Abstract
The pathogenesis of pulmonary arterial hypertension remains undefined. Changes in the expression and effects mediated by a number of vasoactive factors have been implicated to play a role in the onset and progression of the disease. The source of many of these mediators, such as nitric oxide (NO), prostacyclin and endothelin-1 (ET-1), is the pulmonary endothelium. This article focus in the role of nitric oxide in PAH, reviewing the evidence for its involvement in regulation of pulmonary a vascular tone under physiological conditions, the mechanisms by which it can contribute to the pathological changes seen in PAH and strategies for the use of NO as a therapy for treatment of the disease.
Pulmonary arterial hypertension
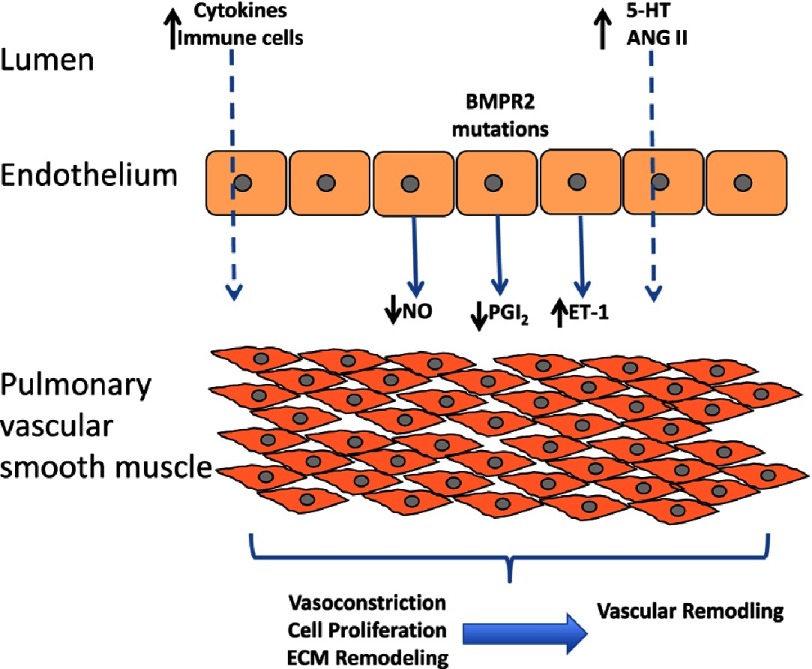
Pulmonary arterial hypertension (PAH) is a rare, debilitating condition with a poor prognosis. While the precise mechanism(s) that mediated the onset and progression of the disease remain undefined, several factors have been implicated in the pathology of PAH. These include endothelial dysfunction, oxidant stress, metabolic dysfunction, immune dysregulation and genetic factors1–9, all of which can contribute to the pulmonary artery vasoconstriction, vascular remodelling and right ventricular failure that are features of the disease (Figure 1).
Figure 1. Schematic diagram of the release of vasoactive factors from the endothelium and their action on the underlying vascular smooth muscle.
Epidemiology of PAH
PAH has an incidence of 15–50 people per million. Initially, median survival was calculated to be only 2.8 years10,11. More recently, data has shown that depending on the presence of co-morbidities the survival 3 years after diagnosis is between 54.4% and 58.2%12. One year survival of PAH has been shown to be influenced by a range of prognostic indicators including renal insufficiency, PAH associated with connective tissue disease, functional class III heart failure, mean right atrial pressure, resting systolic blood pressure, heart rate, 6-minute walk distance, brain natriuretic peptide levels, percentage predicted carbon monoxide diffusion capacity and pericardial effusion on echocardiogram13. There is a predominance of the condition in women, which varies according to the aetiology of the disease14.
Pathogenesis of PAH
The aetiology of PAH is varied, this is reflected in the World Health Organisation’s clinical classification of pulmonary hypertension (Table 1)15. Despite the wide range of causative factors, the lungs of patients with pulmonary hypertension exhibit a range of classical histological changes. These include remodelling of the pulmonary vessels, regions of neovascularisation, fibrotic changes in the vessel wall, thrombus formation and formation of plexiform lesions16. Plexiform lesions are composed of proliferating endothelial cells, matrix proteins and fibroblasts that obliterate the vascular lumen17. The reasons for their formation are poorly understood, however hypoxia, inflammation, shear stress, drugs, viral infections and genetic susceptibility have all been implicated18.
Table 1. WHO classification of pulmonary hypertension.
| Group 1 | Pulmonary arterial hypertension (PAH) |
| Idiopathic (IPAH) | |
| Heritable (HPAH) | |
| Bone morphogenetic protein receptor type 2 (BMPR2) | |
| Activin receptor-like kinase 1 gene (ALK1), endoglin (with or without haemorrhagic telangiectasia) | |
| Unknown | |
| Drug- and toxin-induced | |
| Associated with (APAH): | |
| Connective tissue diseases | |
| Human immunodeficiency virus (HIV) infection | |
| Portal hypertension | |
| Congenital heart disease (CHD) | |
| Schistosomiasis | |
| Chronic haemolytic anaemia | |
| Persistent pulmonary hypertension of the newborn (PPHN) | |
| Group 1′ | Pulmonary veno-occlusive disease (PVOD) and/or pulmonary capillary haemangiomatosis (PCH) |
| Group 2 | Pulmonary hypertension due to left heart diseases |
| Systolic dysfunction | |
| Diastolic dysfunction | |
| Valvular disease | |
| Group 3 | Pulmonary hypertension due to lung diseases and/or hypoxemia |
| Chronic obstructive pulmonary disease (COPD) | |
| Interstitial lung disease (ILD) | |
| Other pulmonary diseases with mixed restrictive and obstructive pattern | |
| Sleep-disordered breathing | |
| Alveolar hypoventilation disorders | |
| Chronic exposure to high altitude | |
| Developmental abnormalities | |
| Group 4 | Chronic thromboembolic pulmonary hypertension (CTEPH) |
| Group 5 | PH with unclear multifactorial mechanisms |
| Haematological disorders: myeloproliferative disorders, splenectomy | |
| Systemic disorders: sarcoidosis, pulmonary Langerhans cell histiocytosis, lymphangioleiomyomatosis, neurofibromatosis, vasculitis | |
| Metabolic disorders: glycogen storage disease, Gaucher disease, thyroid disorders | |
| Others: tumoral obstruction, fibrosing mediastinitis, chronic renal failure on dialysis |
A number of factors and agents responsible for initiating and progressing the increases in pulmonary artery pressure have been suggested. Given the variety of different forms of the disease, it’s not surprising that so many different mediators and mechanisms are believed to be responsible (Table 2), many of which have been reviewed elsewhere1–5,19. At the cellular level dysfunction of the pulmonary endothelium seems to underpin many of the changes seen in PAH. Endothelial cells regulate vascular tone, vascular remodelling and inflammation via the release a range of vasoactive molecules that interact with blood elements and the underlying vascular smooth muscle. These mediators include nitric oxide (NO), prostacyclin and endothelin-1 (ET-1). The role of both ET-1 and prostacyclin has recently been reviewed in this journal2,3. The focus of the present article is on the role of NO in the onset and progression of PAH as well as the use of NO therapies for the alleviation of the clinical symptoms and improving the quality of life of patients with PAH.
Table 2. Causative agents associated with the pathogenesis of PAH.
| Chemical / Drug mediators | Associated conditions |
|---|---|
| Aminorex, | Mutations in bonemorphogenic protein receptor 2 |
| Fenfluramine, | Systemic sclerosis |
| Dexfenfluramine, | HIV infection |
| Cocaine, | Portal hypertension |
| Phenylpropanolamine | Congenital heart disease with left-to-right shunts |
| St. John’s Wort | Recent acute pulmonary embolism |
| Chemotherapeutic agents | Sickle cell disease |
| Serotonin re-uptake inhibitors | |
| Amphetamines | |
| Metamphetamines and L-tryptophan | |
| Exposure to chemicals such as toxic rapeseed oil |
Nitric oxide in the physiology of the pulmonary circulation
As with all other vascular beds, the production of NO by the pulmonary endothelium helps to regulate vascular tone. While a diverse range of endogenous chemical mediators have been identified to stimulate the release of NO from endothelial cells, the frictional force of the flow of blood over the surface of the endothelial cells (shear stress) is the principal physiological stimulus for NO release20.
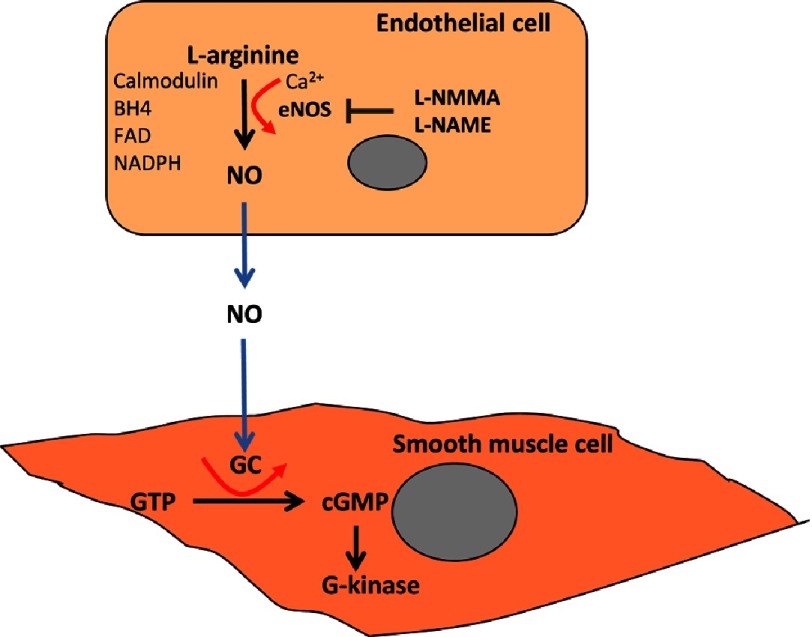
The role of endothelial cells in regulating vascular tone was first reported as the “obligatory role” of the vascular endothelium in mediating relaxation of the underlying smooth muscle in 1980 with the discovery of endothelium-derived relaxing factor (EDRF)21. It was not until 7 years later that the chemical nature of EDRF as NO was identified and the metabolic pathway leading to its synthesis elucidated. Nitric oxide is formed by the enzymatic cleavage of the terminal amino group from the amino acid L-arginine22,23. The enzyme, termed nitric oxide synthase (NOS) was specific for the L isomer, but had no activity against the D isomer of arginine (Figure 2). At approximately the same time, NO was also shown to account for the biological actions of nitrovasodilators, which are capable of generating NO from their chemical structure (so called NO donors)24.
Figure 2. Diagram showing the mechaism of synthesis of nitric oxide from endothelail cells and its action on vascular smooth muscle cells.
The characterisation of the first of a number of inhibitors of NOS, such as L-NG-monomethyl Arginine (L-NMMA), L-NG-Nitroarginine methyl ester (L-NAME) and NG-Methyl-L-arginine (L-NMA), allowed researchers to probe the role of NO in the vascular system, leading to the first demonstration that inhibition of NO increases blood pressure in animals and induces vasoconstriction in human blood vessels25–27. In addition, it was shown that the effects of NO are not confined to the vascular system alone. Besides the endothelial NOS two additional isoforms of the NOS enzyme, a neuronal isoform and an inducible isoform, have been identified. These three isoforms release NO for a variety of different biological functions28. All NOS enzymes bind calmodulin and contain haem and utilise L-arginine and molecular oxygen as substrates and requires the cofactors reduced nicotinamide-adenine-dinucleotide phosphate (NADPH), flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN), and (6R-) 5,6,7,8-tetrahydrobiopterin (BH4). They differ in their distribution, dependence on calcium and the amount of NO that they produce (Table 3)29.
Table 3. Characteristics NOS enzymes.
| Name | Gene | Location | Co-factors |
|---|---|---|---|
| NOS1, nNOS, Neuronal NOS | Chromosome 12 | Neuronal tissue, skeletal muscle | Heam, BH4, NADPH, Ca2 + |
| NOS2, iNOS, Inducible NOS | Chromosome 17 | Immune system Cardiovascular system | Heam, BH4, NADPH, |
| NOS3, eNOS, Endotheial NOS | Chromosome 7 | Endothelium | Heam, BH4, NADPH, Ca2 + |
Most of the biological actions of NO depend on its ability to stimulate the enzyme soluble guanylate cyclase with the subsequent formation of cyclic 3′, 5′ guanosine monophosphate (cGMP)30. This leads to the activation of cGMP-dependent kinases (cGKs), which leads to the activation myosin phosphate and a subsequent release of calcium from intracellular stores allowing smooth muscle cells to relax. cGKs may also activate transcription factors that may change gene expression in the cell and mediate other responses of the cell to NO or alter its response to other stimuli31,32. The bioavailability of NO is influenced by its inactivation by excessive amounts of oxygen-derived species such as superoxide (O2−) or changes in the protective effects of endogenous antioxidants.
Sources of O2− include NADPH oxidases, xanthine oxidase, cytochrome P450 enzymes and from complexes I and III of the electron transport chain in mitochondria. An additional source of O2− may come from uncoupling of NOS, which has been suggested to occur in several pathological conditions33–35. Unless inactivated by oxygen free radicals, the effects of NO are terminated by the breakdown cGMP by phosphodiesterase (PDE) enzymes. There are a number of different isoforms of PDEs that have specific distributions in different organs and vascular beds36.
Interactions between nitric oxide and other endothelium-derived vasoactive mediators
The endothelium was initially regarded simply as monolayer of cells that line the vasculature serving as a barrier between the blood and the vessel wall37, however it is now considered more as an organ in its own right that regulates vascular homeostasis. In addition to NO, the endothelium also releases prostacyclin (PGI2) and ET-1, which also both have a direct effect on the vessel wall38–41. ET-1 is a powerful vasoconstrictor peptide that can be considered as a functional antagonist to the vasodilator effects of NO. ET-1 has also been implicated in the pathogenesis of PAH and inhibition of its effects is a pharmacological target for the treatment of the disease42,43. In contrast, PGI2 is a vasodilator and anti-aggregating substance. The effect of prostacyclin on platelet aggregation are enhanced by the presence of NO44. The effects of PGI2 are mediated via its action on cell surface receptors that in turn stimulate the accumulation of cAMP. Administration of PGI2, has been the most widely used, and to date most successful, therapeutic strategy for the treatment of patients with PAH. The labile nature of PGI2 has meant that such strategies have relied upon the development of stable analogues of PGI2 such as Iloprost, which unfortunately is not orally active and has to be inhaled or given as an I.V. infusion45. There have been some clinical trials that have combined PGI2 analogues or ET-1 receptor antagonists with compounds that promote the effect of NO43. The role of PGI2 in PAH, the therapeutic potential of PGI2 analogues and receptor agonists in the treatment of the disease and potential novel strategies to maximise drug delivery and effect have recently been reviewed in this Journal3.
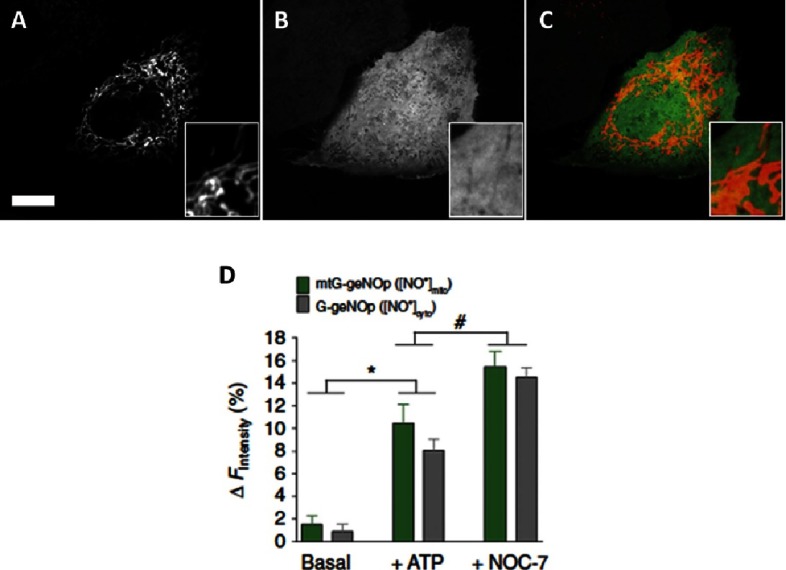
In addition to its direct effects on soluble guanylate cyclase, NO has been implicated to play a role in the regulation of mitochondrial function via its ability to interact with components of the electron transport chain46. NO has also been shown to activate mitochondrial biogenesis via a cGMP-dependent mechanism47. In conditions associated with over-production of NO, such as septic shock, its been shown that low mitochondrial complex I activity is associated with raised nitrite/nitrate concentrations48. The irreversible nature of the inhibition of complex I suggest that it is due to the action of reactive nitrogen species, rather than an inhibitory effect of NO. The direct relevance to how NO regulation of mitochondrial function affects the pulmonary vasculature is currently been investigated. In the fetal lung, it has been shown that interactions between endothelial NOS and mitochondria reduce the levels of oxidative stress and facilitates vasodilation at birth49. The recent development of fluorescent molecular probes specific for the mitochondria and cytosol, that specifically and directly respond to NO have provided the opportunity to obtain a quantifiable and real-time readout of NO dynamics in different cellular compartments (Figure 3)50. This approach may be of use to track the intracellular actions of NO is pulmonary cells.
Figure 3. Confocal images of endothelial cells expressing genetically encoded fluorescent probes that specifically respond to NO in (A) the mitochondria (mtC-geNO), (B) the cytosol (G-geNOp) and (C) a merged image of both.
Scale bar, 10 mm. (D) Maximal average changes in intensity following the addition of ATP or the NO donor NOC-7 in the mitochondrial (green bar) and the cytosol (grey bar).
Role of nitric oxide in the pathophysiology of PAH
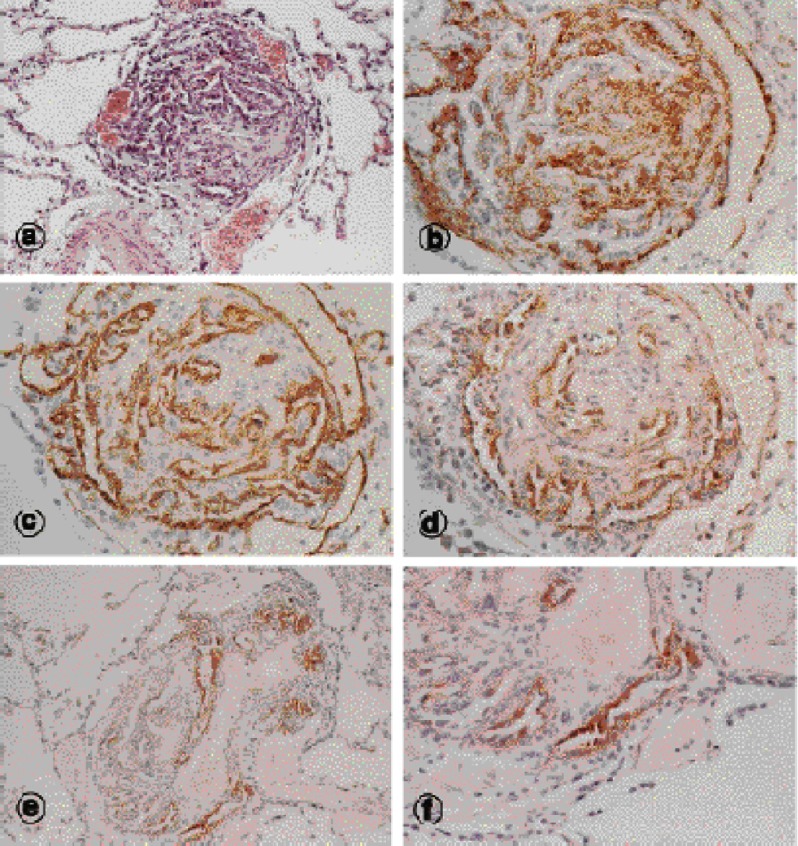
Given the vasodilator effect of NO, it would be easy to speculate that reduced expression or release of NO would be identified in patients with the disease. Indeed there is evidence that pulmonary arteries from patients with PAH due to a variety of reasons have reduced endothelial expression of eNOS51. However there are also studies that have shown no change or even increased expression of eNOS in vessels taken from hypertensive lungs52. Increased expression of endothelial NOS has been specifically localised to plexiform lesions53 (Figure 4). While these studies have been able to show changes in expression of the enzyme responsible for NO synthesis by the endothelium, it remains unclear as to whether this translates to changes in the activity of the enzyme or sensitivity of the vessel wall to the action of NO. Indeed, patients can be classified as responders or non-responders in the ability of inhaled NO to reduce pulmonary vascular resistance (PVR) and mean pulmonary artery pressure (mPAP). Those with a reduction of >30% in PVR or >12% in mPAP were shown to have reduced mortality compared to patients who had responses below these thresholds54–56.
Figure 4. Histological sections of plexiform lesions in pulmonary arteries from patients with PAH (taken from Mason et al.)53.
Reductions in endothelial NOS have been shown to contribute to impaired mitochondrial biogenesis an ovine model of PAH57. Changes in mitochondrial activity in the pulmonary arteries during PAH results in alterations to redox signalling and impaired oxygen sensing. These changes cause activation of transcription factors usually associated with hypoxia, such as hypoxia-induced factor 1a (HIF-1a), which persist even under normoxic conditions58,59. HIF-1a can regulate mitochondrial fission, fusion and metabolism of pulmonary artery smooth muscle cells, which contribute to proliferation of smooth muscle cells and remodelling of the vessel wall60,61. The regulatory role of NO on mitochondria and the activation of these hypoxia-related pathways remain to be determined.
Endogenous inhibitors of NOS enzymes
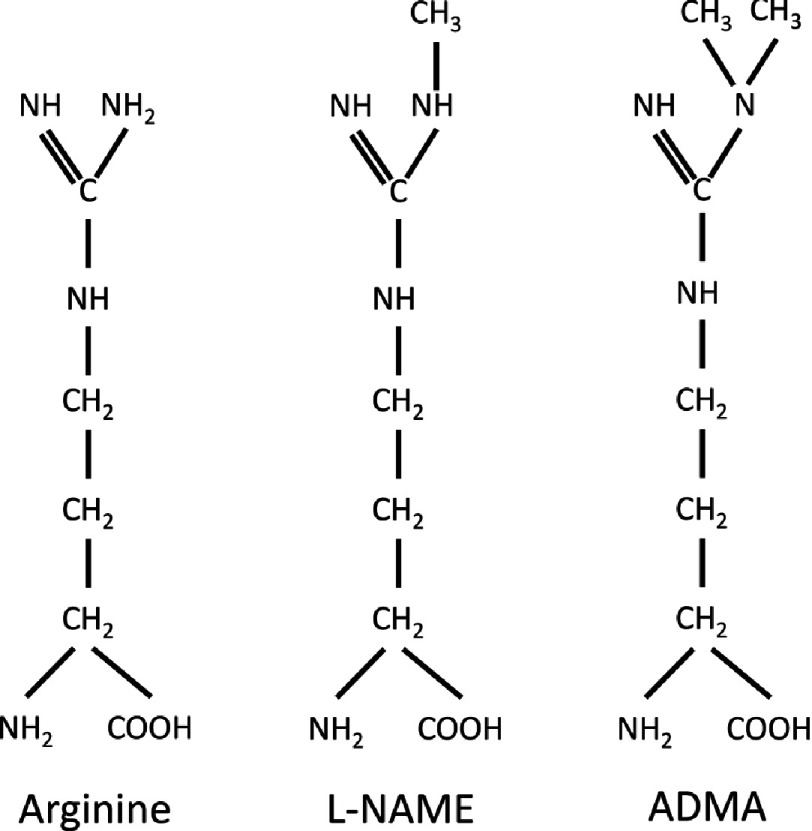
Another potential mechanism for impairment of eNOS is the action of circulating inhibitors of NOS enzymes. The first of these inhibitors to be characterised was asymmetric dimethylarginine (ADMA)56. ADMA is a methyl derivate of arginine, and is produced by the physiological degradation of methylated proteins (Figure 5). Metabolism of ADMA is mediated by the enzyme dimethylarginine dimethylaminohydrolase (DDAH), forming citrulline and dimethylamine62. Loss of DDAH activity leads to accumulation of ADMA and reductions in NO signalling63. Functional polymorphisms in the gene for DDAH2 have associated with increased with increased levels of circulating ADMA, suggesting that there may be an association between genetic variations in the expression of DDAH and cardiovascular risk64,65. ADMA is made in cells by arginine methylation and is released into the cytosol upon protein degradation66. The concentration of ADMA within endothelial cells may be 10–20 fold higher than that seen in the plasma67. Plasma levels of ADMA predict cardiovascular events and mortality and have been associated with a wide range of conditions including hyperlipidemia, hypertension, peripheral arterial disease, chronic renal failure, chronic heart failure, diabetes mellitus type II, preeclampsia and pulmonary hypertension68–71.
Figure 5. Comparisons of the chemical structure of the nitric oxide synthase substrate arginine with the synthetic inhibitor L-NAME and the endogenous inhibitor ADMA of the enzyme.
Nitric oxide as a therapy for PAH
Inhaled NO gas
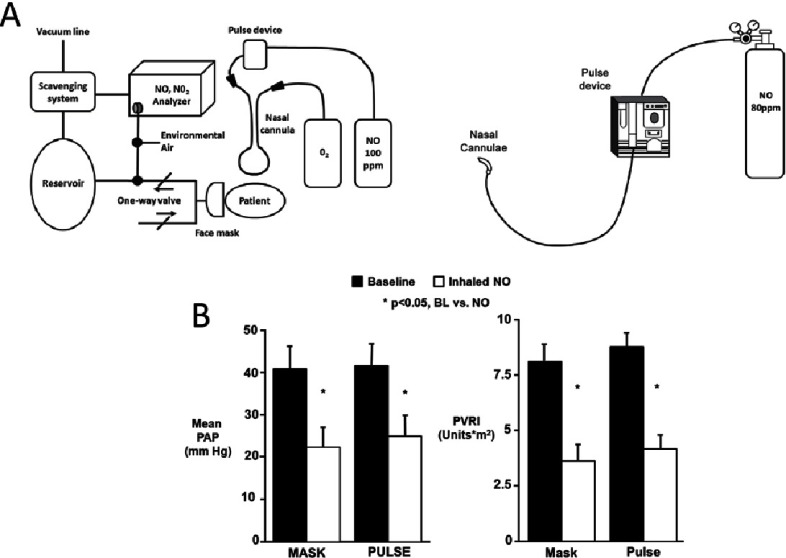
The vasodilator and anti-proliferative actions of NO make it an attractive tool for pharmacological treatment of PAH. Administration of NO gas by inhalation has been shown to be beneficial to patients with PAH, particularly in paediatric cases72–74. However, the usefulness of inhaled NO as a treatment is limited due cost, technical difficulties and the fact that not all patients respond to the therapy. The ability of NO to oxidise haemoglobin to form methaemoglobin, which has been shown to be related the cumulative exposure to the gas, may also limit its effectiveness75. Rapid withdrawal of inhaled NO therapy can also have deleterious effects with levels of oxygenation and pulmonary hypertension returning to levels worse than those seen prior to the commencement of therapy76,77. However, developments in delivery technology have allowed optimisation of inhaled NO therapy by administering pulsed dosing of the gas (Figure 6A)78–80. These studies have shown a reduction in adverse events, no changes in methaemoglobin levels and no reports of syncope79,81. The pulsed delivery of NO was shown to be as effective as continuous therapy in reducing mean pulmonary artery pressure and pulmonary vascular resistance (Figure 6B). The use of inhaled NO therapy has recently been shown to be cost effective for the treatment of infants in a trial of NO therapy for chronic lung disease82. Despite the cost of the therapy, those patients who received inhaled NO had a shorted hospital stay and ventilation period.
Figure 6. (A) New inhaled NO delivery systems that allow pulsed dosing with the gas and (B) data on the reductions achieved with inhaled NO given by either continuous therapy (MASK) or via a pulsed delivery system (PULSE)78.
Phosphodiesterases inhibitors
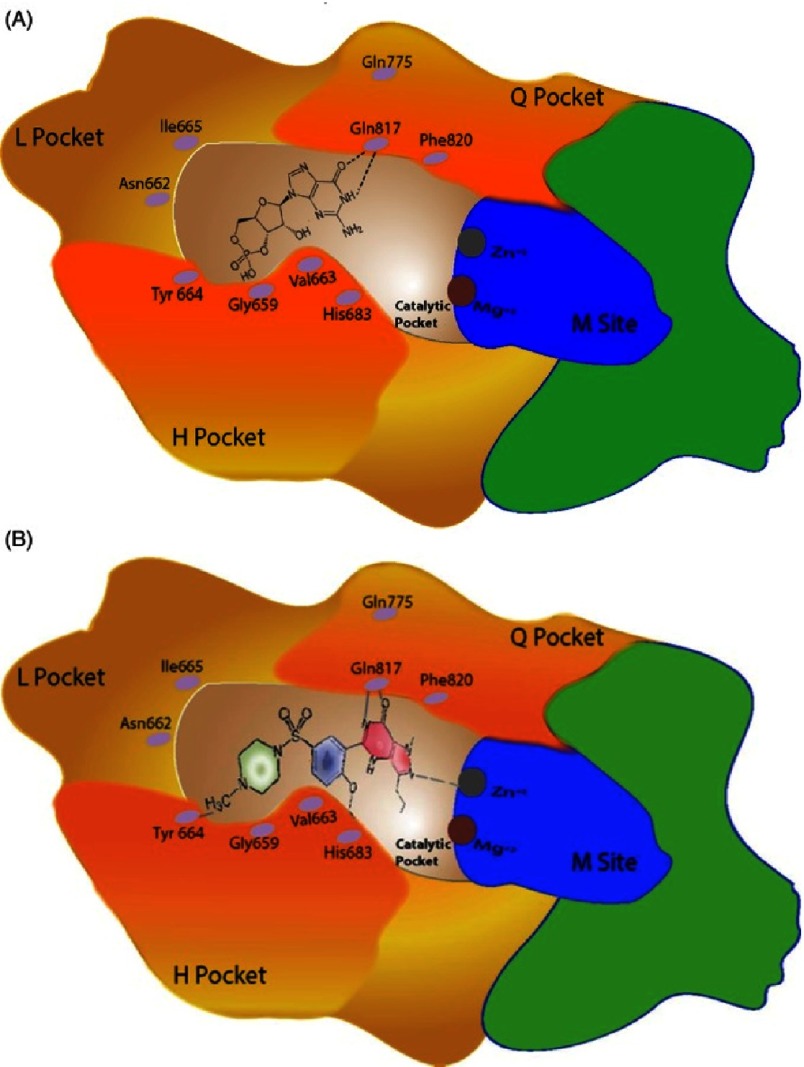
PDEs are responsible for the degradation of cGMP and thus terminating the effects mediated by NO. There currently 12 different isoforms identifed of these enzymes that differ in their 3-dimensional structures and tissue distribution83,84. PDE5 is present in the cardiovascular system; with abundance in the pulmonary vascular smooth muscle and endothelial cells85,86. Inhibition of PDE5 has been shown to mediate anti-proliferative effects on pulmonary artery smooth muscle cells87. The development of PDE5 inhibitors, such as sildenafil, are effective pharmacological agents for the treatment of patients with PAH due to either ability to bind to the PDE5 enzyme, inhibit the breakdown of cGMP and thereby prolong the effect of endogenous NO (Figure 7)88.
Figure 7. Diagram of catalytic Pocket of PDE showing occupation by (A) cGMP and (B) the PDE5 inhibitor sildenafil88.
The SUPER-1 (Sildenafil Use in Pulmonary Arterial Hypertension) trial showed that sildenafil improves the functional capacity and pulmonary haemodynamics in patients with PAH, with only mild adverse effects89. These effects are sustained over a period of 3 years in a follow-up study (SUPER-2)90. In addition to sildenafil, tadalafil and vardenafil, two newer PDE5 inhibitors, are currently being assessed for use in patients with PAH. Tadalafil, which is a once a day oral preparation has been shown to improve exercise capacity, pulmonary haemodynamics and time to clinical worsening, effects that last for up to 1 year in the PHIRST-1 trial (Pulmonary Arterial Hypertension and Response to Tadalafil)91. Similar results have been reported for vardenafil in the EVALUATION (Efficacy and Safety of Vardenafil in the Treatment of Pulmonary Arterial Hypertension) trial, albeit in a much smaller cohort of patients92.
PDE5 is also expressed in the heart and some small studies have suggested that sildenafil is capable of preventing the remodeling of the myocardium seen in heart failure93,94. However, in larger clinical trials this effect of sildenafil was not seen95. It has recently been suggested that this may be due to the expression of PDE9 in myocytes. While PDE5 is found in contractile filaments, where is degrades cGMP produced by the action of NO on the soluble guanylyl cyclase (sGC) receptor, PDE9 is located near the T-tubular’ invaginations of the plasma membrane96. PDE9 in believed to be responsible for the degradation of cGMP produced by atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP) hormones acting on the guanylyl cyclase type A (GC-A) receptor that is located the cardiomyocyte plasma membrane.
cGMP activators
As an alternative to inhibition of the breakdown of cGMP, a new class of drugs are now being developed that directly activates soluble guanylate cyclase, thereby mimicking the effects of NO by increasing levels of cGMP. One such drug is riociguat, which has been assessed for efficacy in the treatment of PAH97. Results of the PATENT-1 (The Pulmonary Arterial Hypertension Soluble Guanylate Cyclase-Stimulator Trial) trial showed an improvement in exercise capacity, pulmonary haemodynamics, N-terminal pro-brain natriuretic peptide levels and time to clinical worsening. While adverse effects were similar to PDE5 inhibitors there was a greater incidence of systemic hypotension and haemoptysis.
Combination therapy
The mainstay of treatment for PAH is still the use of prostacyclin mimetic compounds and more recently endothelin-receptor antagonists and PDE-5 inhibitors. There are potential advantages in using combinations of these drugs to enhance the clinical benefits that can be obtained when the drugs are used in isolation. The use of strategies that combine treatment with PDE-5 inhibitors with PGI2 analogues and/or ET receptor antagonists have recently been comprehensively reviewed43. The results of some of the trials of combination therapy do show some promise, however some results are contradictory. For example the COMPASS-2 (Combination of Bosentan and Sildenafil versus Sildenafil Monotherapy on Pulmonary Arterial Hypertension) trial was able to show an improvement in the 6-minute walk test after 16 weeks of treatment but failed to delay the time to the first morbidity/mortality event98. However, meta-analysis of data from studies that involve combination therapy do indicate that this approach is effective in the treatment of PAH9.
Future therapies
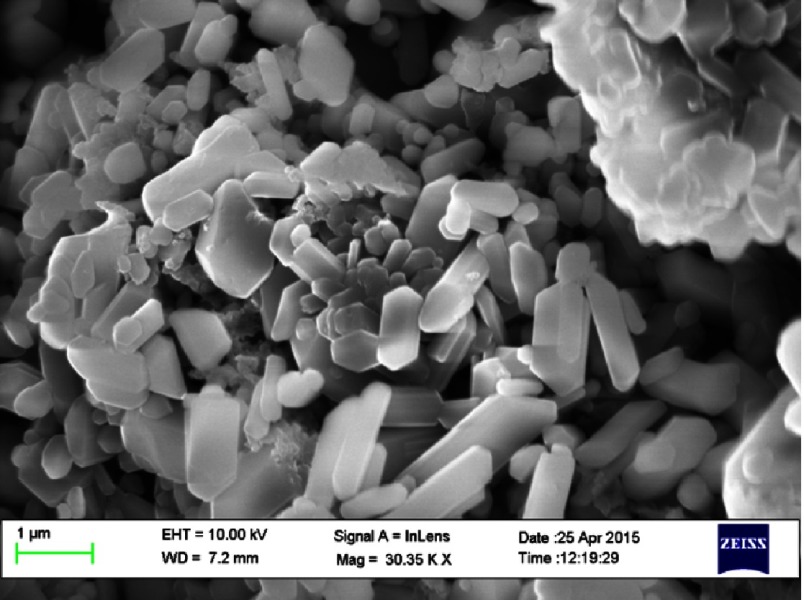
The current pharmacological strategies to augment the supply or duration of the action of NO have a number of limitations mainly relayed to the routes of administration, duration of action and systemic effects. Novel nanoparticles are being developed for the treatment of a number of different conditions (Figure 8)99,100. Of particular interest are fibres that are capable of releasing NO and that are suitable for delivery via inhalation to deliver NO directly to the pulmonary vasculature101,102. These nanofibres have recently been shown to be able to continuously release NO over an 8-hour period and relax pulmonary vessel of rats with pulmonary hypertension in vitro103. These initial observations suggest that nanofibres capable of releasing NO may be of use in the future treatment of patients with PAH.
Figure 8. Scanning electron micrograph on NO-containing nano-particles.
Conclusions
NO plays a significant role in the pulmonary circulation. Changes to its synthesis, release or signalling contribute to the pathogenesis of PAH. While targeting NO in has resulted in advances the treatment of the disease, the quality of life and the prognosis for patients with PAH remains poor. There remains a substantial amount of work to be done in order to optimise strategies that target to NO system or utilise the delivery of NO as a pharmacological agent.
References
- 1.Chakrabarti AM, Mitchell JA, Wort SJ. Progress in the understanding and management of pulmonary arterial hypertension. Global Cardiology Science & Practice. 2015;2015:13. doi: 10.5339/gcsp.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chester AH, Yacoub MH. The role of endothelin-1 in pulmonary arterial hypertension. Global Cardiology Science & Practice. 2014;2014(2):62–78. doi: 10.5339/gcsp.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell JA, Ahmetaj-Shala B, Kirkby NS, Wright WR, Mackenzie LS, Reed DM, et al. Role of prostacyclin in pulmonary hypertension. Global Cardiology Science & Practice. 2014;2014(4):382–93. doi: 10.5339/gcsp.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tonelli AR, Haserodt S, Aytekin M, Dweik RA. Nitric oxide deficiency in pulmonary hypertension: Pathobiology and implications for therapy. Pulm Circ. 2013;3(1):20–30. doi: 10.4103/2045-8932.109911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sitbon O, Morrell N. Pathways in pulmonary arterial hypertension: The future is here. European Respiratory Review: An Official Journal of the European Respiratory Society. 2012;21(126):321–7. doi: 10.1183/09059180.00004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huertas A, Perros F, Tu L, Cohen-Kaminsky S, Montani D, Dorfmuller P, et al. Immune dysregulation and endothelial dysfunction in pulmonary arterial hypertension: A complex interplay. Circulation. 2014;129(12):1332–40. doi: 10.1161/CIRCULATIONAHA.113.004555. [DOI] [PubMed] [Google Scholar]
- 7.Fessel JP, West JD. Redox biology in pulmonary arterial hypertension (2013 Grover Conference Series) Pulm Circ. 2015;5(4):599–609. doi: 10.1086/683814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Austin ED, Loyd JE. The genetics of pulmonary arterial hypertension. Circulation Research. 2014;115(1):189–202. doi: 10.1161/CIRCRESAHA.115.303404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson SR, Brode SK, Mielniczuk LM, Granton JT. Dual therapy in IPAH and SSc-PAH. A qualitative systematic review. Respir Med. 2012;106(5):730–9. doi: 10.1016/j.rmed.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 10.Rich S, Dantzker DR, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Primary pulmonary hypertension. A national prospective study. Annals of Internal Medicine. 1987;107(2):216–23. doi: 10.7326/0003-4819-107-2-216. [DOI] [PubMed] [Google Scholar]
- 11.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Annals of Internal Medicine. 1991;115(5):343–9. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 12.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122(2):156–63. doi: 10.1161/CIRCULATIONAHA.109.911818. [DOI] [PubMed] [Google Scholar]
- 13.Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, et al. Predicting survival in pulmonary arterial hypertension: Insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) Circulation. 2010;122(2):164–72. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 14.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, et al. Pulmonary arterial hypertension in France: Results from a national registry. American Journal of Respiratory and Critical Care Medicine. 2006;173(9):1023–30. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 15.Simonneau G, Galie N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, et al. Clinical classification of pulmonary hypertension. Journal of the American College of Cardiology. 2004;43(12 Suppl S):5S–12S. doi: 10.1016/j.jacc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 16.Tuder RM, Marecki JC, Richter A, Fijalkowska I, Flores S. Pathology of pulmonary hypertension. Clinics in Chest Medicine. 2007;28(1):23–42, vii. doi: 10.1016/j.ccm.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cool CD, Stewart JS, Werahera P, Miller GJ, Williams RL, Voelkel NF, et al. Three-dimensional reconstruction of pulmonary arteries in plexiform pulmonary hypertension using cell-specific markers. Evidence for a dynamic and heterogeneous process of pulmonary endothelial cell growth. The American Journal of Pathology. 1999;155(2):411–9. doi: 10.1016/S0002-9440(10)65137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montani D, Gunther S, Dorfmuller P, Perros F, Girerd B, Garcia G, et al. Pulmonary arterial hypertension. Orphanet Journal of Rare Diseases. 2013;8(1):97. doi: 10.1186/1750-1172-8-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ormiston ML, Upton PD, Li W, Morrell NW. The promise of recombinant BMP ligands and other approaches targeting BMPR-II in the treatment of pulmonary arterial hypertension. Global Cardiology Science & Practice. 2015;2015(4):47. doi: 10.5339/gcsp.2015.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, et al. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91(5):1314–9. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- 21.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288(5789):373–6. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 22.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327(6122):524–6. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 23.Palmer RM, Rees DD, Ashton DS, Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochemical and Biophysical Research Communications. 1988;153(3):1251–6. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- 24.Feelisch M, Noack EA. Correlation between nitric oxide formation during degradation of organic nitrates and activation of guanylate cyclase. European Journal of Pharmacology. 1987;139(1):19–30. doi: 10.1016/0014-2999(87)90493-6. [DOI] [PubMed] [Google Scholar]
- 25.Rees DD, Palmer RM, Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(9):3375–8. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vallance P, Collier J, Moncada S. Nitric oxide synthesised from L-arginine mediates endothelium dependent dilatation in human veins in vivo. Cardiovascular Research. 1989;23(12):1053–7. doi: 10.1093/cvr/23.12.1053. [DOI] [PubMed] [Google Scholar]
- 27.Chester AH, O’Neil GS, Moncada S, Tadjkarimi S, Yacoub MH. Low basal and stimulated release of nitric oxide in atherosclerotic epicardial coronary arteries. Lancet. 1990;336(8720):897–900. doi: 10.1016/0140-6736(90)92269-n. [DOI] [PubMed] [Google Scholar]
- 28.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: Structure, function and inhibition. The Biochemical Journal. 2001;357(Pt 3):593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forstermann U, Sessa WC. Nitric oxide synthases: Regulation and function. Eur Heart J. 2012;33(7):829–37. doi: 10.1093/eurheartj/ehr304. 37a-37d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murad F. The nitric oxide-cyclic GMP signal transduction system for intracellular and intercellular communication. Recent Prog Horm Res. 1994;49:239–48. doi: 10.1016/b978-0-12-571149-4.50016-7. [DOI] [PubMed] [Google Scholar]
- 31.Munzel T, Feil R, Mulsch A, Lohmann SM, Hofmann F, Walter U. Physiology and pathophysiology of vascular signaling controlled by guanosine 3′,5′-cyclic monophosphate-dependent protein kinase [corrected] Circulation. 2003;108(18):2172–83. doi: 10.1161/01.CIR.0000094403.78467.C3. [DOI] [PubMed] [Google Scholar]
- 32.Feil R, Lohmann SM, de Jonge H, Walter U, Hofmann F. Cyclic GMP-dependent protein kinases and the cardiovascular system: Insights from genetically modified mice. Circulation Research. 2003;93(10):907–16. doi: 10.1161/01.RES.0000100390.68771.CC. [DOI] [PubMed] [Google Scholar]
- 33.Stroes E, Kastelein J, Cosentino F, Erkelens W, Wever R, Koomans H, et al. Tetrahydrobiopterin restores endothelial function in hypercholesterolemia. The Journal of Clinical Investigation. 1997;99(1):41–6. doi: 10.1172/JCI119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, et al. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circulation Research. 2001;88(2):E14–22. doi: 10.1161/01.res.88.2.e14. [DOI] [PubMed] [Google Scholar]
- 35.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. The Journal of Clinical Investigation. 2003;111(8):1201–9. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: Molecular regulation to clinical use. Pharmacol Rev. 2006;58(3):488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- 37.Florey The endothelial cell. Br Med J. 1966;2(5512):487–90. doi: 10.1136/bmj.2.5512.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masaki T. The discovery of endothelins. Cardiovascular Research. 1998;39(3):530–3. doi: 10.1016/s0008-6363(98)00153-9. [DOI] [PubMed] [Google Scholar]
- 39.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332(6163):411–5. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 40.Moncada S, Gryglewski R, Bunting S, Vane JR. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976;263(5579):663–5. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- 41.Moncada S, Vane JR. Prostacyclin and the vascular endothelium. Bull Eur Physiopathol Respir. 1981;17(4):687–701. [PubMed] [Google Scholar]
- 42.Ghofrani HA, Humbert M. The role of combination therapy in managing pulmonary arterial hypertension. European Respiratory Review: An Official Journal of the European Respiratory Society. 2014;23(134):469–75. doi: 10.1183/09059180.00007314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Provencher S, Granton JT. Current treatment approaches to pulmonary arterial hypertension. Can J Cardiol. 2015;31(4):460–77. doi: 10.1016/j.cjca.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 44.Radomski MW, Palmer RM, Moncada S. The anti-aggregating properties of vascular endothelium: Interactions between prostacyclin and nitric oxide. British Journal of Pharmacology. 1987;92(3):639–46. doi: 10.1111/j.1476-5381.1987.tb11367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupta V, Ahsan F. Inhalational therapy for pulmonary arterial hypertension: Current status and future prospects. Crit Rev Ther Drug Carrier Syst. 2010;27(4):313–70. doi: 10.1615/critrevtherdrugcarriersyst.v27.i4.20. [DOI] [PubMed] [Google Scholar]
- 46.Cleeter MW, Cooper JM, Darley-Usmar VM, Moncada S, Schapira AH. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Letters. 1994;345(1):50–4. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- 47.Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, et al. Mitochondrial biogenesis in mammals: The role of endogenous nitric oxide. Science. 2003;299(5608):896–9. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- 48.Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360(9328):219–23. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- 49.Konduri GG, Afolayan AJ, Eis A, Pritchard Jr KA, Teng RJ. Interaction of endothelial nitric oxide synthase with mitochondria regulates oxidative stress and function in fetal pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2015;309(9):L1009–17. doi: 10.1152/ajplung.00386.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eroglu E, Gottschalk B, Charoensin S, Blass S, Bischof H, Rost R, et al. Development of novel FP-based probes for live-cell imaging of nitric oxide dynamics. Nat Commun. 2016;7:10623. doi: 10.1038/ncomms10623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. The New England Journal of Medicine. 1995;333(4):214–21. doi: 10.1056/NEJM199507273330403. [DOI] [PubMed] [Google Scholar]
- 52.Xue C, Johns RA. Endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. The New England Journal of Medicine. 1995;333(24):1642–4. doi: 10.1056/NEJM199512143332416. [DOI] [PubMed] [Google Scholar]
- 53.Mason NA, Springall DR, Burke M, Pollock J, Mikhail G, Yacoub MH, et al. High expression of endothelial nitric oxide synthase in plexiform lesions of pulmonary hypertension. The Journal of Pathology. 1998;185(3):313–8. doi: 10.1002/(SICI)1096-9896(199807)185:3<313::AID-PATH93>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 54.Zuckerbraun BS, George P, Gladwin MT. Nitrite in pulmonary arterial hypertension: Therapeutic avenues in the setting of dysregulated arginine/nitric oxide synthase signalling. Cardiovascular Research. 2011;89(3):542–52. doi: 10.1093/cvr/cvq370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malhotra R, Hess D, Lewis GD, Bloch KD, Waxman AB, Semigran MJ. Vasoreactivity to inhaled nitric oxide with oxygen predicts long-term survival in pulmonary arterial hypertension. Pulm Circ. 2011;1(2):250–8. doi: 10.4103/2045-8932.83449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vallance P, Leone A, Calver A, Collier J, Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339(8793):572–5. doi: 10.1016/0140-6736(92)90865-z. [DOI] [PubMed] [Google Scholar]
- 57.Afolayan AJ, Eis A, Alexander M, Michalkiewicz T, Teng RJ, Lakshminrusimha S, et al. Decreased endothelial nitric oxide synthase expression and function contribute to impaired mitochondrial biogenesis and oxidative stress in fetal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2016;310(1):L40–9. doi: 10.1152/ajplung.00392.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ryan J, Dasgupta A, Huston J, Chen KH, Archer SL. Mitochondrial dynamics in pulmonary arterial hypertension. Journal of Molecular Medicine. 2015;93(3):229–42. doi: 10.1007/s00109-015-1263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thebaud B, Bonnet S, et al. An abnormal mitochondrial-hypoxia inducible factor-1alpha-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: Similarities to human pulmonary arterial hypertension. Circulation. 2006;113(22):2630–41. doi: 10.1161/CIRCULATIONAHA.105.609008. [DOI] [PubMed] [Google Scholar]
- 60.Marsboom G, Toth PT, Ryan JJ, Hong Z, Wu X, Fang YH, et al. Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circulation Research. 2012;110(11):1484–97. doi: 10.1161/CIRCRESAHA.111.263848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ryan JJ, Marsboom G, Fang YH, Toth PT, Morrow E, Luo N, et al. PGC1alpha-mediated mitofusin-2 deficiency in female rats and humans with pulmonary arterial hypertension. American Journal of Respiratory and Critical Care Medicine. 2013;187(8):865–78. doi: 10.1164/rccm.201209-1687OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vallance P, Leiper J. Cardiovascular biology of the asymmetric dimethylarginine:dimethylarginine dimethylaminohydrolase pathway. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24(6):1023–30. doi: 10.1161/01.ATV.0000128897.54893.26. [DOI] [PubMed] [Google Scholar]
- 63.Leiper J, Nandi M, Torondel B, Murray-Rust J, Malaki M, O’Hara B, et al. Disruption of methylarginine metabolism impairs vascular homeostasis. Nat Med. 2007;13(2):198–203. doi: 10.1038/nm1543. [DOI] [PubMed] [Google Scholar]
- 64.Weiss SL, Yu M, Jennings L, Haymond S, Zhang G, Wainwright MS. Pilot study of the association of the DDAH2 -449G polymorphism with asymmetric dimethylarginine and hemodynamic shock in pediatric sepsis. PloS One. 2012;7(3):e33355. doi: 10.1371/journal.pone.0033355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Valkonen VP, Tuomainen TP, Laaksonen R. DDAH gene and cardiovascular risk. Vasc Med. 2005;10(Suppl 1):S45–8. doi: 10.1191/1358863x05vm600oa. [DOI] [PubMed] [Google Scholar]
- 66.McBride AE, Silver PA. State of the arg: Protein methylation at arginine comes of age. Cell. 2001;106(1):5–8. doi: 10.1016/s0092-8674(01)00423-8. [DOI] [PubMed] [Google Scholar]
- 67.Teerlink T, Luo Z, Palm F, Wilcox CS. Cellular ADMA: Regulation and action. Pharmacol Res. 2009;60(6):448–60. doi: 10.1016/j.phrs.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kurose I, Wolf R, Grisham MB, Granger DN. Effects of an endogenous inhibitor of nitric oxide synthesis on postcapillary venules. The American Journal of Physiology. 1995;268(6 Pt 2):H2224–31. doi: 10.1152/ajpheart.1995.268.6.H2224. [DOI] [PubMed] [Google Scholar]
- 69.Faraci FM, Brian Jr JE, Heistad DD. Response of cerebral blood vessels to an endogenous inhibitor of nitric oxide synthase. The American Journal of Physiology. 1995;269(5 Pt 2):H1522–7. doi: 10.1152/ajpheart.1995.269.5.H1522. [DOI] [PubMed] [Google Scholar]
- 70.Boger RH. The emerging role of asymmetric dimethylarginine as a novel cardiovascular risk factor. Cardiovascular Research. 2003;59(4):824–33. doi: 10.1016/s0008-6363(03)00500-5. [DOI] [PubMed] [Google Scholar]
- 71.Boger RH, Maas R, Schulze F, Schwedhelm E. Asymmetric dimethylarginine (ADMA) as a prospective marker of cardiovascular disease and mortality—An update on patient populations with a wide range of cardiovascular risk. Pharmacol Res. 2009;60(6):481–7. doi: 10.1016/j.phrs.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 72.Frostell C, Fratacci MD, Wain JC, Jones R, Zapol WM. Inhaled nitric oxide. A selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation. 1991;83(6):2038–47. doi: 10.1161/01.cir.83.6.2038. [DOI] [PubMed] [Google Scholar]
- 73.Pepke-Zaba J, Higenbottam TW, Dinh-Xuan AT, Stone D, Wallwork J. Inhaled nitric oxide as a cause of selective pulmonary vasodilatation in pulmonary hypertension. Lancet. 1991;338(8776):1173–4. doi: 10.1016/0140-6736(91)92033-x. [DOI] [PubMed] [Google Scholar]
- 74.Roberts Jr JD, Fineman JR, Morin 3rd FC, Shaul PW, Rimar S, Schreiber MD, et al. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. The Inhaled Nitric Oxide Study Group. The New England Journal of Medicine. 1997;336(9):605–10. doi: 10.1056/NEJM199702273360902. [DOI] [PubMed] [Google Scholar]
- 75.Salguero KL, Cummings JJ. Inhaled nitric oxide and methemoglobin in full-term infants with persistent pulmonary hypertension of the newborn. Pulm Pharmacol Ther. 2002;15(1):1–5. doi: 10.1006/pupt.2001.0311. [DOI] [PubMed] [Google Scholar]
- 76.Lavoie A, Hall JB, Olson DM, Wylam ME. Life-threatening effects of discontinuing inhaled nitric oxide in severe respiratory failure. American Journal of Respiratory and Critical Care Medicine. 1996;153(6 Pt 1):1985–7. doi: 10.1164/ajrccm.153.6.8665066. [DOI] [PubMed] [Google Scholar]
- 77.Cueto E, Lopez-Herce J, Sanchez A, Carrillo A. Life-threatening effects of discontinuing inhaled nitric oxide in children. Acta Paediatr. 1997;86(12):1337–9. doi: 10.1111/j.1651-2227.1997.tb14909.x. [DOI] [PubMed] [Google Scholar]
- 78.Barst RJ, Channick R, Ivy D, Goldstein B. Clinical perspectives with long-term pulsed inhaled nitric oxide for the treatment of pulmonary arterial hypertension. Pulm Circ. 2012;2(2):139–47. doi: 10.4103/2045-8932.97589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Channick RN, Newhart JW, Johnson FW, Williams PJ, Auger WR, Fedullo PF, et al. Pulsed delivery of inhaled nitric oxide to patients with primary pulmonary hypertension: An ambulatory delivery system and initial clinical tests. Chest. 1996;109(6):1545–9. doi: 10.1378/chest.109.6.1545. [DOI] [PubMed] [Google Scholar]
- 80.Kitamukai O, Sakuma M, Takahashi T, Nawata J, Ikeda J, Shirato K. Hemodynamic effects of inhaled nitric oxide using pulse delivery and continuous delivery systems in pulmonary hypertension. Intern Med. 2002;41(6):429–34. doi: 10.2169/internalmedicine.41.429. [DOI] [PubMed] [Google Scholar]
- 81.Ivy DD, Parker D, Doran A, Parker D, Kinsella JP, Abman SH. Acute hemodynamic effects and home therapy using a novel pulsed nasal nitric oxide delivery system in children and young adults with pulmonary hypertension. The American Journal of Cardiology. 2003;92(7):886–90. doi: 10.1016/s0002-9149(03)00910-x. [DOI] [PubMed] [Google Scholar]
- 82.Zupancic JA, Hibbs AM, Palermo L, Truog WE, Cnaan A, Black DM, et al. Economic evaluation of inhaled nitric oxide in preterm infants undergoing mechanical ventilation. Pediatrics. 2009;124(5):1325–32. doi: 10.1542/peds.2008-3214. [DOI] [PubMed] [Google Scholar]
- 83.Jeon YH, Heo YS, Kim CM, Hyun YL, Lee TG, Ro S, et al. Phosphodiesterase: Overview of protein structures, potential therapeutic applications and recent progress in drug development. Cellular and Molecular Life Sciences: CMLS. 2005;62(11):1198–220. doi: 10.1007/s00018-005-4533-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wallis RM, Corbin JD, Francis SH, Ellis P. Tissue distribution of phosphodiesterase families and the effects of sildenafil on tissue cyclic nucleotides, platelet function, and the contractile responses of trabeculae carneae and aortic rings in vitro. The American Journal of Cardiology. 1999;83(5A):3C–12C. doi: 10.1016/s0002-9149(99)00042-9. [DOI] [PubMed] [Google Scholar]
- 85.Lincoln TM, Hall CL, Park CR, Corbin JD. Guanosine 3′:5′-cyclic monophosphate binding proteins in rat tissues. Proceedings of the National Academy of Sciences of the United States of America. 1976;73(8):2559–63. doi: 10.1073/pnas.73.8.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Corbin JD, Beasley A, Blount MA, Francis SH. High lung PDE5: A strong basis for treating pulmonary hypertension with PDE5 inhibitors. Biochemical and Biophysical Research Communications. 2005;334(3):930–8. doi: 10.1016/j.bbrc.2005.06.183. [DOI] [PubMed] [Google Scholar]
- 87.Wharton J, Strange JW, Moller GM, Growcott EJ, Ren X, Franklyn AP, et al. Antiproliferative effects of phosphodiesterase type 5 inhibition in human pulmonary artery cells. American Journal of Respiratory and Critical Care Medicine. 2005;172(1):105–13. doi: 10.1164/rccm.200411-1587OC. [DOI] [PubMed] [Google Scholar]
- 88.Butrous G. The role of phosphodiesterase inhibitors in the management of pulmonary vascular diseases. Global Cardiology Science & Practice. 2014;2014(3):257–90. doi: 10.5339/gcsp.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. The New England Journal of Medicine. 2005;353(20):2148–57. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 90.Rubin LJ, Badesch DB, Fleming TR, Galie N, Simonneau G, Ghofrani HA, et al. Long-term treatment with sildenafil citrate in pulmonary arterial hypertension: The SUPER-2 study. Chest. 2011;140(5):1274–83. doi: 10.1378/chest.10-0969. [DOI] [PubMed] [Google Scholar]
- 91.Galie N, Brundage BH, Ghofrani HA, Oudiz RJ, Simonneau G, Safdar Z, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119(22):2894–903. doi: 10.1161/CIRCULATIONAHA.108.839274. [DOI] [PubMed] [Google Scholar]
- 92.Jing ZC, Yu ZX, Shen JY, Wu BX, Xu KF, Zhu XY, et al. Vardenafil in pulmonary arterial hypertension: A randomized, double-blind, placebo-controlled study. American Journal of Respiratory and Critical Care Medicine. 2011;183(12):1723–9. doi: 10.1164/rccm.201101-0093OC. [DOI] [PubMed] [Google Scholar]
- 93.Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, et al. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11(2):214–22. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 94.Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: A target of phosphodiesterase-5 inhibition in a 1-year study. Circulation. 2011;124(2):164–74. doi: 10.1161/CIRCULATIONAHA.110.983866. [DOI] [PubMed] [Google Scholar]
- 95.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: A randomized clinical trial. JAMA. 2013;309(12):1268–77. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee DI, Zhu G, Sasaki T, Cho GS, Hamdani N, Holewinski R, et al. Phosphodiesterase 9A controls nitric-oxide-independent cGMP and hypertrophic heart disease. Nature. 2015;519(7544):472–6. doi: 10.1038/nature14332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ghofrani HA, Galie N, Grimminger F, Grunig E, Humbert M, Jing ZC, et al. Riociguat for the treatment of pulmonary arterial hypertension. The New England Journal of Medicine. 2013;369(4):330–40. doi: 10.1056/NEJMoa1209655. [DOI] [PubMed] [Google Scholar]
- 98.McLaughlin V, Channick RN, Ghofrani HA, Lemarie JC, Naeije R, Packer M, et al. Bosentan added to sildenafil therapy in patients with pulmonary arterial hypertension. The European Respiratory Journal. 2015;46(2):405–13. doi: 10.1183/13993003.02044-2014. [DOI] [PubMed] [Google Scholar]
- 99.Milla P, Dosio F, Cattel L. PEGylation of proteins and liposomes: A powerful and flexible strategy to improve the drug delivery. Curr Drug Metab. 2012;13(1):105–19. doi: 10.2174/138920012798356934. [DOI] [PubMed] [Google Scholar]
- 100.Caparrotta TM, Evans M. PEGylated insulin Lispro, (LY2605541)—A new basal insulin analogue. Diabetes Obes Metab. 2014;16(5):388–95. doi: 10.1111/dom.12196. [DOI] [PubMed] [Google Scholar]
- 101.Mansour HM, Rhee YS, Wu X. Nanomedicine in pulmonary delivery. Int J Nanomedicine. 2009;4:299–319. doi: 10.2147/ijn.s4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.El-Sherbiny IM, El-Baz NM, Yacoub MH. Inhaled nano- and microparticles for drug delivery. Global Cardiology Science & Practice. 2015;2015:2. doi: 10.5339/gcsp.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mohamed NA, Ahmetaj-Shala B, Duluc L, Mackenzie LS, Kirkby NS, Reed DM, et al. A new NO-releasing nanoformulation for the treatment of pulmonary arterial hypertension. Journal of Cardiovascular Translational Research. 2016 doi: 10.1007/s12265-016-9684-2. [DOI] [PMC free article] [PubMed] [Google Scholar]