Abstract
BACHGROUND
The functional significance of Wnt antagonist DICKKOPF-4 (DKK4) has not been investigated in renal cancer.
METHODS
We initially found that the expression of DKK4 was significantly higher in renal cancer tissues compared to adjacent normal kidney tissues. To assess the function of DKK4, we established stable DKK4 transfected cells and performed functional analyses including TCF/LEF reporter assay, cell viability, colony formation, apoptosis, cell cycle, invasive capability, wound healing capability and in vivo tumor growth.
RESULTS
The relative TCF/LEF activity was significantly lower in DKK4 transfected cells compared to empty vector, and nuclear beta-catenin expression was decreased in DKK4 transfectants. Also beta-catenin downstream effector proteins, cyclinD1 and c-Myc, showed decreased expression in DKK4 transfectants. However, higher invasiveness and migration was observed in stably transfected DKK4 cells. We also found increased growth of DKK4 transfected tumors in nude mice. Members of the Wnt non-canonical/JNK signaling pathway such as c-Jun expression and phosphorylation, and MMP-2 expression were significantly increased in DKK4 stable trasnfectants.
CONCLUSION
This is the first report to show that DKK4 expression is increased in renal cancer tissues and that DKK4 activates the non-canonical JNK signaling pathway while inhibiting the Wnt-canonical pathway.
Keywords: DKK4, RCC, real-time RT-PCR, MTS assay, colony formation assay, Western blot, apoptosis, cell cycle, FACS, invasion assay, wound healing assay, in vivo study, TCF/LEF reporter assay, non-canonical pathway, canonical pathway
INTRODUCTION
Renal cell carcinoma (RCC) is the third leading cause of death among urological tumors, accounting for 2 % of adult malignancies. 1 Although the rate of detection of incidental RCC has increased with improved diagnostic techniques, metastatic lesions are still found at diagnosis in about 30% of RCC patients.2 Wnt/beta-catenin signaling includes beta-catenin dependent (canonical) and beta-catenin independent (non-canonical) pathways.3-5 The canonical Wnt signaling pathway regulates cell fate and proliferation and this signaling is initiated by binding of Wnt ligands to frizzled (FZD) family receptors and the LRP5/LRP6 co-receptors. Subsequently beta-catenin interacts with members of the lymphoid enhancer factor 1/T-cell factor (LEF1/TCF) family, resulting in a functional transcription factor complex and the expression of downstream target genes. 3, 4 The non-canonical Wnt ligands also bind to FZD family receptors, and ROR2 and RYK co-receptors.4-7 The non-canonical signaling pathways include three pathways (Wnt/Ca2+, Wnt/G protein, and Wnt/PCP signaling pathways) and non-canonical signaling regulates cell polarity and movement.4-7 Among the five Wnt antagonist families (secreted frizzled-related protein (sFRP), Wnt inhibitory factor 1 (Wif1), Xenopus Cerberus, Wise and Dickkopf (DKK) families) the DKK family consists of four main members (DKK1-4) which contain two distinct cysteine-rich domains.3, 8 Our lab has studied several Wnt antagonist genes and these function in renal cancer.9-13 Previously DKK4 has been thought to act as an inhibitor of Wnt/beta-catenin signaling in colorectal cancer.14,15 Recently two groups found that DKK4 expression was increased in colon cancer tissues compared to matched normal colon tissues and DKK4 was induced by activated beta-catenin, although DKK4 itself significantly inhibited TCF/LEF reporter activity in colon cancer cell lines. 16, 17 As far as we know, there have been no reports regarding DKK4 and renal cancer. Therefore we first performed real time RT-PCR to clarify whether DKK4 is up-regulated in human renal cancer tissues compared to matched normal kidney tissues and found that the expression level of DKK4 was significantly higher in renal cancer tissues compared with matched normal kidney tissues. We next performed TCF/LEF reporter assay to confirm DKK4’s effect on the beta-catenin dependent pathway (canonical pathway) and found that the relative TCF/LEF activity was significantly inhibited in DKK4 transfected cells. Also, beta-catenin expression in the nucleus was decreased in DKK4 transfected renal cancer cells compared to empty vector cells, and protein expression of major TCF/beta-catenin down-stream effectors (c-Myc and cyclinD1) was down-regulated in DKK4 transfectants. However, cell invasion and migration ability were higher in DKK4 transfected renal cancer cells. Based on these results, we hypothesized that DKK4 may be functionally oncogenic in renal cancer in spite of the inhibitory effect on beta-catenin dependent pathway. To verify this hypothesis, we examined the effects of DKK4 expression on MTS, colony formation, apoptosis, cell cycle, invasion and migration assays using DKK4 transfected renal cancer cells.
MATERIALS AND METHODS
Clinical Samples
A total of 30 patients (17 male and 13 female) with pathologically confirmed conventional RCC were enrolled in this study (Toho University Hospital, Tokyo, Japan). The mean age of the patients was 60 (range 41-77) (Table 1). They were classified according to the WHO criteria and staged according to the tumor-node-metastasis (TNM) classification. Namely T refers to the size of the renal cancer and whether it has invaded nearby tissue, N refers to whether or not regional lymph nodes are involved, and M whether there is distant metastasis or not. The pathology of all the patients was clear cell renal carcinoma. Samples were obtained from the patients after written informed consent was obtained in Toho University hospital.
Table 1.
Characteristics of renal cancer patients (n=30)
| All patients (n=30) n (%) |
||
|---|---|---|
| age (mean ± SD) years | 60.3 ± 8.5 | |
| gender | male | 17 (57) |
| female | 13 (43) | |
| grade | 1 | 6 (20) |
| 2 | 20 (67) | |
| 3 | 4 (13) | |
| pStage | 1 | 16 (53) |
| 2 | 8 (27) | |
| 3 | 2 (7) | |
| 4 | 4 (13) | |
| pT | 1 | 16 (53) |
| 2 | 9 (30) | |
| 3 | 4 (13) | |
| 4 | 1 (3) | |
| 3+4 | 5 (17) | |
| pN | (-) | 28 (93) |
| (+) | 2 (7) | |
| pM | (-) | 27 (90) |
| (+) | 3 (10) | |
| pathology | clear cell carcinoma | 30 (100) |
Cell culture
Renal cancer cell lines [A-498 (ATCC number; HTB-44), Caki-1 (ATCC number; HTB-46)] were purchased from the American Type Culture Collection (Manassas, VA). The renal cancer cell lines were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum.
Plasmid construction
Plasmids containing the human full-length cDNA fragment of DKK4 (GenBank accession number NM_014420, cat# RC221217) was purchased from Origene (Rockville, MD). This clone (pCMV6-DKK4) expresses the complete DKK4 ORF (open reading frame) with a Tag (MYC/DDK) at the C terminal.
Stable clone establishment
To prepare stable cell lines over-expressing DKK4, we transfected A-498 cells with the pCMV6-DKK4 expression vector encoding DKK4 cDNA using FuGENE HD (Roche Diagnosis, Basel, Switzerland) according to the manufacture’s instructions. Transfected cells were selected by culturing with G418 (150 μg/ml) for two months. Empty vector transfectants were used as controls. Single colonies of stable transfectants were isolated and expanded for further analysis based on the level of DKK4 expression. We selected the top two stable clones which had the highest DKK4 mRNA expression compared to empty vector transfectants. We named these DKK4 clones, clone 1 and 2. DKK4 clone 1 and clone 2 were used for further experiments (MTS, colony formation, invasion, apoptosis, cell cycle analysis, in vivo study). When the cells (stable empty and stable DKK4-clone 1 and clone 2) were confluent, they were trypsinized and re-suspended in media according to the protocol. At the same time, we confirmed the DKK4 expression level of the cells before carrying out the experiments. All experiments were done in triplicate.
Total RNA and protein extraction
Total RNA was extracted from formalin-fixed, paraffin-embedded (FFPE) human renal cancer and matched adjacent non-cancerous normal tissue using a miRNeasy FFPE kit (Qiagen) after micro-dissection. To digest DNA, the Qiagen RNase-Free DNase kit was used. Total RNA were also extracted from cell lines using an RNeasy mini kit (Qiagen). Cells were lysed with RIPA buffer (Pierce, Brebieres, France) containing protease inhibitors (Sigma, St. Louis, MO). We also extracted nuclear protein and cytoplasmic protein separately using a CelLytic NuCLEAR Extraction kit (Sigma-Aldrich, Saint Louis, MI) to confirm beta-catenin expression. Protein quantification was done using a BCA protein assay kit (Pierce, Brebieres, France).
TCF/LEF reporter assay
To monitor the activity of Wnt/beta-catenin signal transduction, we used the TCF/LEF reporter assay with TCF-reporter plasmids (TOPFLASH; containing the wild-type TCF binding site, FOPFLASH; containing a mutant-type TCF binding site) (Millipore, Billerica, MA). The pRL-TK renilla luciferase (Promega, Madison, WI) was co-transfected to normalize transfection efficiency. A-498 renal cancer cells were stimulated with recombinant mouse Wnt3a protein (100ng/mL) (rmWnt3a, #1324-WN, R & D systems, Minneapolis, MN). FuGENE HD (Roche Diagnosis, Basel, Switzerland) was used for transfection according to the manufacture’s instructions. All experiments were performed in triplicate. Luciferase activity was assayed at 48 hours after transfection, using a Dual-Luciferase Reporter Assay system (Promega, Madison, WI).
Cell viability assay
DKK4 or empty stably transfected A-498 cells (empty, DKK4-clone 1, 2) were maintained in medium supplemented with 150 μg/ml G418. Cell viability was measured after 4 days with MTS (CellTiter 96 Aqueous One Solution Cell Proliferation Assay, Promega). To verify the effect of DKK4 on renal cancer cells, we also performed similar MTS assays using another renal cancer cell line (Caki-1). Data are the mean ± S.D. of 6 independent experiments.
Soft Agar Colony formation assay
Soft agar colony formation was assayed with A-498 empty cells and A-498 DKK4 stably transfected cells (clone 1, 2) using a Cell Biolabs CytoSelect Cell Transformation Assay kit. Namely, cells were incubated 7 days in a semi-solid agar media before being solubilized and detected by using the provided MTT solution in a microplate reader (OD570nm). The absorbance was compared between empty vector cells and DKK4 stable transfected cells (clone 1, 2). Data are the mean ± S.D. of 8 independent experiments.
Cell invasion assay
Cell invasion assay was performed with the CytoSelect 24-well cell invasion assay kit as previously described (Cell BioLab, San Diego, CA). The cells (empty and stable DKK4-clone 1, 2) were re-suspended to the upper chamber in triplicate. To verify the effect of DKK4 on renal cancer cell, we performed invasion assays using another renal cancer cell line (Caki-1). Cells migrating through the membrane were stained and counted with a microscope. Five random fields were chosen for each membrane, and the results were expressed as migrated cells quantified at OD 560nm after extraction.
Wound healing assay
The wound healing process begins with tissue matrix remodeling, migration, and eventual closing of the wound area. Therefore this assay is frequently used for assessment of cancer cell migration. Wound healing assay was performed with the CytoSelect 24-well wound healing assay kit as previously described (Cell BioLab, San Diego, CA). To generate a wound field, the cells were cultured until they formed a monolayer around the insert. After removing insert, a 0.9 mm open wound field was left. Cells migrated from either side of the gap. The wound closure was monitored and the percent closure was measured at 10 hours between empty vector and DKK4 clone 1 and clone 2-A-498 cells. To verify the effect of DKK4 on renal cancer cell, we also performed migration assays using another renal cancer cell line (Caki-1). [Percent closure rate (%) = migrated cell surface area/ Total surface area ×100)]
Apoptosis and cell cycle analysis
Cells (empty and DKK4 stably transfected clone 1 and clone 2) were washed twice with 1xPBS and trypsinized. After inactivating trypsin in complete medium, the cells were re-suspended in ice-cold 1x binding buffer (70 μl). Annexin V-FITC solution (10 μl) and 7-AAD viability dye (20 μl) were added to 70 μl of the cell suspensions. After incubation for 15 minutes in the dark, 400 μl of ice-cold 1x binding buffer was added. The apoptotic distribution of the cells in each sample was then determined using a FACS (Cell Lab QUANTA SC, Beckman Coulter, Fullerton, CA). The various phases of cells were determined using a DNA stain (DAPI). Cell populations (G0/G1, S, and G2/M) were measured using fluorescence and contrasted against cell volume. Data are the mean ± S.D. of four independent experiments. To verify the effect of DKK4 on renal cancer cells, we performed apoptosis and cell cycle analysis using another renal cancer cell line (Caki-1).
Quantitative real-time RT-PCR
Quantitative real-time RT-PCR was performed in triplicate with an Applied Biosystems Prism 7500 Fast Sequence Detection System using TaqMan universal PCR master mix according to the manufacture’s protocol (Applied Biosystems Inc., Foster City, CA, USA). The TaqMan probes and primers were purchased from Applied Biosystems. Human GAPDH was used as an endogenous control. Levels of RNA expression were determined using the 7500 Fast System SDS software version 1.3.1 (Applied Biosystems).
Western analysis
Total cell protein (20μg) was used for Western blotting. Samples were resolved in 4-20 % Precise Protein Gels (Pierce, Brebieres, France) and transferred to PVDF membranes (Amersham Biosciences, Fairfield, CT). The membranes were immersed in 0.3% skim milk in TBS containing 0.1% Tween 20 for 1 hour and probed with primary polyclonal and monoclonal antibody against Cyclin D1 (#556470, BD Biosciences), c-Myc (#551102, BD Biosciences), caspase3 (#610322, BD Biosciences), cleaved caspase3 (#9664, Cell Signaling), beta-catenin (#9562, Cell Signaling), Bax (#2772, Cell Signaling), MMP-2 (#4022, Cell Signaling), GAPDH (#2118, Cell Signaling), JNK (#9258, Cell Signaling), phosphorylated JNK (#4668, Cell Signaling), c-Jun (MAB3732, Millipore), phosphorylated c-Jun (#9164, Cell Signaling) overnight at 4°C. To confirm expression of DKK4 in stable transfected cells, we used anti-DKK4 antibody (#TA302466, Origene). Blots were washed in TBS containing 0.1% Tween20 and labeled with horseradish peroxidase (HRP)-conjugated secondary anti-mouse or anti-rabbit antibody (Cell Signaling). Proteins were enhanced by chemiluminescence (Amersham ECL plus Western Blotting detection system, Fairfield, CT) for visualization. The protein expression levels are expressed relative to GAPDH levels.
In vivo study
Groups of six female nude mice (strain BALB/c nude; Charles River Laboratories, Inc., Wilmington, MA), 4-5 weeks old, received subcutaneous injections of 1 × 107 A-498-empty (n=6) or A-498-DKK4 stable transfected cells [clone 1(n=5), clone 2(n=5)] in the right flank area in a volume of 200 μl. Tumor size was determined using calipers once per week for 35 days, and tumor volume was calculated on the basis of width (x) and length (y): x2y/2, where x< y. After the mice were sacrificed, tumors were resected and weighted. In addition, tumor tissues were fixed in 10% formalin, embedded in paraffin, and stained with H&E and DKK4 (#AP1524a, ABGENT, San Diego, CA) for histological examination. Animal experiments were approved by the Animal Studies Subcommittee of the VAMC (protocol # 08-003-01).
Statistical analysis
All statistical analyses were performed using StatView (version 5; SAS Institute Inc., NC). A p-value of < 0.05 was regarded as statistically significant.
RESULTS
DKK4 expression level in renal cancer tissues and adjacent normal kidney tissues
We compared DKK4 mRNA expression levels between renal cancer tissues and matched adjacent normal kidney tissues (n=30) using the expression in each patient normal tissue as reference (expression=1). DKK4 mRNA expression was high in renal cancer tissues compared to matched normal kidney tissues in 19 out of 30 (63.3%) normal kidney and renal cancer paired tissue specimens (Fig. 1).
Figure 1. DKK4 mRNA expression level in human renal cancer tissues and adjacent normal kidney tissues (n= 30).

Expression levels of DKK4 in renal cancer tissues and adjacent normal kidney tissues (30 clear cell carcinoma patients). When the relative DKK4 mRNA expression (DKK4/GAPDH) was higher in renal cancer tissues compared to matched normal renal tissues, the case was included in the category where “the DKK4 expression high in cancer tissues”.
Relationship between DKK4 expression level and clinical characteristics
All patients pathology was clear cell renal carcinoma. DKK4 mRNA expression was classified into two categories based on real-time RT PCR results. Namely, when the relative DKK4 mRNA expression (DKK4/GAPDH) was higher in renal cancer tissues compared to matched normal renal tissues, the case was included in the category where “the DKK4 expression was high in cancer tissues”(Table S1). We investigated the relationship between DKK4 mRNA expression level and clinical factors, including gender, grade, pathologic tumor classification (pT), pathologic lymph node status (pN), pathologic metastasis status (pM) and outcomes (survival and recurrence). There was no significant association between DKK4 expression and clinical parameters except for gender (Supplementary Table. S1).
TCF/LEF reporter assay
The relative TCF/LEF activity was significantly lower in DKK4 transfected cells compared to empty vector (Fig. 2-A).
Figure 2. TCF/LEF reporter assay, establishment of DKK4 transfectants (transient, stable), cell viability and colony formation assay in DKK4 transfectants.

A. TCF/LEF reporter assay. (72 hours transient transfection) B. Expression of DKK4 in stable transfectant (A-498 cells) and transient transfectant (Caki-1 cells) by real-time RT PCR and Western blots. C. Cell viability assay in empty vector and DKK4 transfected cells (A-498-clone 1, clone 2; Caki-1) (MTS assay). D. colony formation assay in empty vector and stably DKK4 transfected cells (clone 1, clone 2).
Establishment of DKK4 stable clones and effect of DKK4 on cell viability, colony forming, and cell invasion
After transfection of A-498 or Caki-1 cells with a pCMV6-DKK4 expression plasmid, the DKK4 expression level was confirmed by real time RT-PCR and Western blotting (Fig.2B). DKK4 protein expression was observed in both DKK4 stably transfected A-498 cells and DKK4 transiently transfected Caki-1 cells. Then cell viability analysis (MTS assay), colony forming assay, and cell invasion assays were performed using stable DKK4 transfectants of A-498 cells or transient DKK4 transfectants of Caki-1 cells. We observed enhanced growth of A-498 and Caki-1 cells after DKK4 transfection by MTS and colony formation assays (Fig. 2C, Fig. 2D). DKK4 also promoted the in vitro invasion and migration ability of A-498 and Caki-1 cells (Fig. 3A, Fig. 3B).
Figure 3. Invasion, wound healing assay and in vivo study with empty vector and DKK4 transfected renal cancer cells.

A. Invasion assay, B. Wound healing assay (A-498 cells-0/ 10 hours; Caki-1 cells-0/8 hours), C. Assessment of promotion of tumor growth in nude mice with empty vector and DKK4 clone 1 and clone 2 cells (C-1). Representative picture of nude mice with DKK4 transfected and empty vector cells are shown (C-2). DKK4 protein expression was confirmed by immunohistochemistry (C-2).
In vivo study
A-498 cells stably transfected with empty vector or the DKK4 gene (stable clone 1 and clone 2) were injected subcutaneously into the right flank of nude mice. Tumor growth was significantly promoted in mice with DKK4 transfected cells compared to those with empty vector transfected cells (Fig. 3C). We performed immunohistochemistry for DKK4 in grown tumors and confirmed that DKK4 protein was highly expressed in DKK4 transfectant tumors (Fig. 3C).
Apoptosis, cell-cycle analyses, and cleaved caspase-3 expression
Apoptosis and cell cycle analysis were performed to investigate whether DKK4 over-expression affected these parameters in renal cancer cells. However we did not find a significant difference in the number of apoptotic cells between DKK4 transfected clones (clone 1 and clone 2) and control cells (Fig. 4A, 4B). We also did not find significant difference in each of the cell cycle phases (G0/G1, S, G2/M) between DKK4 transfectants and controls (data not shown). To verify the effect of DKK4 on renal cancer cells (A-498 cells), we performed apoptosis assays using another renal cancer cell line (Caki-1). Similar to that of A-498 cells, we did not find a significant difference in apoptosis in DKK4 transiently transfected Caki-1 cells. We examined pro-caspase-3 and cleaved caspase-3 expression in DKK4 transfectants (stable clone-2) and controls by Western analysis, but there was no difference of pro-caspase expression between the transfectants and no cleaved caspase-3 was observed in both transfectants (Fig. 4C). This result was also verified in another renal cancer cell line (Caki-1 cells).
Figure 4. Apoptosis assay with stably (A-498) or transient (Caki-1) transfected empty and DKK4 renal cancer cells and protein expression of apoptosis markers.
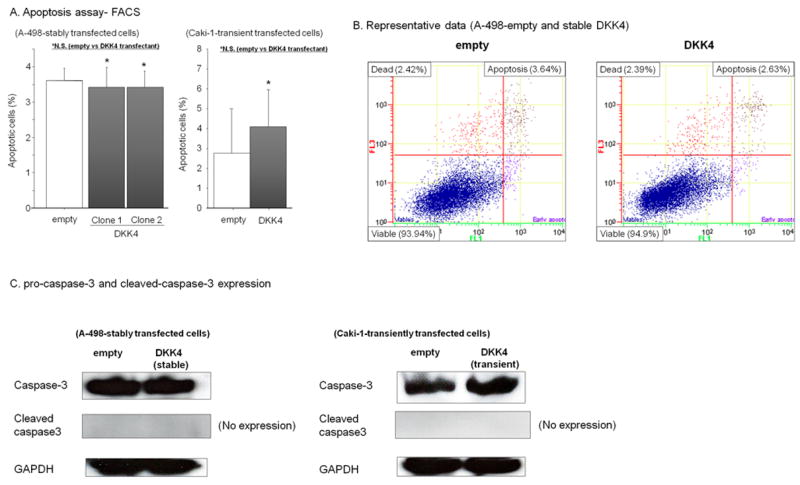
A. Flow cytometry analysis of apoptosis in empty vector and stable DKK4 clone 1 and clone 2 cells and transient DKK4 transfectants of Caki-1 cells. Annexin V-FITC and 7-AAD were measured by flow cytometry. Representative results are also shown (B). Data are the mean ± S.D. of four independent experiments. C. Protein expression of pro-caspase-3 and cleaved caspase3 in empty and DKK4 stable transfectants (A-498) and DKK4 transient transfectants (Caki-1).
Quantitative real-time RT-PCR and Western blotting in DKK4-overexpressing cells
We found decreased TCF/LEF reporter activity in DKK4 transfectants, suggesting that DKK4 affects the Wnt canonical pathway. Therefore we examined the expression of TCF/LEF down-stream effectors such as cyclinD1 and c-Myc by Western blot (Fig. 5A). We found decreased expression of these proteins in DKK4 stable transfectants. Additionally we compared the protein expression of beta-catenin in cytoplasmic and nuclear fractions between empty vector and DKK4 transfectants. Consistent with the TCF/LEF reporter assay results and the decreased TCF/LEF down-stream effector expression, beta-catenin expression in the nucleus was significantly decreased in DKK4 transfectants (Fig. 5B). In contrast to the beta-catenin dependent pathway protein expression, we found tumor growth promotion (in vitro and in vivo) and increased invasion and migration ability in DKK4 transfected cells, suggesting that DKK4 influenced other cancer pathways. Therefore we looked at the expression of genes in the beta-catenin independent pathway, Wnt-JNK. As shown in Fig. 5C, c-JUN expression and phosphorylation was significantly increased in DKK4 stable transfectants. In addition, one of the major down-stream effectors, MMP-2 also showed increased expression in DKK4 transfectants.
Figure 5. Protein expression of signaling pathway genes in empty vector and stable DKK4 transfected A-498 Cells.
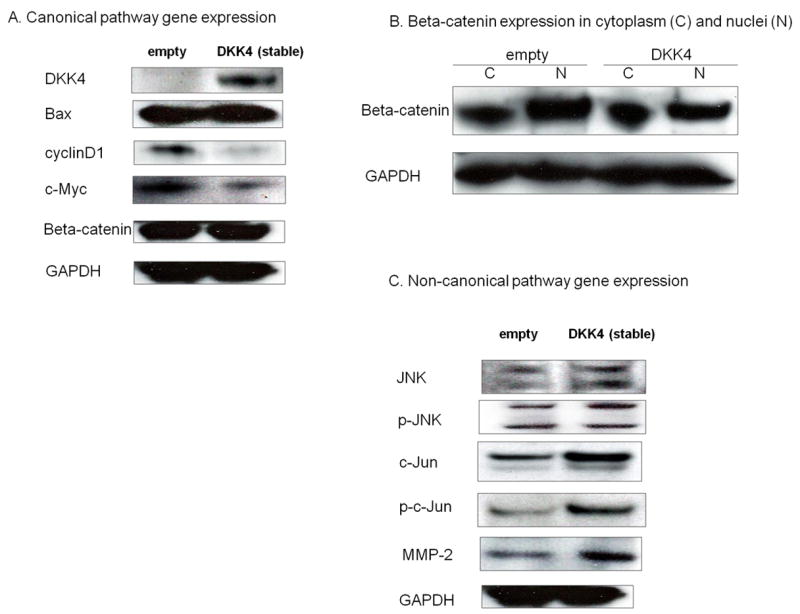
A. Wnt-canonical pathway related genes expression (Western blot assay). B. Beta-catenin expression in cytoplasm and nuclei. C. Non-canonical pathway gene expression (Western blot assay)
Discussion
In this study we found that the expression of DKK4 is up-regulated in renal cancer tissues compared with matched normal kidney tissues. These results suggested that DKK4 may have oncogenic functions in renal cancer. Recently several reports have found that DKK4 expression was high in cancer tissues compared with normal tissues in colon cancers.16,17 Our results are consistent with these previous reports. 16,17
DKK4 is a member of the Wnt antagonist genes and generally has been regarded as an inhibitor of the Wnt canonical pathway.14 Initially in order to investigate the relationship between DKK4 and the canonical pathway, we performed TCF/LEF reporter assays and found that DKK4 inhibited TCF/LEF reporter activity and that beta-catenin expression in the nuclear protein fraction was down regulated in DKK4 transfected renal cancer cells. The expression of c-Myc and cyclin D1, which are major TCF/LEF down-stream effector proteins, were also down-regulated in DKK4 transfected cells. This result means that DKK4 may be involved in the beta-catenin dependent pathway and that DKK4 is a beta-catenin dependent pathway inhibitor as described in previous reports with colon cancer.16,17
In contrast to the inhibitory effect of DKK4 on the beta-catenin dependent Wnt pathway, DKK4 promoted cell proliferation, invasion, and migration in renal cancer cells (A-498, Caki-1). We also observed that DKK4 promoted in vivo tumor growth of A-498 cells. The Wnt signaling pathway involves beta-catenin dependent (canonical) and beta-catenin independent (non-canonical) pathways.4,18 The non-canonical pathway includes three pathways (Wnt/Ca2+, Wnt/G protein, and Wnt/PCP signaling pathways).5 These pathways primarily regulate cell movement.5 The role of non-canonical pathway in renal cancer is less understood. Among these pathways, c-Jun-NH2-kinase (JNK) pathway has been thought to be involved in the non-canonical pathway (Wnt/PCP signaling pathway).5 Non-canonical Wnt signals are transduced through frizzled (FZD) receptors, and small G-proteins (Rho-A, Rac, CDC42) and JNK are involved with dishevelled (Dvl)-dependent effector molecules.4 Generally Rac1 mediates JNK activation. However, the exact role of JNK in Wnt-mediated signaling remains unknown. 19-21 Recently Fukukawa et al found that FZD homologue 10 was highly expressed in synovial sarcoma and caused the activation of the non-canonical Dvl-Rac1-JNK pathway and also caused destruction of the actin cytoskeleton structure through down-regulation of the RhoA activation.22 Activation of JNK is associated with apoptosis in response to various cellular stresses and anti-apoptotic and growth promoting effects.23 Regarding cancers, several reports on the JNK related apoptosis in response to cellular stress have been published.24-28 In renal cancer, JNK related apoptosis has been also reported.29,30 Among DKK family members, DKK3 induces apoptosis in several cancers (prostate, testicular, and breast cancers) through activation of JNK.24-26 DKK1 also plays an important role in the induction of apoptosis via activation of JNK in placental choriocarcinoma and mesothelioma.27,28 Regarding DKK4, there have been no reports showing its relationship with the JNK pathway in cancer. Previous studies with colon cancer have shown that ectopic DKK4 expression increased migration and invasion properties of colon cancer cells, and our results are consistent with this report.16,17 Khatlani et al reported that JNK was activated in non small-cell lung cancer biopsy samples and promoted oncogenesis.23 Wang et al also found that over-expression of active JNK in human breast cancer cells did not cause apoptosis, but enhanced cell migration and invasion.31 In this study, we examined apoptosis by FACS and active caspase-3 protein expression by Western blot analysis in A-498 cells stably transfected with DKK4 or empty vector. We did not find a significant change in apoptotic cell numbers between DKK4 and control transfectants, and also did not find cleaved caspase-3 expression in these cells. These results suggest that DKK4 is not associated with induction of apoptosis. However we did observed significant promotion of cell proliferation and invasion in vitro. We also found that DKK4 transfectants displayed enhanced tumor growth in nude mice compared to control transfectants. In addition to phosphorylation of JNK, expression and phosphorylation of c-Jun were increased in DKK4 transfected A-498 cells. This result is similar to that reported by Wang et al. 31
The JNK pathway includes a number of down-stream proteins. The MMP family plays an important role in cell invasion and metastasis in renal cancer and is mediated by several intracellular pathways. 32-35 Fromigué et al found that atorvastatin (a member of the drug class known as statins) reduced matrix metalloproteinase expression or activity in invading osteosarcoma cells and inhibition of JNK reduced MMP-2 activity.34 Inamoto et al reported that gamma-amino butyric acid (GABA) stimulation significantly increased the expression of MMP-2 and -9 and also increased the invasive activity of renal cancer cells.35 GABA stimulation promoted the phosphorylation of MAPKs, including ERK1/2, JNK, and p38.35 In the present study, we found increased MMP-2 expression in DKK4 transfected A-498 cells. Thus our results suggest that DKK4 promotes the non-canonical pathway by activating JNK, resulting in up-regulation of c-Jun expression and phosphorylation, and increasing MMP-2 expression. Therefore up-regulation of MMP-2 may be involved in the increased growth of DKK4 transfectant tumors in nude mice. Although the exact molecular mechanism as to how DKK4 regulates MMP-2 expression is unknown, our findings indicate that further studies will be warranted on the roles of DKK4 in renal cancer invasion and metastasis.
In conclusion, to our knowledge this is the first report documenting that DKK4 expression is higher in renal cancer tissues compared to matched normal kidney tissues. We examined the role of DKK4 in the canonical and non-canonical Wnt pathways and found that DKK4 inhibited the canonical pathway. Though DKK4 did not induce apoptosis it promoted renal cancer cell proliferation, invasion, and migration probably via the non-canonical JNK pathway, which also increased MMP-2 expression. Thus the current findings contribute important information about the role of DKK4 in renal cancer cells.
Supplementary Material
Acknowledgments
We thank Dr. Roger Erickson for his support and assistance with the preparation of the manuscript. This study was supported by Grants RO1CA130860, RO1CA111470, T32-DK07790 from the NIH, VA Research Enhancement Award Program (REAP), Merit Review grants, and Yamada Science Foundation.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics. 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Bukowski RM. Natural history and therapy of metastatic renal cell carcinoma: the role of interleukin-2. Cancer. 1997;80:1198–1220. doi: 10.1002/(sici)1097-0142(19971001)80:7<1198::aid-cncr3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 3.Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25:7469–7481. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- 4.Katoh M, Katoh M. WNT signaling pathway and stem cell signaling network. Clin Cancer Res. 2007;13:4042–4045. doi: 10.1158/1078-0432.CCR-06-2316. [DOI] [PubMed] [Google Scholar]
- 5.Turashvili G, Bouchal J, Burkadze G, Kolar Z. Wnt signaling pathway in mammary gland development and carcinogenesis. Pathobiology. 2006;73:213–223. doi: 10.1159/000098207. [DOI] [PubMed] [Google Scholar]
- 6.Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430:93–98. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- 7.Lu W, Yamamoto V, Ortega B, Baltimore D. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell. 2004;119:97–108. doi: 10.1016/j.cell.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 8.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 9.Urakami S, Shiina H, Enokida H, et al. Wnt antagonist family genes as biomarkers for diagnosis, staging, and prognosis of renal cell carcinoma using tumor and serum DNA. Clin Cancer Res. 2006;12:6989–6997. doi: 10.1158/1078-0432.CCR-06-1194. [DOI] [PubMed] [Google Scholar]
- 10.Kawamoto K, Hirata H, Kikuno N, Tanaka Y, Nakagawa M, Dahiya R. DNA methylation and histone modifications cause silencing of Wnt antagonist gene in human renal cell carcinoma cell lines. Int J Cancer. 2008;123:535–542. doi: 10.1002/ijc.23514. [DOI] [PubMed] [Google Scholar]
- 11.Hirata H, Hinoda Y, Nakajima K, et al. Wnt antagonist gene DKK2 is epigenetically silenced and inhibits renal cancer progression through apoptotic and cell cycle pathways. Clin Cancer Res. 2009;15:5678–5687. doi: 10.1158/1078-0432.CCR-09-0558. [DOI] [PubMed] [Google Scholar]
- 12.Kawakami K, Hirata H, Yamamura S, et al. Functional significance of Wnt inhibitory factor-1 gene in kidney cancer. Cancer Res. 2009;69:8603–8610. doi: 10.1158/0008-5472.CAN-09-2534. [DOI] [PubMed] [Google Scholar]
- 13.Hirata H, Hinoda Y, Ueno K, Majid S, Saini S, Dahiya R. Role of secreted frizzled-related protein 3 in human renal cell carcinoma. Cancer Res. 2010;70:1896–1905. doi: 10.1158/0008-5472.CAN-09-3549. [DOI] [PubMed] [Google Scholar]
- 14.Sato H, Suzuki H, Toyota M, et al. Frequent epigenetic inactivation of DICKKOPF family genes in human gastrointestinal tumors. Carcinogenesis. 2007;28:2459–2466. doi: 10.1093/carcin/bgm178. [DOI] [PubMed] [Google Scholar]
- 15.Baehs S, Herbst A, Thieme SE, et al. Dickkopf-4 is frequently down-regulated and inhibits growth of colorectal cancer cells. Cancer Lett. 2009;276:152–159. doi: 10.1016/j.canlet.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Pendas-Franco N, Garcia JM, Pena C, et al. DICKKOPF-4 is induced by TCF/beta-catenin and upregulated in human colon cancer, promotes tumour cell invasion and angiogenesis and is repressed by 1alpha, 25-dihydroxyvitamin D3. Oncogene. 2008;27:4467–4477. doi: 10.1038/onc.2008.88. [DOI] [PubMed] [Google Scholar]
- 17.Matsui A, Yamaguchi T, Maekawa S, et al. DICKKOPF-4 and -2 genes are upregulated in human colorectal cancer. Cancer Sci. 2009;100:1923–1930. doi: 10.1111/j.1349-7006.2009.01272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 19.Boutros M, Paricio N, Strutt DI, Mlodzik M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 1998;94:109–118. doi: 10.1016/s0092-8674(00)81226-x. [DOI] [PubMed] [Google Scholar]
- 20.Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC. Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat Neurosci. 2005;8:34–42. doi: 10.1038/nn1374. [DOI] [PubMed] [Google Scholar]
- 21.Kim GH, Han JK. JNK and ROKalpha function in the noncanonical Wnt/RhoA signaling pathway to regulate Xenopus convergent extension movements. Dev Dyn. 2005;232:958–968. doi: 10.1002/dvdy.20262. [DOI] [PubMed] [Google Scholar]
- 22.Fukukawa C, Nagayama S, Tsunoda T, Toguchida J, Nakamura Y, Katagiri T. Activation of the noncanonical Dvl- Rac1-JNK pathway by Frizzled homologue 10 in human synovial sarcoma. Oncogene. 2009;28:1110–1120. doi: 10.1038/onc.2008.467. [DOI] [PubMed] [Google Scholar]
- 23.Khatlani TS, Wislez M, Sun M, et al. c-Jun N-terminal kinase is activated in nonsmall-cell lung cancer and promotes neoplastic transformation in human bronchial epithelial cells. Oncogene. 2007;26:2658–2666. doi: 10.1038/sj.onc.1210050. [DOI] [PubMed] [Google Scholar]
- 24.Abarzua F, Sakaguchi M, Takaishi M, et al. Adenovirusmediated overexpression of REIC/Dkk-3 selectively induces apoptosis in human prostate cancer cells through activation of c-Jun-NH2-kinase. Cancer Res. 2005;65:9617–9622. doi: 10.1158/0008-5472.CAN-05-0829. [DOI] [PubMed] [Google Scholar]
- 25.Tanimoto R, Abarzua F, Sakaguchi M, et al. REIC/Dkk-3 as a potential gene therapeutic agent against human testicular cancer. Int J Mol Med. 2007;19:363–368. [PubMed] [Google Scholar]
- 26.Kawasaki K, Watanabe M, Sakaguchi M, et al. REIC/Dkk- 3 overexpression downregulates P-glycoprotein in multidrug- resistant MCF7/ADR cells and induces apoptosis in breast cancer. Cancer Gene Ther. 2009;16:65–72. doi: 10.1038/cgt.2008.58. [DOI] [PubMed] [Google Scholar]
- 27.Peng S, Miao C, Li J, Fan X, Cao Y, Duan E. Dickkopf-1 induced apoptosis in human placental choriocarcinoma is independent of canonical Wnt signaling. Biochem Biophys Res Commun. 2006;350:641–647. doi: 10.1016/j.bbrc.2006.09.087. [DOI] [PubMed] [Google Scholar]
- 28.Lee AY, He B, You L, et al. Dickkopf-1 antagonizes Wnt signaling independent of beta-catenin in human mesothelioma. Biochem Biophys Res Commun. 2004;323:1246–1250. doi: 10.1016/j.bbrc.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Oya M, Mikami S, Mizuno R, Marumo K, Mukai M, Murai M. c-Jun activation in acquired cystic kidney disease and renal cell carcinoma. J Urol. 2005;174:726–730. doi: 10.1097/01.ju.0000164656.99251.77. [DOI] [PubMed] [Google Scholar]
- 30.Mizuno R, Oya M, Shiomi T, Marumo K, Okada Y, Murai M. Inhibition of MKP-1 expression potentiates JNK related apoptosis in renal cancer cells. J Urol. 2004;172:723–727. doi: 10.1097/01.ju.0000124990.37563.00. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Kuiatse I, Lee AV, Pan J, Giuliano A, Cui X. Sustained c-Jun-NH2-kinase activity promotes epithelial-mesenchymal transition, invasion, and survival of breast cancer cells by regulating extracellular signal-regulated kinase activation. Mol Cancer Res. 2010;8:266–277. doi: 10.1158/1541-7786.MCR-09-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Struckmann K, Mertz K, Steu S, et al. pVHL coordinately regulates CXCR4/CXCL12 andMMP2/MMP9 expression in human clear-cell renal cell carcinoma. J Pathol. 2008;214:464–471. doi: 10.1002/path.2310. [DOI] [PubMed] [Google Scholar]
- 33.Kallakury BV, Karikehalli S, Haholu A, Sheehan CE, Azumi N, Ross JS. Increased expression of matrix metalloproteinases 2 and 9 and tissue inhibitors of metalloproteinases 1 and 2 correlate with poor prognostic variables in renal cell carcinoma. Clin Cancer Res. 2001;7:3113–3119. [PubMed] [Google Scholar]
- 34.Fromigue O, Hamidouche Z, Marie PJ. Blockade of the RhoA-JNK-c-Jun-MMP2 cascade by atorvastatin reduces osteosarcoma cell invasion. J Biol Chem. 2008;283:30549–30556. doi: 10.1074/jbc.M801436200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inamoto T, Azuma H, Sakamoto T, et al. Invasive ability of human renal cell carcinoma cell line Caki-2 is accelerated by gamma-aminobutyric acid, via sustained activation of ERK1/2 inducible matrix metalloproteinases. Cancer Invest. 2007;25:574–583. doi: 10.1080/07357900701522471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


