Abstract
Background and purpose
Endothelial progenitor cells (EPCs) have been extensively investigated as a therapeutic approach for repairing the vascular system in cerebrovascular diseases. Beyond vascular regeneration per se, EPCs may also release factors that affect the entire neurovascular unit. Here, we aim to study the effects of the EPC secretome on oligovascular remodeling in a mouse model of white matter injury after prolonged cerebral hypoperfusion.
Methods
The secretome of mouse EPCs was analyzed with a proteome array. In vitro, the effects of the EPC secretome and its factor angiogenin were assessed on primary oligodendrocyte precursor cells (OPCs) and mature endothelial cells (hCMED/D3). In vivo, mice were subjected to permanent bilateral common carotid artery stenosis, then treated with EPC secretome at 24 hours and at one week, and cognitive outcome was evaluated with the Y maze test together with OPC proliferation/differentiation and vascular density in white matter at 4 weeks.
Results
Multiple growth factors, cytokines and proteases were identified in the EPC secretome including angiogenin. In vitro, the EPC secretome significantly enhanced endothelial and OPC proliferation, and potentiated OPC maturation. Angiogenin was proved to be a key factor since pharmacological blockade of angiogenin signaling negated the positive effects of the EPC secretome. In vivo, treatment with the EPC secretome increased vascular density, myelin and mature oligodendrocytes in white matter, and rescued cognitive function in the mouse hypoperfusion model.
Conclusions
Factors secreted by EPCs may ameliorate white matter damage in the brain by boosting oligovascular remodeling.
Keywords: endothelial progenitor cell, secretome, angiogenesis, oligodendrogenesis, white matter injury, cerebral hipoperfusion, repair
INTRODUCTION
White matter damage caused by chronic cerebral hypoperfusion is a histological feature of stroke and cerebrovascular disease. Recently, it has been proposed that tissue physiopathology is influenced by the dynamic balance between deleterious versus beneficial responses to the initial insult (1), including neuroinflammation, demyelination and cell death but also regenerative capacities such as neurogenesis, angiogenesis and neuroplasticity.
In the context of white matter remodeling, an endogenous pool of oligodendrocyte precursor cells (OPCs) is widely distributed in the adult brain for physiological myelin renewal and for repair under pathological conditions (2). In demyelinating disorders, residual OPCs tend to proliferate and differentiate into oligodendrocytes to alleviate white matter damage (3, 4). However, the regeneration of oligodendrocytes and myelin sheaths might fail due to an insufficient maturation of OPCs. Cell-based therapies are promising therapeutic options for enhancing oligodendrogenesis as bone marrow-derived mesenchymal stem cells (MSCs) and mononuclear cells have been reported to promote myelin repair after intravenous injection into rodent models of demyelination (5, 6). Also, the paracrine effects of transplanted MSC have been reported to promote the OPC maturation and remyelination both in vitro and in vivo (7, 8). Other studies have demonstrated that the administration of endothelial progenitor cells (EPCs) enhances neurogenesis and angiogenesis in mouse models of stroke (9, 10) and EPC-secreted factors promoted cortical vascular remodeling after cerebral ischemia (11), but its regenerative role in white matter damage is unknown. Among these secretome-remodeling factors we have focused on angiogenin, a member of the Ribonuclease A superfamily wich undergoes nuclear translocation in endothelial cells stimulating ribosomal RNA transcription, cell proliferation and growth (12, 13). With known effects on tumor neovascularization and cancer progression (14,15), it has been also described to promote hematopoietic regeneration of stem/progenitor cells (16), being neurotropic and neuroprotective (13). In this regard, mutations in angiogenin have been described in patients with familial and sporadic amyotrophic lateral sclerosis, affecting neuronal survival (13).
In the present study, we used a combination of in vitro culture systems and in vivo mouse model (17) to ask whether EPC-secreted factors (the EPC secretome) can enhance oligovascular proliferation, maturation and repair in damaged white matter. Our results demonstrate for the first time that the treatment with the EPC secretome preserves cognitive function and enhances oligo-angiogenesis in the injured white matter.
MATERIALS AND METHODS
Ethics Statement
Experiments were performed following institutionally approved protocols in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the Directives of the European Union. The manuscript adheres the Transparency and Openness Promotion Guidelines and all data supporting the findings of this study are available from the corresponding authors upon reasonable request.
Cerebral prolonged hypoperfusion model
Cerebral prolonged hypoperfusion was induced by bilateral common carotid artery stenosis (BCAS) using a microcoil in twenty-four male C57Bl/6 mice (2–3 months old, Charles River Institute), as described (17). Surgeries follow-up were performed by an investigator blinded for treatment. The mice were euthanized 28 days after the surgery; see supplementary material.
Protocol for Conditioned Media Production
Mouse EPC cultures were used for the production of conditioned media (CM) in culture for 24h in endothelial basal media (EBM, CC-3156 from Lonza) as described (11), filtered and concentrated using 10 KDa-membrane centrifuge tubes (Sartorius, V0601). Filtered and concentrated fresh EBM was used as vehicle treatment. See the supplementary material.
Secretome Therapy
Under anesthesia (1.5% isoflurane in 70% N2O and 30%O2) each animal received 160 microliters of CM or vehicle intravenously (retroorbital) at 24 hours and at 7 days after BCAS procedure.
5-Bromodeoxyuridine labeling
A cell proliferation marker 5-bromodeoxyuridine (BrdU from Sigma-Aldrich) was dissolved in 0.9% saline (5 mg/ml) and injected intraperitoneally (50 mg/kg body weight) every 12 hours between day 5 and 14 after the BCAS surgery.
Y Maze Test
The Y maze test was performed 28 days after the surgery as described (18, 19). Memory and spontaneous activity indicators were evaluated blindly to treatment, see supplementary material.
Isolation of corpus callosum and western blot
Nine anesthetized mice were transcardially perfused with ice-cold phosphate-buffered saline (PBS). Brains were removed and one half was placed in a brain tissue matrix and sectioned to obtain three 1mm-thick coronal blocks. The corpus callosum was separated from the other half under a microsope as shown in supplementary Figure I, frozen in dry ice and stored at −80°C. For western blot lysates from the corpus callosum region were ultrasonically homogenized in 100–200μl PRO-PREP Protein Extraction Kit (iNtRON Biotechnology). Samples were tested for anti-PDGFR-α, anti-CD31, myelin basic protein (MBP) or anti-β-actin as described in supplementary material.
Immunohistochemistry
Mice were perfused with 4% paraformaldehyde (PFA) in PBS and the brain was postfixed for 24 h in 4% PFA in PBS at 4°C before cryoprotection with 30% sucrose. Frozen brains were cut into 16-μm-thick consecutive coronal sections at 0 to 0.5 mm anterior from the bregma and stained with anti-BrdU, anti-CD31 (for endothelial cells) anti-GST-pi (for mature oligodendrocytes) or anti-PDGFR-α (for oligodendrocyte progenitors) as detailed in supplementary material.
Protein Profile of EPCs Secretome
Conditioned media were collected as described and total protein content measured by the Comassie Protein Assay kit (Thermo Scientific, USA). The protein profile (n=4) was analyzed using the Proteome Profiler Mouse Angiogenesis Array kit (R&D Systems, USA), see supplementary material.
Endothelial Cell Proliferation Assay
To test the angiogenic properties of conditioned media on human cerebral microvascular endothelial cells (hCMEC/D3) we assessed cell proliferation after treatment with conditioned media or Angiogenin (a potent pro-angiogenic factor identified in EPCs secretome) combined with Neomycin (an Angiogenin inhibitor) with the Muse™ Cell Count and Viability Kit, as described (20).
Endothelial Cell Viability Assay
Measurement of the reduction of 3-(4,5-dimethylthiazol- 2-yl)2,5-diphenyl-tetrazolium bromide (MTT) by hCMEC/D3 treated with Neomycin at different doses was performed as described in supplementary material.
Oligodendrocyte Precursor Cell Culture
Primary cultured rat OPCs were prepared according to our previous report (21) from cerebral cortices of 1–2 day old SD rat pups, see supplementary material.
Oligodendrocyte Precursor Cell Viability Assay
OPC viability was assessed by WST reduction assay (Cell Counting Kit-8, Dojindo) which is similar to MTT assay. The cells were incubated with 10% WST solution for 1 hour at 37°C when the absorbance of the culture medium was measured at 450 nm wavelenght with a reference wavelength of 630 nm.
Evaluation of Oligodendrocyte Precursor Cell Differentiation
After OPCs were treated with EPC-CM or vehicle diluted from 1/5 to 1/50 in OPC differentiation media (final concentration of CNTF [ciliary neurotrophic factor), T3 (triiodothyronine), and B27 was 10 ng/mL, 15 nM, and 2%, respectively] for 24 hours, they were further cultured with the same OPC differentiation media for another 5 days.
Immunocytochemistry
Cultured EPCs or OPCs were immunostained for Myelin Basic Protein (MBP) or Angiogenin respectively following Standard procedures detailed in supplementary methods.
EPC-CM western blot for Angiogenin
Thirty-two μl of reduced EPC-CM and EBM-vehicle were loaded in SDS-PAGE (12%) together with Mouse recombinant ANG as positive control, and transferred into PVDF membranes as detailed in supplementary methods.
Statistical Analyses
Normality was assessed by the Shapiro-Wilk test. To assess differences between groups, t-tests and one-way ANOVA followed by Dunnett (bilateral) post-hoc tests were used for normally distributed variables, while the Kruskal-Wallis and Mann-Whitney U-tests were used to explore differences in non-normally distributed variables. Values are expressed and represented as mean ±SD and statistical analysis was conducted by the SPSS 15.0 software. A p value <0.05 was considered statistically significant.
RESULTS
EPC secretome induced endothelial and OPC proliferation and oligodendrocyte maturation in vitro
Mature endothelial cells and OPCs were cultured and exposed to EPC-CM or corresponding vehicle. Our results show that EPC-CM enhanced cell proliferation after treatment in both endothelial and OPC cells (p<0.05; Fig. 1A–B). Additionally, EPC-CM-treated OPCs showed an increase in MBP expression (p<0.05; Fig. 1C) indicating the switch to a more mature oligodendrocyte phenotype.
Figure 1. In vitro Effects of EPCs Conditioned Media (CM) on mature endothelial cells and Oligodendrocyte Progenitors.
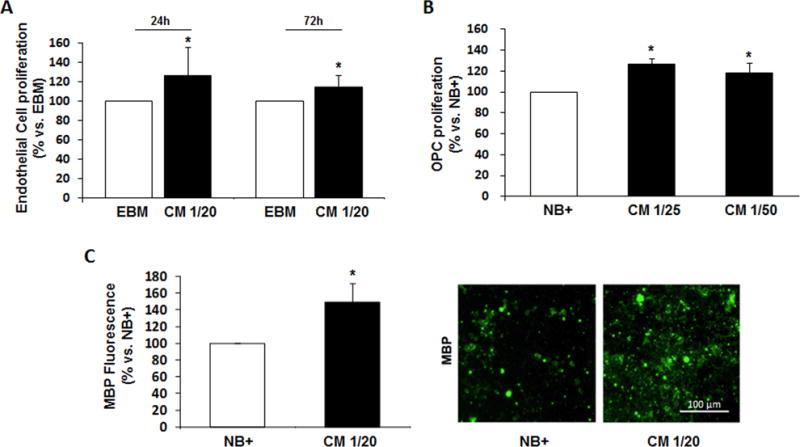
A) The number of viable human endothelial cells (hCMEC/D3) increased at 24 and 72 hours after treatment with CM compared to cells cultured with basal media (EBM). B) OPC proliferation was also observed 24 hours after treatment with CM compared to cells cultured with neurobasal plus 2% B27 (basal media, NB+). C) Cultured OPCs showing maturation markers such as MBP increasing after 24 hours treatment with CM. Tested CM dilutions in basal media are indicated in each graph; n=3–6/group, *p<0.05.
EPC secretome proteomic profile
A proteome array identified 38 proteins, including angiogenin and other promoters of angiogenesis (stromal derived factor-SDF-1, platelet-derived growth factor-PDGFAA/AB/BB, vascular endothelial growth factor VEGF-B, or several matrix metallopreoteinase-MMP), but also some inhibitors (Endostatin or Thrombospondin-2); see Suppl. Table I and Suppl. Fig. II. At the same time, molecules critically involved in the proliferation, maturation and survival of OPCs such as PDGF, insulin-like growth factor-1 (IGF-1), epidermal growth factor (EGF) or Interleukin-1β (IL-1 β) were also detected in the EPC secretome.
Secreted angiogenin as a key factor in the EPC secretome
Western blots demonstrated that angiogenin was present in the EPC-CM (Fig 2A). Immunocytochemistry confirmed that EPCs were indeed positive for angiogenin (Fig. 2B).
Figure 2. Endothelial Progenitor Cells as a source of Angiogenin.
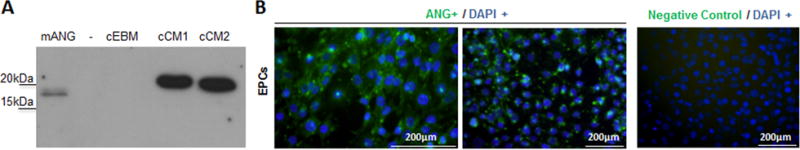
A) ANG immunoblotting where mANG= recombinant mouse ANG, cEBM= concentrated endothelial basal media, cCM1/2= concentrated conditioned media from mouse EPCs. B) Immunocitochemistry images identifying ANG in Endothelial Progenitor Cells.
In parallel, cell cultures treated with recombinant angiogenin at different doses confirmed the induction on endothelial proliferation (p<0.05), but not on OPC cells at the same doses (Figure 3A–B), respectively. Functional assays showed that the effects of the EPC secretome on endothelial proliferation was blocked by the addition of neomycin (10μM), an aminoglycoside antibiotic which blocks angiogenin signaling by inhibiting its nuclear translocation for angiogenesis (p<0.05); however, these effects were not observed in OPC cultures as expected (Fig. 3A–B). No toxic effects of neomycin were observed in endothelial cells (see Suppl. Fig.III).
Figure 3. Study of Angiogenin (ANG) as key factor in Conditioned Media (CM) for endothelial cell proliferation but not for OPCs.
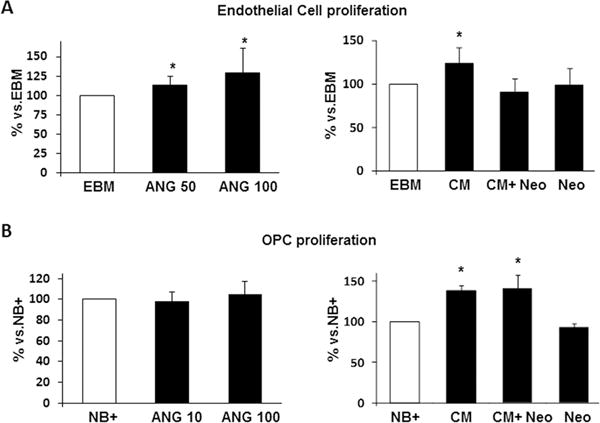
A-B, left panels) Treatment with ANG (50 and 100 ng/mL) induces endothelial cell proliferation dose-dependently but not in OPCs. A-B, right panels) Bar graphs showing that blocking ANG with Neomycin (Neo, 10μM) inhibits CM-induced proliferation in endothelial cells but not in OPCs; CM was diluted 1/20 and 1/5, respectively. Neomycin treatment alone did not reduce cell viability. n=3–6/group, *p<0.05.
EPC secretome therapy improved cognitive function in vivo
Finally, we asked whether these oligovascular effects of the EPC secretome could be observed in vivo (Fig. 4A). Mice were subjected to prolonged cerebral hypoperfusion via permanent bilateral occlusion of the carotid arteries. Cognitive impairment was then measured with the Y maze test. Alterations of entries were significantly increased in EPC-CM-treated mice (75.1 ± 7.5%) compared with vehicle-treated mice (66.3 ± 7.2%) (p<0.05; Fig. 4B). On the other hand, spontaneous activity, shown by the number of total arm entries, was not significantly different (Fig. 4B).
Figure 4. Preclinical model of cerebral hypoperfusion: study design and functional outcome.
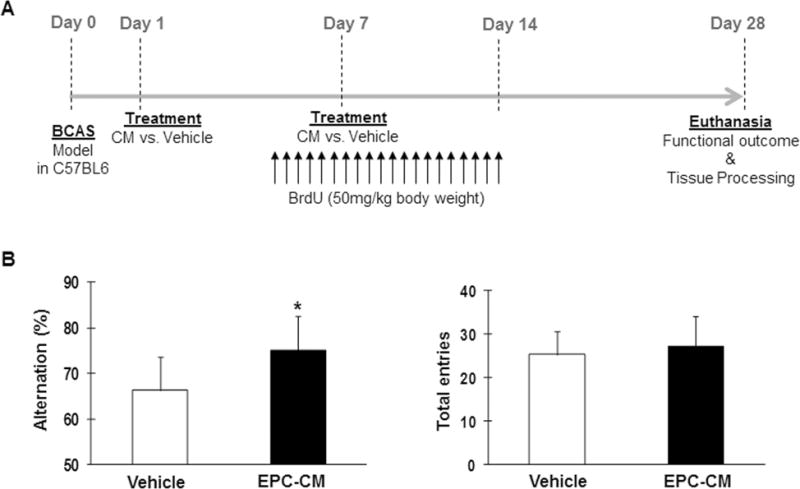
A) Study design: conditioned media from EPCs was administered twenty-four hours and one week after inducing cerebral hypoperfusion with the BCAS model. BrdU was intraperitoneally injected from day 5 to 14 every 12 hours and functional outcome was assessed at day 28 before euthanasia when brains were collected. B) Cell-free conditioned media from EPCs improves cognitive function. Bar graphs showing number of total arm entries and alternation behavior in the Y maze test at day 28 of vehicle-treated (n=10) and EPC-CM-treated (n=8) mice, *p<0.05.
Secretome therapy facilitated oligovascular remodeling in the white matter
We next asked whether these beneficial effects of EPC-CM were associated with white matter remodeling in vivo. Vascular density in the corpus callosum area increased in those mice treated with EPCs-CM (p<0.05, Fig.5A) whereas western blot analysis showed some non-significant increase in CD31 expression (p<0.1, Fig. 5B). Similarly, the presence of OPCs (PDGFR-α marker) was not different between treatments (Fig. 5C) when analyzed by western blot, but the amount of MBP in those areas was significantly increased (by 97%) after EPC-CM treatment; p<0.05, Fig. 5D.
Figure 5. Cell-free conditioned media from EPCs increases endothelial and mature oligodendrocyte markers in the white matter.
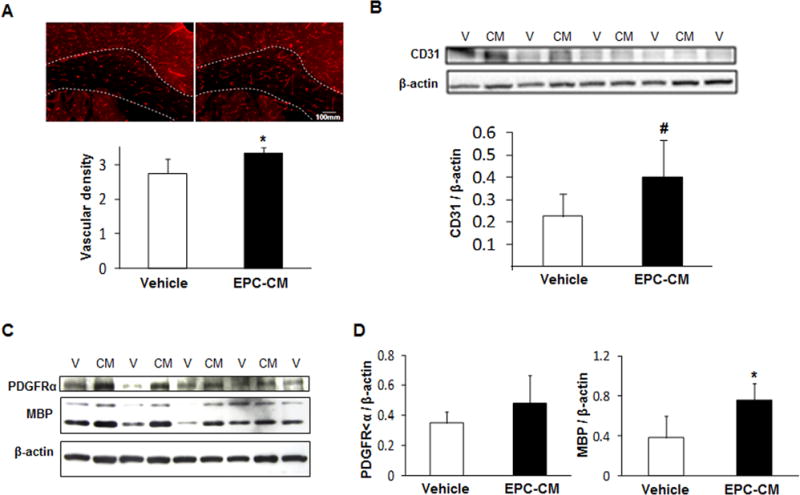
A–B) The corpus callosum was studied for CD31 protein expression (marker for blood vessel) by immunofluorescence or immunoblotting. C–D) Additional immunoblotting for PDGFR-α (marker for OPCs), MBP (marker for mature oligodendrocytes) and β-actin were conducted and quantified; vehicle-treated (n=5) and EPC-CM-treated (n=4) mice, # p<0.1 and *p<0.05.
We then identified OPC proliferation/maturation since the number of total proliferating oligodendrocyte lineage cells increased by 32% in the damaged corpus callosum in mice receiving EPCs-CM (p<0.05, Fig. 6A). Of these, the number of proliferating OPCs (BrdU/PDGFR-a double positive cells) was not different (Fig. 6B) but there was a significant increase in the number of newly generated oligodendrocytes (BrdU/GST-pi double positive cells) in EPC-CM-treated (p<0.05, Fig. 6C), representing about 78% increase. These results suggest that EPC secretome therapy facilitated oligovascular remodeling and maturation of newly generated OPCs after prolonged cerebral hypoperfusion.
Figure 6. Immunohistochemistry study showing the maturation oligodendrocyte lineage cells.
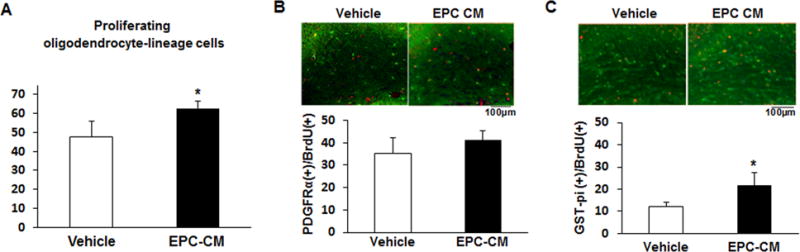
A) Bar graph showing the total number of proliferating oligodendrocyte-lineage cells in the corpus callosum area including both PDGFR-α and GST-pi (precursor and mature oligodendrocytes, respectively) double-stained with 5-bromodeoxyuridine (BrdU). B–C) Representative images of the stained corpus callosum area and bar graphs representing the number of PDGFR-α(+)/BrdU(+) cells (left panel) and GST-pi(+)/BrdU(+) cells (right panel), in vehicle-treated (n=5) and EPC-CM-treated (n=4) mice. *p<0.05.
DISCUSSION
The failure of the myelination/remyelination process attributed to insufficient regenerative responses as well as the suppression of oligodendrocyte maturation, accompanies white mater injury in stroke and cerebrovascular disease. Hence, treatments boosting endogenous myelin repairing responses would be effective to improve patients’ outcome. Our findings in the present study support the therapeutic actions of factors secreted by endothelial progenitor cells highlighting the importance of angiogenin, a known enhancer for angiogenesis. We finally demonstrate the regenerative actions of the EPC secretome in vascular and myelin remodeling in an experimental mouse model of white matter injury.
Cell transplantation is considered a valid therapeutic strategy to repair the injured tissue (22). In this regard, several types of stem and progenitor cells have been successfully tested in preclinical models of stroke, multiple sclerosis or neurodegenerative diseases (23). However, the mechanisms of repair are still under investigation, including direct cell replacement, indirect secretion of nourishing factors or the creation of a biobridge that connects the neurogenic sites of the brain with the injured areas with active remodeling (24,25).
EPCs are bone-marrow derived cells capable of differentiating ex vivo into endothelial-phenotyped cells representing a model for endothelial generation and vascular repair (26, 27). Other studies have demonstrated the benefit of factors secreted by progenitor/stem cells such as VEGF (28) or HGF (8) or interleukin (IL)-8 (29). In this context, the secretome of EPCs is known to stimulate endothelial cell migration, growth and function (30, 31) and to protect from axonal degeneration in cultured cortical neurons exposed to oxygen-glucose deprivation (32). In this regard, intravenously administered EPC-conditioned media enhances vascular remodeling in a cortical model of stroke (11) whereas intramuscular injections of similar products promoted tissue revascularization and recovery in a model of hindlimb ischemia (33).
Following white matter injury, Bai and colleagues have recently shown that conditioned media obtained from MSCs is a valid therapeutic strategy to reverse the effects of inflammatory demyelinating diseases such as experimental autoimmune encephalomyelitis, a model of multiple sclerosis (8). The authors show that the effects of MSCs-conditioned media are dependent on the presence of HGF, accelerating remyelination and functional recovery. To our knowledge, the present study is the first describing recovery from white matter injury after the administration of an EPC secretome in a cell-free approach.
The prolonged hypoperfusion model used in this study causes subcortical white matter injury affecting oligodendrocyte/myelin integrity, without apparent neuronal loss at 1 month (17, 19, 34, 35). Our study demonstrates for the first time the interaction of the EPC secretome with vascular and myelin remodeling by increasing vessel density, the number of proliferating oligodendrocyte lineage cells and enhancing myelination in the corpus callosum. EPCs can be incorporated into neovessels (26, 36) and their restorative actions on vascular remodeling have been widely reported in vivo in stroke models (37–39) or traumatic brain injury (40). At the same time, several nourishing factors have been reported to modulate EPCs function. For example, VEGF-C regulates the proliferation of neural progenitors expressing VEGFR-3 (41) and stimulates the proliferation of EPCs in a hypoxia-ischemia in vivo model (42), whereas VEGF-A strongly promotes OPC migration in a concentration-dependent manner, in vitro (43).
We have identified the presence of several trophic, proteolytic and signaling factors in the EPC-CM that support the therapeutic effects on white matter remodeling reported in this study. It is known that SDF1, VEGF or MMPs could be responsible for maintaining the oligovascular niche in the normal and injured brain (44) and that HGF is essential to accelerate remyelination and recovery in the EAE model (8). We have shown that several of these factors, among others, are present in our therapeutic EPC-CM. The relative contribution of each factor on oligovascular remodeling is unknown, but we pursue on exploring the role of angiogenin as a potent pro-angiogenic molecule that enhances endothelial angiogenesis (12) which can be pharmacologically inhibited by antibiotics such as neomycin or neamine (45, 46). In this regard, we observe that brain endothelial cells proliferated with EPCs secretome treatment being reverted in the presence of neomycin, highlighting the angiogenin-dependent effects of secretome therapy in vascular remodeling. However, we acknowledge that our model systems cannot directly link angiogenin actions in the remodeling brain, especially on oligodendrocyte proliferation, and that other nourishing factors present in the EPCs secretome could be also contributing to the observed white matter repair. Nevertheless, others have suggested that angiogenin may have potent non-vascular effects such as neuroprotection against oxidative stress (47) or hypoxic injury (48).
Importantly, we explore the cognitive function at the end of the study period when spatial working memory dysfunction has been observed (19, 49), allowing the evaluation of any therapeutic intervention. Our results show that the treatment with EPC-CM improves the functional outcome of mice exposed to cerebral hypoperfusion using the Y maze test. Moreover, the scores obtained for alternation rates by EPC-CM treated mice were similar to those scored by sham-operated mice in other studies of the group (18). This finding seems to be selective for the cognitive function and not for motor function. The long-term cognitive status and the effect of prolonged treatments with EPCs secretome deserves to be further evaluated.
Supplementary Material
SUMMARY.
Treatment with the EPC secretome in a mouse model of prolonged cerebral hypoperfusion may improve cognitive function by enhancing oligovascular repair in white matter. These results highlight the importance of EPC-released proteins such as angiogenin in therapeutic strategies for white matter injury after cerebrovascular disease.
Acknowledgments
SOURCES OF FUNDING
This work has been supported by the Miguel Servet program to AR (CPII15/00003), by research grants from Instituto de Salud Carlos III with European Regional Development Funds (PI13/01094, PI16/00981and RD12/0014/0005), by the NIH (R01-NS065089, P201-NS055104), by the Uehara Memorial Foundation and by the Japan Society for the Promotion of Science.
Footnotes
Supplementary Material: Suppl. Table I, Suppl.-Fig.I-Fig.II-Fig.III.
CONFLICTS OF INTEREST/DISCLOSURES
None.
References
- 1.Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med. 2008;14:497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- 2.Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- 3.Zhang R, Chopp M, Zhang ZG. Oligodendrogenesis after cerebral ischemia. Front Cell Neurosci. 2013;7:201. doi: 10.3389/fncel.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maki T, Liang AC, Miyamoto N, Lo EH, Arai K. Mechanisms of oligodendrocyte regeneration from ventricular-subventricular zone-derived progenitor cells in white matter diseases. Front Cell Neurosci. 2013;7:275. doi: 10.3389/fncel.2013.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gordon D, Pavlovska G, Uney JB, Wraith DC, Scolding NJ. Human mesenchymal stem cells infiltrate the spinal cord, reduce demyelination, and localize to white matter lesions in experimental autoimmune encephalomyelitis. J Neuropathol Exp Neurol. 2010;69:1087–1095. doi: 10.1097/NEN.0b013e3181f97392. [DOI] [PubMed] [Google Scholar]
- 6.Garbuzova-Davis S, Rodrigues MC, Mirtyl S, Turner S, Mitha S, Sodhi J, et al. Multiple intravenous administrations of human umbilical cord blood cells benefit in a mouse model of ALS. PLoS One. 2012;7:e31254. doi: 10.1371/journal.pone.0031254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jadasz JJ, Kremer D, Göttle P, Tzekova N, Domke J, Rivera FJ, et al. Mesenchymal stem cell conditioning promotes rat oligodendroglial cell maturation. PLoS One. 2013;8:e71814. doi: 10.1371/journal.pone.0071814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bai L, Lennon DP, Caplan AI, DeChant A, Hecker J, Kranso J, et al. Hepatocyte growth factor mediates mesenchymal stem cell–induced recovery in multiple sclerosis models. Nat Neurosci. 2012;15:862–870. doi: 10.1038/nn.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bai YY, Wang L, Chang D, Zhao Z, Lu CQ, Wang G, et al. Synergistic Effects of Transplanted Endothelial Progenitor Cells and RWJ 67657 in Diabetic Ischemic Stroke Models. Stroke. 2015;46:1938–46. doi: 10.1161/STROKEAHA.114.008495. [DOI] [PubMed] [Google Scholar]
- 11.Rosell A, Morancho A, Navarro-Sobrino M, Martínez-Saez E, Hernández-Guillamon M, Lope-Piedrafita S, et al. Factors secreted by endothelial progenitor cells enhance neurorepair responses after cerebral ischemia in mice. PLoS One. 2013;8:e73244. doi: 10.1371/journal.pone.0073244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao X, Xu Z. Mechanisms of action of angiogenin. Acta Biochim Biophys Sin (Shanghai) 2008;40:619–24. doi: 10.1111/j.1745-7270.2008.00442.x. [DOI] [PubMed] [Google Scholar]
- 13.Thiyagarajan N, Ferguson R, Subramanian V, Acharya KR. Structural and molecular insights into the mechanism of action of human angiogenin-ALS variants in neurons. Nat Commun. 2012;3:1121. doi: 10.1038/ncomms2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bárcena C, Stefanovic M, Tutusaus A, Martinez-Nieto GA, Martinez L, García-Ruiz C, et al. Angiogenin secretion from hepatoma cells activates hepatic stellate cells to amplify a self-sustained cycle promoting liver cancer. Sci Rep. 2015;5:7916. doi: 10.1038/srep07916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshioka N, Wang L, Kishimoto K, Tsuji T, Hu GF. A therapeutic target for prostate cancer based on angiogenin-stimulated angiogenesis and cancer cell proliferation. Proc Natl Acad Sci U S A. 2006;103:14519–24. doi: 10.1073/pnas.0606708103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goncalves KA, Silberstein L, Li S, Severe N, Hu MG, Yang H, et al. Angiogenin Promotes Hematopoietic Regeneration by Dichotomously Regulating Quiescence of Stem and Progenitor Cells. Cell. 2016;166:894–906. doi: 10.1016/j.cell.2016.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ihara M, Taguchi A, Maki T, Washida K, Tomimoto H. A mouse model of chronic cerebral hypoperfusion characterizing features of vascular cognitive impairment. Methods Mol Biol. 2014;1135:95–102. doi: 10.1007/978-1-4939-0320-7_8. [DOI] [PubMed] [Google Scholar]
- 18.Miyamoto N, Maki T, Pham LD, Hayakawa K, Seo JH, Mandeville ET, et al. Oxidative stress interferes with white matter renewal after prolonged cerebral hypoperfusion in mice. Stroke. 2013;44:3516–3521. doi: 10.1161/STROKEAHA.113.002813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maki T, Ihara M, Fujita Y, Nambu T, Miyashita K, Yamada M, et al. Angiogenic and vasoprotective effects of adrenomedullin on prevention of cognitive decline after chronic cerebral hypoperfusion in mice. Stroke. 2011;42:1122–1128. doi: 10.1161/STROKEAHA.110.603399. [DOI] [PubMed] [Google Scholar]
- 20.Carenza E, Barceló V, Morancho A, Montaner J, Rosell A, Roig A. Rapid synthesis of water-dispersible superparamagnetic iron oxide nanoparticles by a microwave-assisted route for safe labeling of endothelial progenitor cells. Acta Biomater. 2014;10:3775–85. doi: 10.1016/j.actbio.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Miyamoto N, Maki T, Shindo A, Liang AC, Maeda M, Egawa N, et al. Astrocytes Promote Oligodendrogenesis after White Matter Damage via Brain-Derived Neurotrophic Factor. J Neurosci. 2015;35:14002–8. doi: 10.1523/JNEUROSCI.1592-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Napoli E, Borlongan CV. Recent Advances in Stem Cell-Based Therapeutics for Stroke. Transl Stroke Res. 2016;7:452–457. doi: 10.1007/s12975-016-0490-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SU, de Vellis J. Stem cell-based cell therapy in neurological diseases: a review. J Neurosci Res. 2009;87:2183–0200. doi: 10.1002/jnr.22054. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Chopp M. Cell-based therapy for ischemic stroke. Expert Opin Biol Ther. 2013;13:1229–1240. doi: 10.1517/14712598.2013.804507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tajiri N, Duncan K, Antoine A, Pabon M, Acosta SA, de la Pena I, et al. Stem cell-paved biobridge facilitates neural repair in traumatic brain injury. Front Syst Neurosci. 2014;8:116. doi: 10.3389/fnsys.2014.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 27.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 28.Horie N, Pereira MP, Niizuma K, Sun G, Keren-Gill H, Encarnacion A, et al. Transplanted stem cell-secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem Cells. 2011;29:274–285. doi: 10.1002/stem.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He T, Peterson TE, Katusic ZS. Paracrine mitogenic effect of human endothelial progenitor cells: role of interleukin-8. Am J Physiol Heart Circ Physiol. 2005;289:H968–972. doi: 10.1152/ajpheart.01166.2004. [DOI] [PubMed] [Google Scholar]
- 30.Di Santo S, Seiler S, Fuchs AL, Staudigl J, Widmer HR. The secretome of endothelial progenitor cells promotes brain endothelial cell activity through PI3-kinase and MAP-kinase. PLoS One. 2014;9:e95731. doi: 10.1371/journal.pone.0095731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Z, von Ballmoos MW, Faessler D, Voelzmann J, Ortmann J, Diehm N, et al. Paracrine factors secreted by endothelial progenitor cells prevent oxidative stress-induced apoptosis of mature endothelial cells. Atherosclerosis. 2010;211:103–109. doi: 10.1016/j.atherosclerosis.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 32.Park KJ, Park E, Liu E, Baker AJ. Bone marrow-derived endothelial progenitor cells protect postischemic axons after traumatic brain injury. J Cereb Blood Flow Metab. 2014;34:357–366. doi: 10.1038/jcbfm.2013.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Santo S, Yang Z, Wyler von Ballmoos M, Voelzmann J, Diehm N, Baumgartner I, et al. Novel cell-free strategy for therapeutic angiogenesis: in vitro generated conditioned medium can replace progenitor cell transplantation. PLoS One. 2009;4:e5643. doi: 10.1371/journal.pone.0005643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shibata M, Ohtani R, Ihara M, Tomimoto H. White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke. 2004;35:2598–2603. doi: 10.1161/01.STR.0000143725.19053.60. [DOI] [PubMed] [Google Scholar]
- 35.McQueen J, Reimer MM, Holland PR, Manso Y, McLaughlin M, Fowler JH, et al. Restoration of oligodendrocyte pools in a mouse model of chronic cerebral hypoperfusion. PloS one. 2014;9:e87227. doi: 10.1371/journal.pone.0087227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friedrich EB, Walenta K, Scharlau J, Nickenig G, Werner N. CD34−/CD133+/VEGFR-2+ Endothelial Progenitor Cell Subpopulation With Potent Vasoregenerative Capacities. Circ Res. 2006;98:e20–5. doi: 10.1161/01.RES.0000205765.28940.93. [DOI] [PubMed] [Google Scholar]
- 37.Fan Y, Shen F, Frenzel T, Zhu W, Ye J, Liu J, et al. Endothelial progenitor cell transplantation improves long-term stroke outcome in mice. Ann Neurol. 2010;67:488–497. doi: 10.1002/ana.21919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morancho A, Ma F, Barceló V, Giralt D, Montaner J, Rosell A. Impaired vascular remodeling after endothelial progenitor cell transplantation in MMP9-deficient mice suffering cortical cerebral ischemia. J Cereb Blood Flow Metab. 2015;35:1547–51. doi: 10.1038/jcbfm.2015.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moubarik C, Guillet B, Youssef B, Codaccioni JL, Piercecchi MD, Sabatier F, et al. Transplanted late outgrowth endothelial progenitor cells as cell therapy product for stroke. Stem Cell Rev. 2011;7:208–220. doi: 10.1007/s12015-010-9157-y. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Li Y, Wang S, Han Z, Huang X, Li S, et al. Transplantation of expanded endothelial colony-forming cells improved outcomes of traumatic brain injury in a mouse model. J Surg Res. 2013;185:441–449. doi: 10.1016/j.jss.2013.05.073. [DOI] [PubMed] [Google Scholar]
- 41.Le Bras B, Barallobre MJ, Homman-Ludiye J, Ny A, Wyns S, Tammela T, et al. VEGF-C is a trophic factor for neural progenitors in the vertebrate embryonic brain. Nat Neurosci. 2006;9:340–348. doi: 10.1038/nn1646. [DOI] [PubMed] [Google Scholar]
- 42.Bain JM, Moore L, Ren Z, Simonishvili S, Levison SW. Vascular endothelial growth factors A and C are induced in the SVZ following neonatal hypoxia-ischemia and exert different effects on neonatal glial progenitors. Transl Stroke Res. 2013;4:158–170. doi: 10.1007/s12975-012-0213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayakawa K, Pham LD, Som AT, Lee BJ, Guo S, Lo EH, et al. Vascular endotelial growth factor regulates the migration of oligodendrocyte precursor cells. J Neurosci. 2011;31:10666–10670. doi: 10.1523/JNEUROSCI.1944-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyamoto N, Pham LD, Seo JH, Kim KW, Lo EH, Arai K. Crosstalk between cerebral endothelium and oligodendrocyte. Cell Mol Life Sci. 2014;71:1055–1066. doi: 10.1007/s00018-013-1488-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu GF. Neomycin inhibits angiogenin-induced angiogenesis. Proc Natl Acad Sci U S A. 95:9791–5. doi: 10.1073/pnas.95.17.9791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ibaragi S, Yoshioka N, Li S, Hu MG, Hirukawa S, Sadow PM, et al. Neamine inhibits prostate cancer growth by suppressing angiogenin-mediated rRNA transcription. Clin Cancer Res. 2009;15:1981–8. doi: 10.1158/1078-0432.CCR-08-2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cho GW, Kang BY, Kim SH. Human angiogenin presents neuroprotective and migration effects in neuroblastoma cells. Mol Cell Biochem. 2010;340:133–41. doi: 10.1007/s11010-010-0410-0. [DOI] [PubMed] [Google Scholar]
- 48.Sebastia J, Kieran D, Breen B, King MA, Netteland DF, Joyce D, et al. Angiogenin protects motoneurons against hypoxic injury. Cell Death Differ. 2009;16:1238–47. doi: 10.1038/cdd.2009.52. [DOI] [PubMed] [Google Scholar]
- 49.Shibata M, Yamasaki N, Miyakawa T, Kalaria RN, Fujita Y, Ohtani R, et al. Selective impairment of working memory in a mouse model of chronic cerebral hypoperfusion. Stroke. 2007;38:2826–32. doi: 10.1161/STROKEAHA.107.490151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


