Abstract
The nervous system integrates information from multiple senses. This multisensory integration already occurs in primary sensory cortices via direct thalamocortical and corticocortical connections across modalities. In humans, sensory loss from birth results in functional recruitment of the deprived cortical territory by the spared senses but the underlying circuit changes are not well known. Using tracer injections into primary auditory, somatosensory, and visual cortex within the first postnatal month of life in a rodent model (Mongolian gerbil) we show that multisensory thalamocortical connections emerge before corticocortical connections but mostly disappear during development. Early auditory, somatosensory, or visual deprivation increases multisensory connections via axonal reorganization processes mediated by non-lemniscal thalamic nuclei and the primary areas themselves. Functional single-photon emission computed tomography of regional cerebral blood flow reveals altered stimulus-induced activity and higher functional connectivity specifically between primary areas in deprived animals. Together, we show that intracortical multisensory connections are formed as a consequence of sensory driven multisensory thalamocortical activity and that spared senses functionally recruit deprived cortical areas by an altered development of sensory thalamocortical and corticocortical connections. The functional-anatomical changes after early sensory deprivation have translational implications for the therapy of developmental hearing loss, blindness, and sensory paralysis and might also underlie developmental synesthesia.
Keywords: cross-modal, deprivation, development, sensory integration, rodent, synesthesia
Introduction
Most behaviorally relevant stimuli activate multiple sensory streams, which have to be integrated for a coherent percept (for review, e.g., Schroeder and Foxe, 2005; Stein and Stanford, 2008; Klemen and Chambers, 2012). This multisensory integration is already present at the level of primary sensory cortices like the primary auditory (A1), somatosensory (S1), and visual cortex (V1) (for review, e.g., Kayser and Logothetis, 2007; Driver and Noesselt, 2008; Bizley et al., 2016). Neurons in these cortices respond to their own ("matched") sensory modality and also to other ("non-matched") modalities and receive convergent anatomical inputs via thalamocortical (from thalamus to cortex) and corticocortical (intracortical) pathways. The cross-modal connectivity at this early processing level provides a reasonable anatomical basis for short-latency multisensory integration processes in primary sensory cortices because the multiple sensory information has to cover only a few synapses from the sensory epithelium to the cortex (Budinger and Scheich, 2009; Henschke et al., 2015). Since short-latency multisensory integration is suited to improve sensory performances, for example, by decreasing reaction times (e.g., humans: Gielen et al., 1983; Molholm et al., 2002; Teder-Salejarvi et al., 2002; Noesselt et al., 2010; animals: Sakata et al., 2004; Gleiss and Kayser, 2012), the establishment of underlying thalamo- and corticocortical connections might also play a crucial role in the development of multisensory behavior (for review, e.g., Stein et al., 2014; Hanganu-Opatz et al., 2015; Murray et al., 2016).
While it is well appreciated that early loss of one sensory modality can lead to enhanced responses and an improved performance in the remaining senses as well as to the functional recruitment of non-deprived cortical areas to different modalities (e.g., humans: Sadato et al., 1996; Lessard et al., 1998; Roder et al., 1999; Finney et al., 2001; Gougoux et al., 2004; animals: Rauschecker et al., 1992; Lomber et al., 2010; Ghoshal et al., 2011; Meng et al., 2015; Hammond-Kenny et al., 2017) it is not well understood how and when individual sensory pathways and in particular connectivity across these pathways change in relation to normal development. So far, previous studies on multisensory connectivity in primary sensory cortices following early sensory loss have paid attention mostly to changes observed in adult individuals and in the deprived cortical area (e.g., Charbonneau et al., 2012; Meredith and Allman, 2012; Chabot et al., 2015; Barone et al., 2013; Karlen et al., 2006; Sieben et al., 2015). We here investigated first the early postnatal development of unisensory and multisensory ("cross-modal" with respect to stimuli; Stein et al., 2010) thalamo- and corticocortical pathways of three primary cortical areas (A1, S1, V1) in normally developing rodents (Mongolian gerbils, Meriones unguiculatus), which are an outbred species and a well-suited model for multisensory and developmental research (Budinger and Scheich, 2009). We then examined how the loss of each one of the three senses (hearing, touch, vision) alters the development of these pathways of all three (deprived and spared) sensory systems.
To examine the thalamocortical and intracortical connections of matched and non-matched sensory modalities we used retrograde tracer injections into A1, S1, and V1. We found that multisensory convergence is first present at the thalamocortical level and then at the intracortical level. Initially, there is an overabundance of multisensory connections followed by an elimination of exuberant connections and refinement. In animals without early auditory, somatosensory, or visual experience we observed an increase of sensory matched as well as non-matched thalamocortical and corticocortical connections mediated mostly by axonal reorganization in non-lemniscal thalamocortical pathways and pathways from primary and secondary cortices. Consistent with the anatomical findings, in vivo single-photon emission computed tomography (SPECT) of cerebral blood flow revealed that sensory deprived animals show altered neuronal responses to sensory stimulations and a higher functional connectivity between spared and deprived primary sensory areas. Together our study demonstrates that the lack of sensory experience has a dramatic effect on the development of thalamic and cortical sensory connections and in particular not only on the pathways underlying the deprived sense but also on pathways of non-deprived senses enabling a functional recruitment of deprived cortical areas by the spared senses.
Materials and methods
Experimental animals
Experiments were performed on 108 Mongolian gerbils (Meriones unguiculatus) of both genders at postnatal days P1 (newborn, somatosensation already active), P9 (before opening of ears and eyes), P15 (after complete ear opening at ~P14), P21 (after complete eye opening at ~P19), P28 (animals weaned, end of critical period), and P120 (adult; for cytoarchitecture only).
Mongolian gerbils have good vision (Jacobs and Deegan, 1994), hearing (in particular of low frequencies: Ryan, 1976), touch (with a particular role of hind paw drumming: Thiessen and Yahr, 1977), and olfaction (Vallejo et al., 2000). There is also multiple anatomical, physiological, and behavioral evidence for cross-modal interactions, even at the level of primary sensory cortices, in this species (e.g., Cahill et al., 1996; Budinger and Scheich, 2009; Kobayasi et al., 2013). At birth, Mongolian gerbils are deaf and blind (Souter et al., 1997) but show first sensorimotor reflexes (Cabana et al., 1993). Gerbils develop slower than rats and mice (Schwentker, 1963), particularly during the activation of the different sensory systems. While there is a partial temporal overlap between the onset of hearing and vision in mice and rats, this is not the case in gerbils enabling a precise investigation of the different sensory influences during development with respect to their onset. In mice, ears open at around P10–12 (Ehret, 1976) and eyes around P12–15 (Fuller and Wimer, 1966). In gerbils, external ear canal and middle ear cavity are completely open at P14 (Finck et al., 1972) and eyes open between P16–20 (average P18.5±1.2d: Wilkinson, 1986; Mowery et al., 2016). We therefore focused on the five above mentioned ages, which span the serial activation of the sensory systems.
The litter size of our animals varied from six to nine pups and was not adjusted; the father was removed from the mother and litter before birth because of his aggressive behavior against surgically treated nestlings. At P28, the young gerbils were separated from the mother. Around the time of ear canal (P14) and eye opening (P16–20), animals were inspected frequently to determine the exact time point of these events. Animals were housed in standard laboratory cages (Tecniplast, Italy; Eurostandard Type IV, 598 × 380 × 200 mm) in air-conditioned rooms (average temperature 22 °C, 12 h light-dark cycle); water and food were available ad libitum.
All experiments were according to the NIH Guide for the Care and Use of Laboratory animals (2011) and the Directive of the European Communities Parliament and Council on the protection of animals used for scientific purposes (2010/63/EU) and were approved by the animal care committee of Sachsen-Anhalt, Germany (number of proposal for animal experimentation: 42502-2-1324 LIN).
Neuroanatomical tracer injections
For tracer injections, 45 normal and 27 deprived gerbils were used; 3 animals per age group, deprivation type and injection site into A1, S1, and V1, respectively. Animals were anesthetized with isoflurane (4 %vol; Baxter, Germany), the cranial skin was locally disinfected, anesthetized (Gingicain, Sanofi-Aventis, Germany), and incised. The skull was exposed by displacement of the skin and muscles, and a small hole was drilled into the skull. Respective craniotomies were performed using stereotaxic information with respect to external landmarks on the skull, such as lambda and bregma, and to other distinct landmarks like characteristic blood vessels of the bone and the brain (Henschke et al., 2015; Radtke-Schuller et al., 2016), which had to be adapted to the smaller size of the young skull and brain. For all animals, an additional post-hoc histological analysis of the injection sites was performed using internal landmarks and cytoarchitectural features (Fig. S1, Tab. S1). In consequence, this study only comprises animals with unequivocally verified injections into A1 (central part, high and low frequencies), S1 (hindlimb area HL), and V1 (monocular and binocular part). Other experimental animals (not listed here), for example, with injection sites even slightly spreading into adjacent cortical areas or the white matter, were not included in this study.
At every experimental age, 18 nl of a 10 % Fluorogold-solution (FG, hydroxystilbamidine; Fluorochrome, LLC, USA; molecular weight: 532.6 Daltons; diluted in distilled water) were injected in 2×9 nl steps over 5 min into A1, S1, and V1 of the left hemisphere using an oil hydraulic nanoliter delivery system (World Precision Instruments, Germany) and fine glass micropipettes (tip diameter 20 µm). We used FG as tracer because of its fast and reliable retrograde transport compared to, for example, dextran amines and viruses, which need transportation and expression times, of up to several weeks (Vercelli et al., 2000; Wickersham et al., 2007). These are, in turn, too long for the scope of the study and the use in very young animals. After the injections, the craniotomies were sealed with bone wax (Ethicon, Johnson & Johnson, Germany), the surgical openings were treated with an anti-inflammatory ointment (Volon A; Dermapharm GmbH, Germany), and the skin was closed with tissue adhesive (Histoacryl; B/Braun, Germany).
Deprivation of early sensory experiences
Somatosensory deprivation was performed at P5 by a bilateral transection of the sciatic nerve of the hindlimb (Wall and Cusick, 1986). At this time point, somatosensation is already active and animals can perceive touch and vibrations (Cabana et al., 1993). We chose this particular kind of somatosensory deprivation because previous studies demonstrated multisensory connections preferentially for the hindlimb and trunk area of the gerbil's somatosensory cortex, which, in turn, might be related to a particular warning behavior (hind paw drumming) of the animals (Budinger et al., 2006). Animals were separated from their mother together with their littermates; then, each animal was anesthetized with isoflurane (4 %vol) and positioned on its side. The skin around the upper femoral joint was locally disinfected, anesthetized, and finally incised along the femur. By displacement of the outer femoral muscles the sciatic nerve became visible, which was then carefully separated from the surrounding tissue and vessels and finally transected. The surgical opening was treated with an anti-inflammatory ointment and closed with surgical silk suture (Johnson & Johnson). The animal was put back with its littermates and 1 h later with its mother. The maturation of each animal was carefully monitored and it turned out that the transection of the sciatic nerve had no major motor consequences except than a partial overflexion of the hind paw fetlocks.
Auditory deprivation was performed by bilateral ototoxic inner hair cell damage (Heydt et al., 2004) at P10, that is before external ear canals and middle ear cavities are completely open (~P14: Finck et al., 1972). For deafening, the skin behind the pinna of the anesthetized animals was locally disinfected and incised. A small hole was drilled into the bulla tympanica and 0.5 µl Gentamycin (1 % in distilled water, 0.0025 % EDTA, 0.00008 % sodium bisulphite; Sigma-Aldrich, Germany) were applied directly onto the round window using a microliter syringe (Hamilton, Switzerland). Thereafter, the bulla was closed with bone wax and the skin incision was treated with an anti-inflammatory ointment and sewed up with surgical silk suture. The animal was put back with its littermates and later on with its mother. At P28, deafness of animals was validated by a lack of startle responses. Therefore, a startle stimulus (50 ms, 120 dB) was delivered to the gerbil in a startle-box system (TSE Systems GmbH, Germany) with or without preceding prepulse stimulus (30 ms, 100 ms before the startle stimulus) at eight different intensities (73–94 dB, 3 dB increments) on a 70 dB white noise background (Bhattacharya et al., 2017). After habituation to the box (3 min), 2 startle trials were followed in pseudo-random order by 10 startle trials and 5 trials at each of the prepulse intensities with stochastically varied intertrial intervals (5–30 s). The maximal startle amplitude was measured by a sensor platform. Only animals, which did not show any sign of acoustic responses, i.e., which were obviously completely deaf, were used for this study.
Visual deprivation was performed at P10 by bilateral enucleation (Chabot et al., 2007), i.e., before vision onset. Eyes open earliest at P16 (Wilkinson, 1986), which is considerably delayed (in our animals towards P19) when the father is absent from the litter (Elwood and Broom, 1978). For enucleation, the eyelid of the anesthetized animal was carefully opened and the eyeball was displaced from its socket using round forceps. Then, the optic nerve and ophthalmic artery were clamped with fine forceps for 2 min. Thereafter, the optic nerve was cut and the eyeball removed. The orbita was filled with absorbable styptic gelatine sponge (Gelastypt, Sanofi-Aventis; Germany) and the eyelid was closed with surgical silk suture.
Histology
Processing
After 3 days of retrograde tracer transport, animals of the age groups P1–15 were reanesthetized with isoflurane (4 %vol) and decapitated. All other animals were reanesthetized with ketamine (20 mg/100 g body weight, ip) and xylazine (1 mg/100 g body weight, ip) and were perfused transcardially with 20 ml of 0.1 M phosphate-buffered saline (PBS, pH = 7.4) followed by 200 ml of 4 % paraformaldehyde (PFA). Then, brains were removed, postfixed overnight in 4 % PFA at 4 °C, and cryoprotected by soaking them in 30 % sucrose in PBS for 48 h. Brains were cut on a cryostat (Leica CM 1950, Germany) into 50 µm thick frontal sections. Every first and second section of each experimental animal was directly mounted on gelatine-coated glass slides for pure FG visualization; out of those, sections in 300 µm intervals were counterstained for cytoarchitecture 1 min using DAPI (4',6-diamidin-2-phenylindoldihydrochlorid; 0.1 % in 0.1 M PBS; Sigma, Germany). Every third section was collected in PBS (free-floating) and stained against GAP43 (rabbit, 1:500, #ab16053, Abcam, United Kingdom; RRID: AB_443303), Caspase-3 (rabbit, 1:500, #Asp175, Cell Signaling, USA; RRID: AB_10665003), and Doublecortin (goat, 1:1000, #sc8066, Santa Cruz Biotechnology, USA; RRID: AB_2088494), respectively (overnight). After the washing and blocking procedure, sections were incubated with the respective secondary Cy3-labeled antibodies (1:500, Dianova, Germany; anti-rabbit #111-165-047, RRID: AB_2338005; anti-goat #305-165-003, RRID: AB_2339464) for 2 h. Finally, sections were washed again, mounted on gelatin-coated slides, and coverslipped with MOWIOL (Fluka, Germany).
In addition, brains of 16 other non-injected animals (two per age group and deprivation type) were used for a detailed cytoarchitectural investigation of the developing gerbil brain (Fig. 1), for a reliable identification of target areas, as well as for the definition of landmark-based volumes of interest (VOIs) in reference MR-images for SPECT experiments. After sectioning on a cryostat, brain sections were directly mounted on gelatine-coated glass slides, counterstained with Nissl (creysl violet), and coverslipped with Merkoglas (Merk; Deutschland).
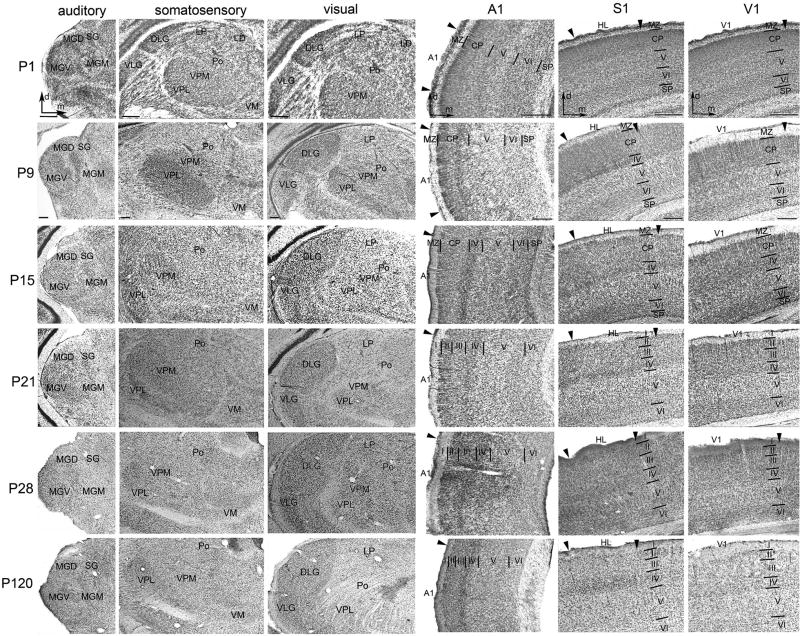
Figure 1.
Nissl-stained frontal sections showing the cytoarchitecture of auditory, somatosensory, and visual thalamic nuclei and of primary cortical areas A1, S1 (HL - hindlimb area), and V1 at all experimental ages (P1–28) and at P120 (adult). Scale bars 200 µm (bars for P9 applicable to all later time points), areal borders are marked by small arrowheads.
(I–VI - cortical layers I–VI, CP - cortical plate, d - dorsal, m - medial, MZ - marginal zone of cortex, SP - subplate of cortex; for other abbreviations see Tab. 1)
Analysis
All sections were thoroughly examined under a combined brightfield and fluorescent microscope (Leica DMRX, Germany) with the appropriate filter sets. FG has (depending on pH) an excitation maximum of 323 nm and an emission maximum of around 540 nm (golden fluorescent; filter set FG, AHF Analysentechnik, Germany), DAPI of 358 nm and 461 nm (blue fluorescent; filter set A, Leica), and Cy3 of 550 nm and 570 nm (red fluorescent; filter set N2.1, Leica). Images were taken with a Nikon D7000 camera mounted on the microscope. Illustrations were arranged using Photoshop (v. 13.0.6).
Cortical areas and subcortical structures were identified on the basis of their architecture and relative location using information derived from extensive anatomical work on gerbils, including stereotaxic atlases of the gerbil brain (Loskota et al., 1974; Radtke-Schuller et al., 2016), and from comparative literature, in particular from stereotaxic brain atlases and descriptions of the mouse and rat (reviewed in Henschke et al., 2015). For young animals, areas were identified on the basis of own extensive anatomical investigations (e.g., Fig. 1) and according to atlases of the developing mouse and rat brain (Paxinos et al., 2006; Ashwell and Paxinos, 2008).
The extent of each injection site was measured using the measure tool of the Neurolucida software (MicroBrightField, USA) in the sections covering the center of each injection site. Width (W, measured parallel to the cortical layers), length (L, measured across the injected layers), and location of each injection site are presented for each experimental animal in Table S1. Average volume (Vi) of injection sites for each experimental age was calculated considering a cylindrical size of the individual injections with Vi = (π/4) × W2 × L (Fig. S2). Average total volume of cortical areas A1, S1, and V1 (VA1,S1,V1) was calculated for each experimental age on the basis of magnetic resonance imaging (MRI) scans of P28 animals (see below) and areal information derived from the gerbil's stereotaxic atlas (Radtke-Schuller et al., 2016). Accordingly, at P28, VA1 = 4.018 mm3, VS1 = 17.838 mm3, VV1 = 10.700 mm3. For the smaller brains of younger animals, correction factors (P1: 0.544, P9: 0.816, P15: 0.960, P21: 0.968), derived from detailed brain size measurements of developing gerbils (Wilkinson, 1986, Fig. 4), were regarded. Cortical thickness of A1, S1, and V1 (Tab. S2) was measured (Neurolucida measuring tool, MicroBrightField) in 5 consecutive sections covering these areas in the left hemisphere of the 16 animals designated to the cytoarchitectural investigation (see above).
Figure 4.
Loss of early sensory experience drastically increases the number of matched and non-matched thalamocortical and corticocortical connections.
(A) Frontal sections showing retrogradely labeled somata in matched and non-matched thalamic nuclei (left and second row) as well as in other primary and secondary sensory cortices (third and right row) following early auditory, somatosensory, and visual deprivation and tracer injections into A1 at P28. Scale bars 200 µm.
(APT - anterior pretectal nucleus; for other abbreviations see Tab. 1 and Fig. 1)
(B) Mean number of retrogradely labeled neurons in sensory matched and non-matched thalamic nuclei following tracer injections into A1 (blue), S1 (red), and V1 (green) listed for normal P28 animals (same as in Fig. 2) and for animals following early sensory deprivation.
(C) Percentage of non-lemniscal inputs on matched thalamocortical connections.
(D) Percentage of non-matched inputs on all sensory thalamocortical connections.
(E) Mean number of retrogradely labeled somata in primary and secondary sensory cortices.
(F) Percentage of inputs from secondary sensory areas on all multisensory corticocortical connections.
(G) Relative increase of multisensory matched and non-matched thalamocortical connections as well as cortical projections from primary and secondary sensory cortices of A1 (blue), S1 (red), and V1 (green) following early auditory, somatosensory, and visual deprivation compared to normal animals (number of labeled cells in deprived animals / number of labeled cells in normal animals).
Error bars are SEM of the total number; stars (color coded as injected areas) indicate significant changes between control animals (P28) and deprived animals (*p = 0.03, KS test; n = 3 for each deprivation type and injection site). Other conventions as in Fig. 2.
After each injection, the numbers of retrogradely labeled neurons (i.e., neurons, which clearly contained the tracer) in all brain structures of interest were counted in two thirds of all sections of each experimental animal (third section was used for immunohistochemistry; see above), summing up to several thousand neurons per animal. According to the scope of this study, mean values of respective labeled neurons for five age groups, three deprivation types, and three injection sites (always n = 3) were calculated for sensory structures of the cortex and thalamus, namely the primary and secondary sensory cortices and the sensory thalamic nuclei (dorsal thalamus, subthalamus, metathalamus). This excludes labeled nuclei of the hypo- and epithalamus and non-sensory thalamic nuclei, i.e., nuclei, which do not primarily process sensory information (e.g., Groenewegen and Witter, 2004; Jones, 2007), and nuclei of lower brainstem structures.
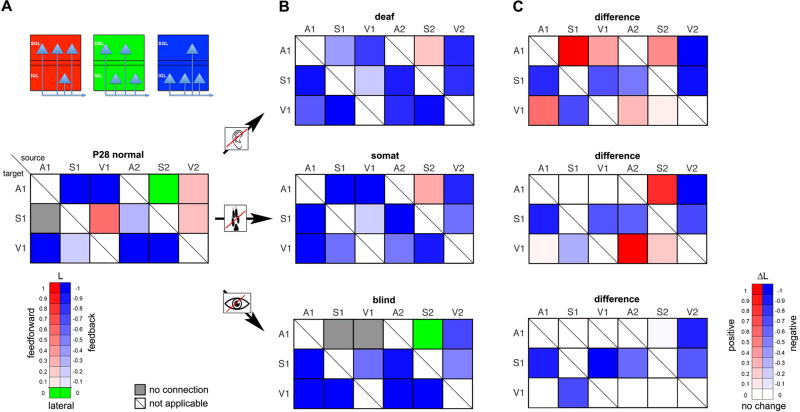
For the laminar distribution of labeled cell bodies in the cortex, a layer index L = (SGL−IGL)/(SGL+IGL) (IGL: number of cells in infragranular, SGL: in supragranular layers) was calculated (e.g., Cappe and Barone, 2005; Budinger et al., 2006; Masse et al., 2017), indicating (together with particular terminal labeling, which was not available in this study using FG) feedback (−1 ≤ L < −0.1), feedforward (0.1 < L ≤ 1), and lateral (i.e., intrinsic) connections (−0.1 ≤ L ≤ 0.1) (Felleman and Van Essen, 1991; Rouiller et al., 1991). The respective values were transformed into a color-coded plot (Fig. 6, Tab. S4).
Figure 6.
The nature of intracortical connections alters due to the loss of early sensory experiences.
(A–B) Illustrated is the putative nature of corticocortical connections based on the laminar distribution of retrogradely labeled neurons (i.e., layer indices L, Tab. S4) in primary and secondary sensory cortices following FG injections into A1, S1, and V1 at P28 in normal animals (A) and in animals after early auditory (deaf), somatosensory (somat), and visual (blind) deprivation (B). Feedforward (red) connections originate mainly in supragranular layers (SGL; 0.1 < L ≤ 1), feedback (blue) in infragranular layers (IGL; −1 ≤ L < −0.1) and lateral (green) connections equally in SGL and IGL (−0.1 ≤ L ≤ 0.1).
(C) Difference maps illustrating that in particular after auditory and somatosensory deprivation, connections turn towards more positive values (red), i.e., originate more in SGL (feedforward-type), whereas after visual deprivations, connections originate more in IGL (feedback-type; blue).
Color coded layer indices were derived from the mean values of retrogradely labeled neurons in primary and secondary sensory areas following FG injections into A1, S1, and V1 at P28 in normal and deprived animals (n = 3 for each experimental condition, see Tab. S4).
For the analysis of DCX and GAP43 staining intensities in sensory thalamus and cortex (Fig. 7), microscopical images of regions of interest were taken as described above always with 10× magnification and 500 ms exposure time. Then, RGB images were converted non-weighted into 8 bit grey-scale images and mean grey values were determined in 3 consecutive sections covering the region of interest in each experimental animal (i.e., n = 9 sections for each structure at each experimental age and for each deprivation type) using the Measurement tool of Image J (v. 1.48g, NIH, USA).
Figure 7.
Changes in connectivity patterns during development and following early sensory deprivation are not due to apoptosis or neurogenesis of projection neurons but rather to axonal remodeling.
(A) Retrogradely labeled neurons do not express CASP (a–c) or DCX (d–e) indicating that there is no apoptosis or neurogenesis of projection neurons.
(a, d) FG-labeled neurons (arrows) in layer V (a) and VI (d) of A1 following FG injection into V1 at P9 (a) and S1 at P15 (d). (b, e) Same sections showing CASP3+ (b) and DCX+ (e) neurons; arrows points to the location of the FG-labeled neurons in a/d. (c, f) Overlay of images revealing that FG-labeled and CASP3+ resp. DCX+ neurons are not co-localized. Scale bar 25 µm.
(B–C) Left side: Doublecortin (DCX) staining intensities in lemniscal (MGV, VPM/VPL, DLG) and non-lemniscal thalamic nuclei (MGD, Po, LP) and primary sensory areas (A1) constantly decrease during early development. Early sensory deprivation usually does not change the DCX expression at P28. Right side: Frontal sections through the auditory thalamus and cortex showing DCX labeling at P1 and P28.
(D–E) Left side: Growth associated protein 43 (GAP43) staining intensities in sensory thalamus and cortex increase until P15 but then decrease in lemniscal thalamic nuclei. In non-lemniscal nuclei and primary areas, GAP43 remains rather high or even increases further. This holds also true for sensory deprived animals. Right side: Frontal sections through the auditory thalamus and cortex showing GAP43 labeling at P1 and P28.
Grey values are mean ± 1 SD; stars (color coded as structures) indicate significant changes between experimental ages or (in some cases) significant differences between normal and deprived animals at P28 (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, Student's t-test; n = 9 sections for each structure at each age and for each deprivation type).
Images show DCX (B, C) and GAP43 (D, E) staining in auditory thalamus (B, D) and primary auditory cortex (A1; C, E) at P1 and P28. Scale bars 200 µm. For abbreviations see Tab. 1 and Fig. 1.
All statistical analyses, including two tailed, unpaired Student's t-test for normally distributed values, non-parametric Kolmogorov-Smirnov (KS) test for small sample sizes, and multivariate non-parametric Scheirer-Ray-Hare (SRH) test (Sachs, 2004) were performed using Microsoft Excel (v. 13) and Real Statistics software (Charles Zaiontz; real-statistics.com).
Single-photon emission computed tomography (SPECT)
Imaging procedure
For SPECT-imaging of regional cerebral blood flow 20 gerbils (P28; 10 normal, 10 somatosensory deprived) were intravenously injected with 99mTc-HMPAO (99mTechnetium hexamethylpropyleneamine oxime). Jugular vein catheter implantation at P27 and preparation of the 99mTc-HMPAO injection solution were essentially done as previously described (Kolodziej et al., 2014). For tracer injection, the catheter was extended by a polyethylene (PE) tube (65 cm, prefilled with 162.5 µl 0.9 % NaCl; BioMedical Instruments, Germany) and connected to a syringe containing 130–150 MBq freshly prepared 99mTc-HMPAO in volumes of 300–380 µl. Injections were made with a perfusion pump (Havard Instruments, USA) at flow rates of 40 µl/min, during which the animals were awake and freely behaving in a sound proof, dark chamber. Animals received either no stimulus (baseline condition, 9 min) or were stimulated acoustically (75 dB SPL white noise bursts, 250 ms on/off, 9 min, free field) or visually (light flashes, 1800 lx at 30 cm, 250 ms on/off, 10 min, strobe LED Eurolite, Germany). Stimulation started when the tracer had filled the PE-extension tube and was about to reach the jugular vein catheter. Stimulation continued for 1 min after tracer injection. The animals were then removed from the chamber and anaesthetized (4–2 % isoflurane, 0.8 % O2) for SPECT/CT imaging. The amounts of tracer remaining in the syringe and in the extension tube were determined using a radionuclide calibrator (Aktivimeter Isomed 2010, Nuklear-Medizin-Technik Dresden GmbH, Germany). Animals were imaged every second day (first baseline, then acoustic and then visual stimulation).
Co-registered head SPECT/CT-scans were made using a four-head NanoSPECT/CT (Mediso, Hungary). CT scans were made at 45 kVp, 177 µA, with 180 projections, 500 ms per projection and 96 µm pixel size, reconstructed (InVivoScope v. 1.43) at isotropic voxel-sizes of 200 µm. SPECT scans were made using ten-pinhole gerbil brain apertures (SCIVIS, Germany) with 1.0 mm pinhole diameters, providing a nominal resolution of 0.7 mm. Twenty-four projections were acquired during a scan time of 1 h. Axial FOV was 29.9 mm. Photopeaks were set to the default values for 99mTc (140 keV +/− 5 %).
Analysis
SPECT images were reconstructed (HiSPECT™, v. 1.4.1876, SCIVIS) at an isotropic voxel size of 167 µm. Using CT landmarks, the co-registered SPECT/CT-images were aligned (MPITool™ software, v6.36, ATV) to a P28 gerbil reference MR obtained at 4.7 T (Bruker BioSpec 47/20, Germany). After alignment, SPECT-brain data sets were cut out from SPECT-head data sets and were global-mean normalized. SPECT data obtained under stimulus-conditions were baseline corrected, i.e., in each animal the normalized data without stimulation were subtracted from the normalized data under stimulus conditions. Voxelwise analyses and landmark-based VOI analyses including statistical analyses (Pearson's Chi-square test for normality; F-test for similar variances; two tailed, unpaired Student's t-test) of control animals versus somatosensory deprived animals and correlation analyses between individual VOIs were done using the Osirix-DICOM viewer (v. 5.8.1), Microsoft Excel (v. 13), and MagnAn-software (v. 2.4, BioCom GbR). Illustrations were made in Photoshop (v. 13.0.6) with images generated in Osirix.
Results
We investigated the development and experience-dependent changes of thalamocortical and corticocortical connections of matched and non-matched sensory inputs by retrograde tracer injections of FG into A1, S1, and V1 of Mongolian gerbils focusing on five ages spanning the serial activation of the sensory systems: P1 (newborn, somatosensation already active), P9 (before ear and eye opening), P15 (shortly after complete ear opening), P21 (shortly after complete eye opening), and P28 (animals weaned, end of critical period).
Typical architecture of sensory thalamic nuclei is already present at birth and of primary sensory cortices at the latest at P21
For a precise identification of thalamic nuclei and cortical areas, reliable injections into A1, S1, and V1, as well as for the definition of landmark-based VOIs in reference MR-images for later SPECT experiments, we first investigated the postnatal cytoarchitectonic development of the gerbil's sensory thalamus and cortex (Figs. 1, 2A).
Figure 2.
Direct inputs from matched and non-matched sensory thalamic nuclei to A1, S1, and V1 are already present at P1, connections with primary and secondary sensory cortices emerge later.
(A) Frontal sections showing injection sites of FG into the primary auditory cortex (A1) at experimental ages P1, P15, and P28 (left row) and retrogradely labeled cell bodies in lemniscal (MGV) and non-lemniscal (MGD, MGM, SG) medial geniculate body (second row), non-matched thalamus (LPMR, Po, LP; arrows; middle row) as well as in S1 and V1 (fourth and right row). Insets show labeled cells enlarged from arrows or white rectangles. Note that there are no multisensory intracortical connections at P1. Scale bars 200 µm and 20 µm (insets). For better identification of the cortical layers, corresponding Nissl-stains are shown (see also Fig. 1).
(HF - hippocampal formation, M1 - primary motor cortex, V2 - secondary visual cortex, wm - white matter; for other abbreviations see Tab. 1 and Fig. 1)
(B) Mean number of retrogradely labeled neurons in sensory matched and non-matched thalamic nuclei following tracer injections into A1 (blue), S1 (red), and V1 (green) listed for all experimental ages.
(C) Percentage of non-lemniscal inputs on matched thalamocortical connections.
(D) Percentage of non-matched inputs on all sensory thalamocortical connections.
(E) Mean number of retrogradely labeled somata in primary and secondary sensory cortices following tracer injections into A1 (blue), S1 (red), and V1 (green).
(F) Percentage of inputs from secondary sensory areas on all multisensory corticocortical connections.
Error bars are SEM of the total number; stars (color coded as injected areas) indicate significant changes between consecutive experimental ages (*p = 0.03, KS test; n = 3 for each experimental age and injection site). Note the different scaling of the x-axes for better visualization of the values. See also Figs. S3 and S4.
At P1, the gerbil's sensory thalamic nuclei are small, but their spatial relationship is already adult-like (P120). The borders of auditory, somatosensory, and visual thalamic nuclei are clearly defined by unstained fiber tracts between the individual nuclei; the sensory nuclei themselves contain very densely packed small neurons. At P15, neurons have gained normal size; at P28, thalamic nuclei nearly reach adult dimensions. Thereby, the borders defined by unstained fiber tracts consistently disappear due to migrating cells.
The primary sensory cortices of a gerbil at P1 can be delineated (from deep to superficial) into subplate (SP), layer VI, layer V, cortical plate (CP; consisting of layers II–IV), and marginal zone (MZ; layer I) (for comparison with other species, e.g., Lopez-Bendito and Molnar, 2003; Clowry et al., 2010; Kanold and Luhmann, 2010). The densely packed neurons of the SP clearly stand out against layer VI, which is more loosely packed. Layer V is defined by large pyramidal cells and the adjacent CP by the radial structure of its neurons. The MZ contains only few neurons. At P1, the gerbil's A1 is approximately 730 µm thick, S1 520 µm, and V1 460 µm (Tab. S2). At the latest at P15 and P21, respectively, layer IV, which contains very densely packed neurons (typical koniocortex), and layer III/II are distinguishable. The MZ develops into layer I approximately between P15 and P21. Notably, layer IV of S1 is already identifiable at P9, consistent with somatosensation being the first active (stimulus-driven) modality. Around P15, the cortex has reached about 95% of its full thickness (compared to P120), being approximately 1340 µm (A1), 1310 µm (S1), and 840 µm (V1) thick (Tab. S2). At P21, when all three senses are active, the cortical layers of A1, S1, and V1 appear adult-like.
In deprived animals, the overall thalamic and cortical cytoarchitecture did not differ from normal P28 animals. In particular, we could not detect signs of neuronal degeneration, such as an increased number of microglia cells in the thalamus or cortex, nor changes in the cortical lamination and/or thickness of deprived animals (Tab. S2).
Multisensory thalamocortical connections develop rapidly but are pruned later on
We next investigated if A1, S1, and V1 received thalamic inputs from non-matched sensory thalamic nuclei and if so, how these connections mature during early postnatal development. We retrogradely labeled thalamic nuclei projecting into A1, S1, and V1 by injections of FG into these cortical areas (Figs. 2A–D, S1, Tab. S1) and for analysis grouped thalamic nuclei into lemniscal (core) and non-lemniscal (non-core) categories (Tab. 1).
Table 1. Sensory thalamic nuclei containing retrogradely labeled somata after injections of FG into the gerbil's A1, S1, and V1.
Listed is the main modality and belonging of theses nuclei to the lemniscal (core) or non-lemniscal (non-core) sensory pathways (e.g., Groenewegen and Witter, 2004; Jones, 2007). Matched nuclei are those, which belong to the same given sensory pathway (e.g., lemniscal and non-lemniscal auditory), non-matched nuclei belong to the other sensory pathways (lemniscal and non-lemniscal somatosensory and visual). In particular some of the non-lemniscal nuclei may already process multisensory information (e.g., Budinger et al., 2006).
| auditory | lemniscal | medial geniculate body (MGB): ventral part (MGV) |
| non-lemniscal | MGB: dorsal part (MGD), medial part (MGM), marginal zone (MZMG), suprageniculate thalamic nucleus (SG) | |
| somatosensory | lemniscal | ventral posterolateral (VPL), ventral posteromedial (VPM) thalamic nucleus |
| non-lemniscal | posterior (Po), ventral anterior (VA), ventromedial (VM), ventrolateral (VL, somatomotor) thalamic nucleus, zona incerta (ZI) | |
| visual | core | dorsal lateral geniculate nucleus (DLG) |
| non-core | laterodorsal (LD), lateral posterior (LP), lateral posterior mediorostral (LPMR), posterior limitans (PLi) thalamic nucleus |
Injections into primary sensory areas resulted in retrogradely labeled neurons in both lemniscal (core) and non-lemniscal (non-core) nuclei of both the respective sensory matched and non-matched pathways, but the fraction of cells in each changed over development. To ensure that this variation is not due to an overall growth of the brain during development, we plotted the average volume of the injection sites (Vi) as percentage of the average total volume of A1, S1, and V1 (VA1,S1,V1) for each experimental age and the overall number of retrogradely labeled cells for each experimental age (Fig. S2). The plots show that over the different age groups, the tracer filled approximately the same fraction of A1, S1, and V1 (2.49 ± 1.99 %) but the overall number of labeled cells considerably decreased, indicating that this decrease and other, more specific changes in cell numbers, are not due to volumetric changes of the injection sites.
A1 injections (Fig. 2A) resulted in retrogradely labeled somata in ipsilateral sensory matched (i.e., lemniscal and non-lemniscal auditory) but also non-matched thalamic nuclei (i.e., lemniscal and non-lemniscal somatosensory and visual) (Fig. 2B blue bars). At P1, labeled neurons were found in lemniscal auditory MGV, but the majority of labeled neurons was found in the non-lemniscal auditory MGD and SG as well as in visual LPMR (Fig. S3 upper two panels). Accordingly, the percentage of non-lemniscal on all auditory inputs (87.3 %; Fig. 2C) and non-matched on all thalamic sensory inputs into A1 (17.1 %; Fig. 2D) was very high compared to later time points. At P9, more cells were found in the MGV than MGD and the overall number of labeled somata in both structures increased. In addition, there were labeled cells in the auditory MGM, visual LD, and somatosensory Po, VM, and ZI (Fig. S3). The percentages of non-lemniscal auditory and non-matched sensory inputs drastically decreased (34.4 % and 3.1 %; Fig. 2C–D). At P15, the number of labeled neurons in auditory MGV, MGD, MGM, SG, and MZMG further increased, likewise in the non-matched nuclei Po, ZI, and LP/LD. At P21 and P28, the overall number of labeled somata in the matched and non-matched sensory thalamus was smaller than at P15. Because of the high inter-animal variability, possibly reflecting different gestational ages and animal differences in this outbred species, not all of the described developmental changes in the amount of labeled neurons reached significance in the non-parametric pairwise KS test (mainly due to the high variance across animals, for multivariate SRH analysis see below); however, the trends were obvious (Figs. 2B, S3). The small percentage of non-lemniscal auditory inputs remained largely constant between P9–21 and then increased towards P28 (47.8 %; Fig. 2C). The percentage of non-matched thalamic inputs remained largely constant in all that time (P9–28) having a value of 4.2% at P28 (Fig. 2D).
Following injections into S1 (hindlimb area HL), we also detected a large number of retrogradely labeled somata in ipsilateral sensory matched and non-matched thalamic nuclei (Fig. 2B red bars, Fig. S3 middle two panels). However, already at P1, we found numerous labeled neurons in the lemniscal (VPL/VPM) and non-lemniscal (VL, Po, VM, VA, ZI) nuclei of the somatosensory pathway, many labeled neurons in core (DLG) and non-core visual thalamic nuclei (LP, LD, LPMR), and some cells in non-lemniscal auditory nuclei (MGD, MGM, MZMG, SG) (Fig. S3). During the following 4 weeks, the overall number of labeled cells in the thalamus continuously decreased; however, there was a second peak of labeled non-matched thalamic nuclei at P15 (Fig. S3). The percentage of labeled non-lemniscal somatosensory nuclei had its maximum on P9 (76.0 %) and shifted towards 64.1 % at P28 (Fig. 2C). The percentage of non-matched inputs was very high at P1 (51.6 %) but then remained largely constant between P9–28 at a lower level (2.8 % at P28; Fig. 2D).
V1 Injections also revealed numerous retrogradely labeled somata in ipsilateral sensory matched and non-matched nuclei of the thalamus (Fig. 2B green bars, Fig. S3 lower two panels). At P1, the core visual nucleus DLG contained the highest number of labeled neurons, followed by the non-lemniscal visual nuclei (LP, LD) (Fig. S3). Until P28, the overall number of labeled cells in the visual thalamic nuclei decreased with varying courses for individual nuclei. There was again a peak of non-matched thalamic nuclei at P15 (Fig. S3). As for S1, the percentage of labeled non-lemniscal to lemniscal visual nuclei had its maximum on P9 (86.5 %) but was rather balanced at P28 (54.7 %; Fig. 2C). The ratio of non-matched to matched sensory thalamic inputs was always very low (1.5 % non-matched connections at P28; Fig. 2D).
Multivariate SHR analysis across the five experimental ages (P1, P9, P15, P21, P28) and the three different locations of the injection sites (A1, S1, V1) always revealed the experimental age as a significant factor (matched connections **p = 0.0039, non-matched *p = 0.0425; Tab. S3 upper panel). The location of the injection sites significantly interacted only with the development of the non-matched thalamocortical connections (*p = 0.0226).
Collectively, our results demonstrate that during the onset of function of the different sensory systems, matched and non-matched thalamocortical projections to primary sensory cortices overlap. This phase includes a transient increase of connections (in particular of non-matched connections around P15; Figs. 2D, S3) and ends with the onset of function of the last modality (vision). In the following phase, which ends with the weaning at P28, exuberant thalamocortical connections are pruned.
Nuclei of the non-lemniscal pathways have individual multisensory cortical projections
Our results show that several thalamic nuclei project to two or all three primary sensory cortices; however, these projections differed for the different thalamic nuclei and experimental ages. To identify the multisensory connectivity patterns of individual thalamic nuclei, we plotted the fraction of targets (A1, S1, V1) of each thalamic nucleus (Fig. 3A). Among the auditory thalamic nuclei, the non-lemniscal MGD projected predominantly to A1 at all ages. In contrast, between P1 and P9 a large fraction of projections from the other non-lemniscal nuclei of the auditory system (MGM, SG, MZMG) also targeted S1 and V1, with multisensory projections remaining from MGM (32.5 % of all outputs) and SG (30.6 %) at P28. The somatosensory non-lemniscal nuclei Po and VM were mostly unisensory, while ZI had a higher multisensory output ratio (28.1 % at P28). The core nucleus of the visual system (DLG) projected always to V1, except at P1, when approximately one third of the labeled neurons sent projections to S1 (Fig. S3 middle right panel). At P1, the non-lemniscal visual nuclei LD and LP (including LPMR) also had strong connections with S1 and A1, which remained for LP for the next 4 weeks (25.7 % at P28) (Fig. 3A).
Figure 3.
During early development, most cross-modal projections to A1, S1, and V1 arise from non-lemniscal thalamic nuclei and secondary sensory cortices.
(A) Fraction of retrogradely labeled cells projecting to A1, S1, and V1 in non-lemniscal sensory thalamic nuclei with substantial connections to two or all three primary sensory areas (normalized to 100 %), listed for all experimental ages. Listed next to the name of each structure is the total minimum and maximum number of labeled cells from all experimental cases per age used for the normalization.
(B) Fraction of connections of retrogradely labeled cells projecting to A1, S1, and V1 in other primary and secondary sensory cortices.
Altogether, most of the sensory thalamic nuclei with projections to multiple different primary cortices belong to the non-lemniscal sensory pathways (Figs. 3A, S3), indicating that they play a key role during multisensory development. In addition, the different projection patterns of the non-lemniscal nuclei suggest that they support different multisensory functions.
Direct connections between primary sensory areas emerge at P15
Our findings show extensive multisensory interconnections at the thalamic level, particularly in very young animals, and a progressive modality-specific refinement of wiring with the onset of sensory experience. Since multisensory processing can also be mediated by direct corticocortical connections between primary sensory areas (for review, e.g., Campi et al., 2010; Stehberg et al., 2014; Henschke et al., 2015; Meredith and Lomber, 2017), we next examined whether such early multisensory convergence and circuit refinement also occurred between primary sensory cortices (Figs. 2A, E, S4).
A1 injections resulted in retrogradely labeled neurons in infragranular layers (IGL) of S1 and V1 at experimental ages P15 (V1 only) and P28 (Fig. 2E blue bars, Fig. S4 upper left panel). In contrast, S1 injections (Fig. 2E red bars, Fig. S4 middle left panel) labeled neurons in IGL as well as supragranular layers (SGL) of V1 and in IGL of A1 from P15 on. At P15, most labeled neurons were in IGL of A1 (75.5 %); at P28, most labeled neurons were in SGL of V1 (83.0 %). Following V1 injections (Fig. 2E green bars, Fig. S4 lower left panel), we detected labeled neurons in IGL and SGL of S1 and A1. Connections from the IGL of A1 appeared first (appearing at P9 and peaking at P15), while S1 connections emerged at P28.
In summary, considerable multisensory corticocortical connections between primary cortical areas exist from P15 on; thus, later than multisensory thalamocortical connections. During the following weeks, both the corticocortical and thalamocortical connections are refined.
Extensive multisensory connections from secondary to primary areas emerge at P9
Higher order sensory areas, e.g., secondary sensory cortices, project to primary sensory areas of other modalities and provide a substrate for multisensory integration (e.g., Paperna and Malach, 1991; Cappe et al., 2009; Banks et al., 2011; Ibrahim et al., 2016). We therefore investigated if the development of these connections precedes or follows connections between primary areas (Figs. 2E–F, 3B, S4).
Following injections into A1, S1, or V1, we found retrogradely labeled neurons in non-matched secondary cortical areas from P9 on with generally higher numbers than for primary areas (Figs. 2E, S4). Labeled neurons were located in IGL and SGL and their numbers increased with age and reached their maximum between P15 and P21 (Fig. S4 right panels). Towards P28, they usually decreased.
Thus, multisensory corticocortical connectivity is present from P9 on. Since from P15 on, primary sensory areas also project across modalities, we investigated which of these pathways is dominant at each age. We plotted the fraction of primary and secondary connections and found that multisensory cortical inputs particularly into A1 and S1 were initially dominated by secondary areas but at P28, following a transient shift towards secondary areas after eye opening at P21, had generally shifted towards primary areas (Figs. 2F, 3B). Accordingly, the percentage of multisensory cortical inputs from secondary sensory areas to primary areas is 75.8 % for A1, 47.2 % for S1, and 59.3 % for V1 (Fig. 2F).
Multivariate SHR analysis across the five experimental ages and the three different locations of the injection sites revealed for connections with primary and secondary sensory cortices that mainly the experimental age (***p = 0.0016 and 0.0013) is the significant factor for the development of intracortical connections (Tab. S3 upper panel). The location of the injection sites only influences the connections between the primary areas themselves (*p = 0.0294).
Together our data demonstrate that connections from secondary areas are more numerous than those among primary areas and that they emerge later than the multisensory thalamocortical connections (P1) but earlier (P9) than connections among primary areas (P15). Both secondary and primary intracortical connections are most numerous around P15; afterwards, exuberant projections are eliminated and refined.
Loss of early sensory experience drastically increases the number of matched and non-matched thalamo- and corticocortical connections
Early sensory experience shapes the development of the matched thalamocortical and intracortical connections underlying a subsequently deprived sense (for review, e.g., Hensch, 2005; Sur and Rubenstein, 2005; Espinosa and Stryker, 2012; Mezzera and Lopez-Bendito, 2016). However, it is largely unknown if and how sensory experience shapes the matched and non-matched thalamocortical and corticocortical connections underlying the other (spared) senses and how experience shapes the non-matched connections of the deprived sense. In order to discern general principles we deprived one of the three main senses and compared the effects of each deprivation. Auditory deprivation was performed by bilateral ototoxic inner hair cell damage at P10, somatosensory deprivation by bilateral axotomy of the sciatic nerve at P5, and visual deprivation by bilateral enucleation at P10. Then, the connectivity patterns of A1, S1, and V1 were examined at P28, i.e., at the end of the last (visual) critical period (Figs. 4–5, S3–4).
Figure 5.
The loss of early sensory experience increases cross-modal connection of most non-lemniscal thalamic nuclei and shifts most cortical connections towards those from secondary areas.
(A) Fraction of retrogradely labeled cells projecting to A1, S1, and V1 in non-lemniscal sensory thalamic nuclei with substantial connections to two or all three primary sensory areas (normalized to 100 %) for normal P28 animals (same as in Fig. 3) and for animals following early loss of auditory, somatosensory, and visual experience. Listed next to the name of each structure is the total minimum and maximum number of labeled cells from all experimental cases per deprivation type used for the normalization.
(B) Fraction of connections of retrogradely labeled cells projecting to A1, S1, and V1 in other primary and secondary sensory cortices for normal P28 animals (same as in Fig. 3) and for animals following early loss of auditory, somatosensory, and visual experience.
Each of these deprivations induced a general increase in matched and non-matched thalamocortical connections, including connections to cortical areas of non-deprived senses (Figs. 4B, G, S3). The largest increase in thalamocortical connections was always seen after somatosensory deprivation (except for V1 after deafening). Generally, we noted differential changes on lemniscal and non-lemniscal pathways.
After auditory deprivation, the increase in matched inputs was due to rather equally higher non-lemniscal and lemniscal projections to A1 and S1 but relatively more non-lemniscal projections to V1 (Fig. 4C). The increase in non-matched thalamic inputs was due to additional projections from non-lemniscal thalamic nuclei of the other senses, for example, from Po, VM, and LP/LPMR to A1, LD/LP and non-lemniscal MGB-divisions to S1, and SG and VM to V1 (Fig. S3). Despite the increase of the number of non-matched thalamic inputs to A1, S1, and V1 after auditory deprivation (significant for S1 and V1; Fig. 4B) the overall percentage of non-matched inputs on all sensory thalamocortical connections increased only for S1, namely from 2.8 % to 14.4 % (Fig. 4D).
Somatosensory deprivation resulted in increased matched inputs to S1 from slightly more lemniscal sources, to V1 from slightly more non-lemniscal sources, and to A1 from equally more non-lemniscal and lemniscal sources (Fig. 4C). Again, the increase in non-matched thalamic projections arose largely from increased projections from non-lemniscal thalamic nuclei, e.g., VM and LP/LPMR to A1, MGB and LD/LPMR to S1, and SG, Po, and VL/VM to V1 (Fig. S3). The overall percentage of non-matched inputs increased for all three primary areas, namely for A1 from 4.2 % to 8.3 %, for S1 from 2.8 % to 8.4 %, and for V1 from 1.5 % to 6.2 % (Fig. 4D).
Following visual deprivation, additional matched inputs originated from rather equally higher non-lemniscal and lemniscal projections to S1 but more non-lemniscal projections to A1 and particularly to V1 (Fig. 4C). Additional non-matched thalamic projections came, among others, from Po, VM, and LP/LPMR to A1, LPMR and SG to S1, and SG, Po, and VL/VM to V1 (Fig. S3). The percentage of non-matched inputs increased only slightly for S1 (from 2.8 % to 4.5 %) but more for A1 (4.2 % to 9.4 %) and V1 (1.5 % to 5.3 %) (Fig. 4D).
Taken together, in deprived animals, many thalamic nuclei have more connections with the cortical area of their own (deprived) modality but also of other (spared) modalities compared to normally developing animals. The increase in thalamic projections is strongest for non-matched thalamic projections and for somatosensory deprivations and is most obvious in V1 (Figs. 4G, S3). Following deprivation, thalamic nuclei such as SG, Po, VM, and LD had more cortical projections across senses (Fig. 5A) indicating a specific recruitment of these nuclei.
We next investigated whether multisensory corticocortical connectivity was also affected by the loss of early sensory experiences. In general, we observed that sensory deprivation led to increased cortical connections from primary and secondary cortical areas (Figs. 4E, G, S4). Similar to its large effect on thalamocortical connections, somatosensory deprivations usually caused the largest increase in cortical projections (mainly from secondary areas) followed by auditory deprivations (mainly from primary areas). As a consequence of the areal and laminar-specific alteration of intracortical connectivity, the ratios of inputs from primary and secondary areas to A1, S1, and V1 changed, thereby shifting towards expanded inputs from secondary areas to A1 and S1, but not V1 (Figs. 4G, 5B).
Multivariate analysis across the three different deprivation types and three different locations of the injection sites revealed for thalamocortical and corticocortical connections that mainly the deprivation type is the significant factor for changing connectivity patterns (thalamocortical ***p = 0.0007 and **p = 0.0011, cortical: ***p = 0.0003 and ***p = 0.0004; Tab. S3 lower panel). The location of the injection site influences the matched thalamocortical connections and the intracortical connections among the primary areas (*p = 0.0462 and *p = 0.0342).
Together we find a general increase in multisensory thalamo- and corticocortical connectivity of the primary sensory cortices following early loss of sensory experiences. This increase is seen in all modalities and for all deprivations, is most pronounced for somatosensory deprivations, and is most evident in V1 (Fig. 4G). The changing ratios of lemniscal/primary sensory and non-lemniscal/secondary sensory inputs (Fig. 4C, F, 5) suggest the existence of a specific mechanism of cross-modal recruitment and compensatory plasticity.
The nature of intracortical connections changes after loss of early sensory experiences
Intracortical connections can originate in infra- (IGL) or supragranular layers (SGL) and these different pathways might signal different aspects of the sensory stimulus (Felleman and Van Essen, 1991; Rouiller et al., 1991). To investigate the changing patterns of multisensory cortical interactions following early sensory deprivation we calculated a layer index (L) (Fig. 6, Tab. S4), which provides (together with the laminar pattern of anterograde terminal labeling) basic information about the type of a projection, such as feedback (majority of labeled cells in IGL), feedforward (majority of labeled cells in SGL), or lateral (also termed intrinsic; equal numbers in IGL and SGL) (Cappe and Barone, 2005; Budinger et al., 2006; Masse et al., 2017).
In normal animals at P28, most connections between primary areas originate mainly from IGL, i.e., the layer indices are negative (L < −0.1), which is indicative for feedback connections (Fig. 6A). Exceptions are the connections from V1 to S1, which originate mainly in SGL (L > 0.1, indicative for feedforward connections). Projections from secondary areas could originate from either IGL (A2 to S1, A2 to V1, S2 to V1; feedback) or SGL (V2 to A1, V2 to S1; feedforward) or equally both (S2 to A1; −0.1 ≤L ≤ 0.1, lateral) (Fig. 6A).
In deprived animals, most of the intracortical connections originated mainly in the IGL (feedback), except for connections from S2 to A1, which were either still lateral or changed to feedforward (Fig. 6B). However, particularly in deaf and somatosensory deprived gerbils, the number of labeled cells in SGL increased for many projections, turning the layer indices towards more positive values (Fig. 6C). In blind gerbils, the projections from S1 and V1 to A1 disappeared; for most remaining connections the number of labeled cells in IGL increased or did not change (Fig. 6C).
Taken together this shows that the nature of cortical connections undergoes profound changes due to early loss of sensory experience. After deprivations, many intracortical connections are more formed by SGL neurons indicating that the connections switch back to a more feedforward-like, stimulus-driven status (e.g., Markov and Kennedy, 2013).
Changes in multisensory connectivity are due to axonal remodeling
The dynamically changing pattern of multisensory convergence during the development of normal and sensory deprived animals could be caused by multiple non-exclusive factors such as generation or elimination of projection neurons or extensive axonal remodeling such as sprouting or pruning (e.g., Innocenti and Price, 2005; Barnes and Finnerty, 2010). To test this we performed immunohistochemistry for markers of cell apoptosis (Cysteinyl-aspartate Specific Protease 3: CASP3; e.g., Roth et al., 2000), neurogenesis (Doublecortin: DCX; e.g., Gleeson et al., 1998), and axonal plasticity (Growth Associated Protein 43: GAP43; e.g., Benowitz and Routtenberg, 1997) (Fig. 7).
Only some CASP3+ neuronal somata were present in the sensory thalamic nuclei and cortical areas of normal and deprived animals (mostly at P1 and P9). None of these neurons was double-labeled with FG indicating that there was no apoptosis of sensory thalamic and cortical projecting neurons (Fig. 7A).
At P1, DCX-labeling showed a high intensity in fibers and cell bodies of the auditory, somatosensory, and visual thalamic nuclei and the primary sensory areas. Until P28, this labeling progressively decreased by 60.4 ± 8.3 % (lemniscal thalamic nuclei), 56.2 ± 9.4 % (non-lemniscal thalamic nuclei), and 56.3 ± 3.4 % (primary cortical areas), respectively (Fig. 7B–C). Since there was no co-localization of DCX and FG in particular neuronal somata (i.e., no double-labeled cells; Fig. 7A), neurons with projections across the modalities were not newly generated.
In contrast to the developmental decrease of DCX staining levels, GAP43 labeling (Fig. 7D–E) progressively increased in the gerbil's lemniscal/core and non-lemniscal/non-core thalamic nuclei and reached its maximum between P9 and P15 (increase of 26 – 116 % for lemniscal and 34 – 61 % for non-lemniscal thalamic nuclei). After P15, the GAP43-expression decreased in the lemniscal nuclei (17.1 ± 6.8 %) whereas the expression in non-lemniscal nuclei remained constantly high or increased even further until P28 (Fig. 7D). The different GAP43-labeling in lemniscal and non-lemniscal thalamic nuclei suggests a different capacity for neuronal plasticity of the lemniscal and non-lemniscal pathway during early development. Whereas this capacity apparently decreases for the lemniscal part of the thalamus, it increases for its non-lemniscal part. In primary sensory cortices, the staining intensity of GAP43 in all three areas was lowest at P1 but increased significantly until P15 (by 54.3 ± 12.8 %); before remaining constant (Fig. 7E).
Sensory deprivations did not change the expression of DCX neither in thalamus (except for VPM/VPL after auditory deprivation; Fig. 7B) or cortex (Fig. 7C). Likewise, GAP43 levels remained constant or slightly increased following sensory deprivation (Fig. 7D–E).
Thus, axonal reorganization processes likely underlie the observed changes in multisensory connectivity during early (normal) development and following early sensory deprivation. Decreasing and increasing numbers of thalamocortical and corticocortical connections can be particularly explained by tangential pruning or sprouting of axonal branches (i.e., across cortical columns and areas), respectively; differences in the laminar distribution of retrogradely cells in the cortex (see layer index) by radial (i.e., across layers, within a column) and/or tangential remodeling of axons. The high concentration of GAP43 in the non-lemniscal thalamic nuclei and primary areas from P15 on suggests a higher potential for axonal remodeling in these regions and might be a major source for cross-modal plasticity during early developmental phases.
Altered sensory-evoked functional connectivity after deprivations reflect increased anatomical connectivity
We have shown that the loss of early sensory experience leads to an increase of multisensory thalamo- and corticocortical connections of the primary sensory cortices. To test how this altered connectivity patterns affect the neuronal processing in the deprived and spared sensory pathways and in particular the functional connectivity between non-deprived and deprived sensory areas we performed SPECT-imaging of regional cerebral blood flow (rCBF) using the flow tracer 99mTechnetium hexamethylpropyleneamine oxime (99mTc-HMPAO). This approach allows the in vivo imaging of brain-wide spatial patterns of neural activity in freely behaving animals (Kolodziej et al., 2014; Bhattacharya et al., 2017). Since we observed the most profound changes in anatomical connectivity after somatosensory deprivations (Figs. 4, S3–4) we compared brain activity to visual and acoustic stimuli (light flashes and noise bursts) in 28 days old normal and somatosensory deprived gerbils.
Upon sensory stimulation, normal and somatosensory deprived animals showed an increased rCBF compared to baseline in the matched sensory pathway but also in structures of the unmatched pathways (Fig. 8, Tab. S5). This was most evident in voxelwise but also VOI-based analysis (Fig. 8A–D); however, deprived animals showed reduced stimulus-induced activation in many of these structures compared to normal animals. Following visual stimulation, this was mainly seen in cortical and subcortical areas of the deprived somatosensory pathway (averaged rCBF decrease of 180 % across all investigated somatosensory structures) but also in most of the spared auditory (−28 %, without right MGB) and visual (−66 %, without left LD/LP, right DLG) cortical and subcortical areas (Fig. 8E). Notable exceptions were some of the spared thalamic nuclei (e.g., LD/LP on the left side, MGB and DLG on the right side; Tab. S5). Likewise, following auditory stimulation, most areas of the deprived somatosensory (−112 %) and spared auditory (−70 %) and visual (−119 %, without V2) pathways displayed a reduced increase in rCBF. Notably, V2 (bilateral) showed a higher rCBF in deprived animals upon acoustic stimulation (Fig. 8F, Tab. S5).
Figure 8.
Stimulus-induced changes in regional cerebral blood flow in sensory cortices differ between normal and somatosensory deprived gerbils.
(A) Mean 99mTc-HMPAO uptake in normal (left) and somatosensory deprived animals (somat; right) under baseline condition (no stimulation) and upon visual stimulation (v-stim). Frontal hemisections through V1 of the right hemisphere (R); normal and somat were aligned at the midline for better comparison. Data are global mean normalized; images are pseudo-colored and fused with a standard MRI of a P28 gerbil for anatomical reference.
(B) Bar graphs for mean tracer uptake in both groups as in A (mean ± 1 SEM; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, Student's t-test).
(C) Difference map of tracer uptake upon stimulation minus baseline (percentage change relative to global mean). Warm colors indicate stimulus-induced increases and cold colors decreases in tracer content relative to baseline conditions. Probability map shows areas of significant differences between both groups (blue: lower stimulus-induced increase or even stimulus-induced decrease in somat compared to normal).
(D) Bar graphs for baseline-corrected mean tracer uptake in both groups as in C.
(E–F) Difference and p-maps upon visual (E) and acoustic (F) stimulation. Conventions as in A and C. Numbers indicate position rostral (+) or caudal (−) to bregma. For further information see Tab. S5.
(G) The linear correlation of tracer content in V1 and S1-hindlimb (HL) area (left hemisphere) is increased in somat (R2 = 0.638) compared to normal animals (R2 = 0.004). See also Tab. S6.
(H) There is a strong linear correlation between the changing anatomical and functional connectivity of primary (psc) and secondary (ssc) sensory cortices in somat. Plotted is the changing, i.e., increasing overall (IGL+SGL) number of neurons connecting psc among each other (blue dots) as well as ssc with psc (green dots) versus the changing, i.e., usually increasing functional connectivity between cortical areas (left hemisphere). Values were derived from Fig. S4 and Tab. S6.
All values are derived from n = 10 normal and n = 10 somat animals.
Since our anatomical measures indicated an altered amount of connectivity, we next sought to confirm that these anatomical rearrangements had functional consequences. We thus analyzed the covariance (correlation) of activity between selected areas upon visual and acoustic stimulation, which is a measure of functional connectivity (e.g., Horwitz et al., 1999) and whether this connectivity would be altered after sensory loss (Fig. 8G, Tab. S6). Generally, the functional connectivity (R2 coefficients) was stronger (i.e., R2 higher) between structures belonging to the same sensory pathway than across modalities. However, specifically the functional connectivity between primary sensory areas was stronger in somatosensory deprived animals than in normal animals. Only a few percent of the stimulus evoked activity in primary sensory cortices of normal animals but up to 64 % of the stimulus evoked activity in the respective areas of somatosensory deprived animals (e.g., R2 for left hemispheric V1/S1-HL upon visual stimulation = 0.638; Tab. S6) could be attributed to direct (linear) correlation (Fig. 8G). In deprived animals, functional connectivity also increased between primary and many secondary areas (e.g., between A1 and S2 upon both stimulations up to R2 = 0.80; Tab. S6). Covariance of activity between secondary areas themselves and between thalamic nuclei and cortical areas did not change in a systematic manner following sensory deprivation.
The changes in functional connectivity mirror our anatomical observations, e.g., that the increased functional connectivity between cortical areas in deprived animals directly correlates with the increased number of connections between them (anatomical connectivity). As the plot of the changing number of projection neurons versus the changing functional connectivity shows (Fig. 8H) this correlation is stronger for connections between primary cortices (R2 = 0.878) than for connections arising from secondary cortices (R2 = 0.228).
Collectively, our SPECT results show that in somatosensory deprived gerbils stimulus-evoked activity in most structures of the deprived and spared sensory pathways is altered upon sensory stimulation (mainly lower than in normal) and the functional connectivity specifically to primary cortices is enhanced.
Discussion
We demonstrate here that during early development, substantial multisensory thalamocortical and intracortical connections to the primary sensory areas exist and that this development can be altered by early sensory loss. This sensory loss has a dramatic effect not only on the pathways underlying the deprived sense but also on pathways serving the non-deprived senses enabling functional recruitment of deprived cortical areas by the spared senses.
We find that during normal development thalamocortical connections to the matched as well to as non-matched primary sensory cortical areas develop first followed by the development of multisensory corticocortical connections between secondary and primary areas and primary areas themselves. Since the three senses develop sequentially, e.g., somatosensory before auditory and vision (gerbil: Bolles and Woods, 1964; Cabana et al., 1993; Mowery et al., 2016), S1 has most matched and non-matched thalamic inputs shortly after birth. Given that sensory systems are active together during development, the eventual connectivity is likely governed by the changing functional competition between sensory inputs during the earliest postnatal developmental phase (for review, e.g., Hensch, 2004; Hensch, 2005). Subsequently, when all three systems are active, a rapid consolidation of unisensory and multisensory pathways takes place characterized by a reduction of exuberant thalamocortical and corticocortical connections. Importantly, our results show that non-lemniscal thalamocortical pathways as well as secondary sensory cortices are the main sources of early multisensory convergence in primary areas. Given that the non-lemniscal identity seems to be the default identity of thalamic neurons (Pouchelon et al., 2014) this suggests that early large-scale cross-connectivity between thalamic and cortical areas that later serve multisensory processing might be the default function of brain networks and that this cross-modal connectivity gets refined by the onset of different sensory experiences.
Early loss of one of the three senses revealed altered thalamocortical and corticocortical connections. The overall number of these connections increases in particular for non-matched connections and especially for those arising from non-lemniscal thalamic nuclei and secondary sensory cortices. Moreover, we find that not only does the number of connections between cortical areas change after sensory deprivations but that the laminar source of intracortical connections changes as well. Corticocortical forward (ascending) connections largely originate in SGL and terminate in granular layer IV, whereas corticocortical feedback (descending) connections originate in IGL and terminate in upper SGL and lower IGL; lateral (intrinsic) connections originate in SGL and IGL and terminate in a columnar fashion across all six cortical layers (Felleman and Van Essen, 1991; Rouiller et al., 1991). Our results on the laminar distribution of retrogradely labeled neurons show, for example, that auditory and somatosensory deprivations result in an increased fraction of projection neurons being located in the SGL than the IGL. Thus, the intracortical connections after such deprivations are now more feedforward suggesting an increased driving (bottom-up) function as compared to dominant IGL (feedback) connections that have a more modulatory (top-down) role (e.g., Markov and Kennedy, 2013; Laramee and Boire, 2014). Interareal sensory pathways initially originate in the SGL and are then remodeled to IGL connections during subsequent development (e.g., Burkhalter, 1993; Batardiere et al., 2002; Berezovskii et al., 2011; Ko et al., 2013); therefore, our findings suggest that deprivations result in immature cortical connectivity between the deprived and non-deprived cortical areas. Altogether, our results show that both thalamocortical and corticocortical alterations might underlie the functional cross-modal recruitment of the deprived and compensatory plasticity of the spared pathways (for review, e.g., Rauschecker, 1995; Pallas, 2001; Bavelier and Neville, 2002; Lee and Whitt, 2015).
The development of early sensory thalamo- and corticocortical connections is not only driven by external stimulus-induced but also by preceding endogenous non-sensory activity (e.g., spontaneous cochlear or retinal waves; Katz and Shatz, 1996; Price et al., 2006; Blankenship and Feller, 2010; Kilb et al., 2011; Hanganu-Opatz et al., 2015). We deprived animals shortly after (somatosensory), shortly before (auditory), and in the midterm before (vision) onset of sensory experience. As a consequence, the formation of connections by preceding spontaneous activity was less complete in the auditory and visual system at the time point of sensory organ deafferentiation (Kotak et al., 2012). This might explain, for example, why effects of somatosensory and auditory deprivation are strongest for the development of connections of the visual system (V1), which is the latest in development and thus most susceptible for plastic changes. Likewise, we find that somatosensory deprivation leads to the highest and visual deprivation to the least increase of matched and non-matched sensory thalamo- and corticocortical connections; therefore, we conclude that with this respect external stimulus-induced activity is most important for unisensory and multisensory sensory pathway formation.
Our results provide evidence that the changes in the thalamo- and corticcortical connectivity patterns, both during normal development and following sensory deprivation, are not due to an apoptosis nor neurogenesis of thalamic and cortical projection neurons but rather to axonal reorganization processes. Increasing numbers of retrogradely labeled thalamocortical and corticocortical neurons might be a particular result of tangential (i.e., across cortical columns and areas) sprouting and growth of their axonal branches including the genesis of new synapses, a changing laminar distribution of labeled cortical neurons may additionally comprise radially orientated (i.e., across layers, within a column) modifications of their axons, all having a variety of molecular mechanisms behind (for review, e.g., Innocenti and Price, 2005; Price et al., 2006; Barnes and Finnerty, 2010; Tropea et al., 2009). The abundance of GAP43, which is highly expressed in neuronal growth cones (e.g., Benowitz and Routtenberg, 1997), in non-lemniscal thalamic nuclei and primary sensory areas indicates that these structures mediate most of the axonal remodeling. Our findings of increased connectivities following early sensory deprivation correlate with the increased density of granular and supragranular dendritic spines, i.e., of the putative targets of enhanced cross-modal thalamic and cortical inputs, in auditory cortex of early blinded or somatic deafferented young rats (Ryugo et al., 1975) and early deafened (but) adult cats (Clemo et al., 2016).
Consistent with our anatomical observations SPECT-imaging shows that in deprived animals stimulus-driven activity is altered and functional connectivity specifically to primary cortices is increased. Since responses to visual and acoustic stimuli decreased in most sensory structures, but the cross-modal inputs to these areas and their functional connectivity increased, the functional anatomical changes may represent a particular form of input refinement into specific subcircuits of the target areas. From the anatomical point of view, a reduced number of postsynaptic targets (i.e., spines of the recipient cells) could explain the reduced activity to intra- and cross-modal sensory stimulation. Indeed, preliminary results from our laboratory show a reduced spine density of apical and basal dendrites of supragranular pyramidal cells in A1, S1, and V1 of sensorily deprived animals compared to normal animals (P28 gerbils; Macharadze et al., in prep.). Another explanation for the reduced activity in deprived animals might be the involvement of specific inhibitory subcircuits in the target areas of cross-modal inputs. This notion is supported by studies in mouse V1, where sound stimulation drives in particular local GABAergic inhibition via direct cross-modal projections from A1, resulting in a reduced overall spike activity and sharpened neuronal orientation preference to concurrent visual stimuli (Iurilli et al., 2012; Ibrahim et al., 2016). Similar to our observations, humans, who experienced a transient period of blindness, show a reduced activation to noise and a subsequent more efficient cortical processing of acoustic stimuli in the auditory cortex than control subjects do (Guerreiro et al., 2016). However, the reduced and probably refined activity seen in our deprived animals at the end of the critical period, could during later development potentially turn into enhanced responses to non-matched stimuli in the deprived sensory pathways as seen in auditory, somatosensory, and visual cortex of several species (e.g., hamster: Izraeli et al., 2002; mouse: Teichert and Bolz, 2017; rat: Piche et al., 2007; ferret: Allman et al., 2009; cat: Meredith and Lomber, 2011). Longitudinal studies, ranging from early postnatal age to adults, may shed further light on that issue.
In adult humans and animals, the loss of a sensory modality leads to functional improvements of remaining senses (for review, e.g., Merabet and Pascual-Leone, 2010; Frasnelli et al., 2011; Renier et al., 2014) including the remarkable echolocalization ability of some blind people (for review, e.g., Kolarik et al., 2014). Several neuronal mechanisms have been suggested to drive this cross-modal (or compensatory) plasticity such as the formation of new pathways or unmasking, strengthening, or remodeling of existing connections (for review e.g., Bavelier and Neville, 2002; Feldman and Brecht, 2005; Barnes and Finnerty, 2010; Kupers and Ptito, 2014). Sensory deprivations in adults can strengthen thalamocortical synapses as well as refine and strengthen intracortical connections of the spared sense (Yu et al., 2012; Klinge et al., 2010; Petrus et al., 2014; Meng et al., 2015; Petrus et al., 2015). In contrast, studies investigating cross-modal connectivity of primary cortices in adult animals following early or congenital sensory loss, draw a conflicting picture of the underlying mechanisms. On the one hand, bilateral neonatal enucleation in mice (Charbonneau et al., 2012) and early deafening (Sanchez-Vives et al., 2006; Meredith and Allman, 2012; Chabot et al., 2015) or congenital deafness (Barone et al., 2013) in cats and ferrets result in only subtle changes of matched and non-matched sensory connections of V1 and A1 when compared to normal individuals. In contrast, adult opossums, which were bilaterally enucleated at early age, show an increase of cross-modal thalamocortical and corticocortical connections of V1 (Karlen et al., 2006). The differences between these studies might be due to timing and severity of the deprivations (e.g., onset, duration, transient, permanent), peculiarities within similar systems (e.g., barrel system versus hindlimb system), or species difference (e.g., developmentally, behaviorally). As a further example and in agreement with our study, which shows a transient peak of non-pruned multimodal thalamocortical and corticocortical connections for the gerbil's A1, S1-HL, and V1 at P15 during normal development, a recent study in rats demonstrated much more projections from V1 to S1-BF (barrel field) at P14 than in adult animals (Bieler et al., 2017). However, a decrease of these projections due to a transient whisker-trimming (P0–P5), compared between transiently deprived and control animals around P20 (Sieben et al., 2015), is in contrast to our findings of generally increasing multisensory connections due to (permanent) deprivation, but actually coincides with the general pruning of these connections towards the end of the critical sensory period. Thus, the interplay between a transient neonatal deprivation, a recovering of stimulus-induced whisker activity, and a general pruning of multisensory connections towards the end of the critical period obviously causes very subtle changes of the connectivity patterns, which appear contradictory at the first glance but indeed are compatible. Altogether, collective results of detailed comparative studies highlight the importance and translational gain of various sensory systems and animal models (see also Meredith and Lomber, 2017).
We show that early sensory loss causes an upregulation of multisensory connections at the end of the critical period, which might be the prerequisite for superior abilities of the remaining senses in adult patients. Thus, our study has translational implications for the management of early acquired or congenital deafness (for review, e.g., Kral and Sharma, 2012), blindness (for review, e.g., Renier et al., 2014; Nys et al., 2015), and sensory paralysis (for review, e.g., Kaas and Collins, 2003; Navarro et al., 2007). Therapeutic approaches that focus on the survival and viability of early multisensory connections at the end of the critical period, for example, by multisensory perceptual training (e.g., Proulx et al., 2014; McGovern et al., 2016), sensory substitution (e.g., Stiles and Shimojo, 2015; Maidenbaum et al., 2014), or, though still prospectively, manipulation of the molecular background (e.g., Berardi et al., 2003; Tropea et al., 2009; Hubener and Bonhoeffer, 2014), might greatly improve the clinical outcome.
Our study might also provide mechanistic insights into unique perceptual phenomena such as synesthesia in particular developmental synesthesia for which competing theories about its origin exist. The cross-activation hypothesis posits that widespread multisensory connections, underlying multisensory associations, are present in early life in human infants (Spector and Maurer, 2009; Ward, 2013), but then are incompletely pruned during maturation (Hubbard et al., 2011). The alternative disinhibited feedback (Grossenbacher and Lovelace, 2001) and re-entrant feedback hypothesis (Smilek et al., 2001) posits that adult synesthetes show a lack of inhibition of normal multisensory connections. Our developmental and deprivation data support the cross-activation theory. Notably, we show that early multisensory connections can be "preserved" or "reactivated" after the loss of a sense and may then very well lead to acquired forms of synesthesia (Afra et al., 2009).
Conclusions
Our study demonstrates that during normal development intracortical multisensory connections are formed as a consequence of sensory driven multisensory thalamocortical activity. After early sensory loss, the functional recruitment of a deprived cortical territory by spared senses is due to changes in the development of sensory thalamocortical and corticocortical connections; non-lemniscal thalamic nuclei and secondary sensory cortices play a pivotal role in this process.
Supplementary Material
Acknowledgments
We like to thank K. Böttger, A. Gürke, D. Montag, J. Stallmann, and D. Vincenz-Zörner for excellent technical assistance. We also thank the three anonymous reviewers for their most helpful comments on the manuscript. This work was supported by the DFG (www.dfg.de) SFB TRR31 (J.U.H., F.W.O., E.B.) and NIH (www.nih.gov) RO1 DC009607 (P.O.K.).
Footnotes
Informed consent
Informed consent was obtained from all individual participants included in the study.
Competing interests
All authors declare that they have no financial, personal, or professional conflict of interest.
Ethical standard
Authors declare that all animal studies have been approved by the appropriate ethics committee (see “Materials and methods”) and have, therefore, been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
References
- Afra P, Funke M, Matsuo F. Acquired auditory-visual synesthesia: A window to early cross-modal sensory interactions. Psychol Res Behav Manag. 2009;2:31–37. doi: 10.2147/prbm.s4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allman BL, Keniston LP, Meredith MA. Adult deafness induces somatosensory conversion of ferret auditory cortex. Proc Natl Acad Sci U S A. 2009;106(14):5925–5930. doi: 10.1073/pnas.0809483106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwell KWS, Paxinos G. Atlas of the rat developing nervous system. Academic Press; San Diego: 2008. [Google Scholar]
- Banks MI, Uhlrich DJ, Smith PH, Krause BM, Manning KA. Descending projections from extrastriate visual cortex modulate responses of cells in primary auditory cortex. Cereb Cortex. 2011;21(11):2620–2638. doi: 10.1093/cercor/bhr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes SJ, Finnerty GT. Sensory experience and cortical rewiring. Neuroscientist. 2010;16(2):186–198. doi: 10.1177/1073858409343961. [DOI] [PubMed] [Google Scholar]
- Barone P, Lacassagne L, Kral A. Reorganization of the connectivity of cortical field DZ in congenitally deaf cat. PLoS ONE. 2013;8(4):e60093. doi: 10.1371/journal.pone.0060093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batardiere A, Barone P, Knoblauch K, Giroud P, Berland M, Dumas AM, Kennedy H. Early specification of the hierarchical organization of visual cortical areas in the macaque monkey. Cereb Cortex. 2002;12(5):453–465. doi: 10.1093/cercor/12.5.453. [DOI] [PubMed] [Google Scholar]
- Bavelier D, Neville HJ. Cross-modal plasticity: where and how? Nat Rev Neurosci. 2002;3(6):443–452. doi: 10.1038/nrn848. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, Routtenberg A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997;20(2):84–91. doi: 10.1016/s0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- Berardi N, Pizzorusso T, Ratto GM, Maffei L. Molecular basis of plasticity in the visual cortex. Trends Neurosci. 2003;26(7):369–378. doi: 10.1016/S0166-2236(03)00168-1. [DOI] [PubMed] [Google Scholar]
- Berezovskii VK, Nassi JJ, Born RT. Segregation of feedforward and feedback projections in mouse visual cortex. J Comp Neurol. 2011;519(18):3672–3683. doi: 10.1002/cne.22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S, Herrera-Molina R, Sabanov V, Ahmed T, Iscru E, Stober F, Richter K, Fischer KD, Angenstein F, Goldschmidt J, Beesley PW, Balschun D, Smalla KH, Gundelfinger ED, Montag D. Genetically Induced Retrograde Amnesia of Associative Memories After Neuroplastin Ablation. Biol Psychiatry. 2017;81(2):124–135. doi: 10.1016/j.biopsych.2016.03.2107. [DOI] [PubMed] [Google Scholar]
- Bieler M, Sieben K, Schildt S, Roder B, Hanganu-Opatz IL. Visual-tactile processing in primary somatosensory cortex emerges before cross-modal experience. Synapse. 2017;71(6) doi: 10.1002/syn.21958. [DOI] [PubMed] [Google Scholar]
- Bizley JK, Jones GP, Town SM. Where are multisensory signals combined for perceptual decision-making? Curr Opin Neurobiol. 2016;40:31–37. doi: 10.1016/j.conb.2016.06.003. [DOI] [PubMed] [Google Scholar]
- Blankenship AG, Feller MB. Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat Rev Neurosci. 2010;11(1):18–29. doi: 10.1038/nrn2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolles RC, Woods PJ. The ontogeny of behaviour in the albino rat. Animal Behaviour. 1964;12(4):427–441. [Google Scholar]
- Budinger E, Heil P, Hess A, Scheich H. Multisensory processing via early cortical stages: Connections of the primary auditory cortical field with other sensory systems. Neuroscience. 2006;143(4):1065–1083. doi: 10.1016/j.neuroscience.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Budinger E, Scheich H. Anatomical connections suitable for the direct processing of neuronal information of different modalities via the rodent primary auditory cortex. Hear Res. 2009;258(1–2):16–27. doi: 10.1016/j.heares.2009.04.021. [DOI] [PubMed] [Google Scholar]
- Burkhalter A. Development of forward and feedback connections between areas V1 and V2 of human visual cortex. Cereb Cortex. 1993;3(5):476–487. doi: 10.1093/cercor/3.5.476. [DOI] [PubMed] [Google Scholar]
- Cabana T, Cassidy G, Pflieger JF, Baron G. The ontogenic development of sensorimotor reflexes and spontaneous locomotion in the Mongolian gerbil (Meriones unguiculatus) Brain Res Bull. 1993;30(3–4):291–301. doi: 10.1016/0361-9230(93)90257-c. [DOI] [PubMed] [Google Scholar]
- Cahill L, Ohl F, Scheich H. Alteration of auditory cortex activity with a visual stimulus through conditioning: a 2-deoxyglucose analysis. Neurobiol Learn Mem. 1996;65:213–222. doi: 10.1006/nlme.1996.0026. [DOI] [PubMed] [Google Scholar]
- Campi KL, Bales KL, Grunewald R, Krubitzer L. Connections of auditory and visual cortex in the prairie vole (Microtus ochrogaster): evidence for multisensory processing in primary sensory areas. Cereb Cortex. 2010;20(1):89–108. doi: 10.1093/cercor/bhp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappe C, Barone P. Heteromodal connections supporting multisensory integration at low levels of cortical processing in the monkey. Eur J Neurosci. 2005;22(11):2886–2902. doi: 10.1111/j.1460-9568.2005.04462.x. [DOI] [PubMed] [Google Scholar]
- Cappe C, Morel A, Barone P, Rouiller EM. The thalamocortical projection systems in primate: an anatomical support for multisensory and sensorimotor interplay. Cereb Cortex. 2009;19(9):2025–2037. doi: 10.1093/cercor/bhn228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabot N, Butler BE, Lomber SG. Differential Modification of Cortical and Thalamic Projections to Cat Primary Auditory Cortex Following Early- and Late-Onset Deafness. J Comp Neurol. 2015;523(15):2297–2320. doi: 10.1002/cne.23790. [DOI] [PubMed] [Google Scholar]
- Chabot N, Robert S, Tremblay R, Miceli D, Boire D, Bronchti G. Audition differently activates the visual system in neonatally enucleated mice compared with anophthalmic mutants. Eur J Neurosci. 2007;26(8):2334–2348. doi: 10.1111/j.1460-9568.2007.05854.x. [DOI] [PubMed] [Google Scholar]
- Charbonneau V, Laramee ME, Boucher V, Bronchti G, Boire D. Cortical and subcortical projections to primary visual cortex in anophthalmic, enucleated and sighted mice. Eur J Neurosci. 2012;36(7):2949–2963. doi: 10.1111/j.1460-9568.2012.08215.x. [DOI] [PubMed] [Google Scholar]
- Clemo HR, Lomber SG, Meredith MA. Synaptic Basis for Cross-modal Plasticity: Enhanced Supragranular Dendritic Spine Density in Anterior Ectosylvian Auditory Cortex of the Early Deaf Cat. Cereb Cortex. 2016;26(4):1365–1376. doi: 10.1093/cercor/bhu225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowry G, Molnar Z, Rakic P. Renewed focus on the developing human neocortex. J Anat. 2010;217(4):276–288. doi: 10.1111/j.1469-7580.2010.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver J, Noesselt T. Multisensory interplay reveals crossmodal influences on 'sensory-specific' brain regions, neural responses, and judgments. Neuron. 2008;57(1):11–23. doi: 10.1016/j.neuron.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret G. Development of absolute auditory thresholds in the house mouse (Mus musculus) J Am Audiol Soc. 1976;1(5):179–184. [PubMed] [Google Scholar]
- Elwood RW, Broom DM. The influence of litter size and parental behaviour on the development of Mongolian gerbil pups. Animal Behaviour. 1978;26(Part 2(0)):438–454. [Google Scholar]
- Espinosa JS, Stryker MP. Development and plasticity of the primary visual cortex. Neuron. 2012;75(2):230–249. doi: 10.1016/j.neuron.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE, Brecht M. Map plasticity in somatosensory cortex. Science. 2005;310(5749):810–815. doi: 10.1126/science.1115807. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1(1):1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Finck A, Schneck CD, Hartman AF. Development of cochlear function in the neonate Mongolian gerbil (Meriones unguiculatus) J Comp Physiol Psychol. 1972;78(3):375–380. doi: 10.1037/h0032373. [DOI] [PubMed] [Google Scholar]
- Finney EM, Fine I, Dobkins KR. Visual stimuli activate auditory cortex in the deaf. Nat Neurosci. 2001;4(12):1171–1173. doi: 10.1038/nn763. [DOI] [PubMed] [Google Scholar]
- Frasnelli J, Collignon O, Voss P, Lepore F. Crossmodal plasticity in sensory loss. Prog Brain Res. 2011;191:233–249. doi: 10.1016/B978-0-444-53752-2.00002-3. [DOI] [PubMed] [Google Scholar]
- Fuller JL, Wimer RE. Biology of the Laboratory Mouse. McGraw-Hill; New York: 1966. Neural, sensory, and motor functions; pp. 609–628. [Google Scholar]
- Ghoshal A, Tomarken A, Ebner F. Cross-sensory modulation of primary sensory cortex is developmentally regulated by early sensory experience. J Neurosci. 2011;31(7):2526–2536. doi: 10.1523/JNEUROSCI.5547-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielen SC, Schmidt RA, Van den Heuvel PJ. On the nature of intersensory facilitation of reaction time. Percept Psychophys. 1983;34(2):161–168. doi: 10.3758/bf03211343. [DOI] [PubMed] [Google Scholar]
- Gleeson JG, Allen KM, Fox JW, Lamperti ED, Berkovic S, Scheffer I, Cooper EC, Dobyns WB, Minnerath SR, Ross ME, Walsh CA. Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell. 1998;92(1):63–72. doi: 10.1016/s0092-8674(00)80899-5. [DOI] [PubMed] [Google Scholar]
- Gleiss S, Kayser C. Audio-visual detection benefits in the rat. PLoS ONE. 2012;7(9):e45677. doi: 10.1371/journal.pone.0045677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gougoux F, Lepore F, Lassonde M, Voss P, Zatorre RJ, Belin P. Neuropsychology: pitch discrimination in the early blind. Nature. 2004;430(6997):309. doi: 10.1038/430309a. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Witter MP. Thalamus. In: Paxinos G, editor. The rat nervous system. San Diego: Elsevier Academic Press; 2004. pp. 407–453. [Google Scholar]
- Grossenbacher PG, Lovelace CT. Mechanisms of synesthesia: cognitive and physiological constraints. Trends Cogn Sci. 2001;5(1):36–41. doi: 10.1016/s1364-6613(00)01571-0. [DOI] [PubMed] [Google Scholar]
- Guerreiro MJ, Putzar L, Roder B. The Effect of Early Visual Deprivation on the Neural Bases of Auditory Processing. J Neurosci. 2016;36(5):1620–1630. doi: 10.1523/JNEUROSCI.2559-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond-Kenny A, Bajo VM, King AJ, Nodal FR. Behavioural benefits of multisensory processing in ferrets. Eur J Neurosci. 2017;45(2):278–289. doi: 10.1111/ejn.13440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanganu-Opatz IL, Rowland BA, Bieler M, Sieben K. Unraveling Cross-Modal Development in Animals: Neural Substrate, Functional Coding and Behavioral Readout. Multisens Res. 2015;28(1–2):33–69. doi: 10.1163/22134808-00002477. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6(11):877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Henschke JU, Noesselt T, Scheich H, Budinger E. Possible anatomical pathways for short-latency multisensory integration processes in primary sensory cortices. Brain Struct Funct. 2015;220(2):955–977. doi: 10.1007/s00429-013-0694-4. [DOI] [PubMed] [Google Scholar]
- Heydt JL, Cunningham LL, Rubel EW, Coltrera MD. Round window gentamicin application: an inner ear hair cell damage protocol for the mouse. Hear Res. 2004;192(1–2):65–74. doi: 10.1016/j.heares.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Tagamets MA, McIntosh AR. Neural modeling, functional brain imaging, and cognition. Trends Cogn Sci. 1999;3(3):91–98. doi: 10.1016/s1364-6613(99)01282-6. [DOI] [PubMed] [Google Scholar]
- Hubbard EM, Brang D, Ramachandran VS. The cross-activation theory at 10. J Neuropsychol. 2011;5(2):152–177. doi: 10.1111/j.1748-6653.2011.02014.x. [DOI] [PubMed] [Google Scholar]
- Hubener M, Bonhoeffer T. Neuronal plasticity: beyond the critical period. Cell. 2014;159(4):727–737. doi: 10.1016/j.cell.2014.10.035. [DOI] [PubMed] [Google Scholar]
- Ibrahim LA, Mesik L, Ji XY, Fang Q, Li HF, Li YT, Zingg B, Zhang LI, Tao HW. Cross-Modality Sharpening of Visual Cortical Processing through Layer-1-Mediated Inhibition and Disinhibition. Neuron. 2016;89(5):1031–1045. doi: 10.1016/j.neuron.2016.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti GM, Price DJ. Exuberance in the development of cortical networks. Nat Rev Neurosci. 2005;6(12):955–965. doi: 10.1038/nrn1790. [DOI] [PubMed] [Google Scholar]
- Iurilli G, Ghezzi D, Olcese U, Lassi G, Nazzaro C, Tonini R, Tucci V, Benfenati F, Medini P. Sound-driven synaptic inhibition in primary visual cortex. Neuron. 2012;73(4):814–828. doi: 10.1016/j.neuron.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izraeli R, Koay G, Lamish M, Heicklen-Klein AJ, Heffner HE, Heffner RS, Wollberg Z. Cross-modal neuroplasticity in neonatally enucleated hamsters: structure, electrophysiology and behaviour. Eur J Neurosci. 2002;15(4):693–712. doi: 10.1046/j.1460-9568.2002.01902.x. [DOI] [PubMed] [Google Scholar]
- Jacobs GH, Deegan JF., 2nd Sensitivity to ultraviolet light in the gerbil (Meriones unguiculatus): characteristics and mechanisms. Vision Res. 1994;34(11):1433–1441. doi: 10.1016/0042-6989(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Jones E. Principles of Thalamic Organization. In: Jones E, editor. The Thalamus. Cambridge: Cambridge University Press; 2007. pp. 87–170. [Google Scholar]
- Kaas JH, Collins CE. Anatomic and functional reorganization of somatosensory cortex in mature primates after peripheral nerve and spinal cord injury. Adv Neurol. 2003;93:87–95. [PubMed] [Google Scholar]
- Kanold PO, Luhmann HJ. The subplate and early cortical circuits. Annu Rev Neurosci. 2010;33:23–48. doi: 10.1146/annurev-neuro-060909-153244. [DOI] [PubMed] [Google Scholar]
- Karlen SJ, Kahn DM, Krubitzer L. Early blindness results in abnormal corticocortical and thalamocortical connections. Neuroscience. 2006;142(3):843–858. doi: 10.1016/j.neuroscience.2006.06.055. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274(5290):1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kayser C, Logothetis NK. Do early sensory cortices integrate cross-modal information? Brain Struct Funct. 2007;212(2):121–132. doi: 10.1007/s00429-007-0154-0. [DOI] [PubMed] [Google Scholar]
- Kilb W, Kirischuk S, Luhmann HJ. Electrical activity patterns and the functional maturation of the neocortex. Eur J Neurosci. 2011;34(10):1677–1686. doi: 10.1111/j.1460-9568.2011.07878.x. [DOI] [PubMed] [Google Scholar]
- Klemen J, Chambers CD. Current perspectives and methods in studying neural mechanisms of multisensory interactions. Neurosci Biobehav Rev. 2012;36(1):111–133. doi: 10.1016/j.neubiorev.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Klinge C, Eippert F, Roder B, Buchel C. Corticocortical connections mediate primary visual cortex responses to auditory stimulation in the blind. J Neurosci. 2010;30(38):12798–12805. doi: 10.1523/JNEUROSCI.2384-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H, Cossell L, Baragli C, Antolik J, Clopath C, Hofer SB, Mrsic-Flogel TD. The emergence of functional microcircuits in visual cortex. Nature. 2013;496(7443):96–100. doi: 10.1038/nature12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayasi KI, Suwa Y, Riquimaroux H. Audiovisual integration in the primary auditory cortex of an awake rodent. Neurosci Lett. 2013;534:24–29. doi: 10.1016/j.neulet.2012.10.056. [DOI] [PubMed] [Google Scholar]
- Kolarik AJ, Cirstea S, Pardhan S, Moore BC. A summary of research investigating echolocation abilities of blind and sighted humans. Hear Res. 2014;310:60–68. doi: 10.1016/j.heares.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Kolodziej A, Lippert M, Angenstein F, Neubert J, Pethe A, Grosser OS, Amthauer H, Schroeder UH, Reymann KG, Scheich H, Ohl FW, Goldschmidt J. SPECT-imaging of activity-dependent changes in regional cerebral blood flow induced by electrical and optogenetic self-stimulation in mice. Neuroimage. 2014;103:171–180. doi: 10.1016/j.neuroimage.2014.09.023. [DOI] [PubMed] [Google Scholar]
- Kotak VC, Pendola LM, Rodriguez-Contreras A. Spontaneous activity in the developing gerbil auditory cortex in vivo involves GABAergic transmission. Neuroscience. 2012;226:130–144. doi: 10.1016/j.neuroscience.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral A, Sharma A. Developmental neuroplasticity after cochlear implantation. Trends Neurosci. 2012;35(2):111–122. doi: 10.1016/j.tins.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupers R, Ptito M. Compensatory plasticity and cross-modal reorganization following early visual deprivation. Neurosci Biobehav Rev. 2014;41:36–52. doi: 10.1016/j.neubiorev.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Laramee ME, Boire D. Visual cortical areas of the mouse: comparison of parcellation and network structure with primates. Front Neural Circuits. 2014;8:149. doi: 10.3389/fncir.2014.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Whitt JL. Cross-modal synaptic plasticity in adult primary sensory cortices. Curr Opin Neurobiol. 2015;35:119–126. doi: 10.1016/j.conb.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessard N, Pare M, Lepore F, Lassonde M. Early-blind human subjects localize sound sources better than sighted subjects. Nature. 1998;395(6699):278–280. doi: 10.1038/26228. [DOI] [PubMed] [Google Scholar]
- Lomber SG, Meredith MA, Kral A. Cross-modal plasticity in specific auditory cortices underlies visual compensations in the deaf. Nat Neurosci. 2010;13(11):1421–1427. doi: 10.1038/nn.2653. [DOI] [PubMed] [Google Scholar]
- Lopez-Bendito G, Molnar Z. Thalamocortical development: how are we going to get there? Nat Rev Neurosci. 2003;4(4):276–289. doi: 10.1038/nrn1075. [DOI] [PubMed] [Google Scholar]
- Loskota WJ, Lomax P, Verity MA. A stereotaxic atlas of the Mongolian gerbil (Meriones unguiculatus) Michigan: Ann Arbor Science; 1974. [Google Scholar]
- Maidenbaum S, Abboud S, Amedi A. Sensory substitution: closing the gap between basic research and widespread practical visual rehabilitation. Neurosci Biobehav Rev. 2014;41:3–15. doi: 10.1016/j.neubiorev.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Markov NT, Kennedy H. The importance of being hierarchical. Curr Opin Neurobiol. 2013;23(2):187–194. doi: 10.1016/j.conb.2012.12.008. [DOI] [PubMed] [Google Scholar]
- Masse IO, Ross S, Bronchti G, Boire D. Asymmetric Direct Reciprocal Connections Between Primary Visual and Somatosensory Cortices of the Mouse. Cereb Cortex. 2017 doi: 10.1093/cercor/bhw239. [DOI] [PubMed] [Google Scholar]
- McGovern DP, Astle AT, Clavin SL, Newell FN. Task-specific transfer of perceptual learning across sensory modalities. Curr Biol. 2016;26(1):R20–21. doi: 10.1016/j.cub.2015.11.048. [DOI] [PubMed] [Google Scholar]
- Meng X, Kao JP, Lee HK, Kanold PO. Visual Deprivation Causes Refinement of Intracortical Circuits in the Auditory Cortex. Cell Rep. 2015;12(6):955–964. doi: 10.1016/j.celrep.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merabet LB, Pascual-Leone A. Neural reorganization following sensory loss: the opportunity of change. Nat Rev Neurosci. 2010;11(1):44–52. doi: 10.1038/nrn2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith MA, Allman BL. Early hearing-impairment results in crossmodal reorganization of ferret core auditory cortex. Neural Plast. 2012;2012:601591. doi: 10.1155/2012/601591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith MA, Lomber SG. Somatosensory and visual crossmodal plasticity in the anterior auditory field of early-deaf cats. Hear Res. 2011;280(1–2):38–47. doi: 10.1016/j.heares.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith MA, Lomber SG. Species-dependent role of crossmodal connectivity among the primary sensory cortices. Hear Res. 2017;343:83–91. doi: 10.1016/j.heares.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzera C, Lopez-Bendito G. Cross-modal plasticity in sensory deprived animal models: From the thalamocortical development point of view. J Chem Neuroanat. 2016;75(PtA):32–40. doi: 10.1016/j.jchemneu.2015.09.005. [DOI] [PubMed] [Google Scholar]
- Molholm S, Ritter W, Murray MM, Javitt DC, Schroeder CE, Foxe JJ. Multisensory auditory-visual interactions during early sensory processing in humans: a high-density electrical mapping study. Brain Res Cogn Brain Res. 2002;14(1):115–128. doi: 10.1016/s0926-6410(02)00066-6. [DOI] [PubMed] [Google Scholar]
- Mowery TM, Kotak VC, Sanes DH. The onset of visual experience gates auditory cortex critical periods. Nat Commun. 2016;7:10416. doi: 10.1038/ncomms10416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MM, Lewkowicz DJ, Amedi A, Wallace MT. Multisensory Processes: A Balancing Act across the Lifespan. Trends Neurosci. 2016;39(8):567–579. doi: 10.1016/j.tins.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro X, Vivo M, Valero-Cabre A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82(4):163–201. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Noesselt T, Tyll S, Boehler CN, Budinger E, Heinze HJ, Driver J. Sound-induced enhancement of low-intensity vision: multisensory influences on human sensory-specific cortices and thalamic bodies relate to perceptual enhancement of visual detection sensitivity. J Neurosci. 2010;30(41):13609–13623. doi: 10.1523/JNEUROSCI.4524-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nys J, Scheyltjens I, Arckens L. Visual system plasticity in mammals: the story of monocular enucleation-induced vision loss. Front Syst Neurosci. 2015;9:60. doi: 10.3389/fnsys.2015.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas SL. Intrinsic and extrinsic factors that shape neocortical specification. Trends Neurosci. 2001;24(7):417–423. doi: 10.1016/s0166-2236(00)01853-1. [DOI] [PubMed] [Google Scholar]
- Paperna T, Malach R. Patterns of sensory intermodality relationships in the cerebral cortex of the rat. J Comp Neurol. 1991;308(3):432–456. doi: 10.1002/cne.903080310. [DOI] [PubMed] [Google Scholar]
- Paxinos G. Atlas of the Developing Mouse Brain: At E17. 5, PO, and P6. Academic press; San Diego: 2007. [Google Scholar]
- Petrus E, Isaiah A, Jones AP, Li D, Wang H, Lee HK, Kanold PO. Crossmodal induction of thalamocortical potentiation leads to enhanced information processing in the auditory cortex. Neuron. 2014;81(3):664–673. doi: 10.1016/j.neuron.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrus E, Rodriguez G, Patterson R, Connor B, Kanold PO, Lee HK. Vision loss shifts the balance of feedforward and intracortical circuits in opposite directions in mouse primary auditory and visual cortices. J Neurosci. 2015;35(23):8790–8801. doi: 10.1523/JNEUROSCI.4975-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piche M, Chabot N, Bronchti G, Miceli D, Lepore F, Guillemot JP. Auditory responses in the visual cortex of neonatally enucleated rats. Neuroscience. 2007;145(3):1144–1156. doi: 10.1016/j.neuroscience.2006.12.050. [DOI] [PubMed] [Google Scholar]
- Pouchelon G, Gambino F, Bellone C, Telley L, Vitali I, Luscher C, Holtmaat A, Jabaudon D. Modality-specific thalamocortical inputs instruct the identity of postsynaptic L4 neurons. Nature. 2014;511(7510):471–474. doi: 10.1038/nature13390. [DOI] [PubMed] [Google Scholar]
- Price DJ, Kennedy H, Dehay C, Zhou L, Mercier M, Jossin Y, Goffinet AM, Tissir F, Blakey D, Molnar Z. The development of cortical connections. Eur J Neurosci. 2006;23(4):910–920. doi: 10.1111/j.1460-9568.2006.04620.x. [DOI] [PubMed] [Google Scholar]
- Proulx MJ, Brown DJ, Pasqualotto A, Meijer P. Multisensory perceptual learning and sensory substitution. Neurosci Biobehav Rev. 2014;41:16–25. doi: 10.1016/j.neubiorev.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Radtke-Schuller S, Schuller G, Angenstein F, Grosser OS, Goldschmidt J, Budinger E. Brain atlas of the Mongolian gerbil (Meriones unguiculatus) in CT/MRI-aided stereotaxic coordinates. Brain Struct Funct. 2016;221(Suppl 1):1–272. doi: 10.1007/s00429-016-1259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP. Compensatory plasticity and sensory substitution in the cerebral cortex. Trends Neurosci. 1995;18(1):36–43. doi: 10.1016/0166-2236(95)93948-w. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B, Korte M, Egert U. Crossmodal changes in the somatosensory vibrissa/barrel system of visually deprived animals. Proc Natl Acad Sci U S A. 1992;89(11):5063–5067. doi: 10.1073/pnas.89.11.5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renier L, De Volder AG, Rauschecker JP. Cortical plasticity and preserved function in early blindness. Neurosci Biobehav Rev. 2014;41:53–63. doi: 10.1016/j.neubiorev.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder B, Teder-Salejarvi W, Sterr A, Rosler F, Hillyard SA, Neville HJ. Improved auditory spatial tuning in blind humans. Nature. 1999;400(6740):162–166. doi: 10.1038/22106. [DOI] [PubMed] [Google Scholar]
- Roth KA, Kuan C, Haydar TF, D'Sa-Eipper C, Shindler KS, Zheng TS, Kuida K, Flavell RA, Rakic P. Epistatic and independent functions of caspase-3 and Bcl-X(L) in developmental programmed cell death. Proc Natl Acad Sci U S A. 2000;97(1):466–471. doi: 10.1073/pnas.97.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouiller EM, Simm GM, Villa AEP, Ribaupierre Yd, Ribaupierre Fd. Auditory corticocortical interconnections in the cat: evidence for parallel and hierarchical arrangement of the auditory cortical areas. Exp Brain Res. 1991;86:483–503. doi: 10.1007/BF00230523. [DOI] [PubMed] [Google Scholar]
- Ryan A. Hearing sensitivity of the mongolian gerbil, Meriones unguiculatis. J Acoust Soc Am. 1976;59(5):1222–1226. doi: 10.1121/1.380961. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Ryugo R, Globus A, Killackey HP. Increased spine density in auditory cortex following visual or somatic deafferentation. Brain Res. 1975;90(1):143–146. doi: 10.1016/0006-8993(75)90689-7. [DOI] [PubMed] [Google Scholar]
- Sachs L. Angewandte Statistik (Applied statistics) Heidelberg: Springer-Verlag; 2004. [Google Scholar]
- Sadato N, Pascual-Leone A, Grafman J, Ibanez V, Deiber MP, Dold G, Hallett M. Activation of the primary visual cortex by Braille reading in blind subjects. Nature. 1996;380(6574):526–528. doi: 10.1038/380526a0. [DOI] [PubMed] [Google Scholar]
- Sakata S, Yamamori T, Sakurai Y. Behavioral studies of auditory-visual spatial recognition and integration in rats. Exp Brain Res. 2004;159(4):409–417. doi: 10.1007/s00221-004-1962-6. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vives MV, Nowak LG, Descalzo VF, Garcia-Velasco JV, Gallego R, Berbel P. Crossmodal audio-visual interactions in the primary visual cortex of the visually deprived cat: a physiological and anatomical study. Prog Brain Res. 2006;155:287–311. doi: 10.1016/S0079-6123(06)55017-4. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Foxe J. Multisensory contributions to low-level, 'unisensory' processing. Curr Opin Neurobiol. 2005;15(4):454–458. doi: 10.1016/j.conb.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Schwentker V. The gerbil. A new laboratory animal, Ill. Vet. 1963;6:5–9. [Google Scholar]
- Sieben K, Bieler M, Roder B, Hanganu-Opatz IL. Neonatal Restriction of Tactile Inputs Leads to Long-Lasting Impairments of Cross-Modal Processing. PLoS Biol. 2015;13(11):e1002304. doi: 10.1371/journal.pbio.1002304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smilek D, Dixon MJ, Cudahy C, Merikle PM. Synaesthetic photisms influence visual perception. J Cogn Neurosci. 2001;13(7):930–936. doi: 10.1162/089892901753165845. [DOI] [PubMed] [Google Scholar]
- Souter M, Nevill G, Forge A. Postnatal maturation of the organ of Corti in gerbils: morphology and physiological responses. J Comp Neurol. 1997;386(4):635–651. [PubMed] [Google Scholar]
- Spector F, Maurer D. Synesthesia: a new approach to understanding the development of perception. Dev Psychol. 2009;45(1):175–189. doi: 10.1037/a0014171. [DOI] [PubMed] [Google Scholar]
- Stehberg J, Dang PT, Frostig RD. Unimodal primary sensory cortices are directly connected by long-range horizontal projections in the rat sensory cortex. Front Neuroanat. 2014;8:93. doi: 10.3389/fnana.2014.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein BE, Burr D, Constantinidis C, Laurienti PJ, Alex Meredith M, Perrault TJ, Jr, Ramachandran R, Roder B, Rowland BA, Sathian K, Schroeder CE, Shams L, Stanford TR, Wallace MT, Yu L, Lewkowicz DJ. Semantic confusion regarding the development of multisensory integration: a practical solution. Eur J Neurosci. 2010;31(10):1713–1720. doi: 10.1111/j.1460-9568.2010.07206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein BE, Stanford TR. Multisensory integration: current issues from the perspective of the single neuron. Nat Rev Neurosci. 2008;9(4):255–266. doi: 10.1038/nrn2331. [DOI] [PubMed] [Google Scholar]
- Stein BE, Stanford TR, Rowland BA. Development of multisensory integration from the perspective of the individual neuron. Nat Rev Neurosci. 2014;15(8):520–535. doi: 10.1038/nrn3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles NR, Shimojo S. Auditory Sensory Substitution is Intuitive and Automatic with Texture Stimuli. Sci Rep. 2015;5:15628. doi: 10.1038/srep15628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur M, Rubenstein JL. Patterning and plasticity of the cerebral cortex. Science. 2005;310(5749):805–810. doi: 10.1126/science.1112070. [DOI] [PubMed] [Google Scholar]
- Teder-Salejarvi WA, McDonald JJ, Di Russo F, Hillyard SA. An analysis of audio-visual crossmodal integration by means of event-related potential (ERP) recordings. Brain Res Cogn Brain Res. 2002;14(1):106–114. doi: 10.1016/s0926-6410(02)00065-4. [DOI] [PubMed] [Google Scholar]
- Teichert M, Bolz J. Simultaneous intrinsic signal imaging of auditory and visual cortex reveals profound effects of acute hearing loss on visual processing. Neuroimage. 2017;159:459–472. doi: 10.1016/j.neuroimage.2017.07.037. [DOI] [PubMed] [Google Scholar]
- Thiessen DD, Yahr P. Mechanisms of territoriality and olfactory communication. Austin and London: University of Texas Press; 1977. The gerbil in behavioral investigations. [Google Scholar]
- Tropea D, Van Wart A, Sur M. Molecular mechanisms of experience-dependent plasticity in visual cortex. Philos Trans R Soc Lond B Biol Sci. 2009;364(1515):341–355. doi: 10.1098/rstb.2008.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo LA, Garrosa M, Al-Majdalawi A, Mayo A, Gayoso MJ. Effects of unilateral deprivation in postnatal development of the olfactory bulb in an altricial rodent, the gerbil (Meriones unguiculatus) Brain Res Dev Brain Res. 2000;122(1):35–46. doi: 10.1016/s0165-3806(00)00050-x. [DOI] [PubMed] [Google Scholar]
- Vercelli A, Repici M, Garbossa D, Grimaldi A. Recent techniques for tracing pathways in the central nervous system of developing and adult mammals. Brain Res Bull. 2000;51(1):11–28. doi: 10.1016/s0361-9230(99)00229-4. [DOI] [PubMed] [Google Scholar]
- Wall JT, Cusick CG. The representation of peripheral nerve inputs in the S-I hindpaw cortex of rats raised with incompletely innervated hindpaws. J Neurosci. 1986;6(4):1129–1147. doi: 10.1523/JNEUROSCI.06-04-01129.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. Synesthesia. Annu Rev Psychol. 2013;64:49–75. doi: 10.1146/annurev-psych-113011-143840. [DOI] [PubMed] [Google Scholar]
- Wickersham IR, Finke S, Conzelmann KK, Callaway EM. Retrograde neuronal tracing with a deletion-mutant rabies virus. Nat Methods. 2007;4(1):47–49. doi: 10.1038/NMETH999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson F. Eye and brain growth in the Mongolian gerbil (Meriones unguiculatus) Behav Brain Res. 1986;19(1):59–69. doi: 10.1016/0166-4328(86)90048-3. [DOI] [PubMed] [Google Scholar]
- Yu X, Chung S, Chen DY, Wang S, Dodd SJ, Walters JR, Isaac JT, Koretsky AP. Thalamocortical inputs show post-critical-period plasticity. Neuron. 2012;74(4):731–742. doi: 10.1016/j.neuron.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.