Abstract
Background
Anabolic-androgenic steroid abuse is implicated in maladaptive behaviors such as impaired cognition in humans. In a rat model, our lab has shown that testosterone decreases preference for a large/uncertain reward in probability discounting. Other studies have shown that androgens decrease dopamine D1 and D2 receptors in the nucleus accumbens shell, a region important for decision-making behavior in probability discounting. Thus, we attempted to restore selection of the large/uncertain reward in testosterone-treated rats by administering the D2 receptor agonist quinpirole or the D1 receptor agonist SKF81297 and testing probability discounting.
Methods
Adolescent male Long-Evans rats were treated chronically with high-dose testosterone (7.5 mg/kg) or vehicle (13% cyclodextrin in water), and tested for probability discounting after injections of saline, 0.1 and 0.5 mg/kg of quinpirole or SKF81297. Rats chose between a small/certain reward (1 sugar pellet, 100% probability) and a large/uncertain reward (4 pellets, decreasing probability: 100, 75, 50, 25, 0%).
Results
Testosterone-treated rats selected the large/uncertain reward significantly less than vehicle-treated controls after saline injection. However, acute injection with 0.1 mg/kg quinpirole increased large/uncertain reward preference in testosterone-treated rats only, indicated by a testosterone x quinpirole interaction. At 0.5 mg/kg, quinpirole increased large/uncertain reward preference in all rats. Acute injection with SKF81297 at 0.1 or 0.5 mg/kg rescued large/uncertain reward preference in testosterone-treated rats by eliminating the difference between groups.
Conclusions
It appears that altered probability discounting behavior in testosterone-treated rats is due to both decreased D1 and D2 receptor function.
Keywords: anabolic agents, dopamine, decision making, operant behavior, food reward
INTRODUCTION
Anabolic-androgenic steroids (AAS) are drugs of abuse associated with maladaptive psychological and behavioral effects such as impaired decision making. Once restricted to elite athletes, AAS use has spread to college and even high-school athletics. Among U.S. high school students, 4–6% of boys have used AAS (Johnston et al. 2013). Additionally, high-school students who reported AAS use also exhibited higher frequencies of risky sex, drinking and driving, carrying a weapon, and not wearing a helmet or seat belt (Middleman et al. 1995). Adverse effects on decision making and risk taking are particularly troubling in these young AAS users, as the neural circuitry underlying cognition is still under development in the adolescent brain (Blakemore and Choudhury 2006). Indeed, we do not know what neurobiological changes underlie AAS effects on decision-making behavior. This was the focus of the present study.
AAS are implicated in cognitive and behavioral dysfunction in both humans and animal subjects (Pope et al. 2013; Wood et al. 2013; Wallin and Wood 2015; Wallin et al. 2015). In particular, AAS have been linked to impaired decision making in humans, with AAS users more likely to engage in risky behaviors such as hazardous alcohol consumption, criminal activity, and poly-substance abuse (Hallgren et al. 2015). However, human studies of AAS use pose a variety of limitations. Animal studies allow us overcome variability in AAS type and dose, and to control for pre-existing individual differences in behavior, including decision-making ability. Using high-dose testosterone to model AAS abuse in male rats, our lab has recently shown that testosterone alters decision making and risk taking in operant behavioral discounting paradigms, where rats respond on 1 of 2 levers to earn food reward (Wood et al. 2013; Cooper et al. 2014; Wallin et al. 2015). One lever offers a small “safe” reward, while the other lever delivers a large reward that is “discounted” or made less desirable by pairing with a cost such as delay, effort, uncertainty, or punishment. With delay discounting, testosterone-treated rats are more willing to wait for a large/delayed reward compared to vehicle-treated controls (Wood et al. 2013). Similarly, testosterone increased willingness to exert physical effort for a large reward in an effort discounting paradigm (Wallin et al. 2015).
To further investigate testosterone effects on decision making, we tested two types of risk taking: physical risk taking (punishment discounting) and risk taking in the context of reward uncertainty (probability discounting, PD). In punishment discounting, testosterone-treated rats were more likely than controls to choose a large reward paired with a mild footshock over a small reward with no shock (Cooper et al. 2014). Importantly, this increase in physical risk taking cannot be attributed to changes in pain perception, as AAS have been shown to have no effect on pain sensitivity in several nociceptive models (Celerier et al, 2003; Tsutsui et al, 2011). In contrast to punishment discounting, testosterone decreased risk taking in PD, with testosterone-treated rats significantly less likely to choose a large/uncertain reward compared to controls (Wallin et al. 2015). These contrasting results show that testosterone affects decision making in a context-dependent manner: increasing tolerance for physical discomfort, but decreasing tolerance for reward uncertainty.
Decision making is exquisitely sensitive to dopamine function in the mesocorticolimbic DA system (Floresco et al. 2008b). Effects of dopaminergic modulations on PD have been well-characterized in previous studies. Dopamine D1 (D1R) and D2 receptor (D2R) antagonists decrease selection of the large/uncertain reward in PD, while dopamine receptor agonists have the opposite effect (Floresco et al. 2008b; St. Onge and Floresco 2009). PD behavior is also dependent on the nucleus accumbens (Acb), particularly the shell subregion of Acb (Stopper and Floresco 2011). In this regard, inactivation of Acb shell decreases selection of the large/uncertain reward in PD, while inactivation of the core subregion has no effect on this task (Stopper and Floresco 2011). Thus, response to reward uncertainty in PD appears to depend on dopamine receptor function in the shell of Acb.
AAS affect density of dopamine receptors throughout the mesolimbic dopamine system. Specifically, AAS decrease D1R and D2R density in Acb shell of male rats (Kindlundh et al. 2001). Therefore, we hypothesize that testosterone may decrease selection of the large/uncertain reward in PD by decreasing D1R and D2R function. To test this, we attempted to rescue PD behavior in testosterone-treated rats by treatment with the D2R agonist quinpirole hydrochloride (quinpirole) or the D1R agonist SKF 81297 (SKF).
METHODS
Animals
32 Male Long-Evans rats (5 weeks of age at the start, Charles River Laboratories, MA) were assigned to receive quinpirole or SKF injections (n=16 each) and were treated chronically with either vehicle or testosterone (n=8/group). Rats were pair-housed with ad libitum access to water under a reversed 14L:10D photoperiod, and performed daily behavioral testing (5 days/week) during the dark phase. Rats remained gonad-intact in order to approximate human AAS use. To maintain a slow rate of growth (3–4 g/day) and facilitate operant responding, rats were food restricted as in our previous studies (Cooper et al. 2014; Wallin and Wood et al. 2015). Vehicle- and testosterone-treated groups did not differ in body weight at the start of the study or throughout testing. Experimental procedures were approved by USC’s Institutional Animal Care and Use Committee and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, 8th Ed (National Research Council, National Academies Press, Washington DC; 2011).
Drug treatments
AAS
As in our previous studies (Cooper et al. 2014; Wallin and Wood 2015), rats received daily injections sc of 7.5 mg/kg testosterone (Steraloids, RI) or vehicle (3% ethanol and 13% cyclodextrin (RBI, MA) 5 d/wk, beginning at 5 weeks of age. Pubertal treatment mirrors patterns of human use, where 4–6% of high school boys in the United States have used AAS (Johnston et al., 2013). Furthermore, AAS have the strongest behavioral effects in rodents when introduced in adolescence (Salas-Ramirez et al., 2008). Injections were delivered immediately before rats were placed in the operant chambers. Rats received injections for at least two weeks prior to behavioral testing, and daily injections continued for the duration of the experiment (Figure 1B). Testosterone was used because it is the endogenous AAS, and accounts for the largest number of adverse analytical findings (55.5%) in urine tests by World Anti-Doping Agency-accredited laboratories (WADA 2012). At 7.5 mg/kg, this dose approximates a heavy steroid dose in humans, and has been used to test effects on rodent discounting behavior (Cooper et al. 2014; Wallin and Wood 2015; Wallin et al, 2015; Wood et al, 2013).
Figure 1.
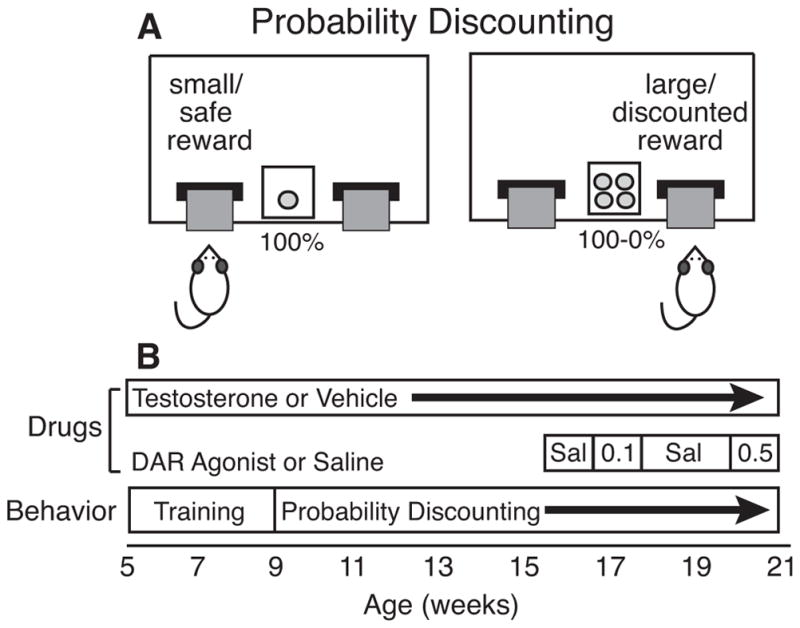
A) Operant chamber for probability discounting. Rats choose between a small/certain reward (1 pellet delivered 100% of the time) and a large/uncertain reward (4 pellets delivered with decreasing probability: 100, 75, 50, 25 and 0%). B) Timeline of drug treatments and behavioral testing relative to age. Sal: Saline; 0.1: 0.1 mg/kg quinpirole or SKF 81297; 0.5: 0.5 mg/kg quinpirole or SKF 81297.
Dopamine receptor agonists
Quinpirole (Sigma-Aldrich, WI) and SKF (Cayman Chemical, MI) were dissolved in 0.9 % saline at a concentration of 0.1 mg/ml. Previous studies have shown that quinpirole induces a transient decrease in locomotor activity in rats (Schindler and Carmona 2002; Eilam and Szechtman 1989). The motor effects of quinpirole dissipate within 60 minutes, while the cognitive effects persist for several hours (Eagle et al. 2014). Thus, rats received saline or dopamine receptor agonist injections ip and were placed separately in clean cages 1 hour prior to behavioral testing. Quinpirole and SKF were each tested at 0.1 and 0.5 mg/kg, starting with the lowest dose. These doses have previously been shown to affect cognition in rats (Boulougouris et al. 2009; Eagle et al. 2014; St Onge and Floresco 2009). Physiologic saline was used as a vehicle control in equivalent volume. Rats continued to receive daily testosterone or cyclodextrin vehicle treatment immediately before training and testing throughout the study.
Training
Rats were trained to respond for 45 mg sucrose pellets (Bio-Serv Inc., Frenchtown, NJ) in operant chambers equipped with 2 retractable levers on either side of a food cup connected to a pellet dispenser, and with a houselight for illumination (Figure 1A). Chambers were enclosed in sound-attenuating boxes with fans for ventilation. Initially, rats learned to respond on each lever to receive a pellet. Next, rats were required to respond within 10 seconds after lever insertion. They were then trained in reward discrimination, where both levers were available. These sessions consisted of 5 blocks of 16 trials each: 8 forced-choice trials followed by 8 free-choice trials. In forced-choice trials, 1 lever was inserted on each trial (4 trials/lever). In free-choice trials, both levers were inserted, and the rat could select either the small reward (1 pellet) or large reward (4 pellets) lever (Figure 1). Each trial lasted 20 seconds, and rats had 10 seconds to make a response before the levers retracted and the trial was counted as an omission. Location of the small and large reward levers (left vs. right) was counterbalanced among rats. All rats were required to complete 80 trials with >80% selection of the large reward lever.
Probability Discounting
Once rats mastered reward discrimination, they were tested for PD as in Wallin et al. (2015). As in reward discrimination, each daily session consisted of 80 trials, divided into 5 blocks of 8 forced-choice and 8 free-choice trials each. Selection of the small/certain reward lever always resulted in delivery of 1 pellet. Selection of the large/uncertain reward lever delivered 4 pellets with decreasing probability on each block (100, 75, 50, 25, and 0%). On rewarded trials, the houselight remained lit for 2 seconds after lever selection while pellets were delivered. On omitted or unrewarded trials the levers retracted and house light turned off for the remainder of the trial.
Once PD behavior stabilized, rats were tested for 3 days with saline injections ip 1 hour prior to daily PD testing (Figure 1B). Next, rats were injected for 5 days with 0.1 mg/kg quinpirole or SKF prior to daily PD testing. Subsequently, rats were injected for 10 days with saline until their operant behavior in PD stabilized and large reward preference in both groups returned to the level of initial saline treatment. Finally, rats were tested for PD with 0.5 mg/kg injections of dopamine receptor agonist for 5 days.
Open field
We have previously shown that icv administration of testosterone decreases locomotion (Peters and Wood 2004). To test if chronic systemic testosterone in the current study inhibits locomotion, and to determine if testosterone and quinpirole have a synergistic effect to suppress motor activity, rats were tested for activity in an open field on the first days of saline, 0.1 mg/kg and 0.5 mg/kg dopamine receptor agonist injections. Rats were tested individually 20 minutes after ip injection of saline or agonist, 40 minutes before operant testing. In the open field test, each rat was introduced for 3 minutes into a white box (32×32 inches) with a lined grid floor of 4×4 inch squares. Open field sessions were videotaped and analyzed and for motor activity, measured by total number of lines crossed by all four paws in each 3-minute session.
Data analysis
Large/uncertain reward preference was defined as the percent of completed free-choice trials/block in which rats chose the large/uncertain reward lever. Large/uncertain reward preference in each probability block was averaged for vehicle- and testosterone-treated rats over the last 3 days of testing with saline and each dose of quinpirole or SKF. Data from quinpirole- and SKF-treated rats were analyzed separately. Initially, large/uncertain reward preference was compared by a three-factor mixed ANOVA with testosterone treatment as the between-subjects factor and probability block and dopamine receptor agonist treatment (saline, 0.1, and 0.5 mg/kg quinpirole or SKF) as repeated measures. When there was a significant interaction, follow-up ANOVAs were completed to compare the effect of each agonist dose on large/uncertain reward preference relative to behavior after initial saline treatment, as in Wallin-Miller et al. (2017).
To investigate sensitivity to reward delivery and omission in PD, Win-Stay and Lose-Shift behavior was analyzed on a trial-by-trial basis (as in Wallin et al. 2015 and Stopper and Floresco 2011). A Win-Stay occurred when the rat received the large reward (win), and responded on the large reward lever again in the following trial (stay). A Lose-Shift occurred when the rat received no pellets from the large reward lever (loss), and selected the small reward on the following trial (shift). Win-Stay and Lose-Shift ratios were computed as the number of times each behavior occurred divided by the total number of wins or losses, respectively. Additionally, Total Shift ratio was analyzed to determine the overall tendency to switch between levers regardless of previous trial outcomes. Total Shift ratio was calculated as:
Win-Stay, Lose-Shift, and Total Shift ratios from each session were averaged for vehicle- and testosterone-treated rats under each dopamine receptor agonist treatment and compared by RM-ANOVA with testosterone treatment as the between-subjects factor and dopamine receptor agonist (0.0, 0.1, and 0.5 mg/kg) as the repeated measure.
In the open field test, number of lines crossed was averaged for each group (vehicle and testosterone) under each agonist treatment. Data were analyzed by RM-ANOVA with testosterone as the between-subjects factor and dopamine receptor agonist (0.0, 0.1, and 0.5 mg/kg quinpirole or SKF) as the repeated measure. Post-hoc analysis compared lines crossed under each agonist treatment to saline by Student’s t-test with the Bonferroni correction for multiple comparisons.
RESULTS
D2R Agonist: Quinpirole
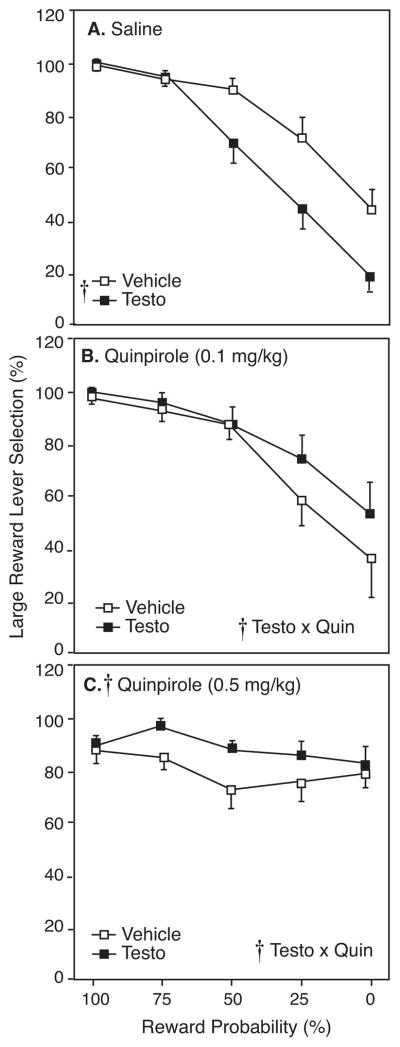
Figure 2A compares selection of the large/uncertain reward lever by testosterone- and vehicle-treated rats during 8 free-choice trials in each probability block during the initial 3 days of saline injection. There was a main effect of probability block (F4,56=54.04, p<0.05), such that all rats decreased their choice of the large/uncertain reward lever as the chance of receiving the large reward decreased. This main effect of probability block on large/uncertain reward preference was seen in every measure of probability discounting throughout the study. As in our previous study (Wallin et al. 2015), testosterone-treated rats exhibited a lower preference for the large/uncertain reward lever than vehicle-treated controls (F1,14=8.03, p<0.05). For instance, in the 25% and 0% probability blocks, vehicle-treated rats chose the large/uncertain reward on 76.3% ± 8.6% and 47.2% ± 9.8% of trials, compared to testosterone-treated rats at 43.8 ± 8.1% and 17.5% ± 6.0%, respectively.
Figure 2.
Large reward lever selection (mean±SEM) by testosterone- (Testo; closed squares) and vehicle-treated rats (Veh; open squares) in response to A) saline, B) 0.1 mg/kg, or C) 0.5 mg/kg quinpirole (Quin) as a percentage of completed free-choice trials during each probability block with probability discounting. Crosses indicate significant main effects or interaction (p<0.05) by RM-ANOVA.
Figure 2 presents the effect of the D2R agonist quinpirole on preference for the large/uncertain reward lever in testosterone- and vehicle-treated rats at 0.1 (Figure 2B) and 0.5 mg/kg (Figure 2C). By three-factor mixed ANOVA, there was a significant main effect of quinpirole on large/uncertain lever preference (F2,26=5.23, p<0.05). While there was no main effect of testosterone (F1,13=0.034, p>0.05), there was a significant interaction of testosterone and quinpirole (F2,26=9.53, p<0.05), indicating that quinpirole affected testosterone- and vehicle-treated rats differently. Finally, this analysis also showed a significant testosterone x quinpirole x probability block interaction (F8,104=2.77, p<0.05), such that quinpirole affected testosterone- and vehicle-treated rats differently across the probability blocks.
Because the initial three-factor mixed ANOVA revealed significant interactions, behavior from each dose was subsequently compared to behavior after saline, as in Wallin-Miller et al. (2017). There was no main effect of 0.1 mg/kg quinpirole on large reward lever selection (F1,13=3.50, p>0.05). However, there was a significant interaction of testosterone and quinpirole (F1,13=16.27, p<0.05), where 0.1 mg/kg quinpirole increased large reward selection in testosterone-treated rats only. There was also a significant testosterone x quinpirole x probability block interaction (F4,52=3.21, p<0.05). Specifically, 0.1 mg/kg quinpirole injections had the strongest effect on preference for the large/uncertain reward lever in testosterone-treated rats later probability blocks (Figure 2B).
In contrast, comparing behavior after 0.5 mg/kg quinpirole to behavior after saline revealed increased large/uncertain reward selection in all rats, indicated by a main effect of quinpirole (Figure 2C; F1,14=8.63, p<0.05). Furthermore, there was a significant testosterone x quinpirole interaction (F1,14=12.98, p<0.05), and a quinpirole x probability block interaction (F4,56=30.37, p<0.05). Thus, the effect of 0.5mg/kg quinpirole on large reward preference was dependent on probability block, and induced a “flattening” of the discounting curve in both testosterone- and vehicle-treated rats. Specifically, post-hoc analyses Student’s t-test with Bonferroni correction revealed that 0.5 mg/kg quinpirole significantly decreased large reward preference in the 100% probability block relative to saline injections (t(14)=3.12, p<0.01), but increased large reward preference in the 0% probability block (t(14)=6.23, p<0.01).
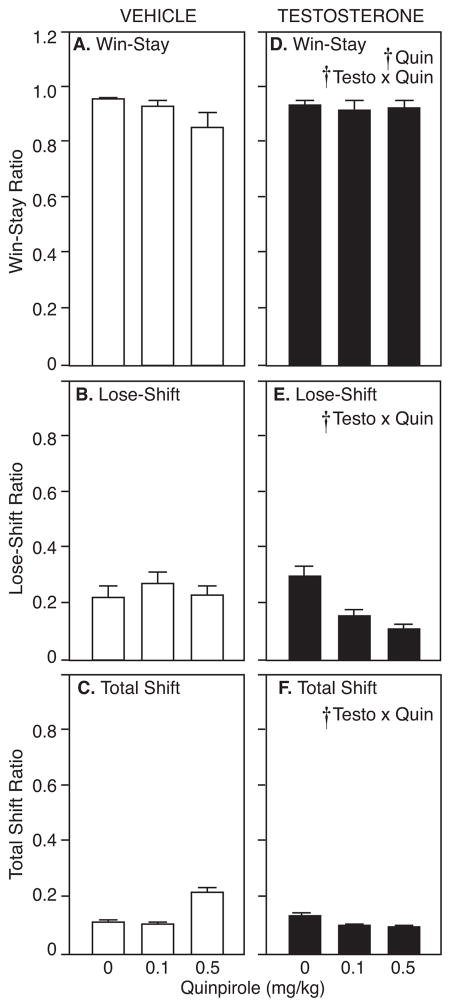
Win-Stay and Lose-Shift ratios, measuring sensitivity to reward delivery and omission, respectively, are shown in Figure 3. As in previous studies (Stopper et al. 2012), vehicle-treated rats are more likely to “stay” after a win than to “shift” after a loss, indicating greater sensitivity to reward delivery than to reward omission (t(14)=20.79, p<0.05). Averaging Win-Stay and Lose-Shift ratios across probability block for vehicle-treated rats after saline injections, the mean Win-Stay ratio for vehicle-treated rats was 0.96±0.01 and the Lose-Shift ratio was 0.21±0.05. Interestingly, quinpirole affected vehicle- and testosterone-treated rats differently. For Win-Stay ratios, there was no main effect of testosterone (F1,14=0.19, p>0.05). However, there was a significant main effect of quinpirole to decrease the Win-Stay ratio (F2,28=9.74, p<0.05), particularly in vehicle-treated rats (indicated by a testosterone x quinpirole interaction; F2,28=5.29, p<0.05; Figure 3A). For Lose-Shift ratios, there was no main effect of testosterone (F1,14=0.024, p>0.05) and no main effect of quinpirole (F2,28=1.79, p>0.05). However, there was a significant interaction of testosterone and quinpirole (F2,28=3.71, p<0.05), with quinpirole decreasing Lose-Shift ratios in testosterone-treated rats without affecting Lose-Shift behavior in vehicle-treated controls (Figure 3B). Furthermore, quinpirole significantly increased Total Shift ratio only in vehicle-treated rats, as indicated by a testosterone x quinpirole interaction (F2,28 =5.41, p<0.05; Figure 3C).
Figure 3.
A/C) Win-stay, B/D) Lose-shift, and C/E) Total Shift ratios (mean±SEM) by vehicle- (left) and testosterone-treated rats (right) during probability discounting in response to saline, 0.1, and 0.5 mg/kg quinpirole. Crosses indicate significant main effects or interaction (p<0.05) by RM-ANOVA.
D1R Agonist: SKF 81297
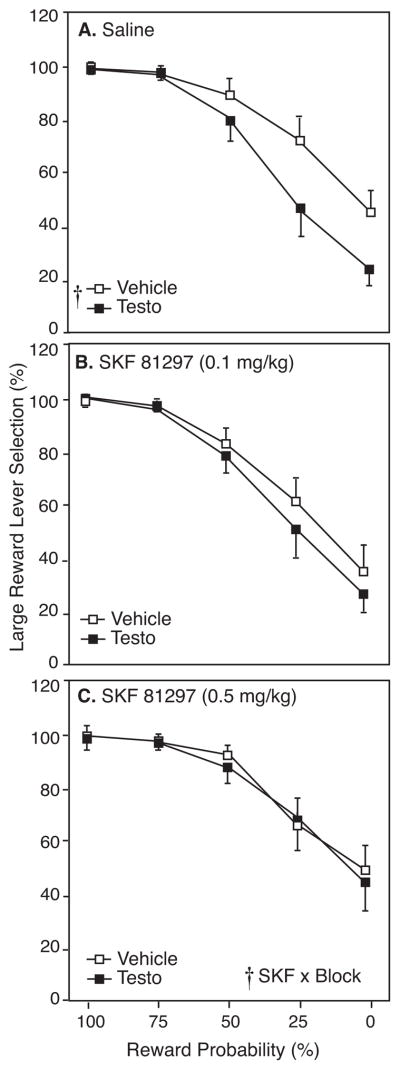
Figure 4A compares selection of the large/uncertain reward lever by vehicle- and testosterone-treated rats during 8 free-choice trials in each probability block during the initial 3 days of saline injection. As shown in Figure 2A and in our previous study (Wallin et al. 2015), testosterone-treated rats exhibited a lower preference for the large/uncertain reward lever than vehicle-treated controls (F1,18=4.96, p<0.05). In the 25% and 0% probability blocks, vehicle-treated rats chose the large/uncertain reward on 70.7 ± 7.4% and 43.7 ± 4.9% of trials, while testosterone-treated rats selected it on 47.2 ± 10.9% and 21.9 ± 4.3% of trials, respectively.
Figure 4.
Large reward lever selection (mean±SEM) by testosterone- (Testo; closed squares) and vehicle-treated rats (Veh; open squares) in response to A) saline, B) 0.1 mg/kg, or C) 0.5 mh/kg SKF 81297 treatment (SKF) as a percentage of completed free-choice trials during each probability block with probability discounting. Cross indicates significant main effects or interaction (p<0.05) by RM-ANOVA.
Figure 4 also presents the effect of the D1R agonist SKF on preference for the large/uncertain reward lever in testosterone- and vehicle-treated rats at 0.1 (Figure 4B) and 0.5 mg/kg injections (Figure 4C). By three-factor mixed ANOVA there was a significant main effect of SKF on large/uncertain lever preference (F2,20=5.96, p<0.05) and a significant SKF x block interaction (F8,80=4.03, p<0.05) such that SKF most strongly affected the later probability blocks. However, there was no main effect of testosterone (F1,10=1.84, p>0.05), and no significant interaction of testosterone with SKF (F2,20=0.87, p>0.05).
As with quinpirole, large/uncertain reward preference of vehicle- and testosterone-treated rats after each SKF dose was subsequently compared to preference after saline injections. There was no main effect of 0.1 mg/kg SKF on decision making behavior. However, this dose eliminated the effect of testosterone on preference for the large/uncertain reward lever (F1,18=1.17, p>0.05), suggesting that SKF, like quinpirole, diminishes differences in decision making between testosterone- and vehicle-treated rats. Unlike quinpirole, the high dose of SKF did not significantly increase preference for the large/uncertain reward in all rats compared to behavior after saline injections (F1,10=1.12, p>0.05). However, the 0.5 mg/kg dose of SKF also rescued behavior in testosterone-treated rats, bringing large/uncertain reward preference up to that of vehicle-treated controls (F1,10=1.97, p>0.05; Figure 4C).
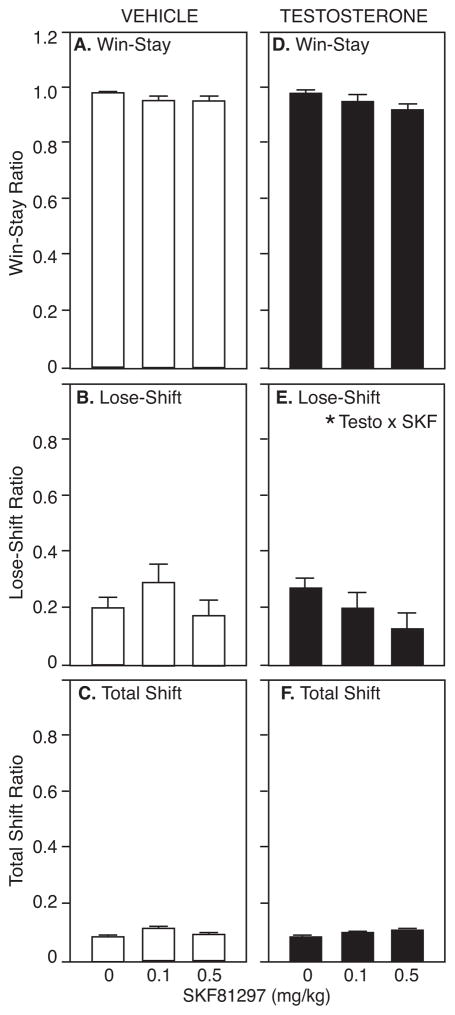
SKF effects on Win-Stay, Lose-Shift, and Total Shift ratios are shown in Figure 5. Win-Stay and Lose-Shift ratios after saline were similar to those shown in Figure 3. Win-Stay ratios were 0.98±0.01 for vehicle-treated rats and 0.97±0.01 for testosterone-treated rats, while Lose-Shift ratios were 0.19±0.04 and 0.27±0.04, respectively. For Win-Stay ratio, there was no main effect of testosterone (F1,10=2.66, p>0.05) or SKF (F1,10=1.77, p>0.05) and no significant interaction (F1,10=0.56, p>0.05; Figure 5A). For Lose-Shift ratio, there was no main effect of testosterone (F1,10=0.51, p>0.05) and no main effect of SKF (F2,9=2.84, p>0.05). However, there was a trend toward an interaction of testosterone with SKF on Lose-Shift ratio (F2,9=3.27, p=0.08; Figure 5B). Although this trend did not reach significance, the pattern of SKF effects on Lose-Shift ratios in vehicle- and testosterone-treated rats mirrors that seen with quinpirole. As seen in Figures 3B and 5B, both agonist treatments increased the Lose-Shift ratio in vehicle-treated rats and decreased the Lose-Shift ratio in testosterone-treated rats. However, Total Shift ratio was not influenced by either testosterone (F1,10=0.27, p>0.05) or SKF treatment (F2,9=5.41, p>0.05; Figure 3C).
Figure 5.
A/C) Win-stay, B/D) Lose-shift, and C/E) Total Shift ratios (mean±SEM) by vehicle- (left) and testosterone-treated rats (right) during probability discounting in response to saline, 0.1, and 0.5 mg/kg SKF 81297. Asterisk indicates p=0.08 by RM-ANOVA.
Open Field
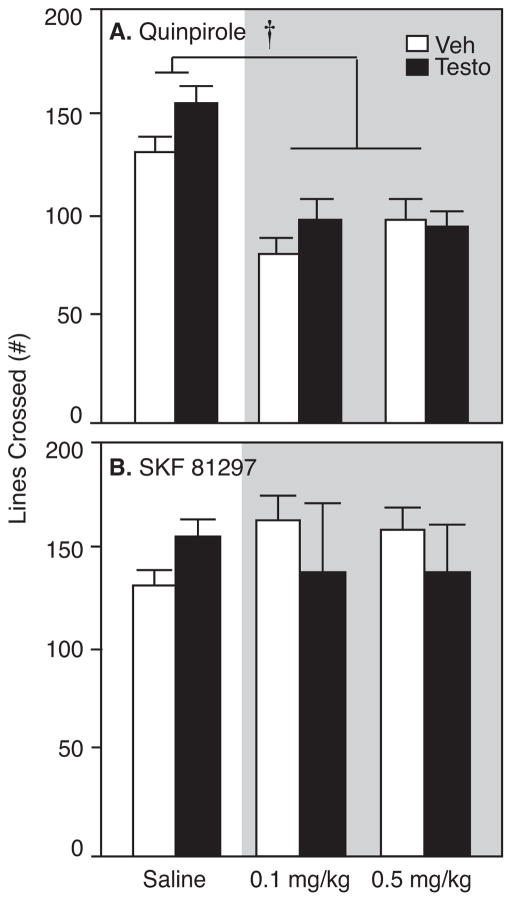
Figure 6 compares locomotor activity in an open field 20 minutes after injections with saline, 0.1, and 0.5 mg/kg dopamine receptor agonists. By RM-ANOVA, there was no main effect of testosterone on motor activity (F1,6=0.004, p>0.05) and no interaction with dopamine receptor agonists (F4,24=1.31, p>0.05). However, there was a significant effect of agonist on motor activity in open field, measured by number of lines crossed, (F4,24=6.58, p<0.05). Post-hoc analysis by Student’s t-test with Bonferroni correction for multiple comparisons showed that both 0.1 and 0.5 mg/kg quinpirole significantly decreased motor activity in testosterone- and vehicle-treated rats (0.1 mg/kg: t(14)=8.64, p<0.05; 0.5 mg/kg: t(14)=5.14, p<0.05). Neither dose of SKF affected motor activity (0.1 mg/kg: t(14)=0.05, p>0.05; 0.5 mg/kg: t(14)=0.30, p>0.05).
Figure 6.
Number of line crossings (mean±SEM) by testosterone- (Testo; closed bars) and vehicle-treated rats (Veh; open bars) after saline, 0.1, and 0.5 mg/kg of quinpirole or SKF 81297 during a 3-minute open field test. Asterisks indicate significant effects of both 0.1 and 0.5 mg/kg quinpirole relative to saline by Student’s t-test with Bonferonni correction for multiple comparisons (both p<0.01)
DISCUSSION
The present study determined the impact of dopamine receptor agonists on decision-making behavior of testosterone- and vehicle-treated rats in PD. As shown previously in Wallin et al. (2015), chronic testosterone treatment significantly decreased preference for the large/uncertain reward relative to vehicle-treated controls. Our hypothesis that acute injections of D1R or D2R agonists would rescue testosterone-treated rats’ behavior in PD was supported. Low dose (0.1 mg/kg) injections of either agonist increased large reward lever preference in testosterone-treated rats, bringing them up to the level of vehicle-treated controls. This suggests that the decreased preference for the large/uncertain reward in testosterone-treated rats is due, at least in part, to decreased dopamine receptor function. Consistent with increased large reward preference, both quinpirole and SKF decreased the Lose-Shift ratio in testosterone-treated rats, suggesting that the dopamine receptor agonists rescued behavior by decreasing loss sensitivity in the testosterone-treated group. These results indicate abnormal function of the dopamine system in AAS-treated rats, corresponding with the maladaptive cognitive and behavioral effects observed in animal studies of AAS and human AAS users.
Testosterone’s effects on large/uncertain reward preference cannot be attributed to changes in satiety or motor activity. Body weights of testosterone- and vehicle-treated rats did not differ throughout the study, and there is no effect of high-dose testosterone on 24-hr food intake in rats fed ad libitum (Wood et al. 2013). Furthermore, as we reported previously in Wallin et al. (2015), neither vehicle nor testosterone groups reach satiety when the large reward is delivered with 100% probability across all blocks during reward discrimination. With regard to motor activity, we have previously shown that icv testosterone decreased locomotor activity in male hamsters (Peters and Wood 2004). However, in the current study, systemic testosterone treatment did not affect locomotion in the open field test. Although quinpirole transiently reduced locomotor activity within 20 minutes, there was no interaction of quinpirole and testosterone. Furthermore, quinpirole did not interfere with responses on the large reward lever at 100% probability when rats were tested for PD 60 minutes after injection. Thus, testosterone’s effects on large reward preference are consistent with changes in decision-making behavior.
Decision making is dependent upon the mesocorticolimbic dopamine system, a distributed network of brain circuitry involving both cortical and subcortical regions. Within this system, dopaminergic neurons in the midbrain ventral tegmental area project to Acb, basolateral amygdala (BLA), and to regions of prefrontal cortex (PFC) including orbital frontal cortex, anterior cingulate cortex and medial PFC (Swanson, 1982). Glutamatergic connections link PFC to ventral tegmental area, BLA, and Acb, and also project from BLA to Acb (Swanson 1982; Beckstead et al. 1993, reviewed in Nestler 2000; and Kauer and Malenka 2007).
To understand how AAS alter behavior, it is useful to consider AAS effects in the context of previous studies on the neurobiology decision making. A large body of literature has shown that decision-making behavior in PD is particularly dependent upon the PFC, BLA, Acb, and the connections between these regions. Like AAS, inactivation of BLA or Acb decreases preference for the large/uncertain reward in PD (Ghods-Sharifi et al. 2009; Stopper and Floresco 2011). Disconnection of BLA from Acb also decreases large/uncertain reward preference (St. Onge et al. 2012), suggesting that subcortical connections between these two regions are important for driving selection of large, probabilistic rewards. In contrast, inactivation of medial PFC increases preference for the large/uncertain reward in PD (St. Onge and Floresco 2010). Likewise, disconnecting projections from the PFC to BLA increases large/uncertain reward preference (St. Onge et al. 2012), suggesting that top-down regulation by the PFC mitigates the subcortical drive for large reward selection and biases choice towards small/certain rewards. A balance between these opposing drives is important for optimal decision making.
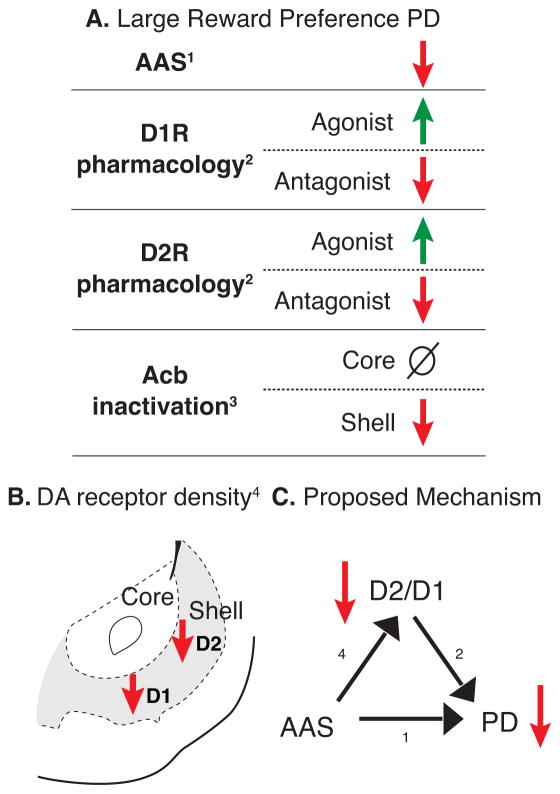
Decision making is also regulated by dopamine function within the mesolimbic dopamine system. By comparing testosterone’s influence on PD to the known effects of dopamine manipulations, we can make inferences about the neural mechanisms underlying AAS effects on decision making. For instance, systemic administration of D1R or D2R agonists increases selection of the large reward in PD, while dopamine receptors antagonists decrease large reward selection (Floresco et al. 2008b and 2008c; St. Onge and Floresco 2009; Orsini et al. 2015a). Figure 7A compares the effects of testosterone on large/uncertain reward preference in PD with the results of other experimental manipulations of this system. Like systemic antagonism of D1R or D2R, AAS decrease preference for the large/uncertain reward in PD (Wallin et al. 2015). Conversely, systemic stimulation with a D1R or D2R agonist increases selection of the large/uncertain reward in PD (St. Onge and Floresco 2009). Thus, we proposed that dopamine receptor stimulation would rescue the large/uncertain reward preference of testosterone-treated rats. This hypothesis was supported, as systemic treatment with either a D1R or D2R agonist eliminated the behavioral difference between testosterone- and vehicle-treated rats in PD.
Figure 7.
A) Effects of various manipulations to increase (green arrows) or decrease (red arrows) large reward preference in probability discounting (PD; St. Onge and Floresco, 2009; Stopper and Floresco, 2011; Wallin et al., 2015). B) AAS decrease dopamine D1 (D1R) and D2 (D2R) receptors in the shell region of the nucleus accumbens (Kindlundh et al., 2001). C) Proposed mechanism for AAS effect on PD. AAS may decrease large reward preference in PD via decreased D1R and/or D2R density in Acb shell.
The decreased large/uncertain reward preference in testosterone-treated rats may be due to effects on dopamine receptors in Acb. The shell of Acb is particularly important to PD, as inactivation of this subregion decreases preference for the large/uncertain reward and decreases the Win-Stay ratio (Stopper and Floresco 2011). Like Acb shell inactivation, local D1R antagonism in Acb decreases large/uncertain reward preference, but does so via an increase in Lose-Shift ratio (Stopper et al. 2012). In this way, testosterone treatment resembles D1R antagonism in Acb. In both the current study and previous work (Wallin et al. 2015), there was a trend toward increased Lose-Shift ratio in testosterone-treated rats. Relevant to this finding is previous work by Kindlundh et al. (2001), showing that AAS alter dopamine receptor density in various brain regions. For example, autoradiography showed that the AAS nandrolone decanoate decreases both D1R and D2R density in Acb shell of male rats (Figure 7B). Because AAS decrease dopamine receptor densities in Acb shell (Kindlundh et al. 2001), and both testosterone and systemic dopamine receptor antagonism decrease large/uncertain reward preference in PD (St. Onge and Floresco 2009; Wallin et al. 2015), AAS may alter decision-making behavior by decreasing D1R and D2R function in the Acb shell (Figure 7C). Comparing the effects of quinpirole and SKF on PD behavior, it may be that stimulation of D2R was more effective than treatment with a D1R agonist, in part, because levels of D2R in the Acb shell are nearly twice those of D1R (Kindlundh et al. 2001). In terms of hormonal mechanisms, AAS may act through classical androgen receptors, through classical estrogen receptors after conversion to estrogen via the aromatase enzyme, or through non-genomic mechanisms (Mermelstein et al, 1996; Vaudevan et al, 2005). Since the mesolimbic dopamine system has few classical steroid receptors (Creutz and Kritzer, 2004), it is likely that the effects of AAS on Acb are transduced through non-genomic receptors. In this regard, classical androgen receptors are not required for androgen self-administration (Sato et al, 2010). This proposed mechanism provides a neural substrate for the impaired decision making associated with AAS abuse, and suggests fundamental alterations of the mesolimbic dopamine circuitry of human AAS users.
Interestingly, testosterone effects on decision making are consistent with BLA manipulations in both PD and punishment discounting. In PD, either D1R antagonism within, or lesion of BLA will decrease preference for the large/uncertain reward (Larkin et al. 2016; Ghods-Sharifi et al. 2009), similar to testosterone in the current study and previously (Wallin et al. 2015). However, in punishment discounting, BLA lesions have the opposite effect on decision making—increasing preference for a large reward paired with a chance of footshock (Orsini et al. 2015b). This is also consistent with testosterone effects on punishment discounting, as we have previously shown that testosterone increases choice of the large reward in spite of footshock (Cooper et al. 2014). These corresponding effects suggest that AAS may affect decision making in human users via alteration of BLA function. Indeed, human AAS users show abnormal amygdala function, including decreased connectivity between the amygdala and regions of the PFC (Westlye et al. 2017).
Dopamine transmission in the medial PFC is also important for decision making and Win-Stay and Lose-Shift behavior in PD (Orsini et al. 2015a). D1R antagonism in the medial PFC resembles testosterone treatment, decreasing preference for the large/uncertain reward and increasing the Lose-Shift ratio. In contrast, D2R agonism in the medial PFC results in a flattening of the discounting curve in PD and a decrease in the Win-Stay ratio (St. Onge et al. 2011). This corresponds to the effects of systemic quinpirole in the current study, as the 0.5 mg/kg dose of quinpirole flattened the discounting curve and decreased the Win-Stay ratio. In particular, in vehicle-treated rats, the combination of a reduced Win-Stay ratio combined with no change in the Lose-Shift ratio resulted in an increase in the Total Shift ratio with quinpirole. Thus, in vehicle treated rats, the flattening of the discounting curve can be attributed to a general increase in shifting between levers, regardless of reward outcome. In contrast, quinpirole decreased the Lose-Shift ratio in testosterone-treated rats without affecting the Total Shift ratio, indicating a true decrease in loss sensitivity. This shows that vehicle- and testosterone-treated rats respond differently to D2R stimulation. Furthermore, it suggests that quinpirole rescues decision-making behavior in testosterone-treated rats by normalizing medial PFC dopamine function and decreasing Lose-Shift behavior only when it is abnormally high (in testosterone-treated rats). If so, the implication is that AAS impair decision making in humans via alterations in loss sensitivity due to decreased D2R function in the medial PFC. Local administration of dopamine receptor agonists to specific brain regions of testosterone-treated rats during PD can determine the site of action.
Recent findings from human and animal studies support the hypothesis that AAS impair cognition via effects on the PFC. In addition to decision making, dopamine function in medial PFC underlies executive functions such as cognitive flexibility (Floresco 2013). Cognitive flexibility, or the ability to update behavior in response to changing task requirements, is impaired by medial PFC inactivation and D1R or D2R antagonism in the medial PFC (Floresco et al. 2006 and 2008a). We have recently shown that testosterone impairs cognitive flexibility in our animal model of AAS abuse (Wallin and Wood 2015). Similarly, in a recent fMRI study of human subjects, AAS users exhibited decreased connectivity between regions of the PFC and amygdala (Westlye et al. 2017). Alteration of PFC function is particularly disturbing in the context of adolescent AAS users, as prefrontal cortical circuitry is still under development (Blakemore and Choudhury 2006). Furthermore, impaired executive function is associated with a variety of maladaptive behaviors and psychiatric disorders (Floresco et al. 2009). This highlights the potential of AAS to cause a broad variety of behavioral and cognitive impairments. Therefore, future studies of AAS effects on medial PFC will be important for understanding how AAS change the brain to ultimately alter cognition and behavior.
Acknowledgments
This work was funded by NIH R01-DA029613 to RIW
Footnotes
Conflicts of Interest: All authors declare no conflicts of Interest.
References
- Aubele T, Kritzer MF. Gonadectomy and hormone replacement affects in vivo basal extracellular dopamine levels in the prefrontal cortex but not motor cortex of adult male rats. Cerebral Cortex. 2010;21(1):222–232. doi: 10.1093/cercor/bhq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead RM, Domesick VB, Nauta WJ. Neuroanatomy. Birkhäuser; Boston: 1993. Efferent connections of the substantia nigra and ventral tegmental area in the rat; pp. 449–475. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Choudhury Development of the adolescent brain: implications for executive function and social cognition. Journal of child psychology and psychiatry. 2006;47(3–4):296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Castañé A, Robbins TW. Dopamine D2/D3 receptor agonist quinpirole impairs spatial reversal learning in rats: investigation of D3 receptor involvement in persistent behavior. Psychopharmacology (Berl) 2009;202:611–620. doi: 10.1007/s00213-008-1341-2. [DOI] [PubMed] [Google Scholar]
- Célérier E, Yazdi MT, Castañé A, Ghozland S, Nyberg F, Maldonado R. Effects of nandrolone on acute morphine responses, tolerance and dependence in mice. European Journal of Pharmacology. 2003;465(1):69–81. doi: 10.1016/s0014-2999(03)01462-6. [DOI] [PubMed] [Google Scholar]
- Cooper SE, Goings SP, Kim JY, Wood RI. Testosterone enhances risk tolerance without altering motor impulsivity in male rats. Psychoneuroendocrinology. 2014;40:201–212. doi: 10.1016/j.psyneuen.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutz LM, Kritzer MF. Mesostriatal and mesolimbic projections of midbrain neurons immunoreactive for estrogen receptor beta or androgen receptors in rats. Journal of Comparative Neurology. 2004;476:348–362. doi: 10.1002/cne.20229. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Noschang C, d’Angelo LS, Noble CA, Day JO, Dongelmans ML, Theobald DE, Mar AC, Urcelay GP, Morein-Zamir S, Robbins TW. The dopamine D2/D3 receptor agonist quinpirole increases checking-like behaviour in an operant observing response task with uncertain reinforcement: a novel possible model of OCD. Behavioural Brain Research. 2014;264:207–229. doi: 10.1016/j.bbr.2013.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilam D, Szechtman H. Biphasic effect of D-2 agonist quinpirole on locomotion and movements. European Journal of Pharmacology. 1989;161(2–3):151–157. doi: 10.1016/0014-2999(89)90837-6. [DOI] [PubMed] [Google Scholar]
- Floresco SB. Prefrontal dopamine and behavioral flexibility: shifting from an “inverted-U” toward a family of functions. Frontiers in Neuroscience. 2013;7:62. doi: 10.3389/fnins.2013.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behavioural Brain Research. 2008a;190(1):85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Ghods-Sharifi S. Amygdala-prefrontal cortical circuitry regulates effort-based decision making. Cerebral Cortex. 2007;17(2):251–260. doi: 10.1093/cercor/bhj143. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Maric TL. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology. 2006;31(2):297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Maric TL, Ghods-Sharifi S. Dopaminergic and glutamatergic regulation of effort-and delay-based decision making. Neuropsychopharmacology. 2008b;33(8):1966–1979. doi: 10.1038/sj.npp.1301565. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Onge JR, Ghods-Sharifi S, Winstanley CA. Cortico-limbic-striatal circuits subserving different forms of cost-benefit decision making. Cognitive, Affective, & Behavioral Neuroscience. 2008c;8(4):375–389. doi: 10.3758/CABN.8.4.375. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Zhang Y, Enomoto T. Neural circuits subserving behavioral flexibility and their relevance to schizophrenia. Behavioural Brain Research. 2009;204(2):396–409. doi: 10.1016/j.bbr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Ghods-Sharifi S, St Onge JR, Floresco SB. Fundamental contribution by the basolateral amygdala to different forms of decision making. Journal of Neuroscience. 2009;29:5251–5259. doi: 10.1523/JNEUROSCI.0315-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren M, Pope HG, Jr, Kanayama G, Hudson JI, Lundin A, Kollman H. Anti-Social Behaviors Associated with Anabolic-Androgenic Steroid Use among Male Adolescents. European Addiction Research. 2015;21:321–326. doi: 10.1159/000433580. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use: 2012 Overview, Key findings on adolescent drug use. Ann Arbor: Institute for Social Research, The University of Michigan; 2013. [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nature Reviews Neuroscience. 2007;8(11):844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kindlundh A, Lindblom J, Bergström L, Wikberg JE, Nyberg F. The anabolic-androgenic steroid nandrolone decanoate affects the density of dopamine receptors in the male rat brain. European Journal of Neuroscience. 2001;13(2):291–296. doi: 10.1046/j.0953-816x.2000.01402.x. [DOI] [PubMed] [Google Scholar]
- Larkin JD, Jenni NL, Floresco SB. Modulation of risk/reward decision making by dopaminergic transmission within the basolateral amygdala. Psychopharmacology. 2016;233(1):121–136. doi: 10.1007/s00213-015-4094-8. [DOI] [PubMed] [Google Scholar]
- Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. Journal of Neuroscience. 1996;16:595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleman AB, Faulkner AH, Woods ER, Emans SJ, Durant RH. High-Risk Behaviors Among High School Students in Massachusetts Who Use Anabolic Steroids. Pediatrics. 1995;96(2 Pt 1):268–272. [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nature Reviews Neuroscience. 2001;2(2):119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Orsini CA, Moorman DE, Young JW, Setlow B, Floresco SB. Neural mechanisms regulating different forms of risk-related decision-making: Insights from animal models. Neuroscience & Biobehavioral Reviews. 2015a;58:147–167. doi: 10.1016/j.neubiorev.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Trotta RT, Bizon JL, Setlow B. Dissociable roles for the basolateral amygdala and orbitofrontal cortex in decision-making under risk of punishment. Journal of Neuroscience. 2015b;35(4):1368–1379. doi: 10.1523/JNEUROSCI.3586-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters KD, Wood RI. Androgen dependence in hamsters: overdose, tolerance, and potential opioidergic mechanisms. Neuroscience. 2005;130(4):971–981. doi: 10.1016/j.neuroscience.2004.09.063. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Wood RI, Rogol A, Nyberg F, Bowers L, Bhasin S. Adverse health consequences of performance-enhancing drugs: An Endocrine Society scientific statement. Endocrine Reviews. 2013;35(3):341–375. doi: 10.1210/er.2013-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Pardo M, Yohn SE, López-Cruz L, San Miguel N, Correa M. Behavioral Neuroscience of Motivation. Springer International Publishing; 2015. Mesolimbic dopamine and the regulation of motivated behavior; pp. 231–257. [DOI] [PubMed] [Google Scholar]
- Salas-Ramirez KY, Montalto PR, Sisk CL. Anabolic androgenic steroids differentially affect social behaviors in adolescent and adult male Syrian hamsters. Hormones & Behavior. 2008;53(2):378–385. doi: 10.1016/j.yhbeh.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato SM, Johansen J, Jordan CL, Wood RI. Membrane, not nuclear, androgen receptor mediates androgen reinforcement. Psychoneuroendocrinology. 2010;35(7):1063–1073. doi: 10.1016/j.psyneuen.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler CW, Carmona GN. Effects of dopamine agonists and antagonists on locomotor activity in male and female rats. Pharmacololgy Biochemistry & Behavior. 2002;72:857–863. doi: 10.1016/s0091-3057(02)00770-0. [DOI] [PubMed] [Google Scholar]
- Simon NW, Gilbert RJ, Mayse JD, Bizon JL, Setlow B. Balancing risk and reward: a rat model of risky decision making. Neuropsychopharmacology. 2009;34(10):2208–2217. doi: 10.1038/npp.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Montgomery KS, Beas BS, Mitchell MR, LaSarge CL, Mendez IA, Bañuelos C, Vokes CM, Taylor AB, Haberman RP, Bizon JL. Dopaminergic modulation of risky decision-making. Journal of Neuroscience. 2011;31(48):17460–17470. doi: 10.1523/JNEUROSCI.3772-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Onge JR, Abhari H, Floresco SB. Dissociable contributions by prefrontal D1 and D2 receptors to risk-based decision making. Journal of Neuroscience. 2011;31(23):8625–8633. doi: 10.1523/JNEUROSCI.1020-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Onge JR, Floresco SB. Dopaminergic modulation of risk-based decision making. Neuropsychopharmacology. 2009;34(3):681–697. doi: 10.1038/npp.2008.121. [DOI] [PubMed] [Google Scholar]
- St Onge JR, Floresco SB. Prefrontal cortical contribution to risk-based decision making. Cerebral Cortex. 2010;20(8):1816–1828. doi: 10.1093/cercor/bhp250. [DOI] [PubMed] [Google Scholar]
- St Onge JR, Stopper CM, Zahm DS, Floresco SB. Separate prefrontal-subcortical circuits mediate different components of risk-based decision making. Journal of Neuroscience. 2012;32(8):2886–2899. doi: 10.1523/JNEUROSCI.5625-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopper CM, Floresco SB. Contributions of the nucleus accumbens and its subregions to different aspects of risk-based decision making. Cognitive, Affective, & Behavioral Neuroscience. 2011;11(1):97–112. doi: 10.3758/s13415-010-0015-9. [DOI] [PubMed] [Google Scholar]
- Stopper CM, Khayambashi S, Floresco SB. Receptor-specific modulation of risk-based decision making by nucleus accumbens dopamine. Neuropsychopharmacology. 2012;38(5):715–728. doi: 10.1038/npp.2012.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Research Bulletin. 1982;9(1):321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Tsutsui KT, Wood RI, Craft RM. Anabolic androgenic steroid effects on nociception and morphine antinociception in male rats. Pharmacology Biochemistry & Behavior. 2011;99(3):500–508. doi: 10.1016/j.pbb.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan N, Kow LM, Pfaff D. Integration of steroid hormone initiated membrane action to genomic function in the brain. Steroids. 2005;70(5–7):388–396. doi: 10.1016/j.steroids.2005.02.007. [DOI] [PubMed] [Google Scholar]
- WADA. Anti-Doping Testing Figures Report. 2012 www.wada-ama.org.
- Wallin KG, Alves JM, Wood RI. Anabolic androgenic steroids and decision making: Probability and effort discounting in male rats. Psychoneuroendocrinology. 2015;57:84–92. doi: 10.1016/j.psyneuen.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin KG, Wood RI. Anabolic-androgenic steroids impair set-shifting and reversal learning in male rats. European Neuropsychopharmacology. 2015;25(4):583–590. doi: 10.1016/j.euroneuro.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin-Miller KG, Chesley JR, Castrillon J, Wood RI. Sex differences and hormone effects on ethanol-enhanced risk taking in rats. Drug and Alcohol Dependence. 2017;174:137–144. doi: 10.1016/j.drugalcdep.2017.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlye LT, Kaufmann T, Alnæs D, Hullstein IR, Bjørnebekk A. Brain connectivity aberrations in anabolic-androgenic steroid users. NeuroImage: Clinical. 2017;13:62–69. doi: 10.1016/j.nicl.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RI, Armstrong A, Fridkin V, Shah V, Najafi A, Jakowec M. Roid rage in rats? Testosterone effects on aggressive motivation, impulsivity and tyrosine hydroxylase. Physiology & Behavior. 2013;110:6–12. doi: 10.1016/j.physbeh.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]