Abstract
Retinal degeneration 3 protein (RD3) binds to retinal membrane guanylyl cyclase (RetGC) and suppresses the basal activity of RetGC in photoreceptor cells that opposes the allosteric activation of the cyclase by GCAP proteins. Mutations in RD3 that disrupt its inhibition of RetGC are implicated in human retinal degenerative disorders. Here we report both backbone and sidechain NMR assignments for the RD3 protein (BMRB accession no. 27305).
Keywords: Retinal degeneration protein 3, retinal guanylyl cyclase, RetGC, RD3, GCAP
Biological Context
Cyclic GMP is a key second messenger in visual phototransduction (Fu & Yau, 2007). Light activation of photoreceptors causes hydrolysis of cGMP by a phosphodiesterase (PDE6) that in turn causes the closure of cyclic nucleotide gated ion channels, which generates a neural signal. To recover the photoreceptor from excitation, the cGMP becomes replenished by a retinal membrane guanylyl cyclase (RetGC) (Dizhoor et al, 1994; Lowe et al, 1995), controlled by guanylyl cyclase activating proteins, GCAPs (Koch & Dell’Orco, 2015), and retinal degeneration 3 (RD3) protein (Azadi et al, 2010). RetGC binding to RD3 prevents cyclase activation by GCAPs (Peshenko et al, 2016), and promotes accumulation of RetGC in the outer segment (Azadi et al, 2010; Zulliger et al, 2015). A nonsense mutation deleting RD3 causes severe congenital blindness, Leber’s congenital amaurosis (LCA) type 12 (Friedman et al, 2006), and mutations in RetGC affecting its interactions with GCAPs and RD3 have been linked to LCA type 1 (Zulliger et al, 2015). Based on mutational analysis (Peshenko et al, 2016), the cyclase-binding domain involves the central portion of RD3 while the N- and C-proximal regions are not essential for the RetGC1 binding and inhibition. In this study, we report NMR assignments of the RD3 protein as a first step toward determining the atomic level structure of RD3 and its interaction with RetGC.
Methods and Experiments
Preparation of RD3-D
A cDNA of Homo sapiens RD3 (residues 1-195, called RD3) and deletion construct (residues 18-161, called RD3-D) generated by PCR amplification using Phusion Flash polymerase (Thermo Fisher) were each subcloned into pET-11d vector (Novagen/Calbiochem) that produced recombinant RD3 and RD3-D protein without any affinity tags. Uniformly 15N-labeled and 13C,15N-labeled samples of RD3 and RD3-D were expressed in E. coli as described previously (Peshenko et al, 2016) using M9 minimal media. RD3-D was purified from inclusion bodies using urea extraction (Peshenko et al, 2016) followed by Phenyl-Sepharose, anion exchange (HiTrap Q HP, GE Healthcare), and Superdex-200 column chromatography. Purified protein was more than 95% pure based on SDS-PAGE.
NMR spectroscopy
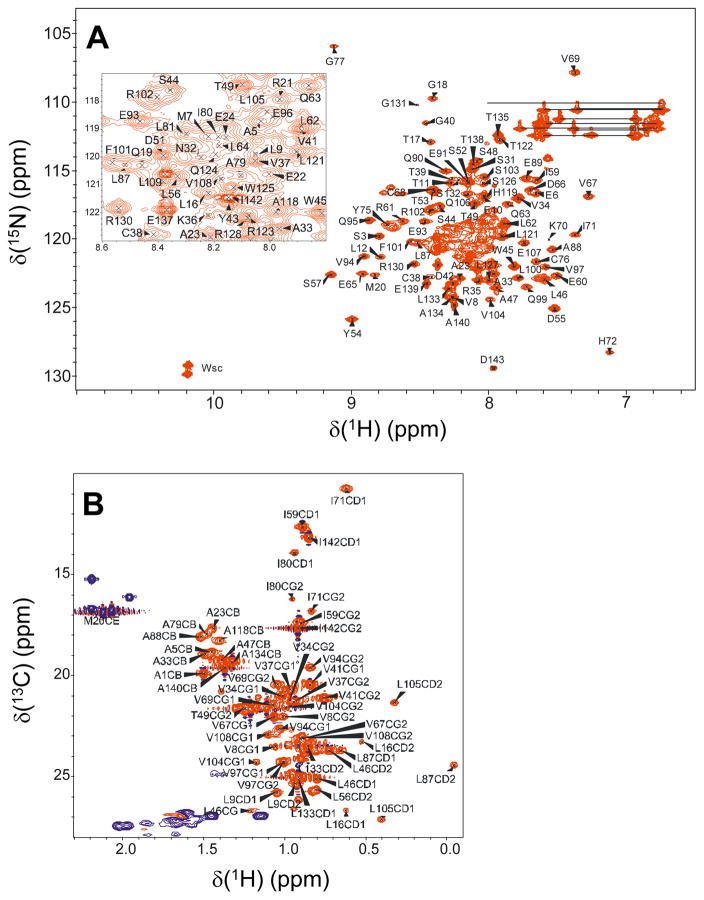
Samples of RD3-D for NMR analysis (prepared as described above) were exchanged into a buffer containing 5 mM Tris-d11 (pH 7.4) with 3 mM DTT-d10, 0.04% w/v NaN3, 50 μM EDTA-d12, and 93% H2O/7% D2O. The final protein concentration was 0.9 mM. All NMR experiments were performed at 23°C on a Bruker Avance 600 MHz spectrometer equipped with a four channel interface and triple resonance cryogenic (TCI) probe. The 15N-1H HSQC spectrum (Fig. 1 A) was recorded with 256 × 2048 complex points for 15N(F1) and 1H(F2), respectively. Assignment of backbone resonances was obtained by analyzing the following spectra: HNCA, HNCACB, CBCA(CO)NH, HNCO (Ikura et al, 1990). The assignment of side chain resonances was obtained by analyzing HCCH-TOCSY, CCCONH, HCCCONH, 13C-edited NOESY, 15N-edited NOESY, 15N-edited TOCSY, and CT-HSQC (Fig. 1 B). The NMR data were processed using NMRPipe (Delaglio et al, 1995) and analyzed using Sparky NMRFAM (Lee et al, 2015).
Fig. 1.
Two-dimensional NMR spectra 1H,15N-HSQC (A) and 1H,13C-HSQC (B) of 15N,13C-labeled RD3-D recorded at 600-MHz 1H frequency and at 23 °C. The inset in panel A shows an expanded view of assignments from the spectrally crowded central region. Side chain amide resonances of Asn and Gln are connected with solid lines. Indole side chain resonances are marked as Wsc. Representative backbone assignments are indicated by residue labels; complete assignments are available as BMRB accession no. 27305.
Assignments and Data Deposition
Two-dimensional NMR spectra of RD3-D (15N-1H HSQC (Fig. 1A) and constant-time 13C-1H HSQC (Fig. 1B)) are presented to illustrate representative NMR assignments of backbone and side-chain methyl resonances, respectively. NMR assignments were based on 3D heteronuclear NMR spectra recorded from 13C/15N-labeled RD3-D. The full-length RD3 protein (residues 1-195) was not soluble enough for NMR structural analysis and the first 17 residues from the N-terminus and final 35 residues at the C-terminus were predicted to be unstructured (Molday et al, 2014; Shen et al, 2009). A deletion construct (residues 18-161, called RD3-D) is soluble enough for NMR structural analysis and binds to RetGC with a dissociation constant in the submicromolar range, like the full-length RD3 protein. The NMR spectra of RD3-D exhibited well-dispersed peaks with uniform intensities indicative of a stably folded structure. A few amide resonances exhibited noteworthy downfield shifts, including S57, S74, G77 and S98 that are flanked by nearby aromatic rings (Y54, Y75 and F101 respectively), which may explain the large ring current shifts (Fig. 1A). Ring current shifted methyl resonances assigned to residues L87 and L105 (Fig. 1C) suggest these methyl groups are near aromatic residues in the hydrophobic core. More than 86% of the backbone resonances (1HN, 15N, 13Cα, 13Cβ, and 13CO) and methyl side-chain resonances were assigned. The unassigned residues (marked by an asterisk in Fig. 2) had severely overlapped backbone amide resonances and/or weak NMR intensities that obscured their assignment. The chemical shift assignments (1H, 15N, 13C) of RD3-D have been deposited in the BioMagResBank (http://www.bmrb.wisc.edu) under accession number 27305.
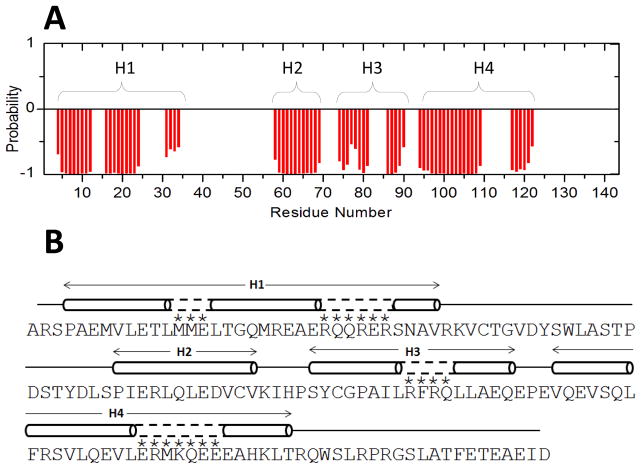
Fig. 2.
Primary and secondary structures of RD3. (A) TALOS+ ANN-secondary structure probability plotted as a function of residue number. (B) Secondary structure elements (cylinder for helix and line for random coil) were calculated on the basis of chemical shift index and sequential NOE patterns. Residues marked with an asterisk are not assigned but are predicted to have helical structure (dashed lines) based on a secondary structure prediction from the amino acid sequence.
The secondary structure of RD3-D was calculated based on the chemical shift index (Wishart et al, 1992) of each assigned amino acid residue and ANN-Secondary structure prediction using TALOS+ (Shen et al, 2009) (Fig. 2A). RD3-D contains four α-helices named H1 (residues 4-24), H2 (residues 58-69), H3 (residues 74-90), and H4 (residues 94-122) depicted by cylinders in Fig. 2B. The secondary structure of RD3-D is consistent with a four helix bundle formed by two helical hairpins connected in a parallel fashion. Preliminary NMR-derived distance restraints inferred from NOESY spectra suggest that residues M20 and E22 in H1 are less than 5 Å away from L87 and A88 in H3, which places H1 and H3 in close proximity. Also, residue L16 in H1 is less than 5 Å away from S98 in H4, which places H1 close to H4 as well. These distance restraints suggest that the two helical hairpins might be connected in a parallel fashion. The NMR assignments of RD3-D presented here are an important first step toward determining its full three-dimensional structure.
Acknowledgments
We thank Jeff Walton for technical support and help with NMR experiments. Work supported by NIH grants (EY012347) to J.B.A and (EY011522) to A.M.D.
References
- Azadi S, Molday LL, Molday RS. RD3, the protein associated with Leber congenital amaurosis type 12, is required for guanylate cyclase trafficking in photoreceptor cells. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21158–21163. doi: 10.1073/pnas.1010460107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeiffer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- Dizhoor AM, Lowe DG, Olsevskaya EV, Laura RP, Hurley JB. The human photoreceptor membrane guanylyl cyclase, RetGC, is present in outer segments and is regulated by calcium and a soluble activator. Neuron. 1994;12:1345–1352. doi: 10.1016/0896-6273(94)90449-9. [DOI] [PubMed] [Google Scholar]
- Friedman JS, Chang B, Kannabiran C, Chakarova C, Singh HP, Jalali S, Hawes NL, Branham K, Othman M, Filippova E, Thompson DA, Webster AR, Andréasson S, Jacobson SG, Bhattacharya SS, Heckenlively JR, Swaroop A. Premature truncation of a novel protein, RD3, exhibiting subnuclear localization is associated with retinal degeneration. American journal of human genetics. 2006;79:1059–1070. doi: 10.1086/510021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Yau KW. Phototransduction in mouse rods and cones. Pflugers Archiv: European journal of physiology. 2007;454:805–819. doi: 10.1007/s00424-006-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikura M, Kay LE, Bax A. A novel approach for sequential assignment of 1H, 13C, and 15N spectra of proteins: heteronuclear triple-resonance three-dimensional NMR spectroscopy. Application to calmodulin. Biochemistry. 1990;29:4659–4667. doi: 10.1021/bi00471a022. [DOI] [PubMed] [Google Scholar]
- Koch KW, Dell’Orco D. Protein and Signaling Networks in Vertebrate Photoreceptor Cells. Frontiers in molecular neuroscience. 2015;8:67. doi: 10.3389/fnmol.2015.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Tonelli M, Markley JL. NMRFAM-SPARKY: enhanced software for biomolecular NMR spectroscopy. Bioinformatics. 2015;31:1325–1327. doi: 10.1093/bioinformatics/btu830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe DG, Dizhoor AM, Liu K, Gu Q, Spencer M, Laura R, Lu L, Hurley JB. Cloning and expression of a second photoreceptor-specific membrane retina guanylyl cyclase (RetGC), RetGC-2. Proc Natl Acad Sci USA. 1995;6:5535–5539. doi: 10.1073/pnas.92.12.5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molday LL, Jefferies T, Molday RS. Insights into the role of RD3 in guanylate cyclase trafficking, photoreceptor degeneration, and Leber congenital amaurosis. Frontiers in molecular neuroscience. 2014;7:44. doi: 10.3389/fnmol.2014.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peshenko IV, Olshevskaya EV, Dizhoor AM. Functional Study and Mapping Sites for Interaction with the Target Enzyme in Retinal Degeneration 3 (RD3) Protein. J Biol Chem. 2016;291:19713–19723. doi: 10.1074/jbc.M116.742288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Delaglio F, Cornilescu G, Bax A. TALOS+: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J Biomol NMR. 2009;44:213–223. doi: 10.1007/s10858-009-9333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart DS, Sykes BD, Richards FM. The chemical shift index: a fast and simple method for the assignment of protein secondary structure through NMR spectroscopy. Biochemistry. 1992;31:1647–1651. doi: 10.1021/bi00121a010. [DOI] [PubMed] [Google Scholar]
- Zulliger R, Naash MI, Rajala RV, Molday RS, Azadi S. Impaired association of retinal degeneration-3 with guanylate cyclase-1 and guanylate cyclase-activating protein-1 leads to leber congenital amaurosis-1. J Biol Chem. 2015;290:3488–3499. doi: 10.1074/jbc.M114.616656. [DOI] [PMC free article] [PubMed] [Google Scholar]