Abstract
Linking individuals in primary care settings with substance use disorders (SUD) to SUD treatment has proven to be challenging, despite the widespread use of Screening, Brief Intervention, and Referral to Treatment (SBIRT). This paper reports findings from a pilot study that examined the efficacy of the Recovery Management Checkups intervention, adapted for primary care settings (RMC-PC), for assertively linking and engaging patients from Federally Qualified Health Centers into SUD treatment. Findings showed that patients in the RMC-PC intervention (n=92) had significantly higher rates of SUD treatment entry and received more days of SUD treatment compared with those who receive the usual SBIRT referral (n=50). Receipt of RMC-PC had both direct and indirect effects, partially mediated through days of SUD treatment, on reducing days of drug use at 6-months post-intake. RMC-PC is a promising intervention to address the need for more assertive methods for linking patients in primary care to SUD treatment.
Keywords: substance use disorder, primary care, treatment referral, linkage, recovery
Introduction
For individuals who are identified as in need of treatment for substance use disorders (SUD), successful linkage to and engagement in SUD treatment are paramount to achieving and sustaining recovery. There is a well-developed body of evidence demonstrating that SUDs often constitute a chronic condition marked by cycles of recovery, relapse, and repeated treatments that may span many years before reaching stable recovery.1,2 Longitudinal analyses consistently suggest that the earlier initiation and longer duration of treatment are associated with a greater likelihood of sustained abstinence.3,4
Although SUDs are similar to other chronic diseases with regard to rates of relapse and treatment compliance,5 other aspects of SUDs present barriers to treatment utilization. Substance misuse is often embedded within a lifestyle that is transient and chaotic, leading to physical and social instability, and may also involve criminal behavior. Furthermore, these aspects often lead to alienation from friends and family members. Programs designed to manage other chronic medical conditions or serious illness, such as heart disease, cancer, and dementia, commonly enlist the support of family and friends as caregivers to help manage the patient’s condition and interactions with health care providers;6,7 however, the social isolation, secrecy, and stigma accompanying years of substance use may limit the involvement of typical caregivers for individuals with SUD.8 Thus, there is a need for interventions that can effectively engage individuals with SUD into care and provide ongoing monitoring and early re-intervention as needed.
Federally Qualified Healthcare Centers as Promising Venues for Linking Individuals to SUD Treatment
Efforts to engage individuals with SUD in treatment have recently focused on primary health care services, where individuals with SUD who may not otherwise access SUD treatment on their own or through other channels (i.e., criminal justice system, employer) can be identified.9-12 In particular, Federally Qualified Health Centers (FQHC) are primary health care providers that receive grants to enhance Medicare and Medicaid reimbursement rates and increase access to medications at reduced rates.13 FQHCs are required to serve an underserved area or population, offer a sliding fee scale, provide comprehensive services, and have an ongoing quality assurance program. As such, their patient populations include a disproportionate number of low-income individuals, African Americans, and Hispanics, who typically experience more barriers to accessing SUD services.14,15
The National Association of Community Health Centers currently recommends annual screening of 100% of FQHC patients for alcohol and substance use problems.16 Of the 24,295,946 patients served by FQHCs in 2015, only 117,043 (0.5%) received any SUD diagnosis or treatment (https://bphc.hrsa.gov/uds/datacenter.aspx). Given this low rate of SUD assessment and referral, in 2016 the Health Resources and Services Administration made Screening, Brief Intervention and Referral to Treatment (SBIRT) utilization rates one of its formal annual performance criteria.
Yet to-date, findings on use of SBIRT to identify and intervene with patients with SUD in primary care settings have yielded weak evidence of its effectiveness.17-19 Studies have shown overall identification rates of only 0.5 to 5.0% of patients in need of SUD treatment.20,21 Findings from recent studies, including a meta-analysis of SBIRT models, have found that the referral to treatment components have little effect on increasing linkage to SUD treatment or treatment utilization.22,23 Even in cases where patients are identified and referred to on-site SUD treatment or “behavioral health services,” these are typically underequipped, overburdened, and unable to fully address the volume of patients who need these services.24,25 Moreover, current efforts to incorporate behavioral health services within FQHCs have focused more on mental health services than on SUD services.26,27 In one national study, FQHCs were less likely to use standardized procedures for screening, referral, information tracking, and follow-up for SUD services than for mental or other health services.28 Further, the co-location of SUD treatment in FQHCs is unlikely to always be feasible or cost-effective; however, the alternative of referring these patients to external agencies is still hampered by numerous financial, administrative and human service barriers to coordinating care.29
Recovery Management Checkups (RMC)
Given the weak findings on the efficacy of SBIRT for linking individuals in primary care to SUD treatment, more assertive interventions are needed to engage this population into SUD treatment. One such assertive model is the Recovery Management Checkups (RMC). The RMC model is based on the public health theory that long-term monitoring through regular checkups and early (re)intervention will facilitate early detection of relapse, reduce the time to treatment re-entry, and, consequently, improve long-term outcomes.30 This approach does not rely on participants having to initiate help-seeking. Rather, these regularly scheduled quarterly checkups are pro-active and may be conducted either face-to-face or by phone. They include quarterly assessments and personalized feedback for participants on the status of their SUD recovery and current risks over extended periods of time. RMC utilizes specialized staff, Linkage Managers, who use motivational interviewing, problem solving around barriers to treatment, and assertive linkage (e.g., making appointments, providing transportation, and negotiating access). It also includes on-going contact with patients to ensure that they engage in treatment and follow through on continuing care recommendations.
The RMC model has been evaluated and shown to be effective in three randomized trials in which individuals recruited from SUD treatment and jail settings received quarterly checkups from 2 to 4 years.4,31-33 Across the 3 trials, RMC was used to provide ongoing monitoring, early re-intervention and, when indicated, linkage back to SUD treatment for over 1,300 patients. In the longest trial, which included quarterly checkups for 4 years, patients assigned to RMC were significantly more likely (p<.05) than those assigned to a control group to enter SUD treatment sooner (13 vs. 45 months d=-0.61), enter treatment at any time (70% vs. 51% any admissions, d=.50), and stay in treatment longer (112 vs. 79 days, d=0.23.31 The latter is important because process analyses show that only those who stayed in treatment 10 or more days significantly reduced their substance use.34 Moreover, the size of these effects increased over time with repeated quarterly exposures to RMC, and RMC participants also reported significantly more total days of abstinence (1,026 vs. 932 days, d=+0.24) and fewer past-month SUD symptoms (89 vs. 126 symptom-months, d=-0.27) relative to the comparison sample that received usual care.
Following on these findings of RMC as an effective intervention for linking individuals into SUD treatment and helping them to sustain recovery through repeated checkups, this paper reports on findings from a pilot study in which the RMC intervention was adapted for use in primary care settings (RMC-PC) and tested as to its efficacy for linking individuals with SUDs in FQHCs into SUD treatment. Further, the study examines both the direct effect and indirect effect of RMC-PC on substance use outcomes as mediated by receipt of SUD treatment.
Methods
Overview of the Study Design
Data come from a pilot study conducted as part of a larger SBIRT implementation grant being conducted in multiple FQHCs by the state of Illinois. All sites received training on SBIRT, implemented it as part of their standard procedures, and included a sample of their clients for a 6-month follow-up assessment study (conducted by the lead author). It quickly became apparent that the referral to treatment component was not leading to a high level of actual treatment utilization. The state therefore contracted with [the lead author and developer of the RMC] to conduct a pilot study to examine whether the RMC intervention could be used to increase the rates of treatment utilization beyond those obtained with SBIRT only.
The quasi-experimental study design compares patients who were recruited before (SBIRT as usual comparison) and after (RMC-PC) implementation of the RMC pilot study in a subset of 3 FQHC sites that were participating in the SBIRT implementation project. First, the comparison group comprised patients in these sites who were screened and determined to be at moderate or higher substance use severity on the screening instruments (described below); they were then referred to SUD treatment using SBIRT as usual procedures and were recruited between August 2012 and September 2015 to participate in the follow-up for the overall SBIRT project. Second, the RMC-PC pilot study was conducted these same 3 FQHC sites with patients who similarly had been screened, determined to be at moderate or higher substance use severity, and then referred to SUD treatment. The RMC group was recruited into the follow-up study between June 2014 and February 2016 (recruitment intervals varied across sites).
Of the 167 people who were recruited for both groups between August 2012 and February 2016, 1 died and 142 completed their follow-up for a completion rate of 86% (142/[167-1]) – with no difference between groups. The analysis sample used in this study was subset to those participants who had completed their intake and 6-month post-intake assessments. All data was collected using the SAMHSA/CSAT Government Performance Reporting Act (GPRA) instrument35 at intake, discharge, and 6 months post-intake. All procedures were reviewed and approved by the Chestnut Health Systems Institutional Review Board.
Procedures
Initial Screening
As part of their routine procedures, FQHC staff performed initial screening with all study participants following the same SBIRT procedures. All FQHC patients were screened annually for alcohol or drug problems in a two-stage process. First, patients were asked about the frequency/amount of their alcohol and drug use in the past year. If they were drinking frequently (weekly) or heavily (5+ drinks/day for male, 3+ drinks/day for female), or reported any drug use, they were screened using the Alcohol Use Disorder Identification Test (AUDIT)36 and the Drug Abuse Screening Test (DAST).37 They were then classified based on the highest severity on either measure and provided with a range of interventions.
Brief Intervention, Brief Treatment and Referral to Treatment
Patients in the normal range (0-8 on AUDIT and 0 on DAST) received no further intervention. Patients with low severity (8-15 on AUDIT or 1-2 on DAST) received a brief intervention on-site, which utilized motivational interviewing and the Brief Negotiated Interview approach, which consists of the following steps: 1) raise the subject, in a respectful manner; 2) provide personalized feedback on health risks and consequences; 3) enhance motivation to change, using reflective listening and patient empowerment; 4) negotiate and advise on next steps, using a nonjudgmental and patient-centered approach; and 5) summarize specific goals and review follow-up plan.38 Patients with moderate severity (16-19 on AUDIT or 3-5 on DAST) received a referral to Brief Treatment that consists of 1 to 6 sessions based on the World Health Organization model of motivational interviewing linked to screener data.39 Patients with high severity (20+ on the AUDIT or 6+ on the DAST) were presumed to have SUD and received a referral to treatment for a more comprehensive clinical assessment and treatment along a continuum of care (outpatient, intensive outpatient, residential, and medication-assisted treatment options) by programs licensed by the state/federal government and professionally accredited.
When making usual referrals for SUD treatment, the FQHC staff reported that they routinely provided the patient with the address/contact information for the SUD treatment provider and an appointment card, and called or mailed reminders of upcoming appointments. FQHC staff also reported that they only rarely accompanied individuals to the appointment, worked with other agencies to ensure the patient shows to the appointment, or contacted the agency for data on attendance, updates on progress or to speak with family members to ensure attendance.
Study Recruitment
Following the above procedures, FQHC staff identified 167 patients who were in need of SUD treatment, offered them a referral to SUD treatment (brief or regular), and asked these patients if they would be interested in participating in the follow-up study. This included 59 people during the “SBIRT as usual” comparison period and 108 during the RMC-PC pilot study. Consent to be contacted by research staff for the 6-month follow-up interview was obtained at the time of the baseline screening and GPRA assessment. Six individuals who initially gave consent at this time declined to participate when contacted for the follow-up. Of the remainder, 6 month follow-up interviews were completed on 142 people (88%) – 50 in the SBIRT as usual comparison and 92 in the RMCPC group.
Recovery Management Checkups – Primary Care (RMC-PC)
Using standard motivational interviewing techniques,40 the Linkage Manager contacted participants by phone and discussed with them the benefits of going to treatment, engaged in problem solving about their expressed barriers to treatment, and provided assertive linkage (e.g., making appointments, providing transportation, and negotiating access). For patients who initially refused the referral to SUD treatment at the FQHC, the Linkage Manager explored the benefits, consequences, and/or inconveniences of the patient’s current substance use as well as explored the patient’s motivation for treatment. Using the techniques of empathy and reflection, the Linkage Manager explored the issues and barriers patients identified, including stigma or concerns about how treatment participation would affect their employment status or family relationships. Using open-ended questions, the Linkage Manager explored not only reasons the patient opted out of the treatment referral but also the potential benefits of treatment. The Linkage Manager sought to develop discrepancy between how the patient currently perceived his/her situation and stated goals, and used the technique of “rolling with resistance” to enhance treatment motivation. The Linkage Manager assured the patient that the decision was up to him/her regarding treatment, thereby empowering the patient in the decision process and encouraging “change talk.”
After participants entered SUD treatment, the Linkage Manager implemented an Engagement and Retention Protocol designed to improve retention rates. Specifically, for participants entering detox, the Linkage Manager either called or visited them daily until they moved to the next level of care. After entering treatment (either residential or outpatient), the protocol included a combination of phone calls and face-to-face visits during the first 14 days. If at any point during treatment, a participant threatened to leave or failed to show for an appointment, the treatment staff would contact the Linkage Manager to arrange an intervention to re-engage the individual in treatment. For those participants who declined the treatment option, the Linkage Manager and participant agreed upon an Alternative Action plan, which included various behaviors the individual planned to engage in to reduce or stop their substance use and other high-risk activities, such as unsafe sexual activity or involvement in illegal activity. These check-ups continued on a quarterly basis through the duration of the study.
Using these methods, 99% of the RMC-PC group agreed to go to treatment, and 90% actually showed to treatment intake. Based on the linkage manager’s final service logs, of the 108 RMC-PC participants (including those who did not participate in the 6-month follow-up study): 1 (1%) could not be found; 1 (1%) refused a referral; 9 (8%) agreed to the referral, but never showed to treatment; and 97 (90%) showed to treatment intake.
Outcome Measures
The primary outcomes are whether the participant received any SUD treatment (yes or no) and the days of SUD treatment received within 6 months (range: 0 to 182 days). The secondary outcomes are the change in the days of alcohol use and drug use (month 6 minus baseline; possible range from -30 to +30 days). The latter were calculated separately and combined. For the combined alcohol and other drug (AOD) use measure, the days of use were summed and then capped at 30 days.
Analyses
Data were subset to the 142 people with 6-month follow-up interviews. Odds ratios were computed to compare the percentage of the RMC-PC group over the SBIRT as usual quasi-experimental comparison group for any treatment. Days of treatment received and change in days of alcohol and drug use in the past 30 days were compared across the two groups using Cohen’s effect size d, and evaluated with a t-test. Days of treatment were based on the discharge data; the change scores for AOD use were based on the past 30 days at 6 months minus the past 30 days at intake. Lastly, a path model was constructed to evaluate the direct and indirect effects of receipt of the RMC-PC intervention (vs. SBIRT as usual) on days of any alcohol or drug use, with days of SUD treatment received as a possible mediator, based on the regression coefficients and percentage of variance explained of the outcome variable. All analyses were conducted using IBM SPSS version 23.
Results
Characteristics of Study Sample
Characteristics of the follow-up study sample overall and by group (SBIRT as usual and RMC-PC) are shown in Table 1. Overall, a majority were male (61%) and African American (77%), with an average age of 50.3 years. Approximately one half (51%) had a high school degree or more education, although few were currently employed (16%). Most (90%) reported substance use at least weekly in the 30 days prior to the baseline interview; this includes weekly use of opioids (54%), alcohol (36%), marijuana (29%), and stimulants (22%). Patients with moderate to high scores on the AUDIT (mean of 7.0) and DAST (mean of 5.4), received referrals to SUD treatment; 65% were referred to regular treatment and 35% to brief treatment. Over half reported that their health was only fair (34%) or poor (17%). Although 89% reported having engaged in some illegal activity in the past 30 days, only 6% reported having been arrested in the prior 30 days.
Table 1.
Participant Characteristics in the 30 days Prior to Intake by Condition
| Total (n=142) | SBIRT as Usuala (n=50) | SBIRT+RMC-PCb (n=92) | Chi-sq. /F-value | p-value | |
|---|---|---|---|---|---|
| Female | 39% | 42% | 37% | 0.35 | 0.591 |
| Hispanic | 17% | 28% | 11% | 6.61 | 0.018 |
| Race: | |||||
| African American | 77% | 68% | 82% | 6.65 | 0.070 |
| Caucasian | 8% | 6% | 9% | ||
| Other | 15% | 26% | 10% | ||
| Mixed | 1% | 2% | 1% | ||
| Age: Mean (SD) | 50.3 (8.9) | 41.4 (11.6) | 48.5 (10.5) | 13.60 | 0.000 |
| Currently Employed | 16% | 26% | 11% | 5.89 | 0.035 |
| High School Education or higher | 51% | 51% | 51% | 0.00 | 1.000 |
| Weekly Substance Use | |||||
| Alcohol or other drug use | 91% | 78% | 98% | 15.31 | 0.000 |
| Alcohol use | 36% | 46% | 30% | 3.41 | 0.070 |
| Marijuana use | 29% | 34% | 26% | 0.99 | 0.338 |
| Opioid use | 54% | 18% | 73% | 39.14 | 0.000 |
| Stimulant use (including cocaine) | 22% | 20% | 23% | 0.15 | 0.832 |
| Other drug use | 1% | 0% | 2% | 1.10 | 0.541 |
| AUDITc Mean (SD) | 7.0 (9.5) | 10.1 (10.1) | 5.3 (8.7) | 8.75 | 0.004 |
| DASTd Mean (SD) | 5.4 (2.7) | 4.1 (2.7) | 6.2 (2.4) | 20.54 | 0.000 |
| Referral to: | |||||
| Brief Treatment | 35% | 46% | 28% | 4.51 | 0.042 |
| Regular Treatment | 65% | 54% | 72% | ||
| Health Status | |||||
| Excellent | 5% | 6% | 4% | 6.09 | 0.202 |
| Very Good | 9% | 10% | 8% | ||
| Good | 36% | 26% | 41% | ||
| Fair | 34% | 32% | 35% | ||
| Poor | 17% | 26% | 12% | ||
| Any illegal activity | 89% | 74% | 97% | 16.75 | 0.000 |
| Any arrest | 6% | 4% | 7% | 0.39 | 0.713 |
Bold indicates p < 0.05
SBIRT: Screening, Brief Intervention, and Referral to Treatment
RMC-PC: Recovery Management Checkups for Primary Care
AUDIT: Alcohol Use Disorder Test
DAST: Drug Abuse Screening Test
Relative to the SBIRT as usual comparison group, participants who received RMC-PC were less likely to be employed and more likely to be Hispanic, older, weekly users of any alcohol or other drugs, weekly users of opioids, and engaged in illegal activity. They had lower scores on the AUDIT and higher scores on the DAST. Although a majority of both groups received a referral to regular treatment, a greater proportion of the RMC group was referred to regular treatment, reflecting their overall higher severity.
Treatment Utilization
As shown in Table 2, relative to the SBIRT as usual comparison group, participants who received RMC-PC were significantly more likely to have received any treatment within 6 months post-intake (14% vs. 64%, OR=10.98, p<.001, 95% CI=4.44 to 27.16) and to receive more total days of treatment during this time (10.38 vs. 63.24 days, d=0.98, p<.0001). In both cases the distributions are zero saturated and right skewed; thus the medians were examined, which also significantly differed by group (0 vs. 78.5 days).
Table 2.
Days of Treatment, Alcohol and Drug Use by Condition
| Time | Total
|
% / Mean by Condition
|
Comparisons (RMC-PC v SBIRT)
|
|||||
|---|---|---|---|---|---|---|---|---|
| Mean | S.D. | SBIRT as Usuala | SBIRT+RMC-PCb | OR /Cohen’s d | X2 /t-test | p-value | ||
|
| ||||||||
| N | N=142 | N=50 | N=92 | |||||
| Treatment Reported at Dischargec | Received Any AOD Treatmentd | 46% | 14% | 64% | 10.98 | 32.73 | 0.000 | |
| Days of any AOD Treatmentd | 44.63 | 53.75 | 10.38 | 63.24 | 0.98 | 7.38 | 0.000 | |
|
| ||||||||
| Days Used Alcohole | Intake | 7.42 | 10.36 | 8.68 | 6.73 | -0.19 | -1.07 | 0.285 |
| Follow-up | 2.74 | 6.05 | 3.66 | 2.24 | -0.23 | -1.34 | 0.182 | |
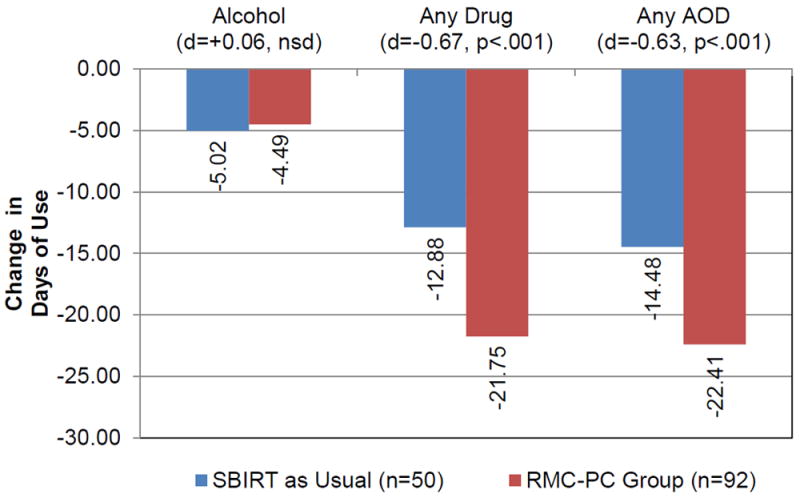
| Change | -4.68 | 8.96 | -5.02 | -4.49 | 0.06 | 0.34 | 0.737 | |
|
| ||||||||
| Days Used Illicit Drugs or Misused Prescription Medicatione | Intake | 21.40 | 11.77 | 15.98 | 24.35 | 0.71 | 3.91 | 0.000 |
| Follow-up | 2.67 | 6.64 | 2.82 | 2.60 | -0.03 | -0.19 | 0.853 | |
| Change | -18.67 | 13.34 | -12.88 | -21.75 | -0.67 | -3.85 | 0.000 | |
|
| ||||||||
| Days Used Alcohol or Other Drugse | Intake | 24.87 | 9.42 | 20.72 | 27.12 | 0.68 | 3.51 | 0.001 |
| Follow-up | 5.25 | 8.94 | 6.24 | 4.71 | -0.17 | -0.91 | 0.363 | |
| Change | -19.62 | 12.60 | -14.48 | -22.41 | -0.63 | -3.63 | 0.000 | |
SBIRT: Screening, Brief Intervention, and Referral to Treatment
RMC-PC: Recovery Management Checkups for Primary Care
Data collected at discharge from treatment or 6-month interview – whichever comes first.
AOD Treatment: alcohol or other drug use treatment, including outpatient, intensive outpatient, inpatient, or methadone.
Out of 30 days at intake and follow-up, change calculated as follow-up minus intake; range = -30 to +30
Changes in Substance Use
Table 2 also shows the days of using alcohol, illicit drugs (including misuse of prescription drugs), and alcohol or other drugs at intake and follow-up and the change score (post minus pre) by condition. For alcohol use there was a small significant difference in the days of alcohol use at follow-up; however after subtracting the baseline differences the change scores were not significantly different (-5.02 vs. -4.49 days, d=0.06, t(1)=0.34, p=.737). For drug use, the RMC-PC group started significantly higher and ended slightly lower, yielding significantly greater reductions (post-pre) in past-month days of any illicit drug use (-12.88 vs. -21.75 days, d=-0.67, t(1)=-3.85, p<.001). This difference was primarily driven by greater reductions in opioid use (-3.14 vs. -18.61, d=-1.08), t(1)=-8.10, p<.001). When alcohol and other drug use (AOD) are combined, the RMC-PC group started significantly higher, ended slightly lower and produced a significantly greater reduction in past-month days of AOD use (-14.47 vs, -22.41, d=-0.63), t(1)= -3.63, p<.001). Figure 1 shows that both SBIRT and RMC-PC were associated with reduced days of use, but that RMCPC reductions were greater for illicit drugs and combined AOD use.
Figure 1.
Change in Days of Use by Condition
Direct and Indirect Effects of RMC-PC
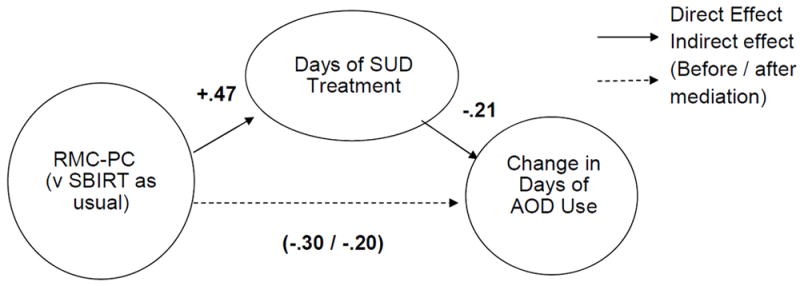
To further explore the relationships among RMC-PC, receipt of treatment, and substance use outcomes, path analysis was conducted, as shown by standardized path coefficients in Figure 2. To control for the baseline differences between the two groups, the dependent variable is modeled as changes in days of AOD use (post-pre) within subjects. Consistent with the bivariate findings above, there are statistically and clinically significant paths from being in the RMC-PC group to days of SUD treatment (standardized p= +0.47, 22% of variance) and changes in days of any AOD use (standardized p= -0.20, 4% of variance). Relative to the comparison group, being in the RMC-PC group is associated with a 6-day increase in the days of treatment and a further reduction of 3.78 days of AOD use. The days of SUD treatment also had a direct effect on changes in days of AOD use (standardized p=-0.21, 4% of variance). This means that every 53.75 days of SUD treatment received is associated with a further reduction of 2.65 days of AOD use.
Figure 2.
Path Analysis of Mediation Effect
Adding days of SUD treatment to the model reduces the direct effect of RMC-PC on the change in AOD use along the bottom path from -.3 to -.2 and is “partially” mediating (i.e., explaining) the effect of RMC-PC on changes in AOD use. Thus based on the combined AOD use model (as shown), RMC-PC has statistically significant effects (p < 0.05) -.20 directly and -.10 indirectly (via days of treatment). Even after controlling for the indirect effects of RMC-PC through SUD treatment, the remaining direct effect of RMC-PC is the equivalent of a further reduction of 2.52 days of any AOD use and close to the effect of treatment. This pattern of findings is slightly stronger when predicting the change in days of any drug use separately (data not shown). The direct and indirect effects of RMC-PC on change in days of alcohol use are not statistically significant.
Post Hoc Examination of Group Non-Equivalence
Although both groups were recruited from the same FQHC sites using the same methods, the latter cohort (RMC-PC) was associated with some significant changes in case mix as shown in Table 1. The most clinically significant of these was the sharp increase in the number of weekly opioid users and its associated increase in DAST scores and overall severity. In general the small and uneven sample sizes (n=50 comparison, n= 92 in RMC-PC) in this pilot do not provide sufficient power to conduct robust subgroup or covariate analysis. However, to address concerns about group non-equivalence, post hoc analysis were conducted for the subset of patients who reported weekly or more frequent opioid use at baseline (n’s =14 and 67, respectively). These show that there are still robust effects on the percent who received any AOD treatment (21% vs. 81%, Odds Ratio=15.25, p<.001) and the days of AOD treatment received (22.21 vs. 81.03 days, Cohen’s d= 1.10, p<.001).
Discussion
This study showed that, relative to use of SBIRT as usual procedures for screening individuals in FQHCs for SUD and referring those in need to treatment, an assertive linkage and engagement intervention, the RMC-PC, significantly increased the number of patients who received any SUD treatment and the days of treatment received across a range of SUD treatment modalities (i.e., residential, intensive outpatient, medication-assisted treatment). In addition, the study findings provide preliminary evidence on the beneficial effects of RMC-PC on changes in drug use, both directly and indirectly (via increased treatment received). This latter finding is particularly important as evidence to date on the effectiveness of SBIRT has been weaker for individuals who have drug use disorders, rather than alcohol use disorders.41 There was a lack of a marginal direct or indirect effect of RMC-PC on changes in days of alcohol use, however, both groups reduced alcohol use at levels similar to what has been consistently found in the literature.42-44
Although limited in size, the post hoc analyses showed that the effect of RMC was even stronger for the subgroup that was using opioids at least weekly. This finding is significant, given the current national opioid crisis, in which increases in use of heroin and misuse of prescription opioids have led to rapidly increasing rates of death due to overdose from opioids or heroin.45 Consistent trends have been seen in Illinois, where this study was conducted; from 2013 – 2015; deaths due to heroin overdose increased by 45% and those due to prescription opioid-overdose by 72%.46 Concurrently, the state also expanded methadone treatment capacity, with gradual increases in the percentage of treatment admissions reporting primary use of opioids (27.8% to 29.0%).47 As reflected in this pilot study, the RMC group, which was mainly recruited over this time period, had a significantly higher proportion of opioid users, compared with the SBIRT as usual group that was recruited earlier in time. Although policy initiatives and funding have been directed to increasing the capacity for medication-assisted treatment for individuals with opioid use disorders,48 assertive means for linking such individuals to treatment and providing ongoing support for treatment engagement and retention will be critical to the success of these efforts to ameliorate this crisis in opioid use and associated mortality.
Together these findings demonstrate the feasibility of the RMC-PC as an assertive and robust linkage model for drug-using patients in primary care settings that effectively increased their participation in SUD treatment, leading to significantly greater reductions in drug use and any AOD use. RMC-PC has the potential to engage publicly insured and low-income patients identified within FQHCs as needing SUD treatment, who often perceive numerous barriers to accessing SUD treatment and may lack the skills or motivation for negotiating health care systems to surmount these barriers on their own. Moreover, the RMC-PC intervention may be particularly useful to surmount the pessimistic and negative views of SUD treatment based on past experiences that are commonplace among those who are resistant to entering treatment; such attitudinal barriers are more prevalent among African Americans and Hispanics relative to whites,14 similar to the pilot study sample composition.
As this is a preliminary quasi-experimental pilot study of the efficacy of the RMC-PC intervention, the findings are necessarily limited by the small and uneven sample sizes and lack of randomization to the SBIRT as usual and RMC-PC groups, resulting in several significant differences between groups at baseline. Although a detailed analysis of covariance is beyond the scope of this pilot study, it is noteworthy that after sub-setting to the weekly or more frequent opioid users, the RMC-PC group was still associated with higher rates of entering treatment and days of treatment. Another limitation was the reliance on GPRA measures, which lack published psychometrics and detailed measures of SUD, treatment experiences, and self-help involvement. Lastly, the RMC intervention was delivered in three clinic sites operated by two FQHCs agencies and RMC-PC was provided by research staff. A robust test of RMC effectiveness requires a fully powered experimental design with in-depth measurement that will enable examination of effects across sites and is able to examine the relation of SUD severity with treatment and substance use outcomes. Ideally RMC-PC should also be tested in a larger clinical trial using standardized measures with a more diverse range of clinics and FQHC providers as well as utilizing non-research staff who are trained to provide RMC-PC.
Implications for Behavioral Health
The substantial risks of untreated substance use have been well established, including high rates of physical and mental health morbidity and premature death.49 Further, untreated SUDs exact a high cost from society, since individuals with chronic drug use disorders, who do not receive SUD treatment, use significantly more inpatient and emergency health services, resulting in substantially higher medical costs, compared with those who receive SUD treatment.50,50,51 This is likely to become even more pressing as a result of the current rise in opioid use disorders and associated increase in overdose-related deaths. Within the context of changing policies on health care delivery, and particularly the relationship of SUD treatment with primary health care services, there should be a priority on establishing and implementing evidence-based interventions. Assertive linkage interventions that effectively engage individuals in primary care settings into SUD treatment have the potential to increase access to behavioral health services among patients who need these services.
Acknowledgments
This paper was supported by grant no. TI023455 from the Substance Abuse and Mental Health Service Administration’s (SAMHSA) Center for Substance Abuse Treatment (CSAT) to the Illinois Department of Human Services’ (IDHS) Division of Alcoholism and Substance Abuse (DASA). The authors thank Brittany Bolender, Rod Funk, Cheryl Peterson and Belinda Willis for their assistance preparing the manuscript. The opinions are those of the authors and do not reflect official positions of the government. Comments and questions can be addressed to Dr. Scott.
Footnotes
Conflict of Interest Statement
The authors report no conflicts of interest.
Contributor Information
Christy K Scott, Lighthouse Institute, Chestnut Health Systems, Chicago, IL 221 W. Walton, Chicago, IL 60610; Phone: (312) 664-4321; Fax: (312)664-4324; cscott@chestnut.org.
Christine E. Grella, Chestnut Health Systems, Chicago, IL 221 W. Walton, Chicago, IL 60610; Phone: (310) 267-5451; Fax: (310) 473-7885; cegrella@chestnut.org.
Michael L. Dennis, Chestnut Health Systems, 448 Wylie Drive, Normal, IL 61761; Phone: (309)451-7801; Fax: (309)451-7765; mdennis@chestnut.org.
Lisa Nicholson, Chestnut Health Systems, Chicago, IL 221 W. Walton, Chicago, IL 60610; Phone: (312) 664-4321; Fax: (312)664-4324; lnicholson@chestnut.org.
References
- 1.Dennis M, Scott CK. Managing addiction as a chronic condition. Addiction Science & Clinical Practice. 2007;4(1):45–55. doi: 10.1151/ascp074145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott CK, Dennis ML, Laudet A, et al. Surviving drug addiction: The effect of treatment and abstinence on mortality. American Journal of Public Health. 2011;101(4):737–744. doi: 10.2105/AJPH.2010.197038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hser YI, Evans L, Grella C, et al. Long-term course of opioid addiction. Harvard Review of Psychiatry. 2015;23(2):76–89. doi: 10.1097/HRP.0000000000000052. [DOI] [PubMed] [Google Scholar]
- 4.Scott CK, Dennis ML, Foss MA. Utilizing recovery management checkups to shorten the cycle of relapse, treatment reentry, and recovery. Drug and Alcohol Dependence. 2005;78(3):325–338. doi: 10.1016/j.drugalcdep.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLellan AT, Lewis DC, O’Brien CP, et al. Drug dependence: A chronic medical illness. The Journal of the American Medical Association. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- 6.Martire LINKAGE MANAGER. Lustig AP, Schulz R, et al. Is it beneficial to involve a family member? A meta-analysis of psychosocial interventions for chronic illness. Health Psychology. 2004;23(6):599–611. doi: 10.1037/0278-6133.23.6.599. [DOI] [PubMed] [Google Scholar]
- 7.Hartmann M, Bäzner E, Wild B, et al. Effects of interventions involving the family in the treatment of adult patients with chronic physical diseases: A meta-analysis. Psychotherapy & Psychosomatics. 2010;79(3):136–148. doi: 10.1159/000286958. [DOI] [PubMed] [Google Scholar]
- 8.Scott CK, Dennis ML. Recovery Management Checkups with adult chronic substance users. In: Kelly JF, White WL, editors. Addiction Recovery Management: Theory, Research and Practice, Current Clinical Psychiatry. New York, NY: Springer Science+Business Media, LLC; 2011. pp. 87–101. [Google Scholar]
- 9.Cucciare MA, Timko C. Bridging the gap between medical settings and specialty addiction treatment. Addiction. 2015;110(9):1416–1420. doi: 10.1111/add.12977. [DOI] [PubMed] [Google Scholar]
- 10.Cucciare MA, Coleman EA, Timko C. A conceptual model to facilitate transitions from primary care to specialty substance use disorder care: a review of the literature. Primary Health Care Research & Development. 2015;16(5):492–505. doi: 10.1017/S1463423614000164. [DOI] [PubMed] [Google Scholar]
- 11.Ducharme LJ, Chandler RK, Harris AHS. Implementing effective substance abuse treatments in general medical settings: Mapping the research terrain. Journal of Substance Abuse Treatment. 2016;60:110–118. doi: 10.1016/j.jsat.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samet JH, Friedmann P, Saitz R. Benefits of linking primary medical care and substance abuse services: patient, provider, and societal perspectives. Archives of Internal Medicine. 2001;161(1):85–91. doi: 10.1001/archinte.161.1.85. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Department of Health and Human Services HIT. [December 5, 2016];What are Federally qualified health centers (FQHCs)? Available online at http://www.hrsa.gov/healthit/toolbox/RuralHealthITtoolbox/Introduction/qualified.html.
- 14.Otiniano Verissimo AD, Grella CE. Influence of gender and race/ethnicity on perceived barriers to help-seeking for alcohol or drug problems. Journal of Substance Abuse Treatment. 2017;75:54–61. doi: 10.1016/j.jsat.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells K, Klap R, Koike A, et al. Ethnic disparities in unmet need for alcoholism, drug abuse, and mental health care. American Journal of Psychiatry. 2001;158:2027–2032. doi: 10.1176/appi.ajp.158.12.2027. [DOI] [PubMed] [Google Scholar]
- 16.Benson DS, Townes PJ, Dobbs D. Quality Management Plan: A practical, patient-centered template. Bethesda MD: National Association of Community Health Centers; 2011. [Google Scholar]
- 17.D’Onofrio G, Bernstein SL. Screening, brief intervention and referral of emergency department patients with unhealthy drug use: Efficacious or not? Evidence Based Mental Health. 2015;18(4):e8. doi: 10.1136/eb-2014-102037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saitz R, et al. Commentaries on Glass. SBIRT is the answer? Probably not. Addiction. 2015;110(9):1416–1420. doi: 10.1111/add.12986. [DOI] [PubMed] [Google Scholar]
- 19.Saitz R, Alford PA, Bernstein J, et al. Screening and brief intervention for unhealthy drug use in primary care settings: Randomized clinical trials are needed. Journal of Addiction Medicine. 2010;4(3):123–130. doi: 10.1097/ADM.0b013e3181db6b67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babor TF, McRee BG, Kassebaum PA, et al. Screening, Brief Intervention, and Referral to Treatment (SBIRT): Toward a public health approach to the management of substance abuse. Substance Abuse. 2007;28(3):7–30. doi: 10.1300/J465v28n03_03. [DOI] [PubMed] [Google Scholar]
- 21.Madras BK, Compton WM, Avula D, et al. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: Comparison at intake and 6 months later. Drug and Alcohol Dependence. 2009;99(1):280–295. doi: 10.1016/j.drugalcdep.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glass JE, Hamilton AM, Powell BJ, et al. Specialty substance use disorder services following brief alcohol intervention: A meta-analysis of randomized controlled trials. Addiction. 2015;110(9):1404–1415. doi: 10.1111/add.12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim TW, Bernstein J, Cheng DM, et al. Receipt of Addiction Treatment as a Consequence of a Brief Intervention for Drug Use in Primary Care: A Randomized Trial. Addiction. 2016 doi: 10.1111/add.13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abraham AJ, Knudsen HK, Rieckmann T, et al. Disparities in access to physicians and medications for the treatment of substance use disorders between publicly and privately funded treatment programs in the United States. Journal of Studies on Alcohol and Drugs. 2013;74:258–265. doi: 10.15288/jsad.2013.74.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weisner CM, Mertens J, Tam TW, et al. Factors affecting the initiation of substance abuse treatment in an HMO. Addiction. 2001;96(5):705–716. doi: 10.1046/j.1360-0443.2001.9657056.x. [DOI] [PubMed] [Google Scholar]
- 26.Collins C, Hunson DL, Munger R, et al. Evolving models of behavioral health integration in primary care. New York: Milbank Memorial Fund; 2010. [Google Scholar]
- 27.Chaple M, Sacks S, Randell J, et al. A technical assistance framework to facilitate the delivery of integrated behavioral health services in Federally Qualified Health Centers (FQHCs) Journal of Substance Abuse Treatment. 2016;60:62–69. doi: 10.1016/j.jsat.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Kessler R, Miller BF, Kelly M, et al. Mental health, substance abuse, and health behavior services in patient-centered medical homes. American Board of Family Medicine. 2014;27(5):637–644. doi: 10.3122/jabfm.2014.05.140021. [DOI] [PubMed] [Google Scholar]
- 29.Andrews CM. The relationship of state Medicaid coverage to Medicaid acceptance among substance abuse providers in the United States. Journal of Behavioral Health Services & Research. 2014;41:460–472. doi: 10.1007/s11414-013-9387-2. [DOI] [PubMed] [Google Scholar]
- 30.Scott CK, Dennis ML. Recovery Management Check-ups: An Early Re-Intervention Model. Lighthouse Institute; Chicago, IL: 2003. Retrieved on 12/19/16 from http://chestnut.org/Lighthouse-Institute/Bookstore/Product-Details/manuals/recovery-management-check-ups. [Google Scholar]
- 31.Dennis ML, Scott CK. Four-year outcomes from the Early Re-Intervention Experiment (ERI) with recovery management checkups (RMC) Drug and Alcohol Dependence. 2012;121(1):10–17. doi: 10.1016/j.drugalcdep.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dennis ML, Scott CK, Funk R. An experimental evaluation of recovery management checkups (RMC) for people with chronic substance use disorders. Evaluation and Program Planning. 2003;26(3):339–352. doi: 10.1016/S0149-7189(03)00037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott CK, Dennis ML, Lurigio AJ. The effects of specialized probation and Recovery Management Check-Ups (RMCs) on treatment participation, substance use, HIV-risk behaviors, and recidivism among female offenders: Main findings of a three-year experiment using subject by intervention interaction analysis. Journal of Experimental Criminology. doi: 10.1007/s11292-016-9281-z. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scott CK, Dennis ML. Results from two randomized clinical trials evaluating the impact of quarterly recovery management checkups with adult chronic substance users. Addiction. 2009;104(6):959–971. doi: 10.1111/j.1360-0443.2009.02525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atanda R, Podrasky-Mattia L, Benton A. Developing a data collection system. Evaluation and Program Planning. 2005;28(3):335–339. [Google Scholar]
- 36.Babor TF, Higgins-Biddle JC, Saunders JB, et al. AUDIT: The alcohol use disorders identification test guidelines for use in primary care. Second Edition. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- 37.Skinner HA. The drug abuse screening test. Addictive Behaviors. 1982;7:363–371. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- 38.D’Onofrio G, Pantalon MV, Degutis LC, Larkin GL, O’Connor PG, Fiellin D. BNI Training Manual For Opioid Dependent Patients in the ED. New Haven, CT: Yale University School of Medicine; 2009. [Google Scholar]
- 39.World Health Organization. Brief Intervention: The ASSIST-linked brief intervention for hazardous and harmful substance use manual for use in primary care. Geneva, Switzerland: WHO Press; 2010. [December 19 2016]. Available online at http://apps.who.int/iris/bitstream/10665/44321/1/9789241599399_eng.pdf. [Google Scholar]
- 40.Center for Substance Abuse Treatment. Enhancing Motivation for Change in Substance Abuse Treatment Treatment Improvement Protocol (TIP) Series, No 35 Report No : SMA13-4212. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. [PubMed] [Google Scholar]
- 41.Hingson R, Compton WM. Screening and brief intervention and referral to treatment for drug use in primary care: Back to the drawing board. The Journal of the American Medical Association. 2014;312(5):488–489. doi: 10.1001/jama.2014.7863. [DOI] [PubMed] [Google Scholar]
- 42.Dawson DA, Grant BF, Stinson FS, et al. Estimating the effect of help-seeking on achieving recovery from alcohol dependence. Addiction. 2006;101(6):8245–834. doi: 10.1111/j.1360-0443.2006.01433.x. [DOI] [PubMed] [Google Scholar]
- 43.Kadden R, Carbonari J, Litt M, et al. Matching Alcoholism Treatments to Client Heterogeneity: Project MATCH Three-Year Drinking Outcomes. Alcoholism: Clinical and Experimental Research. 1998;22(6):1300–1311. doi: 10.1111/j.1530-0277.1998.tb03912.x. [DOI] [PubMed] [Google Scholar]
- 44.Weisner C, Matzger H, Kaskutas LA. How important is treatment? One-year outcomes of treated and untreated alcohol-dependent individuals. Addiction. 2003;98(7):901–911. doi: 10.1046/j.1360-0443.2003.00438.x. [DOI] [PubMed] [Google Scholar]
- 45.Rudd RA, Aleshire N, Zibbell JE, et al. Increases in drug and opioid overdose deaths — United States, 2000–2014. Centers for Disease Control and Prevention Morbidity and Mortality Weekly Report. 2016 Jan 1;64(50):1378–1382. doi: 10.15585/mmwr.mm6450a3. Downloaded from: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6450a3.htm. [DOI] [PubMed] [Google Scholar]
- 46.Tuyet M. State Opioid Crisis Response Advisory Council: Data from the Illinois Department of Public Health. Presentation on January 21, 2017. [Google Scholar]
- 47.Kirby D. State Opioid Crisis Response Advisory Council: Data from the Illinois Department of Human Services, Division of Alcoholism and Substance Abuse. Presentation on January 21, 2017. [Google Scholar]
- 48.Volkow ND, Frieden TR, Hyde PS, et al. Medication-assisted therapies: Tackling the opioid-overdose epidemic. New England Journal of Medicine. 2014;370:263–266. doi: 10.1056/NEJMp1402780. [DOI] [PubMed] [Google Scholar]
- 49.Degenhardt L, Whiteford HA, Ferrari AJ, et al. Global burden of disease attributable to illicit drug use and dependence: Findings from the Global Burden of Disease Study 2010. The Lancet. 2013;382:1564–1574. doi: 10.1016/S0140-6736(13)61530-5. [DOI] [PubMed] [Google Scholar]
- 50.French MT, McGeary KA, Chitwood DD, et al. Chronic illicit drug use, health services utilization, and the cost of medical care. Social Science and Medicine. 2000;50(12):1703–1713. doi: 10.1016/s0277-9536(99)00411-6. [DOI] [PubMed] [Google Scholar]
- 51.Parthasarathy S, Weisner CM. Five-year trajectories of health care utilization and cost in a drug and alcohol treatment sample. Drug and Alcohol Dependence. 2005;80(2):231–240. doi: 10.1016/j.drugalcdep.2005.04.004. [DOI] [PubMed] [Google Scholar]


