Abstract
Purpose
To determine whether measures of intraocular pressure (IOP) variation are independently associated with the risk of developing open-angle glaucoma (OAG).
Design
A population-based, longitudinal study.
Methods
3,666 Latinos free of OAG at the baseline of the Los Angeles Latino Eye Study were followed up four years later. Maximum IOP, standard deviation (SD) of IOP, range of IOP and mean IOP were derived from six readings obtained at the two visits. OAG diagnosis at each visit was based on the consensus of experts who had access to all clinical examination data from that visit. Multivariate logistic regression was performed.
Results
Maximum, SD, and range of IOP were all associated with risk of developing OAG, even after adjustment for mean IOP. Maximum IOP provided the best fit to the data and other IOP measures were not associated with OAG risk in the model that had included maximum IOP. The effect of IOP variation varied by the level of IOP. Among participants with higher IOPs (≥15 mmHg), only higher levels of maximum IOP were associated with a higher OAG risk (P<0.05), while SD and range of IOP were not associated with OAG risk. Among participants with lower IOPs (<15 mmHg), higher levels of maximum, SD, and range of IOP were all associated with a higher risk of developing OAG (Ps<0.05). Mean IOP was associated with OAG risk only in participants with higher IOPs and not in those with lower IOPs. Results were similar when participants were stratified as <18 and ≥18 mmHg.
Conclusions
IOP variation was an independent risk factor for OAG. Maximum IOP was the most consistent IOP measure for predicting OAG risk across the entire spectrum of IOPs, possibly by capturing the effect of IOP variation among persons with relative lower IOPs as well mean IOP effects in those with higher IOPs.
INTRODUCTION
Elevated intraocular pressure (IOP) is an established important risk factor for open-angle glaucoma (OAG) and lowering diurnal IOP is the main therapeutic target. However, OAG occurs across the entire spectrum of IOP including IOPs in the statistically normal range1. In addition, it is well known that not all individuals with elevated IOP develop OAG and some individuals with “controlled” IOPs still experience progressive damage.2
Because IOP fluctuates over short-term (transient change in IOP occurring over the course of the day, e.g., diurnal change) and long-term (change in IOP occurring over months or years, e.g., inter-visit change) periods, there have been studies characterizing various features of IOP that may predict the development and progression of glaucoma beyond that of mean IOP.3, 4 In a hospital-based study,5 patients diagnosed with OAG were found to have a higher “amplitude” (range) of diurnal IOP fluctuation than patients admitted for conditions other than glaucoma or suspected of glaucoma. Contrary to other studies, Liu et al.6 found that diurnal-to-nocturnal increase in habitual IOP was significantly less in the untreated glaucoma patients than in the age-matched healthy individuals. As for long-term variation in IOP, a number of studies have examined its effect on the risk of developing OAG among individuals with ocular hypertension (Table 1).7–10 In two studies,9, 10 IOP variation was not associated with OAG risk. In the other two studies,7, 8 IOP variation was marginally associated with risk of OAG in univariate analysis (Ps=0.092 and 0.063 respectively) but did not remain significant in multivariate analyses that included mean IOP, possibly due to the correlation between IOP variation and mean IOP.8 However, in the Collaborative Initial Glaucoma Treatment Study (CIGTS), greater IOP variation (standard deviation - SD and range of IOP) and maximum IOP were found to be more predictive of visual field loss than mean IOP, particularly among glaucoma patients treated medically.11 In addition, the Advanced Glaucoma Intervention Study (AGIS) found that greater IOP variation predicted higher risk of glaucomatous progression among patients with low mean IOP but not among patients with high mean IOP.12, 13 It is possible that the association between IOP variation and the risk of developing OAG may be present among individuals with low IOP but not among those with high IOP. Furthermore, longitudinal data from non-human primates have been studied to determine the impact of maximum IOP and other measures of variation of IOP,14 and maximum IOP was found to be the best predictor of structural changes in the position of the disc and retinal nerve fiber layer thickness. It was postulated that either maximum IOP may be the most damaging to the optic disc, or that it captures both the contribution from mean IOP and the contribution of more variable IOP. In addition, recent studies suggest that the impact of IOP fluctuation/variation on optic nerve head may be modified by biomechanical properties of lamina cribrosa and peripapillary sclera.15 In light of the inconsistent findings from previous studies, potential correlations between different IOP measures, and the suspected variations in individual susceptibility to IOP and/or IOP variation, more studies are needed to elucidate how the dynamic component of IOP contributes to the development of OAG among different populations.
Table 1.
Variation in Intraocular Pressure and the Risk of Developing Open-angle Glaucoma in Prospective Cohort Studies.
| Study | Diagnostic Innovations in Glaucoma Study(DIGS)7 | Malmo Ocular Hypertension Study8 | European Glaucoma Prevention Study9 | Ocular Hypertension Treatment Study (OHTS)10 |
|---|---|---|---|---|
| Sample population | 252 eyes of 126 untreated ocular hypertensive patients. 47 eyes of 31 subjects developed OAG during follow-up | 90 patients with ocular hypertension: 44 were randomized to treatment with: placebo and 46 to timolol. 37 of them developed OAG. | 1,077 patients with IOP ≥22 mmHg, who were randomized to treatment with dorzolamide or placebo. Among them, 12 developed OAG. | 819 patients with ocular hypertension randomized either to topical ocular hypotensive medication or to close observation without treatment. Among them, 104 developed OAG. |
| Duration of follow-up | 7.1 years on average | 8.5 years on average | 5 years on average | 6.5 years (median) |
| Mean age | 56.3 years | 62 years | 57.0 years36 | 56.1 years37 |
| Race/ethnicity | 94% NHW, 3% AA, 2% Hispanic, 2% Asian | 100% NHW | 99.8% NHW | 70% NHW, 24.7% AA, 3.5% Hispanic37 |
| Average IOP during follow-up | 24.4 mmHg | 22.7 mmHg | 19.7 mmHg | 21.4 mmHg |
| IOP variation measurement | SD of all available IOP measurements | Mean daily range of IOP | SD of follow-up IOPs | Patient-specific variance of IOP |
| Association with IOP variation | No significant association | No significant association | No significant association | Inverse association |
| Association with mean IOP | Significant association | Significant association | Significant association | - |
Abbreviations: AA=African American, IOP=intraocular pressure, NHW=non-Hispanic white, OAG=open-angle glaucoma, SD=standard deviation.
The purpose of the present investigation was to evaluate in a population-based sample of Latinos whether measures of IOP variation (SD and range of IOP) were associated with risk of developing OAG independent of mean IOP, and whether maximum IOP, a more clinically usable (easy to calculate and interpret) measure that may capture the effects of both elevated mean IOP and greater IOP variation, was a better predictor of the risk of developing OAG than mean IOP. In addition, we explored these associations separately among individuals with relatively lower mean IOPs and those with higher mean IOPs.
METHODS
Study Design and Population
The Los Angeles Latino Eye Study (LALES) is a population-based cohort study of eye disease in self-identified Latinos aged 40 years and older living in 6 census tracts in the city of La Puente, Los Angeles County, California. Details of the study design, methods, and baseline data have been reported elsewhere.16 Briefly, the baseline examination was performed from 2000 to 2003 with four-year follow-up examination performed from 2004 to 2008. All eligible participants of the baseline LALES examination were invited to return for a home interview and a clinical examination. Similar questionnaire and examination procedures were used for both the baseline and the follow-up studies. Trained ophthalmologists and technicians performed a comprehensive ocular examination using standardized protocols, which included visual field (VF) testing, IOP measurement, and simultaneous stereoscopic fundus photography of the optic disc. Specifically, participants came to the LALES clinic between 8:00 am and 4:00 pm for scheduled eye exam. There was no significant difference in the hours of eye exam between individuals who did develop OAG and those who did not (P>0.10). At each visit, three consecutive readings of IOP were obtained through the Goldmann applanation tonometer (Haag-Streit, Bern, Switzerland) by a certified ophthalmic technician who was unaware of the participant’s glaucoma status. Goldmann tonometry was performed first in the right eye then in the left eye, and then was repeated two more times in the same order within minutes. In addition, an interviewer-administered questionnaire was used to assess ocular and medical histories, and laboratory testing was performed to obtain objective diagnostic criteria.
The study protocol was approved by the Institutional Review Board/Ethics Committee at the University of Southern California and adhered to the recommendations of the Declaration of Helsinki. Written, informed consent was obtained from all participants.
Diagnosis of OAG and definitions of incidence of OAG
Detailed descriptions of all OAG diagnosis-related testing, and the definition and determination of the diagnosis have been previously reported.17 In short, participants’ peripheral vision was tested using the Humphrey Automated Field Analyzer II (Carl Zeiss Meditech, Dublin, CA). VF was evaluated using a Swedish Interactive Threshold Algorithm (SITA) Standard C24. Optic nerve findings were assessed from the simultaneous stereoscopic optic disc photographs using a stereoscopic viewer (Asahi viewer, Pentax, Englewood, CO). An expert consensus method was used for OAG diagnosis based on history and clinical examination data. Definite or probable OAG was defined as the presence of an open angle and (1) evidence of characteristic or compatible glaucomatous optic disc damage on stereo fundus photography in at least one eye; and/or (2) congruent, characteristic, or compatible glaucomatous VF abnormality. IOP level was not considered in establishing the diagnosis of OAG.
The incidence of OAG was defined as the presence of definite or probable OAG in either eye or both eyes at the four-year follow-up examination among participants who did not have any evidence of glaucomatous VF abnormality, evidence of glaucomatous optic disc damage at the baseline, or a combination thereof. The mean, maximum, SD, and range of all IOP readings from both the baseline and the follow-up were not calculated and not presented to the glaucoma specialists during glaucoma diagnosis.
Statistical Analyses
Only participants who completed an in-home questionnaire at the baseline and had a clinical ophthalmology examination and reliable glaucoma data at both the baseline and the four-year follow-up were included. Details of risk factor measurements have been reported previously.18–23 We have previously investigated how baseline socio-demographic and lifestyle factors, medical history, physiological measurements, and ocular factors predicted the development of OAG.18 In the present analysis, four IOP related variables - the mean IOP, maximum IOP, SD and range of IOPs were assessed for their association with the development of OAG during a four-year follow-up period.
Incidence of OAG was dichotomized into yes/no categories. One eye of each participant was selected based on the following criteria: if the participant had only one eye diagnosed with OAG, then that eye was selected; if both eyes were glaucomatous or non-glaucomatous, the eye with the worse mean deviation on Humphrey VF testing was selected.
Four different IOP variables were assessed as independent variables in regression analyses:
Mean IOP was the average of six IOP readings measured at the baseline visit and the four-year follow-up visit;
Maximum IOP was the highest IOP recorded at either the baseline or follow-up visit;
SD of IOP was the standard deviation of all IOP readings obtained at either visit; and
Range of IOP was the absolute difference between the highest and the lowest IOP recorded at either visit.
Correlations of SD and range of IOP with mean IOP were assessed using Pearson’s correlation coefficient and explored in detail using scatter plots locally weighted scatter-plot smoothing (LOWESS) plots24 without assuming that these relationships are linear. LOWESS plots were generated with a bandwidth of 0.60 and Cleveland’s tricube weighting function.
Because the follow-up visit of all participants was conducted in 4 years, time to event (survival) analysis could not be performed. Instead, univariate and multivariate Logistic regression models were used to assess the association between measures of IOP and the risk of developing OAG. In the multivariable logistic regression analyses, the following previously-identified baseline risk factors were included as covariates: age, axial length, central cornea thickness, waist to hip ratio and lack of vision insurance. Akaike Information Criterion (AIC) and max-rescaled R2 were estimated from PROC LOGISTIC procedure in SAS 9.4 (SAS Institute Inc., Cary, NC) to compare the overall goodness of the fit and predictive power of each model. Smaller value of AIC and greater value of max-rescaled R2 indicate better models. Further, to identify significant independent predictors among the four IOP measurements, multivariate logistic regression analyses with forward stepwise selection was performed with a P ≤0.20 criterion for entry into the model and P≤0.05 for retention in the model. During the model selection, the order of introducing IOP measures in the multivariate model was determined automatically based on improvement in the overall fit of the model. All reported P values are two-sided with a significance level set at P≤0.05.
RESULTS
Among the 5,907 living eligible participants who had completed an in-home questionnaire and a clinical ophthalmology examination at the baseline, 4,538 (77%) completed the four-year follow-up clinical exam. Among them, 599 (13%) participants did not have complete data for glaucoma diagnosis. Detailed comparison of sociodemiogaphic and clinical characteristics at baseline between the 3,939 participants with reliable glaucoma data at both visits and the other living eligible participants (N=1,968) without reliable glaucoma at both visits have been reported previously.18, 25 Among the remaining 3,939 participants with reliable glaucoma data at both visits, 167 had been diagnosed with OAG at the baseline, 86 reported having IOP-lowering treatment either at the baseline or the follow-up, and 20 did not have Goldmann IOP measurements at the baseline, therefore only the remaining 3,666 participants who were free of OAG at the baseline and did not receive any IOP-lowering treatments were included in the current analyses. Compared with the 273 individuals excluded, these remaining participants were more likely to be younger, employed, married or living with a partner, have more years of education, less likely to have health insurance, a family history of glaucoma, diabetes, and hypertension, and had lower mean, maximum, SD, and range of IOPs from both visits (Supplemental Table 1, available at AJO.com).
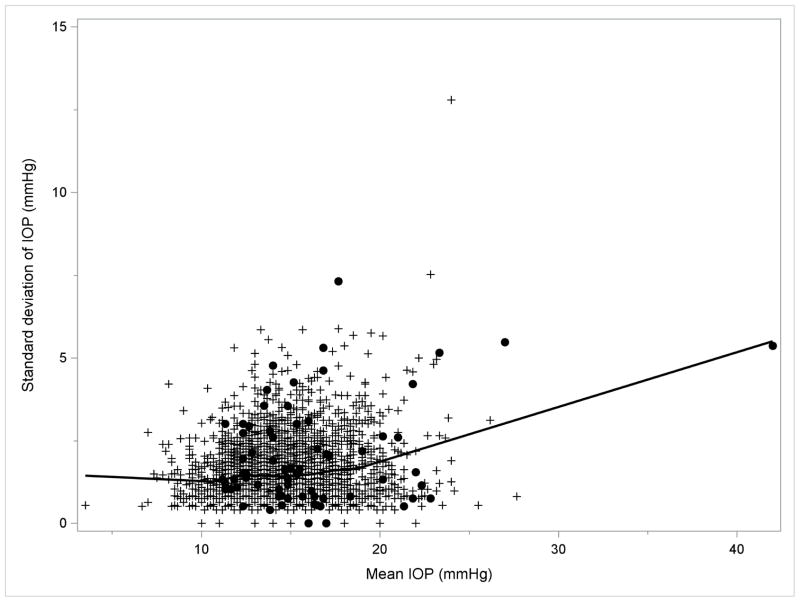
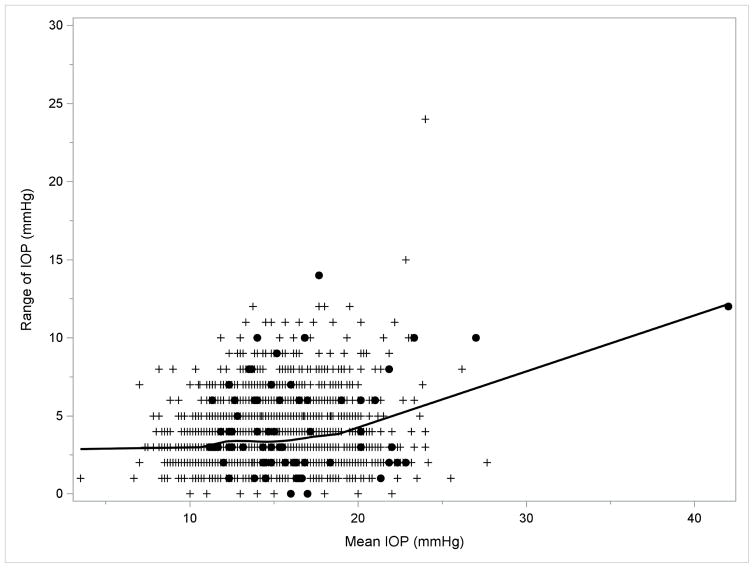
Overall, in the LALES, the mean, maximum, SD, and range of IOP were 14.4±2.7, 16.2±3.0, 1.46±0.89, and 3.41±1.88 mmHg, respectively. For a better assessment of the independent contributions of each IOP measure to OAG risk, we also evaluated the correlation of maximum, SD, and range of IOP with mean IOP, the most commonly used IOP measure. Maximum IOP was highly correlated with mean IOP (Pearson correlation coefficient – r =0.945, P<0.001) and moderately correlated with both SD and range of IOP (r=0.446 and 0.451 respectively, P<0.001). SD and range of IOP were highly correlated with each other (r=0.974, P<0.001), and both measures were in much weaker correlation with mean IOP (r=0.156 and 0.150 respectively, Ps<0.001). As shown in Figures 1a and 1b, SD and range of IOP remained relatively consistent (r between SD of IOP and mean IOP =0.109; r between range of IOP and mean IOP=0.104, Ps<0.001) among participants with low mean IOP (<19 mmHg), and only increased slightly when mean IOP was 19 mmHg or greater(corresponding r = 0.234 and 0.219, P <0.001 and P=0.001 respectively).
FIGURE 1.
LOWESS Plots Demonstrating the Correlation of (1a, left) Standard Deviation and (1b, right) Range of Intraocular Pressures with Mean Intraocular Pressure in the Los Angeles Latino Eye Study. Abbreviations: IOP = intraocular pressure; LOWESS = locally weighted scatterplot smoothing. Filled circles indicate participants who developed open-angle glaucoma; plus sign indicate participants who did not develop open-angle glaucoma.
Incidence of OAG occurred among 73 of the study participants. Table 2 presents the multivariate assessment of the association between the three measures of IOP variation and risk of developing OAG. The mean, maximum, SD, and range of IOP among participants who developed OAG were 16.1±4.6, 18.2±5.2, 2.00±1.52, and 4.37±3.03 mmHg, respectively, higher than those among participants who did not develop OAG (14.4±2.6, 16.1±2.9, 1.45±0.87, and 3.39±1.84 mmHg, respectively). These differences remain statistically significant after adjustment for baseline risk factors including age, axial length, lack of vision insurance, waist to hip ratio, and central corneal thickness. Because of the reported IOP reduction after cataract surgery,26 we also performed analyses with further adjustment for history of cataract surgeries and analyses excluding individuals with a history of cataract surgery; our results did not change substantially. Furthermore, SD and range of IOP remained associated with risk of developing OAG even after adjustment for mean IOP (Table 2), the most commonly used measure of IOP. Maximum IOP was not assessed with further adjustment for mean IOP due to its high correlation with mean IOP. When comparing models with different IOP measures, maximum IOP provided better fit to the observed data than SD and range of IOP, as well as mean IOP based on values of AIC and Cox-Snell R2. In addition, maximum IOP provided similar fit as SD/range of IOP combined with mean IOP. Further analysis by stepwise selection identified maximum IOP as the only significant, independent predictor of OAG development. In other words, mean, SD, and range of IOP were no longer a significant predictor of OAG incidence in a multivariate model that had already included maximum IOP.
Table 2.
Different Measures of Intraocular Pressure and the Four-year Risk of Developing Open-Angle Glaucoma in the Los Angeles Latino Eye Study
| Measures of IOP variationa | Participants without OAG (N=3,593)b | Participants with OAG (N=73)b | OR (95% CI)c per mmHg higher | Pc | AICc | Max-rescaled Cox-Snell R2 |
|---|---|---|---|---|---|---|
| Mean IOP | 14.4±2.6 | 16.1±4.6 | 1.18 (1.09–1.28) | <0.001 | 603.9 | 0.161 |
| SD of IOP | 1.45±0.87 | 2.00±1.52 | 1.50 (1.25–1.79) | <0.001 | 605.2 | 0.159 |
| SD of IOP, additionally adjusted for mean IOPd | - | - | 1.35 (1.12–1.63) | 0.002 | 597.3 | 0.174 |
| Range of IOP | 3.39±1.84 | 4.37±3.03 | 1.22 (1.11–1.34) | <0.001 | 607.1 | 0.156 |
| Range of IOP, additionally adjusted for mean IOPd | - | - | 1.15 (1.04–1.27) | 0.005 | 598.7 | 0.172 |
| Maximum IOP | 16.1±2.9 | 18.2±5.2 | 1.18 (1.11–1.27) | <0.001 | 597.5 | 0.171 |
Abbreviations: AIC=Akaike information criterion; CI=confidence interval; IOP=intraocular pressure; OAG=open angle glaucoma; OR=odds ratio; SD=standard deviation.
Based on six daytime measures – three at the baseline and three at the 4 year follow-up visit.
Average ± standard deviation of measures of IOP.
Estimated from logistic regression models adjusting for age, axial length, lack of vision insurance, waist to hip ratio, and central corneal thickness at the baseline.
Mean IOP was included as a covariate in the logistic regression as well.
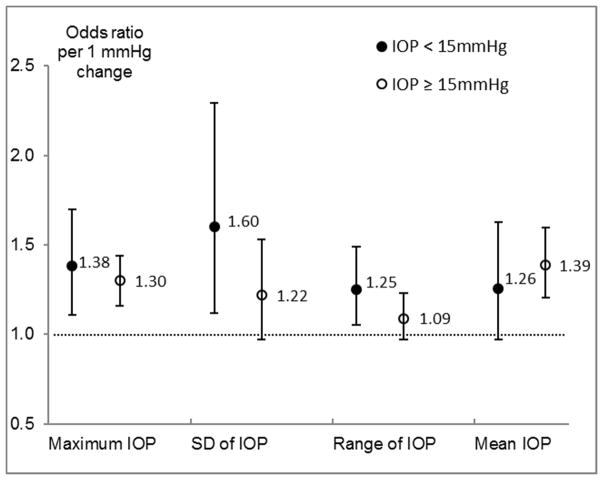
Given previous reports of the effect of IOP variation being limited to individuals with low mean IOP,13 we further examined the relationship between measurements of IOP variation and the development of OAG for participants with different levels of IOP (Figure 2). Among participants with mean IOP of less than 15 mmHg (median level for OAG cases), greater maximum, SD, and range of IOP were all associated with a higher risk of developing OAG (P<0.05), but mean IOP was not significantly associated with the risk of developing OAG. Adjustment for mean IOP did not change the association of SD and range of IOP with OAG risk substantially. A nonlinear dose response relationship was observed between SD/range of IOP and the 4-year risk of developing OAG (Table 3). Risk of developing OAG was substantially higher among individuals with greater variation in IOP (SD > 3 mmHg or range > 6 mmHg) compared with individuals with moderate or small variation in IOP. Among participants with a mean IOP of 15 mmHg or greater (Figure 2), higher levels of mean and maximum IOP were associated with a higher risk of developing OAG, while SD and range of IOP were associated with risk of developing OAG without adjustment for mean IOP (data not shown) but were not associated with OAG risk after adjustment for mean IOP (Figure 2 and Table 3). Conversely, with SD or range of IOP in the regression model, mean IOP remained associated with risk of developing OAG with a slightly reduced magnitude of association (data not shown). Similar results were observed when participants were stratified into two groups defined as mean IOP <18 mmHg and mean IOP ≥18 mmHg (data not shown), or as maximum IOP <17 mmHg and maximum IOP ≥17 mmHg (Supplemental Figure 1 and Supplemental Table 2, available at AJO.com).
FIGURE 2.
Odds Ratios (95% Confidence Intervals)1 of Developing Open Angle Glaucoma Associated with Different Measures of Intraocular Pressure Stratified by Intraocular Pressure Level. Abbreviations: IOP = intraocular pressure; LOWESS = locally weighted scatterplot smoothing. Abbreviations: IOP = intraocular pressure; SD = standard deviation
1Odds ratio were estimated from logistic regression models adjusting for age, axial length, lack of vision insurance, waist to hip ratio, and central corneal thickness at the baseline. Estimates for SD and range of IOP were additionally adjusted for mean IOP.
Table 3.
Levels of Variation in Intraocular Pressure and the Four-year Risk of Developing Open-angle Glaucoma Stratified by Mean Intraocular Pressure
| IOP <15 mmHg |
IOP ≥15 mmHg |
|||||||
|---|---|---|---|---|---|---|---|---|
| N | Incidence rate | OR (95% CI)a | Pa | N | Incidence rate | OR (95% CI)a | Pa | |
| SD of IOP | ||||||||
| ≤1 mmHg | 832 | 1.0% | 1.00 (reference) | - | 493 | 2.6% | 1.00 (reference) | - |
| >1, ≤2 mmHg | 974 | 1.8% | 1.77 (0.75–4.19) | 0.19 | 592 | 1.2% | 0.35 (0.12–0.97) | 0.043 |
| >2, ≤3 mmHg | 309 | 1.9% | 2.10 (0.71–6.23) | 0.18 | 242 | 2.5% | 0.96 (0.34–2.68) | 0.93 |
| >3 mmHg | 96 | 6.3% | 5.85 (1.81–18.92) | 0.003 | 128 | 7.8% | 1.80 (0.67–4.82) | 0.24 |
| Range of IOP | ||||||||
| ≤2 mmHg | 841 | 1.1% | 1.00 (reference) | - | 514 | 2.7% | 1.00 (reference) | - |
| >2, ≤4 mmHg | 928 | 1.7% | 1.62 (0.70–3.74) | 0.26 | 538 | 1.3% | 0.38 (0.14–1.04) | 0.061 |
| >4, ≤6 mmHg | 344 | 1.7% | 1.66 (0.57–4.80) | 0.35 | 276 | 2.2% | 0.86 (0.31–2.36) | 0.76 |
| >6 mmHg | 98 | 6.1% | 5.37 (1.70–16.97) | 0.004 | 127 | 7.1% | 1.50 (0.55–4.11) | 0.43 |
Abbreviations: CI=confidence interval; IOP=intraocular pressure; OR=odds ratio; SD=standard deviation.
Estimated from logistic regression models adjusting for baseline variables including age, axial length, lack of vision insurance, waist to hip ratio, central corneal thickness, and mean IOP.
To explore the sources of IOP variation captured by the six IOP readings obtained at two visits, we found that long-term (between two visits) variation of mean IOP was the stronger contributor to our measures of IOP variation than short-term (within-day) fluctuation of IOP (Supplemental Table 3, available at AJO.com). On average, the long-term variation of mean IOP, calculated as the difference between the mean IOP at the baseline and mean IOP at the follow-up, was 0.08±4.48 mmHg for incident OAG cases, similar to that for those who did not develop OAG (0.15±2.83 mmHg; P for difference=0.89). When its magnitude and direction were considered, long-term variation of mean IOP in either direction (IOP increased or decreased between visits) was associated with an increased risk of OAG (Supplemental Table 4, available at AJO.com). Short-term (within-day) fluctuation of IOP also seemed to be associated with higher risk of OAG; however, this association was not statistically significant.
DISCUSSION
In this study, we investigated the role of measures of IOP variation in predicting the development of OAG among a population-based cohort of Latinos. To our knowledge, this is the first such investigation in a population-based sample.
Results from our investigation suggest that greater IOP variation, measured by SD and range of IOPs, was associated with a higher risk of developing OAG among individuals with lower IOP (<15 mmHg), but was not an important independent risk factor among individuals with higher IOP (≥15 mmHg). Among individuals with higher IOP, mean and maximum IOP were better predictors of OAG incidence than SD and range of IOPs. This is consistent with results from the AGIS 12, 13 that greater long-term IOP fluctuation (defined based on SD of IOP at all visits) was associated with a higher risk of VF progression among OAG patients with low mean IOP (mean IOP, 10.8 mmHg) but not among those with high mean IOP (mean IOP, 20.6 mmHg). This observed difference in the effect of IOP variation suggests that disparate findings from previous studies7, 27, 28 may be explained by between-study differences in their participants’ IOP level. For example, Hong et al. reported27 that among glaucoma patients who maintained their IOPs below 18 mmHg after a triple procedure (mean IOP was between 10 and 12 mmHg), progressive VF loss was more common among patients with greater long-term IOP variation (defined as SD in postoperative IOPs > 2 mmHg) than among patients with less variation (SD ≤2 mmHg). In the EMGT in which the mean IOP was 18.5 mmHg28, IOP fluctuation was not associated with glaucoma progression. Among the untreated ocular hypertensive participants in the DIGS (mean IOP during follow-up was 24.3 mmHg)7 and the Malmo Ocular Hypertension Study (mean IOP was 22.7 mmHg)8, long-term IOP fluctuations was not associated with the conversion from ocular hypertension to glaucoma. However, contrary to the hypothesis that the effect of IOP variation on glaucoma development/progression may be more pronounced among individuals with low IOP, the CIGTS11 found that greater inter-visit variation in IOP was associated with worse VF deterioration among individuals randomized to topical medications who had a mean IOP of 17.1–18.3 mmHg, but not among individuals randomized to trabeculectomy who had a low mean IOP of 13.8–14.4 mmHg. It is possible that factors other than mean IOP level may also influence the effect of IOP variation.
It remains unclear how variation in IOP increases the risk of OAG development and/or progression. A recent study of vascular smooth muscle cells29 found that variable mechanical stretch imposed on cells can lead to the reorganization of cytoskeletal and mitochondrial networks as well as changes in mitochondrial membrane potential, ATP levels and general metabolism. Observations from in vitro studies of human neuroblastoma cells also suggest that cyclic shear stress, which might mimic cell exposure conditions associated with variation in IOP, may lead to more elastic cytoskelectal structure30 and cause more cells with fragmented DNA than steady shear stress31. So it is possible that abnormal fluctuations in IOP may cause mechanical stresses that overwhelm the compensatory mechanisms in the eye and then damage optic nerve cells through altered cytoskeletal and mitochondrial functions.13 A few other hypotheses have also been proposed to explain why the effect of IOP variation on OAG development or progression may be more pronounced among individuals with low IOP4, such as the exponentially-diminishing-return hypothesis. It has also been proposed that percentage of IOP variation may be a better measure of glaucoma risk than absolute IOP variation4 and the finding of the effect of IOP variation being more pronounced among individuals with low IOP may be explained by the higher percentage of IOP variation. In LALES, the association between percent of change in IOP (defined as the ratio of IOP range to mean IOP multiplied by 100) and the risk of developing OAG remained more pronounced among individuals with lower IOP (data not shown).
Three variables have been used most often to assess or take into account fluctuation/variation in IOP measurements - the peak/maximum, range and SD of multiple IOP measurements,7, 8, 11–13, 27, 28, 32 and it remains unclear which measures best capture the effect of IOP variation. Range and maximum are more sensitive to outliers and the number of measurements than SD and mean. On the other hand, range and maximum of IOP may reflect extreme IOP values that may be more important in assessing fluctuation/variation33 and can be easily calculated and interpreted, while SD is only appropriate if data are normally distributed. In addition, all three variables are in varying degrees of correlation with the mean level of IOP, making it difficult to distinguish the effects of IOP variation from the effect of IOP elevation.34 In the LALES, the correlation of SD and range of IOP with mean IOP was higher among individuals with a mean IOP of 19 mmHg or greater (r=0.23 and 0.22 respectively) and lower among individuals with lower mean IOP (r= 0.11 and 0.10 respectively). This finding is consistent with observations from some previous studies:7, 13, 28, 32 stronger correlations between mean IOP and IOP variation (r≥0.35) were reported by studies with higher mean IOP7, 28, 32; and weaker correlation (r=0.16) was reported by the AGIS13 whose participants had a lower mean IOP. The weaker correlation of IOP variation with mean IOP at lower IOP levels, as observed in the LALES and AGIS, allows for a better chance of capturing the contribution of IOP variation to OAG risk/progression independent from that of mean IOP.
In terms of predictability of glaucomatous damages of various IOP variables, Gardiner et al.14 found that peak IOP provided the best predictability of structural change measured by the mean position of the disc from confocal scanning laser tomography and retinal nerve fiber layer thickness from spectral domain ocular coherence tomography. Consistently, in the LALES, we found that maximum IOP was the most consistent predictor of OAG incidence over the entire range of IOP. Given that (a) SD and range of IOP were the better predictors of OAG development at lower IOP levels and (b) mean IOP was the better predictor at higher IOP levels (than SD and range of IOP), it is reasonable to postulate14 that both greater IOP variations and higher mean IOP can lead to glaucomatous damages among different individuals and maximum IOP captures the contributions from both sources for a population as a whole.
One of the major factors limiting research on the impact of IOP fluctuation/variation on OAG development and/or progression is the lack of feasible method for monitoring IOP continuously and assessing IOP fluctuation/variation directly.3, 4 In clinical practice, IOP is most often measured during regular office hours when a patient comes for visit. Such discrete IOP measures are inadequate for capturing the variation in IOP35, because for a significant number of individuals IOP peaks during nocturnal/sleep periods.35–37 Alternatively, variation in IOP can be estimated using measures of IOP changes over longer periods of time, often from multiple visits that are months or years apart.7, 11–13, 27, 28, 32, 35 However, compared to short-term fluctuation, long-term variation in IOP may reflect the combined effects from a different set of physiological and environmental factors. In addition to the lack of consensus on how to measure IOP fluctuation/variation clinically, there is no therapeutic target for IOP fluctuation/variation in order to prevent glaucomatous damage. In a study by Hong et al27, greater VF deterioration was found for patients with SD> 2 mmHg compared to patients with SD ≤2 mmHg. In the AGIS,12 eyes with a SD of IOP ≥ 3 mmHg experienced significant VF progression over the course of follow-up, while eyes with a SD of IOP <3 mmHg did not. Like these two clinic-based studies, our population-based study also found that among individuals with lower IOP, risk of developing OAG was higher when SD of IOP was great than 3 mmHg or when range of IOP was greater than 6 mm Hg, suggesting that controlling SD of IOP at 3 mmHg or under or range of IOP at 6 mmHg or under may be a reasonable target for preventing the development and/or progression of OAG.
Our study has a number of limitations. Firstly, this study only included Latino participants from Los Angeles County; therefore, our results may be not applicable to other populations. Secondly, we did not continuously measure IOP over an extended period, so we were unable to assess precisely the short-term fluctuation of IOP and the relationship between short-term fluctuation and long-term variation of IOP. The IOP variation that we assessed based on six IOP measurements from two study visits may not represent the exact variation in IOP that the study participants experienced. Because of the short interval (within minutes) between the measurements taken within the same day and the long-duration (4 years) between the two visits, the IOP variation we evaluated was attributed mostly to long-term variation of IOP occurred between visits and to a lesser extent to short-term fluctuation in IOP. Nonetheless, our exploratory analysis showed both short-term fluctuation and long-term variation of IOP seemed to increase the risk of developing OAG. Further studies with better measurements of both short-term and long-term variation in IOP are needed to confirm these findings. Thirdly, IOP measurements were not obtained under identical conditions or identical time for each participant and the between-individual differences in IOP variation that we observed may be influenced by diurnal fluctuation and seasonal variations in IOP and by random differences in the participants’ physical condition. However, these limitations would have led to non-differential measurement errors and therefore biased our results towards null, indicating that the positive associations we observed between measures of IOP variations and the risk of developing OAG could have been stronger. Also, the three IOP readings from the baseline were not masked during glaucoma diagnosis at the baseline and the three IOP readings from the follow-up examination were not masked during glaucoma diagnosis at the follow-up. Even though our glaucoma specialists were specifically instructed to exclude IOP in establishing the diagnosis of OAG, it is still possible that individuals with higher IOP may be more likely to be falsely classified as having OAG than individuals with lower IOP. However, this potential bias is unlikely to play a substantial role in our findings based on the following observations: 1) the positive associations that we observed between measures of IOP variation and OAG risk remained among individuals with low IOP (<15 mmHg); 2) an increased risk of OAG was observed among individuals whose IOP decreased between visits, i.e. whose maximum IOP occurred at the baseline and therefore remained unknown to the glaucoma specialists during the follow-up diagnosis, because they had access to only the data from the follow-up examination; and 3) when vertical cup-to-disk ratio, a more subjective measure than an overall glaucoma diagnosis, was used as the outcome, similar results were found with SD and range of IOP identified as independent risk factors and maximum IOP remained as the best-fitting IOP measure (data not shown).
In summary, our data demonstrate that greater IOP variation may be damaging to the optic nerve among individuals with relatively lower IOP, and maximum IOP may be the most consistent predictor of the development of OAG as it reflects both mean IOP level and IOP variation. Further studies are needed to explore practical approaches for capturing the true characteristics of IOP variation in a large number of individuals and assess dose-response relationships between IOP variation and the risk of development and/or progression of OAG.
Supplementary Material
Acknowledgments
Financial Support: National Institutes of Health Grants NEI EY-11753 and an unrestricted grant from the Research to Prevent Blindness, New York, New York. The sponsor or funding organization had no role in the design or conduct of this research.
Funding/Support: This work was supported by grant EY-11753 from the National Eye Institute, National Institutes of Health, Bethesda, Maryland, and an unrestricted Departmental grant from Research to Prevent Blindness, New York, NY 10022.
Abbreviations and Acronyms
- ATP
Adenosine triphosphate
- AGIS
Advanced Glaucoma Intervention Study
- AIC
Akaike Information Criterion
- DIGS
Diagnostic Innovations in Glaucoma Study
- EMGT
Early Manifest Glaucoma Trial
- IOP
Elevated intraocular pressure
- LALES
Los Angeles Latino Eye Study
- OAG
Open angle glaucoma
- SITA
Swedish Interactive Threshold Algorithm
- VF
Visual field
Footnotes
Conflict of Interest: No conflict of interest exists for any author.
Supplemental Material available at AJO.com
Financial Disclosures: No financial disclosures.
Other acknowledgements: See Appendix 1 for members/affiliations of the Los Angeles Latino Eye Study
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kamal D, Hitchings R. Normal tension glaucoma - a practical approach. Br J Ophthalmol. 1998;82(7):835–40. doi: 10.1136/bjo.82.7.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collaer N, Zeyen T, Caprioli J. Sequential office pressure measurements in the management of glaucoma. J Glaucoma. 2005;14(3):196–200. doi: 10.1097/01.ijg.0000159125.34241.79. [DOI] [PubMed] [Google Scholar]
- 3.Singh K, Shrivastava A. Intraocular pressure fluctuations: how much do they matter? Curr Opin Ophthalmol. 2009;20(2):84–7. doi: 10.1097/icu.0b013e328324e6c4. [DOI] [PubMed] [Google Scholar]
- 4.Singh K, Sit AJ. Intraocular pressure variability and glaucoma risk: complex and controversial. Arch Ophthalmol. 2011;129(8):1080–1081. doi: 10.1001/archophthalmol.2011.66. [DOI] [PubMed] [Google Scholar]
- 5.Tajunisah I, Reddy S, Fathilah J. Diurnal variation of intraocular pressure in suspected glaucoma patients and their outcome. Graefes Arch Clin Exp Ophthalmol. 2007;245(12):1851–7. doi: 10.1007/s00417-007-0681-7. [DOI] [PubMed] [Google Scholar]
- 6.Liu JHK, Zhang X, Kripke DF, Weinreb RN. Twenty-four-hour intraocular pressure pattern associated with early glaucomatous changes. Invest Ophthalmol Vis Sci. 2003;44(4):1586–90. doi: 10.1167/iovs.02-0666. [DOI] [PubMed] [Google Scholar]
- 7.Medeiros FA, Weinreb RN, Zangwill LM, et al. Long-term intraocular pressure fluctuations and risk of conversion from ocular hypertension to glaucoma. Ophthalmology. 2008;115(6):934–40. doi: 10.1016/j.ophtha.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bengtsson B, Heijl A. Diurnal IOP fluctuation: not an independent risk factor for glaucomatous visual field loss in high-risk ocular hypertension. Graefes Arch Clin Exp Ophthalmol. 2005;243(6):513–8. doi: 10.1007/s00417-004-1103-8. [DOI] [PubMed] [Google Scholar]
- 9.Miglior S, Torri V, Zeyen T, Pfeiffer N, Vaz JC, Adamsons I. Intercurrent factors associated with the development of open-angle glaucoma in the European Glaucoma Prevention Study. Am J Ophthalmol. 2007;144(2):266–275e1. doi: 10.1016/j.ajo.2007.04.040. [DOI] [PubMed] [Google Scholar]
- 10.Gao F, Miller JP, Miglior S, et al. A joint model for prognostic effect of biomarker variability on outcomes: long-term intraocular pressure (IOP) fluctuation on the risk of developing primary open-angle glaucoma (POAG) JP journal of biostatistics. 2011;5(2):73–96. [PMC free article] [PubMed] [Google Scholar]
- 11.Musch DC, Gillespie BW, Niziol LM, Lichter PR, Varma R. Intraocular pressure control and long-term visual field loss in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology. 2011;118(9):1766–1773. doi: 10.1016/j.ophtha.2011.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nouri-Mahdavi K, Hoffman D, Coleman AL, et al. Predictive factors for glaucomatous visual field progression in the Advanced Glaucoma Intervention Study. Ophthalmology. 2004;111(9):1627–35. doi: 10.1016/j.ophtha.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Caprioli J, Coleman AL. Intraocular pressure fluctuation: a risk factor for visual field progression at low intraocular pressures in the Advanced Glaucoma Intervention Study. Ophthalmology. 2008;115(7):1123–1129e3. doi: 10.1016/j.ophtha.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 14.Gardiner SK, Fortune B, Wang L, Downs JC, Burgoyne CF. Intraocular pressure magnitude and variability as predictors of rates of structural change in non-human primate experimental glaucoma. Exp Eye Res. 2012;103(0):1–8. doi: 10.1016/j.exer.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Downs JC. Optic nerve head biomechanics in aging and disease. Exp Eye Res. 2015;133:19–29. doi: 10.1016/j.exer.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varma R, Paz SH, Azen SP, et al. The Los Angeles Latino Eye Study: design, methods, and baseline data. Ophthalmology. 2004;111(6):1121–1131. doi: 10.1016/j.ophtha.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Varma R, Ying-Lai M, Francis BA, et al. Prevalence of open-angle glaucoma and ocular hypertension in Latinos: The Los Angeles Latino Eye Study. Ophthalmology. 2004;111(8):1439–1448. doi: 10.1016/j.ophtha.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 18.Jiang X, Varma R, Wu S, et al. Baseline risk factors that predict the development of open-angle glaucoma in a population: the Los Angeles Latino Eye Study. Ophthalmology. 2012;119(11):2245–53. doi: 10.1016/j.ophtha.2012.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doshi V, Ying-Lai M, Azen SP, Varma R. Sociodemographic, family history, and lifestyle risk factors for open-angle glaucoma and ocular hypertension: the Los Angeles Latino Eye Study. Ophthalmology. 2008;115(4):639–647e2. doi: 10.1016/j.ophtha.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 20.Memarzadeh F, Ying-Lai M, Chung J, Azen SP, Varma R Los Angeles Latino Eye Study G. Blood pressure, perfusion pressure, and open-angle glaucoma: the Los Angeles Latino Eye Study. Invest Ophthalmol Vis Sci. 2010;51(6):2872–7. doi: 10.1167/iovs.08-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chopra V, Varma R, Francis BA, Wu J, Torres M, Azen SP. Type 2 diabetes mellitus and the risk of open-angle glaucoma: the Los Angeles Latino Eye Study. Ophthalmology. 2008;115(2):227–32e1. doi: 10.1016/j.ophtha.2007.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Francis BA, Varma R, Chopra V, Lai M-Y, Shtir C, Azen SP. Intraocular pressure, central corneal thickness, and prevalence of open-angle glaucoma: the Los Angeles Latino Eye Study. Am J Ophthalmol. 2008;146(5):741–6. doi: 10.1016/j.ajo.2008.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuzin AA, Varma R, Reddy HS, Torres M, Azen SP. Ocular biometry and open-angle glaucoma: the Los Angeles Latino Eye Study. Ophthalmology. 2010;117(9):1713–1719. doi: 10.1016/j.ophtha.2010.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chambers JM, Cleveland WS, Kleiner B, Tukey PA. Graphical methods for data analysis. Boston, MA: Duxbury Press; 1983. [Google Scholar]
- 25.Varma R, Wang D, Wu C, et al. Four-year incidence of open-angle glaucoma and ocular hypertension: the Los Angeles Latino Eye Study. Am J Ophthalmol. 2012;154(2):315–325e1. doi: 10.1016/j.ajo.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mansberger SL, Gordon MO, Jampel H, et al. Reduction in intraocular pressure after cataract extraction: the Ocular Hypertension Treatment Study. Ophthalmology. 2012;119(9):1826–31. doi: 10.1016/j.ophtha.2012.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong S, Seong GJ, Hong YJ. Long-term intraocular pressure fluctuation and progressive visual field deterioration in patients with glaucoma and low intraocular pressures after a triple procedure. Arch Ophthalmol. 2007;125(8):1010–3. doi: 10.1001/archopht.125.8.1010. [DOI] [PubMed] [Google Scholar]
- 28.Bengtsson B, Leske MC, Hyman L, Heijl A. Fluctuation of intraocular pressure and glaucoma progression in the Early Manifest Glaucoma Trial. Ophthalmology. 2007;114(2):205–209. doi: 10.1016/j.ophtha.2006.07.060. [DOI] [PubMed] [Google Scholar]
- 29.Bartolak-Suki E, Imsirovic J, Parameswaran H, et al. Fluctuation-driven mechanotransduction regulates mitochondrial-network structure and function. Nat Mater. 2015;14(10):1049–57. doi: 10.1038/nmat4358. [DOI] [PubMed] [Google Scholar]
- 30.Edwards ME, Wang SSS, Good TA. Role of viscoelastic properties of dfferentiated SH-SY5Y human neuroblastoma cells in cyclic shear stress injury. Biotechnol Prog. 2001;17(4):760–767. doi: 10.1021/bp010040m. [DOI] [PubMed] [Google Scholar]
- 31.Wostyn P, De Groot V, Audenaert K, De Deyn PP. Are intracranial pressure fluctuations important in glaucoma? Med Hypotheses. 2011;77(4):598–600. doi: 10.1016/j.mehy.2011.06.043. [DOI] [PubMed] [Google Scholar]
- 32.Bergeå B, Bodin L, Svedbergh B. Impact of intraocular pressure regulation on visual fields in open-angle glaucoma. Ophthalmology. 1999;106(5):997–1004. doi: 10.1016/S0161-6420(99)00523-0. [DOI] [PubMed] [Google Scholar]
- 33.Varma R, Hwang L-J, Grunden JW, Bean GW. Inter-visit intraocular pressure range: an alternative parameter for assessing intraocular pressure control in clinical trials. Am J Ophthalmol. 2008;145(2):336–42. doi: 10.1016/j.ajo.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Tu YK, Kellett M, Clerehugh V, Gilthorpe MS. Problems of correlations between explanatory variables in multiple regression analyses in the dental literature. Br Dent J. 2005;199(7):457–61. doi: 10.1038/sj.bdj.4812743. [DOI] [PubMed] [Google Scholar]
- 35.Sultan MB, Mansberger SL, Lee PP. Understanding the importance of IOP variables in glaucoma: a systematic review. Surv Ophthalmol. 2009;54(6):643–662. doi: 10.1016/j.survophthal.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Liu JHK, Weinreb RN. Monitoring intraocular pressure for 24 h. Br J Ophthalmol. 2011;95(5):599–600. doi: 10.1136/bjo.2010.199737. [DOI] [PubMed] [Google Scholar]
- 37.Mosaed S, Liu JHK, Weinreb RN. Correlation between office and peak nocturnal intraocular pressures in healthy subjects and glaucoma patients. Am J Ophthalmol. 2005;139(2):320–4. doi: 10.1016/j.ajo.2004.09.062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.