Abstract
Candidate drugs to counter intracellular polymerization of deoxygenated sickle hemoglobin (Hb S) continue to represent a promising approach to mitigating the primary cause of the pathophysiology associated with sickle cell disease (SCD). One such compound is the naturally occurring antisickling agent, 5-hydroxymethyl-2-furfural (5-HMF), which has been studied in the clinic for the treatment of SCD. As part of our efforts to develop novel efficacious drugs with improved pharmacologic properties, we structurally modified 5-HMF into 12 ether and ester derivatives. The choice of 5-HMF as a pharmacophore was influenced by a combination of its demonstrated attractive hemoglobin modifying and antisickling properties, well-known safety profiles, and its reported nontoxic major metabolites. The derivatives were investigated for their time- and/or dose-dependent effects on important antisickling parameters, such as modification of hemoglobin, corresponding changes in oxygen affinity, and inhibition of red blood cell sickling. The novel test compounds bound and modified Hb and concomitantly increased the protein affinity for oxygen. Five of the derivatives exhibited 1.5- to 4.0-fold higher antisickling effects than 5-HMF. The binding mode of the compounds with Hb was confirmed by X-ray crystallography and, in part, helps explain their observed biochemical properties. Our findings, in addition to the potential therapeutic application, provide valuable insights and potential guidance for further modifications of these (and similar) compounds to enhance their pharmacologic properties.
Keywords: hemoglobin, sickle cell disease, aromatic aldehydes, furfural, polymerization, antisickling, oxygen affinity, adduct formation, crystal structure
Graphical abstract
INTRODUCTION
Sickle cell disease (SCD) is caused by a single point mutation of βGlu6 in normal hemoglobin (Hb) to βVal6 in sickle Hb (Hb S).1,2 The primary pathophysiology of the disease involves polymerization of Hb S into insoluble fibers under low oxygen (O2) saturation, which is exacerbated by the inherent low affinity of sickle red blood cells (RBCs) for oxygen, presumably due to high concentrations of 2,3-diphosphoglycerate and/or sphingosine phosphate in sickle RBCs.3–6 The insoluble Hb S fibers then cause the RBCs to form rigid sickled cells that occlude small blood vessels, triggering several secondary pathological consequences, such as painful crises, organ damage, oxidative stress, hemolysis, impaired microvascular blood flow, morbidity, and mortality.7–10
Aromatic aldehydes can allosterically modify Hb to the high-O2-affinity form.6,11,12 Consequently, several candidates, such as vanillin and its derivatives (INN compounds, Tucaresol, and Valeresol), 5-HMF, and most recently, GBT-440 have been established to increase the oxygen affinity of Hb S to counter polymerization, thereby providing a potential therapeutic intervention for SCD.6,11–19 Vanillin, Tucaresol, Valeresol, and 5-HMF have undergone phase I and/or II studies,6,16,20–24 while GBT-440 is currently undergoing a phase III clinical studies for the treatment of SCD (clinicaltrials.gov, NCT03036813), suggesting a viable route to treat this disease with aromatic aldehydes. The pharmacologic effect of these compounds primarily involves formation of a Schiff-base interaction between the aldehyde moiety of the compound and the N-terminal amine of αVal1 of the liganded Hb and, along with other protein interactions, stabilizes the R-state Hb in the R2 conformation, thus increasing Hb affinity for oxygen.6,11–15 Consistently, the synthetic derivatives, such as Tucaresol and GBT-440, with apparent increased protein interactions have demonstrated both enhanced potency and improved pharmacokinetic properties than the parent vanillin.13,16,17 Unlike the above-mentioned aromatic aldehydes, there are other classes of aromatic aldehydes that bind Hb and preferentially stabilize the T-state relative to the R-state resulting in a decrease in Hb affinity for oxygen.11,12,25,26 These compounds as expected promote sickling.
5-HMF undergoes extensive oxidative metabolism of the aldehyde moiety resulting in a short plasma half-life (~1 h) and low bioavailability that significantly reduces its pharmacologic effect.14,20,21,27,28 This observation, as well as the relatively low potency of 5-HMF, which would necessitate high doses, likely contributed to the termination of the phase II clinical trials. Based on our previous success in derivatizing the antisickling lead vanillin,13,29 we sought to derivatize the nontoxic 5-HMF, a superior and more promising antisickling lead than vanillin, to increase its interactions with the protein, which we envisaged would enhance or improve its potency and metabolic profiles. Using the crystallographic binding mode of 5-HMF to liganded Hb as a guide, we synthesized or obtained 12 derivatives of 5-HMF that incorporate different substituents at the alcohol moiety for potential additional interactions with the protein. Subsequently, we investigated the compounds in vitro for their time- and/or concentration-dependent pharmacodynamic/metabolic profiles, using the following parameters: Hb binding/modification properties; allosteric effects; and antisickling activities, using normal (AA) or sickle (SS) blood. One representative compound was also studied for its atomic interactions with Hb using X-ray crystallography.
EXPERIMENTAL SECTION
Materials and General Procedure
Normal whole blood was obtained from healthy adult donors at the Virginia Commonwealth University after informed consent, in accordance with regulations of the IRB for Protection of Human Subjects. Hb was purified from discarded normal blood samples using standard procedures.30 Leftover blood samples from patients with homozygous SS blood were obtained and utilized, based on an approved IRB protocol at the Children’s Hospital of Philadelphia, with informed consent.
All other reagents used in the syntheses and functional assays were purchased from Sigma-Aldrich (St. Louis, MO) and ThermoFisher Scientific (Waltham, MA) and utilized without additional purification. 5-(Phenoxymethyl)-2-furan carbaldehyde (5-PMFC), 5-((2-nitrophenoxy)methyl)-2-furan carbaldehyde (5-NMFC), and 5-((4-chlorophenoxy)methyl)-2-furan carbaldehyde (5-CMFC) were obtained from Aldrich and used without further purification. 1H NMR and 13C NMR spectra were obtained on a Bruker 400 MHz spectrometer and tetramethylsilane (TMS) was used as an internal standard. Peak positions are given in parts per million (δ). Column chromatography was performed on silica gel (grade 60 mesh; Bodman Industries, Aston, PA). Routine thin-layer chromatography (TLC) was performed on silica gel GHIF plates (250 µm, 2.5 × 10 cm; Analtech Inc., Newark, DE). MS spectra were obtained from a PerkinElmer Flexar UHLPC with AxION 2 Time of Flight (TOF) Mass Spectrometer, and the molecular weight of the compounds was within 0.05% of calculated values. Infrared spectra were obtained on a Thermo Nicolet iS10 FTIR. Purity of the compounds was determined by HPLC using a Varian Microsorb 100-5 C18 column (250 × 4.6 mm) and a Prostar 325 UV–vis (254 nm) as the detector.
UPLC–MS data were obtained using Waters Acquity H-Class UPLC coupled to a TQD ESI-MS detector and a PDA detector.
Synthesis of Compounds
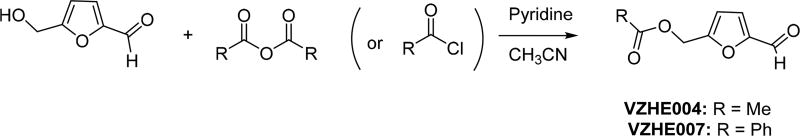
(5-Formylfuran-2-yl)methyl Acetate (VZHE004)
The synthesis of VZHE004 was carried out as per a previously reported procedure.31 5-HMF (63 mg, 0.5 mmol) and acetic anhydride (102 mg, 1 mmol) were dissolved in 2 mL of acetonitrile, and the mixture was cooled to 0 °C. Pyridine (79 mg, 1 mmol) was added slowly. The resultant reaction mixture was stirred at 0 °C for 30 min and then at r.t. for 15 h. The reaction mixture was diluted with EtOAc (15 mL) and then washed with 0.1 N HCl (5 mL × 3), 10% NaHCO3 (5 mL × 2), and brine (5 mL). The organic layer was then dried over Na2SO4. After filtration and concentration, the resultant crude oil was purified with silica gel column chromatography and eluted with the solvent system of EtOAc/hexanes = 1:4 to give 52 mg of the product as a colorless oil, and the yield was 62%. 1H NMR (CDCl3): δ 9.64 (s, 1H), 7.20 (d, J = 3.52 Hz, 1H), 6.58 (d, J = 3.56 Hz, 1H), 5.12 (s, 2H), 2.11 (s, 3H). 13C NMR (CDCl3): δ 177.92, 170.41, 155.61, 153.08, 121.58, 112.66, 57.96, 20.80.
tert-Butyl((5-formylfuran-2-yl)methyl) Carbonate (VZHE006)
5-HMF (1.26 g, 10 mmol) and ditert-butyl dicarbonate (Boc2O) (4.36 g, 20 mmol) were dissolved in 40 mL of CH2Cl2, and the mixture was cooled to 0 °C. Pyridine (1.58 g, 20 mmol) was added slowly. The resultant reaction mixture was stirred at 0 °C for 30 min and then at r.t. for 15 h. The reaction mixture was diluted with EtOAc (150 mL) and then washed with 0.1 N HCl (50 mL × 3), 10% NaHCO3 (50 mL × 2), and brine (50 mL). The organic layer was then dried over Na2SO4. After filtration and concentration, the resultant crude oil was purified with silica gel column chromatography and eluted with the solvent system of EtOAc/hexanes = 1:6 to give 1.8 g of the product as a colorless oil, and the yield was 80%. IR (cm−1): 2984.4, 1740.9, 1678.7, 1524.8, 1369.4, 1272.5, 1250.6, 1153.2, 1085.9, 854.1, 812.1, 791.8, 766.4. 753.5. 1H NMR (CDCl3): δ 9.65 (s, 1H), 7.20 (d, J = 3.56 Hz, 1H), 6.61 (d, J = 3.52 Hz, 1H), 5.11 (s, 2H), 1.50 (s, 9H). 13C NMR (CDCl3): δ 177.89, 155.15, 153.00, 152.88, 121.14, 112.57, 83.21, 60.19, 27.73. MS: [M + Na]+ calcd 249.0739; found 249.0762.
(5-Formylfuran-2-yl)methyl Benzoate (VZHE007)
5-HMF (122 mg, 1 mmol) and benzoyl chloride (280 mg, 2 mmol) were dissolved in 4 mL of CH2Cl2. The mixture was cooled to 0 °C and triethylamine (202 g, 2 mmol) was added slowly. The resultant reaction mixture was stirred at 0 °C for 30 min and then at r.t. for 15 h. After filtration, the reaction mixture was diluted with EtOAc (15 mL) and then washed with 0.1 N HCl (5 mL × 3), 10% NaHCO3 (5 mL × 2), and brine (5 mL). The organic layer was then dried over Na2SO4. After filtration and concentration, the resultant crude oil was purified with silica gel column chromatography and eluted with the solvent system of EtOAc/hexanes = 1:8 to give 200 mg of the product as a white solid, and the yield was 90%. M.p.: 50–51 °C. IR (cm−1): 3122.5, 2852.2, 1708.7, 1671.1, 1253.2, 812.6, 773.2, 701.4. 1H NMR (CDCl3): δ 9.66 (s, 1H), 8.05 (m, 2H), 7.58 (t, J = 7.54 Hz, 1H), 7.45 (t, J = 7.76 Hz, 2H), 7.23 (d, J = 3.52 Hz, 1H), 6.68 (d, J = 3.52 Hz, 1H), 5.42 (s, 2H). 13C NMR (CDCl3): δ 177.83, 165.94, 155.56, 152.97, 133.44, 129.85, 129.35, 128.49, 121.56, 112.73, 58.25. MS: [M + Na]+ calcd 253.0477; found 253.0554.
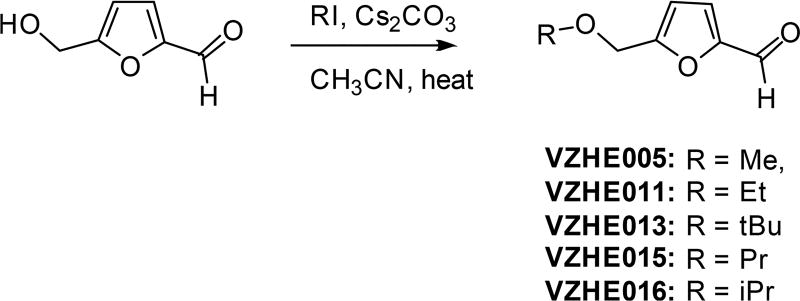
5-(Methoxymethyl)-2-furan Carboxadehyde (VZHE005)
The synthesis of VZHE005 was carried out as per a previously reported procedure.32 5-HMF (126 mg, 1.0 mmol) was dissolved in acetonitrile (5 mL), and MeI (426 mg, 3.0 mmol) was added and followed by Cs2CO3 (488 mg, 1.5 mmol). The reaction mixture was heated at 50 °C for 2 days. The reaction mixture was then filtered, concentrated, and purified with silica gel chromatography with EtOAc/hexanes = 1:8 as the eluent. The product was obtained as a light yellow oil, 67 mg, yield of 48%. HPLC purity: 98.6% (retention time: 3.72 min; 40% H2O and 60% MeCN over 30 min at 254 nm on Varian C18 column). 1H NMR (CDCl3): δ 9.63 (s, 1H), 7.21 (d, J = 3.48 Hz, 1H), 6.53 (d, J = 3.52 Hz, 1H), 4.49 (s, 2H), 3.43 (s, 3H). 13C NMR (CDCl3): δ 177.85, 158.47, 152.87, 121.76, 111.26, 66.76, 58.85.
5-(Ethoxymethyl)-2-furan Carboxadehyde (VZHE011)
The synthesis of VZHE011 was carried out as per previously reported procedure.32 5-HMF (122 mg, 1.0 mmol) was dissolved in acetonitrile (5 mL), and EtI (468, 3.0 mmol) was added and followed by Cs2CO3 (488, 1.5 mmol). The reaction mixture was heated at 50 °C for 2 days. The reaction mixture was then filtered, concentrated and purified with silica gel chromatography with EtOAc: Hexanes = 1:8 as the eluent. The product was obtained as a light yellow oil, 77 mg, yield of 50%. HPLC purity: 95.0% (retention time: 4.01 min; 40% H2O and 60% MeCN over 30 min at 254 nm on Varian C18 column). 1H NMR (CDCl3): δ 9.62 (s, 1H), 7.21 (d, J = 3.52 Hz, 1H), 6.52 (d, J = 3.56 Hz, 1H), 4.54 (s, 2H), 3.60 (q, J = 7.00 Hz, 2H), 1.25 (t, J = 6.98 Hz, 3H). 13C NMR (CDCl3): δ 177.83, 158.95, 152.77, 121.91, 111.08, 66.77, 64.92, 15.19.
5-(tert-Butoxymethyl)furan-2-carbaldehyde (VZHE013)
5-HMF (100 mg, 0.82 mmol) was dissolved in DCM (5 mL), and Boc2O (0.89 g, 4.10 mmol) and Mg(ClO4)2 (36 mg, 0.16 mmol) were added. The reaction mixture was refluxed for 2 days. The reaction mixture was then filtered, concentrated, and purified with silica gel chromatography with EtOAc/hexanes = 1:8 as the eluent. The product was obtained as a light yellow oil, 58 mg, yield of 36%. HPLC purity: 95.4% (retention time: 4.78 min; 40% H2O and 60% MeCN over 30 min at 254 nm on Varian C18 column). IR: 2974.8, 1676.9, 1188.4, 1019.9, 803.7. 1H NMR (CDCl3): δ 9.59 (s, 1H), 7.20 (d, J = 3.48 Hz, 1H), 6.49 (d, J = 3.48 Hz, 1H), 4.49 (s, 2H), 1.28 (s, 9H). 13C NMR (CDCl3): δ 177.61, 160.33, 152.39, 122.24, 110.30, 74.45, 57.31, 27.47. MS: [M + Na]+ calcd 205.0841; found 205.0903.
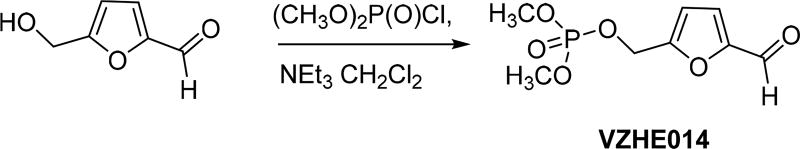
(5-Formylfuran-2-yl)methyl Dimethyl Phosphate (VZHE014)
5-HMF (183 mg, 1.5 mmol) and dimethyl chlorophosphate (217 mg, 1.5 mmol) were dissolved in 4 mL of CH2Cl2. The mixture was cooled to 0 °C, and pyridine (130 mg, 1.65 mmol) was added slowly. The resultant reaction mixture was stirred at 0 °C for 30 min and then at rt for 15 h. After filtration, the reaction mixture was diluted with EtOAc (15 mL) and then washed with 0.1 N HCl (5 mL × 3), 10% NaHCO3 (5 mL × 2), and brine (5 mL). The organic layer was then dried with Na2SO4. After filtration and concentration, the resultant crude oil was purified with column and eluted with the solvent system of EtOAc/hexanes = 1:2 and then EtOAc to give 180 mg of the product as a colorless oil, and the yield was 51%. IR: 2959.34, 1675.9, 1525.5, 1267.5, 1008.8, 845.0, 754.0. 1H NMR (CDCl3): δ 9.66 (s, 1H), 7.23 (d, J = 3.52 Hz, 1H), 6.67 (d, J = 3.56 Hz, 1H), 5.10 (d, J = 9.04 Hz, 2H), 3.78 (d, J = 11.2 Hz, 6H). 13C NMR (CDCl3): δ 177.95, 155.17 (d, 3JP–C = 7.15 Hz), 153.17, 121.56, 112.70, 60.89 (d, 2JP–C = 4.83 Hz), 54.68 (d, 2JP–C = 5.96 Hz).31P NMR (CDCl3): δ, 1.07. MS: [M + Na]+ calcd 257.0191; found 257.0219
5-(Propoxymethyl)-2-furan Carboxadehyde (VZHE015)
The synthesis of VZHE015 was carried out as per a previously reported procedure.32 5-HMF (126 mg, 1.0 mmol) was dissolved in acetonitrile (5 mL), and PrI (510 mg, 3.0 mmol) was added and followed by Cs2CO3 (488 mg, 1.5 mmol). The reaction mixture was heated to relux for 2 days. The reaction mixture was then filtered, concentrated, and purified with silica gel chromatography with EtOAc/hexanes = 1:8 as the eluent. The product was obtained as a light yellow oil, 94 mg, yield of 56%. 1H NMR (CDCl3): δ 9.62 (s, 1H), 7.21 (d, J = 3.52 Hz, 1H), 6.52 (d, J = 3.52 Hz, 1H), 4.54 (s, 2H), 3.49 (t, J = 6.68 Hz, 2H), 1.63 (m, 2H), 0.93 (t, J = 7.42 Hz, 2H). 13C NMR (CDCl3): δ 177.69, 158.93, 152.56, 121.91, 110.90, 72.96, 64.99, 22.82, 10.46.
5-(Isopropoxymethyl)-2-furan Carboxadehyde (VZHE016)
The synthesis of VZHE016 was carried out as per a previously reported procedure.32 5-HMF (126 mg, 1.0 mmol) was dissolved in acetonitrile (5 mL), and i-PrI (510 mg, 3.0 mmol) was added and followed by Cs2CO3 (488 mg, 1.5 mmol). The reaction mixture was stirred at rt for 4 days. The reaction mixture was then filtered, concentrated, and purified with silica gel chromatography with EtOAc/hexanes = 1:8 as the eluent. The product was obtained as a light yellow oil, 60 mg, yield of 40%. 1H NMR (CDCl3): δ 9.61 (s, 1H), 7.21 (d, J = 3.52 Hz, 1H), 6.52 (d, J = 3.52 Hz, 1H), 4.54 (s, 2H), 3.73 (heptet, J = 6.12 Hz, 1H), 1.22 (d, J = 6.08 Hz, 6H). 13C NMR (CDCl3): δ 177.83, 159.57, 152.66, 122.19, 110.83, 72.31, 63.68, 22.11.
Hemoglobin Modification, Oxygen Equilibrium, and Antisickling Studies Using Human Sickle Blood
The 12 compounds, VZHE004, VZHE006, VZHE007, VZHE005, VZHE011, VZHE013, VZHE014, VZHE015, VZHE016, 5-PMFC, 5-CMFC, 5-NMFC, and the parent compound (positive control) 5-HMF were investigated for their abilities to prevent hypoxia-induced RBC sickling (RBC morphology study), to increase sickle Hb oxygen affinity (oxygen equilibrium curve; OEC study), and to modify sickle Hb (adduct formation study) as previously published.14,15 Briefly, blood suspensions from a subject with homozygous SCD (hematocrit: 20%) were incubated under air in the absence or presence of 1, 2, and 5 mM concentration of test compounds at 37 °C for 1 h. Following, the suspensions were incubated under hypoxic condition (4% oxygen/96% nitrogen) at 37 °C for 2 h. Aliquot samples were fixed with 2% glutaraldehyde solution without exposure to air, and then subjected to microscopic morphological analysis. The residual samples were washed in phosphate-buffered saline and hemolyzed in hypotonic lysis buffer for subsequent analyses.
For the OEC study, approximately 100 µL aliquot samples from clarified lysate obtained from the antisickling study were added to 4 mL of 0.1 M potassium phosphate buffer, pH 7.0, in a cuvette and subjected to hemoximetry analysis using Hemox Analyzer (TCS Scientific Corp.) to assess P50 shifts. Finally, for the Hb adduct formation study, clarified lysates, also from the above antisickling study, were subjected to cation-exchange HPLC (Hitachi D-7000 Series, Hitachi Instruments, Inc., San Jose, CA), using a weak cation-exchange column (Poly CAT A: 30 mm × 4.6 mm, Poly LC, Inc., Columbia, MD).
Time-Dependent Adduct Formation Studies Using Normal Human Whole Blood
The compounds VZHE004, VZHE006, VZHE007, VZHE005, VZHE011, VZHE013, VZHE014, VZHE015, VZHE016, 5-PMFC, 5-NMFC, and the control 5-HMF were used to conduct time-dependent studies on Hb adduct formation using normal whole blood. The study was performed in a 96-well deepwell (Thermo Scientific) plate, where each compound at 2 mM concentration was added to 600 µL of whole blood (30% hct) and incubated at 37 °C for 24 h with shaking (at 140 rpm). At 0.5, 1, 2, 4, 6, 8, and 24 h time intervals, a 75 µL aliquot of blood was removed from each well using a multichannel pipet and added to respective tubes containing 75 µL of Na cyanoborohydride and borohydride mix (1:1 v/v 50 mM stock) to terminate the Schiff-base reaction, fix the Schiff-base adducts, and reduce the free reactive aldehyde.33 We have previously optimized these conditions. After mixing, the tubes were stored immediately at −80 °C until ready for analysis to determine Hb adduct formation using cation-exchange HPLC (Hitachi D-7000 Series, Hitachi Instruments, Inc., San Jose, CA) as previously described.14,15 The observed Hb adduct values in %Hb were plotted as a function of time [hours]; peak adduct concentration was obtained by visual inspection, and area under the curve, AUC(0–24), was obtained by linear trapezoidal rule across the entire 24-h measurement period.
Crystallization, Data Collection, and Structure Determination of Liganded Hb in Complex with VZHE004
A freshly made solution of VZHE004 or VZHE005 in DMSO was added to carbon monoxide ligated Hb (30 mg/mL protein) at Hb tetramer–compound molar ratio of 1:10 to form the COHb-compound complex, and then crystallized using 10–20% PEG6000, 100 mM Hepes, pH 7.4. Cherry-red needle crystals formed in 1–3 days for VZHE004 and were used to collect X-ray diffraction data at 100 K using a Molecular Structure Corporation (MSC) X-Stream Cryogenic Cooler System (The Woodlands, TX), an R-Axis IV image plate detector, and a Rigaku Micro-Max-007 generator (40 kV and 20 mA). The crystals were first cryoprotected with 80 µL of mother liquor mixed with 62 µL of 50% PEG6000. The data set was processed with the D*trek software (Rigaku) and the CCP4 suite of programs.34 The crystal is in the space group P212121 with cell constant of 62.64, 83.32, and 104.95.
The crystal structure of COHb in complex with VZHE004 was determined by a molecular replacement method with Phenix,35,36 using the native R2-state Hb crystal structure (PDB code 1BBB) as a search model.37 The structure was refined using both Phenix and CNS.35,36,38 The final refined structure at 1.85 Å contained four CO molecules bound at all four distal heme sites, two VZHE004 bound at the α-cleft, and several water molecules with final Rfactor and Rfree of 17.9% and 22.3%, respectively.
Model building and correction were carried out using COOT.39 The atomic coordinates and structure factor of VZHE004 in complex with carbon monoxide hemoglobin have been deposited in the RCSB Protein Data Bank with accession code 5URC. Detailed crystallographic and refinement parameters are reported in Table 1.
Table 1.
Data Collection and Refinement Statistics of Carbonmonoxy Hb in Complex with VZHE004a
| Data Collection Statistics | |
| space group | P212121 |
| unit cell a, b, c (Å) | 62.64, 83.32, 104.95 |
| resolution (Å) | 29.32–1.85 (1.92–1.85) |
| unique reflections | 47163 |
| redundancy | 4.28 (3.71) |
| completeness (%) | 99.1 (96.5) |
| average I/σ(I) | 21.5 (6.5) |
| Rmergeb(%) | 3.8 (17.3) |
| Refinement Statistics | |
| resolution (Å) | 29.32–1.85 (1.97–1.85) |
| No. of reflections | 47094 (7239) |
| Rwork (%) | 17.9 (28.8) |
| Rfreec(%) | 22.3 (36.0) |
| rmsd bonds (Å) | 0.007 |
| rmsd angles (deg) | 1.7 |
| dihedral angles | |
| most favored (%) | 96.64 |
| allowed (%) | 3.0 |
| average B (Å2)/atom | |
| all atoms | 28.4 |
| protein | 24.2 |
| hemes | 21.6 |
| VZHE004 | 37.1 |
| PDB ID code | 5URC |
Numbers in parentheses are for the highest resolution shell.
Rmerge = ΣhklΣi|Ii(hkl) − 〈I(hkl)〉|/ΣhklΣiIi(hkl).
Rfree was calculated from 5% randomly selected reflection for cross-validation. All other measured reflections were used during refinement.
Statistical Analyses
All functional and biological assays evaluating antisickling properties, Hb modification, and oxygen affinity changes were conducted in three biological replicates. Results are reported as mean values with standard deviations, from triplicate analyses.
RESULTS
Design and Synthesis of Novel Derivatives of 5-HMF
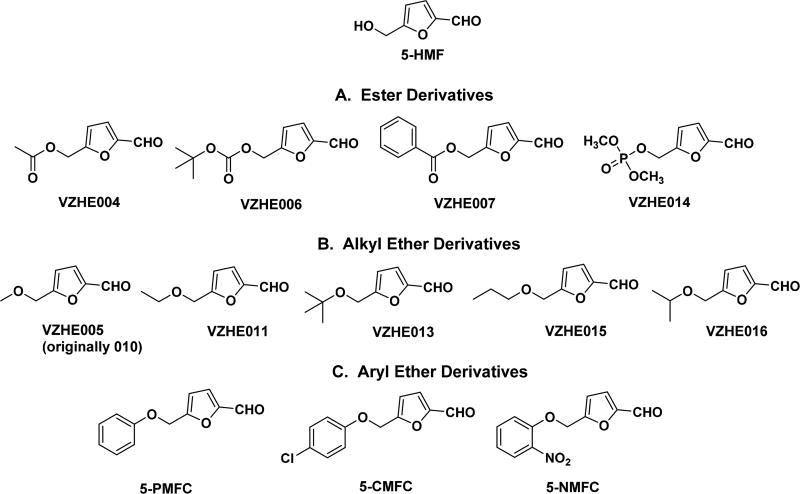
Our group and others have previously developed several pharmacologically improved derivatives of the antisickling compound vanillin or benzaldehydes,6,13–19 prompting us to employ a similar strategy to structurally modify 5-HMF (Figure 1), a better and more promising lead antisickling compound than vanillin. The crystal structure of 5-HMF in complex with liganded Hb (PDB code 1QXE) shows two molecules of the compound bound symmetrically at the α-cleft, with the aldehyde group of each molecule making Schiff-base interactions with the α1 and α2 N-terminal Val1 amines, respectively.14,15 The 5-alcohol and the furan ring oxygen of 5-HMF make direct and/or water-mediated hydrogen-bond interactions with the protein. These interactions help stabilize the high-O2-affinity R-state Hb. From the crystallographic binding mode of 5-HMF, it appeared that introducing alkyl or aryl substituents at the alcohol moiety of 5-HMF could result in additional hydrophobic interactions with the α-cleft pocket residues αAla130, αSer131, and αThr134 that may increase the compounds’ binding affinity and result in positive allosteric activity. Two derivatives, the methyl ester VZHE004 and the methyl ether VZHE005 (Figure 1), were first synthesized and then tested for their effect on Hb oxygen affinity (P50 shift or oxygen equilibrium curve; OEC), Hb modification (adduct formation), and antisickling effect (RBC morphology) as previously described.14,15 The positive pharmacologic outcome prompted us to study several other derivatives, including the esters VZHE006, VZHE007, and VZHE014; the alkyl ethers VZHE011, VZHE013, VZHE015, and VZHE016; and the aryl ethers 5-PMFC, 5-CMFC, and 5-NMFC (Figure 1). The synthetic pathways adopted for the preparation of nine of the above compounds (VZHE analogues) are outlined in Schemes 1–4, and detailed syntheses are described in the Experimental Section. The aryl ether compounds (5-PMFC, 5-CMFC, and 5-NMFC) were available commercially and purchased from Sigma. All compounds were tested for their effect on Hb oxygen affinity, Hb modification, sickle RBC morphology, and the X-ray crystallographic binding interactions of VZHE004 with Hb determined.
Figure 1.
Derivatives of 5-hydroxymethyl-2-furfural (5-HMF).
Scheme 1.
Scheme 4.
Novel Derivatives Modified Hb and Increased Its Affinity for Oxygen with Homozygous Sickle Blood
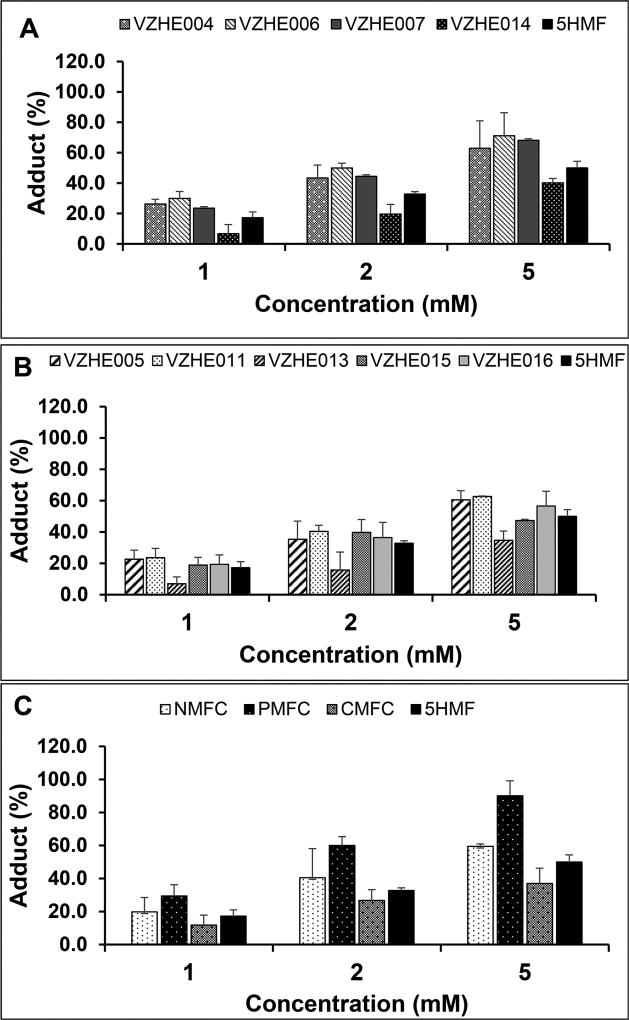
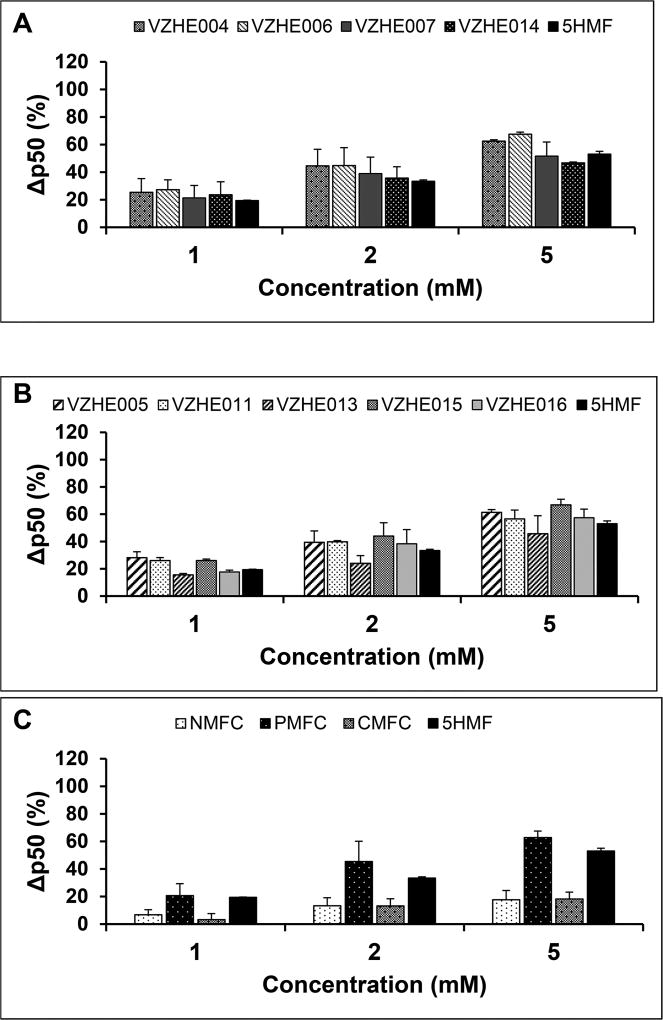
Aromatic aldehydes that modify Hb and increase the protein affinity for oxygen are expected to prevent hypoxia-induced polymerization with concomitant RBC sickling. Thus, all compounds were tested for their effect on Hb modification and Hb oxygen affinity at 1, 2, and 5 mM as previously reported,14,15 and the results are summarized in Table 2 (for the 2 mM drug concentration) and Figures 2 and 3 (for all concentrations). We observed a concentration-dependent effect on Hb S modification (Figure 2a–c) and Hb oxygen affinity (P50 shift) (Figure 3a–c). For the majority of compounds, across all three groups, the esters VZHE004, VZHE006, and VZHE007; the alkyl ethers VZHE005, VZHE011, VZHE015, and VZHE016; and the aryl ether 5-PMFC, there appears to be a direct correlation between the two critical biochemical effects. With few exceptions, most of the derivatives, including VZHE004, VZHE006, VZHE007, VZHE005, VZHE011, VZHE015, VZHE016, and 5-PMFC exhibited similar or greater Hb modification and P50 shift than 5-HMF, especially at the lower concentrations of 1 and 2 mM. However, 5-NMFC and 5-CMFC, which though either showed better than or comparable adduct formation to 5-HMF, respectively, unexpectedly resulted in significantly less P50 shift than 5-HMF at all three concentrations (Table 2, Figures 2c and 3c). This observation (would be further discussed) is likely due to binding of the compounds to both liganded and unliganded Hb, which could lead to stabilization of R-state and/or T-state Hb affecting the direction and magnitude of the P50 shift.
Table 2.
Hemoglobin Adduct Formation, Oxygen Equilibrium, and Antisickling Studies Using Homozygous Sickle Red Blood Cells at 2 mM Test Compound Concentrationa
| compound | modified Hb (%)b |
ΔP50 (%)c | inhibition of sickling (%)d |
|---|---|---|---|
| 5-HMF | 32.8 ± 1.6 | 33.4 ± 0.9 | 25.7 ± 4.4 |
| Ester Derivatives | |||
| VZHE004 | 43.5 ± 8.4 | 44.6 ± 11.9 | 26.3 ± 4.4 |
| VZHE006 | 50.0 ± 3.0 | 44.8 ± 12.9 | 71.6 ± 0.1 |
| VZHE007 | 44.5 ± 6.0 | 38.9 ± 11.9 | 10.0 ± 5.9 |
| VZHE014 | 19.7 ± 6.3 | 35.7 ± 8.1 | 8.4 ± 5.9 |
| Alkyl Ether Derivatives | |||
| VZHE005 | 35.3 ± 11.6 | 39.4 ± 8.3 | 35.3 ± 6.1 |
| VZHE011 | 40.4 ± 3.9 | 39.8 ± 0.8 | 58.8 ± 7.0 |
| VZHE013 | 15.7 ± 4.3 | 24.0 ± 5.6 | 7.4 ± 6.9 |
| VZHE015 | 39.7 ± 8.2 | 44.0 ± 9.7 | 62.9 ± 6.2 |
| VZHE016 | 36.4 ± 9.7 | 38.4 ± 10.4 | 22.5 ± 8.8 |
| Aryl Ether Derivatives | |||
| 5-NMFC | 40.5 ± 17.6 | 13.4 ± 5.7 | 10.3 ± 0.5 |
| 5-PMFC | 60.53 ± 5.3 | 45.4 ± 14.6 | 94.7 ± 0.1 |
| 5-CMFC | 26.7 ± 6.5 | 13.2 ± 5.2 | 2.0 ± 1.9 |
All studies were conducted with SS cells suspensions (20% hematocrit) incubated with 2 mM of each test compound; the results are the mean values ± SD for three separate experiments (biological replicates). The final concentration of DMSO was <2% in all samples, including in control samples.
Hb S adduct values obtained from HPLC elution patterns of hemolysate after incubation of compounds with SS cells.
Scheme 2.
Scheme 3.
Figure 2.
Concentration-dependent modification of Hb S by test compounds. Hemolysates from SS RBCs, which had been incubated with 0, 1, 2, or 5 mM of test compounds, were subjected to cation-exchange HPLC to determine the degrees of drug-modified Hb S adducts. Most ester and alkyl ether derivatives demonstrated similar or higher levels of modification (Hb S adducts) as 5-HMF (a,b). The two aryl ether derivatives 5-NMFC and 5-PMFC, especially the latter, showed superior Hb modification at all three experimental concentrations (c). Untreated control samples (not shown) had no modified hemoglobin. Mean values and standard deviations from three biological replicates are reported.
Figure 3.
Degree of shift in oxygen equilibra (ΔP50) by test compounds. Aliquot samples from hemolysates were subjected to hemoximetry analysis using the Hemox Analyzer (TCS Scientific Corp.) to assess change in P50 (ΔP50), relative to the untreated control samples. In accordance with observations on the levels of Hb modification, both ester and alkyl ether derivatives showed similar or slight improvements of P50 shift over 5-HMF (a,b). The aryl ether 5-PMFC showed better P50 shift than 5-HMF, while the other two aryl ethers 5-NMFC and 5-CMFC showed lower degrees of shift than 5-HMF (c). ΔP50 values were calculated by normalizing to untreated control samples. Data reported are mean values and standard deviations, from three replicate experiments.
Among the esters, VZHE004, VZHE006, and VZHE007 exhibited similar and significantly higher adduct formation than the phosphate ester VZHE014 (Table 2, Figure 2a). Nonetheless, all four compounds showed potent P50 shifts that are similar or slightly better than 5-HMF (Table 2, Figure 3a). With the exception of the ethyl ether VZHE013, which showed weaker adduct formation and P50 shift than 5-HMF, the other four ethers VZHE005, VZHE011, VZHE015, and VZHE016 were comparable or slightly more potent than 5-HMF (Table 2, Figures 2b and 3b). In both the ester and alkyl ether class of compounds, there appears to be no correlation between the substituent size and activity. In the aryl ethers, 5-PMFC without any substitution on the phenyl ring is significantly more potent in modifying Hb or increasing Hb affinity for oxygen than the analogues 5-CMFC and 5-NMFC with chloro- or nitro- substitution, respectively (Table 2, Figures 2c and 3c).
Novel Derivatives Demonstrated Improved in Vitro Antisickling with Homozygous Sickle Blood
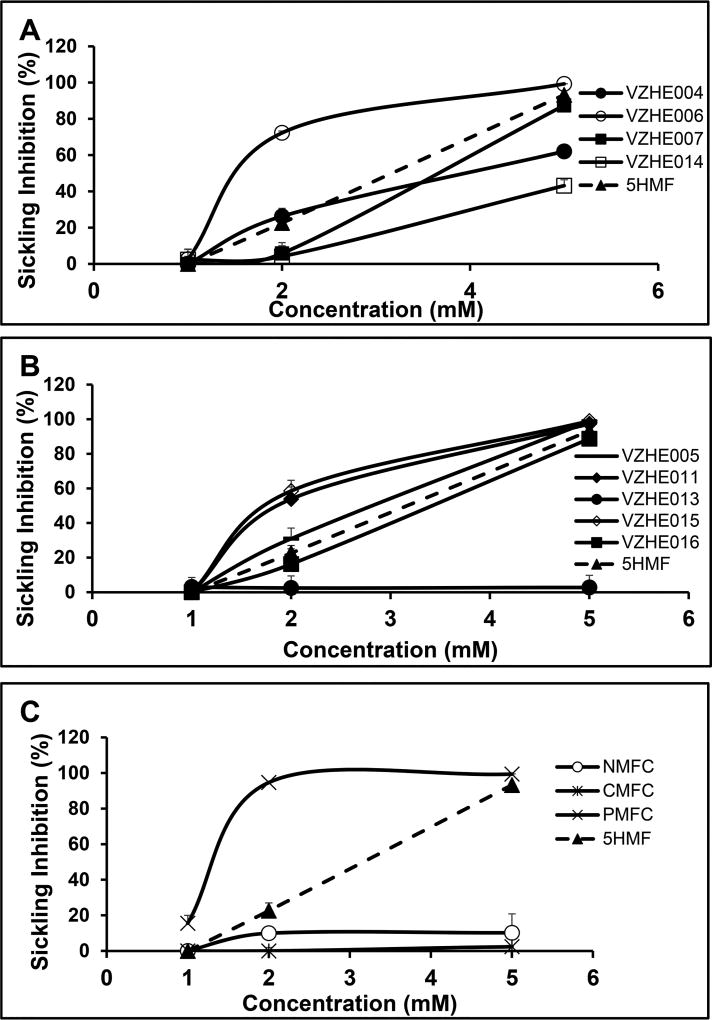
To investigate whether improvement in Hb oxygen affinity by several of the compounds also translated to their ability to prevent RBC sickling, all 12 compounds and 5-HMF were subjected to an in vitro sickling assay under hypoxic conditions using SS blood from SCD patients as previously described.14,15 Expectantly, the results of the antisickling study (using aliquot samples from the same incubation assay for determining Hb adduct and OEC shifts) demonstrated in the most part a dose-dependent inhibition of RBC sickling (Figures 4 and S1), consistent with the primary mechanism of action, i.e., by modifying Hb and increasing the protein affinity for oxygen. At the lowest concentration of 1 mM, almost all compounds, including 5-HMF, showed only minimal antisickling effect, except 5-PMFC, which inhibited RBC sickling of approximately 17% (Figures 4 and S1). At 2 mM concentration, the ester VZHE006, the three alkyl ethers VZHE005, VZHE011, and VZHE015, and the aryl ether 5-PMFC showed significant antisickling potency (35–95% RBC sickling inhibition) when compared to 5-HMF (26%), with 5-PMFC showing the most potent effect of ~95% sickling inhibition (Table 2, Figures 4 and S1). At 5 mM concentrations, more than half of the compounds inhibited over 95% RBC sickling; exception being VZHE004 (60%), VZHE014 (43%), VZHE016 (<10%), 5-NMFC (<10%), and 5-CMFC (<10%) (Figures 4 and S1). The observation that no significant antisickling activity was observed at 1 mM for most of the compounds even though they significantly increased Hb affinity for O2 suggests that there may be a nonlinear relationship between drug-induced (intramolecular) effects on allosteric O2-binding to Hb and the prevention of HbS polymerization by reducing intermolecular HbS interactions; it appears that a certain threshold of P50 shift is required for the antisickling effect to manifest itself. As expected from their minimal effect on Hb oxygen affinity, 5-NMFC and 5-CMFC (ΔP50 of <20%) showed almost no antisickling effect (<10%) at all three doses (Table 2, Figures 4c and S1c). What is most surprising is that VZHE013, which showed %P50 shifts of 16, 24, and 46 at 1, 2, and 5 mM (Figure 3b), respectively, and expected to translate into significant antisickling effect, showed less than 5% sickling inhibition at all three concentrations (Table 2, Figures 4b and S1b). The explanation behind this observation is not clear.
Figure 4.
Line charts showing dose-dependent inhibition of SS cell sickling from experiments. Briefly, hematocrit-normalized SS RBCs were incubated with 0, 1, 2, or 5 mM test compounds under 4% oxygen gas at 37 °C for 2 h, after which aliquot samples were obtained, fixed, and analyzed for degrees of sickling. The superior effects of VZHE006 (a) and 5-PMFC (c) over 5-HMF (dotted lines) are clearly demonstrated at 2 mM concentrations. Improved properties of VZHE011 and VZHE015 are also shown (b). Sickling inhibition, reported as mean values from three replicates, was calculated by normalizing to untreated control samples, as detailed in Table 2.
Although the P50 shifts of the esters VZHE004, VZHE006, VZHE007, and VZHE014 are comparable, VZHE006 exhibits roughly 3-fold antisickling potency over the other three esters and 5-HMF at 2 mM concentration (Table 2, Figures 4a and S1a). At 5 mM, VZHE006 remains the most potent (>95%), followed by VZHE007 at ~83%, and then VZHE004 (~60%) and VZHE014 (~40%) (Table 2, Figures 4a and S1a). These observations, we speculate, may be due to a dual mechanism of antisickling effect that involve increasing the oxygen affinity of Hb, as well as direct stereospecific polymer destabilization. Such dual antisickling mechanism of action has previously been proposed for vanillin derivatives.11,13
Among the alkyl ethers, with the exception of VZHE013, which showed very little antisickling activity at all concentrations, the rest exhibited over 95% antisickling effect at 5 mM (Table 2, Figures 4b and S1b). At 2 mM concentration, VZHE011 and VZHE015 are more potent (>54% inhibition) than 5-HMF (~23%), VZHE005 (~31%), and VZHE016 (~16%). Like the esters, there appears to be no direct correlation between structure and antisickling activity of the alkyl ether class of compounds.
Within the aryl ethers, 5-PMFC exhibits the most potent antisickling effect (~95% vs ~23% by 5-HMF at 2 mM), while 5-CMFC and 5-NMFC with substitution on the phenyl ring were among the least potent compounds (<10%) (Table 2, Figures 4c and S1). The fact that 5-CMFC and 5-NMFC show significant adduct formation but very little P50 shifts and antisickling activities highly suggest that these compounds not only bind to liganded Hb and stabilize the R-state, but also bind to unliganded Hb and confer significant stability to the T-state that ultimately led to a smaller increase in Hb affinity for oxygen. To fully understand the effect of substitution on the aryl ring of these ethers on Hb allosteric activity would require extensive SAR studies in the future.
An important observation is that the compounds display different antisickling profiles with increasing concentration (Figure 4). 5-HMF and the ether derivatives VZHE005 and VZHE016 show linear relationships between sickling inhibition and the compounds’ concentration in the medium. Similar observation has previously been reported for 5-HMF.14 Impressively, the aryl ether 5-PMFC and the ester VZHE006 show very rapid antisickling effect when the concentration is increased from 1 to 2 mM, leveling off to almost ~100% RBC inhibition, although slower for VZHE006. Thus, these two compounds, especially 5-PMFC, are capable of eliciting maximum antisickling effect at the relatively low compound concentration of 2 mM or even less. Other derivatives, such as VZHE011 and VZHE013, also show similar antisickling profile as VZHE006 but with significantly slower rate of RBC inhibition from 1 to 2 mM (Figure 4). The esters VZHE007 and VZHE014, however, show a lag in antisickling effect from 1 to 2 mM and only after 2 mM is there any significant biological effect (Figure 4). The other compounds, the alkyl ether VZHE013 and the aryl ethers 5-NMFC and 5-CMFC, barely showed any antisickling activity over the range of the three concentrations (Figure 4). This observation, taken in conjunction with the adduct formation and P50 shift results, as noted above, clearly suggests multiple mechanisms of antisickling action by these compounds.
Aryl Ethers Showed Improved in Vitro Metabolic Profile
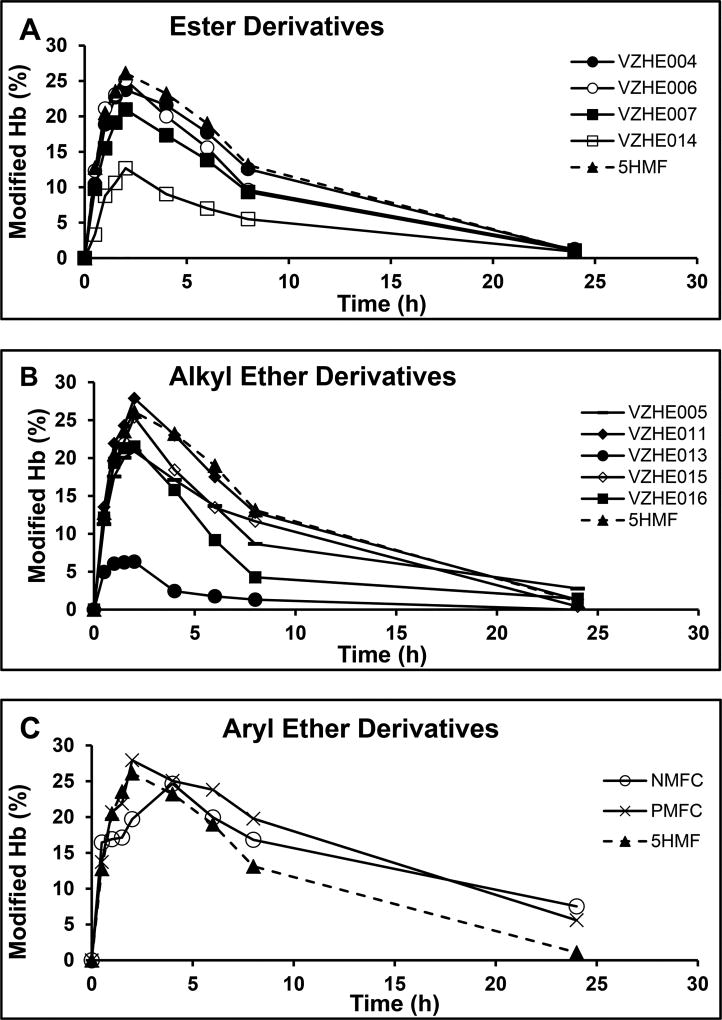
One of our goals was to improve on the antisickling potency of 5-HMF, which we seem to have accomplished to some extent. As noted above, 5-HMF suffers from oxidative metabolism that leads to short half-life and suboptimal bioavailability.14,20,21,27,28 Thus, a second goal of ours was to improve on the pharmacokinetic properties of 5-HMF through derivatization to reduce the apparent rapid oxidative metabolism of the aldehyde into the inactive acid analogue. We therefore conducted in vitro time-dependent Hb modification studies with the derivatives (2 mM drug concentration) using freshly drawn normal whole blood to determine whether the structural modifications, in addition to the observed improved functional/biological activity, have also translated into improvement in the compounds duration of action. Blood contains enzymes that are known to metabolize aromatic aldehydes into their corresponding nonactive acid analogues and is a good predictor of the metabolic stability of aromatic aldehyde.27,28 The result shows all compounds to exhibit maximum Hb modification at 2–4 h and then declined toward the baseline during the 24 h experiment (Figure 5). Most of the compounds, including 5-HMF, declined rapidly from the high of 20–28% adduct formation to ~2% at 24 h corresponding to, ~8% remaining activity. However, the aryl ethers 5-NMFC and 5-PMFC at 24 h still showed significant Hb modification of ~7% that correspond to ~29% remaining activity.
Figure 5.
Time-dependent modification of Hb A in normal blood incubated with test compounds. Normal blood from healthy donors was incubated with 2 mM test molecules for 24 h, during which aliquot samples were collected, reduced, and stored until batch analysis by cation exchange HPLC. Levels of modified Hb peaked between 2 and 4 h for all compounds followed by a decline, suggesting that the compounds are subject to metabolism by RBC enzymes. For the esters and alkyl ethers, there was ~92% decline in adduct formation at 24 h, similar to 5-HMF (a,b), while the aryl ethers showed only ~70% decline (c).
This time-dependent Hb adduct profiles reflect the initial binding of the various compounds to the Hb molecule, followed by slow dissociation from Hb (Schiff-base interaction) and subsequent metabolic degradation in RBCs. The different profiles suggest that most of the ester and alkyl ether derivatives may have similar or improved binding rates to Hb relative to 5-HMF as indicated by their increased peak adduct formation, while their terminal decline and AUC24h (Table 3) are quite similar to 5-HMF, suggesting that the unbinding rate from Hb is not very different.
Table 3.
Peak Hb A Modifications (%) and AUC24h (%·h) of Test Compounds Incubated at 2 mM Concentration with Blood from Healthy Donorsa
| compound | peak Hb 5 A modification (%) | AUC24h (%·h) |
|---|---|---|
| 5-HMF | 26.1 | 272.2 |
| Ester Derivatives | ||
| VZHE004 | 23.8 | 256.4 |
| VZHE006 | 25.1 | 227.6 |
| VZHE007 | 21.0 | 203.4 |
| VZHE014 | 12.7 | 116.1 |
| Alkyl Ether Derivatives | ||
| VZHE005 | 21.0 | 212.5 |
| VZHE011 | 27.9 | 271.5 |
| VZHE013 | 6.3 | 36.7 |
| VZHE015 | 25.4 | 230.7 |
| VZHE016 | 15.8 | 153.0 |
| Aryl Ether Derivatives | ||
| 5-NMFC | 24.7 | 350.9 |
| 5-PMFC | 27.9 | 383.7 |
All studies were conducted with AA cells suspensions (30% hematocrit) incubated with 2 mM of each test compound, and values are reported from a single experiment. Hb A modification values were obtained from HPLC analyses of hemolysates at the end of the assay. Peak adduct concentration was obtained by visual inspection and area under the curve, AUC(0–24) was obtained by the linear trapezoidal rule across the entire 24-h measurement period.
However, the aryl ester derivatives, while showing similar peak adduct formation, demonstrate a more gradual terminal decline and enhanced AUC relative to 5-HMF as well as residual Hb adduct at 24 h of up to 7.5% (compared to 1.1% for 5-HMF). This suggests that their binding rates to Hb may be similar, but their unbinding rates to Hb much slower than 5-HMF, resulting in improved Hb affinity. Alternatively (or additionally), they may be more resistant to metabolic degradation than 5-HMF, as well as the ester and alkyl ether derivatives.
Structural Study Showed VZHE004 Binds to the α-cleft of Hemoglobin
Derivatization of vanillin and its analogues have been shown to lead to more potent antisickling compounds, and X-ray crystallography has played an important role in driving this structure-based drug design effort.11,13 Based on 5-HMF’s mode of binding to liganded Hb, we structurally modified this lead compound into several derivatives that we anticipated would increase further interactions with Hb. To confirm our hypothesis, we attempted crystallization experiments for VZHE004 and VZHE005 with liganded Hb and were successfully able to determine the crystal structure of VZHE004 in complex with CO-liganded Hb in the R2-state conformation. The structure was solved using molecular replacement with the native R2-state Hb structure (PDB code 1BBB) and refined to 1.85 Å. Structural statistics are summarized in Table 1, and the structure deposited in the Protein Databank (PDB) with the ID code 5URC.
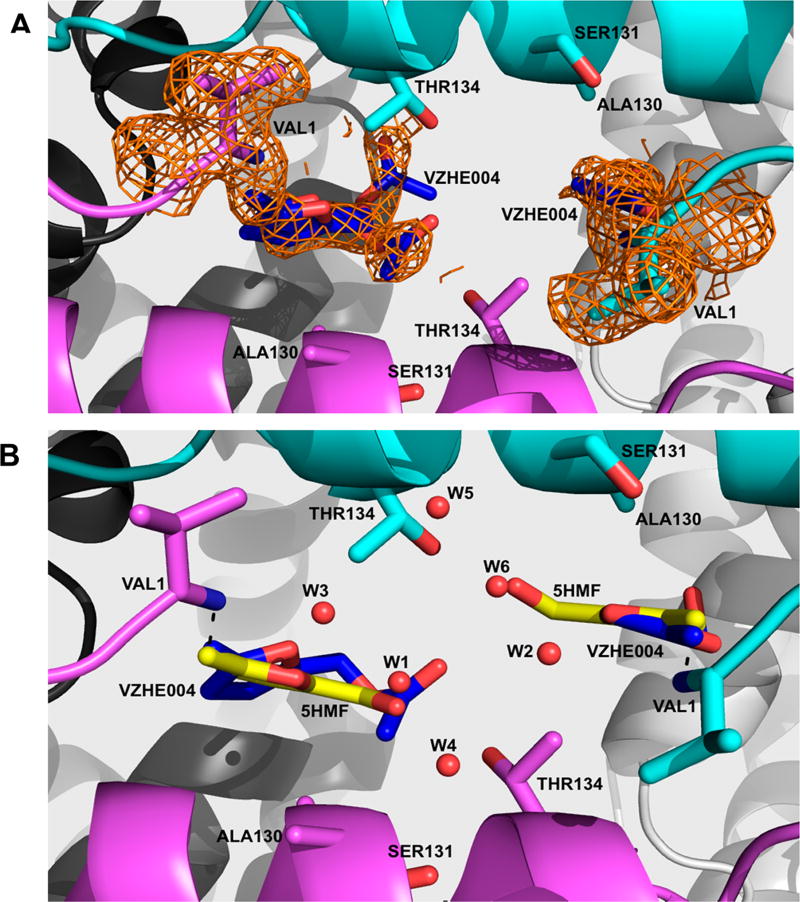
The overall tetrameric structure is indistinguishable (rmsd ≈ 0.4 Å) from 1BBB or the R2 structure in complex with 5-HMF (PDB code 1QXE). Like 5-HMF we also observed a pair of VZHE004 molecules covalently bound to the N-terminal αVal1 amines (Schiff-base interaction) in a symmetry-related fashion at the α-cleft (Figure 6). The electron density of VZHE004 was relatively weak especially at the methyl acetate position (Figures 6a and S2). As such, the compound was refined as 5-HMF at the α1Val1 site, while the full VZHE004 was modeled at the α2Val1 binding site but with the methyl acetate refined in two alternate conformations (Figure 6a). However, to make sure that the disorder is not due to partial hydrolytic cleavage of the ester, Hb and VZHE004 were incubated for 3 days. The UPLC–MS analysis of the incubated solution showed intact VZHE004, suggesting that the observed electron density is likely due to disorder (see Supporting Information and Figure S3).
Figure 6.
Crystal structure of carbon monoxide Hb in complex with VZHE004 in the R2-state conformation. Hb subunits are in sticks or ribbons (α1-subunit in cyan, α2-subunit in magenta, β1-subunit in gray, and β2-subunit in white). Water molecules are in red spheres. (a) Pair of VZHE004 molecules (blue sticks) bound at the α-cleft of Hb making Schiff-base interactions with the αVal1N. Superposed on the bound VZHE004 and the N-terminal αVal1 is the final electron-density map with coefficients 2Fo–Fc shown at the 0.6σ level. Note that the compound was refined as 5-HMF at the α1Val1 site (due to apparent disorder at the methyl acetate position), while the full VZHE004 was modeled at the α2Val1 binding site but with the methyl acetate refined in two alternate conformations. (b) VZHE004 (blue sticks) and 5-HMF from the crystal structure 1QXE (bright yellow sticks) superposed on each other showing similar binding modes. Also, shown in Figure S2 is the initial Fo–Fc map (before any ligand was built in the model).
A hallmark of 5-HMF binding is a direct and/or water-mediated hydrogen-bond interactions that involve the 5-hydroxyl oxygen or the furan oxygen with the protein, which in addition to the Schiff-base interaction explained the potent functional and antisickling activities of 5-HMF when compared to furfural, 5-ethylfurfural, 5-methylfurfural, and vanillin.11,13–15 It is apparent from our structure comparative analysis that VZHE004 and 5-HMF bind and make similar interactions with the R2 liganded Hb that tie the two α-subunits together through direct and/or hydrogen-bond interactions with the hydroxyl of αThr134 and αSer131 (Figure 6), providing atomic level explanation of the compounds’ ability to increase Hb affinity for oxygen. The methyl acetate moiety appears to make hydrophobic interactions with αAla130, αSer131, and αThr134; however, the apparent disorder suggests these interactions to be weak. We expect the other compounds to bind similarly as VZHE004, including making hydrophobic interactions with αAla130, αSer131, and αThr134 as the pocket size is big enough to accommodate even the bulkier substituents. We also expect similar disorder at the alcohol substitution; however, derivatives with bulkier substituents should forge stronger interactions with αAla130, αSer131, and αThr134 and show decreased disorder. These differences in binding interactions and the fact that these compounds may also bind to both liganded and unliganded Hb and affect Hb allosteric properties in opposite directions may in part explain the differences in functional/biological activities of these compounds.
DISCUSSION
We have previously shown that aromatic aldehydes affect their high-O2-affinity and/or antisickling activities by forming Schiff-base interaction with the N-terminal αVal1 amines of the R2-state conformation of Hb, and that the pharmacological potency of this class of compounds depends on both the number and type of noncovalent interactions with the protein.11,13–15 Vanillin was one of the earliest aromatic aldehydes to be studied for its antisickling activity,40,41 but low potency and poor pharmacokinetic properties as a result of rapid metabolism of the aromatic aldehyde prevented its development in the clinic. Further modification of vanillin and its analogues yielded significantly improved pharmacologic agents, with some of the derivative compounds subsequently undergoing preclinical or clinical studies.11,13,16–19,22 Based on the successful derivatization of vanillin into better pharmacologic compounds, we employed a similar strategy to derivatize 5-HMF; a more potent candidate molecule with better pharmacokinetic properties than vanillin. The choice of 5-HMF as a pharmacophore was also influenced by its well-known safety profiles and already characterized metabolites.
We hypothesized, based on the binding mode of 5-HMF to liganded Hb, that adding substituents at the alcohol moiety will lead to further interactions with the protein that could potentially enhance its functional and biological effect. We first synthesized and tested two compounds, the ester VZHE004 and alkyl ether VZHE005, which expectantly showed improved allosteric and antisickling activities, prompting us to study several other ester and ether analogues (Figure 1). Our study showed the compounds bind Hb as proposed, resulting in modification and increasing oxygen affinity of the protein with concomitant inhibition of RBC sickling. Importantly, several of the compounds including VZHE005, VZHE011, VZHE006, VZHE015, and 5-PMFC at 2 mM showed 1.5 - to 4.0-fold improvement in antisickling activity compared to 5-HMF, with 5-PMFC being the most potent, almost reaching 95% potency compared to the ~26% by 5-HMF at 2 mM (Table 2). It is also worth pointing out, 5-PMFC, as well as VZHE006, VZHE011, and VZHE015 in varying extents, are capable of eliciting significant antisickling effect at relatively low compound concentration (Figures 4 and S1).
The superior functional and/or antisickling activity of several of these compounds when compared to 5-HMF could in part be due to the additional protein interactions afforded by the alcohol substituents (with αAla130, αSer131, and αThr134) as suggested by the crystal structure of Hb in complex with VZHE004. Manually docking our most potent compound 5-PMFC based on VZHE004 or 5-HMF binding mode, suggests that the phenyl group of 5-PMFC will make stronger hydrophobic interactions with αAla130, αSer131, and αThr134, which in part might explain this compound’s superior functional and biological activities. Likewise, subtle differences in binding interactions and/or disorder of the ester or ether substituents of the compounds could also explain their functional/biological activity differences. As noted above, certain vanillin derivatives have been reported to exhibit dual antisickling activity by increasing the oxygen affinity of Hb and stereospecifically inhibiting polymer stabilization.11,13 The SAR presented here appears to suggest a similar dual mechanism of action by the 5-HMF derivatives, especially when we consider that some of the compounds, despite comparable Hb modification and P50 shifts, exhibit significant variation in their antisickling potencies.
It is notable that the substituted aryl ethers 5-NMFC and 5-CMFC although significantly modify Hb, unlike the unsubstituted aryl analogue 5-PMFC, did not translate into higher P50 shift or antisickling effect (Table 2, Figures 2–4). We have previously shown that aromatic aldehydes that form Schiff-base adduct with the N-terminal Val1 nitrogen and modify Hb may exhibit different allosteric behaviors (increase or decrease Hb affinity for oxygen), with the direction and magnitude of the allosteric shift dependent on preferential binding and/or stabilization of one Hb state (R or T) over the other.11,25,26 For example, 5-HMF binds to both liganded and unliganded Hb, but significantly weaker to the latter; thus preferentially stabilizing the R-state and increasing Hb oxygen affinity.11,15 It is likely that 5-NMFC and 5-CMFC bind and confer more stabilization to the T-state than 5-PMFC, in part explaining the significant differences in their P50 and antisickling activities. In general, we expect all compounds to bind to both liganded and unliganded Hb to generate Hb adduct; however, differences in their binding affinities may result in different stabilization effects on the R or T state, partly explaining the observed differences in their allosteric and antisickling activities.
The antisickling activity of aromatic aldehydes is primarily due to the Schiff-base interactions between the aldehyde moiety and the N-terminal αVal1 amines of Hb. Competing with this reaction are several enzymatic reactions; principally metabolism of the aldehyde into the pharmacologically inactive acid, such as by aldehyde dehydrogenase, aldehyde oxidase in the liver, blood, and other tissues. This is especially true for 5-HMF, which undergoes extensive oxidative metabolism that reduces its pharmacologic effect.14,20,21,27,28 Vanillin has an even poorer metabolism profile than 5-HMF; nonetheless, some of its derivatives exhibit improved pharmacokinetic properties as a consequence of their resistance to extensive metabolism.16,17,23 By derivatizing 5-HMF, we therefore anticipated improvement in the compounds’ pharmacokinetic properties by decreasing metabolism of the aldehyde moiety. However, it appears that, like 5-HMF, the esters and alkyl ethers regardless of the substitution at the 5-hydroxyl position also undergo significant and rapid metabolism in whole blood (Figure 5). Although, not yet tested, other metabolic routes, including the liver, could even play a bigger role in further reducing the pharmacologic activity of these compounds. The aryl ethers, as shown by 5-PMFC and 5-NMFC, exhibit some resistance to metabolism in whole blood, suggesting that these derivatives may afford better protection against oxidative metabolism. Additionally, 5-PMFC also showed the most potent antisickling effect. We speculate that therapeutic efficacy could be obtained with relatively low levels of modification of liganded Hb S, as evidenced by patients with varying levels of fetal Hb who show mild disease symptoms,42 though perhaps, in theory, millimolar concentrations would still be required of our current most potent compound. Recent evidence, however, suggests that, indeed, micromolar plasma concentrations of an analogous molecule with higher efficacy could be therapeutic.17,43 These positive recent reports encourage the utility SAR studies to optimize promising candidate compounds. Our current results with 5-PMFC, in conjunction with the crystal structure of VZHE004, suggest an important start, and the right directions for further targeted structural modifications to attain desirable pharmacologic results. The crystal structure of VZHE004 suggests that the α-cleft binding site could accommodate bulkier substituents at the alcohol moiety (5-position) of 5-HMF that would lead to stronger hydrophobic interactions with the protein. We believe that careful SAR studies by varying the substituent on the phenyl ring of 5-PMFC, including the size, position, and type, would lead to an increase in antisickling potency. Another potential modification to increase potency and pharmacokinetic properties is incorporating a hydroxyl moiety on the furan ring at the ortho position of the aldehyde group. Such structural modifications have been shown in vanillin derivatives to stabilize the Schiff-base interaction between the aromatic aldehyde and the protein to improve their pharmacologic profiles.16,17
CONCLUSION
Over the last several years, 5-HMF has been one of the most promising antisickling lead candidate drugs and has provided a proof-of- principle guide for similar Hb-targeting antisickling agents. In this study, we demonstrate that derivatization of 5-HMF has led to a newer generation of analogues with greater potency and/or improved in vitro metabolic profile in whole blood, with 5-PMFC clearly the most superior. However, even the 4-fold increase in potency of some of these compounds may not be enough to translate into an effective therapeutic dose because of the large amount of compound that would be required to modify the large amount of Hb in vivo. While this study did not lead to a compound to take into the clinic, it is important to note that it has identified a particularly advantageous structural moiety (aryl ether) that could be manipulated to further improve on the pharmacologic profile of this important lead compound.
Supplementary Material
Acknowledgments
This work was supported by NIH/NIMHD grant MD009124 (to M.K.S.) and NIH/NHLBI grant K01HL103186 (to O.A.). Structural biology resources were provided in part by NIH grant CA16059 to the VCU Massey Cancer Center.
Footnotes
ASSOCIATED CONTENT
- Additional experimental and characterization details (PDF)
The authors declare no competing financial interest.
References
- 1.Ingram VM Abnormal human haemoglobins. III. The chemical difference between normal and sickle cell haemoglobins. Biochim. Biophys. Acta. 1959;36:402–411. doi: 10.1016/0006-3002(59)90183-0. [DOI] [PubMed] [Google Scholar]
- 2.Pauling L, Itano HA. Sickle cell anemia, a molecular disease. Science. 1949;109:543. [PubMed] [Google Scholar]
- 3.Jensen FB. The dual roles of red blood cells in tissue oxygen delivery: oxygen carriers and regulators of local blood flow. J. Exp Biol. 2009;212:3387–3393. doi: 10.1242/jeb.023697. [DOI] [PubMed] [Google Scholar]
- 4.Rogers SC, Ross JG, d’Avignon A, Gibbons LB, Gazit V, Hassan MN, McLaughlin D, Griffin S, Neumayr T, Debaun M, DeBaun MR, Doctor A. Sickle hemoglobin disturbs normal coupling among erythrocyte O2 content, glycolysis, and antioxidant capacity. Blood. 2013;121:1651–1662. doi: 10.1182/blood-2012-02-414037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Berka V, Song A, Sun K, Wang W, Zhang W, Ning C, Li C, Zhang Q, Bogdanov M, Alexander DC, Milburn MV, Ahmed MH, Lin H, Idowu M, Zhang J, Kato GJ, Abdulmalik OY, Zhang W, Dowhan W, Kellems RE, Zhang P, Jin J, Safo M, Tsai AL, Juneja HS, Xia Y. Elevated sphingosine-1-phosphate promotes sickling and sickle cell disease progression. J. Clin. Invest. 2014;124:2750–2761. doi: 10.1172/JCI74604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Safo MK, Kato GJ. Therapeutic strategies to alter the oxygen affinity of sickle hemoglobin. Hematol. Oncol. Clin. North Am. 2014;28:217–231. doi: 10.1016/j.hoc.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Habara A, Steinberg MH. Minireview: Genetic basis of heterogeneity and severity in sickle cell disease. Exp. Biol. Med (London, U. K.) 2016;241:689–696. doi: 10.1177/1535370216636726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akinsheye I, Klings ES. Sickle cell anemia and vascular dysfunction: the nitric oxide connection. J. Cell. Physiol. 2010;224:620–625. doi: 10.1002/jcp.22195. [DOI] [PubMed] [Google Scholar]
- 9.De Franceschi L. Pathophisiology of sickle cell disease and new drugs for the treatment. Mediterr. J Hematol. Infect. Dis. 2009;1:e2009024. doi: 10.4084/MJHID.2009.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turhan A, Weiss LA, Mohandas N, Coller BS, Frenette PS. Primary role for adherent leukocytes in sickle cell vascular occlusion: a new paradigm. Proc. Natl. Acad. Sci. U. S. A. 2002;99:3047–3051. doi: 10.1073/pnas.052522799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Safo MK, Ahmed MH, Ghatge MS, Boyiri T. Hemoglobin-ligand binding: Understanding Hb function and allostery on atomic level. Biochim. Biophys. Acta, Proteins Proteomics. 2011;1814:797–809. doi: 10.1016/j.bbapap.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Safo MK, Bruno S. Allosteric Effectors of Hemoglobin: Past, Present and Future. In: Mozzarelli A, Bettati S, editors. Chemistry and Biochemistry of Oxygen Therapeutics: From Transfusion to Artificial Blood. John Wiley & Sons, Ltd; 2011. pp. 285–300. [Google Scholar]
- 13.Abdulmalik O, Ghatge MS, Musayev FN, Parikh A, Chen Q, Yang J, Nnamani IN, Danso-Danquah R, Eseonu DN, Asakura K, Abraham DJ, Venitz J, Safo MK. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2011;D67:920–928. doi: 10.1107/S0907444911036353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdulmalik O, Safo MK, Chen Q, Yang J, Brugnara C, Ohene-Frempong K, Abraham DJ, Asakura T. 5-Hydroxymethyl-2-Furfural Modifies Intracellular Sickle Haemoglobin and Inhibits Sickling of Red Blood Cells. Br. J. Haematol. 2005;128:552–561. doi: 10.1111/j.1365-2141.2004.05332.x. [DOI] [PubMed] [Google Scholar]
- 15.Safo MK, Abdulmalik O, Danso-Danquah R, Burnett JC, Nokuri S, Joshi GS, Musayev FN, Asakura T, Abraham DJ. Structural basis for the potent antisickling effect of a novel class of five-membered heterocyclic aldehydic compounds. J. Med. Chem. 2004;47:4665–4676. doi: 10.1021/jm0498001. [DOI] [PubMed] [Google Scholar]
- 16.Arya R, Rolan PE, Wootton R, Posner J, Bellingham AJ. Tucaresol increases oxygen affinity and reduces haemolysis in subjects with sickle cell anaemia. Br. J. Haematol. 1996;93:817–821. doi: 10.1046/j.1365-2141.1996.d01-1744.x. [DOI] [PubMed] [Google Scholar]
- 17.Oksenberg D, Dufu K, Patel MP, Chuang C, Li Z, Xu Q, Silva-Garcia A, Zhou C, Hutchaleelaha A, Patskovska L, Patskovsky Y, Almo SC, Sinha U, Metcalf BW, Archer DR. GBT440 increases haemoglobin oxygen affinity, reduces sickling and prolongs RBC half-life in a murine model of sickle cell disease. Br. J. Haematol. 2016;175:141–153. doi: 10.1111/bjh.14214. [DOI] [PubMed] [Google Scholar]
- 18.Keidan AJ, Franklin IM, White RD, Joy M, Huehns ER, Stuart J. Effect of BW12C on oxygen affinity of haemoglobin in sickle-cell disease. Lancet. 1986;1:831–834. doi: 10.1016/s0140-6736(86)90941-4. [DOI] [PubMed] [Google Scholar]
- 19.Beddell CR, Goodford PJ, Kneen G, White RD, Wilkinson S, Wootton R. Substituted benzaldehydes designed to increase the oxygen affinity of human haemoglobin and inhibit the sickling of sickle erythrocytes. Br. J. Pharmacol. 1984;82:397–407. doi: 10.1111/j.1476-5381.1984.tb10775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato GJ, Lawrence MP, Mendelsohn LG, Saiyed R, Wang X, Conrey AK, Starling JM, Grimes G, Taylor JG, McKew J, Minniti CP, Stern W. Phase 1 clinical trial of the candidate anti-sickling agent Aes-103 in adults with sickle cell anemia. Blood. 2013;122:1009. [Google Scholar]
- 21.Stern W, Mathews D, McKew J, Shen X, Kato GJ. A Phase 1, First-in-Man, Dose-Response Study of Aes-103 (5-HMF), an Anti-Sickling, Allosteric Modifier of Hemoglobin Oxygen Affinity in Healthy Norman Volunteers. ASH Annual Meeting Abstracts. 2012;120:3210. [Google Scholar]
- 22.Oder E, Safo MK, Abdulmalik O, Kato GJ. New developments in anti-sickling agents: can drugs directly prevent the polymerization of sickle haemoglobin in vivo? Br. J. Haematol. 2016;175:24–30. doi: 10.1111/bjh.14264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rolan PE, Mercer AJ, Wootton R, Posner J. Pharmacokinetics and pharmacodynamics of tucaresol, an antisickling agent, in healthy volunteers. Br. J. Clin. Pharmacol. 1995;39:375–380. doi: 10.1111/j.1365-2125.1995.tb04465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rolan PE, Parker JE, Gray SJ, Weatherley BC, Ingram J, Leavens W, Wootton R, Posner J. The pharmacokinetics, tolerability and pharmacodynamics of tucaresol (589C80; 4[2-formyl-3-hydroxyphenoxymethyl] benzoic acid), a potential anti-sickling agent, following oral administration to healthy subjects. Br. J. Clin. Pharmacol. 1993;35:419–425. doi: 10.1111/j.1365-2125.1993.tb04160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abraham DJ, Safo MK, Boyiri T, Danso-Danquah RE, Kister J, Poyart C. How allosteric effectors can bind to the same protein residue and produce opposite shifts in the allosteric equilibrium. Biochemistry. 1995;34:15006–15020. doi: 10.1021/bi00046a007. [DOI] [PubMed] [Google Scholar]
- 26.Boyiri T, Safo MK, Danso-Danquah RE, Kister J, Poyart C, Abraham DJ. Bisaldehyde allosteric effectors as molecular ratchets and probes. Biochemistry. 1995;34:15021–15036. doi: 10.1021/bi00046a008. [DOI] [PubMed] [Google Scholar]
- 27.Parikh A, Venitz J. Novel In-Vitro Target-Site Drug Disposition (TSDD)/Pharmacodynamic (PD) Model for 5-Hydroxymethyl Furfural (5-HMF) in Human Whole Blood. Clin. Pharmacol. Ther. 2014;95:S83–S84. [Google Scholar]
- 28.Godfrey VB, Chen LJ, Griffin RJ, Lebetkin EH, Burka LT. Distribution and metabolism of (5-hydroxymethyl)furfural in male F344 rats and B6C3F1 mice after oral administration. J. Toxicol. Environ. Health, Part A. 1999;57:199–210. doi: 10.1080/009841099157764. [DOI] [PubMed] [Google Scholar]
- 29.Nnamani IN, Joshi GS, Danso-Danquah R, Abdulmalik O, Asakura T, Abraham DJ, Safo MK. Pyridyl derivatives of benzaldehyde as potential antisickling agents. Chem. Biodiversity. 2008;5:1762–1769. doi: 10.1002/cbdv.200890165. [DOI] [PubMed] [Google Scholar]
- 30.Safo MK, Abraham DJ. X-ray crystallography of hemoglobins. Methods Mol. Med. 2003;82:1–19. doi: 10.1385/1-59259-373-9:001. [DOI] [PubMed] [Google Scholar]
- 31.Coelho JA, Trindade AF, Andre V, Duarte MT, Veiros LF, Afonso CA. Trienamines derived from 5-substituted furfurals: remote epsilon-functionalization of 2,4-dienals. Org. Biomol. Chem. 2014;12:9324–9328. doi: 10.1039/c4ob01759e. [DOI] [PubMed] [Google Scholar]
- 32.Viil I, Bredihhin A, Maeorg U, Vares L. Preparation of potential biofuel 5-ethoxymethylfurfural and other 5-alkoxymethylfurfurals in the presence of oil shale ash. RSC Adv. 2014;4:5689–5693. [Google Scholar]
- 33.Davies R, Hedebrant U, Athanassiadis I, Rydberg P, Tornqvist M. Improved method to measure aldehyde adducts to N-terminal valine in hemoglobin using 5-hydroxymethylfurfural and 2,5-furandialdehyde as model compounds. Food Chem. Toxicol. 2009;47:1950–1957. doi: 10.1016/j.fct.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 34.Winn MD, Ballard CC, Cowtan KD, Dodson EJ, Emsley P, Evans PR, Keegan RM, Krissinel EB, Leslie AG, McCoy A, McNicholas SJ, Murshudov GN, Pannu NS, Potterton EA, Powell HR, Read RJ, Vagin A, Wilson KS. Overview of the CCP4 suite and current developments. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2011;67:235–242. doi: 10.1107/S0907444910045749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Echols N, Grosse-Kunstleve RW, Afonine PV, Bunkoczi G, Chen VB, Headd JJ, McCoy AJ, Moriarty NW, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Adams PD. Graphical tools for macromolecular crystallography in PHENIX. J. Appl. Crystallogr. 2012;45:581–586. doi: 10.1107/S0021889812017293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adams PD, Afonine PV, Bunkoczi G, Chen VB, Echols N, Headd JJ, Hung LW, Jain S, Kapral GJ, Grosse Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner RD, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. The Phenix software for automated determination of macromolecular structures. Methods. 2011;55:94–106. doi: 10.1016/j.ymeth.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silva MM, Rogers PH, Arnone A. A third quaternary structure of human hemoglobin A at 1.7-A resolution. J. Biol. Chem. 1992;267:17248–17256. [PubMed] [Google Scholar]
- 38.Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr., Sect. D: Biol. Crystallogr. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 39.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abraham DJ, Mehanna AS, Wireko FC, Whitney J, Thomas RP, Orringer EP. Vanillin, a potential agent for the treatment of sickle cell anemia. Blood. 1991;77:1334–1341. [PubMed] [Google Scholar]
- 41.Zaugg RH, Walder JA, Walder RY, Steele JM, Klotz IM. Modification of hemoglobin with analogs of aspirin. J. Biol. Chem. 1980;255:2816–2821. [PubMed] [Google Scholar]
- 42.el-Hazmi MA. Heterogeneity and variation of clinical and haematological expression of haemoglobin S in Saudi Arabs. Acta Haematol. 1992;88:67–71. doi: 10.1159/000204654. [DOI] [PubMed] [Google Scholar]
- 43.Metcalf B, Chuang C, Dufu K, Patel MP, Silva-Garcia A, Johnson C, Lu Q, Partridge JR, Patskovska L, Patskovsky Y, Almo SC, Jacobson MP, Hua L, Xu Q, Gwaltney SL, Yee C, Harris J, Morgan BP, James J, Xu D, Hutchaleelaha A, Paulvannan K, Oksenberg D, Li Z. Discovery of GBT440, an Orally Bioavailable R-State Stabilizer of Sickle Cell Hemoglobin. ACS Med. Chem. Lett. 2017;8:321–326. doi: 10.1021/acsmedchemlett.6b00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.