Abstract
Despite previous findings of therapeutic effects for heart rate variability biofeedback (HRVB) on asthma, it is not known whether HRVB can substitute either for controller or rescue medication, or whether it affects airway inflammation. Sixty-eight paid volunteer steroid naïve study participants with mild or moderate asthma were given three months of HRVB or a comparison condition consisting of EEG alpha biofeedback with relaxing music and relaxed paced breathing (EEG+), in a two-center trial. All participants received a month of intensive asthma education prior to randomization. Both treatment conditions produced similar significant improvements on the methacholine challenge test (MCT), asthma symptoms, and asthma quality of life (AQOL). MCT effects were of similar size to those of enhanced placebo procedures reported elsewhere, and were 65% of those of a course of a high-potency inhaled steroid budesonide given to a sub-group of participants following biofeedback training. Exhaled nitric oxide decreased significantly only in the HRVB group, 81% of the budesonide effect, but with no significant differences between groups. Participants reported becoming more relaxed during practice of both techniques. Administration of albuterol after biofeedback sessions produced a large improvement in pulmonary function test results, indicating that neither treatment normalized pulmonary function as a potent controller medication would have done. Impulse oscillometry showed increased upper airway (vocal cord) resistance during biofeedback periods in both groups. These data suggest that HRVB should not be considered an alternative to asthma controller medications (e.g., inhaled steroids), although both biofeedback conditions produced some beneficial effects, warranting further research, and suggesting potential complementary effects. Various hypotheses are presented to explain why HRVB effects on asthma appeared smaller in this study than in earlier studies.
INTRODUCTION
Various medical treatments are used in asthma therapy and have been advocated by accepted treatment guidelines published in the report of the National Asthma Education and Prevention Program, Expert Panel 3 (National Heart Lung and Blood Institute (NHLBI), 2007). While effective to varying degrees most of these therapies share disadvantages of high cost and patient concerns over side effects, factors that greatly limit adherence (Milgrom et al., 1996; Strunk, 2002; Williams, 2004; World Health Organization, 2003) and, hence, are associated with suboptimal outcomes (Milgrom et al., 1996; Williams, 2004). Asthma patients frequently seek alternative and complementary treatments (Langmack, 2001), including several types of breathing exercises (Freitas et al., 2013). Heart rate variability biofeedback (HRVB) is an intervention involving breathing at about six breaths/min, in a way that maximizes respiratory sinus arrhythmia (RSA), while bringing RSA into phase synchrony with breathing and maximizing baroreflex gain (Lehrer et al., 2013; Lehrer et al., 2003; Vaschillo, Lehrer, Rishe, & Konstantinov, 2002; Vaschillo, Vaschillo, & Lehrer, 2006). In a study of optimally medicated study participants with mild through severe asthma (Lehrer et al., 2004), the addition of HRVB improved pulmonary function and asthma symptoms. It also decreased the frequency of asthma exacerbations while reducing the dose of asthma medication needed for adequate symptom control. Although HRVB appeared to be clinically effective, that study did not examine whether it might best be used either to supplement or replace ‘controller’ or ‘rescue’ medications. In standard clinical practice, anti-inflammatory agents such as inhaled corticosteroids are used as controller medications, to prevent exacerbations, while bronchodilator medication, such as albuterol, is used as a rescue medication, to provide immediate relief when symptoms occur (National Heart Lung and Blood Institute (NHLBI), 2007). Given our prior results showing that HRVB resulted in decreased medication requirements, including for controller medications (Lehrer et al., 2004), the primary aim of the current study was to determine whether HRVB can function as a controller treatment in asthma therapy.
A general measure of the presence and severity of asthma is reactivity of the airways to various inhaled challenges. The methacholine challenge test (MCT) assesses the sensitivity of the airways to an inhaled parasympathetic stimulant, such that people without asthma do not show a decrease in pulmonary function at a standard dose, while asthma patients do, with greater sensitivity to a lower dose related to more severe asthma (Brusasco & Crimi, 2001; Davis & Cockcroft, 2012; Hewitt, 2008; James & Ryan, 1997). Because airways reactivity is a central characteristic of asthma and is influenced by airways inflammation, this measure was taken as our primary outcome measure. The MCT also indirectly reflects airways inflammation, the underlying disease process that is thought to produce heightened airway irritability (NHLBI, 2007), as well as other factors such as changes in autonomic nervous system activity and alterations in breathing dynamics that mechanically affect airway smooth muscle contractility (Brusasco, Crimi, & Pellegrino, 1998). It is possible that HRVB has a direct effect on reducing inflammation. It directly stimulates respiratory sinus arrhythmia (RSA) (Berntson et al., 1997; Lehrer et al., 2003; Vaschillo et al., 2006), which reflects vagus nerve function. Vagus nerve stimulation is known to decrease inflammation (Pavlov & Tracey, 2006). We measured airways inflammation by measuring exhaled nitric oxide(Alving, Weitzberg, & Lundberg, 1993; Barnes & Liew, 1995; Deykin, Massaro, Drazen, & Israel, 2002; Guo, Wang, Xing, & Wang, 2016; Malmberg, Pelkonen, Haahtela, & Turpeinen, 2003; Silkoff, Stevens, Pak, Bucher-Bartelson, & Martin, 1999)
Another possible pathway for HRVB effects on asthma may be through strengthening of processes that modulate autonomic reactivity. Increases in parasympathetic activity causes increased bronchial tone and can lead to bronchoconstriction (Aboussafy, Campbell, Lavoie, Aboud, & Ditto, 2005; Feldman, Lehrer, Hochron, & Schwartz, 2002; Lehrer et al., 1997). RSA appears to reflect a regulatory component of vagus nerve activity (Porges, 1995, 2001, 2007). There is evidence that slow breathing decouples RSA from tonic vagal effects such as decreased mean heart rate (Song & Lehrer, 2003). Also, HRVB stimulates the baroreflex (Lehrer et al., 2003; Vaschillo et al., 2002), which has modulatory effects on blood pressure and autonomic responses to various stimuli (Eckberg & Sleight, 1992; Grrishma, Gaur, Velkumary, Subramanian, & Gurunandan, 2015; Van Buren, Kasbergen, Gispen, & De Wildt, 1998). If HRVB modulates autonomic reactivity throughout the body, including the airways, HRVB may therefore improve airway autonomic tone and decrease susceptibility to an asthma exacerbation (Lehrer et al., 2004).
In order to minimize possible experimenter bias effects and to conduct the study according to the most rigorous standards used in asthma research, we conducted a two-center trial. The two centers were Rutgers, where we have done many studies of HRVB and therefore have considerable expertise with the method, but also may be invested in the method with the elevated potential for experimental bias, and National Jewish Health, an international center for asthma research with little prior experience or investment in HRVB.
We also examined asthma control from a questionnaire and daily records of symptoms and twice daily peak flow records, asthma quality of life, and the proportion of inhaled steroid effects achievable by biofeedback in a subset of patients with mild or moderate persistent asthma. We studied patients who had not taken inhaled steroid medication for at least one month in order to assess the effects of HRVB as a substitute for controller medication, and to measure the proportion of the steroid effect achieved by biofeedback when inhaled steroids are administered after a course of biofeedback.
METHODS
The IRB’s of both Robert Wood Johnson Medical School (RWJMS) and National Jewish Health (NJH) approved all study procedures. We used a modified intent-to-treat analysis, including data from all randomized participants, with least squares estimates used for participant dropouts and missing data. Participants signed informed consent statements prior to study procedures and were reimbursed for expenses and inconveniences. In the “long” protocol described below patients were paid $535 for attending all sessions, and in the “short” protocol $435, with proportionately less for missed sessions.
Participants
Following 516 telephone screenings, sixty-eight volunteers were randomized (Fig. 1). We recruited non-smoking (within the past year and < 10 pack-year history) adults (age 18–75) with physician-diagnosed mild or moderate persistent asthma for at least one year. Inclusion criteria involved lack of asthma control (a score of ≤ 19 on the Asthma Control Test (Nathan et al., 2004; Schatz et al., 2007; Schatz et al., 2006), or at least one of the following during a two week period prior to screening and again in a two-week period during a one-month asthma education run-in period that occurred prior to randomization: 1) rescue use of B2-agonist ≥ 2/week, or 2) waking due to asthma ≥ once/week.
Fig. 1.
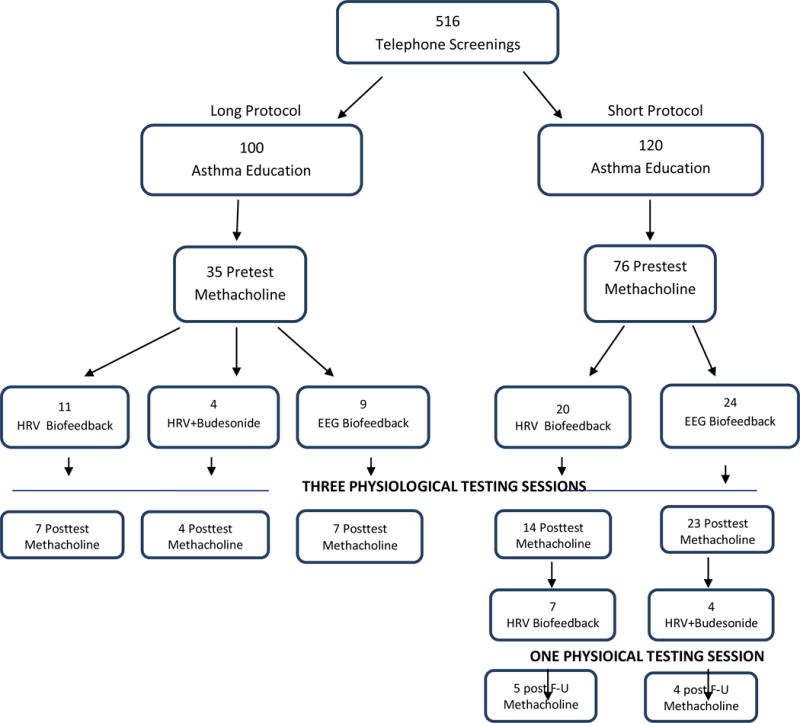
CONSORT DIAGRAM
Exclusions were: 1) inhaled steroid medication or other anti-inflammatory agents or any other medications affecting the respiratory or autonomic nervous systems within the past month, or anti-IgE medications within the past six months; 2) severe asthma (FEV1 < 60% predicted at screening, >1 course of oral steroids within the past three months, more than four night-time asthma awakenings in the previous two weeks or ≥ six puffs of rescue medication per day for more than six consecutive days in the past month, a history of non-responsiveness to B2-agonists, a history after age 12 or within the past 10 years of life-threatening asthma, or ≥ two inpatient asthma hospitalizations within the past year). MCTs were rescheduled if the forced experiatory volume in the first second from maximum vital capacity (FEV1) was < 60% expected on the day of testing, and study volunteers were excluded if this occurred repeatedly; 3) comorbid severe physical or mental diseases, 4) inability to read and write English or Spanish (training and assessments were done in both), 5) plans to leave the area within four months, or 6) inability or unwillingness to carry out study procedures. Withdrawal and abstention throughout study participation from certain other medications was required: 72 hours prior to screening for xanthines, long-acting anticholinergics, or long-acting β2-agonists, and 48 hours for short-acting anticholinergics.
PROCEDURE
Primary outcome measure: methacholine challenge test
MCTs were given before and after treatment. The pre-treatment MCT also was part of the screening procedure for study admission, as described below.
The procedure is summarized in Table 1. This was a three-phase study.
TABLE 1.
Session content
| Visit # | Visit Content | ||
|---|---|---|---|
|
| |||
| Long Protocol | Short Protocol | HRVB | EEG+ |
|
| |||
| 1 to 6 | 1 to 4 | Asthma education | Asthma Education |
| 7 | 6 | methacholine challenge test | methacholine challenge test |
| 8 | 7 | 6/min shallow breathing + PT | 15/min breathing + PT, music selection |
| 9 to 10 | 8 | determine resonance frequency | Relaxed breathing (RB) |
| 10 to 12 | 9 | Training in longer exhalation than inhalation, pursed lips breathing. diaphragmatic paced resonance frequency breathing, HRVB Introduce home trainer | RB, listen, in weekly sequence, to 1st, 2nd, and 3rd choice music, EEG alpha feedback introduce home trainer |
| 13 | 10 | Practice HRVB + PT | RB, relaxing music, EEG biofeedback, + PT |
| 14 to 16 | 11 | Practice HRVB (+ BUD n = 4 in long protocol) | RB, relaxing music, EEG biofeedback, |
| 17 | 12 | Practice HRVB + PT (+ BUD n = 4 in long protocol) | RB, relaxing music, EEG biofeedback, + PT |
| 18 | 13 | Methacholine challenge test | Methacholine challenge test |
| 14–15 | HRVB + BUD (short protocol) | ||
| 16 | BUD + Methacholine challenge test (short protocol) | ||
PT = psychophysiological test; RB = relaxed paced breathing; HRVB = heart rate variability biofeedback; EEG+ = treatment condition with EEG biofeedback, music, and RB BUD = Budesonide
Phase 1
The initial 4-weeks were devoted to asthma self-treatment education, consistent with recommendations of the National Asthma Education Program (Sheffer & Taggart, 1993), while baseline symptoms and pulmonary function were assessed. Participants were also trained to complete daily symptom questionnaires and to take twice daily peak flow measures, and were taught to follow a standard asthma action plan, including use of albuterol and, where necessary, oral steroids.
Phase 2. Biofeedback
Participants who successfully completed this phase were then randomized into two groups for 10 weeks of training in one of two biofeedback methods: HRVB, as described elsewhere (Lehrer, 2012; Lehrer et al., 2013), and EEG+ (Table 1) which included instructions to increase and decrease EEG alpha from the right frontal to occipital areas (F4 to Oz), to listen regularly to relaxing music (Lehrer et al., 1994), and to do paced breathing at the baseline rate of spontaneous breathing observed during a nondemanding task, as described below. Similar, although less detailed, EEG biofeedback and music conditions have been used in our previous research with no significant effects on asthma (Lehrer et al., 2004). The paced breathing control was used in order to provide a similar instructional procedure to HRVB without triggering the baroreflex, stretching the airways, or stimulating vagal activity. Participants in both conditions were given a personal home trainer device (StressEraser, Helicor, New York), and were instructed to practice their technique at home twice daily for 20 min. For the HRVB group the device provided instantaneous heart rate variability biofeedback. For the EEG+ group, the device had been altered so as only to give prompts for paced breathing at the individual’s baseline rate, rather than providing biofeedback. Although the control condition was designed to be moderately relaxing, our previous research had shown that relaxation instruction has minimal effects on asthma (Lehrer et al, 1994). For both groups, the device also recorded practice time for each practice session. This information was downloaded at each laboratory session. Spirometry was performed at the first training session, mid-training, and at the last training session. Participants were not specifically instructed to use the methods they had learned in order to ameliorate symptoms of an asthma exacerbation, since the primary purpose of the study was to examine the effects of HRVB as an asthma controller rather than as a rescue procedure.
An MCT was given after the asthma education run-in, prior to randomization, to assess subject eligibility and to serve as a baseline, and was repeated after the last treatment session. Participants also attended three testing sessions, one at the very beginning of treatment, within days of the pre-test MCT, one at mid-treatment, and one at the end, approximately three months after the first test session and a few days before the final MCT. MCT’s were spaced approximately three months apart. In each psychophysiological test session we measured physiological activity in response to three 5-min tasks: The first was a five-minute nondemanding ‘plain vanilla’ baseline task (Jennings, Kamarck, Stewart, Eddy, & Johnson, 1992) involving counting the number of squares of a specific color flashed on a computer screen for several seconds each. The second five-minute task consisted of practicing one of the study’s treatments (HRVB or EEG+). The third five-minute task was a repeat of the vanilla baseline task. Each psychophysiological test session consisted of events in the following order:
A spirometry assessment
Questionnaires
The three five-minute tasks, with assessment of forced oscillation variables immediately after each task, and 10 minutes of practicing the study intervention prior to the second five-minute assessment
A post-training spirometry assessment
Administration of two puffs of albuterol
After 15 minutes, a post-albuterol spirometry assessment
Phase 3. Budesonide comparison
A sub-sample of eight HRVB participants were given a four-week course of budesonide (720 mcg/day). In the long protocol this was given to four participants in the second half of treatment (after the mid-treatment assessment, described below), between the first and the second MCT. In the short protocol budesonide was given to four participants after the post-test MCT (Table 1), and a third MCT was given a month later. We did not power the study to prove the significance of the budesonide effects, but, since the effect of budesonide tends to be large and fairly stable (Jatakanon, Kharitonov, Lim, & Barnes, 1999; Jonasson, Carlsen, Jonasson, & Mowinckel, 2000), we rather used it to estimate the proportion of the steroid effect achievable by biofeedback. We calculated the percent of the MCT PC20FEV1 budesonide effect achieved by HRVB over the 10 weeks of treatment.
Short and long protocols
Twenty participants were randomized to the “long protocol”, which consisted of 10 weekly treatment sessions, including three combined testing and treatment sessions, at the beginning, middle, and end of treatment (Table 1). Because of excessive participant burden, we changed to a “short protocol” with fewer sessions over the same period of time: three asthma education sessions instead of four, six instead of ten treatment sessions including three with assessments at the beginning, middle, and end of treatment, with telephone contacts during nonvisit weeks to assess symptoms, gather data collected at home, and reinforce the need for regular practice of the treatment method. Forty-four participants were assigned to the short protocol.
Primary outcome measure: methacholine challenge tests (MCTs)
MCTs were given according to the standard American Thoracic Society protocol (Crapo et al., 2000), using a Rosenthal French dosimeter (Laboratory for Applied Immunology, Baltimore, MD). We presented successive doubling doses of methacholine from 0.31 mg/mL. Spirometry was conducted after each dose, and the test was terminated when FEV1 fell to or below 20% of the value after a placebo inhalation of saline. If this criterion was not reached after a dose of 25 mg/mL, the test was terminated at that point.
Secondary outcome measures
Exhaled nitric oxide (eNO)
eNO was measured at the beginning of each psychophysiological testing session using a (NIOX MINO®, Aerocrine, Morrisville, NC), following the standardized procedure recommended by Silkoff and colleagues (Silkoff et al., 1999).
Daily symptoms and peak flows
We collected a daily diary of asthma symptoms and PEFR data (from a Piko-1® electronic peak flow meter, Ferraris, Louisville, CO) (Fonseca et al., 2005), from the first asthma education session through the last psychophysiological test session. Participants took PEFR readings upon awakening and during the time period between 4–7 PM, before taking asthma medications that day or at least 2 hours following the last dose of albuterol, and during asthma symptoms. Accuracy of the PEF readings were checked regularly against electronic records from the spirometer as described by Irvin et al (Irvin, Martin, Chinchilli, Kunselman, & Cherniack, 1997). The participants were instructed to bring the meter to each laboratory visit, to report readings during scheduled telephone contacts, and to telephone the study’s asthma physician for readings <20% below “personal best.”
Rescue albuterol use
A Doser® (Meditrak, Inc., Hudson, MS) was used to monitor rescue use of albuterol. Prior to dispensing a Doser to participants, quality assurance testing was performed as recommended in the Doser manual. After Doser data were downloaded at each laboratory session, study participants were queried by staff about number of albuterol doses, and Doser data were compared with the participant’s report of albuterol use in their daily diaries. Since the Doser sometimes recorded false actuations when accidentally activated in a pocket, carry bag, or pocketbook, the staff and participant discussed all discrepancies between diary and Doser data to arrive at the most accurate possible number of canister actuations.
Questionnaires given during the three psychophysiological test sessions
At each test session we administered two questionnaires assessing asthma symptoms: 1)The Asthma Control Test (ACT), which has five items and a total score range of 1–25, with scores of 19 or less indicating poor asthma control (Nathan et al., 2004; Schatz et al., 2006) and 2) Juniper’s Asthma Quality of Life Questionnaire with Standardized Activities (AQOL), containing 32 items, each with a range of 1–7, assessing the extent to which asthma does not impair activities of daily life or produce unpleasant symptoms (Juniper, Buist, Cox, Ferrie, & King, 1999). Both scales are widely used in asthma research, and are accepted as stable through test-retest procedures and measures of internal reliability, and are accepted as valid by face validity and relationship to physiological and other symptom measures of asthma condition (Juniper, Guyatt, Ferrie, & Griffith, 1993; Nathan et al., 2004; Rowe & Oxman, 1993; Schatz et al., 2006).
Pulmonary assessment
We used a Jaeger Masterscreen unit (Hoechberg, Germany) for spirometry and impulse oscillometry to assess pulmonary function at the beginning of each session and at the end, just before and 15 minutes after taking two post-session puffs of albuterol. We used standard procedures for spirometry (Miller et al., 2005). Patients were coached to do three exhalations through a calibrated pneumotach with maximum force from total lung capacity. We measured FEV1) and FEV1/FVC (full vital capacity), with predicted values adjusted for age, sex, height, and race.
Airway impedance and compliance were assessed using the Jaeger Masterscreen device immediately after each of the 5-minute baseline vanilla tasks and 5-minute biofeedback task in the three psychophysiological test sessions. We instructed the participant to inhale to maximum vital capacity through a tube with a calibrated pneumotach. Because IOS assessments are sometimes variable, affected by glottal closure, tongue placement, etc., we took three 1-minute IOS assessments after each 5-minute task, and analyzed the minimum of the three values (indicating the ‘healthiest’ assessment). We report here measures of lower-airway obstruction characteristic of asthma (resistance at 5 Hz), upper airway obstruction characteristic of vocal cord tension (resistance at 35 Hz), a common site for emotional effects on the airways, and lung reactance using the area in the reactance graph between 5 Hz and resonance frequency of the lung (AX) (Beydon et al., 2007). Our previous research (Lehrer et al, 2004) had found this procedure more sensitive than spirometry in reflecting biofeedback-induced airways improvements.
Measures of treatment integrity
Heart rate variability and respiration rate
Heart rate variability and respiration rate were measured using a J&J Engineering C2+ system. Raw EKG data were collected and stored at approximately 1000 samples/sec from leads placed on the wrists, or, where necessary for better recording, on the right wrist and left leg. Respiration was recorded at approximately 20 samples/sec from a strain gauge attached around the waist at approximately the level of the xiphoid process. Data were edited manually to eliminate artifact and arrhythmic heartbeats using the WinCPRS program (Absolute Aliens Oy, Turku, Finland). This program yielded outputs of respiration rate and both low frequency (0.05 – 0.15 Hz) and high frequency (0.15 – 0.4 Hz). We analyzed these data for three tasks: 1) A five-minute pre-biofeedback ‘plain vanilla’ baseline task (Jennings et al., 1992) involving counting the number of squares of a specific color flashed on a computer screen for several seconds each; 2) five minutes of practicing one of the study’s treatments (HRVB or EEG+); and 3) a five-minute post-biofeedback vanilla baseline.
If participants were appropriately trained, then low frequency HRV should increase and high frequency decrease in the HRVB group during practice of biofeedback, while respiration rate was expected to drop to approximately six breaths/min (Lehrer, et al, 2003). In the EEG+ group respiration rate during biofeedback periods was expected to approximate that during baseline (vanilla task) periods, without large increases is low frequency HRV.
Additionally, in order to assess the relaxation experienced by participants during practice of their respective techniques, we administered the Smith R-States Inventory (SRSI) (Smith, 2001) for relaxation experiences after each of the 5-minute tasks. This is a well-validated factor-analytically derived scale assessing varieties of relaxation experiences. We expected both treatment conditions to have relaxing effects.
Treatment credibility
Treatment credibility was assessed at each test session using The Credibility/Expectancy Scale(Deviliya & Borkovec, 2000), a validated nine-point scale commonly used in other studies of behavioral interventions.
STATISTICAL ANALYSIS
Methacholine challenge tests
Airway reactivity in MCT’s was assessed as the dose of methacholine that produced a 20% decrease in FEV1 (PC20FEV1). Due to repeated measures and the longitudinal design of our study, we used the generalized estimating equation (GEE) regression analysis (McCulloch, Searle, & Neuhaus, 2001) to compare the changes in PC20FEV1 between HRVB and EEG+. We assessed the changes in PC20FEV1 using a log linear regression model, assuming that these measures followed a gamma distribution due to right-skewness. Treatment (HRVB vs. EEG+), sessions (Pre vs. Post MCT) and Treatments × Sessions interactions were included in the statistical model as independent variables. Correlations between repeated measurements of the same person were adjusted in the variance estimates using the robust sandwich approach (McCulloch et al., 2001). Patients with PC20FEV1 ≥ 25 mg/mL were scored as 25 in this analysis.
Measures taken during physiological test sessions
Mixed model analyses were used to study the changes in eNO, the ACT, and the AQOL scale. As fixed effects, we tested effects for Treatment (HRVB vs. EEG+), Sessions (pre-asthma education and the three physiological test sessions for the Asthma Control Test, and just the three physiological test sessions for eNO and the AQOL scale), and the Treatments × Sessions interaction. A random intercept was included to account for the correlation between repeated measurements of the same person. Where appropriate, normalizing transformations were used.
For heart rate variability, respiration rate, impulse oscillometry data, and the SRSI relaxation inventory, we used mixed models analyses including Treatment (HRVB vs. EEG+) as a between-groups measure, and both Sessions (first, middle, last), Tasks (pre-biofeedback vanilla, biofeedback, post- biofeedback vanilla) and the interactions of these factors (up to their three-way interaction). Correlations between repeatedly measured outcomes were modeled using two levels of random effects: one for person and the other for sessions within person. This structure assumes stronger within-person correlations between measurements in the same session than between sessions. Linear contrasts were made to compare the changes between treatments, between sessions, and between tasks, where applicable. Statistical significance was defined as p < 0.05. Analyses were carried out using Proc Genmod and Proc Mixed, as appropriate, in SAS version 9.4. Age, sex, height, and weight were entered as covariates for impulse oscillometry analyses.
Spirometry data were analyzed using two mixed models repeated measures analyses, with Treatment as a between-groups factor and either Pre-biofeedback/Post-biofeedback, or Post-biofeedback (also considered as Pre-albuterol)/Post-albuterol and Session (the three psychophysiological test sessions) as a repeated measure.
Measures taken daily
For episodes of poor asthma control (EPACs), home peak flows, symptom free days, and albuterol use, we analyzed data for three time periods: during the pre-intervention asthma education period, during the first half of the treatment period (between the first and second psychophysiological test sessions), and during the last half of the treatment period (between the second and third psychophysiological test sessions). Home practice time was analyzed as average minutes/day of practice from data recorded on the StressEraser during the first and last halves of the training period.
Episodes of poor asthma control (EPACs) were analyzed using a random effect logistic regression analysis. An EPAC was counted in the presence of any of the following: 1) occurrence of any symptoms: wheeze, cough, activity limitations, and sleep disturbance; 2) any albuterol use; 3) a single daily peak flow reading (AM, PM, or during symptoms) of ≤ 20% of the highest value during the measurement period (about a month); or 4) use of steroids. A score of 1 or 0 was given for each day in the study.
For albuterol use, we divided the number of puffs reported at each session by the number of days from the previous session, yielding a measure of average puffs per day. Because the modal number of puffs per day was 0, we used the GEE regression analysis for this variable as well. Data for each day were entered to the analysis, with the three Sessions, as described above, treated as a repeated measure, and Treatment (HRVB vs. EEG+) as a between-groups measure.
Protocol differences
For PC20FEV1 and the Asthma Control Test, we also separately examined differences between the two protocols (Short vs. Long) and two sites (Rutgers vs. National Jewish Health), by including one of these variables as factors in the regression analysis. We tested the main effects along with interactions of Site × Treatment Condition, and Site × Treatment Condition × Session. We also tested the main effect for Protocol (short vs. long), along with interactions of Protocol × Treatment Condition, and Protocol × Treatment Condition × Session.
RESULTS
As shown in the CONSORT diagram, a large proportion of volunteers failed screening (Fig. 1), primarily because of recent ICS use or smoking. Of 68 participants randomized to begin treatment, 55 remained in the study through the post-test methacholine challenge. Our dropout rate of 19% is approximately the same as our previous mind-body asthma studies (Lehrer et al., 1994; Lehrer, Hochron, McCann, Swartzman, & Reba, 1986; Lehrer et al., 2004). Participant characteristics are summarized in Table 2. There were no significant between-treatment-group pretest differences on any of the outcome measures.
Table 2.
Baseline characteristics of randomized participants
| Variable | Treatment groups | Total (n = 68) | |
|---|---|---|---|
| HRVB (n = 35) | EEG+ (n = 33) | ||
|
| |||
| Demographics | |||
| Age (years), median (Q1, Q3) | 38 (26,45) | 31 (26,48) | 34 (26,46) |
| Male, n (%) | 12 (34%) | 10 (30%) | 22 (32%) |
| Race n (%) | |||
| White | 17 (49%) | 21 (64%) | 38 (56%) |
| Black | 5 (14%) | 5 (15%) | 10 (14%) |
| Mixed | 5 (14%) | 6 (18%) | 11 (16%) |
| Asian | 4 (13%) | 1 (3%) | 5 (8%) |
| Undisclosed/Other | 4 (13%) | 0 (0%) | 4 (6%) |
| Ethnic Hispanic n (%) | 4 (13%) | 8 (24%) | 12 (19%) |
| BMI, median (Q1, Q3) | 28.53 (24.78,31.65) | 27.41 (22.32,31.57) | 28.27 (22.93,31.57) |
| Asthma characteristics | |||
| Self-report measures | |||
| Participant reported inhaled short-acting beta- agonist use ≥3 times/week, n (%) | 3 (10%) | 7 (21%) | 10 (16%) |
| Other self-report measures (Q1, Q3) | |||
| ACT score (max possible range: 5–25) | 20 (17.5,22.5) | 20 (16.5,23) | 20 (18,22) |
| ACT≤19, n(%) | 18 (51%) | 17 (52%) | 35 (51%) |
| AQOL (item mean, max possible range: 1–7) | 5.75 (4.97,6.41) | 5.88 (5.41,6.56) | 5.83 (5.25,6.47) |
| Physiological measures | |||
| Lung function, median (Q1, Q3) | |||
| PC20(mg/mL) | 1.66 (1.10,5.0) | 1.40 (0.51,3.35) | 1.46 (0.79, 5.0) |
| FEV1 (L) | 2.79 (2.20,3.19) | 3.01 (2.46,3.43) | 2.89 (2.24,3,36) |
| %-predicted FEV1* | 86.7 (68.7,97.9) | 84.1 (71.8,99.8) | 85.9 (71.0,98.0) |
| FVC (L) | 3.78 (3.19,4.47) | 4.11 (3.42,4.98) | 4.04 (3.31,4.78) |
| %-predicted FVC* | 102.2 (82.8,108.7) | 103.7 (90.0,109.8) | 103.0 (87.5,108.7) |
| Peak flow (L/sec) | 6.90 (5.75,7.90) | 7.66 (6.26,9.14) | 7.27 (6.01,8.42) |
| %-predicted peak flow* | 92.2 (80.7,99.9) | 92.6 (80.2,114.7) | 92.2 (80.7,107) |
| eNO (ppb), median (Q1, Q3) | 35.0 (20.0, 61.0) | 43.0 (84.0, 24.0) | 40.5 (21.0, 71.0) |
Predicted values of Hankenson.
Q1 = first quartile, Q3 = third quartile, ACT=Asthma Control Test, AQOL = Asthma Quality of Life, eNO = exhaled nitric oxide
Primary outcome variable: Methacholine challenge test results
Both groups improved significantly, with no significant differences between groups, and with a moderate effect size (Table 3). No differences were found between sites (RWJMS vs. NJH) or between the Short and Long protocols (Table 4). The biofeedback effect for HRVB was approximately 65% of the budesonide effect (Post - budesonide : Pre budesonide ratio = 3.524, 95% CI = 1.933–6.424).
Table 3.
Primary outcome measure: PC20FEV1 values from methacholine challenge tests
| HRV | EEG + | Between group difference | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Session | Mean | 95% CIL | 95% CIU | Mean | 95%CIL | 95%CIU | HRV/EEG+ Ratio |
95%CIL |
95%CIU |
|||
| Pre | 1.6368 | 0.9856 | 2.7184 | 2.0319 | 1.3531 | 3.0513 | 1.2413 | 0.648 | 2.3781 | |||
| Post | 4.8749 | 2.2857 | 10.397 | 4.3039 | 2.4783 | 7.4741 | 0.8829 | 0.3458 | 2.2537 | |||
|
| ||||||||||||
| Ratio | 95%CIL | 95% CIU | P | Ratio | 95%CIL | 95%CIU | p | Ratio | 95%CIL | 95%CIU | p | |
| Post/Pre | 2.9782 | 1.393 | 6.3675 | <0.005 | 2.1181 | 1.2133 | 3.6977 | <0.009 | 2.0364 | 1.3194 | 6.2015 | n.s. |
|
| ||||||||||||
| Cohen’s D | 0.47 | 0.41 | 0.17 | |||||||||
GEE regression analyses were computed using the SAS Genmod procedure, using a log linear regression model and a gamma distribution.
CIL and CIU are, respectively, lower and upper confidence limits.
Table 4.
Effect of Protocol and Site on PC20FEV1 and the Asthma Control Test
| FACTORS | df | ASTHMA CONTROL TEST | PC20FEV1 | ||
|---|---|---|---|---|---|
|
| |||||
| χ2 | P | χ2 | P | ||
| Protocol | 1 | 0 | n.s. | 0 | n.s. |
| Study condition * Protocol | 1 | 0.72 | n.s. | 0.72 | n.s. |
| Session * Protocol | 1 | 0.01 | n.s. | 0.01 | n.s. |
| Session * Study condition* Protocol | 1 | 0.35 | n.s. | 0.35 | n.s. |
|
| |||||
| Site | 1 | 0.01 | n.s. | 0.01 | n.s. |
| Session * Site | 1 | 2.24 | n.s. | 2.24 | n.s. |
| Study condition * Site | 1 | 2.3 | n.s. | 2.3 | n.s. |
| Session * Study condition * Site | 1 | 0.01 | n.s. | 0.01 | n.s. |
Secondary outcome measures: Symptoms (Table 5 and Fig. 2)
Table 5.
TREATMENT EFFECTS ON ASTHMA SYMPTOMS, eNO, HOME PEAK FLOWS, AND ALBUTEROL USE
| Statistic | Session | Session × Group | Session | Session × Group |
|---|---|---|---|---|
| Asthma Control Testa | Asthma Quality of Lifeb | |||
| F | 3.83 | 8.71 | 13.20 | 13.20 |
| d.f. | 3,100 | 2,69 | 2,137 | 2,137 |
| P | <.03 | <.0004 | <.0001 | <.0001 |
| EPACs | Symptom-free days | |||
| F | 13.20 | 3.47 | 3.47 | 0 |
| d.f. | 2,137 | 2,69 | 2,69 | 3,100 |
| P | <.0001 | <.04 | <.04 | n.s. |
| AM Peak Flow | PEF Variability (log) | |||
| F | 40.5 | 4.95 | 8.71 | 0.65 |
| d.f. | 2,96 | 2,96 | 2,69 | 2,69 |
| P | <0.0001 | <0.01 | <.0004 | n.s. |
| Log eNO | PRACTICE TIME | |||
| F | 3.47 | 0 | 0 | 24.78 |
| d.f. | 2,110 | 1,41 | 1,41 | 1,41 |
| P | <.04 | n.s. | n.s. | <.0001 |
| Albuterol Puffs/Dayc | ||||
| F | 14.07 | 5.51 | ||
| d.f. | 2,143 | 2,143 | ||
| P | <.0001 | <.005 | ||
Sessions refer to the three psychophysiological testing sessions.
An additional measure was taken at study entry for the Asthma Control Test.
For normalization, statistics on the AQOL were computed on the square of total score.
Albuterol puffs/day was analyzed using a random effects Poisson model, with number of observation days for each Session as an offset term.
Fig 2. Symptom measures.
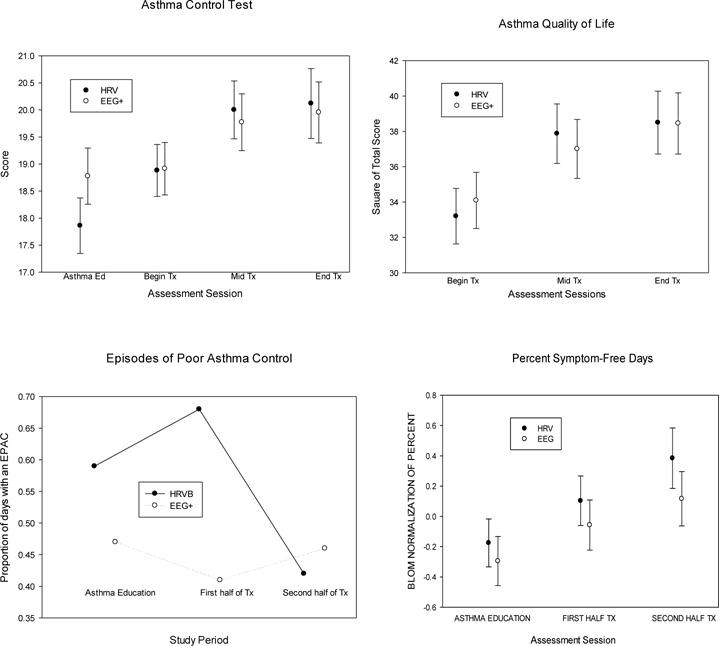
Error bars are standard errors. HRVB refers to heart rate variability biofeedback, EEG+ to the combination of EEG biofeedback, listening to relaxing music, and doing paced breathing at the individual’s resting rate, averaging about 15 breaths/min.
Asthma control as measured by the Asthma control Test improved significantly across sessions, averaged across groups, with a moderate to low effect size, d = 0.467. The Sessions × Treatment Conditions interaction is not significant, indicating no differences between groups across treatment sessions. There is no significant main or interaction effect involving Site or Protocol (Table 4). Mean improvement in the HRVB group from the first to last test session (M = 1.17) is 47% of the budesonide effect from the last pre-budesonide test session to the post-budesonide session (M = 2.50). Because the Asthma Control Test was also given prior to the asthma education run-in, we also could measure the average change after asthma education: (M = 1.58 in the HRVB group, which is larger than the subsequent HRVB effect and 63% of the budesonide effect).
Scores on the AQOL test improved significantly across sessions, but without any significant between-group differences, with medium to low effect sizes for both groups.
We found a significant decrease in number of EPACs from the asthma education to the late treatment period (between the second and third psychophysiological test sessions) across both groups. The effect is significant for the HRVB group but not for EEG+, although baseline levels were higher in the HRVB group, suggesting regression to the mean. There were no significant between-treatment-group differences for percent symptom free days, although, averaged across groups, it increased, with a significant main effect for Session, but a small effect size, d = 0.276.
Secondary outcomes: home peak flows (Table 5 and Fig 2)
AM peak flows improved significantly during the training averaged across groups. Peak flow variability, calculated as the standard deviation of AM, PM, and measures taken during asthma symptoms, decreased significantly during the training period averaged across groups. Effect sizes were small for changes between the asthma education period and the last half of treatment in both the HRVB group, d = .28, and the EEG+ group.
There was a significant decrease in albuterol use averaged across time periods and treatment groups, with a significantly greater decrease in the EEG+ group, from a higher initial baseline level. The end-of-treatment rate of use was approximately identical in the two groups d = .0.2.
Exhaled nitric oxide (eNO)
We found no significant between-group treatment effects for eNO across all sessions, with a trivial effect size, Cohen’s d = .178 for changes between the first and last assessment sessions (Fig. 3, Table 5). However, eNO decreased significantly from the first to last assessment sessions in the HRVB group, t(64) = 2.57, p < .02, d = .624 (medium to high effect size), but not in the EEG+ group, t(64) = 1.07, p = n.s., d = .238 (trivial effect size). There was a progressive decrease across sessions, averaged across treatment conditions, with a significant Session effect. Compared with the effect after budesonide, the change in the HRVB group from the first to last assessment session (mean log decrease = .249 log ppb) was 81% of that achieved in this group by a month of budesonide, compared with the last observation before exposure to the drug (mean log decrease after budesonide = .309 log ppb).
Fig 3. Lung function tests at home and during physiological test sessions.
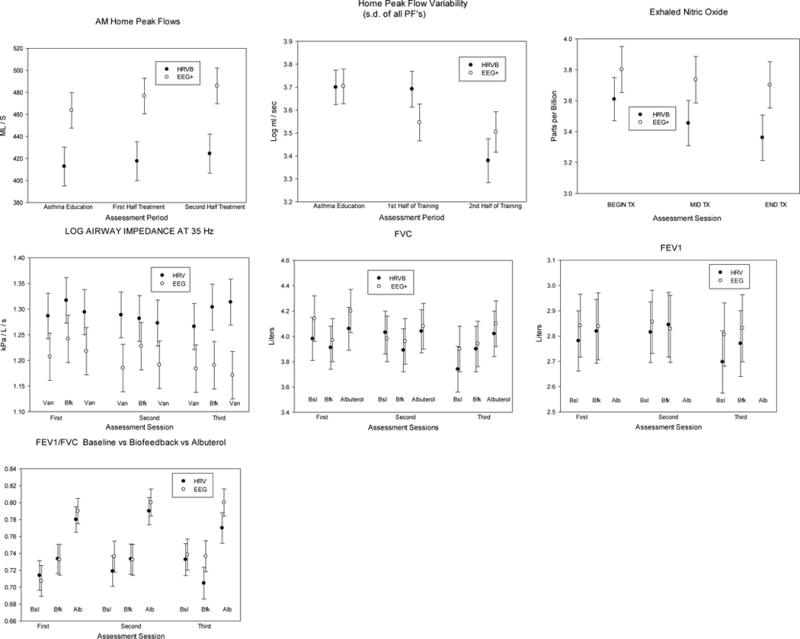
Error bars are standard errors. FVC refers to forced vital capacity, and FEV1 to the volume of air exhaled in the first second of a forced expiratory maneuver from maximum vial capacity.
Secondary outcome measure: spirometry (Table 6 and Fig. 4)
Table 6.
SPIROMETRY DURING psychophysiological test SESSIONS: MIXED MODELS MAIN EFFECTS AND INTERACTIONS
| Measure | Effect | SESSION | PREPOST BFK | PREPOST BFK × SESSION | GROUP × SESSION | GROUP × SESSION | GROUP × PREPOST BFK × SESSION | PREPOST ALBUTEROL |
|---|---|---|---|---|---|---|---|---|
| FEV1 | F | 2,92 | 0.97 | 0.35 | 0.98 | 1.2 | .01 | 312.63 |
| Df | 2,105 | 1,67 | 2,97 | 2,105 | 1,67 | 2,97 | 1,65 | |
| P | 0.06 | n.s. | n.s. | n.s. | n.s. | n.s. | <.0001 | |
| PEF | F | 0.98 | 1.06 | 1 | 0.86 | 0.75 | 0.51 | 173.36 |
| Df | 2,105 | 1,67 | 2,97 | 2,105 | 1,67 | 2,97 | 1,65 | |
| P | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | <.0001 | |
| FEV1/FVC | F | 1.82 | 0.16 | 4.04 | 1.17 | 0.01 | 1.77 | 175.33 |
| Df | 2,105 | 1,67 | 2,97 | 2,105 | 1,67 | 2,97 | 1,65 | |
| P | n.s. | n.s. | 0.03 | n.s. | n.s. | n.s. | <.0001 | |
| FVC | F | 3.9 | 0.09 | 7.13 | 0.94 | 2.18 | 1.46 | 48.38 |
| Df | 2,105 | 1,67 | 2,97 | 2,105 | 1,67 | 2,97 | 1,65 | |
| P | 0.03 | n.s. | 0.002 | n.s. | n.s. | n.s. | <.0001 |
Data are from mixed models repeated measures analyses. Sessions refers to the three psychophysiological testing sessions during the treatment protocol. “Prepost bfk” refers to spirometry conducted at the beginning of the session vs. after the post-biofeedback vanilla baseline task. “Prepost albuterol” refers to the post-biofeedback vanilla task vs. 15 minutes after a dose of albuterol.
Fig. 4. Measures of treatment integrity: Heart Rate Variability, Respiratory Frequency, Relaxation Experience and Practice Time.
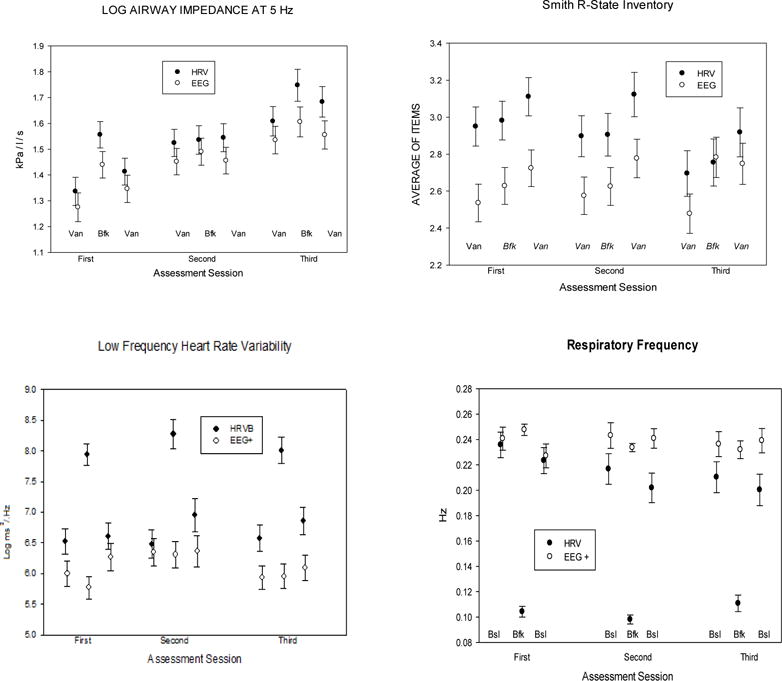
Error bars are standard errors.
There were few spirometry differences during biofeedback training in either group during psychophysiological test sessions. FEV1/FVC tended to increase during biofeedback in both treatment conditions, although it fell during the last session in the HRVB group. At the end of the psychophysiological testing sessions (after biofeedback and control periods) we found significant improvements after taking albuterol in all spirometry variables (FEV1, FVC, FEV1/FVC) between pre- and 15 minutes post-albuterol, p < .0001, with no differences between treatment groups or testing sessions.
Secondary outcome measures: forced oscillation (Table 7 and Fig 4.)
Table 7.
Forced Oscillation Pneumography, HRV, and Respiration Frequency During psychophysiological test sessions
| Assessment Period × Group | Task | Task × Group | ||
|---|---|---|---|---|
| AX | F(2,64) | 2.36 | 2.17 | 0.09 |
| p | n.s. | n.s. | n.s. | |
| R5 Hz | F(2,64) | 0.72 | 2.16 | 2.01 |
| p | n.s. | n.s. | n.s. | |
| R35 Hz | F(2,64) | 0.23 | 4.64 | 1.67 |
| p | n.s. | <.02 | n.s. | |
| Log low frequency HRV | F(2,64) | .57 | 34.78 | 46.74 |
| p | n.s. | <.0001 | <.0001 | |
| Log high frequency HRV | F(2,64) | .41 | 4.83 | 10.5 |
| p | n.s. | <.02 | <.0001 | |
| Log resp. frequency | F(2,64) | 1.76 | 59.42 | 58.07 |
| p | n.s. | <.0001 | <.0001 | |
| SRSI (Relaxation) | F(2,137) | 2.25 | 13.20 | 0.35 |
| p | n.s. | <.0001 | n.s. |
Note — Pulmonary values all are adjusted for age, gender, height, and weight
For AX and airway resistance at 5 Hz there are no significant main effects for tasks or sessions, and no significant interactions with treatment condition. For resistance at 35 Hz (reflecting upper airway, or vocal cord tension) a significant Task effect reflects a possible increase in upper airway obstruction during biofeedback across conditions.
Treatment integrity (Table 7 and Fig. 4)
Relaxation experiences were assessed from the normalized ranks (Blom noralization) of the total score of the SRSI. We found a significant effect for Task, but no interaction with Treatment Condition, and no effect involving Sessions. Participants reported increasing relaxation throughout each session, from the first vanilla task through the treatment task to the post-treatment vanilla task.
When done correctly HRV biofeedback produces very large increases in low frequency HRV (0.05–0.15 Hz) and decreases in high frequency HRV (0.15 – 0.4 Hz), while respiration rate slows to about six breaths/min (0.1 Hz). These conditions were met during biofeedback periods in the psychophysiological test sessions.
There were no differences between groups in treatment credibility. For the second and third psychophysiological test sessions, the main effect for Treatment Condition was F(1,40) = 2.73 and the Treatment Condition × Session effect was F(1,40) = 0.43. Credibility did not change significantly across the two assessment sessions, F(1,40) = 0.43. On the 9 point scale the modal score was 8.4, M = 7.5, s.d. = 1.3.
Treatment credibility
Both treatments were highly credible. Averaging across items, the modal rating was over 8 in a 9-point scale of credibility. There are no significant differences between treatments or across sessions.
DISCUSSION
Overall we found that HRVB cannot serve as an alternative to asthma controller medication, although some therapeutic effects occurred in both treatment conditions. Both biofeedback treatments decreased airway inflammation and sensitivity to methacholine with a moderate effect size that was close to 65% of the budesonide effect, and with a statistically significant decrease only for HRVB. This probably is not a Hawthorne effect, reflecting participation in a research study, because the pre-intervention assessment was taken after a fairly intensive asthma education program, during which many research procedures occurred. The increase of approximately one doubling dose of methacholine, or 65% of the budesonide effect, and an improvement in the ACT approximating 80% of the budesonide effect suggest that the role of HRVB in asthma therapy deserves further investigation. Nevertheless, this study produced little evidence for a specific HRVB effect on asthma. Both groups showed significant improvement for home peak flow readings, EPACs, and symptom-free days, with no between-groups differences, suggesting a nonspecific effect for biofeedback and/or paced breathing types of interventions, across the two treatment conditions. The biofeedback effects in this study are of similar size to the large placebo effects found on other literature on the Asthma Control Test and eNO (Smith et al., 2015) and PC20FEV1 (Kemeny et al., 2007).
Perhaps biofeedback may have something in common with placebo responses, but, because of their size, these effect should not be dismissed as trivial. Although across measures the effect size produced by both procedures is small to moderate, the effect on eNO, which may reflect inflammation, an important causative mechanism in asthma, is large, and specific to HRVB. Whether or not the mechanism is the specific physiological effects of HRVB or EEG+, it may be important to evaluate how to capitalize on these effects in treating asthma, particularly in view of the real or perceived adverse effects of validated pharmacological interventions and the large prevalence of nonadherence to medical asthma regimens. Indeed it may be useful to determine how biofeedback may be used to enhance effects of suggestion or other behavioral processes that may help asthma. Large therapeutic effects on asthma have been found for “enhanced” placebo presentations (Wise et al., 2009), greater than simple ones. Clinically significant suggestion effects on asthma also have been found throughout the literature for measures of pulmonary function in asthma (Isenberg, Lehrer, & Hochron, 1992). Might it not be useful, therefore, to use biofeedback as an enhanced method of suggestion, which may be more acceptable both to doctors and patients than giving a pill known to be pharmacologically ineffectives? We know that biofeedback has marked psychophysiological effects, and, as described below, may yet be found to have validated effects on asthma. Nevertheless, although both biofeedback procedures in this study produced a similar effect that may be of clinical significance, caution is warranted because the large albuterol effects after biofeedback sessions suggest that biofeedback did not normalize asthma control and hence leave patients at elevated risk for asthma exacerbations. This study found no evidence that biofeedback can safely substitute for either controller or reliever medicine.
More investigation is needed in order to understand why, apparently paradoxically, upper airway obstruction appeared in increase immediately after biofeedback periods in both groups, as reflected in forced oscillation resistance at 35 Hz (Hafez, Abu-Bakr, & Mohamed, 2015) This occurred despite the fact that both procedures were accompanied by relaxation experiences, and increased vocal cord tension often reflects emotional tension (Hoyte, 2013; Wamboldt & Balkissoon, 2008). Collateral data are needed perhaps from EMG and vocal quality measures to verify whether this indeed occurred. Note, however, that reported relaxation did not suddenly increase after the biofeedback procedures, but gradually increased over the course of the session, suggesting a slower effect over time.
The diminished effects of HRVB in this compared with previous studies of HRVB for asthma may be explained by a number of factors. Participants had less severe asthma than in previous studies, with 30% of participants having MCT PC20FEV1 > 8 mg/ml, the usual threshold for asthma, although they had other objective aspects of asthma such as a bronchodilator effect. Perhaps because we only recruited participants with relatively mild asthma, a floor effect occurred, below which improvement was unmeasurable. The asthma education run-in prior to randomization may have accentuated this floor effect as evidenced by a substantial improvement in asthma symptoms. Additionally, participants in this study were not encouraged to use their biofeedback training to avert symptoms during exacerbations, as had been done in our previous study, thus eliminating possible immediate effects of biofeedback on overall asthma control. Conceivably the modulation of acute asthma exacerbations in the previous study may have contributed to the more robust chronic effect in other studies. Also this study did not allow us to assess possible interactions between HRV biofeedback and ICS medications, which may have been a factor contributing to improvement in the previous study. It is possible that, through regular practice of deep breathing, the technique for inhaling medications may have improved, thus improving the medication effect. Finally, no previous studies used paced breathing as a component in a control condition. As our data show, this procedure also has beneficial effects on asthma, thus minimizing between-group differences in this study. It also is possible that the highly controlled nature of this study, conducted in two separate centers, gives a more generalizable assessment of HRVB’s effects on asthma: i.e., as less effective than previous research would suggest. However, in view of previous positive findings, further biofeedback research in asthma appears justified, e.g., as a complement to ICS, with more severe asthma, or an alternative or complement to rescue medication.
The relatively modest biofeedback effects in this study did not result from poor technique in training HRV biofeedback. The greatly increased low frequency HRV and slowed respiration rate in the HRVB group are identical to those from previous studies of this technique (Lehrer et al, 2003, 2004). Also both treatments were rated as highly credible, even though they were no more effective than a credible placebo.
In conclusion, HRVB should not be used as a substitute for inhaled steroid medication for treating asthma. Its possible effects on asthma symptoms, inflammation, and pulmonary function deserve further evaluation, either as a specific treatment or as an avenue for investigating clinically significant placebo effects. It may be useful as a complementary, but not alternative, treatment for asthma.
Acknowledgments
This research was funded by National Institutes of Health – National Heart Lung and Blood Institute grant #R01 HL089495, and at the National Jewish site was supported by NIH/NCATS Colorado CTSI Grant Number UL1 TR001082. Contents are the sole responsibility of the authors and do not necessarily represent official NIH views. The study was approved by the institutional review boards of Rutgers Robert Wood Johnson Medical School # 0220080228 and National Jewish Health # 17604.
ABBREVIATIONS
- ACT
Asthma Control Test
- AQOL
Juniper’s Asthma Quality of Life Questionnaire with Standardized Activities
- AX
a forced oscillation measure of lung reactance
- EEG+
the comparison condition, comprising EEG biofeedback, relaxing music, and relaxed paced breathing at ~ 15 breaths/min
- EPAC
episodes of poor asthma control
- eNO
Exhaled nitric oxide
- FEV1
quantity of air exhaled in the first second of a forced expiratory maneuver from maximum vital capacity
- FVC
total air exhaled in a forced expiratory maneuver from maximum vital capacity
- GEE
generalized estimating equation
- HRV
heart rate variability
- HRVB
heart rate variability biofeedback
- ICS
inhaled corticosteroids
- IOS
impulse oscillometry system
- LS mean
least square mean
- MCT
methacholine challenge test
- NJH
National Jewish Health
- PC20FEV1
the dose of methacholine at which a 20% decrease in FEV1 occurs
- PEF
peak expiratory flow during a forced airway maneuver
- RSA
Respiratory sinus arrhythmia
- RWJMS
Robert Wood Johnson Medical School
Footnotes
No author has a financial conflict of interest with material in this paper.
Clinical Trial Registration: NCT02766374
References
- Aboussafy D, Campbell TS, Lavoie K, Aboud FE, Ditto B. Airflow and autonomic responses to stress and relaxation in asthma: The impact of stressor type. International Journal of Psychophysiology. 2005;57(3):195–201. doi: 10.1016/j.ijpsycho.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Alving K, Weitzberg E, Lundberg JM. Increased amount of nitric oxide in exhaled air of asthmatics. European Respiratory Journal. 1993;6:1368–1370. [PubMed] [Google Scholar]
- Barnes PJ, Liew FY. Nitric oxide and asthmatic inflammation. Immunology Today. 1995;16(3):128–130. doi: 10.1016/0167-5699(95)80128-6. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufman PG, Malik M, van der Molen MW. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Beydon N, Davis SD, Lombardi E, Allen JL, Arets HG, Aurora P, Young Children Pulmonary Function, T An official American Thoracic Society/European Respiratory Society statement: pulmonary function testing in preschool children. American Journal of Respiratory & Critical Care Medicine. 2007;175(12):1304–1345. doi: 10.1164/rccm.200605-642ST. [DOI] [PubMed] [Google Scholar]
- Brusasco V, Crimi E. Methacholine provocation test for diagnosis of allergic respiratory diseases. Allergy. 2001;56(12):1114–1120. doi: 10.1034/j.1398-9995.2001.00148.x. [DOI] [PubMed] [Google Scholar]
- Brusasco V, Crimi E, Pellegrino R. Airway hyperresponsiveness in asthma: not just a matter of airway inflammation. Thorax. 1998;53(11):992–998. doi: 10.1136/thx.53.11.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, Sterk PJ. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. American Journal of Respiratory & Critical Care Medicine. 2000;161(1):309–329. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- Davis BE, Cockcroft DW. Past, present and future uses of methacholine testing. Expert Review of Respiratory Medicine. 2012;6(3):321–329. doi: 10.1586/ers.12.29. [DOI] [PubMed] [Google Scholar]
- Deviliya GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. Journal of Behavior Therapy & Experimental Psychiatry. 2000;31(2):73–86. doi: 10.1016/s0005-7916(00)00012-4. [DOI] [PubMed] [Google Scholar]
- Deykin A, Massaro AF, Drazen JM, Israel E. Exhaled nitric oxide as a diagnostic test for asthma: online versus offline techniques and effect of flow rate. American Journal of Respiratory & Critical Care Medicine. 2002;165(12):1597–1601. doi: 10.1164/rccm.2201081. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, Sleight P. Human baroreflexes in health and disease. Gloustershire, U.K.: Clarendon Press; 1992. [Google Scholar]
- Feldman JM, Lehrer PM, Hochron SM, Schwartz GE. Defensiveness and individual response stereotypy in asthma. Psychosomatic Medicine. 2002;64(2):294–301. doi: 10.1097/00006842-200203000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca JA, Costa-Pereira A, Delgado L, Silva LN, Magalhaes M, Castel-Branco MG, Vaz M. Pulmonary function electronic monitoring devices: a randomized agreement study. Chest. 2005;128(3):1258–1265. doi: 10.1378/chest.128.3.1258. [DOI] [PubMed] [Google Scholar]
- Freitas DA, Holloway EA, Bruno SS, Chaves GS, Fregonezi GA, Mendonca KP. Breathing exercises for adults with asthma. Cochrane Database of Systematic Reviews. 2013;(10):CD001277. doi: 10.1002/14651858.CD001277.pub3. [DOI] [PubMed] [Google Scholar]
- Grrishma B, Gaur GS, Velkumary S, Subramanian SK, Gurunandan U. Assessment of Cardiovascular Autonomic Functions and Baroreceptor Reactivity in Women with Premenstrual Syndrome. Indian Journal of Physiology & Pharmacology. 2015;59(2):148–154. [PubMed] [Google Scholar]
- Guo Z, Wang Y, Xing G, Wang X. Diagnostic accuracy of fractional exhaled nitric oxide in asthma: a systematic review and meta-analysis of prospective studies. Journal of Asthma. 2016;53(4):404–412. doi: 10.3109/02770903.2015.1101132. [DOI] [PubMed] [Google Scholar]
- Hafez MR, Abu-Bakr SM, Mohamed AA. Forced oscillometry track sites of airway obstruction in bronchial asthma. Annals of Allergy, Asthma, & Immunology. 2015;115(1):28–32. doi: 10.1016/j.anai.2015.04.017. [DOI] [PubMed] [Google Scholar]
- Hewitt DJ. Interpretation of the “positive” methacholine challenge. American Journal of Industrial Medicine. 2008;51(10):769–781. doi: 10.1002/ajim.20631. [DOI] [PubMed] [Google Scholar]
- Hoyte FCL. Vocal cord dysfunction. Immunology and Allergy Clinics of North America. 2013;33(1):1–22. doi: 10.1016/j.iac.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Irvin CG, Martin RJ, Chinchilli VM, Kunselman SJ, Cherniack RM. Quality control of peak flow meters for multicenter clinical trials. The Asthma Clinical Research Network (ACRN) American Journal of Respiratory & Critical Care Medicine. 1997;156(2 Pt 1):396–402. doi: 10.1164/ajrccm.156.2.9609054. [DOI] [PubMed] [Google Scholar]
- Isenberg SA, Lehrer PM, Hochron S. The effects of suggestion and emotional arousal on pulmonary function in asthma: a review and a hypothesis regarding vagal mediation. Psychosomatic Medicine. 1992;54(2):192–216. doi: 10.1097/00006842-199203000-00006. [DOI] [PubMed] [Google Scholar]
- James A, Ryan G. Testing airway responsiveness using inhaled methacholine or histamine. Respirology. 1997;2(2):97–105. doi: 10.1111/j.1440-1843.1997.tb00061.x. [DOI] [PubMed] [Google Scholar]
- Jatakanon A, Kharitonov S, Lim S, Barnes PJ. Effect of differing doses of inhaled budesonide on markers of airway inflammation in patients with mild asthma. Thorax. 1999;54(2):108–114. doi: 10.1136/thx.54.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JR, Kamarck T, Stewart C, Eddy M, Johnson P. Alternate cardiovascular baseline assessment techniques: vanilla or resting baseline. Psychophysiology. 1992;29(6):742–750. doi: 10.1111/j.1469-8986.1992.tb02052.x. [DOI] [PubMed] [Google Scholar]
- Jonasson G, Carlsen KH, Jonasson C, Mowinckel P. Low-dose inhaled budesonide once or twice daily for 27 months in children with mild asthma. Allergy. 2000;55(8):740–748. doi: 10.1034/j.1398-9995.2000.00661.x. [DOI] [PubMed] [Google Scholar]
- Juniper EF, Buist AS, Cox FM, Ferrie PJ, King DR. Validation of a standardized version of the Asthma Quality of Life Questionnaire. Chest. 1999;115:1265–1270. doi: 10.1378/chest.115.5.1265. [DOI] [PubMed] [Google Scholar]
- Juniper EF, Guyatt GH, Ferrie PJ, Griffith LE. Measuring quality of life in asthma. American Review of Respiratory Disease. 1993;147(4):832–838. doi: 10.1164/ajrccm/147.4.832. [DOI] [PubMed] [Google Scholar]
- Kemeny ME, Rosenwasser LJ, Panettieri RA, Rose RM, Berg-Smith SM, Kline JN. Placebo response in asthma: a robust and objective phenomenon. Journal of Allergy & Clinical Immunology. 2007;119(6):1375–1381. doi: 10.1016/j.jaci.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Langmack EL. Complimentary and alternative therapies for asthma. In: Szefler SJ, Leung DYM, editors. Severe Asthma, Pathogenesis and Clinical Management. 2nd. New York: Marcel Dekker; 2001. pp. 221–238. [Google Scholar]
- Lehrer PM. Biofeedback therapy for asthma. In: Anbar R, editor. Functional Respiratory Disorders. New York: Springer Science and Business; 2012. [Google Scholar]
- Lehrer PM, Hochron SM, Mayne T, Isenberg S, Carlson V, Lasoski AM, Rausch L. Relaxation and music therapies for asthma among patients prestabilized on asthma medication. Journal of Behavioral Medicine. 1994;17(1):1–24. doi: 10.1007/BF01856879. [DOI] [PubMed] [Google Scholar]
- Lehrer PM, Hochron SM, Mayne T, Isenberg S, Lasoski AM, Carlson V, Porges S. Relationship between changes in EMG and respiratory sinus arrhythmia in a study of relaxation therapy for asthma. Applied Psychophysiology and Biofeedback. 1997;22(3):183–191. doi: 10.1023/a:1026263826106. [DOI] [PubMed] [Google Scholar]
- Lehrer PM, Hochron SM, McCann B, Swartzman L, Reba P. Relaxation decreases large-airway but not small-airway asthma. Journal of Psychosomatic Research. 1986;30(1):13–25. doi: 10.1016/0022-3999(86)90061-9. [DOI] [PubMed] [Google Scholar]
- Lehrer PM, Vaschillo B, Zucker T, Graves J, Katsamanis M, Aviles M, Wamboldt FS. Protocol for heart rate variability biofeedback training. Biofeedback. 2013;41(3):98–109. [Google Scholar]
- Lehrer PM, Vaschillo EG, Vaschillo B, Lu SE, Eckberg DL, Edelberg R, Hamer RM. Heart rate variability biofeedback increases baroreflex gain and peak expiratory flow. Psychosomatic Medicine. 2003;65(5):796–805. doi: 10.1097/01.psy.0000089200.81962.19. [DOI] [PubMed] [Google Scholar]
- Lehrer PM, Vaschillo EG, Vaschillo B, Lu SE, Scardella A, Siddique M, Habib RH. Biofeedback treatment for asthma. Chest. 2004;126(2):352–361. doi: 10.1378/chest.126.2.352. [DOI] [PubMed] [Google Scholar]
- Malmberg LP, Pelkonen AS, Haahtela T, Turpeinen M. Exhaled nitric oxide rather than lung function distinguishes preschool children with probable asthma. Thorax. 2003;58:494–499. doi: 10.1136/thorax.58.6.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch CE, Searle SR, Neuhaus JM. Generalized, linear, and mixed models. New York: John Wiley & Sons, Inc; 2001. [Google Scholar]
- Milgrom H, Bender BG, Ackerson L, Bowry P, Smith B, Rand C. Noncompliance and treatment failure in children with asthma. Journal of Allergy and Clinical Immunology. 1996;98:1051–1057. doi: 10.1016/s0091-6749(96)80190-4. [DOI] [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Force AET. Standardisation of spirometry. European Respiratory Journal. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, Pendergraft TB. Development of the asthma control test: a survey for assessing asthma control. Journal of Allergy & Clinical Immunology. 2004;113(1):59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- National Heart Lung and Blood Institute (NHLBI) Expert panel report 3 : Guidelines for the diagnosis and management of asthma: Full report. Washington, DC: National Asthma Education and Prevention Program, Department of Health and Human Services; 2007. [Google Scholar]
- Pavlov VA, Tracey KJ. Controlling inflammation: the cholinergic anti-inflammatory pathway. Biochemical Society Transactions. 2006;34(Pt 6):1037–1040. doi: 10.1042/BST0341037. [DOI] [PubMed] [Google Scholar]
- Porges SW. Orienting in a defensive world: mammalian modifications of our evolutionary heritage. A Polyvagal Theory. Psychophysiology. 1995;32:301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: phylogenetic substrates of a social nervous system. International Journal of Psychophysiology. 2001;42:123–146. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74(2):116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe BH, Oxman AD. Performance of an asthma quality of life questionnaire in an outpatient setting. American Review of Respiratory Disease. 1993;148(3):675–681. doi: 10.1164/ajrccm/148.3.675. [DOI] [PubMed] [Google Scholar]
- Schatz M, Mosen DM, Kosinski M, Vollmer WM, Magid DJ, O’Connor E, Zeiger RS. Validity of the Asthma Control Test completed at home. American Journal of Managed Care. 2007;13(12):661–667. [PubMed] [Google Scholar]
- Schatz M, Sorkness CA, Li JT, Marcus P, Murray JJ, Nathan RA, Jhingran P. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. Journal of Allergy & Clinical Immunology. 2006;117(3):549–556. doi: 10.1016/j.jaci.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Sheffer AL, Taggart VS. The National Asthma Education Program. Expert panel report guidelines for the diagnosis and management of asthma. National Heart, Lung, and Blood Institute. Medical Care. 1993;31(3 Suppl):MS20–28. [PubMed] [Google Scholar]
- Silkoff PE, Stevens A, Pak J, Bucher-Bartelson B, Martin RJ. A method for the standardized offline collection of exhaled nitric oxide. Chest. 1999;116(3):754–759. doi: 10.1378/chest.116.3.754. [DOI] [PubMed] [Google Scholar]
- Smith JC. Advances in ABC Relaxation: Applications and inventories. New York, NY: Springer Publishing Co; US; 2001. Advances in ABC Relaxation Theory: The 14 + 1 map; pp. 3–32. [Google Scholar]
- Smith LJ, Kalhan R, Wise RA, Sugar EA, Lima JJ, Irvin CG, American Lung Association Asthma Clinical Research, C. Effect of a soy isoflavone supplement on lung function and clinical outcomes in patients with poorly controlled asthma: a randomized clinical trial. JAMA. 2015;313(20):2033–2043. doi: 10.1001/jama.2015.5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song HS, Lehrer PM. The effects of specific respiratory rates on heart rate and heart rate variability. Applied Psychophysiology & Biofeedback. 2003;28(1):13–23. doi: 10.1023/a:1022312815649. [DOI] [PubMed] [Google Scholar]
- Strunk RC, Ford JG, Taggart V. Reducing disparities in asthma care: Priorities for research–National Heart, Lung, and Blood Institute workshop report. Journal of Allergy and Clinical Immunology. 2002;109:229–237. doi: 10.1067/mai.2002.120950. [DOI] [PubMed] [Google Scholar]
- Van Buren T, Kasbergen CM, Gispen WH, De Wildt DJ. In vivo cardiovascular reactivity and baroreflex activity in diabetic rats. Cardiovascular Research. 1998;38(3):763–771. doi: 10.1016/s0008-6363(98)00038-8. [DOI] [PubMed] [Google Scholar]
- Vaschillo EG, Lehrer PM, Rishe N, Konstantinov M. Heart rate variability biofeedback as a method for assessing baroreflex function: a preliminary study of resonance in the cardiovascular system. Applied Psychophysiology & Biofeedback. 2002;27(1):1–27. doi: 10.1023/a:1014587304314. [DOI] [PubMed] [Google Scholar]
- Vaschillo EG, Vaschillo B, Lehrer PM. Characteristics of resonance in heart rate variability stimulated by biofeedback. Applied Psychophysiology & Biofeedback. 2006;31(2):129–142. doi: 10.1007/s10484-006-9009-3. [DOI] [PubMed] [Google Scholar]
- Wamboldt FS, Balkissoon RC. Vocal cord dysfunction. In: Castro M, Kraft M, editors. Clinical asthma. Philadelphia: Elsevier; 2008. pp. 345–350. [Google Scholar]
- Williams LK, Pladevall M, Xi H, Peterson EL, Joseph C, Lafata JE, Ownby DR, Johnson CC. Relationship between adherence to inhaled corticosteroids and poor outcomes among adults with asthma. Journal of Allergy and Clinical Immunology. 2004;114:1288–1293. doi: 10.1016/j.jaci.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bartlett SJ, Brown ED, Castro M, Cohen R, Holbrook JT, American Lung Association Asthma Clinical Research, C. Randomized trial of the effect of drug presentation on asthma outcomes: the American Lung Association Asthma Clinical Research Centers. Journal of Allergy & Clinical Immunology. 2009;124(3):436–444. 444e431–438. doi: 10.1016/j.jaci.2009.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Adherence to long-term therapies: evidence for action 2003 [Google Scholar]


