Abstract
Background
Parkinson’s disease (PD) is divided into postural instability gait difficulty (PIGD) and tremor-dominant (TD) subtypes. Increasing evidence has suggested that the GABAergic neurotransmitter system is involved in the pathogenesis of PD.
Purpose
To evaluate the differences of GABA levels between PD motor subtypes using MEGA-PRESS.
Study type
Cohort
Subjects
PD patients were classified into PIGD (n = 13) and TD groups (n = 9); sixteen age- and sex- matched healthy controls were also recruited. All subjects were right-handed.
Sequence
All subjects underwent an MRS scan including MEGA-PRESS at 3.0T.
Assessment
The detected GABA signal also contains signal from macromolecules (MM) and homocarnosine, so it is referred as to GABA+. GABA+ levels and Creatine (Cr) levels were quantified in the left basal ganglia (BG) using Gannet 2.0 by Tao Gong.
Statistical tests
Differences in GABA+ levels among three groups were analyzed using analysis of covariance. The relationship between GABA levels and unified Parkinson's disease rating scale (UPDRS) was also analyzed.
Results
GABA+ levels were significantly lower in left BG regions of PD patients compared with healthy controls (p < 0.001). In PD patients, the GABA concentration was lower in the TD group than PIGD group (p = 0.019). Cr levels in PIGD and TD were lower than controls (p = 0.020; p = 0.002). A significant negative correlation was found in PIGD between GABA levels and UPDRS (r = −0.572, p = 0.041), while no correlation was found in TD (r = −0.339, p = 0.372).
Data conclusion
Low BG GABA levels in PD patients, and differences between PIGD/TD patients, suggest that GABAergic dysfunction may play an important role in the pathogenesis of Parkinson’s disease.
Keywords: Parkinson’s disease, GABA, MEGA-PRESS, Postural instability gait difficulty, Tremor
INSTRUCTION
Parkinson’s disease (PD) can be divided into two motor subtypes (1), postural instability gait difficulty (PIGD) and tremor-dominant (TD), depending on whether tremor or balance and gait disturbances are the most pronounced symptoms. Gamma-aminobutyric acid (GABA) is the main inhibitory neurotransmitter in the human brain. Increasing evidence has suggested that the GABAergic neurotransmitter system is involved in the pathogenesis of PD (2).
Proton (1H) magnetic resonance spectroscopy (1H-MRS) is a noninvasive technique that permits measurements of neurometabolites in the healthy or diseased human brain in vivo, including various degenerative diseases (3–6). The low concentration of GABA and the presence of overlapping signals from more concentrated metabolites render GABA measurements ambiguous using conventional 1H-MRS at 3.0 T (7). As a result, a spectral editing technique, MEscher-GArwood Point Resolved Spectroscopy (MEGA-PRESS (8)), is widely used to remove overlapping signals, allowing observations of GABA levels in healthy and diseased patients (9–11).
The basal ganglia play a major role in the regulation of human movement: the major projection from the substantia nigra is to nuclei of the basal ganglia; and the basal ganglia circuitry processes the signals that flow from the cortex, allowing the correct execution of voluntary movements, as dramatically manifest in Parkinson's disease (12). In basal ganglia, the majority of the neurons uses GABA as the neurotransmitter and has inhibitory effects on their targets. Previous studies have demonstrated altered GABA levels in the basal ganglia of PD patients compared to healthy volunteers (13,14). Thus, the aims of this study were to: i) evaluate differences in GABA levels between motor subtypes of PD in basal ganglia using MEGA-PRESS; and ii) determine the relationship between GABA levels and Unified Parkinson’s disease rating scale (UPDRS) in PD patients.
MATERIALS AND METHODS
Subjects
Twenty three patients with a clinical diagnosis of idiopathic PD (14 women and 9 men aged 45–79 years) according to the United Kingdom (UK) PD Brain Bank criteria (15). and 16 age- and sex-matched healthy controls (9 women and 7 men aged 46–70 years) were recruited in this study (Table 1). All subjects were right-handed. The local ethics board approved this study and participants provided written informed consent before study initiation.
Table 1.
Demographic, MRS and Segmentation Data for PIGD, TD, and control subjects
| PIGD | TD | Controls | P Value | |
|---|---|---|---|---|
| No.(female/male) | 13(8/5) | 9(6/3) | 16(9/7) | 0.789 |
| Age, y(mean ± SD) | 59.8±8.4 | 58.8±7.7 | 66±9.9 | 0.123 |
| Duration, y(mean ± SD) | 3.3±1.7 | 3.8±3.4 | --- | 0.582 |
| H-Y stage | 2.2±0.3 | 2.1±0.4 | --- | 0.206 |
| UPDRS (part III) | 30.2±7.7 | 32.1±9.9 | --- | 0.614 |
| GABA+ levels (IU) | 1.36±0.18 | 1.15±0.16 | 1.56±0.23 | 0.000a |
| Cr levels (IU) | 7.54±1.30 | 7.05±0.87 | 8.87±1.41 | 0.008a |
| GABA+ fitting errors (%) | 4.93±1.31 | 6.00±1.81 | 5.57±1.24 | 0.213 |
| GM/ (GM+WM) | 0.45±0.04 | 0.48±0.04 | 0.48±0.04 | 0.284 |
| CSF (%) | 5.7±1.7 | 6.4±1.6 | 5.0±1.5 | 0.214 |
H-Ystage = the Hoehn and Yahr stage; UPDRS = Unified Parkinson’s disease rating scale; SD = standard deviation; GM = gray matter; WM = white matter; CSF = cerebrospinal fluid; IU= institutional units.
Significant differences between groups are tested by analysis of covariance, adjusting for GM / (GM + WM+CSF) with P < 0.05 accepted as significant.
All the patients were assessed by a neurologist (Y.X.) who has six years’ experience in diagnosing movement disorders. The Hoehn and Yahr (H–Y) stage and the motor part (part III) of the UPDRS were used to assess the severity of illness during an “off” phase (at least 12 h off medicine).
We excluded patients with H-Y stage 4–5, Mini Mental State Examination score (MMSE) < 24/30, history of Deep Brain Stimulation, motor or neurological comorbidities affecting test performance, claustrophobia, medical or psychiatric conditions preventing the subject from undergoing an MRI examination.
PD patients were divided into PIGD, TD or indeterminate based on the method used by Jankovic et al (1) as the average global tremor score (UPDRS items 16 and 20–21: right and left arm tremor by history; rest tremor of either face, lips, or chin, all 4 limbs; postural or action tremor of both arms by examination. Total score divided by 8) / the average global ‘PIGD’ score (UPDRS items 13–15, 29, 30: walking, freezing, and falls by history; postural instability and gait by examination. Total score / 5). The TD subtype was defined as the ratios of 1.5 or more; whereas PIGD subtype as the ratios ≤ 1.0 and indeterminate subtype as the ratios between 1.0 and 1.5. In addition, patients with a zero in the average global ‘PIGD’ score were classified as TD; patients with a zero in the mean global tremor score were classified as PIGD. Subjects with an indeterminate subtype were excluded from further analysis.
MRI/MRS study protocol
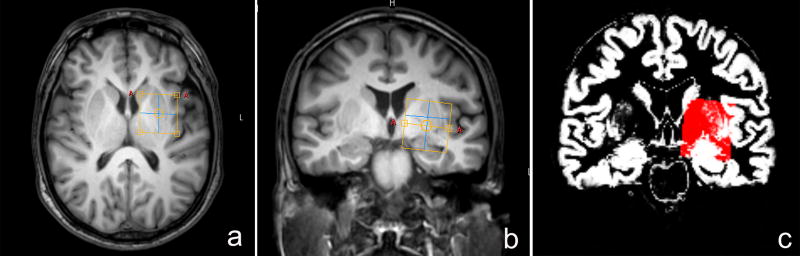
All the data were acquired using 3.0 T MR scanner (Philips Achieva TX, Best, The Netherlands) with an 8-channel phased-array head coil. Three-dimensional turbo field echo T1-weighted images were acquired using the following parameters: TR/TE = 8.2/3.7 ms; slice thickness =1 mm; matrix size = 256 × 256; field of view = 24 × 24 cm2; flip angle = 8°. The T1 images were used for MRS voxel placement in the region of the left BG and tissue segmentation. The MRS voxel was placed on axial images between the Sylvian fissure and the lateral ventricle, with the anterior border aligned with the medial midpoint of caudate nucleus, the sagittal superior border along the floor of the lateral ventricle body. Coronal and sagittal images were used to adjust the position of the ROI in order to maximize inclusion of basal ganglia structures while avoiding the lateral ventricle, as shown in Figure 1.
Fig. 1.
The position of voxels and corresponding segmentation data. T1-weighted TFE images show single-voxel placements centered on basal ganglia in the axial (a), coronal (b) projections. The corresponding results of brain segmentation are shown in the BG(c) – gray matter in white and white matter in black.
MEGA-PRESS was performed to detect GABA signals with the following parameters (16): TR/TE = 2000/68 ms; 256 signal averages; acquisition bandwidth 2000 Hz; voxel size = 3 × 3 × 2 cm3 and scan duration 8 min 48 s. Chemical Shift Selective Suppression (CHESS) was used for water suppression. MEGA-PRESS allows the GABA signal to be separated from other overlapping signals by making use of the J-coupling between GABA’s C-3 protons at 1.89 ppm and the C-4 protons at 3.01 ppm. During the scan, two spectra are obtained: every even acquisition (edit-OFF) is a regular PRESS sequence, whereas during odd acquisitions (edit-ON) a narrow-bandwidth (88 Hz FWHM) 14-ms sinc-Gaussian RF pulse is applied to the resonance peak at 1.89 ppm. The majority of peaks in the ON and OFF spectra, including overlapping Cr and choline (Cho) signals are unaffected by the editing pulses, so subtraction of the OFF from the ON spectra removes them, retaining a well-defined GABA signal at 3.01 ppm. Unsuppressed water signals were also recorded with the same parameters (except with 4 signal averages) as an internal concentration reference.
MRS data processing and quantification
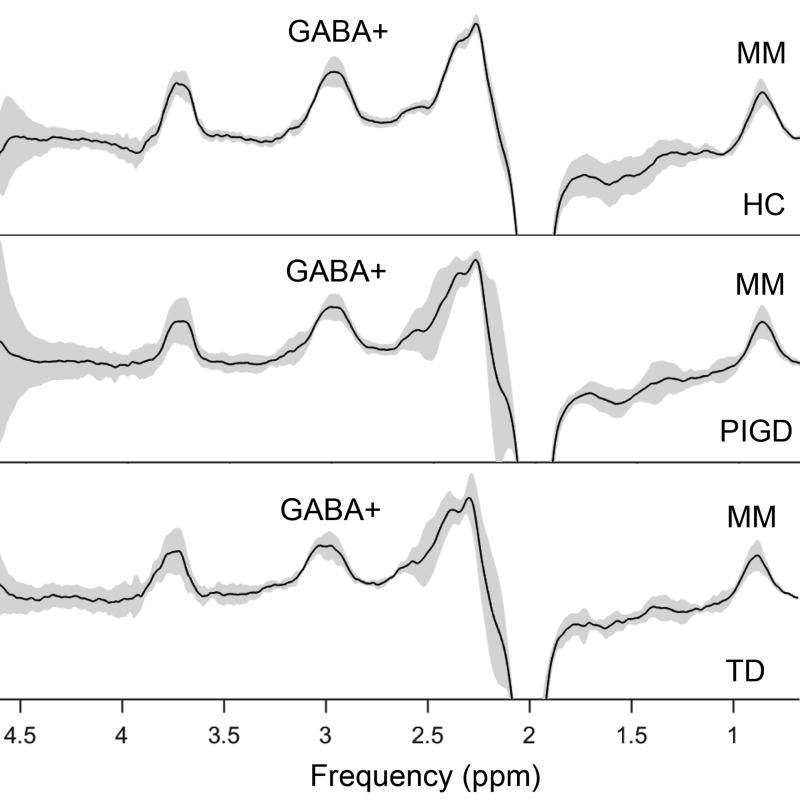
The GABA signal detected at 3 ppm also contains signal from macromolecules (MM) and homocarnosine (17), hence, the detected signal is referred to as GABA+ rather than GABA. GABA+ levels were quantified in the BG (Fig.1a, 1b) using the Matlab-based (The Mathworks, Natick, MA) analysis toolkit, Gannet 2.0 (18) (Fig.2). Gannet has two modules, GannetLoad and GannetFit, capable of processing MEGA-PRESS data and provide quantitative metrics of the GABA peak, as shown in the previous studies (16,19). Only spectra with a relative fitting error (FitError) of GABA+ generated by GannetFit below 10% were included in the final statistical analysis.
Fig. 2.
The mean (plus/minus standard deviation) GABA-edited spectra from the MEGA-PRESS sequence in BG of healthy controls (HC), PIGD and TD patients.
Water-scaled Cr concentrations can be estimated in institutional units (i.u) from metabolite peak amplitudes according to the following formula:
| (1) |
ICr and Iw are the averaged raw Cr and water signals, respectively, [H2O] is the brain water concentration (55,550 mmol/l), VISw is the water visibility (0.65). The relaxation adjustment terms T1W_Cr and T2W_Cr are given by the equation: T1W_Cr = [1−exp (−TR / T1W)] / [1−exp (−TR / T1Cr)]; T2W_Cr = exp (−TE / T2W) / exp (−TE / T2Cr), where TR is the repetition time, TE is the echo time, T1 and T2 are relaxation times. T1W and T2W of water, respectively, are 1.1 s and 0.095 s (20). T1Cr and T2Cr of Cr, respectively, are 1.38 s and 0.15 s (21). Hw / HCr is 2/3, defined as the number of protons contributing to the water signal and the Cr signal at 3.0 ppm.
Segmentation of T1-weighted images was performed using SPM8 (Fig.1c). The GM fractions (GM / (GM + WM + CSF) were estimated as the ratio of GM volume to the sum of GM, White matter (WM) volumes and cerebrospinal fluid (CSF) in the ROIs.
Statistical Analysis
Results are presented as mean ± standard deviation (SD). Statistical analysis was performed to determine differences of GABA+ levels and Cr levels among PIGD, TD, and controls using analysis of covariance (ANCOVA), adjusting for GM fractions and least significant difference (LSD) tests. The differences in sex, age, GABA+ fitting errors and GM fractions between the aforementioned three groups were determined using analysis of variance (ANOVA). Student’s t-test was used to compare the disease duration, H-Y stage, and UPDRS between PIGD and TD. Pearson correlation coefficients were used to assess the presence of linear associations between GABA+ levels and UPDRS. A p-value less than 0.05 was considered as significant. Statistical analyses were conducted using SPSS 22.0 (Chicago, IL, USA).
RESULTS
The ANOVA revealed no statistical differences in age (p = 0.847) and sex distribution (p = 0.123) (Table 1) between the three groups. No statistical differences were found in duration (p = 0.582), H-Y stage (p = 0.206) and UPDRS (p = 0.214) between PIGD and TD groups.
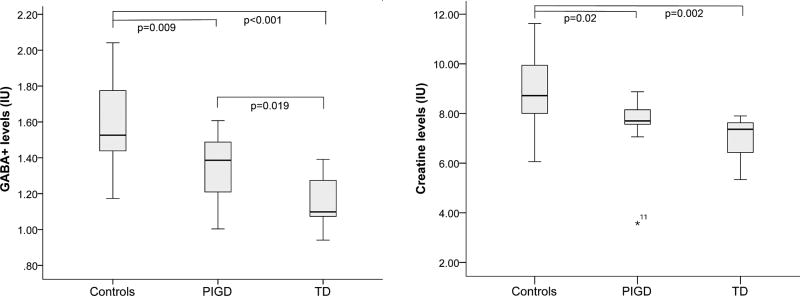
No differences among PIGD, TD and controls were observed in voxel segmentation (p = 0.284, see Table 1). GABA+ levels were significantly lower in BG regions of PD patients compared with healthy controls (p < 0.001). In PD patients, the GABA+ concentration was lower in the TD group than PIGD group (p = 0.019) (Fig.3).
Fig. 3.
GABA+ and Cr levels in controls, PIGD and TD groups using MEGA-PRESS. IU= institutional units. ANCOVA, adjusting for GM / (GM + WM+ CSF), and least significant difference (LSD) tests.
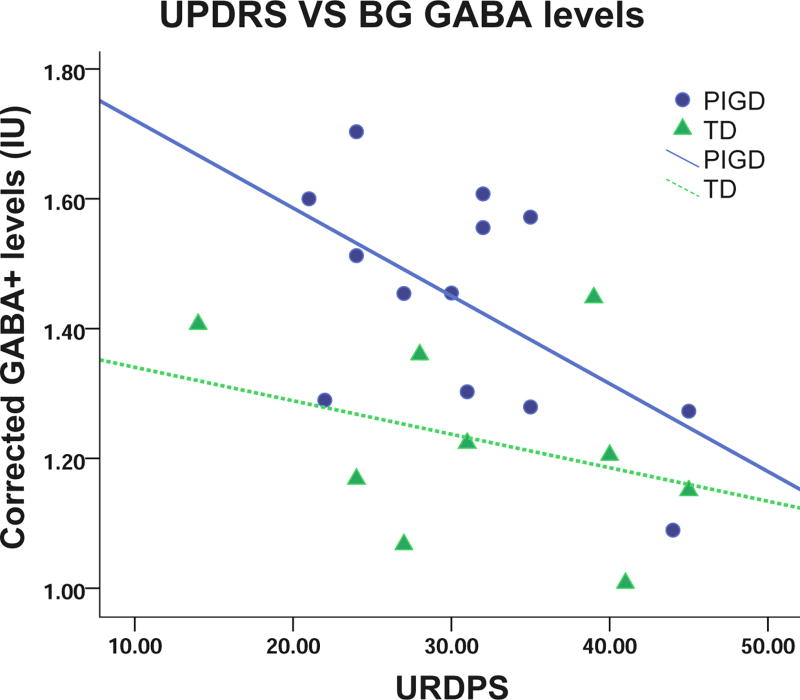
Table 1 shows Cr levels in PIGD and TD group were lower than healthy controls (p = 0.020 and 0.002, respectively). Cr level in TD group was not significantly lower than PIGD group (p = 0.440). There is a negative correlation in whole PD patients between GABA+ levels and UPDRS (r = −0.425, p = 0.048). A significant negative correlation was found in PIGD between GABA+ levels and UPDRS (r = −0.572, p = 0.041), while no significant correlation was observed in TD (r = −0.339, p = 0.372), as shown in Fig.4.
Fig. 4.
Relationship between GABA+ levels and UPDRS of PIGD (r = −0.572, p = 0.041) and TD (r = −0.339, p = 0.372) patients. A significant negative relationship is seen between GABA levels and UPDRS in PIGD, while none is seen in TD. IU= institutional units.
DISCUSSION
This study has shown that GABA+ levels were reduced in the left BG region of PD patients compared with age- and sex-matched healthy controls. We further demonstrated significant differences in GABA+ levels between the major motor sub-types of PD and GABA+ correlation with UPDRS. Basal ganglia Cr levels were also reduced in PD patients compared with healthy controls.
In previous human studies, GABA levels in the striatum or putamen have been reported to be either reduced (22), unaltered (14) or elevated (13,23) in PD compared with healthy controls. Similarly, either elevated BG GABA levels (24,25) or reduced left striatum GABA levels (26) have been reported in animal studies. Our study showed reduced GABA+ levels in a BG region, which includes the striatum. Relative to healthy controls, the differing GABA+ levels in PD patients between our study and literature could be due to several reasons. Firstly, a larger VOI (included striatum, globus pallidus, part of thalamus, and other gray- and white-matter structures) was chosen for our study (3 × 3 × 2 cm3) because of the different data acquisition strategy. Indeed, thalamic GABA levels in references (22) and (14) are significantly reduced. Secondly, there are differences between methodologies – our study is closest in methodology to (14). And in this study, water was used as reference to quantify GABA levels rather than Cr (24,27). Thirdly, cohort factors, such as demographics, inclusion criteria and effects of PD treatment can influence GABA levels (2). Lastly, there is a concern that high CSF content in PD BG voxels might contribute to lower GABA levels because of brain atrophy. However, the voxel CSF fraction is small (~6%) in all groups and group differences in CSF fraction are small and non-significant. Additionally, we have performed CSF correction on the water-referenced GABA+ measurements to mitigate such effects (which does not substantially change the statistical outcomes of the study).
The reduced GABA levels in our study might indicat that the pathogenesis of PD may be related to GABAergic neuronal loss or dysfunction. These results are in line with the GABA-collapse hypothesis recently proposed by Blaszczyk (28,29). PD is a severe multisystemic neurodegenerative disorder of the nervous system, whose clinical symptoms reflect the progression of the most advanced GABA pathology.
We further demonstrated significant differences in GABA+ levels between the major motor sub-types of PD. PIGD progresses more rapidly than TD (30,31), however, we found that the BG GABA+ levels in TD were significantly lower than PIGD. This may indicate that TD and PIGD differ in terms of central pathophysiological mechanism.
One previous study found no significant relationships between GABA levels in pons or putamen regions (using a 7T MR scanner) and the UPDRS (13). In our study, however, we found a significant negative relationship between BG GABA+ levels and UPDRS of PD patients, indicating that altered GABA levels relate to symptom type and severity. The significant relationship between GABA levels and UPDRS was mainly driven by the PIGD group probably because of the smaller number of PD patients in the TD group or limited range of GABA values, consequently, reduced statistical power to detect a correlation.
Altered Cr levels have previously been found in the putamen portion of BG, while other regions showed no changes (32,33). It has also been demonstrated that Cr can be elevated in PD patients after acute administration of L-DOPA (34). Given that Cr levels in PD patients were significantly different to healthy controls, in agreement with previous studies (34,35), Cr may not be an appropriate choice for internal reference, especially for the calculation of GABA levels in BG region of PDs (34,36).
The study has several limitations, including study design and data acquisition strategy. Firstly, the sample size in each group is rather small for a clinical study of this kind, especially for the TD group. Therefore, these results should be viewed as preliminary, and a larger sample size should be recruited to confirm the results. Secondly, it is always possible that group differences in metabolite levels arise as a result of relaxation differences (either in the metabolite or water signals) between groups. Future research estimating relaxation values (T1 and T2) in subjects with PD would be beneficial. Thirdly, the VOI was relatively large (3 × 3 × 2 cm3), comprising striatum, thalamus and globus pallidus structures. The larger VOI was necessary to increase the signal-to-noise ratio of the GABA signal (due to the low intensity of GABA MRS signals available at 3 T) and remains a limitation of MEGA-PRESS technique (7). The large VOI reduces the specificity of results; therefore, the results of our study may not be representative of individual structures of BG. Fourthly, the GABA+ levels detected by MEGA-PRESS represent GABA plus co-edited macromolecules (MM) and homocarnosine. New methods for detecting “pure GABA” (37) should be employed for future studies of PD patients, but remain extremely sensitive to experimental instability (38). Fifthly, only left BG regions were studied, basing that decision on handedness (all subjects are right-handed), rather than considering laterality with respect to the predominantly affected side. This remains a concern in spite of one previous study suggesting that there are no differences of major metabolites in PD patients between contralateral and ipsilateral sides (34).
We demonstrated, in a preliminary fashion, differences in GABA+ between PIGD and TD, and a correlation between GABA levels and UPDRS in PIGD/TD patients. Low BG GABA+ levels in PD patients, and differences between PIGD/TD patients, suggest that GABAergic dysfunction may play an important role in the pathogenesis of Parkinson’s disease.
Acknowledgments
We would like to thank all volunteers and patients for their participation.
Funding
This project was funded by National Natural Science Foundation of China (81671668; 81371534), Major research project of Shandong province (2016ZDJS07A16) and Shandong province focus on research and development programs (2015GGH318020). This study applies tools developed under NIH R01 EB016089 and P41 EB015909; RAEE also receives salary support from these grants.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Jankovic J, McDermott M, Carter J, et al. Variable expression of Parkinson's disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology. 1990;40(10):1529–1534. doi: 10.1212/wnl.40.10.1529. [DOI] [PubMed] [Google Scholar]
- 2.Dydak U, Edmondson DA, Zauber SE. Magnetic Resonance Spectroscopy in Parkinsonian Disorders. 2016:71–102. [Google Scholar]
- 3.Almuqbel M, Melzer TR, Myall DJ, et al. Metabolite ratios in the posterior cingulate cortex do not track cognitive decline in Parkinson's disease in a clinical setting. Parkinsonism & related disorders. 2016;22:54–61. doi: 10.1016/j.parkreldis.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Guo Z, Liu X, Hou H, et al. (1)H-MRS asymmetry changes in the anterior and posterior cingulate gyrus in patients with mild cognitive impairment and mild Alzheimer's disease. Comprehensive psychiatry. 2016;69:179–185. doi: 10.1016/j.comppsych.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Moon CM, Kim BC, Jeong GW. Effects of donepezil on brain morphometric and metabolic changes in patients with Alzheimer's disease: A DARTEL-based VBM and (1)H-MRS. Magnetic resonance imaging. 2016;34(7):1008–1016. doi: 10.1016/j.mri.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 6.Wu G, Shen YJ, Huang MH, Xing Z, Liu Y, Chen J. Proton MR Spectroscopy for Monitoring Pathologic Changes in the Substantia Nigra and Globus Pallidus in Parkinson Disease. AJR American journal of roentgenology. 2016;206(2):385–389. doi: 10.2214/AJR.14.14052. [DOI] [PubMed] [Google Scholar]
- 7.Mullins PG, McGonigle DJ, O'Gorman RL, et al. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. NeuroImage. 2014;86:43–52. doi: 10.1016/j.neuroimage.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR in biomedicine. 1998;11(6):266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 9.Grewal M, Dabas A, Saharan S, Barker PB, Edden RA, Mandal PK. GABA quantitation using MEGA-PRESS: Regional and hemispheric differences. Journal of magnetic resonance imaging : JMRI. 2016;44(6):1619–1623. doi: 10.1002/jmri.25324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lange T, Ko CW, Lai PH, Dacko M, Tsai SY, Buechert M. Simultaneous detection of valine and lactate using MEGA-PRESS editing in pyogenic brain abscess. NMR in biomedicine. 2016;29(12):1739–1747. doi: 10.1002/nbm.3660. [DOI] [PubMed] [Google Scholar]
- 11.Menschikov PE, Semenova NA, Ublinskiy MV, et al. (1)H-MRS and MEGA-PRESS pulse sequence in the study of balance of inhibitory and excitatory neurotransmitters in the human brain of ultra-high risk of schizophrenia patients. Doklady Biochemistry and biophysics. 2016;468(1):168–172. doi: 10.1134/S1607672916030029. [DOI] [PubMed] [Google Scholar]
- 12.Blandini F, Nappi G, Tassorelli C, Martignoni E. Functional changes of the basal ganglia circuitry in Parkinson's disease. Progress in neurobiology. 2000;62(1):63–88. doi: 10.1016/s0301-0082(99)00067-2. [DOI] [PubMed] [Google Scholar]
- 13.Emir UE, Tuite PJ, Oz G. Elevated pontine and putamenal GABA levels in mild-moderate Parkinson disease detected by 7 tesla proton MRS. PloS one. 2012;7(1):e30918. doi: 10.1371/journal.pone.0030918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dharmadhikari S, Ma R, Yeh CL, et al. Striatal and thalamic GABA level concentrations play differential roles for the modulation of response selection processes by proprioceptive information. NeuroImage. 2015;120:36–42. doi: 10.1016/j.neuroimage.2015.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. Journal of neurology, neurosurgery, and psychiatry. 1988;51(6):745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu B, Yang H, Gao F, et al. Investigation of brain GABA+ in primary hypothyroidism using edited proton MR spectroscopy. Clinical endocrinology. 2016 doi: 10.1111/cen.13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothman DL, Behar KL, Prichard JW, Petroff OA. Homocarnosine and the measurement of neuronal pH in patients with epilepsy. Magnetic resonance in medicine. 1997;38(6):924–929. doi: 10.1002/mrm.1910380611. [DOI] [PubMed] [Google Scholar]
- 18.Edden RA, Puts NA, Harris AD, Barker PB, Evans CJ. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. Journal of magnetic resonance imaging : JMRI. 2014;40(6):1445–1452. doi: 10.1002/jmri.24478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z, Zhang A, Zhao B, et al. GABA+ levels in postmenopausal women with mild-to-moderate depression: A preliminary study. Medicine. 2016;95(39):e4918. doi: 10.1097/MD.0000000000004918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wansapura JP, Holland SK, Dunn RS, Ball WS., Jr NMR relaxation times in the human brain at 3.0 tesla. Journal of magnetic resonance imaging : JMRI. 1999;9(4):531–538. doi: 10.1002/(sici)1522-2586(199904)9:4<531::aid-jmri4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Srinivasan R, Ratiney H, Lu Y, Chang SM, Nelson SJ. Comparison of T(1) and T(2) metabolite relaxation times in glioma and normal brain at 3T. Journal of magnetic resonance imaging : JMRI. 2008;28(2):342–350. doi: 10.1002/jmri.21453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerlach M, Gsell W, Kornhuber J, et al. A post mortem study on neurochemical markers of dopaminergic, GABA-ergic and glutamatergic neurons in basal ganglia-thalamocortical circuits in Parkinson syndrome. Brain research. 1996;741(1–2):142–152. doi: 10.1016/s0006-8993(96)00915-8. [DOI] [PubMed] [Google Scholar]
- 23.Kish SJ, Rajput A, Gilbert J, et al. Elevated γ-aminobutyric acid level in striatal but not extrastriatal brain regions in Parkinson's disease: Correlation with striatal dopamine loss. Annals of Neurology. 1986;20(1):26–31. doi: 10.1002/ana.410200106. [DOI] [PubMed] [Google Scholar]
- 24.Heo H, Ahn JB, Lee HH, et al. Neurometabolic profiles of the substantia nigra and striatum of MPTP-intoxicated common marmosets: An in vivo proton MRS study at 9.4 T. NMR in biomedicine. 2016 doi: 10.1002/nbm.3686. [DOI] [PubMed] [Google Scholar]
- 25.Buchanan RJ, Darrow DP, Meier KT, et al. Changes in GABA and glutamate concentrations during memory tasks in patients with Parkinson's disease undergoing DBS surgery. Frontiers in human neuroscience. 2014;8:81. doi: 10.3389/fnhum.2014.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao HC, Zhu H, Song CY, et al. Metabolic changes detected by ex vivo high resolution 1H NMR spectroscopy in the striatum of 6-OHDA-induced Parkinson's rat. Molecular neurobiology. 2013;47(1):123–130. doi: 10.1007/s12035-012-8336-z. [DOI] [PubMed] [Google Scholar]
- 27.Dydak U, Jiang YM, Long LL, et al. In vivo measurement of brain GABA concentrations by magnetic resonance spectroscopy in smelters occupationally exposed to manganese. Environmental health perspectives. 2011;119(2):219–224. doi: 10.1289/ehp.1002192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blaszczyk JW. Parkinson's Disease and Neurodegeneration: GABA-Collapse Hypothesis. Frontiers in neuroscience. 2016;10:269. doi: 10.3389/fnins.2016.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blaszczyk JW. Nigrostriatal interaction in the aging brain: new therapeutic target for Parkinson's disease. Acta neurobiologiae experimentalis. 2017;77(1):106–112. doi: 10.21307/ane-2017-041. [DOI] [PubMed] [Google Scholar]
- 30.Burn DJ, Rowan EN, Allan LM, Molloy S, O'Brien JT, McKeith IG. Motor subtype and cognitive decline in Parkinson's disease, Parkinson's disease with dementia, and dementia with Lewy bodies. Journal of neurology, neurosurgery, and psychiatry. 2006;77(5):585–589. doi: 10.1136/jnnp.2005.081711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alves G, Larsen JP, Emre M, Wentzel-Larsen T, Aarsland D. Changes in motor subtype and risk for incident dementia in Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2006;21(8):1123–1130. doi: 10.1002/mds.20897. [DOI] [PubMed] [Google Scholar]
- 32.Kickler N, Krack P, Fraix V, et al. Glutamate measurement in Parkinson's disease using MRS at 3 T field strength. NMR in biomedicine. 2007;20(8):757–762. doi: 10.1002/nbm.1141. [DOI] [PubMed] [Google Scholar]
- 33.Weiduschat N, Mao X, Beal MF, Nirenberg MJ, Shungu DC, Henchcliffe C. Usefulness of proton and phosphorus MR spectroscopic imaging for early diagnosis of Parkinson's disease. Journal of neuroimaging : official journal of the American Society of Neuroimaging. 2015;25(1):105–110. doi: 10.1111/jon.12074. [DOI] [PubMed] [Google Scholar]
- 34.Mazuel L, Chassain C, Jean B, et al. Proton MR Spectroscopy for Diagnosis and Evaluation of Treatment Efficacy in Parkinson Disease. Radiology. 2016;278(2):505–513. doi: 10.1148/radiol.2015142764. [DOI] [PubMed] [Google Scholar]
- 35.Hattingen E, Magerkurth J, Pilatus U, et al. Phosphorus and proton magnetic resonance spectroscopy demonstrates mitochondrial dysfunction in early and advanced Parkinson's disease. Brain : a journal of neurology. 2009;132(Pt 12):3285–3297. doi: 10.1093/brain/awp293. [DOI] [PubMed] [Google Scholar]
- 36.Tomiyasu M, Aida N, Shibasaki J, et al. In vivo estimation of gamma-aminobutyric acid levels in the neonatal brain. NMR in biomedicine. 2017;30(1) doi: 10.1002/nbm.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edden RA, Puts NA, Barker PB. Macromolecule-suppressed GABA-edited magnetic resonance spectroscopy at 3T. Magnetic resonance in medicine. 2012;68(3):657–661. doi: 10.1002/mrm.24391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edden RA, Oeltzschner G, Harris AD, et al. Prospective frequency correction for macromolecule-suppressed GABA editing at 3T. Journal of magnetic resonance imaging : JMRI. 2016;44(6):1474–1482. doi: 10.1002/jmri.25304. [DOI] [PMC free article] [PubMed] [Google Scholar]