Abstract
Objective
To investigate the relationship between spinal cord damage and specific motor function in participants with incomplete spinal cord injury (iSCI).
Design
single-blinded cross-sectional study design.
Setting
University setting research laboratory.
Participants
Fourteen individuals with chronic cervical iSCI (1 female and 13 males, average age = 43 ± 12 years old).
Interventions
Not applicable.
Main Outcome Measures
Axial T2 MRI of spinal cord damage was performed in 14 participants with iSCI. Each participants’ damage was processed for total damage quantification, lateral corticospinal tract (LCST) and gracilis fasciculus (GF) analysis. Plantarflexion and knee extension were quantified using an isokinetic dynamometer. Walking ability was assessed using a 6-minute walk test.
Results
Total damage was correlated with plantarflexion, knee extension, and distance walked in 6 minutes. Right LCST damage was correlated with right plantarflexion and right knee extension, while left LCST damage was correlated with left sided measures. Right and left GF damage were not correlated with the motor output measures.
Conclusions
MRI measures of spinal cord damage were correlated to motor function, and this measure appears to have spatial specificity to descending tracts, which may offer prognostic value following spinal cord injury.
Keywords: spinal cord injury, magnetic resonance imaging, lateral corticospinal tract, Spinal Cord Toolbox
The potential to recover at least some standing or basic walking ability in patients with motor incomplete spinal cord injury (iSCI) is variable and depends on a number of factors including, but not limited to, the severity of the injury, age, and the spatial extent and location of the spinal cord lesion.1 While the recovery of volitional walking ability is often a primary goal in rehabilitation, there remains a paucity of available evidence towards predicting which patients will, and to which extent, recover walking function.2
Conventional magnetic resonance imaging (MRI) in the acute stages after SCI can help define the spatial extent of a lesion.1,3–11 Sagittal T2-weighted imaging is commonly used clinically to provide estimates of the extent of damage (as identified by signal hyperintensity on T2-weighted images) along the superior-inferior axis and can be used to assign patients into qualitative prognostic categories (i.e. fair, poor).4,5,7 Recently, the amount of spared spinal cord tissue bridging, measured using sagittal T2-weighted imaging of spinal cord damage, proved to be predictive of neurological and functional recovery after SCI.12 However, two-dimensional (2D) sagittal sequences typically lack spatial information in the axial plane,13 making measurement of the highly specific somatotopic organization of spinal cord pathways a challenge. Standard axial T2-weighted images using 2D sequences have yet to demonstrate prognostic value for motor recovery.7 This may be due to challenges with quantifying the spatial extent and precise location of the spinal cord damage secondary to poor spatial coverage along the superior-inferior axis and/or partial volume artifacts.13
Continual advancements in MR technology such as high-resolution three-dimensional (3D) T2-weighted sequences and the open-source spinal cord image processing application, the Spinal Cord Toolbox, are, however, providing a means to quantitatively assess spinal cord damage and its relation to the spinal cord pathways.14,15 Based on a standard spinal cord anatomical atlas, a spinal cord template-based white-matter (WM) pathway atlas has been created for use within the Spinal Cord Toolbox.16 The WM atlas was created using probabilistic modeling and validated using five healthy controls, yet its clinical utility in individuals with spinal cord injury has not been explored.16
In this pilot study, we take advantage of these recent innovations and explore the validity of using the WM atlas and a novel semi-automated method for quantifying the extent and spatial location of the cross-sectional spinal cord damage in participants with iSCI. Before the predictive usefulness of such approach can be explored, the correlational value to specific motor function in a cross-sectional design must be established.
The purpose of this single blind cross-sectional study was to investigate the relationship between spinal cord damage and specific motor function in 14 participants with iSCI. We hypothesized that overall total spinal cord damage would be negatively correlated with motor output (ankle plantarflexion and knee extension) and walking ability. We then investigated how the extent of damage to two specific white matter tracts were related to these measures. As the lateral corticospinal tract (LSCT) conveys descending motor signals and the gracile fasciculus (GF) conveys sensory information during walking, we hypothesized that the extent of damage to the LCST, but not the GF, would be negatively correlated to motor output in an ipsilesional manner. Finally, since both motor output and sensory feedback are important for walking performance, we hypothesized that damage to these tracts would be negatively correlated with walking ability.
Methods
Participant characteristics
Participants were recruited from an academic hospital’s spinal cord injury database, in accordance with Institutional Review Board approval and the Declaration of Helsinki. Fourteen individuals with chronic cervical iSCI participated (1 female and 13 males, average age = 43 ± 12 years old), and other data from these participants have been previously reported.17 See Table 1 for details on the participant characteristics, including time since injury.
Table 1.
Participant Characteristics
| Participant # | Gender | Age (years) | Height (cm) | Weight (kg) | Time since Injury (years) | Mechanism of Injury | Level of Injury | ASIA Impairment Scale |
|---|---|---|---|---|---|---|---|---|
| iSCI1 | M | 53 | 185 | 82.1 | 6 | MVC | C5 | C |
| iSCI2 | M | 57 | 178 | 79.4 | 8 | Cycling Injury | C3 | D |
| iSCI3 | F | 52 | 157 | 83.9 | 0.5 | MVC | C6 | D |
| iSCI4 | M | 31 | 178 | 72.6 | 4 | Diving Injury | C5 | D |
| iSCI5 | M | 28 | 188 | 95.3 | 3 | Diving Injury | C5 | D |
| iSCI6 | M | 50 | 168 | 83.9 | 2 | Fall from Height | C6 | D |
| iSCI7 | M | 30 | 193 | 115.7 | 4 | Diving Injury | C6 | C |
| iSCI8 | M | 27 | 191 | 86.2 | 4 | Skiing Injury | C5 | C |
| iSCI9 | M | 32 | 173 | 81.7 | 5 | MVC | C7 | D |
| iSCI10 | M | 45 | 175 | 59.0 | 4 | ATV Injury | C3 | D |
| iSCI11 | M | 36 | 185 | 73.5 | 2 | Motorcycle Injury | C6 | C |
| iSCI12 | M | 50 | 178 | 72.6 | 5 | Fall from Height | C4 | D |
| iSCI13 | M | 64 | 175 | 108.9 | 4 | Fall | C5 | D |
| iSCI14 | M | 52 | 175 | 70.3 | 31 | Diving injury | C5 | D |
MVC = Motor Vehicle Collision, ATV = All Terrain Vehicle
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research according to the Declaration of Helsinki, including obtaining informed consent.
Image Acquisition
Imaging of the cervical spinal cord was performed with a 3.0 T Siemens (Munich, Germany) Prisma magnetic resonance scanner equipped with a 64-channel head/neck coil. With the assistance of trained study personnel, the patients were transferred to the scanner bed and placed supine. Following localizer scans, a high-resolution 3D image of the cervical and upper thoracic spine was acquired using a T2-weighted single slab 3D turbo spin echo sequence with a slab selective, variable excitation pulse (SPACE, TR=1500 ms, TEeff=115 ms, echo train length=78, flip angle=90°/140°, effective resolution=0.8 × 0.8 × 0.8 mm3, interpolated resolution=0.8 × 0.4 × 0.4 mm3).15,18 Patient comfort and safety was monitored throughout the imaging session.
Image Processing
An assessor blinded to the clinical history and experimental measures of the patients performed image processing using the open-source Spinal Cord Toolbox.14 The first step in the processing pipeline was the registration of the images to the MNI-Poly-AMU T2-weighted spinal cord template. To accomplish this, a binary mask of the spinal cord including the lesion was generated to identify the spinal cord in the image. To permit accurate registration of the lesioned spinal cord images to the template, the superior and inferior boundaries of the spinal cord lesion were identified, and the spinal cord within the boundaries of the lesion was filled with the average voxel intensity from the non-lesioned cord. The images were then straightened along the spinal cord and non-linearly registered to the template. The transformation from this registration was then applied to the lesioned spinal cord images to register the lesion images to the template. To quantify the extent of axial cord lesion, the maximum values within the boundaries of the lesion were then projected onto the axial plane, and the images were thresholded based on the voxel intensities within a non-lesioned 1 cm axial cross-section of the spinal cord immediately superior to the lesion. A threshold of two standard deviations above the mean was used to define lesioned tissue versus non-lesioned tissue. The extent of spinal cord damage was then quantified in the axial plane as the ratio of the spinal cord that was lesioned across the total cord and within the right and left LCST and GF using the WM atlas (i.e., damage = lesioned area/(lesioned area + non-lesioned area)). The images from each step of the processing and analysis pipeline were manually inspected for quality assurance, see Figure 1.
Figure 1.
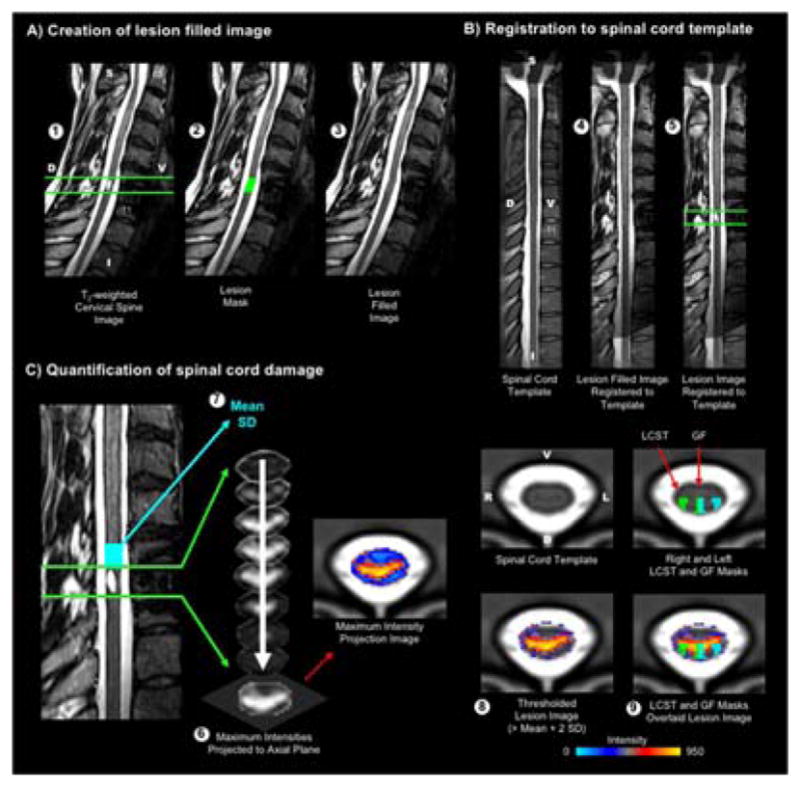
Methods. A) Prior to registering the images to the MNI-Poly-AMU spinal cord template, the superior and inferior boundaries (shown in green) of the spinal cord lesion were identified (1), and the lesioned cord was then filled (2 and 3) to permit accurate registration of the lesioned spinal cord images to the template. B) The lesion filled image was then straightened along the spinal cord and registered to the spinal cord template (4). The transformation from this registration was then applied to the lesioned spinal cord images to register the lesion images to the template (5). C) To quantify the extent of axial cord lesion, the maximum values within the boundaries of the lesion were then projected onto the axial plane to create a maximum intensity projection image (6). The mean and standard deviation (SD) of the voxel intensities were then calculated within a non-lesioned 1 cm axial cross-section of the spinal cord immediately superior to the lesion (shown in light blue) (7). The maximum intensity projection image was then thresholded at two standard deviations above the mean to define the lesion (8). The extent of spinal cord damage was then quantified in the axial plane as the ratio of the spinal cord that was lesioned across the total cord and within the right and left lateral corticospinal tracts (LCST) and gracile fasciculi (GF) (9). Participant iSCI11 is shown. The right and left LCST and GF are shown in green and light blue, respectively. D = dorsal, V = ventral, S = superior, I = inferior.
Plantarflexion Torque
All torque measurements were performed under the supervision of a licensed physical therapist that was blinded to the imaging findings. For the right and left isometric ankle plantarflexion torque measurement, participants were sat in a comfortable position with ankles at neutral, knees flexed at 20°, and hips flexed at 75°. Each participant’s trunk was secured in the testing seat using both lap belts and shoulder straps, while the participant was instructed to rest their hands on the handle bars (near the hips), but to not grip down. Maximum volitional torque was quantified following three maximal isometric contractions of ankle plantarflexion, each contraction held 3–4 seconds duration in an isokinetic dynamometer, each ankle tested separately (Biodex Rehabilitation System v3, Shirley NY USA). Verbal encouragement to facilitate maximum torque production was provided during each trial.19 Torque traces were monitored online using a biofeedback screen.
Knee Extension Torque
Right and left knee extension torque measurement proceeded in a similar fashion as the ankle torque testing (above). Participants were secured in sitting with the feet unsupported, knees flexed at 90°, and hips flexed at 75°, with each knee tested separately in an isokinetic dynamometer (Biodex Rehabilitation System v3, Shirley NY USA).
6-Minute Walk Test
Each participant completed the standard over ground 6-minute walk test,20 under the supervision of a licensed physical therapist who was blinded to the imaging findings. Participants were allowed to use assistive devices and braces as necessary and were instructed to walk at their regular, self-selected gait velocity, while the distance walked within the 6-minute time frame was recorded. The 6-minute walk test has been shown to be a valid and reliable measure in the iSCI population20,21.
Statistical Analysis
All statistical analyses of the data were performed using IBM SPSS (Version 21, Armonk, NY, USA). All data were tested for normality using Kolmogorov-Smirnov (KS) statistical analyses. As the identification of the superior and inferior borders of the spinal cord lesion was performed manually, we assessed the inter-rater reliability of identifying the lesion borders between two independent raters using intra-class correlation coefficients (ICC 2,1). Pearson correlations were chosen to examine linear relationships between the spatial distribution of spinal cord damage and plantarflexion torque production, knee extension torque production, and distance walked in 6 minutes. Significance was set at an alpha = 0.05.
Results
All data met assumptions of normality using KS statistical analyses. Potential confounding variables (age, height, weight, time since injury, and level of injury) were not significantly correlated with walking ability. Individual data are presented in Tables 2 and 3.
Table 2.
Individual Participant Data – Spinal Cord Damage Measures
| Participant # | Total Damage | Right LCST Damage | Left LCST Damage | Right GF Damage | Left GF Damage |
|---|---|---|---|---|---|
| iSCI1 | 0.98 | 1.00 | 1.00 | 1.00 | 1.00 |
| iSCI2 | 0.54 | 0.77 | 0.18 | 0.71 | 0.58 |
| iSCI3 | 0.87 | 0.69 | 1.00 | 1.00 | 0.94 |
| iSCI4 | 0.82 | 0.96 | 0.35 | 0.83 | 0.56 |
| iSCI5 | 0.79 | 1.00 | 0.33 | 1.00 | 1.00 |
| iSCI6 | 0.11 | 0.00 | 0.00 | 0.00 | 0.00 |
| iSCI7 | 0.97 | 1.00 | 1.00 | 1.00 | 1.00 |
| iSCI8 | 0.75 | 0.88 | 0.88 | 0.50 | 0.50 |
| iSCI9 | 0.10 | 0.00 | 0.00 | 0.00 | 0.00 |
| iSCI10 | 0.22 | 0.57 | 0.00 | 0.08 | 0.23 |
| iSCI11 | 0.62 | 0.71 | 0.29 | 0.69 | 0.77 |
| iSCI12 | 0.17 | 0.04 | 0.00 | 0.35 | 0.48 |
| iSCI13 | 0.91 | 1.00 | 1.00 | 0.87 | 1.00 |
| iSCI14 | 0.78 | 0.50 | 0.88 | 0.31 | 0.31 |
LCST = Lateral corticospinal tract. GF = Gracile Fasciculi. Damage = lesioned area/(lesioned area + non-lesioned area)
Table 3.
Individual Participant Data – Motor Output and Walking Ability
| Participant # | Left Plantarflexion Torque (Nm) | Right Plantarflexion Torque (Nm) | Left Knee Extension Torque (Nm) | Right Knee Extension Torque (Nm) | 6 Minute Walk Distance (m) |
|---|---|---|---|---|---|
| iSCI1 | 45.52 | 30.03 | 27.84 | 16.94 | 63.60 |
| iSCI2 | 82.93 | 64.45 | 101.95 | 100.13 | 186.90 |
| iSCI3 | 86.00 | 93.00 | 69.00 | 103.00 | 147.00 |
| iSCI4 | 44.35 | 29.56 | 57.17 | 63.98 | 134.18 |
| iSCI5 | 149.05 | 72.98 | 172.60 | 71.49 | 408.60 |
| iSCI6 | 104.91 | 133.29 | 112.20 | 127.80 | 412.10 |
| iSCI7 | 52.80 | 12.69 | 24.20 | 20.09 | 49.00 |
| iSCI8 | 12.66 | 29.73 | 11.78 | 40.13 | 29.95 |
| iSCI9 | 83.56 | 61.26 | 90.43 | 52.45 | 430.60 |
| iSCI10 | 68.29 | 55.46 | 132.00 | 70.00 | 165.00 |
| iSCI11 | 80.84 | 36.13 | 47.29 | 18.63 | 98.20 |
| iSCI12 | 92.40 | 95.61 | 96.87 | 122.65 | 244.40 |
| iSCI13 | 49.25 | 57.85 | 66.70 | 60.90 | 166.00 |
| iSCI14 | 37.70 | 47.59 | 37.61 | 41.29 | 241.00 |
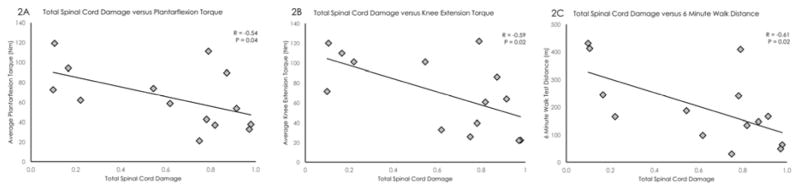
High inter-rater reliability was present for the identification the superior and inferior borders of the spinal cord lesion (Intra-class correlation coefficients (ICC 2,1) = 0.998 for both superior and inferior borders). Total spinal cord damage was significantly correlated with averaged plantarflexion torque (r = −0.54, p < 0.05), averaged knee extension torque (r = −0.59, p < 0.05), and distance walked in 6 minutes (r = −0.61, p < 0.05), see Figure 2.
Figure 2.
Total spinal cord damage was significantly correlated with averaged plantarflexion torque (left), averaged knee extension torque (middle), and distance walked in 6 minutes (right).
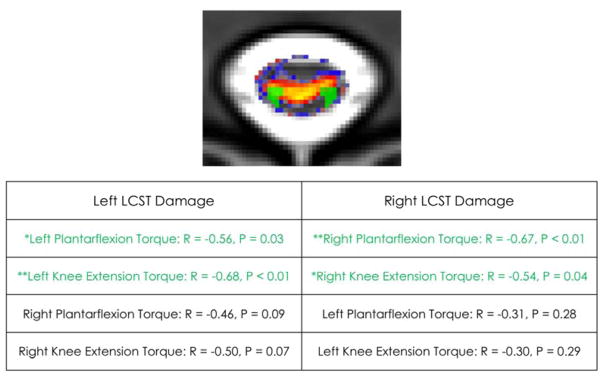
Right sided LCST damage was significantly correlated with the right sided motor output measures (right plantarflexion torque: r = −0.67, p < 0.01, right knee extension torque: r = −0.54, p < 0.05) but not significantly correlated with left sided motor output measures, see Figure 3. Left sided LCST damage was significantly correlated with the left sided motor output measures (left plantarflexion torque: r = −0.56, p< 0.05, left knee extension torque: r = −0.68, p < 0.01), but not significantly correlated with right sided motor output measures (see Figure 3).
Figure 3.
The lateral corticospinal tract (LCST) damage is depicted in green in participant iSCI11 (top). Correlation table for LCST damage and torque output (bottom).
Both right and left LCST damage was significantly correlated with distance walked in 6 minutes (right: r = −0.64, p = 0.01, left: r = −0.60, p = 0.02). The right sided GF damage was not significantly correlated with right sided motor output measures (right plantarflexion torque: r = −0.41, p = 0.14, right knee extension torque: r = −0.31, p = 0.27) and similarly, the left sided GF damage was not significantly correlated with motor output measures (left plantarflexion torque: r = −0.01, p = 0.97, left knee extension torque: r = −0.22, p = 0.45). A trend was found when correlating right and left GF damage to distance walked in 6 minutes (r = −0.51, p = 0.06 for both sides).
Discussion
Results of this preliminary study demonstrate the potential value of high resolution 3D imaging sequences and advanced analysis methods to quantify the spatial extent and location of spinal cord damage. There appears to be spatial specificity of the measure, which adds a level of validation in using the WM atlas in individuals with spinal cord injury. Right LCST damage was significantly correlated with each of the right-sided motor output measures, and left LCST damage was significantly correlated with the left-sided motor output measures. Furthermore, the measure appears to be tract-specific as the LCST damage was significantly correlated with its ipsilateral motor output, while the GF damage was not.
Previous work, using transcranial magnetic stimulation, demonstrated a correlation between the integrity of the lateral corticospinal tract and clinical measures of walking in the iSCI population22. In accordance with our MRI-based work, both the right and left lateral corticospinal tract damage measures were significantly correlated with distance walked in 6 minutes. A recent case study involving isolated spinal cord dorsal column damage due to a spinal tumor, the patient reported gait clumsiness amongst other symptoms.23 Similarly, animal studies show that isolated dorsal column lesions contribute to poorer performance on measures of walking.24 While not statistically significant, we found our dorsal column measure, that is GF damage, showed a negative linear trend with walking ability. In our participants, the fact that dorsal column injury was not in isolation likely contributed to the lack of statistical significance, as other ascending and descending pathways are involved with locomotion.25 GF conveys proprioception and light touch information from the lower limbs and provides important feedback during walking. Disruption of sensory feedback from injury could certainly result in gait deficits. In addition to GF, the spinothalamic and spinocerebellar tracts also convey sensory feedback to the brain. The lack of a statistical significance between GF damage and locomotion in the present sample may be due to compensation from these other sensory pathways. Future studies with larger population of patients with varying levels of motor and sensory deficits could quantitatively assess the relationship between spinal cord tract damage and specific motor and sensory deficits.
Our approach of targeting the damage in specific spinal cord regions is similar to recent literature using the Spinal Cord Toolbox and the advanced spinal cord MR imaging techniques of diffusion tensor imaging (DTI) and magnetization transfer imaging (MTR) in patients with degenerative cervical myelopathy26. These researchers found specific white matter changes rostral to the spinal cord compression26. It is plausible that applying a measure of region-specific and tract-specific damage quantification in the acute stage may be used to predict motor deficits, which could improve the clinical assessment and management of patients with spinal cord disorders.
Limitations
An inherent limitation of this cross-sectional design is that causal inference for spinal cord damage measure and deficits in motor output cannot be determined. With only one female participant and our male participant who sustained his spinal cord injury 31 years ago, our research sample certainly could have been more homogenous. A larger, prospective study involving participants with varying levels of motor impairment is warranted to investigate the prognostic value of this MRI measure of spinal cord damage for predicting motor output following spinal cord injury. Other variables such as spasticity may have influenced the results of motor output and ambulation as it has been demonstrated in people with neuromuscular disorders27,28 however we did not quantitatively assess spasticity in this present study. Because our pilot study was exploratory in nature, we made necessary assumptions that our spinal cord damage measured was valid, yet MR artefact could have played a role in terms of accuracy and sensitivity considering the wide range of cord damage in our participants classified as AIS D (0.54 – 0.91). The Spinal Cord Toolbox and WM atlas were created and validated using healthy control imaging data. Although our preliminary results suggest that these image processing tools may be valid for use in participants with spinal cord injury, further research is needed to corroborate these findings. For future studies, a larger sample size will allow us to build more advanced predictive models to better assess the interaction between damage variables and function. A larger, prospective study tracking participants with varying levels of motor impairment in longitudinal fashion is warranted. Such investigation would aim to identify the prognostic value of this MRI measure of spinal cord damage for motor output and recovery following spinal cord injury.
Conclusion
MRI measures of spinal cord damage were significantly correlated with motor output in a tract-specific manner, and this measure also appears to have spatial specificity. With future research, this imaging method may offer prognostic value following spinal cord injury.
Highlights.
Structural MRI of spinal cord damage is negatively correlated with walking output in people with motor incomplete spinal cord injury.
Lateral corticospinal tract damage is related to specific lower extremity motor deficits, in an ipsilesional manner, in these participants.
Acknowledgments
This research was conceptualized and carried out at Northwestern University, Feinberg School of Medicine, Department of Physical Therapy and Human Movement Sciences
We thank all participants for their willingness to take part in this study. James M. Elliott is supported by the NIH award 1 R01HD079076-01A1, entitled “Neuromuscular Mechanisms Underlying Poor Recovery from Whiplash Injuries”. Andrew C. Smith is supported by the NIH Extramural Loan Repayment Program for Clinical Researchers funded by the National Institute of Neurological Disorders and Stroke, and by the Foundation for Physical Therapy Promotion of Doctoral Studies programs. Kenneth A. Weber II is supported by the Interdisciplinary Research Training in Pain and Substance Use Disorders T32DA035165 funded by the National Institute on Drug Abuse.
List of Abbreviations
- iSCI
incomplete spinal cord injury
- MRI
magnetic resonance imaging
- 2D
two dimensional
- 3D
three dimensional
- WM
white matter
- LCST
lateral corticospinal tract
- GF
gracile fasciculus
- T
Tesla
- KS
Kolmogorov-Smirnov
- ICC
intra-class correlation
- DTI
diffusion tensor imaging
- MTR
magnetization transfer ratio
Footnotes
Conflict of Interest Statement: Elliott and Parrish - Relevant financial activities outside the submitted work: board membership, consultancy, other (Pain ID LLC), payment for lectures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wilson JR, Grossman RG, Frankowski RF, et al. A clinical prediction model for long-term functional outcome after traumatic spinal cord injury based on acute clinical and imaging factors. J Neurotrauma. 2012;29(13):2263–2271. doi: 10.1089/neu.2012.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ditunno PL, Patrick M, Stineman M, Ditunno JF. Who wants to walk? Preferences for recovery after SCI: a longitudinal and cross-sectional study. Spinal Cord. 2008;46(7):500–506. doi: 10.1038/sj.sc.3102172. [DOI] [PubMed] [Google Scholar]
- 3.Flanders A, Schaefer D, Doan H, Mishkin M, Gonzalez C, Northrup B. Acute cervical spine trauma: correlation of MR imaging findings with degree of neurologic deficit. Radiology. 1990;177:25–33. doi: 10.1148/radiology.177.1.2399326. [DOI] [PubMed] [Google Scholar]
- 4.Flanders A, Spettell C, Tartaglino L, Friedman D, Herbison G. Forecasting motor recovery after cervical spinal cord injury: value of MR imaging. Radiology. 1996;201:649–655. doi: 10.1148/radiology.201.3.8939210. [DOI] [PubMed] [Google Scholar]
- 5.Boldin C, Raith J, Fankhauser F, Haunschmid C, Schwantzer G, Schweighofer F. Predicting neurologic recovery in cervical spinal cord injury with postoperative MR imaging. Spine (Phila Pa 1976) 2006;31(5):554–559. doi: 10.1097/01.brs.0000201274.59427.a4. [DOI] [PubMed] [Google Scholar]
- 6.Lundell H, Barthelemy D, Skimminge a, Dyrby TB, Biering-Sørensen F, Nielsen JB. Independent spinal cord atrophy measures correlate to motor and sensory deficits in individuals with spinal cord injury. Spinal Cord. 2011;49(1):70–75. doi: 10.1038/sc.2010.87. [DOI] [PubMed] [Google Scholar]
- 7.Bozzo A, Marcoux J, Radhakrishna M, Pelletier J, Goulet B. The role of magnetic resonance imaging in the management of acute spinal cord injury. J Neurotrauma. 2011;28(8):1401–1411. doi: 10.1089/neu.2009.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burns AS, Marino RJ, Flanders AE, Flett H. Clinical Diagnosis and Prognosis Following Spinal Cord Injury. 1. Vol. 109. Elsevier B.V; 2012. [DOI] [PubMed] [Google Scholar]
- 9.Ellingson BM, Salamon N, Holly LT. Imaging techniques in spinal cord injury. World Neurosurg. 2014;82(6):1351–1358. doi: 10.1016/j.wneu.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scivoletto G, Tamburella F, Laurenza L, Torre M, Molinari M. Who is going to walk? A review of the factors influencing walking recovery after spinal cord injury. Front Hum Neurosci. 2014;8:141. doi: 10.3389/fnhum.2014.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyanji F, Furlan J, Aarabi B, Arnold P, Fehlings M. Acute cervical traumatic spinal cord injury: MR imaging findings correlated with neurologic outcome - prospective study with 100 consecutive patients. Radiology. 2007;243(3):820–827. doi: 10.1148/radiol.2433060583. [DOI] [PubMed] [Google Scholar]
- 12.Huber E, Lachappelle P, Sutter R, Curt A, Freund P. Are midsagittal tissue bridges predictive of outcome after cervical spinal cord injury? Ann Neurol. 2017;81(5):740–748. doi: 10.1002/ana.24932. [DOI] [PubMed] [Google Scholar]
- 13.Martin AR, Aleksanderek I, Cohen-Adad J, et al. Translating state-of-the-art spinal cord MRI techniques to clinical use: A systematic review of clinical studies utilizing DTI, MT, MWF, MRS, and fMRI. Neuro Image Clin. 2016;10:192–238. doi: 10.1016/j.nicl.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Leener B, Lévy S, Dupont SM, et al. SCT: Spinal Cord Toolbox, an open-source software for processing spinal cord MRI data. Neuroimage. 2017;145:24–43. doi: 10.1016/j.neuroimage.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Lichy MP, Wietek BM, Mugler JP, et al. Magnetic resonance imaging of the body trunk using a single-slab, 3-dimensional, T2-weighted turbo-spin-echo sequence with high sampling efficiency (SPACE) for high spatial resolution imaging: initial clinical experiences. Invest Radiol. 2005;40(12):754–760. doi: 10.1097/01.rli.0000185880.92346.9e. [DOI] [PubMed] [Google Scholar]
- 16.Lévy S, Benhamou M, Naaman C, Rainville P, Callot V, Cohen-Adad J. White matter atlas of the human spinal cord with estimation of partial volume effect. Neuroimage. 2015;119:262–271. doi: 10.1016/j.neuroimage.2015.06.040. [DOI] [PubMed] [Google Scholar]
- 17.Smith AC, Weber KA, Parrish TB, et al. Ambulatory function in motor incomplete spinal cord injury: A magnetic resonance imaging study of spinal cord edema and lower extremity muscle morphometry. Spinal Cord. 2017;55:672–678. doi: 10.1038/sc.2017.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mugler JP, Bao S, Mulkern RV, et al. Optimized single-slab three-dimensional spin-echo MR imaging of the brain. Radiology. 2000;216(3):891–899. doi: 10.1148/radiology.216.3.r00au46891. [DOI] [PubMed] [Google Scholar]
- 19.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81(4):1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 20.Scivoletto G, Tamburella F, Laurenza L, Foti C, Ditunno JF, Molinari M. Validity and reliability of the 10-m walk test and the 6-min walk test in spinal cord injury patients. Spinal Cord. 2011;49(6):736–740. doi: 10.1038/sc.2010.180. [DOI] [PubMed] [Google Scholar]
- 21.van Hedel HJ, Wirz M, Dietz V. Assessing walking ability in subjects with spinal cord injury: validity and reliability of 3 walking tests. Arch Phys Med Rehabil. 2005;86(2):190–196. doi: 10.1016/j.apmr.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Barthélemy D, Willerslev-Olsen M, Lundell H, Biering-Sørensen F, Nielsen JB. Assessment of transmission in specific descending pathways in relation to gait and balance following spinal cord injury. Prog Brain Res. 2015;218:79–101. doi: 10.1016/bs.pbr.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Aiyer SN, Shetty AP, Kanna R, Maheswaran A, Rajasekaran S. Isolated dorsal column dysfunction due to an intraspinal Osteolipoma - Case report and review of literature. J Clin Orthop trauma. 2016;7(Suppl 1):2–4. doi: 10.1016/j.jcot.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fagoe ND, Attwell CL, Eggers R, et al. Evaluation of Five Tests for Sensitivity to Functional Deficits following Cervical or Thoracic Dorsal Column Transection in the Rat. PLoS One. 2016;11(3):e0150141. doi: 10.1371/journal.pone.0150141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Côté M-P, Detloff MR, Wade RE, Lemay MA, Houlé JD. Plasticity in ascending long propriospinal and descending supraspinal pathways in chronic cervical spinal cord injured rats. Front Physiol. 2012;3:330. doi: 10.3389/fphys.2012.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin AR, De Leener B, Cohen-Adad J, et al. 163 Microstructural MRI Quantifies Tract-Specific Injury and Correlates With Global Disability and Focal Neurological Deficits in Degenerative Cervical Myelopathy. Neurosurgery. 2016;63(Suppl 1):165. [Google Scholar]
- 27.Pau M, Coghe G, Corona F, Marrosu MG, Cocco E. Effect of spasticity on kinematics of gait and muscular activation in people with Multiple Sclerosis. J Neurol Sci. 2015;358(1–2):339–344. doi: 10.1016/j.jns.2015.09.352. [DOI] [PubMed] [Google Scholar]
- 28.Bar-On L, Molenaers G, Aertbeliën E, Monari D, Feys H, Desloovere K. The relation between spasticity and muscle behavior during the swing phase of gait in children with cerebral palsy. Res Dev Disabil. 2014;35(12):3354–3364. doi: 10.1016/j.ridd.2014.07.053. [DOI] [PubMed] [Google Scholar]