Abstract
Background
Recent studies have demonstrated the effectiveness of family-centered, pediatric weight management programs in reducing childhood obesity. Yet, programs to optimize the care of low-income children with obesity are needed. We sought to examine the comparative effectiveness of two, potentially scalable pediatric weight management programs delivered to low-income children in a clinical or community setting.
Materials and Methods
The Clinic and Community Approaches to Healthy Weight trial is a randomized trial in two communities in Massachusetts that serve a large population of low-income children and families. The two-arm trial compares the effects of a pediatric weight management program delivered in the Healthy Weight Clinics of two federally qualified health centers (FQHC) to the Healthy Weight and Your Child programs delivered in two YMCAs. Eligible children are 6 to 12 years old with a body mass index (BMI) ≥ 85th percentile seen in primary care at the two FQHCs. Both programs are one-year in duration and have at least 30 contact hours throughout the year. Measures are collected at baseline, 6 months, and 1 year. The main outcome is 1-year change in BMI (kg/m2) and percent change of the 95th percentile (%BMIp95).
Conclusion
The Clinic and Community Approaches to Healthy Weight trial seeks to 1) examine the comparative effects of a clinical and community based intervention in improving childhood obesity, and 2) inform the care of >7 million children with obesity covered by the Children’s Health Insurance Program or Medicaid.
Keywords: Childhood obesity treatment, Clinical and Community Approaches, randomized controlled trial
Introduction
The high prevalence of childhood obesity places a significant burden on morbidity, quality of life, health care utilization, and costs. While childhood overweight and obesity prevalence may have plateaued in some population subgroups, overall rates remain at historically high levels and racial/ethnic and socioeconomic disparities appear to be widening.(1–5) Many factors contribute to the intractability of obesity but promising approaches for reduction are emerging including multi-sector, collaborative interventions across settings where children spend their time.
In recent years, several innovative and effective approaches have emerged to prevent and treat childhood obesity in both clinical and community settings. For example, in 2011, the Massachusetts Department of Public Health (MDPH) along with academic and community partners launched the MA Childhood Obesity Research Demonstration (MA CORD) project, a multifaceted initiative to prevent childhood obesity among low-income children(6–8). Through clinical, community, and environmental approaches, MA CORD favorably shifted the mean body mass index (BMI) curve among 2-12 year old children in a large community in MA (9–11). In addition to MA CORD, there have been other innovations in childhood obesity prevention and management. Members of our research team have successfully implemented Healthy Weight Clinics (HWC) in federally qualified community health centers (FQHC), shown to be effective in improving BMI and obesity-related health care quality using enhanced electronic health records (EHR), clinical decision supports, community health workers, text messages, and self-guided behavior change support(9, 12, 13). Members of our team have also developed effective family-centered, pediatric weight management programs such as the Healthy Weight and Your Child program (HWYC) based on the Mind Exercise Nutrition… Do It (MEND) program, which is being delivered in 19 YMCAs across the US currently and will be expanded to 36 YMCAs by the end of the year.(14, 15) An important next step for optimizing childhood obesity prevention and care is to develop strategies to improve the care of low-income children with overweight or obesity and in whom far greater energy deficits will be necessary to achieve improvements in BMI than those achieved by community and environmental approaches alone.
To accomplish this goal, we designed the Clinic and Community Approaches to Healthy Weight study to examine the comparative effectiveness of two pediatric weight management programs - (1) Healthy Weight Clinics based in federally qualified community health centers, and (2) the YMCA’s Healthy Weight and Your Child program- in improving body mass index outcomes among children. We hypothesize that both programs will result in clinically significant improvements in overweight and obesity outcomes and could offer two approaches for pediatric weight management for low-income children covered by the Children’s Health Insurance Program (CHIP) or Medicaid. In this article we describe the rationale, design, and evaluation approach of the trial (Figure 1).
Figure 1.

Logic Model of the Clinic and Community Approaches to Healthy Weight (MA-CORD 2.0) Trial
Materials and Methods: Intervention Development
Overview of study design (Figure 2)
Figure 2.
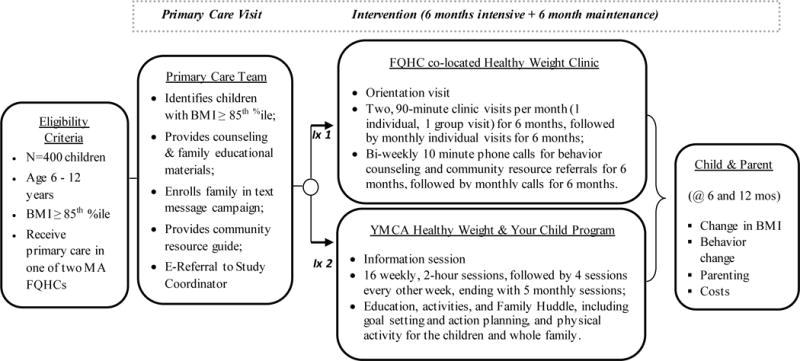
Study Design and intervention components of the Clinic and Community Approaches to Healthy Weight (MA-CORD 2.0) Trial
This study is a randomized trial being conducted in two FQHCs and two regional YMCAs serving the same communities in in Massachusetts. The trial was designed and is being implemented in consultation with a diverse advisory board of 20 clinical, public health, community partners, Medicaid officials, and parent stakeholders. Children ages 6-12 years old with a BMI ≥ 85th percentile are enrolled at the time of their primary care visits to each of the two participating FQHCs and randomly assigned to one of 2 arms: 1) pediatric weight management in a Healthy Weight Clinic within the FQHCs or 2) pediatric weight management at a local YMCA delivering the Healthy Weight and Your Child Program. The primary intention-to-treat analysis will examine the comparative effects of pediatric weight management delivered in Healthy Weight Clinics compared to the YMCA’s Healthy Weight and Your Child program on reduced age-associated BMI gain over a 6-month and 1-year period and percent change of the 95th percentile (%BMIp95). Additionally, we aim to improve specific dietary, physical activity, sleep, and sedentary behaviors and we will compare intervention costs. All study activities were approved by the Institutional Review Board at the Massachusetts Department of Public Health. The trial has also been recorded in the clinicaltrials.gov national registry of randomized trials.
The trial was designed and is being implemented in consultation with a diverse advisory board of 20 clinical, public health, community partners, Medicaid officials, and parent stakeholders. We have completed semi-structured interviews with the 26 stakeholders focusing on 1) feasibility of past obesity treatment strategies in health centers and communities 2) preferred settings for obesity treatment 3) gaps and successes in childhood weight management programs 4) what treatment packages would be appealing to payers and 5) obtaining their feedback on the proposed interventions.
Selection of Participating Communities
The intervention FQHCs were chosen because they met the following criteria:
Provide pediatric care to a minimum of 1,500 children annually, ages 6-12 years of age;
Reach a population with high eligibility for Medicaid and Children’s Health Insurance Program (CHIP) and in a community where >50% of students are eligible for free or reduced meals in the National School Lunch Program;
Use a fully-functional electronic health record for all pediatric care;
Are members of and contribute electronic data to the Community Health Information Association (CHIA) Data Reporting and Visualization System (DRVS), a web-based, central data repository(16, 17);
Have an existing co-located Healthy Weight Clinic; and
Have a local YMCA implementing the Healthy Weight and Your Child Program in 2016.
Randomization and Blinding
We use a simple randomization by FQHC site, where each participant has a 50% chance to be in either intervention arm. The randomization is conducted a priori, and a set of numbered, opaque envelopes is prepared for each intervention FQHC and stored by the study coordinators. After consent has been obtained and the parent completes a baseline assessment, the coordinator reveals randomization assignment. This activity preserves blinding of the coordinators during the baseline assessment. Further assessments, at six months and 1-year, are conducted by study coordinators blinded to intervention assignment.
Eligibility and Recruitment
Eligibility criteria is assessed by both the referring primary care provider and study coordinators and includes: 1) child is age 6.0 through 12.9 years at enrollment, 2) child’s BMI meets or exceeds the 85th percentile for age and sex, and 3) parent can speak English or Spanish. We exclude: 1) children who do not have at least one parent or legal guardian who is able to follow study procedures for 1-year, 2) families who plan to leave their FQHC within the study time frame, 3) families for whom the intervention is inappropriate as determined by the primary care clinician, e.g., due to emotional or cognitive difficulties, 4) children with chronic conditions who take medications that substantially interfere with growth or physical activity participation, and 5) children who have a sibling enrolled in the study.
Recruitment began in December 2016 and will continue for one year (Figure 3). Children are referred to the study by their primary care provider by either an electronic referral or by an electronic fax during any health care visit where a height and weight is obtained. At the time of referral, parents are given a study fact sheet by their provider. After the referral is made, parents are mailed an introductory letter and fact sheet by the study team. A minimum of 5 days later a bilingual study coordinator contacts parents by phone. The coordinator explains that we are conducting a research study to examine various strategies to improve the care that is provided for children who require weight management. The coordinator obtains verbal informed consent from the parent and administers a 20-minute baseline survey. After the parent completes the survey, the coordinator opens a uniquely identified opaque envelope that reveals the child’s randomization status. Families are provided with $25 for completing each of the baseline, 6 month and 12 month surveys, as well as up to three $25 attendance incentives.
Figure 3.
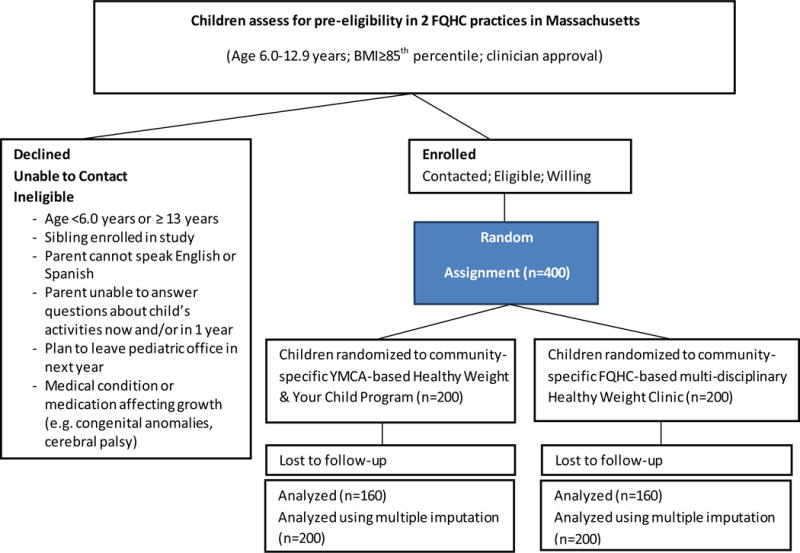
Clinic and Community Approaches to Healthy Weight (MA-CORD 2.0) Randomized Controlled Trial Planned Participant Flow Chart
Intervention arms
Children are randomized to one of two intervention arms: weight management at either a Healthy Weight Clinic in their FQHC or the Healthy Weight and Your Child program at their local YMCA. Table 1 outlines the components for each of the intervention arms. Each of the two intervention groups receive an intensive 6-month intervention, followed by a 6-month maintenance period that delivers ≥ 30 hours of contact time over the 1-year intervention period consistent with the current USPSTF guidelines to provide ≥ 26 hours of intervention(18).
Table 1.
Overview of the Healthy Weight Clinic and Healthy Weight and Your Child Program
| Healthy Weight Clinic | Healthy Weight and Your Child | |
|---|---|---|
| Location | Federally Qualified Health Center (Clinic- based) | YMCA (Community-based) |
| Staff | Multi-disciplinary team:
|
YMCA trained group leaders
|
| Program Structure |
|
|
| Intervention contact hours over 1-year study period | 30 contact hours
|
50 contact hours
|
| Visit structure |
|
|
| Curriculum | Based on Next Steps Guide:
|
Adapted from MEND (Mind, Exercise, Nutrition, Do It!)
|
Common Intervention Components
Children in both arms are exposed to quality of care improvements in their FQHC which includes primary care provider weight management training and text messages to participating families for self-guided behavior change support. Parents are given the option to sign-up for the text message campaign at enrollment, described in detail elsewhere(19). Parents receive messages 2-3 times a week with educational tips about weight-related behaviors and how to make healthy changes, as well as messages that support social and emotional wellness, and promote community resource utilization.
Healthy Weight Clinic (HWC)
The HWC provides a staged, comprehensive, multidisciplinary team intervention for treatment of children with a BMI ≥ 85th percentile. The team includes a pediatrician, community health worker and dietitian with access to behavioral/mental health providers as needed. The HWC team has been trained to deliver motivational interviewing and behavioral modification techniques to engage families in setting and following through on healthy eating and activity goals. Visits alternate between group visits with other children and families in the program and individual visits for the first six months and in the second six months the visits will be individual. To guide the content of each group HWC visit, we developed an interactive curriculum modeled after the Next Steps Practitioner’s Guide for Themed Follow Up Visits, a resource developed by The American Academy of Pediatrics and National Institute for Children’s Health Quality to assist providers in pediatric weight management.(20) Topics for group visits include: understanding health, healthy eating, healthy drinks, physical activity, bullying, sleep and screens, reading food labels and eating out of the home. Individual visits are aimed at the main areas of concern for the family. Goal setting and motivational interviewing techniques are used in the individual visits.
During the first 6 months of the intervention the community health worker or dietitian makes biweekly phone calls to the family on weeks they do not have an in person visit. During the second 6 months, they provide once monthly calls. During the phone calls they 1) provide behavioral counseling using motivational interviewing techniques and 2) provide them with contacts of community resources that can aid the family in behavioral change or address social determinants of health.
YMCA Healthy Weight and Your Child (HWYC)
The YMCA of the USA has worked with the two local YMCAs to train staff to implement the nationally licensed and standardized HWYC program. Two YMCA group leaders, one with a background and experience in public health and the second with a history of working with children and families, provide support, education and activities during every session, including: a Family Huddle which incorporates goal setting and action planning, a parent discussion, and 60 minutes of physical activity for the children the last 30 minutes of which is for the whole family. The program is delivered over 12 months, which includes 16 weekly sessions, followed by 4 sessions delivered every other week and concluding with 5 monthly sessions, for a total of 25 in-person sessions. Sessions are 2 hours in length and include a group of about 8-15 children and their caregivers.
Quality Assurance and Observations in the Healthy Weight Clinics and YMCA’s Healthy Weight and Your Child
To ensure the program is being conducted per protocol and that it is patient-centered we conduct quarterly observations by members of the research staff and patient advisors guided by an observation tool we created. The observations focus on ensuring the program works logistically (visit length, attendance), that the leaders are engaging and following the curriculum, and that the program is patient-centered and weight sensitive. During these quarterly visits, research staff also calibrate the scales and stadiometers.
Outcome Measures
The primary outcome is change in BMI in kg/m2 and percent change of the 95th percentile (%BMIp95). (21) Height, weight and BMI measurements are collected by staff in the Healthy Weight Clinics and the YMCAs at enrollment and throughout the program. Obesity behavioral outcomes and parenting around these behaviors including screen time, physical activity, sleep duration and diet are collected in surveys at baseline, 6 months and 1-year (Table 2). We also collect cost data (including formal and informal health care sector costs and non-health care sector costs) and qualitative information from parents and stakeholders that are incorporated into the implementation of each program.
Table 2.
Outcomes used for Evaluation of the Clinic and Community Approaches to Healthy Weight (MA-CORD 2.0 Trial)
| Behavior | Intervention goal | Measures and Validity Relationships |
|---|---|---|
| Sleep | ||
| Duration of sleep | Increase sleep duration to 10 hours/day | Parent report of average amount of daily sleep their children obtained; associated with childhood BMI.(6, 23) |
| Parenting around sleep | Goes to bed in the same place each night Has a regular bedtime routine |
Parent report if child goes to bed in the same place each night. Parent report if child has calming bedtime routine. (24) |
| Regular Bed Time | Regular bedtime on most days | Parent report of typical bedtime on weekday and weekend days. (6) |
| Sleep Habits | Not sleeping next to or near a screen based device most days. | Parent report of the frequency child sleeps next to or near a screen based device. (6) |
| Screen time | ||
| Duration of screen time | Limiting screen-viewing time to < 2 hours/day | Parent report of average daily hours spent watching TV or videos; playing video games; and using the computer on weekday and weekend days; associated with child BMI. (6) |
Parenting around screen time
|
Parent sets screen time limits for child. Family does not watch screen during meals. No use of screen based devices at bed time most days. |
Parent agreement with screen time limit setting. Parent agreement with family screen watching during meals. Parent agreement of use of screen based device when falling asleep. (25, 26) |
| TV in room where child sleeps | No TV in room where child sleeps | Presence of TV in bedroom; associated with BMI in children. (6) |
| Physical Activity | ||
| Number of days per week the child is active | Increasing time active to > 1 hour per day. | Parent report of days active at least one hour or more in the last week.(6) |
Parenting around Physical Activity
|
Parent models healthy physical activity and provides more opportunities for child to be active. | Parent agreement with being physically active. Parent agreement with whether child is taken to places they can be active.(25, 27) |
| Diet and diet quality | ||
| Sugar sweetened beverages | Lower daily intake of beverages with sugar added | Parent report using questions from a validated semi-quantitative child food frequency questionnaire. Associated with BMI. (28) |
| Fast Food Intake | Lower weekly intake of fast food meals | Modified question adapted from the Project VIVA Study; associated with BMI. (29) |
Parenting around nutrition
|
Increase access to healthy food Parent modeling of healthy food habits |
Parent reported access to healthy foods in household and parental modeling (30) |
Adverse Outcomes
|
No binge eating or dietary restraint symptoms. | Parent report of binge eating or dietary restraint symptoms (31–35) |
Statistical Analysis
We will examine baseline distributions of participant characteristics by intervention arm and by FQHC. Changes in BMI and percent change of the 95th percentile (%BMIp95) will be compared between the intervention groups using an intention-to-treat analysis. Mixed, fixed and random effects longitudinal models will be used. Primary fixed effect predictors will include: Intervention Group and Site, Time (assessed at baseline, 6 months and 1 year), and the Intervention Group and Site X Time interaction. Fixed covariates include: child race/ethnicity, parental income and parental education. Other covariates may be included based on bivariate analyses. Random terms will be included for participants’ intercepts and slope of change, and permitted to be correlated. We will perform a noninferiority test of child BMI to rule out that the BMI change difference for the HYWC is not appreciably inferior to that of the standard Healthy Weight clinic intervention as we hypothesize both interventions will be equally effective. The noninferiority test will be a “two one-sided test” (TOST) or similar technique after specifying a minimal interaction effect considered to be nontrivial (see below). We will perform multiple imputation using chained equations to impute missing outcomes for the participants who do not have a BMI measure at the 1-year outcome. Residuals from models will be checked for conformance to significance test assumptions of normality and homoscedasticity, and data transformations applied if necessary.
Sample Size/Power Analysis
We computed power for a noninferiority test, in which we stipulated a minimal value favoring the standard intervention as indicating “appreciable” inferiority of the new intervention. We plan to enroll 400 children into the study, with half assigned to each intervention group (Figure 3). Based on prior work, we expect over 90% to have follow-up BMI measures, but will use a conservative 80% for power calculations (n=160 per arm, 320 Total N), and will also compute power in the unlikely case that follow-up retention is as low as 70% (n=140 per arm, 280 total). We computed power for 2 different dependent variables: change in BMI and change in BMI z-scores, in both cases assuming a 5% Type I error rate. For change in BMI, we considered a mean change difference of 1 kg/m2 as the border of “appreciable” inferiority as this was the difference noted in the most effective trials evaluated by the USPSTF with ≥52 hours of contact (18), and used a within arm change score standard deviation (SD) of 2 estimated from previous work(12). Given these specifications, if we assume the actual mean BMI population difference is approximately 0.4, power was computed to be 85% for N=320 and 81% for N=280. Although our outcome will be change in BMI and percent change of the 95th percentile (%BMIp95), clinically important weight loss associated with cardiometabolic benefits has been estimated to be a change in 0.2 BMI z-score units (18), so we estimate non-inferiority based on this estimate. Thus, we considered a mean change difference of 0.2 units of BMI z-scores as the border of “appreciable” inferiority, and used a within arm change score SD of 0.3 estimated from previous work(12). Given these specifications, if we assume the actual population mean difference is approximately 0.1 BMI z-score units, power was computed to be 91% for N=320 and 87% for N=280.
Discussion
Overall, this study is poised to improve obesity-related care for low-income children by comparatively testing two evidence-based programs for childhood obesity management, one in a clinical setting and one in the community. It is also possible that the interaction between clinic and community providers will provide qualitative support for models of community-integrated health(22). We anticipate that the two models will provide a foundation for widely disseminated models of clinical and community based obesity treatment for low-income children covered by the Children’s Health Insurance Program (CHIP) or Medicaid.
There are limitations of our study, one of which is generalizability. Both FQHCs have had weight management programs before and have two clinician champions dedicated to childhood obesity. They also serve a predominantly Hispanic and low-income population, perhaps making the results of our study less generalizable among other racial/ethnic or SES groups. Second, as with any survey and behavioral intervention, parents could exaggerate children’s improvements in their healthful behaviors, however, BMI, an objective measure, will be our main outcome. Finally, the study timeline does not allow us to examine outcomes beyond one year to assess the maintenance of any intervention effects.
If successful, this project will provide two examples of successful weight management programs for children that could be disseminated and adopted elsewhere, with comparative effectiveness and cost-effectiveness analyses to help health care decision-makers and payers evaluate the benefits of the programs and consider reimbursement.
Acknowledgments
The authors thank the families, institutions, faculty, research staff, and students that are participating in the MA-CORD 2.0 study.
Funding: This study was supported by the Centers for Disease Control and Prevention National Center for Chronic Disease Prevention and Health Promotion (Award no.: U18DP006259). Dr. Fiechtner’s is supported by grant number K12HS022986 from the Agency for Healthcare Research and Quality. Dr. Sharifi is supported by grants K08 HS024332 from the Agency for Healthcare Research and Quality. Dr. Taveras is supported by grant K24 DK10589 from the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Centers for Disease Control, Agency for Healthcare Research and Quality, the National Institutes of Health, or any other funders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests: The authors have no competing interests to declare.
ClinicalTrials.gov: NCT03012126
Bibliography
- 1.Olds T, Maher C, Zumin S, Peneau S, Lioret S, Castetbon K, et al. Evidence that the prevalence of childhood overweight is plateauing: data from nine countries. International journal of pediatric obesity : IJPO : an official journal of the International Association for the Study of Obesity. 2011 Oct;6(5–6):342–60. doi: 10.3109/17477166.2011.605895. [DOI] [PubMed] [Google Scholar]
- 2.Madsen KA, Weedn AE, Crawford PB. Disparities in peaks, plateaus, and declines in prevalence of high BMI among adolescents. Pediatrics. 2010 Sep;126(3):434–42. doi: 10.1542/peds.2009-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of Obesity Among Adults and Youth: United States, 2011–2014. Hyattsville, MD: 2015. [PubMed] [Google Scholar]
- 4.Wang Y, Baker JL, Hill JO, Dietz WH. Controversies regarding reported trends: has the obesity epidemic leveled off in the United States? Advances in Nutrition: An International Review Journal. 2012;3(5):751–2. doi: 10.3945/an.112.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang YC, Gortmaker SL, Taveras EM. Trends and racial/ethnic disparities in severe obesity among US children and adolescents, 1976–2006. Pediatric Obesity. 2011;6(1):12–20. doi: 10.3109/17477161003587774. [DOI] [PubMed] [Google Scholar]
- 6.Davison KK, Falbe J, Taveras EM, Gortmaker S, Kulldorff M, Perkins M, et al. Evaluation overview for the Massachusetts childhood obesity research demonstration (MA-CORD) project. Childhood Obesity. 2015;11(1):23–36. doi: 10.1089/chi.2014.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taveras EM, Blaine RE, Davison KK, Gortmaker S, Anand S, Falbe J, et al. Design of the Massachusetts Childhood Obesity Research Demonstration (MA-CORD) study. Child Obes. 2015 Feb;11(1):11–22. doi: 10.1089/chi.2014.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foltz JL, Belay B, Dooyema CA, Williams N, Blanck HM. Childhood Obesity Research Demonstration (CORD): the cross-site overview and opportunities for interventions addressing obesity community-wide. Child Obes. 2015 Feb;11(1):4–10. doi: 10.1089/chi.2014.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taveras EM, Perkins M, Anand S, Woo Baidal JA, Nelson CC, Kamdar N, et al. Clinical effectiveness of the massachusetts childhood obesity research demonstration initiative among low-income children. Obesity. 2017;25(7):1159–66. doi: 10.1002/oby.21866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franckle RL, Falbe J, Gortmaker S, Barrett JL, Giles C, Ganter C, et al. Student obesity prevalence and behavioral outcomes for the Massachusetts Childhood Obesity Research Demonstration project. Obesity. 2017 Jul;25(7):1175–82. doi: 10.1002/oby.21867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woo Baidal JA, Nelson CC, Perkins M, Colchamiro R, Leung-Strle P, Kwass JA, et al. Childhood obesity prevention in the women, infants, and children program: Outcomes of the MA-CORD study. Obesity. 2017 Jul;25(7):1167–74. doi: 10.1002/oby.21865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taveras EM, Marshall R, Sharifi M, Avalon E, Fiechtner L, Horan C, et al. Comparative Effectiveness of Clinical-Community Childhood Obesity Interventions: A Randomized Clinical Trial. JAMA Pediatr. 2017 Aug 07;171(8):e171325. doi: 10.1001/jamapediatrics.2017.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taveras EM, Marshall R, Kleinman KP, Gillman MW, Hacker K, Horan CM, et al. Comparative effectiveness of childhood obesity interventions in pediatric primary care: a cluster-randomized clinical trial. JAMA Pediatr. 2015 Jun;169(6):535–42. doi: 10.1001/jamapediatrics.2015.0182. [DOI] [PubMed] [Google Scholar]
- 14.Kolotourou M, Radley D, Gammon C, Smith L, Chadwick P, Sacher PM. Long-term outcomes following the MEND 7-13 child weight management program. Childhood Obesity. 2015;11(3):325–30. doi: 10.1089/chi.2014.0092. [DOI] [PubMed] [Google Scholar]
- 15.Butte NF, Hoelscher DM, Barlow SE, Pont S, Durand C, Vandewater EA, et al. Efficacy of a community- versus primary care-centered program for childhood obesity: TX CORD RCT. Obesity (Silver Spring) 2017 Jul 13; doi: 10.1002/oby.21929. [DOI] [PubMed] [Google Scholar]
- 16.Centers Massachusetts Leauge of Community Health Centers. Community Health Information Association 2017. Available from: http://www.massleague.org/Programs/HealthInformationTechnology/CHIA.php.
- 17.Azara Healthcare. Azara DRVS 2017. Available from: http://www.azarahealthcare.com/solutions/azara-drvs/
- 18.Grossman DC, Bibbins-Domingo K, Curry SJ, Barry MJ, Davidson KW, Doubeni CA, et al. Screening for obesity in children and adolescents: US Preventive Services Task Force recommendation statement. Jama. 2017;317(23):2417–26. doi: 10.1001/jama.2017.6803. [DOI] [PubMed] [Google Scholar]
- 19.Price S, Ferisin S, Sharifi M, Steinberg D, Bennett G, Wolin KY, et al. Development and implementation of an interactive text messaging campaign to support behavior change in a childhood obesity randomized controlled trial. Journal of health communication. 2015;20(7):843–50. doi: 10.1080/10810730.2015.1018582. [DOI] [PubMed] [Google Scholar]
- 20.Fanburg J, Rogers V, Dedekian M, Cooke E, Anand S, Homer C, editors. American Academy of Pediatrics. Next Steps: A Practitioner’s Guide For Themed Follow-up Visits For Their Patients. Elk Grove Village, IL: 2013. [Google Scholar]
- 21.Freedman DS, Butte NF, Taveras EM, Goodman AB, Ogden CL, Blanck HM. The limitations of transforming very high body mass indexes into z-scores among 8.7 million 2-to 4-year-old children. The Journal of Pediatrics. 2017 doi: 10.1016/j.jpeds.2017.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dietz WH, Solomon LS, Pronk N, Ziegenhorn SK, Standish M, Longjohn MM, et al. An integrated framework for the prevention and treatment of obesity and its related chronic diseases. Health affairs. 2015;34(9):1456–63. doi: 10.1377/hlthaff.2015.0371. [DOI] [PubMed] [Google Scholar]
- 23.Center for Disease Control and Prevention. Behavioral risk factor surveillance system survey questionnaire. US Department of Health and Human Services; Atlanta, Georgia: 2001. pp. 22–3. [Google Scholar]
- 24.Meitzer LJ, Avis KT, Biggs S, Reynolds AC, Crabtree VM, Bevans KB. The Children’s Report of Sleep Patterns (CRSP): a self-report measure of sleep for school-aged children. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine. 2013;9(3):235. doi: 10.5664/jcsm.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larios SE, Ayala GX, Arredondo EM, Baquero B, Elder JP. Development and validation of a scale to measure Latino parenting strategies related to children’s obesigenic behaviors. The parenting strategies for eating and activity scale (PEAS) Appetite. 2009;52(1):166–72. doi: 10.1016/j.appet.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaughn A, Hales D, Ward DS. Measuring the physical activity practices used by parents of preschool children. Medicine and science in sports and exercise. 2013;45(12):2369. doi: 10.1249/MSS.0b013e31829d27de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davison KK, Li K, Baskin ML, Cox T, Affuso O. Measuring parental support for children’s physical activity in white and African American parents: the Activity Support Scale for Multiple Groups (ACTS-MG) Preventive Medicine. 2011 Jan;52(1):39–43. doi: 10.1016/j.ypmed.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention (CDC) National Center for Health Statistics. National Health and Nutrition Examination Survey Questionnaire. Hyattsville, MD: U.S. Department of Health and Human Services; 2015. [Google Scholar]
- 29.Taveras EM, Gillman MW, Kleinman KP, Rich-Edwards JW, Rifas-Shiman SL. Reducing racial/ethnic disparities in childhood obesity: the role of early life risk factors. JAMA pediatrics. 2013 Aug 01;167(8):731–8. doi: 10.1001/jamapediatrics.2013.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musher-Eizenman D, Holub S. Comprehensive Feeding Practices Questionnaire: validation of a new measure of parental feeding practices. Journal of pediatric psychology. 2007;32(8):960–72. doi: 10.1093/jpepsy/jsm037. [DOI] [PubMed] [Google Scholar]
- 31.Van Strien T, Frijters JE, Bergers G, Defares PB. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. International journal of eating disorders. 1986;5(2):295–315. [Google Scholar]
- 32.Caccialaza R, Nicholls D, Cena H, Maccarini L. Validation of the Dutch eating behaviour questionnaire parent version (DEBQ-P) in the Italian population: A screening tool to detect differences in eating behaviour. European Journal of Clinical Nutrition. 2004;58(9):1217. doi: 10.1038/sj.ejcn.1601949. [DOI] [PubMed] [Google Scholar]
- 33.Braet C, Van Strien T. Assessment of emotional, externally induced and restrained eating behaviour in nine to twelve-year-old obese and non-obese children. Behav Res Ther. 1997 Sep;35(9):863–73. doi: 10.1016/s0005-7967(97)00045-4. [DOI] [PubMed] [Google Scholar]
- 34.Russell H, Oliver C. The assessment of food-related problems in individuals with Prader-Willi syndrome. British Journal of Clinical Psychology. 2003;42(4):379–92. doi: 10.1348/014466503322528928. [DOI] [PubMed] [Google Scholar]
- 35.Johnson WG, Grieve FG, Adams CD, Sandy J. Measuring binge eating in adolescents: adolescent and parent versions of the questionnaire of eating and weight patterns. International Journal of Eating Disorders. 1999;26(3):301–14. doi: 10.1002/(sici)1098-108x(199911)26:3<301::aid-eat8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]


