Abstract
This prospective study aimed to address changes in inflammatory response between different aged populations of patients who sustained burn and inhalation injury. Plasma and bronchoalveolar lavage (BAL) samples were collected from 104 patients within 15 hours of their estimated time of burn injury. Clinical variables, laboratory parameters, and immune mediator profiles were examined in association with clinical outcomes. Older patients were at higher odds for death after burn injury (odds ratio (OR) = 7.37 per 10 years, p=0.004). In plasma collected within 15 hours after burn injury, significant increases in the concentrations of interleukin 1 receptor antagonist (IL-1RA), interleukin 2 (IL-2), interleukin 4 (IL-4), interleukin 6 (IL-6), granulocyte colony-stimulating factor (G-CSF), interferon-gamma-induced protein 10 (IP-10) and monocyte chemoattractant protein 1 (MCP-1) (p<0.05 for all) were observed in the ≥65 group. In the BAL fluid, MCP-1 was increased three-fold in the ≥65 group. This study suggests that changes in certain immune mediators were present in the older cohort, in association with in-hospital mortality.
Keywords: Burn, Inhalation Injury, Aging, Inflammation, Immune Mediators
1. Introduction
According to the National Burn Repository, there are over 40,000 burn admissions every year. In an epidemiological study using this repository’s data, it was determined that 14% of these patients are over the age of 65 years (1). Burn injury in the elderly carries increased risk of death and morbidity (2–10). The relationship between advanced age and poorer outcomes in burn injury is characterized in the Baux Score, used commonly to predict mortality risk among burn patients. The Baux score may be tabulated by adding the age of the patient and the percentage of total body surface area (%TBSA) affected by the burn (11), indicating the relatively linear relationship between aging and worsened survival in this setting. The reason why prognosis is so poor in elderly burn patients has proven to be elusive, despite associated morbidity and mortality. Of the organ systems at risk for failure after burn and inhalation injury, the lungs are particularly vulnerable and are usually one of the first organs to fail, a phenomenon increased by inhalation injury (12). The presence of inhalational injury adds an additional 17 points to the Baux equation, and is known as the Revised Baux Score, illustrating the relative importance of not just aging but inhalational injury as contributors to mortality in the setting of burn injury.
Previously, we have evaluated the inflammatory response to burn and inhalation injury, both systemically and in lung, via measurements in plasma and bronchoalveolar lavage (BAL) fluid (2, 13, 14). We observed significant differences in concentrations of both pro- and anti-inflammatory immune mediators that varied with severity of inhalation injury and mortality. To the author’s knowledge, there is no report in the literature that specifically addresses differences in the immune response between various age groups in burn patients.
Advanced chronological age is associated with increased mortality, but reasons for these observations are still incompletely understood (2, 3, 5–7). We hypothesized older patients with burn and inhalation injury would exhibit alterations in their systemic and pulmonary inflammatory responses in comparison to younger patients, in association with worse clinical outcomes and increased mortality.
2. Materials and Methods
2.1 Patient selection and sample collection
From January 2007 to April 2015, blood samples were collected from 104 patients admitted to the burn intensive care unit at Loyola University Medical Center within 15 hours of burn injury.
Patients were excluded from the study for the following reasons: age less than 18 years, malignancy, immunosuppressive medication, or known autoimmune disease. Diagnostic Bronchoscopy and BAL were performed by a standardized protocol upon admission to the burn intensive care unit in patients where inhalation injury was suspected by history and/or physical findings (15). BAL fluid that was not required for routine clinical analysis was obtained for use in research. All BAL samples were collected before aerosolized pulmonary medications (e.g. heparin) were administered. The bronchoscope was directed into a subsegment of the right middle lobe and wedged; the first 50 mL aliquot of saline was instilled, and the aspirate discarded. Subsequent 50 mL aliquots were instilled into the same subsegment, and immediately aspirated with gentle hand aspiration into sterile syringes. These were immediately transferred into sterile 50 mL conical tubes, placed on ice, and transported to the laboratory for additional processing. An average of 8.3 mL (standard deviation of 5.4 mL) of BAL fluid was collected for research. Blood samples were collected at the same time as bronchoscopy. Samples were collected an average of 7.6 hours (standard deviation of 3.6 hours) after injury. This study and associated consent documents were approved by Institutional Review Board.
2.2 Variables
Clinical variables were collected including age, sex, race/ethnicity, % total body surface area (%TBSA) burn, grade of inhalation injury, mechanism of injury, Baux score (Age+%TBSA), Revised Baux Score (Age+%TBSA+17), admission sepsis-related organ failure assessment (SOFA) score, lowest partial pressure of oxygen in arterial blood to fraction of inspired oxygen (P:F) ration in the first 48 hours, admission % carboxyhemoglobin in the blood, initial 24 and 72 hour fluid requirements (11, 16). The degree of inhalation injury was determined using a standardized bronchoscopic scoring system based on Abbreviated Injury Score criteria (grade 0–4, no visible injury, mild, moderate, severe and massive injury) (3, 17). Transfusion of blood products was not included in the analysis because a median of 0 units (IQR 0–5) of any blood product was transfused in our cohort. Outcomes including development of the acute respiratory distress syndrome (ARDS, defined by Berlin criteria (18)), sepsis (defined as an increase in SOFA score of 2 points or more representing life-threatening organ dysfunction (19)) as well as 28-day ventilator-free days, 28-day ICU-free days and in-hospital mortality were also collected (11, 16).
2.3 Sample processing and cytokine studies
Wash buffer of phosphate buffered saline (PBS) with a carrier protein of 5% fetal bovine serum and 1% Pen-Strep-Glutamine was added to the BAL sample at a volume of 50% of the recovered sample as previously described (14). This was gently mixed then strained through sterile 100 micron nylon cell strainers (BD Biosciences, Bedford, MA) to remove soot and mucus. To separate the cellular component from the fluid, the strained BAL sample was centrifuged at 1200 RPM for 5 minutes. The supernatant was then aliquoted and frozen at -80 degrees Celsius. Blood samples were collected in sodium citrate vacutainers then centrifuged at 600xG for 10 minutes to separate the plasma for aliquoting and storage at -80 degrees Celsius.
Cytokine concentrations in BAL fluid and plasma were measured by Bio-Rad Multiplex Assays (Hercules, CA) according to manufacturer protocol (20). All samples were assayed in duplicate and the results analyzed using the Bio-Plex manager software, version 6.1. Total protein content in BAL fluid was measured as previously published by Lowry et al. (21). The total protein in BAL was utilized to standardize cytokine values measured given the variability in yield of the BAL procedure.
2.4 Statistical Analysis
Patient demographics, outcomes, and analyte concentrations were assessed for normality and parametric or nonparametric tests applied where appropriate. Specifically, parametric data were analyzed by the Student’s t-test or one-way analysis of variance (with Bonferroni’s post-test) and non-parametric data were analyzed by the Mann Whitney test or Kruskal-Wallis test (with Dunn’s post-test). Normally distributed continuous variables of parametric tests are reported as mean with standard deviation, and non-parametric data are reported as median with 25th and 75th percentiles. Otherwise dichotomous variables were compared with Chi-squared and Fisher’s exact tests, and are reported as a number and percent. Logistic regression was performed, where indicated, to adjust for the effects of relevant confounders.
Outliers were defined as values three standard deviations from the mean. Samples from patients who demonstrated three or more analytes as outliers were examined in sensitivity analyses and are shown in Supplemental Tables 1 and 2. Age groups were defined <50 years, 50–64 years, and ≥65 years. The ≥65 years cohort was chosen due to its relationship with Medicare eligibility as well as the World Health Organization (WHO) and National Institutes of Health (NIH) definition of “elderly”. The two younger cohorts were chosen in order to make the groups more similar in size. When comparing levels of immune mediators in plasma and BAL between the three different age groups, multivariable linear regression adjusting for age group, sex, race, %TBSA and inhalation injury grade was used. Adjusted p-values are for the age group ≥65 years compared to the referent group <50 years.
The performance of each trauma index in predicting ARDS development was evaluated in logistic regression using the area under the receiver operating characteristic curve (AUC ROC). The nonparametric approach of DeLong et al. was used to compare the ROC curves against the reference trauma index with the best area under the ROC (22). Statistical analyses were calculated with SAS Version 9.1 (SAS Institute Inc., Cary, NC) and corresponding graphs created with GraphPad Prism 5 for Windows (GraphPad Software, La Jolla, CA). A difference between observed variables was considered significant for p<0.05.
3. Results
3.1 Clinical characteristics and comparisons
There were 104 samples available for analysis and 64.4% were male and 61.5% were white. As expected, flame burn was the predominant mechanism of injury, comprising 94.2% (n=98) of the cohort. After fiberoptic bronchoscopy, it was determined that 87 patients (83.7%) had visible inhalation injury (grade 1–4). The median %TBSA was 12.8% (interquartile range 1.0–30.0%). Approximately half of the cohort was under 50 years old, 25% were aged 50–64 years and 22% were 65 years or older. The patient demographic and clinical characteristics by age group are listed in Table 1. The only clinical parameter that was different between age groups was the Baux Score. There were no differences in initial oxygen requirement or fluid needs by age group.
Table 1.
Patient Demographics and Characteristics
| Characteristic | Total (n=104) |
<50 years (n=55) |
50–64 years (n=26) |
>65 years (n=23) |
p-value |
|---|---|---|---|---|---|
| Sex (male), n (%) | 67 (64.4) | 37 (67.3) | 17 (65.4) | 13 (56.5) | 0.66 |
| Race (white), n (%) | 64 (61.5) | 29 (52.7) | 18 (69.2) | 17 (73.9) | 0.14 |
| #Admission SOFA Score, median (IQR) | 5 (4–8) | 4 (4–7) | 6 (4–8) | 7 (4–9) | 0.19 |
| Mechanism (flame), n (%) | 98 (94.2) | 52 (94.6) | 24 (92.3) | 22 (95.7) | 0.87 |
| Inhalation Injury (Yes), n (%) | 87 (83.7) | 44 (80.0) | 23 (88.5) | 20 (87.0) | 0.56 |
| Inhalation Injury Grade, median (IQR) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0.55 |
| TBSA, % median (IQR) | 12.8 (1.0–30.0) |
16.0 (1.0–31.0) |
10.3 (1.6–40.0) |
7.0 (0–15.0) |
0.21 |
| *Revised Baux Score, median (IQR) | 93.9 (75.1–121.2) |
91.7 (76.0–118.2) |
104.3 (72.5–136.5) |
93.7 (69.4–115.4) |
0.40 |
| Baux Score | 83.8 (66.0–104.5) |
67.0 (51.0–93.0) |
84.8 (78.0–114.0) |
99.5 (89.0–106.0) |
<0.001 |
| Lowest PaO2:FiO2 ratio (initial 48 hours), median | 182 (131.8–253.8) |
196.0 (136.0–277.5) |
143.0 (105.0–200.0) |
182.7 (157.5–217.5) |
0.12 |
| % COHB, median (IQR) | 5.7 (2.5–13.4) |
5.0 (1.6–10.9) |
5.0 (2.6–13.7) |
9.0 (3.5–40.0) |
0.10 |
| 24 hr fluid resuscitation (cc/kg), median (IQR) | 79.5 (38.8–156.1) |
81.8 (42.2–146.6) |
68.4 (34.9–208.3) |
99.7 (40.1–146.9) |
0.92 |
| 72 hr fluid resuscitation (cc/kg), median (IQR) | 194.7 (112.7–323.0) |
196.1 (99.7–310.8) |
186.9 (142.9–365.3) |
194.7 (91.5–264.3) |
0.42 |
| Blood products transfused (units)┴, median (IQR) | 0 (0–5) |
0 (0–6) |
0 (0–3) |
0 (0–4) |
0.44 |
Revised Baux Score = Age + TBSA + 17 * (inhalation injury, 1=yes, 0= no).
n=84 for this characteristic. TBSA, total body surface area; COHB, carboxyhemoglobin; IQR, interquartile range; SOFA, sequential organ failure assessment; PaO2:FiO2, ratio of arterial oxygen partial pressure to fractional inspired oxygen; Inhalational injury score is based on bronchoscopy visualization and ranges from 0–4 (no injury, mild, moderate, severe, and massive injury).
Blood products = packed red blood cells, fresh frozen plasma, and platelets
3.2 Outcomes
The case-rate of sepsis and ARDS was not different between age groups; however, approximately 72% of both the <50 and 50–64 year age groups developed ARDS, compared to less than half of the ≥65 year age group. Utilization of resources was similar between age groups with similar 28-day ventilator-free days and 28-day ICU-free days (Table 2).
Table 2.
Outcomes
| Characteristic | Total (n=104) |
<50 years (n=55) |
50–64 years (n=26) |
>65 years (n=23) |
p-value |
|---|---|---|---|---|---|
| Sepsis | 20 (19.4) |
12 (21.8) |
2 (7.7) |
6 (27.3) |
0.18 |
| 28-day Ventilator-Free Days | 3.5 (0.0–23.5) |
6.0 (0.024.0) | 6.5 (0.0–25.0 |
0.0 (0.0–19.0) |
0.36 |
| 28-day ICU-Free Days | 0 (0–15) | 0.0 (0.0–16.0) |
1.0 (0.0–19.0) |
0.0 (0.0–9.0) |
0.47 |
| Acute Respiratory Distress Syndrome | 69 (67.0) | 40 (72.7) | 18 (72.0) | 11 (47.8) | 0.08 |
| In-Hospital Death | 22 (21.4) | 6 (10.9) | 6 (23.1) | 10 (45.5) | 0.004 |
Revised Baux Score = Age + TBSA + 17 * (inhalation injury, 1=yes, 0= no).
TBSA, total body surface area; IQR, interquartile range. Inhalational injury score is based on bronchoscopy visualization and ranges from 0–4 (no injury, mild, moderate, severe, and massive injury).
In-hospital death was associated with age ≥65 (p = 0.004). In the fully adjusted model with race, sex, %TBSA and inhalation injury grade, each 10 year incremental increase in age was associated with an odds ratio (OR) of 7.37 (95% CI 2.49–21.87) for in-hospital death.
3.3 Inflammatory mediators in BAL
Results for immune mediator concentrations in the BAL fluid are reported in Tables 3 and 4. Of the 27 mediators measured, 17 were detectable in the BAL fluid. MCP-1 was the only mediator found to be different in the ≥65 group. We found a 3- and 9-fold elevation in MCP-1 levels in the ≥65 age group in comparison to the <50 age group and the 50–64 age group, respectively (p<0.05). The levels of this chemokine showed age-dependent difference regardless of whether values were assessed relative to fluid concentration (Table 3) or when normalized to total protein concentration (Table 4).
Table 3.
Immune BAL Mediator Levels between Age Groups (pg/ml fluid)
| Immune Mediator |
Total (n=91) |
<50 years (n=50) |
50–64 years (n=21) |
>65 years (n=20) |
p-value | p-value* |
|---|---|---|---|---|---|---|
| IL-1β | 20.3 (2.8–105.4) |
13.7 (2.2–88.6) |
53.0 (7.4–133.7) |
15.4 (2.5–71.3) |
0.40 | 0.72 |
| IL-1RA | 219.8 (108.1–476.3) |
215.2 (91.2–729.7) |
247.1 (107.0–479.9) |
219.6 (153.5–358.5) |
0.98 | 0.66 |
| IL-2 | 0.7 (0.0–3.0) |
0.6 (0.0–3.5) |
1.1 (0.1–2.3) |
0.7 (0.0–3.8) |
0.75 | 0.98 |
| IL-4 | 1.1 (0.04–2.9) |
1.2 (0.1–2.9) |
1.1 (0.0–3.2) |
1.1 (0.0–2.9) |
0.95 | 0.42 |
| IL-6 | 175.5 (52.2–517.5) |
243.9 (50.9–541.5) |
136.4 (40.4–386.4) |
178.8 (66.5–318.1) |
0.64 | 0.32 |
| IL-8 | 1943.5 (527.1–5816.8) |
1776.9 (263.8–4969.2) |
2393.3 (391.6–8067.3) |
2347.7 (984.6–4068.7) |
0.60 | 0.28 |
| IL-10 | 7.3 (3.0–13.7) |
7.4 (3.0–20.4) |
8.1 (3.1–11.7) |
5.6 (2.7–8.9) |
0.45 | 0.09 |
| IL-12 | 11.2 (0.0–27.8) |
15.6 (0.5–34.0) |
6.9 (0.0–21.2) |
9.9 (0.0–21.6) |
0.34 | 0.28 |
| IL-13 | 3.1 (1.4–5.3) |
3.1 (1.1–5.3) |
3.1 (1.9–5.4) |
3.2 (1.2–4.3) |
0.83 | 0.73 |
| IL-17 | 0.0 (0.0–10.0) |
0.0 (0.0–11.8) |
0.0 (0.0–8.3) |
0.0 (0.0–0.6) |
0.61 | 0.20 |
| Eotaxin | 42.1 (0.8–78.8) |
45.5 (0.0–78.3) |
27.8 (7.4–69.3) |
42.3 (0.0–105.9) |
0.96 | 0.44 |
| G-CSF | 127.7 (56.3–390.7) |
169.5 (66.0–415.2) |
163.3 (62.7–323.2) |
85.1 (46.2–337.1) |
0.52 | 0.81 |
| GM-CSF | 7.9 (0.0–42.3) |
14.9 (0.1–51.1) |
4.2 (0.0–30.5) |
7.6 (0.0–68.3) |
0.58 | 0.72 |
| IFN-γ | 35.9 (8.0–92.7) |
27.6 (7.3–110.7) |
36.6 (0.7–82.0) |
41.5 (12.1–89.6) |
0.80 | 0.65 |
| IP-10 | 1072.8 (405.3–2101.5) |
1117.8 (436.8–2317.5) |
760.6 (388.7–1773.1) |
1019.7 (433.2–1829.3) |
0.69 | 0.97 |
| MCP-1 | 123.4 (47.0–374.8) |
92.8 (34.8–266.7) |
81.5 (47.5–233.2) |
351.2 (123.4–1417.1) |
0.003 | 0.05 |
| TNF-α | 17.4 (0.2–65.8) |
23.6 (3.7–62.8) |
22.6 (0.0–88.0) |
11.7 (0.0–65.8) |
0.75 | 0.69 |
Data represented as median with 25%–75% interquartile range; all values are pg/ml; Univariable comparison of continuous variables by Kruskal-Wallis test.
Multivariable linear regression adjusting for age group, sex, race, % TBSA, and inhalation injury grade. Adjusted p-value is for the age group over 65 years compared to referent group <50 years. IL, interleukin; IL-1RA, interleukin-1 receptor antagonist; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN-γ, interferon-gamma; IP-10, interferon-gamma-inducible protein 10; MCP-1, monocyte chemoattractant protein 1; TNF-α, tumor necrosis factor alpha
Table 4.
Immune BAL Mediator Levels between Age Groups: Normalized to Protein Levels. (pg/mg protein)
| Immune Mediator | Total (n=91) |
<50 years (n=50) |
50–64 years (n=21) |
>65 years (n=20) |
p-value | p-value* |
|---|---|---|---|---|---|---|
| IL-1β | 21.4 (4.1–86.3) |
13.8 (3.3–77.0) |
51.6 (16.3–97.1) |
19.6 (4.1–75.9) |
0.26 | 0.10 |
| IL-1RA | 194.1 (104.7–534.5) |
169.0 (90.6–459.9) |
284.6 (112.1–680.2) |
188.1 (119.4–329.6) |
0.67 | 0.93 |
| IL-2 | 0.8 (0.0–2.4) |
0.8 (0.0–2.3) |
0.8 (0.1–1.4) |
0.9 (0.0–3.1) |
0.71 | 0.33 |
| IL-4 | 0.9 (0.04–2.2) |
1.0 (0.08–2.2) |
0.6 (0.0–2.1) |
0.9 (0.0–2.2) |
0.66 | 0.71 |
| IL-6 | 166.8 (62.7–427.5) |
181.3 (60.9–446.0) |
155.6 (45.0–402.7) |
147.9 (72.5–436.8) |
0.91 | 0.32 |
| IL-8 | 1735.6 (516.8–4501.0) |
1227.7 (436.6–4704.3) |
2394.6 (412.7–5362.1) |
2036.5 (1080.6–2751.9) |
0.48 | 0.23 |
| IL-10 | 6.1 (3.3–13.4) |
6.0 (3.4–16.6) |
7.4 (4.0–12.3) |
4.2 (2.7–8.5) |
0.29 | 0.08 |
| IL-12 | 12.3 (0.1–29.4) |
17.1 (4.0–33.5) |
6.2 (0.0–21.2) |
9.1 (0.0–16.5) |
0.12 | 0.11 |
| IL-13 | 2.6 (1.5–5.3) |
2.3 (1.5–4.4) |
3.3 (1.7–5.6) |
2.5 (1.2–4.7) |
0.31 | 0.53 |
| IL-17 | 0.0 (0.0–7.6) |
0.0 (0.0–8.0) |
0.0 (0.0–11.0) |
0.0 (0.0–0.4) |
0.48 | 0.32 |
| Eotaxin | 31.5 (0.0–70.2) |
35.6 (0.0–65.5) |
32.6 (9.6–101.5) |
26.1 (0.0–64.2) |
0.71 | 0.57 |
| G-CSF | 112.5 (47.6–281.0) |
102.8 (48.8–352.0) |
138.0 (49.5–273.0) |
121.9 (41.0–236.2) |
0.76 | 0.32 |
| GM-CSF | 7.3 (0.0–47.7) |
16.5 (0.3–50.1) |
4.4 (0.0–22.0) |
7.3 (0.0–51.2) |
0.42 | 0.21 |
| IFN-γ | 28.1 (8.4–75.5) |
23.2 (8.6–71.8) |
27.2 (0.0–69.4) |
39.3 (11.4–105.4) |
0.49 | 0.30 |
| IP-10 | 932.1 (313.6–1832.4) |
968.1 (361.1–2270.2) |
987.7 (250.2–1659.7) |
584.4 (308.0–1105.3) |
0.87 | 0.92 |
| MCP-1 | 125.5 (38.1–403.5) |
83.6 (28.8–257.9) |
33.9 (416.4) |
308.8 (131.9–983.4) |
0.003 | 0.06 |
| TNF-α | 163.6 (0.14–47.7) |
17.5 (3.1–49.3) |
15.4 (0.0–79.2) |
15.3 (0.0–29.5) |
0.54 | 0.37 |
Data represented as median with 25%–75% interquartile range; all values are pg/mg; Univariable comparison of continuous variables by Kruskal-Wallis test.
Multivariable linear regression adjusting for age group, sex, race, % TBSA, and inhalation injury grade. Adjusted p-value is for the age group over 65 years compared to referent group <50 years. IL, interleukin; IL-1RA, interleukin-1 receptor antagonist; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN-γ, interferon-gamma; IP-10, interferon-gamma-inducible protein 10; MCP-1, monocyte chemoattractant protein 1; TNF-α, tumor necrosis factor alpha
Multivariable analyses for an association between the immune mediators in the BAL samples with in-hospital mortality are shown in Tables 5 and 6. No associations were found between plasma or BAL cytokine levels and in-hospital death (Table 5), however, when evaluating BAL fluid cytokines normalized to total protein concentration (Table 6) there was an association found between BAL fluid MCP-1 levels and in-hospital death (p=0.03). Each incremental increase of 100 pg/mg protein of admission MCP-1 in BAL fluid was associated with an OR of 1.16 (95% CI 1.01–1.32) for in-hospital death. No interaction/effect modification was found between age categories and MCP-1 and the odds risk was attenuated in the analysis stratified to burn patients 65 years or older. A Hosmer-Lemeshow test showed that the model fit the data well (p=0.71). Receiving operator characteristic (ROC) plots to evaluate the previously discussed data sets for a predictor of in-hospital mortality yielded no strong predictors identified when the data was adjusted appropriately.
Table 5.
Unadjusted and Adjusted Association between Immune Mediator in BAL (pg/mL fluid) and In-Hospital Death
| Immune Mediator | Unadjusted OR (95% CI) |
p-value | Fully adjusted OR (95% CI) |
p-value |
*Stratified to Age >65 (95% CI) |
p-value |
|---|---|---|---|---|---|---|
|
| ||||||
| IL-1β, 100 pg/ml | 0.99 (0.86–1.16) |
0.96 | 1.03 (0.84–1.26) |
0.77 | 0.95 (0.62–1.46) |
0.83 |
| IL-1RA, 100 pg/ml | 0.99 (0.92–1.07) |
0.83 | 0.98 (0.89–1.10) |
0.82 | 0.98 (0.81–1.20) |
0.87 |
| IL-6, 100 pg/ml | 1.01 (0.96–1.06) |
0.81 | 1.01 (0.88–1.16) |
0.87 | 1.15 (0.88–1.50) |
0.30 |
| IL-8, 100 pg/ml | 0.99 (0.98–1.01) |
0.25 | 0.98 (0.97–1.01) |
0.08 | 0.97 (0.88–1.06) |
0.51 |
| IL-10, 25 pg/ml | 0.23 (0.04–1.23) |
0.09 | 0.12 (0.01–1.91) |
0.13 | 0.01 (0.01–14.82) |
0.16 |
| IL-12, 100 pg/ml | 0.81 (0.26–2.56) |
0.72 | 0.71 (0.05–11.06) |
0.80 | 0.08 (0.01–130.83) |
0.51 |
| IL-13, pg/ml | 0.99 (0.88–1.11) |
0.80 | 0.86 (0.62–1.20) |
0.86 | 0.86 (0.60–1.24) |
0.42 |
| IL-17, pg/ml | 0.97 (0.92–1.02) |
0.21 | 1.00 (0.90–1.12) |
0.94 | 0.93 (0.73–1.18) |
0.54 |
| Eotaxin, 25 pg/ml | 1.10 (0.90–1.32) |
0.36 | 1.31 (0.89–1.92) |
0.18 | 3.73 (0.63–22.18) |
0.15 |
| G-CSF, 100 pg/ml | 0.89 (0.75–1.06) |
0.89 | 0.73 (0.43–1.23) |
0.23 | 0.84 (0.57–1.22) |
0.36 |
| GM-CSF, 100 pg/ml | 1.03 (0.31–3.40) |
0.96 | 0.23 (0.01–5.56) |
0.40 | 0.23 (0.01–13.49) |
0.48 |
| IFN-γ, 100 pg/ml | 1.02 (0.54–1.89) |
0.96 | 0.87 (0.26–3.00) |
0.83 | 1.36 (0.32–5.75) |
0.67 |
| IP-10, 100 pg/ml | 0.99 (0.98–1.02) |
0.95 | 1.01 (0.98–1.05) |
0.57 | 0.99 (0.94–1.04) |
0.67 |
| MCP-1, 25 pg/ml | 1.01 (0.99–1.02) |
0.21 | 1.08 (0.98–1.19) |
0.13 | 1.03 (0.99–1.06) |
0.14 |
| TNF-α, 100 pg/ml | 0.82 (0.40–1.67) |
0.58 | 0.69 (0.14–3.47) |
0.65 | 0.63 (0.10–4.16) |
0.63 |
Multivariable linear regression adjusting for age group, sex, race, %TBSA and inhalation injury grade was used for the fully adjusted model.
This model was then stratified to the subjects ≥ 65 years.
IL, interleukin; IL-1RA, interleukin-1 receptor antagonist; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN-γ, interferon-gamma; IP-10, interferon-gamma-inducible protein 10; MCP-1, monocyte chemoattractant protein 1; TNF-α, tumor necrosis factor alpha
Table 6.
Unadjusted and Adjusted Association between Immune Mediator in BAL (pg/mg protein) and In-Hospital Death
| Immune Mediator | Unadjusted OR (95% CI) |
p-value | Fully adjusted OR (95% CI) |
p-value |
*Stratified to Age >65 (95% CI) |
p-value |
|---|---|---|---|---|---|---|
|
| ||||||
| IL-1β, pg/mg | 0.91 (0.67–1.23) |
0.53 | 0.91 (0.60–1.39) |
0.67 | 0.86 (0.45–1.66) |
0.65 |
| IL-1RA, pg/mg | 0.94 (0.83–1.07) |
0.33 | 0.91 (0.71–1.16) |
0.43 | 0.96 (0.79–1.18) |
0.71 |
| IL-2, pg/mg | 0.76 (0.54–1.08) |
0.12 | 0.55 (0.28–1.10) |
0.09 | 0.64 (0.32–1.27) |
0.20 |
| IL-4, pg/mg | 0.77 (0.53–1.13) |
0.19 | 0.50 (0.17–1.45) |
0.20 | 0.53 (0.20–1.41) |
0.20 |
| IL-6, 100 pg/mg | 1.02 (0.96–1.09) |
0.53 | 1.09 (0.81–1.47) |
0.57 | 2.13 (0.86–5.31) |
0.10 |
| IL-8, 100 pg/mg | 0.99 (0.98–1.01) |
0.34 | 0.99 (0.96–1.01) |
0.19 | 0.99 (0.94–1.04) |
0.74 |
| IL-10, 25 pg/mg | 0.11 (0.01–1.10) |
0.06 | 0.01 (0.01–7.16) |
0.18 | 0.01 (0.01–7.96) |
0.13 |
| IL-12, pg/mg | 0.99 (0.98–1.01) |
0.45 | 0.98 (0.94–1.02) |
0.28 | 0.97 (0.91–1.04) |
0.44 |
| IL-13, pg/mg | 0.98 (0.88–1.09) |
0.98 | 0.93 (0.70–1.23) |
0.93 | 0.93 (0.66–1.31) |
0.67 |
| IL-17, pg/mg | 0.97 (0.91–1.03) |
0.26 | 0.92 (0.75–1.13) |
0.44 | 0.91 (0.67–1.23) |
0.55 |
| Eotaxin, pg/mg | 1.45 (0.74–2.84) |
0.28 | 2.24 (0.73–6.87) |
0.16 | 11.09 (0.47–262.90) |
0.14 |
| G-CSF, pg/mg | 0.91 (0.75–1.09) |
0.28 | 0.73 (0.46–1.16) |
0.18 | 0.81 (0.46–1.45) |
0.48 |
| GM-CSF, pg/mg | 0.96 (0.71–1.29) |
0.78 | 0.87 (0.42–1.77) |
0.69 | 0.83 (0.24–2.87) |
0.76 |
| IFN-γ, pg/mg | 0.84 (0.41–1.74) |
0.64 | 0.26 (0.05–1.34) |
0.11 | 0.57 (0.10–3.22) |
0.53 |
| IP-10, pg/mg | 1.00 (0.99–1.02) |
0.86 | 1.00 (0.98–1.03) |
0.88 | 1.01 (0.98–1.03) |
0.71 |
| MCP-1, pg/mg | 1.01 (0.97–1.05) |
0.67 | 1.16 (1.01–1.32) |
0.03 | 1.11 (0.97–1.28) |
0.14 |
| TNF-α, pg/mg | 0.54 (0.19–1.55) |
0.25 | 0.48 (0.06–3.99) |
0.49 | 0.67 (0.01–160.7) |
0.88 |
Multivariable linear regression adjusting for age group, sex, race, %TBSA and inhalation injury grade was used for the fully adjusted model.
This model was then stratified to the subjects ≥ 65 years.
IL, interleukin; IL-1RA, interleukin-1 receptor antagonist; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN-γ, interferon-gamma; IP-10, interferon-gamma-inducible protein 10; MCP-1, monocyte chemoattractant protein 1; TNF-α, tumor necrosis factor alpha
3.4 Inflammatory mediators in plasma
The panel of immune mediator levels in the plasma of burn patients suspected of having inhalation injury demonstrated multiple cytokines/chemokines that differed between age groups. Of the 27 mediators in the multiplex kit, we were able to detect 17 in the plasma. IL-2 and IP-10 decreased by 50% in the ≥65 cohort, while IL-6 showed a 2.5 fold increase (p<0.05 for all). When comparing only the younger (<50 years) and older (≥65 years) groups, we showed changes in IL-1RA, IL-4, IL-6, G-CSF, IP-10 and MCP-1 levels (p<0.05 in all). Among the most impressive age dependent differences in plasma concentrations were IL-6, G-CSF and MCP-1 which had a 4-, 2- and 2-fold increase, respectively, from younger to older subjects. The level of IL-6 was elevated from 83.9 pg/mL in younger subjects to 352.5 pg/mL in older subjects (p<0.001). In the younger cohort of burn patients, G-CSF was 47.9 pg/mL and 91.9 pg/mL in the older cohort while plasma levels of MCP-1 rose from 128.6 g/mL to 224.6 pg/mL. In general there was a trend towards increase in immune mediators with increasing age (Table 7).
Table 7.
Immune Plasma Mediator Levels between Age Groups (pg/ml fluid)
| Immune Mediator | Total (n=91) |
<50 years (n=50) |
50–64 years (n=21) |
>65 years (n=20) |
p-value | p-value* |
|---|---|---|---|---|---|---|
| IL-1β | 3.3 (1.8–4.8) |
3.6 (2.3–4.8) |
3.1 (1.8–4.5) |
3.4 (1.5–4.9) |
0.82 | 0.53 |
| IL-1RA | 306.5 (158.8–734.6) |
281.9 (136.9–549.6) |
319.6 (222.6–815.8) |
554.3 (166.9–1497.7) |
0.14 | 0.01 |
| IL-2 | 6.1 (2.9–11.5) |
5.5 (1.9–11.4) |
8.9 (5.4–22.0) |
4.0 (2.3–9.8) |
0.03 | 0.30 |
| IL-4 | 4.3 (2.0–9.3) |
5.1 (2.1–9.9) |
5.0 (2.1–12.4) |
3.4 (1.9–4.8) |
0.18 | 0.02 |
| IL-6 | 123.6 (45.1–352.5) |
83.9 (35.0–225.7) |
135.5 (56.0–264.8) |
352.5 (54.2–912.8) |
0.04 | <0.001 |
| IL-8 | 29.0 (16.7–61.2) |
28.2 (14.1–61.2) |
23.8 (14.1–53.0) |
34.9 (26.9–78.9) |
0.14 | 0.37 |
| IL-10 | 12.6 (7.1–19.2) |
12.3 (60–19.3) |
13.4 (5.0–19.8) |
14.5 (9.8–17.4) |
0.79 | 0.80 |
| IL-12 | 12.9 (7.6–23.7) |
13.2 (7.8–24.2) |
13.8 (7.9–35.3) |
10.8 (6.8–17.1) |
0.57 | 0.31 |
| IL-13 | 4.5 (3.1–6.0) |
4.6 (2.9–6.1) |
5.1 (3.8–8.4) |
4.0 (2.2–5.0) |
0.20 | 0.33 |
| IL-17 | 18.3 (5.4–53.0) |
21.3 (0.0–70.1) |
20.4 (8.3–94.7) |
15.2 (0.0–29.4) |
0.26 | 0.05 |
| Eotaxin | 164.0 (115.7–223.9) |
159.2 (111.4–201.5) |
143.8 (114.2–233.4) |
179.5 (138.0–316.9) |
0.29 | 0.10 |
| G-CSF | 50.9 (34.1–131.3) |
47.9 (33.6–119.9) |
46.7 (32.3–111.0) |
91.9 (43.5–341.2) |
0.09 | 0.001 |
| GM-CSF | 22.5 (7.8–45.3) |
18.9 (5.3–40.4) |
29.9 (10.3–59.9) |
24.5 (15.8–36.9) |
0.45 | 0.33 |
| IFN-γ | 163.6 (101.2–284.0) |
157.3 (99.3–286.0) |
219.2 (99.2–292.6) |
131.6 (106.1–274.6) |
0.94 | 0.85 |
| IP-10 | 694.2 (436.7–1204.8) |
597.6 (396.8–857.5) |
1136.2 (433.1–1548.8) |
827.1 (535.5–1650.4) |
0.02 | 0.01 |
| MCP-1 | 144.3 (61.1–323.4) |
128.6 (59.3–274.4) |
113.8 (54.2–329.2) |
224.6 (98.2–793.8) |
0.12 | 0.002 |
| TNF-α | 55.6 (29.0–130.9) |
48.9 (26.7–85.0) |
82.5 (43.4–145.0) |
61.3 (32.9–167.8) |
0.33 | 0.07 |
Data represented as median with 25%–75% interquartile range; all values are pg/ml; Univariable comparison of continuous variables by Kruskal-Wallis test.
Multivariable linear regression adjusting for age group, sex, race, % TBSA, and inhalation injury grade. Adjusted p-value is for the age group over 65 years compared to referent group <50 years.
We further evaluated whether there were any associations between plasma levels of these immune mediators and in-hospital mortality. Our results for this evaluation can be found in Table 8. Evaluating the data without adjusting for age, sex, race, %TBSA and inhalation injury grade, there is an association between in-hospital mortality and three cytokines, IL-1RA (p=0.001), IL-6 (p=0.003) and IL-10 (p=0.02). These associations, however, were not significant in the multivariable model adjusted for clinical factors.
Table 8.
Unadjusted and Adjusted Association between Immune Mediator in Plasma and In Hospital Death
| Immune Mediator | Unadjusted OR (95% CI) |
p-value | Fully adjusted OR (95% CI) |
p-value |
*Stratified to Age >65 (95% CI) |
p-value |
|---|---|---|---|---|---|---|
|
| ||||||
| IL-1β, pg/ml | 1.04 (0.16–9.92) |
0.68 | 1.08 (0.75–1.56) |
0.67 | 0.88 (0.53–1.44) |
0.60 |
| IL-1RA, 100 pg/ml | 1.07 (1.03–1.11) |
0.001 | 1.12 (0.95–1.31) |
0.17 | 1.44 (0.82–2.51) |
0.20 |
| IL-2, pg/ml | 0.99 (0.95–1.05) |
0.94 | 1.11 (0.98–1.24) |
0.09 | 2.16 (0.18–26.59) |
0.55 |
| IL-4, pg/ml | 0.91 (0.80–1.03) |
0.12 | 1.07 (0.85–1.35) |
0.54 | 1.68 (0.16–17.51) |
0.42 |
| IL-6, 100 pg/ml | 1.21 (1.07–1.40) |
0.003 | 1.15 (0.93–1.44) |
0.20 | 1.15 (0.91–1.45) |
0.24 |
| IL-8, pg/ml | 1.01 (0.99–1.01) |
0.17 | 1.00 (0.98–1.01) |
0.73 | 1.07 (0.10–11.25) |
0.40 |
| IL-10, 25 pg/ml | 1.59 (1.08–2.34) |
0.02 | 2.18 (0.84–5.62) |
0.10 | 1.91 (0.63–5.73) |
0.25 |
| IL-12, pg/ml | 0.97 (0.93–1.02) |
0.23 | 1.01 (0.94–1.08) |
0.88 | 0.06 (0.02–1.60) |
0.09 |
| IL-13, pg/ml | 0.97 (0.87–1.09) |
0.61 | 1.02 (0.78–1.35) |
0.86 | NS | ┼NS |
| IL-17, pg/ml | 0.99 (0.98–1.01) |
0.39 | 1.02 (0.99–1.04) |
0.22 | 1.69 (0.21–23.69) | 0.32 |
| Eotaxin, pg/ml | 1.00 (0.99–1.01) |
0.33 | 0.99 (0.99–1.01) |
0.44 | 0.68 (0.05–9.59) | 0.19 |
| G-CSF, pg/ml | 1.17 (0.93–1.47) |
0.18 | 0.99 (0.99–1.00) |
0.65 | 1.10 (0.09–13.98) | 0.78 |
| GM-CSF, pg/ml | 1.01 (0.99–1.02) |
0.44 | 1.02 (0.97–1.06) |
0.44 | 2.30 (0.15–34.38) | 0.61 |
| IFN-γ, 100 pg/ml | 0.99 (0.40–1.40) |
0.97 | 1.27 (0.65–2.46) |
0.49 | 1.04 (0.45–2.62) | 0.93 |
| IP-10, 100 pg/ml | 1.07 (0.99–1.15) |
0.07 | 1.12 (0.98–1.26) |
0.09 | 1.14 (0.96–1.35) | 0.13 |
| MCP-1, 10 pg/ml | 1.14 (0.99–1.32) |
0.08 | 0.97 (0.76–1.23) |
0.80 | 0.88 (0.66–1.19) | 0.42 |
| TNF-α, 100 pg/ml | 1.14 (0.69–1.87) |
0.62 | 0.98 (0.36–2.62) |
0.96 | 0.88 (0.32–2.44) | 0.81 |
Multivariable linear regression adjusting for age group, sex, race, %TBSA and inhalation injury grade was used for the fully adjusted model.
This model was then stratified to the subjects’ ≥ 65 years.
┼NS, not significant. IL, interleukin; IL-1RA, interleukin-1 receptor antagonist; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN-γ, interferon-gamma; IP-10, interferon-gamma-inducible protein 10; MCP-1, monocyte chemoattractant protein 1; TNF-α, tumor necrosis factor alpha
Area under the curve receiver operating characteristics (AUC ROC) between immune mediators and in-hospital death are shown in Table 9. The strongest predictor for in-hospital death with an AUC ROC of 0.84 (95% CI 0.73–0.95) was IL-1RA (Figure 1). In examining all candidate biomarkers and clinical characteristics, the best model in variable selection by stepwise logistic regression was a two-variable model with IL-1RA and %TBSA. The addition of %TBSA did improve discrimination with an AUC ROC of 0.87 (95% CI 0.78–0.97), and the Hosmer-Lemeshow test showed that the model fit the data well (p=0.59).
Table 9.
Receiver Operating Characteristics for each plasma immune mediator in predicting In-Hospital death
| Immune Mediator | Area Under the Curve | 95% CI |
|---|---|---|
|
| ||
| IL-1β | 0.56 | 0.40–073 |
| IL-1RA | 0.84 | 0.73–0.95 |
| IL-2 | 0.47 | 0.33–0.62 |
| IL-4 | 0.63 | 0.49–0.78 |
| IL-6 | 0.70 | 0.55–0.86 |
| IL-8 | 0.65 | 0.50–0.81 |
| IL-10 | 0.67 | 0.53–0.81 |
| IL-12 | 0.58 | 0.44–0.72 |
| IL-13 | 0.52 | 0.36–0.68 |
| IL-17 | 0.52 | 0.38–0.65 |
| Eotaxin | 0.65 | 0.53–0.78 |
| G-CSF | 0.59 | 0.42–0.76 |
| GM-CSF | 0.52 | 0.35–0.68 |
| IFN-γ | 0.49 | 0.33–0.66 |
| IP-10 | 0.61 | 0.44–0.79 |
| MCP-1 | 0.64 | 0.48–0.79 |
| TNF-α | 0.52 | 0.35–0.69 |
IL, interleukin; IL-1RA, interleukin-1 receptor antagonist; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN-γ, interferon-gamma; IP-10, interferon-gamma-inducible protein 10; MCP-1, monocyte chemoattractant protein 1; TNF-α, tumor necrosis factor alpha
Figure 1. Receiver Operating Characteristics Curve (ROC) for Plasma IL-1RA.
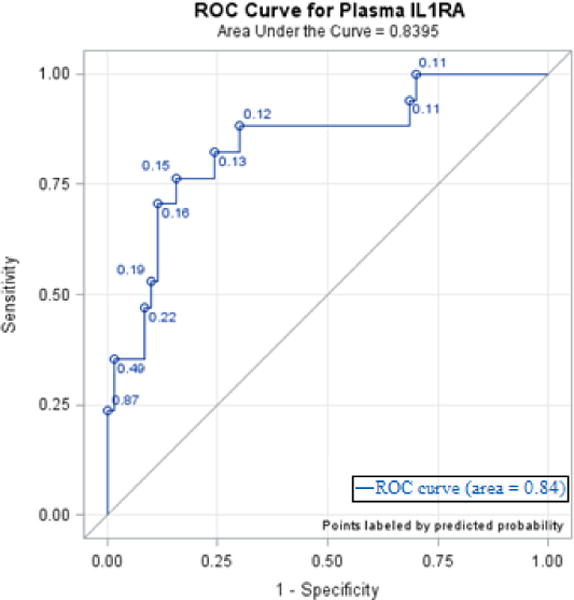
Figure 1 shows a receiver operating characteristics curve for plasma interleukin 1 receptor antagonist, IL-1RA. The area under the curve (AUC) is 0.84.
4. Discussion
The findings in the present study is similar to reports published by our laboratory and others (2, 13, 14, 23–25).There is little doubt that inhalation injury causes heightened morbidity and mortality, and it has been shown in previous reports that the concentrations of certain immune mediators in the BAL fluid are increased with inhalation injury (14, 26). To our knowledge, the particular concentrations of different immune mediators have not been stratified on an age basis. The novelty of this study was to evaluate changes of particular immune mediators in the elderly population of burn/inhalation injury patients. Our results show that there is an altered response of immune mediators in the elderly when compared to different age groups, suggesting a state of post-injury immune dysregulation.
The increase of MCP-1 in the BAL sample of burn patients who are ≥65 years of age is an interesting but not an unexpected finding. Previous papers have shown that this immune mediator is increased in states of systemic inflammation and tissue injury (2, 14, 27). It is a regulator of monocyte/macrophage migration, particularly into areas of the body that are stressed or damaged, and may also affect other organs in a systemic manner. It has been long understood that increased age is associated with increased mortality after burn; hence, one would anticipate that a mediators such as MCP-1 would be increased in concentration that theoretically could assist with the recovery process. This information begs the question of whether, perhaps, the immune cells that are recruited into the site of interest are not as effective at performing their pro- and anti-inflammatory activities, namely to initiate and resolve pulmonary inflammation, in the elderly as they are in the young. If correct, then perhaps for this reason the concentration of this chemoattractant would be higher. This is speculation and would require further research in order to produce an answer.
Our previous work reveal that there is a pulmonary immune hyporesponsiveness associated with mortality in patients following burn and inhalation injury (13). In that paper by Davis et al., the patient populations are stratified into those that survive and those that do not. Advanced age was again found to be associated with mortality, however, when the group was analyzed as whole, concentrations of both pro- and anti-inflammatory mediators were lower in the non-survivor group. It is interesting, therefore, that we have found mostly an increase in immune mediators in the elderly who are also at the highest risk for mortality, relative to younger subjects. There are multiple possible explanations for these findings, and reflect the complex nature of the systemic inflammatory response to injury. Elevated levels of IL-1RA, for example, have been shown to be associated with increased mortality (14). IL-1RA is anti-inflammatory and may be a contributing factor to a deranged inflammatory response leading to poorer outcomes. However, in animal models of sepsis, IL-1RA deficient mice exhibit evidence of worsened inflammation (28). This illustrates the delicate balance of this immune mediator that is required for an adequate immune response (29). Our study found elevated IL-1RA in the plasma as a predictor of in-hospital death. Perhaps overexpression of this particular anti-inflammatory factor may result in an ineffective immune response, which may then lead to increased susceptibility to infection and, in turn, mortality.
Another interesting result of our study is that many of the immune mediators that have been shown in previous papers to be associated with mortality were also found to be elevated in the elderly population when compared to our younger cohort, specifically IL-1RA, IL-4, IL-6, and MCP-1 (30–34). It has been proposed that when the IL-1β/IL-1RA ratio is reduced, patients have an association with worse prognosis (35, 36). It is re-assuring that data from the present study are consistent with previous published works, and open the opportunity for further research into more mechanistic approaches defining the precise roles of these particular immune mediators in the lung and vascular compartments. With a greater understanding of the impact of advanced age on the response to tissue injury, our work may serve as diagnostically valuable biomarkers or therapeutic targets to promote immune homeostasis and recovery.
As mentioned earlier, prior studies have shown an increase in immune mediator concentration in the BAL fluid associated with worsening grade of inhalational injury (14). In the current study, even after adjusting for worsening grade of inhalational injury, we still saw a trend of increased inflammatory mediators in the BAL fluid in the elderly population. This may show that the elderly, regardless of the magnitude of the systemic insult, continue to have a deranged inflammatory responses to injury when compared to the young. In fact, when analyzing the clinical outcomes of the elderly relative to the younger cohort, we found that the rate of ARDS was less although the rate of mortality was higher. Although not statistically significant but it trended close to significance (p=0.08). This may, of course, be a consequence of the heightened rate of early mortality in the elderly and perhaps this population did not have time to develop ARDS before death. However, it may also be possible that the elderly experience a dysregulated inflammatory response distinct from the systemic response occurring in the younger cohort.
We acknowledge several weaknesses of our study. First, our results are subject to considerable selection bias, as we only selected patients who were suspected of having an associated inhalation injury. Second, our data are limited to the first 15 hours after injury, and analysis of later time points may prove useful in determining which factors may contribute or change in response to subsequent complications, such as pneumonia, sepsis, or multiple organ failure. Undoubtedly, such comparisons will be the target of future research, especially with a greater number of study patients as may be afforded by collaboration with other centers. Finally, the sample size of our cohort is limited and may therefore be underpowered to detect a difference in our multivariable analyses so any insignificant associations may have been due to limitations in sample size. A future expanded multi-institutional study may assist with further conclusions by providing a larger population to study the complex nature of aging and the physiological response to trauma such as burn injuries.
5. Conclusion
In this study, we have shown that there is a dysregulated inflammatory response in the older cohort when compared to a younger cohort in patients with burn and inhalation injuries. Many immune mediators in the plasma, such as IL-1RA, IL-2, IL-4, IL-6, G-CSF, IP-10, and MCP-1, were significantly elevated. Previous research in our laboratory and those of others has shown that a rise in the concentration of these mediators is associated with increased mortality after burn injury (2, 13, 14). Perhaps, finding a consistent trend in elevation when cohorts are stratified by age may be a contributing factor to the increased risk of mortality in the elderly with burn. Our observed statistically significantly increase in one immune mediator in the BAL fluid, namely MCP-1, is a factor that has been shown to be heightened in the lungs of non-survivors of burn/inhalation injury (2, 14). This may be another factor contributing to worsened prognosis with age. The next step will be a larger study to evaluate changes in immune mediator response in the elderly.
Supplementary Material
Highlights.
Altered inflammatory response in the older cohort when compared to younger cohort
3-fold increase in MCP-1 in BAL fluid samples from the elderly cohort
4-fold, 2-fold and 2-fold increase of IL-6, G-CSF and MCP-1 respectively in the plasma in the elderly cohort
An association between concentration of MCP-1 in BAL fluid and in-hospital death
IL-1RA in the plasma fits an ROC model to be a predictor for in-hospital death
Acknowledgments
The authors have no conflicts of interest to disclose. The work herein was supported in part by NIH AG018859 (EJK), GM115257 (EJK), R21AA023193 (EJK), R24 AA019661 (ELB) and K23 AA024503 (MA). We would also like to acknowledge and thank the staff of the Burn ICU at Loyola University Medical Center and the University of Colorado Anchutz Medical Center as well as the Colorado Pulmonary Alcohol Research Collective (CoPARC).
Abbreviations
- BAL
bronchoalveolar lavage
- OR
odds ratio
- IL-1RA
interleukin 1 receptor antagonist
- IL-1β
interleukin 1 beta
- IL-2
interleukin 2
- IL-4
interleukin 4
- IL-6
interleukin 6
- IL-8
interleukin 8
- IL-10
interleukin 10
- IL-12
interleukin 12
- IL-13
interleukin 13
- IL-17
interleukin 17
- G-CSF
granulocyte colony-stimulating factor
- IP-10
interferon-gamma-induced protein 10
- MCP-1
monocyte chemoattractant protein 1
- AUC
area under the curve
- ROC
receiver operating characteristics
- %TBSA
percent total body surface area
- ICU
intensive care unit
- COHB
carboxyhemoglobin
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- IFN-γ
interferon-gamma
- TNF-α
tumor necrosis factor alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pham TN, Kramer CB, Wang J, Rivara FP, Heimbach DM, Gibran NS, Klein MB. Epidemiology and outcomes of older adults with burn injury: an analysis of the National Burn Repository. Journal of burn care & research: official publication of the American Burn Association. 2009;30:30–36. doi: 10.1097/BCR.0b013e3181921efc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis CS, Janus SE, Mosier MJ, Carter SR, Gibbs JT, Ramirez L, Gamelli RL, Kovacs EJ. Inhalation injury severity and systemic immune perturbations in burned adults. Annals of surgery. 2013;257:1137–1146. doi: 10.1097/SLA.0b013e318275f424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hassan Z, Wong J, Bush J, Bayat A, Dunn K. Assessing the severity of inhalation injuries in adults. Burns. 2010;36:212–216. doi: 10.1016/j.burns.2009.06.205. [DOI] [PubMed] [Google Scholar]
- 4.Navar PD, Saffle JR, Warden GD. Effect of inhalation injury on fluid resuscitation requirements after thermal injury. The American Journal of Surgery. 1985;150:716–720. doi: 10.1016/0002-9610(85)90415-5. [DOI] [PubMed] [Google Scholar]
- 5.Enkhbaatar P, Pruitt BA, Suman O, Mlcak R, Wolf SE, Sakurai H, Herndon DN. Pathophysiology, research challenges, and clinical management of smoke inhalation injury. The Lancet. 2016;388:1437–1446. doi: 10.1016/S0140-6736(16)31458-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bessey PQ, Phillips BD, Lentz CW, Edelman LS, Faraklas I, Finocchiaro MA, Kemalyan NA, Klein MB, Miller SF, Mosier MJ. Synopsis of the 2013 annual report of the national burn repository. Journal of Burn Care & Research. 2014;35:S218–S234. doi: 10.1097/BCR.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 7.Pruitt B, Mason A. Epidemiological, demographic, and outcome characteristics of burn injury. Total Burn Care London: D Herndon Ed, Saunders Co. 1996;13 [Google Scholar]
- 8.Latenser BA, Miller SF, Bessey PQ, Browning SM, Caruso DM, Gomez M, Jeng JC, Krichbaum JA, Lentz CW, Saffle JR. National Burn Repository 2006: a ten-year review. Journal of Burn Care & Research. 2007;28:635–658. doi: 10.1097/BCR.0B013E31814B25B1. [DOI] [PubMed] [Google Scholar]
- 9.Klein MB, Goverman J, Hayden DL, Fagan SP, McDonald-Smith GP, Alexander AK. Benchmarking outcomes in the critically injured burn patient. Annals of surgery. 2014;259 doi: 10.1097/SLA.0000000000000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hazeldine J, Lord JM, Hampson P. Immunesenescence and inflammaging: A contributory factor in the poor outcome of the geriatric trauma patient. Ageing research reviews. 2015;24:349–357. doi: 10.1016/j.arr.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Osler T, Glance LG, Hosmer DW. Simplified Estimates of the Probability of Death After Burn Injuries: Extending and Updating the Baux Score. Journal of Trauma and Acute Care Surgery. 2010;68:690–697. doi: 10.1097/TA.0b013e3181c453b3. [DOI] [PubMed] [Google Scholar]
- 12.Shirani KZ, Pruitt BA, Jr, Mason AD., Jr The influence of inhalation injury and pneumonia on burn mortality. Annals of surgery. 1987;205:82. doi: 10.1097/00000658-198701000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis CS, Albright JM, Carter SR, Ramirez L, Kim H, Gamelli RL, Kovacs EJ. Early pulmonary immune hyporesponsiveness is associated with mortality after burn and smoke inhalation injury. Journal of burn care & research: official publication of the American Burn Association. 2012;33:26–35. doi: 10.1097/BCR.0b013e318234d903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albright JM, Davis CS, Bird MD, Ramirez L, Kim H, Burnham EL, Gamelli RL, Kovacs EJ. The acute pulmonary inflammatory response to the graded severity of smoke inhalation injury. Critical Care Medicine. 2012;40:1113–1121. doi: 10.1097/CCM.0b013e3182374a67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ettensohn DB, Jankowski MJ, Redondo AA, Duncan PG. Bronchoalveolar lavage in the normal volunteer subject: 2. safety and results of repeated BAL, and use in the assessment of intrasubject variability. Chest. 1988;94:281–285. doi: 10.1378/chest.94.2.281. [DOI] [PubMed] [Google Scholar]
- 16.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Medicine. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 17.Endorf FW, Gamelli RL. Inhalation injury, pulmonary perturbations, and fluid resuscitation. Journal of burn care & research. 2007;28:80–83. doi: 10.1097/BCR.0B013E31802C889F. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson ND, Fan E, Camporota L, Antonelli M, Anzueto A, Beale R, Brochard L, Brower R, Esteban A, Gattinoni L. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive care medicine. 2012;38:1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 19.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM. The third international consensus definitions for sepsis and septic shock (sepsis-3) Jama. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan SS, Smith MS, Reda D, Suffredini AF, McCoy JP. Multiplex bead array assays for detection of soluble cytokines: Comparisons of sensitivity and quantitative values among kits from multiple manufacturers. Cytometry Part B: Clinical Cytometry. 2004;61B:35–39. doi: 10.1002/cyto.b.20021. [DOI] [PubMed] [Google Scholar]
- 21.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. Journal of biological chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- 22.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988:837–845. [PubMed] [Google Scholar]
- 23.Parsons PE, Eisner MD, Thompson BT, Matthay MA, Ancukiewicz M, Bernard GR, Wheeler AP, Network, N. A. R. D. S. C. T. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Critical Care Medicine. 2005;33:1–6. doi: 10.1097/01.ccm.0000149854.61192.dc. [DOI] [PubMed] [Google Scholar]
- 24.Carsin H, Assicol M, Feger F, Roy O, Pennacino I, Bever H, Ainaud P, Bohuon C. Evolution and significance of circulating procalcitonin levels compared with IL-6, TNFα and endotoxin levels early after thermal injury. Burns. 1997;23:218–224. doi: 10.1016/s0305-4179(96)00124-6. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez JL, Miller CG, Garner WL, Till GO, Guerrero P, Moore NP, Corridore M, Normolle DP, Smith DJ, Remick DG. Correlation of the local and systemic cytokine response with clinical outcome following thermal injury. J Trauma. 1993;34:684–694. doi: 10.1097/00005373-199305000-00011. discussion 694–685. [DOI] [PubMed] [Google Scholar]
- 26.Dehne MG, Sablotzki A, Hoffmann A, Mühling J, Dietrich FE, Hempelmann G. Alterations of acute phase reaction and cytokine production in patients following severe burn injury. Burns. 2002;28:535–542. doi: 10.1016/s0305-4179(02)00050-5. [DOI] [PubMed] [Google Scholar]
- 27.Nacionales DC, Szpila B, Ungaro R, Lopez MC, Zhang J, Gentile LF, Cuenca AL, Vanzant E, Mathias B, Jyot J. A detailed characterization of the dysfunctional immunity and abnormal myelopoiesis induced by severe shock and trauma in the aged. The Journal of Immunology. 2015;195:2396–2407. doi: 10.4049/jimmunol.1500984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frank JA, Pittet JF, Wray C, Matthay MA. Protection from experimental ventilator-induced acute lung injury by IL-1 receptor blockade. Thorax. 2008;63:147–153. doi: 10.1136/thx.2007.079608. [DOI] [PubMed] [Google Scholar]
- 29.Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine & growth factor reviews. 2002;13:323–340. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 30.Guo Y, Dickerson C, Chrest FJ, Adler WH, Munster AM, Winchurch RA. Increased levels of circulating interleukin 6 in burn patients. Clinical immunology and immunopathology. 1990;54:361–371. doi: 10.1016/0090-1229(90)90050-z. [DOI] [PubMed] [Google Scholar]
- 31.Damas P, Ledoux D, Nys M, Vrindts Y, De Groote D, Franchimont P, Lamy M. Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Annals of surgery. 1992;215:356. doi: 10.1097/00000658-199204000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nylen ES, Whang KT, Snider RH, Steinwald PM, White JC, Becker KL. Mortality is increased by procalcitonin and decreased by an antiserum reactive to procalcitonin in experimental sepsis. Critical Care Medicine. 1998;26:1001–1006. doi: 10.1097/00003246-199806000-00015. [DOI] [PubMed] [Google Scholar]
- 33.Jeschke MG, Chinkes DL, Finnerty CC, Kulp G, Suman OE, Norbury WB, Branski LK, Gauglitz GG, Mlcak RP, Herndon DN. The pathophysiologic response to severe burn injury. Annals of surgery. 2008;248:387. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gauglitz GG, Song J, Herndon DN, Finnerty CC, Boehning D, Barral JM, Jeschke MG. CHARACTERIZATION OF THE INFLAMMATORY RESPONSE DURING ACUTE AND POST-ACUTE PHASES AFTER SEVERE BURN. Shock. 2008;30:503–507. doi: 10.1097/SHK.0b013e31816e3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vindenes HA, Ulvestad E, Bjerknes R. Concentrations of cytokines in plasma of patients with large burns: their relation to time after injury, burn size, inflammatory variables, infection, and outcome. The European journal of surgery. 1998;164:647–656. doi: 10.1080/110241598750005525. [DOI] [PubMed] [Google Scholar]
- 36.Um JY, Jeong HJ, Park RK, Hong SH, Kim H. Aspects of gene polymorphisms in cerebral infarction: inflammatory cytokines. Cellular and molecular life sciences. 2005;62:824–833. doi: 10.1007/s00018-004-4267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


