superior ability to destroy cancer cells by inhibiting proliferation with minimal or reduced toxicity in unaffected normal cells. The naturally available extracts killed the cancer cells by cytotoxicity, apoptosis, overexpressing reactive oxygen species, mitochondrial dysfunction effects, or altering key signaling pathways and mRNA levels.2,5–8,10,11,19,20,28,29 Following the completion of a particular chemotherapeutic cycle, patients often complain of fatigue, nausea, malaise, diarrhea, mucositis, pain, rashes, infections, headache, and neurological problems. Unlike synthetic chemotherapeutics, naturally available extracts can be combined with the synthetic anticancer drugs to minimize adverse effects following its administration.12,17,47
Anticancer Pathways Induced by Natural Extracts
Naturally available extracts played a role in inhibiting cancer cell proliferation by upregulating or downregulating certain pathways to prevent abnormal cell proliferation. The pathways targeted by naturally available extracts include NFkB, Akt/p13, and VEGF. Lee et al13,14 explored the ability of alpha tomatine to inhibit NFkB activation by decreasing nuclear levels of NFkB/p65 and p50 transcription factors and inhibiting nuclear translocation. Edderkaoui et al6 reported that ellagic acid inhibits the NFkB pathway and that embellin inhibits STAT3 activity via reduced expression of apoptotic inhibitors, namely, survivin and XIAP. Embellin prevents the anti-apoptotic activity of XIAP by blocking the interaction between XIAP and caspases and also reduces surviving protein expression.6 Torres et al28 reported that graviola prevented cancer cell proliferation by inhibiting NFkB activity.28 Meng et al19 proved that ECMS arrested cancer cell proliferation by downregulating NFkB signaling and the p13/Akt pathway. Kim et al11,12 demonstrated suppression of the Akt pathway by proanthrocyanidin from cranberry extracts. The Akt pathway is also known as the P13K pathway, which favors survival and growth following the signals peripheral to the cells. Akt suppression will prevent the proliferation and migration of abnormally multiplying cells, thereby arresting the progress of proliferating cancer cells. In 2013, Pan et al22 reported that aloin and aloe vera extract exhibit anticancer properties by downregulating VEGF-induced cell viability. VEGF aids in angiogenesis. VEGF downregulation prevents abnormal cell proliferation and carcinogenesis. In 2014, Arumugam et al 2 demonstrated that neem leaves exhibited anticancer properties by upregulating the P53 tumor suppressor, mainly functioning to promote apoptosis in multiplying cells. The anticancer property of neem leaves is accomplished by upregulating P53.
Cancer Cells Treated With Natural Extracts Exhibited Apoptotic Effects at the G2M and G1 Phases
Naturally available extracts exhibited apoptotic and antiproliferative activities and caused cell cycle arrest in cancer cells at the G2M phase of the cell cycle. The G2M checkpoint inspects DNA after replication and guides the cells to safely enter mitosis. If DNA damage is present at this stage, the cell cycle is delayed by checkpoint inhibition, and DNA repair is initiated. If the damage is beyond repair, the cells are eliminated by apoptosis or are transitioned into a nonreplicative state. The G1 and S checkpoints monitor the integrity of DNA before replication. Following the treatment of cancer cells with naturally available extracts, p21 and p53 are overexpressed.48 p21 and p53 play essential roles in cell regulation. p21 is an inhibitor of cyclin/cyclin-dependent kinase complexes and interacts with other regulators of signal transduction. p21 induction is mediated by both p53 and p53-independent mechanisms and is essential for the onset of both G1 and G2 cell cycle arrest in the damage response and cell senescence.
MTT Analysis Is the Most Preferred Assay to Determine Cell Viability/Cytotoxicity
In a majority of the studies (13 out of 20), cell viability was determined using the MTT assay.1,2,7,10,14,15,17,19,22,23,25,34 The MTT assay utilizes dimethylthiazolium diphenyltetrazolium bromide in a colorimetric analysis to evaluate cellular metabolic activity. The yellow tetrazole is reduced to purple formazan in living cells. The intensity of the color can be estimated using a spectrophotometer.49 Tetrazolium dye reduction is dependent on NADPH-dependent oxidoreductase enzymes that are largely present in the cytosolic compartment of the cell. Rapidly dividing cells exhibit high rates of MTT reduction, which could explain why MTT analysis is commonly employed to estimate cancer cell viability. The other assays to determine cancer cell viability include MTS, Brdu, MMP, trypsinisation, wound healing assay, matrigel tube formation assay, Western blot analysis, brine shrimp lethality assay, scratch assay, ATPase activity assay, transmission electron microscopy, reverse transcription polymerase chain reaction, cell colony forming analysis, and carcinogenesis bioassay.2,4,8,11,29
Cell Apoptosis Analysis
The apoptotic activities of the cancer cell lines were evaluated by annexin V propidium iodide assays, acridine orange-ethidium bromide assays, and flow cytometry assays. Techniques including ELISA, Western blot, flow cytometry analysis, immunoblot assays, e-staining, DNA cell cycle analysis, trypan blue dye exclusion analysis, dual reporter luciferase assay, immunofluorescence assay, transwell assay, reverse transcription polymerase chain reaction, and scratch assays were also employed. The annexin V propidium iodide assay was most commonly employed to determine the cytotoxicity of naturally available extracts in cancer cells as it is cost effective, stable, and has the ability to prevent entry of dye into viable cells.50,51 The ability of propidium iodide to enter a cell depends on its membrane permeability and an intact plasma membrane. Early apoptotic and viable cells do not take up the stain.52–54 The integrity of the plasma and nuclear membranes is reduced in late apoptotic phase and necrotic cells. In these cells, the dye passes through the membranes, enters nucleic acids, and exhibits red fluorescence.55 However, conventional annexin V propidium iodide may also reveal false positive results in approximately 40% cases, and propidium iodide staining of RNA is noted in the cytoplasmic compartment.56 In flow cytometry analysis, the production of reactive oxygen species was monitored with 2′7′-dichloro-dihydroflurescein diacetate and a mitochondrial superoxide indicator. The fluorescence was monitored by flow cytometry or laser confocal microscopy. Although the results can be obtained in a short time, apoptotic cells and apoptotic bodies can overlap in the micronucleus during measurements. Cytotoxic activity was also determined using acridine orange-ethidium bromide staining of cancer cells that were incubated with naturally available extracts.22 Acridine orange emission changes from yellow to orange or red fluorescence as the pH is reduced in the acidic vacuoles of living cells. Acridine orange emits yellow fluorescence when it binds RNA and green fluorescence when it binds DNA. Nuclei emit yellowish-green fluorescence in normal conditions, whereas dead cells are stained red with ethidium bromide. The cell cytotoxicity of naturally available extracts was determined by analyzing the fluorescent changes in the treated cancer cells.
Time Frame of Cytotoxicity in Cancer Cells
A cytotoxic effect was induced in cancer cell lines within 24 to 72 hours on treatment with naturally available extracts. Naturally available extracts contain polyphenolic components that favor cell cycle arrest at the G1-S phase where the integrity of DNA is monitored before replication and at the G2M phase where DNA integrity is assessed within 48 hours after replication. Therefore, naturally available extracts prevent the progression of abnormally proliferating cells to mitosis by inducing apoptosis or senescence within 72 hours.
Types of Cell Lines Treated With Naturally Available Extracts
The cell lines were cultured in DMEM, 10% FBS, and RPMI1640. In the majority of studies (70%), the cell lines were cultured in DMEM,1,2,4,5,7,11,14,19,23,28,30,34 whereas only a few cells were cultured in RPMI1640 and 10% FBS. DMEM is a modification of basal Eagle medium and contains a 4-fold increase in the concentrations of amino acids and vitamins as well as additional supplementary components, such as glycine, serine, and ferric nitrate. The initial DMEM formula consists of approximately 1000 mg/L of glucose. High-glucose DMEM is an additional modification of DMEM and contains 4500 mg/L of glucose. DMEM and FBS were found to be ideal candidates for cell culture studies.
Substantial studies with natural extracts in oral cancer have not been performed to date; therefore, the efficacy of natural extracts in oral cancer can be evaluated using cancer cell lines and accomplishing associated clinical research. Comparative studies of naturally available extracts, combinations of different naturally available extracts, and assessments of their effectiveness in in vivo studies should also be performed.
Limitations
A few limitations in the present review should be noted. Only studies published in English were included; therefore, numerous recent studies published in other languages were not reported. Limited research has been performed on effective dosage and route; the frequency of the administration of the different types of extracts individually or in combination; and the most effective naturally available extracts when combined with routinely used anticancer agents.
Conclusions
Based on the results, it is evident that naturally available extracts exhibit good potency to prevent cancer progression. In most of the studies, naturally available extracts inhibited the proliferative activity of cancer cells.
An increase in number of cancer subjects indicates a deliberate need for a better selective, effective, and economic mode of treatment. Natural extracts hold great potential to provide nontoxic alternatives for the treatment of cancer. Natural extracts are a mixture of pharmacologically active compounds that may target abnormally proliferating cells without toxic effects on normal cells. The complete scientific and clinical evaluation of potential natural extracts is essential to advance these products to mainstream cancer therapies and provide alternate, safer, and reasonable complementary treatments.
Footnotes
Author Contributions: Study concept: Marin Abraham, Roopa S. Rao, Dominic Augustine
Study design: S. V. Sowmya, Vanishri C. Haragannavar, Roopa S. Rao
Data acquisition: Dominic Augustine, Roopa S. Rao, Shwetha Nambiar
Quality control of data and algorithm: Kavitha Prasad, Vanishri C. Haragannavar, Shwetha Nambiar, Roopa S. Rao
Data analysis and interpretation: S. V. Sowmya, Vanishri C. Haragannavar, Shwetha Nambiar
Manuscript preparation: Kamran Habib Awan, Shankargouda Patil
Manuscript editing: Dominic Augustine, Roopa S. Rao
Manuscript review: Dominic Augustine, Roopa S. Rao, Shankargouda Patil
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: This study did not warrant ethical review board clearance as no human subjects were involved.
References
- 1. Akimoto M, Iizuka M, Kanematsu R, Yoshida M, Takenaga K. Anticancer effect of ginger extract against pancreatic cancer cells mainly through reactive oxygen species-mediated autotic cell death. PLoS One. 2015;10:e0126605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arumugam A, Agullo P, Boopalan T, et al. Neem leaf extract inhibits mammary carcinogenesis by altering cell proliferation, apoptosis, and angiogenesis. Cancer Biol Ther. 2014;15:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bai Y, Zhao F, Li Y, Wang L, Fang XJ, Wang CY. Ginkgo biloba extract induce cell apoptosis and G0/G1 cycle arrest in gastric cancer cells. Int J Clin Exp Med. 2015;8:20977–20982. [PMC free article] [PubMed] [Google Scholar]
- 4. Bassa LM, Jacobs C, Gregory K, Henchey E, Ser-Dolansky J, Schneider SS. Rhodiola crenulata induces an early estrogenic response and reduces proliferation and tumorsphere formation over time in MCF7 breast cancer cells. Phytomedicine. 2016;23:87–94. [DOI] [PubMed] [Google Scholar]
- 5. Bishayee A, Mandal A, Bhattacharyya P, Bhatia D. Pomegranate exerts chemoprevention of experimentally induced mammary tumorigenesis by suppression of cell proliferation and induction of apoptosis. Nutr Cancer. 2016;68:120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Edderkaoui M, Lugea A, Hui H, et al. Ellagic acid and embelin affect key cellular components of pancreatic adenocarcinoma, cancer, and stellate cells. Nutr Cancer. 2013;65:1232–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fan H, Tian W, Ma X. Curcumin induces apoptosis of HepG2 cells via inhibiting fatty acid synthase. Target Oncol. 2014;9:279–286. [DOI] [PubMed] [Google Scholar]
- 8. Giménez DG, Prado EG, Rodríguez TS, Arche AF, De la Puerta R. Cytotoxic effect of the pentacyclic oxindole alkaloid mitraphylline isolated from Uncaria tomentosa bark on human Ewing’s sarcoma and breast cancer cell lines. Planta Med. 2010;76:133–136. [DOI] [PubMed] [Google Scholar]
- 9. Hsu YL, Kuo PL, Lin LT, Lin CC. Asiatic acid, a triterpene, induces apoptosis and cell cycle arrest through activation of extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways in human breast cancer cells. J Pharmacol Exp Ther. 2005;313:333–344. [DOI] [PubMed] [Google Scholar]
- 10. Kala R, Shah HN, Martin SL, Tollefsbol TO. Epigenetic-based combinatorial resveratrol and pterostilbene alters DNA damage response by affecting SIRT1 and DNMT enzyme expression, including SIRT1-dependent γ-H2AX and telomerase regulation in triple-negative breast cancer. BMC Cancer. 2015;15:672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim KK, Singh AP, Singh RK, et al. Anti-angiogenic activity of cranberry proanthocyanidins and cytotoxic properties in ovarian cancer cells. Int J Oncol. 2012;40:227–235. [DOI] [PubMed] [Google Scholar]
- 12. Kim SM, Lee SY, Yuk DY, et al. Inhibition of NF-kappaB by ginsenoside Rg3 enhances the susceptibility of colon cancer cells to docetaxel. Arch Pharm Res. 2009;32:755–765. [DOI] [PubMed] [Google Scholar]
- 13. Lee H, Ko JH, Baek SH, et al. Embelin inhibits invasion and migration of MDA-MB-231 breast cancer cells by suppression of CXC chemokine receptor 4, matrix metalloproteinases-9/2, and epithelial-mesenchymal transition. Phytother Res. 2016;30:1021–1032. [DOI] [PubMed] [Google Scholar]
- 14. Lee ST, Wong PF, Cheah SC, Mustafa MR. Alpha-tomatine induces apoptosis and inhibits nuclear factor-kappa B activation on human prostatic adenocarcinoma PC-3 cells. PLoS One. 2011;6:e18915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ling H, Lu LF, He J, Xiao GH, Jiang H, Su Q. Diallyl disulfide selectively causes checkpoint kinase-1 mediated G2/M arrest in human MGC803 gastric cancer cell line. Oncol Rep. 2014;32:2274–2282. [DOI] [PubMed] [Google Scholar]
- 16. Liu N, Yang HL, Wang P, et al. Functional proteomic analysis revels that the ethanol extract of Annona muricata L. Induces liver cancer cell apoptosis through endoplasmic reticulum stress pathway. J Ethnopharmacol. 2016;189:210–217. [DOI] [PubMed] [Google Scholar]
- 17. Liu TG, Huang Y, Cui DD, et al. Inhibitory effect of ginsenoside Rg3 combined with gemcitabine on angiogenesis and growth of lung cancer in mice. BMC Cancer. 2009;9:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mantena SK, Sharma SD, Katiyar SK. Berberine, a natural product, induces G1-phase cell cycle arrest and caspase-3-dependent apoptosis in human prostate carcinoma cells. Mol Cancer Ther. 2006;5:296–308. [DOI] [PubMed] [Google Scholar]
- 19. Meng LY, Liu HR, Shen Y, Yu YQ, Tao X. Cochinchina momordica seed extract induces G2/M arrest and apoptosis in human breast cancer MDA-MB-231 cells by modulating the PI3K/Akt pathway. Asian Pac J Cancer Prev. 2011;12:3483–3488. [PubMed] [Google Scholar]
- 20. Elias ST, Salles PM, de Paula JE, et al. Cytotoxic effect of Pouteria torta leaf extract on human oral and breast cancer cell lines. J Can Res Ther. 2013;9;601–606. [DOI] [PubMed] [Google Scholar]
- 21. Narrima P, Paydar M, Looi CY, et al. Persea declinata (Bl.) Kosterm bark crude extract induces apoptosis in MCF-7 cells via G0/G1 cell cycle arrest, Bcl-2/Bax/Bcl-xl signaling pathways, and ROS generation. Evid Based Complement Alternat Med. 2014;2014:248103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pan Q, Pan H, Lou H, Xu Y, Tian L. Inhibition of the angiogenesis and growth of aloin in human colorectal cancer in vitro and in vivo. Cancer Cell Int. 2013;13:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Samal SK, Routray S, Veeramachaneni GK, Dash R, Botlagunta M. Ketorolac salt is a newly discovered DDX3 inhibitor to treat oral cancer. Sci Rep. 2015;5:9982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shrivastava A, Kuzontkoski PM, Groopman JE, Prasad A. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol Cancer Ther. 2011;10:1161–1172. [DOI] [PubMed] [Google Scholar]
- 25. Sodde VK, Lobo R, Kumar N, Maheshwari R, Shreedhara CS. Cytotoxic activity of Macrosolen parasiticus (L.) danser on the growth of breast cancer cell line (MCF-7). Pharmacogn Mag. 2015;11(suppl 1):S156–S160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sun Y, Wang X, Zhou Q, et al. Inhibitory effect of emodin on migration, invasion and metastasis of human breast cancer MDA-MB-231 cells in vitro and in vivo. Oncol Rep. 2015;33:338–346. [DOI] [PubMed] [Google Scholar]
- 27. Ye H, Ye L, Kang H, et al. HIT: linking herbal active ingredients to targets. Nucleic Acid Res. 2011;39:D1055–D1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Torres MP, Rachagani S, Purohit V, et al. Graviola: a novel promising natural-derived drug that inhibits tumorigenicity and metastasis of pancreatic cancer cells in vitro and in vivo through altering cell metabolism. Cancer Lett. 2012;323:29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xiao X, Chen B, Liu X, et al. Diallyl disulfide suppresses SRC/Ras/ERK signaling-mediated proliferation and metastasis in human breast cancer by up-regulating miR-34a. PLoS One. 2014;9:e112720. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30. Yen YH, Farooqi AA, Li KT, et al. Methanolic extracts of Solieria robusta inhibits proliferation of oral cancer Ca9-22 cells via apoptosis and oxidative stress. Molecules. 2014;19:18721–18732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhong G, Wan F. An outline on the early pharmaceutical development before Galen [in Chinese]. Zhonghua Yi Shi Za Zhi. 1999;29:178–182. [PubMed] [Google Scholar]
- 32. Zhou Y, Shu F, Liang X, et al. Ampelopsin induces cell growth inhibition and apoptosis in breast cancer cells through ROS generation and endoplasmic reticulum stress pathway. PLoS One. 2014;9:e89021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhu X, Wang J, Ou Y, Han W, Li H. Polyphenol extract of Phyllanthus emblica (PEEP) induces inhibition of cell proliferation and triggers apoptosis in cervical cancer cells. Eur J Med Res. 2013;18:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhu X, Wang K, Zhang K, et al. Ziyuglycoside II inhibits the growth of human breast carcinoma MDA-MB-435 cells via cell cycle arrest and induction of apoptosis through the mitochondria dependent pathway. Int J Mol Sci. 2013;14:18041–18055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Castiglioni A. A History of Medicine. New York, NY: AA Knopf; 1941. [Google Scholar]
- 36. Dev S. Ancient-modern concordance in ayurvedic plants: some examples. Environ Health Perspect. 1999;107:783–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Newman DJ, Cragg GM, Snader KM. The influence of natural products upon drug discovery. Nat Prod Rep. 2000;17:215–234. [DOI] [PubMed] [Google Scholar]
- 38. Wermuth CG. The Practice of Medicinal Chemistry. 2nd ed Cambridge, MA: Academic Press; 2003. [Google Scholar]
- 39. Zhen CF. The Chen Zhi Fan Collectanea of Medical History. Beijing, China: Pecking University Medical Press; 2004. [Google Scholar]
- 40. Newman DJ. Natural products as leads to potential drugs: an old process or the new hope for drug discovery? J Med Chem. 2008;51:2589–2599. [DOI] [PubMed] [Google Scholar]
- 41. Koehn FE, Carter GT. The evolving role of natural products in drug discovery. Nat Rev Drug Discov. 2005;4:206–220. [DOI] [PubMed] [Google Scholar]
- 42. Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Asif M. A brief study of toxic effects of some medicinal herbs on kidney. Adv Biomed Res. 2012;1:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. [DOI] [PubMed] [Google Scholar]
- 45. Boudin F, Nie JY, Bartlett JC, Grad R, Pluye P, Dawes M. Combining classifiers for robust PICO element detection. BMC Med Inform Decis Mak. 2010;10:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Obstet Gynecol. 2010;115:1063–1070. [DOI] [PubMed] [Google Scholar]
- 47. Li XL, Wang CZ, Mehendale SR, Sun S, Wang Q, Yuan CS. Panaxadiol, a purified ginseng component, enhances the anti-cancer effects of 5-fluorouracil in human colorectal cancer cells. Cancer Chemother Pharmacol. 2009;64:1097–1104. [DOI] [PubMed] [Google Scholar]
- 48. Luk SCW, Siu SWF, Lai CK, Wu YJ, Pang SF. Cell cycle arrest by a natural product via G2/M checkpoint. Int J Med Sci. 2005;2:64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271–277. [DOI] [PubMed] [Google Scholar]
- 50. Fried J, Perez AG, Clarkson BD. Flow cytofluorometric analysis of cell cycle distributions using propidium iodide. Properties of the method and mathematical analysis of the data. J Cell Biol. 1976;71:172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bacso Z, Everson RB, Eliason JF. The DNA of annexin V-binding apoptotic cells is highly fragmented. Cancer Res. 2000;60:4623–4628. [PubMed] [Google Scholar]
- 52. Vermes I, Haanen C, Steffens-Nakken H, Reutellingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J Immunol Methods. 1995;184:39–51. [DOI] [PubMed] [Google Scholar]
- 53. Vermes I, Haanen C, Reutelingsperger C. Flow cytometry of apoptotic cell death. J Immunol Methods. 2000;243:167–190. [DOI] [PubMed] [Google Scholar]
- 54. Darzynkiewicz Z, Bruno S, Del Bino G, et al. Features of apoptotic cells measured by flow cytometry. Cytometry. 1992;13:795–808. [DOI] [PubMed] [Google Scholar]
- 55. Faleiro L, Lazebnik Y. Caspases disrupt the nuclear-cytoplasmic barrier. J Cell Biol. 2000;151:951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rieger AM, Hall BE, Luong LT, Schang LM, Barreda DR. Conventional apoptosis assays using propidium iodide generate a significant number of false positives that prevent accurate assessment of cell death. J Immunol Methods. 2010;358:81–92. [DOI] [PubMed] [Google Scholar]