Abstract
Parkinson's disease manifests as a progressive movement disorder with underlying degeneration of dopaminergic neurons in the substantia nigra, consequent depletion of dopamine levels, and the accumulation of Lewy bodies in the brain. Since α-synuclein (α-Syn) protein is the major component of Lewy bodies, mouse models expressing wild type or mutant SNCA/α-Syn genes provide a useful tool to investigate canonical characteristics of the disease. We evaluated a mouse model (denoted M20) that expresses human wild type SNCA gene. The M20 mice showed abnormal locomotor behavior and reduced species-specific home cage activity. However, the direction of behavioral changes was task specific. In comparison with their control littermates, the M20 mice exhibited shorter grip endurance, and longer times to traverse elevated beams, but they descended the vertical pole faster and stayed longer on the accelerated rod than the control mice. The M20 mice were also impaired in burrowing and nest building activities. These results indicate a possible role of α-Syn in motor coordination and the motivation to perform species-specific behaviors in the pre-symptomatic model of synucleinopathy.
Keywords: α-synuclein, mouse model, motor behavior, burrowing, nest building, Parkinson's disease
1. Introduction
Parkinson disease (PD) is one of the most common neurodegenerative disorders affecting approximately 1% of elderly people (Tanner, 1992; Varma and Sen, 2015). The disease typically manifests itself between the sixth and seventh decade of life and is predominantly characterized by movement disorders that encompass uncontrollable resting tremors, muscular rigidity, bradykinesia, and postural instability (Dietz, 2001; Khan, et al., 2003). These disorders can be accompanied by other behavioral symptoms, such as depression, sleep disorders, olfactory dysfunction, and progressing cognitive decline (Oertel, et al., 2001; Owen, et al., 1993; Yang, et al., 2016; Zhang, et al., 2016). Pathologically, the disease is characterized by degeneration of dopaminergic neurons in the substantia nigra pars compacta, with consequent depletion of dopamine levels in striatal projections and in other brain regions (Cheesman, et al., 2005; Cools, et al., 2001; Lewis, et al., 2005; Mehler-Wex, et al., 2006). The main histopathological features of PD are Lewy bodies and Lewy neurites, proteinaceous fibrillar cytoplasmic inclusions composed mainly of α-Synuclein (α-Syn) protein (Emmer, et al., 2011; Giasson, et al., 2002; Goedert, 2001; Graham and Sidhu, 2010; Oaks, et al., 2013; Paumier, et al., 2013; Uchihara and Giasson, 2016; van der Putten, et al., 2000). PD is predominantly a sporadic disease of unknown pathogenesis, with no mechanism-driven therapeutic interventions available at present. Symptomatic therapy based on administration of dopamine precursor L-DOPA is successful (Jimenez-Shahed, 2016; LeWitt, 2016). Unfortunately, negative side effects and decreasing efficacy of long-term treatments (Ceravolo, et al., 2016; Nonnekes, et al., 2016) present serious constraints to these symptomatic therapeutic approaches.
The identification of familial forms of PD, with the mutations in many genes including SNCA/α-Syn, Parkin, DJ-1, and LRRK2/dardarin (reviewed by Klein and Schlossmacher, 2007), created opportunities to generate genetic animal models replicating pathological and phenotypic facets of the disease. These single gene defects, however, are relatively rare and represent only about 10% of all PD cases (Klein and Schlossmacher, 2007; Recchia, et al., 2004). Although the etiology of sporadic cases of PD is unknown, both idiopathic and sporadic cases are characterized by the loss of dopaminergic neurons and Lewy pathology. α-Syn protein has been shown to be the major component of Lewy pathology in PD and other neurodegenerative synucleinopathies (Emmer, et al., 2011; Giasson, et al., 2002; Goedert, 2001; Graham and Sidhu, 2010; Oaks, et al., 2013; Paumier, et al., 2013; Peelaerts, et al., 2015; Uchihara and Giasson, 2016; van der Putten, et al., 2000). Consequently, the generation of mouse models expressing wild type or mutant α-Syn (Giasson, et al., 2002; Magen and Chesselet, 2010; Magen, et al., 2012; Rockenstein, et al., 2002) provided an experimental tool to replicate and investigate canonical characteristics of the disease, like the loss of dopaminergic neurons and consequent deterioration of the motor system (reviewed by Chesselet and Richter, 2011). The models expressing wild-type α-Syn are notably interesting since they replicate the prevalent sporadic cases of the disease, and the simple over expression of wild-type human α-Syn due to the duplication or triplication of the SNCA gene is sufficient to cause PD and related disorders (Chartier-Harlin, et al., 2004; Singleton, et al., 2003).
In the present study we characterized the motor phenotype of a mouse model over-expressing wild type human α-Syn gene driven by the murine PrP promoter, denoted M20 (Giasson, et al., 2002). The original general characterization of hemizygous and homozygous M20 mice revealed no overt neurological phenotypes or α-Syn inclusions in the brain at ages up to 24 and 14 months, respectively (Giasson, et al., 2002). Interestingly, another mouse model expressing human wild-type α-Syn under Thy1 promoter (Thy1-αSyn) (Rockenstein, et al., 2002) presents early and progressive deterioration of motor function evaluated in beam traversing, inverted grid, and pole tests (Fleming, et al., 2004). These mice also showed impairment in the response to stimulus removal and deterioration of fine motor skills at the age of 6 months (Fleming, et al., 2004). Following, in our study we characterized the motor behavior of the M20 model in detail given that the differences in the promoter used to drive transgene expression (mouse PrP versus Thy-1) and in the mouse genetic background might affect observed phenotypes (Janus, et al., 2016; Wahlsten, et al., 2006) and brain pathology of mouse models (Lehman, et al., 2003; Magara, et al., 1999; Seabrook, et al., 2004). Such phenotypic characterization of the M20 model is timely as the mice have recently been used in many α-Syn prion-like transmission studies (Ayers, et al., 2015; Rutherford and Giasson, 2015; Rutherford, et al., 2017; Sacino, et al., 2014; Sacino, et al., 2014; Sorrentino, et al., 2017) and they are currently being used in many ongoing studies.
Here we report that 6-month-old M20 mice showed differences in their motor behavior in a battery of tests including inverted wire, pole descending, beam traversing, and rotarod tests, as well as in species-specific home cage burrowing activity as compared to their non-transgenic (nTg) littermates.
2. Methods
2.1 Mice
Males and females transgenic (Tg) mice expressing wild type human α-Syn (M20 line, (Giasson, et al., 2002) and their non-transgenic (nTg) littermates were used in the study. Only heterozygous Tg mice were used. The level of human α-Syn expression in the cortex of the M20 mice is 5.6 ± 0.07 over the endogenous mouse α-Syn level (Giasson, et al., 2002). The expression pattern of human α-Syn in these mice have previously been reported using immunoblotting and immunocytochemical analyses (Emmer, et al., 2011; Giasson, et al., 2002; Sacino, et al., 2014). Human α-Syn in these mice is widely expressed at similar levels throughout the CNS neuronaxis and, in the naïve state, these mice do not develop α-Syn pathological inclusions. The mice were maintained on hybrid C3;B6 genetic background, were not homozygous for the recessive retinal degeneration (rd), and were bred in house in the Animal Care facility of the University of Florida, Gainesville. Twenty two mice [12 nTg (3♂:9♀) and 10 M20 (3♂:7♀)] were used in the study. Mice were housed in same-sex groups of 2 - 4 under standard laboratory conditions (12:12h light/dark cycle, lights on at 0600 h) with room temperature of 22°C, and water and food available ad libitum. All tests were performed during the light phase of the cycle between 09:00 and 14:00 h in accordance with AAALAC and institutional guidelines. Institutional Animal Care and Use Committee of University of Florida approved all experimental protocols used in the study.
2.2 Experimental design
The mice were transferred to the behavioural lab at 3 months of age. Their body weight was recorded upon arrival, and once per month afterwards up to the age of 7 months when all tests were concluded. In order to facilitate individual recognition of mice, their tails were individually marked with black dots, using non-toxic, waterproof permanent marker (Sharpie), corresponding to ear-punch markings of the mice within each cage. The tail marking was reapplied once per week afterwards to maintain its visibility. The mice were handled using hand-cupping method throughout the study. In this method, mice were scooped up by the experimenter's cupped hands and were transferred to a testing apparatus without physical restraint (Hurst and West, 2010). Prolonged physical restraint or picking mice up by their tail increases anxiety (Hurst and West, 2010), which confounds the performance in behavioral tests. The behavioral tests were administered sequentially; with SHIRPA overall screen first (age of testing ∼6.5 months), followed by inverted wire (IW) test (∼6.5 months of age), pole test (∼6.6 months), grip test (∼6.6 months), beam traversing test (∼6.7 months), rotarod (RR) test (6.9 months), and burrowing and nest building test (∼7.0 months).
2.3 Behavioral tests
Primary neurological and sensorimotor examination (SHIRPA, SmithKline Beecham Pharmaceuticals; Harwell, MRC Mouse Genome Centre and Mammalian Genetics Unit; Imperial College School of Medicine at St Mary's; Royal London Hospital, St Bartholomew's and the Royal London School of Medicine; Phenotype Assessment). The SHIRPA screen (Rogers, et al., 1997) involves a series of short tests assessing the physical conditions of a mouse, including: (1) body position in a cage, respiration, tremor, transfer arousal, palprebral closure, piloerection, gait, and tail elevation; (2) reflexes -pinna reflex, trunk curl, limb grasping, visual placing, negative geotaxis, and righting reflex.
Inverted Wire Test
The test, also known as Mesh Grip Test, assesses four-limb neuromuscular strength and grip endurance (Yao, et al., 2011). A mouse was placed in the center of the wire mesh (27 × 28 cm wire grid with wires 0.9 cm apart) that was held horizontally. The firm grip of the legs was induced by the gentle horizontal movement of the grid 2 – 3 times, followed by inverting it and holding it ∼40 cm above an open larger cage padded with a sponge covered by 2 layers of soft padding (Protection Plus Disposable Underpads, Medline Industries, Inc.) to cushion any falls. All mice received one session of habituation to the testing conditions, followed by three days of testing with one trial per day. A trial ended when the mouse fell from the grid or at the cut off time of 100 seconds.
Pole Descending Test
The test assesses motor coordination of mice while descending a vertical pole (Chesselet, et al., 2012; Fleming, et al., 2004). A mouse was placed head up near the top of a vertical wooden pole (50 cm long, 1 cm in diameter, wrapped by firmly glued plastic mesh to facilitate grip and preventing sliding). The times to turn head down and descend the pole were measured by a stopwatch and recorded separately. The cut off time of the test was 120 seconds. All mice were habituated to the test in two consecutive days with 3 trials per day, followed by a one-day test with 3 trials. The average durations to turn to head down position and the time to descent the pole are reported.
Grip Strength Test
The test is widely used as a non-invasive method to evaluate mouse limb strength. It is based on the natural tendency of a mouse to grasp a bar or a grid when briefly suspended by a tail. We used a horizontal bar version of the test, where the mouse grasps the horizontal bar (a toothpick) with its forelimbs. A mouse was picked up by the base of a tail and gently lowered towards the horizontal bar until it grasped the bar. The hind legs were then supported by an experimenter's hand and the mouse was gently pulled backward until it released a grip. The maximum pull-force at the time the animal released the grip was recorded on a horizontally mounted scale (Pesola Spring Scale model 40300) equipped with a drag pointer. The test was administered in two consecutive days with three trials per day. The averaged maximum score across the two tests was used for the analysis.
Beam Traversing test
The test assesses a mouse's ability to maintain balance while traversing a narrow beam to reach the safety of a goal cage (Carter, et al., 1999). Two types of square aluminum 110cm-long beams; 2.5 and 1.7 cm wide, respectively, were used in the test. Both beams were fitted with 0.62 cm square obstacles that were perpendicular to the width of a beam and spaced at 10 cm intervals. The beam was elevated 50 cm above the surface of a testing bench. The start end was lit by a 40 W lamp fixed ∼25 cm above the beam, while the opposite goal end was aligned with a 4.5 cm circular opening to the goal cage. The goal cage was filled with fresh cage bedding mixed with the bedding taken from the home cage of a tested mouse, and contained a square of pressed cotton (Nestlet) placed in the middle. A strong white 50 cm wide surface liner (Fisherbrand™) was suspended 40 cm below the beam to catch a mouse in the case of a fall. Two days before the test, the mice were habituated to the testing conditions using a wide (2.5 cm) beam. During habituation, each mouse was placed on a beam facing the entrance to the goal cage at ∼10 cm from the entrance. Upon the entrance to the cage, a mouse was allowed to explore it for ∼30 s. A second and third trial was administered, increasing the distance of placement on a beam to 20 and 40 cm from the cage entrance, respectively. On the second day, each mouse was given 2 trials being released from the start end of the wide beam. In the case a mouse did not traverse the beam and did not enter the goal box, additional training trials (up to 3 in total) were administered. Following the habituation, the mice were tested during 4 consecutive days, traversing the wider (2.5 cm) beam during the first two days, followed by two days of traversing the narrower (1.7 cm) beam. Each day of testing comprised of two trials. The recorded measures included: latency to traverse the beam, time to reach the goal cage, number of pauses, turns in the direction, and foot slips. The fall from the beam terminated the test. The cut off time of the test was set to 120s.
Rotarod test
The test evaluates neurological deficits in the motor coordination of rodents (Dunham and Miya, 1957). In this test a mouse is challenged to stay upright on a horizontal rod that gradually accelerates along its long axis. The mice were trained in squads of 4 in the Rotamex-5 apparatus (Columbus Inst. OH). The apparatus was fitted with a mouse rod (spindle) 3 cm in diameter, which has a knurled surface of parallel ridges along its long axis to ensure secure grip. The rod was mounted 44 cm above the surface. Two days before the test, all mice were given three, 5-min habituation trials; the first with constant (4 rpm), and the 2nd and 3rd with accelerating (4 to 20 rpm) rotation of the rod. The mice were tested in three consecutive days, with three, 5-min trials (inter-trial-interval of 40–50 min) and gradual acceleration of the rod from 4 to 40 rpm within the 5-min trial. An array of infrared beams recorded the upright position of a mouse on the rod. In the case of passive rotation (a mouse loses its balance, but clings to the rod and recovers balance after a full rotation), the latency to the first passive rotation was recorded and reported along with the total time to fall and the rotation speed at fall.
Burrowing and nest building activity
Although mice burrow spontaneously (Dudek, et al., 1983), the burrowing test was preceded by a habituation trial that acclimated mice to the novel conditions of the test and burrowing activity (Deacon, 2006). A day before the test, all cage mates were moved as a group from their home cage to a larger rat cage (26 × 48 × 20 cm) fitted with a burrow consisted of a plastic white tube, 20 cm long and 7.5 cm in diameter, which was raised 2.5 cm by two screws bolted at the open end. The lower end of the burrow was closed off. The tube contained ∼200g of standard mouse food pellets. Also, a commercially available, pressed 2.5 cm square cotton wafer (Nestlet, Ancare) was placed in the cage. The cage water bottle was fitted with longer (11 cm) water tube allowing the mice easy access to water. The burrowing test was administered the following day. Three hours before the onset of the dark phase of the LD cycle, mice were placed individually in rat cages containing a burrow, filled with ∼200g of food pellets. A nestlet and two additional food pellets were placed in the middle of the cage floor, covered by a thin layer of bedding. The amount of food pellets displaced (initial food pellets weight (∼200 g) minus the weight of the pellets left in a tube) after the first 2 h of the test was recorded and the test continued overnight with the remaining pellets left in a burrow. The following morning, the mice were returned to their home cages and the weight of the pellets left in the burrow was recorded. The burrowing activity was expressed as the percent of food pellets displaced after 2 h and overnight. The nest quality was assessed on a 1 to 5 scale: with 1 - nestlet no shredded, no nest; 2 – nestlet shredded, scattered on the floor; 3 – nestlet mostly shredded, flat nest; 4 – nestlet shredded, bowl nest; 5 – nestlet shredded, nest in a burrow.
2.4 Data analysis
Departures from normal distribution were checked using Kolmogorov-Smirnov (K-S) goodness of fit test. General linear model of factorial ANOVA (Statistical Package for Social Sciences, SPSS v.23, Inc. Chicago) with genotype (M20 versus nTg) as between subject, and test sessions as within subject factors, was used to analyze the data. When necessary, degrees of freedom were adjusted by Greenhouse-Geisser epsilon correction for the heterogeneity of variance. Comparisons between two independent groups were done using Student t-test. Comparisons against chance performance were done using Kolmogorov-Smirnov one sample t-test. The comparisons of scores obtained on ordinal scale of measurement (SHIRPA screen, nest quality scale) were performed using non-parametric Mann-Whitney U test for two independent samples. The critical α level was set to 0.05, and all values in the text and figures represent means ± SEM. Due to limitation of space only significant results pertaining to the main experimental factors and interactions are reported.
3. Results
3.1 Body weight
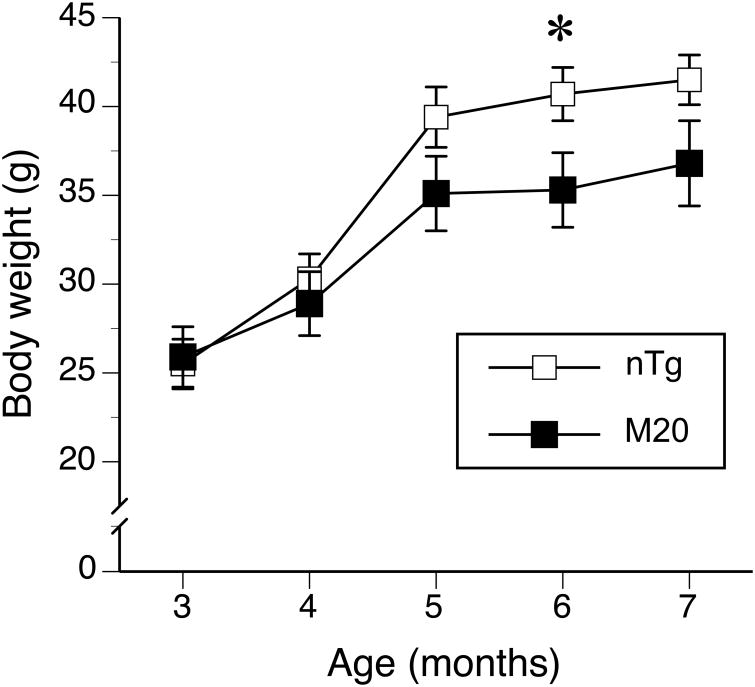
Both M20 Tg and their nTg littermates exhibited an increase in body weight between the age of 3 and 7 months (F(2,37) = 195.7, p < 0.001; dfs adjusted by Greenhouse-Geisser epsilon correction). Although, the body weight of M20 mice was not significantly different from the nTg mice (F(1,20) = 1.7, p = 0.2), they gained weight at slower rate than nTg mice (F(2,37) = 8.5, p = 0.001, age body weight × genotype interaction effect). The comparisons of the differences at each age revealed that M20 mice showed significantly lower body weight only at the age of 6 months (t(20) = 2.2, p < 0.05, Fig. 1). The phenotypic evaluation of the mice took place between the 6 and 7 month of the mice's age and at that age the M20 mice showed steadily lower body weight than the controls (Fig. 1). Since, the body weight was not randomly distributed and independent of the genotype (r2 = 0.19, p < 0.05, df = 22), it could not be used as a covariate in the analyses of data. The lower body weight of M20 mice indicated shared variance with the genotype, which violated the assumptions of the analysis of covariance (Miller and Chapman, 2001; Tabachnick and Fidell, 1989). We report, however, the association of the body weight with the performance of the mice in each behavioral test separately to provide an insight of the effect of body weight on mice performance in a test.
Figure 1. Transgenic M20 mice over-expressing wild type human α-Syn showed lower body weight than their non-transgenic littermates.
The body weight of M20 (n = 10) and nTg (n = 12) mice was recorded between 3 and 7 months of age. The M20 mice showed a slower body weight gain over time, especially within the last 2 months (5-7 months of age) of the study (p = 0.001, age by genotype interaction). Data are expressed as the mean ± SEM. * p < 0.05.
3.2 Effects of α-Syn expression on physical condition and reflexes of mice (SHIRPA screen)
The M20 did not differ from nTg littermates in most measures evaluating physical conditions and reflexes. All mice showed normal respiration and active exploration of a novel cage and performed frequent rearings. Their gait was normal, with no noticeable tremor, their eyes were fully opened and tail horizontally extended during walking. The pinna reflex, the extension of forelimbs during visual placing and righting reflex were present and comparable between the M20 and their control littermates. However, the negative geotaxis reflex differentiated the genotypes (Mann-Whitney U = 33.5, nnTg = 12; nM20 = 10, p < 0.05, two-tailed test). Eleven out of 12 nTg mice turned and climbed upwards on an inclined wire grid, with only one nTg mouse freezing after turning. On the other hand, only 5 out of 10 M20 mice showed the negative geotaxis reflex, two turned but froze, two failed to turn, and one M20 mouse did not move within the 30 sec cut off limit.
Inverted wire test
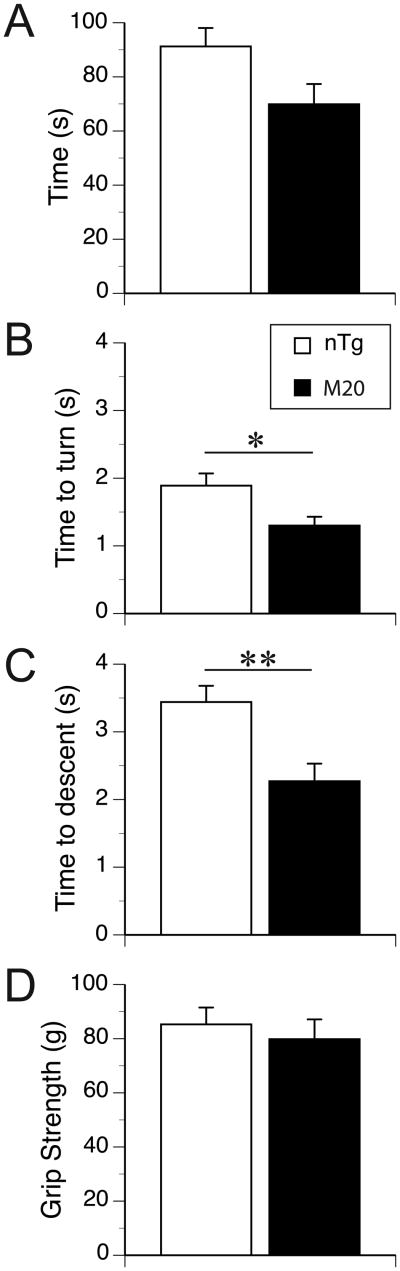
Overall the M20 mice showed significantly shorter times of grip endurance than the nTg counterparts (F(1,20) = 4.5, p < 0.05, Figure 2A). The endurance scores were comparable across all three days of testing (F(1,28) = 1.3, p = 0.3, dfs adjusted by Greenhouse-Geisser epsilon correction), with no significant interaction between test days and genotypes. The performance in this test was not affected by the differences in the body weight of mice.
Figure 2. Evaluation of M20 and nTg control mice in inverted wire, pole descending and grip tests.
(A) The M20 mice (n = 10) showed shorter times of grip to inverted wire grid as compared to their nTg littermates (n = 12) (p < 0.05). (B and C) Pole descending test; after being placed at the top of 50-cm vertical pole, the M20 mice showed shorter latencies of head-down turns than nTg control (B, p < 0.05). Also, their descent time was shorter than the descent of the control mice (C, p < 0.01). (D) The grip strength of the forelimbs of both M20 and nTg mice was comparable. Data are expressed as the mean ± SEM. * p < 0.05; ** p < 0.01.
3.3 Effects of α-Syn expression on motor behavior
Pole descending test
The time of head-down turns at the top of the pole, and the consequent descent time from a vertical pole for the M20 and nTg mice are shown in Fig 2B and C, respectively. The M20 mice turned head-down faster and also descended the pole faster than nTg mice (t(20) = 2.6, p < 0.02, Fig. 2B and t(20) = 3.3, p < 0.01, Fig. 2C, respectively). Neither measure was significantly affected by the body weight (data not shown).
Grip Strength test
The strength of the forelimbs was comparable between the M20 and nTg mice (Fig. 2D), and it was not affected by the differences in the body weight (data not shown).
Beam traversing test
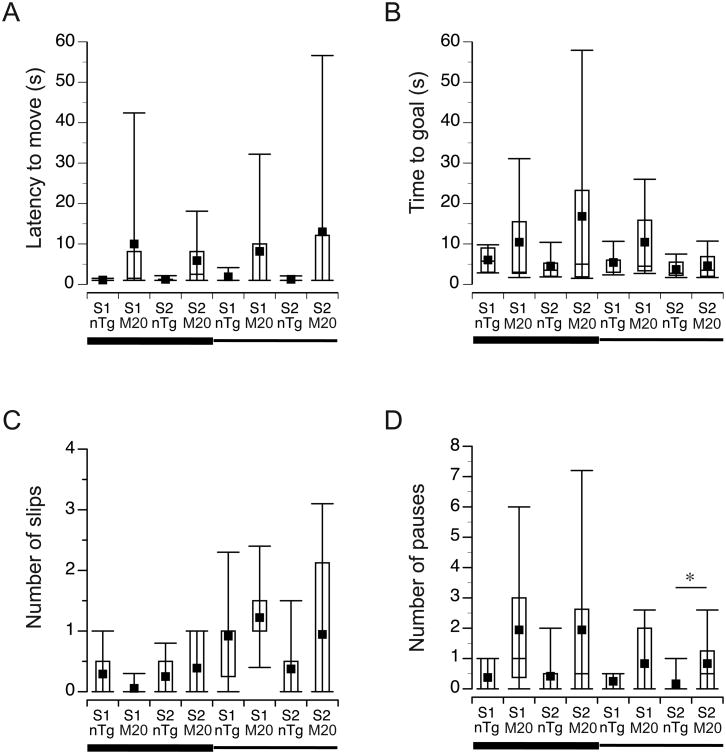
None of the variables (latency to move, time to the goal, number of slips, pauses, and falls) were affected by the body weight of mice (data not shown). Although, the M20 mice showed longer latencies to begin traversing the beams (Fig. 3A), the effect of the genotype was not significant (F(1,19) = 3.6, p = 0.08). Similarly, the time to reach the goal cage of M20 mice tended to be longer than the time of nTg littermates (9.9 ± 2.4 sec versus 4.9 ± 2.1 sec, respectively), but the difference was not significant (F(1,19) = 2.4, p = 0.1, Fig. 3B). Since the completion of the test relies on spontaneous behavior of mice to traverse the beam, the scores yielded by this test are usually more variable and more labile to changes in procedures or laboratory conditions (Janus and Welzl, 2010; Wahlsten, et al., 2003). Therefore, we presented the results using box plot graphs, which depict first quartile, median, mean, third quartile, and interquartile range (Fig. 3) to reflect the nature of the scores obtained in the test. No significant interactions between the beam type and the genotype of mice were found in the analyses of the latency to move or the time to reach the goal. Since the time to traverse the beam depends not only on the speed of walking, but also on the fluidity of movements and limb coordination, we analyzed additionally the number of slips and pauses exhibited by mice during the test. These analyses revealed that the M20 and nTg mice showed comparable levels of slips during traversing both the wide and the narrow beams (Fig. 3C), and all mice slipped more often when traversing the narrow beam (Z = -3.31, p = 0.001, Wilcoxon Signed Ranks test, Fig. 3C). Also, the overall number of pauses was comparable between M20 and nTg mice when traversing the wide (Mann-Whitney U = 33.5, nnTg = 12; nM20 = 10, p = 0.07, two-tailed test) and the narrow (Mann-Whitney U = 39.5, nnTg = 12; nM20 = 10, p = 0.16, two-tailed test) beams (Fig. 3D). However, The post-hoc analysis of pauses revealed that M20 mice paused more frequently than nTg littermates while traversing the narrow beam during the second session of the test (Mann-Whitney U = 28.0, nnTg = 12; nM20 = 10, p < 0.05, two-tailed test, Fig. 3D). The comparison of pauses on the wide and the narrow beams within each genotype revealed comparable performance for nTg (Z = -1.27, p = 0.21, Wilcoxon Signed Ranks test) and for M20 (Z = -1.95, p = 0.051, Wilcoxon Signed Ranks test) mice. The rate of pauses, but not slips, was positively associated with the longer time to reach the goal box during traversing the wide beam for both M20 and nTg mice (r = 0.87, n = 10, p = 0.001 and r = 0.96, n = 12, p < 0.001, respectively), but only for nTg mice during traversing of the narrow beam (r = 0.58, n = 12, p < 0.05). None of the tested mice fell off the beam during the entire test.
Figure 3. Locomotor evaluation in beam-traversing test.
M20 and nTg mice (n = 10 and n = 12, respectively) were tested on wide and narrow beams in two consecutive days (sessions); each session consisting of two trials. Data from the two trials were averaged and are presented as box plots, including; first quartile, median (horizontal line in the central box), mean (black square), third quartile (the central box represents an interquartile range). The whiskers on the bottom and the top represent the 10th and the 90th percentile of the scores. S denotes session. The horizontal wide and narrow lines below x-axis represent the 2.5-cm and 1.7cm wide beams, respectively. * p < 0.05.
Rotarod test
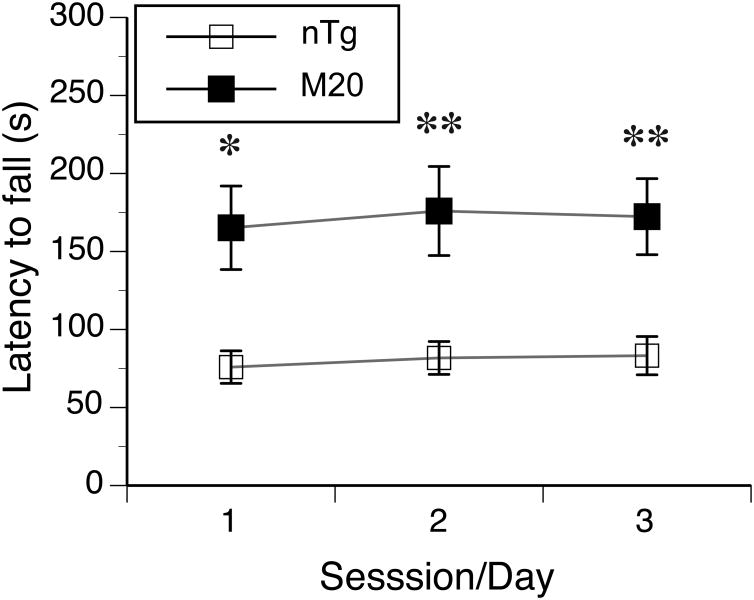
One M20 female was removed from rotarod test because it was persistently jumping off the rod. Since the latency to fall from the rotating rod was significantly associated with its revolution (rpm) at the fall (Pearson correlation coefficients for both M20 and nTg mice varied between 0.99 to 1.0 for the 3 sessions of the test, all ps < 0.001), we report only the latency to fall (Fig. 4). Overall, the latency (averaged over the 3 test sessions) to fall from the accelerating rod was 80.3 s for nTg and 171.2 s for M20 mice, which corresponded to 14 and 25 rpm of rotating speed, respectively. Overall, M20 mice stayed significantly longer on the accelerated rod than their nTg littermates (F(1,19) = 13.2, p < 0.01, Fig. 4). The latency to fall was not significantly different between the 3 sessions of the test within each genotype, and no significant interaction between the genotype and testing sessions was observed. Post-hoc analysis revealed that the genotypes differed significantly at each day of testing (Fig. 4). Although the associations between the body weight and the latencies to fall were negative within each genotype in all three sessions, none of the associations reached significance at α = 0.05.
Figure 4. Transgenic M20 mice showed longer latencies to fall from the accelerated rod than nTg littermates in rotarod test.
The mice (n = 10 and n = 12 for M20 and nTg, respectively) were tested in 3 consecutive days (sessions), with three trials per session. Data from the three trials within each session were averaged and are presented as the mean ± SEM. * p < 0.05; ** p < 0.01.
3.4 Effects of α-Syn expression on species-specific home cage behavior
Burrowing and nest building behavior
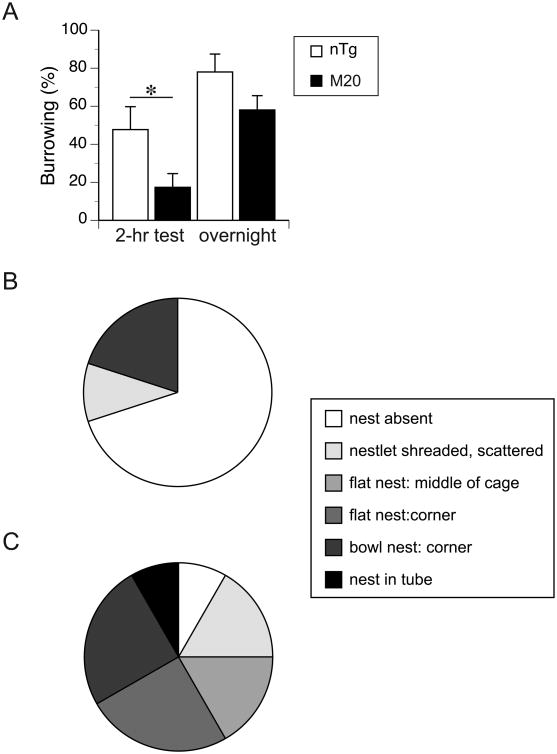
All mice were engaged in removing food pellets from the tubes shortly after being introduced to the testing cages. The M20 mice, however, displaced significantly fewer pellets than the control nTg littermates after the first 2-hour period of the test (t(18) = 2.2, p < 0.05, Fig. 5A). They also tended to remove fewer pellets than nTg mice overnight (18 hr after the onset of the test); however, the difference was not significant at α = 0.05 (Fig. 5A). The M20 mice also built less elaborated nests as compared to their nTg control littermates (Fig. 5B). Out of the 12 nTg mice, four built either bowl-shaped nests in the corner of the cage or a nest inside an empty burrowing tube, another 3 mice building flat nests in the cage corners (∼ 58% in total), and only 1 nTg mouse did not shred the nestlet at all (Fig. 5C). In contrast, none of the M20 mice build a nest in a burrowing tube, only 2 mice build a bowl-shaped nest in the corner of the cage and 7 out of the 10 mice did not shred the nestlets at all during the test (Fig. 5C). These differences in the nests quality between nTg and M20 mice were significant (Mann-Whitney U = 24.5, nnTg = 12; nM20 = 10, p < 0.02, two-tailed test).
Figure 5. M20 mice showed reduced burrowing activity and nest building behavior.
(A) M20 mice (n = 10) displaced fewer pellets from the burrow than nTg littermates (n = 12) after 2 hours from the onset of the test. The reduced burrowing of M20 mice continued throughout the rest of the test duration. (B) The majority of the M20 mice (7 out of 10) did not shred nestlets and build nests. (C) Most of the nTg mice (9 out of 12) shredded nestles and built the nest that was confined either in the middle or corners of the cage, or in the burrowing tube, Only one nTg mouse did not build a nest during the test. The differences in the nest quality between the genotypes were significant (p < 0.02, Mann-Whitney test).
4. Discussion
There is a general consensus, based on the available models that α-Syn biological alterations lead to motor dysfunction (Emmer, et al., 2011; Giasson, et al., 2002; Goedert, 2001; Graham and Sidhu, 2010; Oaks, et al., 2013; Paumier, et al., 2013; Uchihara and Giasson, 2016; van der Putten, et al., 2000). In humans, these motor dysfunctions are primarily due to dopaminergic deficiencies in the substantia nigra par compacta and striatum that is accompanied by neuronal α-Syn aggregation, but many additional regions of the CNS are also affected (Uchihara and Giasson, 2016). Detailed accounts of pathologies and behavioral deficits in α-Syn animal models are presented in several scholarly reviews (Beal, 2010; Chesselet and Richter, 2011; Fernagut and Chesselet, 2004; Magen and Chesselet, 2010). The over expression of full-length wild type human α-Syn gene generally manifests less severe pathological phenotypes in these models (reviewed by Magen and Chesselet, 2010). However, the simple over expression of wild-type human α-Syn due to the duplication or triplication of the SNCA gene is sufficient to cause PD and related disorders (Chartier-Harlin, et al., 2004; Singleton, et al., 2003). Furthermore, recent studies indicate that α-Syn expression can be altered by β2-adrenoreceptor activation, which can be a risk factor for PD (Mittal, et al., 2017). Thus, models of wild-type human α-Syn over expression present a useful tool to study the causative factors underlying PD (Edwards, et al., 2010; Gatto, et al., 2010).
Our results showed that over expression of wild-type α-Syn under the murine PrP promoter, which is relatively uniform across the neuroaxis (Emmer, et al., 2011; Giasson, et al., 2002; Graham and Sidhu, 2010; Oaks, et al., 2013; Paumier, et al., 2013; Sacino, et al., 2014; van der Putten, et al., 2000), decreased the rate of body weight gain of the mice within the first 6 months of age and modulated their motor behavior in a task-specific manner. Overall, the general physical conditions, including responses to novel environment, basic reflexes, as well as the physical strength of front paws were comparable between M20 and nTg control mice. Interestingly, the lower body weight did not affect the performance of M20 mice in the battery of administered tests; however, the direction of changes differentiating the M20 mice from their control littermates was task specific. Specifically, while the M20 mice showed shorter grip endurance in the inverted wire test, and longer pauses resulting in longer times to traverse narrow elevated beams as compared to their nTg littermates, the results which suggests impairments in performance in these task, faster times to descend the vertical pole (Fig. 2B and C) and longer latencies to fall off the accelerated rod (Fig. 4) might suggest improved performance. It has to be noted, however, that another line of α-Syn transgenic mouse line, denoted M7, that also expresses human α-Syn and that was generated with the same construct as the M20 line showed no significant impairment on the rotarod task at 7 month of age: nTg = 306.2 +/- 11.0 (n = 12), M7 homozygous = 316.4 +/- 12.0 (n = 12) (Giasson, et al., 2002). The most parsimonious explanation of this discrepancy might be that the M7 mice express about 2 fold less human α-Syn than the M20 line and that the rotarod test was performed at only at one time point for the M7 line. Overall, our observation that the M20 mice began to show lower body weight than their control littermates at about 4 months of age is comparable with the changes in the body weight of Thy1 α-Syn mice that showed lower body weight at a similar age (Chesselet, et al., 2012). Earlier studies using male only mice reported lower body weight in Thy 1 α-Syn model even earlier, at of 2 months age (Fleming, et al., 2004). Similarly to M20 mice, the Thy1 α-Syn mice showed impairment in movement coordination while traversing elevated beams (Chesselet, et al., 2012; Fleming, et al., 2004). However, both the times to orient head down and descend the vertical pole were significantly longer in Thy1 α-Syn male mice as compared to their controls (Fleming, et al., 2004), which is in contrast to the results observed in M20 mice in the present study. Also, the performance on the inverted wire grid differed between the two models. Despite the differences between the recorded parameters (the latency to fall in the present study, and step distance in the case of Thy1 α-Syn mice (Fleming, et al., 2004)) the Thy1 α-Syn mice showed lower step distance, indicating their lower activity. Our qualitative observations indicate that M20 mice were falling sooner, in most cases due to increased locomotor activity, off the inverted wire grid. Also, the similarity between the two models can be drawn when comparing home cage, species-specific behavior. Our results provide compelling evidence of impaired burrowing activity and nest building behavior. Similar results were demonstrated in a series of elegant experiments showing that Thy1 α-Syn mice were impaired in cotton manipulation and adhesive removal (Fleming, et al., 2004) as well as nest building (Chesselet, et al., 2012), at the ages comparable to the age at which the M20 mice were tested. The observed differences between the M20 and Thy-1 α-Syn mice might be due to the differences in protocols and mouse husbandry between different laboratories (Janus and Welzl, 2010; Wahlsten, 2001; Wahlsten, et al., 2003) or due to the use of different promoters (Janus, 2016). Differences in the relative expression of α-Syn in transgenic mice generated using the Thy and PrP promotes are possible, although both promoters reportedly result in wide spread CNS neuronal expression.
The result demonstrating that M20 mice showed consistently longer latencies than their nTg littermates to fall off the rotating rod is surprising, but this behavior of M20 mice was reliable and repeatable; they usually stayed on the rod about 70 s longer than nTg littermates (Fig. 4). This behavior seems to be a characteristic phenotypic feature of the M20 model, at least at the age of 6-7 months, since we observed it in a pilot experiment as well as in an independent unpublished study (data not shown). Unfortunately the Thy1 α-Syn mice were not evaluated in the rotarod test (Chesselet, et al., 2012; Fleming, et al., 2004; Magen, et al., 2012). However, a different model, over expressing human wild-type α-Syn under the control of the platelet-derived growth factor-β (PDGF-β), showed significant impairment in the rotarod test; with Tg α-Syn mice showing shorter latencies to fall off the rod (Masliah, et al., 2000). Interestingly, yet another model, over expressing the mutated human A53T α-Syn (α-SynA53T) gene under the murine PrP promoter, showed significantly longer latencies of falling off the accelerated rod as compared to their control counterparts at the age of 9 months (Guerreiro, et al., 2017). An explanation suggesting that the lower body weight might facilitate longer latencies in the rotarod test (Guerreiro, et al., 2017) is unlikely in the case of the M20 model because the body weight was not associated with the rotarod performance of the mice. A possible behavioural factor underlying longer latencies to fall off the rod observed in some models over expressing α-Syn might be related to their reduced anxiety as compared to α-Syn knockout or nTg controls (George, et al., 2008). The Tg α-Syn A53T mice (Giasson, et al., 2002) were evaluated in plus maze and open field, and showed reduced locomotor habituation, while spending a higher proportion of time in the open arms of the plus maze (George, et al., 2008). The same model, evaluated in an independent study in both rotarod and plus maze, demonstrated shorter latencies to fall, despite showing lower anxiety levels in the plus maze test at the age of 12 months and up (Oaks, et al., 2013).
At present, it might be difficult to reconcile the observed phenotypic discrepancies observed in rotarod test between various models over expressing α-Syn. The usual suspects related to different mutations, prompters, gene copy numbers and idiosyncratic to each laboratory testing protocols (Janus and Welzl, 2010; Wahlsten, et al., 2003), or the differences in the handling of mice before testing (Hurst and West, 2010; Janus, et al., 2016) that might affect the phenotypes of mice should always be considered, however, in the case of M20 mice the longer latencies of fall off the accelerated rod were observed repeatedly in our lab (University of Florida), regardless of the amount of pre-experimental handling, as well as independently at University of Pennsylvania (Giasson, unpublished data). Alternatively, the expression of human wild-type α-Syn in a mouse results in more benign pathological phenotype than the expression of mutated α-Syn. The lack of overt loss of dopaminergic neurons, as established in M20 mice (Giasson, et al., 2002), might likely model the early stages of idiopathic PD (Chesselet, 2008). Detailed characterization of Thy1 α-Syn mice revealed that the mice showed elevated levels of extracellular dopamine in the straitum around 6 months of age, which coincided with their increased activity in the open field (Lam, et al., 2011). Future studies will establish whether the same phenotype can be observed in M20 mice, and whether the increased level of dopamine in the striatum might affect the motor behaviour of the mice in the rotarod test. Although the link between the mutations in α-Syn and PD is well established, the normal function of α-Syn, including its role in neuronal functioning that affects behaviour, is not fully elucidated. α-Syn is redundant with β-Syn in the CNS, but interestingly deletion of both of these synuclein proteins does not affect brain basic functions or survival in double-knockout mice (Chandra, et al., 2004). Although dopamine levels were decreased by 20%, its uptake and release from synaptic terminals was normal, suggesting that synucleins may not be critical for neurotransmitter release, but may significantly contribute to the regulation or maintenance of presynaptic function (Chandra, et al., 2004). Increased levels of dopamine at the early stages of PD often lead to increased general activity, impairments in set-shifting, task switching or probabilistic reversal learning (Cools, et al., 2001; Cools, et al., 2001; Partiot, et al., 1996; Peterson, et al., 2009; Taylor, et al., 1990), and tasks that implicate basal ganglia and frontostriatum circuits (Taylor, et al., 1990), including nucleus accumbens (Groenewegen, et al., 1996), and manifest behaviourally by the increase in repetitive behaviours, which in mice might include longer latencies to fall off the rotating rod (Rothwell, et al., 2014). In conclusion, a parsimonious explanation of persistently longer latencies to fall off the accelerated rod suggests that the increased levels of dopamine at stages preceding overt motor dysfunction might inadvertently affect fronto-striato circuits with consequent increases in repetitive behaviours. The comparative studies between lines expressing wild type (pre-symptomatic mild pathology) and mutated (progressing pathology with loss of dopaminergic neurons) lines of α-Syn transgenic mice should elucidate whether longer latencies of falling off the rod might present an early pathological biomarker of functional dysfunction of striatum - basal ganglia circuits.
Highlights.
M20 mice expressing human wild type SNCAα-Syn gene had lower body weight
The physical development and reflexes of the M20 mice were not compromised
Motor behavior of M20 mice was compromised in the wire and beam traversing tests
M20 mice increased their performance in the vertical pole and rota-rod tests
M20 mice showed impaired home cage, species specific burrowing and nest building
Acknowledgments
The study was supported in part by a grant NS089622 to B.I.G., and in part by internal CTRND, UF funds. We would like to thank Ms. Hurara Khan and Mr. Santiago Arminiana for their assistance in the preparation of behavioral experiments.
The manuscript and the presented results have not been published before and the manuscript is not considered for publication elsewhere.
Footnotes
Disclosure statement: The authors declare no conflict of interests.
All experiments were performed in accordance with AAALAC and institutional UF IACUC guidelines.
All authors have reviewed the contents of the manuscript, approved its contents and validated the accuracy of the data.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ayers JI, Fromholt S, Sinyavskaya O, Siemienski Z, Rosario AM, Li A, Crosby KW, Cruz PE, DiNunno NM, Janus C, Ceballos-Diaz C, Borchelt DR, Golde TE, Chakrabarty P, Levites Y. Widespread and efficient transduction of spinal cord and brain following neonatal AAV injection and potential disease modifying effect in ALS mice. Mol Ther. 2015;23(1):53–62. doi: 10.1038/mt.2014.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal MF. Parkinson's disease: a model dilemma. Nature. 2010;466(7310):S8–10. doi: 10.1038/466S8a. [DOI] [PubMed] [Google Scholar]
- Carter RJ, Lione LA, Humby T, Mangiarini L, Mahal A, Bates GP, Dunnett SB, Morton AJ. Characterization of progressive motor deficits in mice transgenic for the human Huntington's disease mutation. Journal of Neuroscience. 1999;19(8):3248–3257. doi: 10.1523/JNEUROSCI.19-08-03248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceravolo R, Rossi C, Del Prete E, Bonuccelli U. A review of adverse events linked to dopamine agonists in the treatment of Parkinson's disease. Expert Opin Drug Saf. 2016;15(2):181–198. doi: 10.1517/14740338.2016.1130128. [DOI] [PubMed] [Google Scholar]
- Chandra S, Fornai F, Kwon HB, Yazdani U, Atasoy D, Liu X, Hammer RE, Battaglia G, German DC, Castillo PE, Sudhof TC. Double-knockout mice for alpha- and beta-synucleins: effect on synaptic functions. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(41):14966–14971. doi: 10.1073/pnas.0406283101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destee A. Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet. 2004;364(9440):1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- Cheesman AL, Barker RA, Lewis SJ, Robbins TW, Owen AM, Brooks DJ. Lateralisation of striatal function: evidence from 18F-dopa PET in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2005;76(9):1204–1210. doi: 10.1136/jnnp.2004.055079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesselet MF. In vivo alpha-synuclein overexpression in rodents: a useful model of Parkinson's disease? Exp Neurol. 2008;209(1):22–27. doi: 10.1016/j.expneurol.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesselet MF, Richter F. Modelling of Parkinson's disease in mice. Lancet Neurol. 2011;10(12):1108–1118. doi: 10.1016/S1474-4422(11)70227-7. [DOI] [PubMed] [Google Scholar]
- Chesselet MF, Richter F, Zhu C, Magen I, Watson MB, Subramaniam SR. A progressive mouse model of Parkinson's disease: the Thy1-aSyn (“Line 61”) mice. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2012;9(2):297–314. doi: 10.1007/s13311-012-0104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or impaired cognitive function in Parkinson's disease as a function of dopaminergic medication and task demands. Cereb Cortex. 2001;11(12):1136–1143. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. Mechanisms of cognitive set flexibility in Parkinson's disease. Brain. 2001;124(Pt 12):2503–2512. doi: 10.1093/brain/124.12.2503. [DOI] [PubMed] [Google Scholar]
- Deacon RM. Burrowing in rodents: a sensitive method for detecting behavioral dysfunction. Nature protocols. 2006;1(1):118–121. doi: 10.1038/nprot.2006.19. [DOI] [PubMed] [Google Scholar]
- Dietz V. Gait disorder in spasticity and Parkinson's disease. Adv Neurol. 2001;87:143–154. [PubMed] [Google Scholar]
- Dudek BC, Adams N, Boice R, Abbott ME. Genetic influences on digging behaviors in mice (Mus musculus) in laboratory and seminatural settings. Journal of Comparative Psychology. 1983;97(3):249–259. [PubMed] [Google Scholar]
- Dunham NW, Miya TS. A note on a simple apparatus for detecting neurological deficit in rats and mice. J Am Pharm Assoc. 1957;46:208–209. doi: 10.1002/jps.3030460322. [DOI] [PubMed] [Google Scholar]
- Edwards TL, Scott WK, Almonte C, Burt A, Powell EH, Beecham GW, Wang L, Zuchner S, Konidari I, Wang G, Singer C, Nahab F, Scott B, Stajich JM, Pericak-Vance M, Haines J, Vance JM, Martin ER. Genome-wide association study confirms SNPs in SNCA and the MAPT region as common risk factors for Parkinson disease. Ann Hum Genet. 2010;74(2):97–109. doi: 10.1111/j.1469-1809.2009.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmer KL, Waxman EA, Covy JP, Giasson BI. E46K human alpha-synuclein transgenic mice develop Lewy-like and tau pathology associated with age-dependent, detrimental motor impairment. J Biol Chem. 2011;286(40):35104–35118. doi: 10.1074/jbc.M111.247965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernagut PO, Chesselet MF. Alpha-synuclein and transgenic mouse models. Neurobiology of disease. 2004;17(2):123–130. doi: 10.1016/j.nbd.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Fleming SM, Salcedo J, Fernagut PO, Rockenstein E, Masliah E, Levine MS, Chesselet MF. Early and progressive sensorimotor anomalies in mice overexpressing wild-type human alpha-synuclein. J Neurosci. 2004;24(42):9434–9440. doi: 10.1523/JNEUROSCI.3080-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto NM, Rhodes SL, Manthripragada AD, Bronstein J, Cockburn M, Farrer M, Ritz B. alpha-Synuclein gene may interact with environmental factors in increasing risk of Parkinson's disease. Neuroepidemiology. 2010;35(3):191–195. doi: 10.1159/000315157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S, van den Buuse M, San Mok S, Masters CL, Li QX, Culvenor JG. Alpha-synuclein transgenic mice exhibit reduced anxiety-like behaviour. Exp Neurol. 2008;210(2):788–792. doi: 10.1016/j.expneurol.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VM. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron. 2002;34(4):521–533. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- Goedert M. Alpha-synuclein and neurodegenerative diseases. Nature reviews. 2001;2(7):492–501. doi: 10.1038/35081564. [DOI] [PubMed] [Google Scholar]
- Graham DR, Sidhu A. Mice expressing the A53T mutant form of human alpha-synuclein exhibit hyperactivity and reduced anxiety-like behavior. J Neurosci Res. 2010;88(8):1777–1783. doi: 10.1002/jnr.22331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV. The nucleus accumbens: gateway for limbic structures to reach the motor system? Prog Brain Res. 1996;107:485–511. doi: 10.1016/s0079-6123(08)61883-x. [DOI] [PubMed] [Google Scholar]
- Guerreiro PS, Coelho JE, Sousa-Lima I, Macedo P, Lopes LV, Outeiro TF, Pais TF. Mutant A53T alpha-Synuclein Improves Rotarod Performance Before Motor Deficits and Affects Metabolic Pathways. Neuromolecular Med. 2017;19(1):113–121. doi: 10.1007/s12017-016-8435-5. [DOI] [PubMed] [Google Scholar]
- Hurst JL, West RS. Taming anxiety in laboratory mice. Nat Methods. 2010:1–2. doi: 10.1038/nmeth.1500. [DOI] [PubMed] [Google Scholar]
- Janus C, Welzl H. Mouse models of neurodegenerative diseases: criteria and general methodology. Methods in molecular biology. 2010;602:323–345. doi: 10.1007/978-1-60761-058-8_19. [DOI] [PubMed] [Google Scholar]
- Janus C, Hernandez C, deLelys V, Roder H, Welzl H. Better Utilization of Mouse Models of Neurodegenerative Diseases in Preclinical Studies: From the Bench to the Clinic. Methods Mol Biol. 2016;1438:311–347. doi: 10.1007/978-1-4939-3661-8_18. [DOI] [PubMed] [Google Scholar]
- Janus C, Welzl H. Neurodegenerative Diseases and Dementia. In: Yong-Kyu K, Gerwitz J, editors. Animal Models of Behavior Genetics Research. New York: Springer; 2016. [Google Scholar]
- Jimenez-Shahed J. A review of current and novel levodopa formulations for the treatment of Parkinson's disease. Ther Deliv. 2016;7(3):179–191. doi: 10.4155/tde.15.96. [DOI] [PubMed] [Google Scholar]
- Khan NL, Graham E, Critchley P, Schrag AE, Wood NW, Lees AJ, Bhatia KP, Quinn N. Parkin disease: a phenotypic study of a large case series. Brain : a journal of neurology. 2003;126(Pt 6):1279–1292. doi: 10.1093/brain/awg142. [DOI] [PubMed] [Google Scholar]
- Klein C, Schlossmacher MG. Parkinson disease, 10 years after its genetic revolution: multiple clues to a complex disorder. Neurology. 2007;69(22):2093–2104. doi: 10.1212/01.wnl.0000271880.27321.a7. [DOI] [PubMed] [Google Scholar]
- Lam HA, Wu N, Cely I, Kelly RL, Hean S, Richter F, Magen I, Cepeda C, Ackerson LC, Walwyn W, Masliah E, Chesselet MF, Levine MS, Maidment NT. Elevated tonic extracellular dopamine concentration and altered dopamine modulation of synaptic activity precede dopamine loss in the striatum of mice overexpressing human alpha-synuclein. J Neurosci Res. 2011;89(7):1091–1102. doi: 10.1002/jnr.22611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman EJ, Kulnane LS, Gao Y, Petriello MC, Pimpis KM, Younkin L, Dolios G, Wang R, Younkin SG, Lamb BT. Genetic background regulates beta-amyloid precursor protein processing and beta-amyloid deposition in the mouse. Human molecular genetics. 2003;12(22):2949–2956. doi: 10.1093/hmg/ddg322. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Slabosz A, Robbins TW, Barker RA, Owen AM. Dopaminergic basis for deficits in working memory but not attentional set-shifting in Parkinson's disease. Neuropsychologia. 2005;43(6):823–832. doi: 10.1016/j.neuropsychologia.2004.10.001. [DOI] [PubMed] [Google Scholar]
- LeWitt PA. New levodopa therapeutic strategies. Parkinsonism Relat Disord. 2016;22(1):S37–40. doi: 10.1016/j.parkreldis.2015.09.021. [DOI] [PubMed] [Google Scholar]
- Magara F, Muller U, Li ZW, Lipp HP, Weissmann C, Stagljar M, Wolfer DP. Genetic background changes the pattern of forebrain commissure defects in transgenic mice underexpressing the beta-amyloid-precursor protein. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(8):4656–4661. doi: 10.1073/pnas.96.8.4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magen I, Chesselet MF. Genetic mouse models of Parkinson's disease The state of the art. Prog Brain Res. 2010;184:53–87. doi: 10.1016/S0079-6123(10)84004-X. [DOI] [PubMed] [Google Scholar]
- Magen I, Fleming SM, Zhu C, Garcia EC, Cardiff KM, Dinh D, De La Rosa K, Sanchez M, Torres ER, Masliah E, Jentsch JD, Chesselet MF. Cognitive deficits in a mouse model of pre-manifest Parkinson's disease. Eur J Neurosci. 2012;35(6):870–882. doi: 10.1111/j.1460-9568.2012.08012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science (New York, NY. 2000;287(5456):1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- Mehler-Wex C, Riederer P, Gerlach M. Dopaminergic dysbalance in distinct basal ganglia neurocircuits: implications for the pathophysiology of Parkinson's disease, schizophrenia and attention deficit hyperactivity disorder. Neurotox Res. 2006;10(3-4):167–179. doi: 10.1007/BF03033354. [DOI] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol. 2001;110(1):40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Mittal S, Bjornevik K, Im DS, Flierl A, Dong X, Locascio JJ, Abo KM, Long E, Jin M, Xu B, Xiang YK, Rochet JC, Engeland A, Rizzu P, Heutink P, Bartels T, Selkoe DJ, Caldarone BJ, Glicksman MA, Khurana V, Schule B, Park DS, Riise T, Scherzer CR. beta2-Adrenoreceptor is a regulator of the alpha-synuclein gene driving risk of Parkinson's disease. Science (New York, NY. 2017;357(6354):891–898. doi: 10.1126/science.aaf3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonnekes J, Timmer MH, de Vries NM, Rascol O, Helmich RC, Bloem BR. Unmasking levodopa resistance in Parkinson's disease. Mov Disord. 2016;31(11):1602–1609. doi: 10.1002/mds.26712. [DOI] [PubMed] [Google Scholar]
- Oaks AW, Frankfurt M, Finkelstein DI, Sidhu A. Age-dependent effects of A53T alpha-synuclein on behavior and dopaminergic function. PLoS One. 2013;8(4):e60378. doi: 10.1371/journal.pone.0060378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel WH, Hoglinger GU, Caraceni T, Girotti F, Eichhorn T, Spottke AE, Krieg JC, Poewe W. Depression in Parkinson's disease. An update. Adv Neurol. 2001;86:373–383. [PubMed] [Google Scholar]
- Owen AM, Beksinska M, James M, Leigh PN, Summers BA, Marsden CD, Quinn NP, Sahakian BJ, Robbins TW. Visuospatial memory deficits at different stages of Parkinson's disease. Neuropsychologia. 1993;31(7):627–644. doi: 10.1016/0028-3932(93)90135-m. [DOI] [PubMed] [Google Scholar]
- Partiot A, Verin M, Pillon B, Teixeira-Ferreira C, Agid Y, Dubois B. Delayed response tasks in basal ganglia lesions in man: further evidence for a striato-frontal cooperation in behavioural adaptation. Neuropsychologia. 1996;34(7):709–721. doi: 10.1016/0028-3932(95)00143-3. [DOI] [PubMed] [Google Scholar]
- Paumier KL, Sukoff Rizzo SJ, Berger Z, Chen Y, Gonzales C, Kaftan E, Li L, Lotarski S, Monaghan M, Shen W, Stolyar P, Vasilyev D, Zaleska M, W DH, Dunlop J. Behavioral characterization of A53T mice reveals early and late stage deficits related to Parkinson's disease. PLoS One. 2013;8(8):e70274. doi: 10.1371/journal.pone.0070274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelaerts W, Bousset L, Van der Perren A, Moskalyuk A, Pulizzi R, Giugliano M, Van den Haute C, Melki R, Baekelandt V. alpha-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature. 2015;522(7556):340–344. doi: 10.1038/nature14547. [DOI] [PubMed] [Google Scholar]
- Peterson DA, Elliott C, Song DD, Makeig S, Sejnowski TJ, Poizner H. Probabilistic reversal learning is impaired in Parkinson's disease. Neuroscience. 2009;163(4):1092–1101. doi: 10.1016/j.neuroscience.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recchia A, Debetto P, Negro A, Guidolin D, Skaper SD, Giusti P. Alpha-synuclein and Parkinson's disease. FASEB J. 2004;18(6):617–626. doi: 10.1096/fj.03-0338rev. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Mallory M, Hashimoto M, Song D, Shults CW, Lang I, Masliah E. Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the platelet-derived growth factor and Thy-1 promoters. J Neurosci Res. 2002;68(5):568–578. doi: 10.1002/jnr.10231. [DOI] [PubMed] [Google Scholar]
- Rogers DC, Fisher EM, Brown SD, Peters J, Hunter AJ, Martin JE. Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm Genome. 1997;8(10):711–713. doi: 10.1007/s003359900551. [DOI] [PubMed] [Google Scholar]
- Rothwell PE, Fuccillo MV, Maxeiner S, Hayton SJ, Gokce O, Lim BK, Fowler SC, Malenka RC, Sudhof TC. Autism-associated neuroligin-3 mutations commonly impair striatal circuits to boost repetitive behaviors. Cell. 2014;158(1):198–212. doi: 10.1016/j.cell.2014.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford NJ, Giasson BI. The A53E alpha-synuclein pathological mutation demonstrates reduced aggregation propensity in vitro and in cell culture. Neuroscience letters. 2015;597:43–48. doi: 10.1016/j.neulet.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford NJ, Dhillon JS, Riffe CJ, Howard JK, Brooks M, Giasson BI. Comparison of the in vivo induction and transmission of alpha-synuclein pathology by mutant alpha-synuclein fibril seeds in transgenic mice. Human molecular genetics. 2017;26(24):4906–4915. doi: 10.1093/hmg/ddx371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacino AN, Brooks M, McKinney AB, Thomas MA, Shaw G, Golde TE, Giasson BI. Brain injection of alpha-synuclein induces multiple proteinopathies, gliosis, and a neuronal injury marker. J Neurosci. 2014;34(37):12368–12378. doi: 10.1523/JNEUROSCI.2102-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacino AN, Brooks M, Thomas MA, McKinney AB, Lee S, Regenhardt RW, McGarvey NH, Ayers JI, Notterpek L, Borchelt DR, Golde TE, Giasson BI. Intramuscular injection of alpha-synuclein induces CNS alpha-synuclein pathology and a rapid-onset motor phenotype in transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(29):10732–10737. doi: 10.1073/pnas.1321785111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabrook TJ, Iglesias M, Bloom JK, Spooner ET, Lemere CA. Differences in the immune response to long term Abeta vaccination in C57BL/6 and B6D2F1 mice. Vaccine. 2004;22(29-30):4075–4083. doi: 10.1016/j.vaccine.2004.03.061. [DOI] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson's disease. Science (New York, NY. 2003;302(5646):841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Sorrentino ZA, Brooks MMT, Hudson V, 3rd, Rutherford NJ, Golde TE, Giasson BI, Chakrabarty P. Intrastriatal injection of alpha-synuclein can lead to widespread synucleinopathy independent of neuroanatomic connectivity. Mol Neurodegener. 2017;12(1):40. doi: 10.1186/s13024-017-0182-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. Second. New York: HarperCollinsPublishers, Inc; 1989. [Google Scholar]
- Tanner CM. Occupational and environmental causes of parkinsonism. Occup Med. 1992;7(3):503–513. [PubMed] [Google Scholar]
- Taylor AE, Saint-Cyr JA, Lang AE. Subcognitive processing in the frontocaudate “complex loop”: the role of the striatum. Alzheimer Dis Assoc Disord. 1990;4(3):150–160. doi: 10.1097/00002093-199040300-00003. [DOI] [PubMed] [Google Scholar]
- Taylor AE, Saint-Cyr JA, Lang AE. Memory and learning in early Parkinson's disease: evidence for a “frontal lobe syndrome”. Brain Cogn. 1990;13(2):211–232. doi: 10.1016/0278-2626(90)90051-o. [DOI] [PubMed] [Google Scholar]
- Uchihara T, Giasson BI. Propagation of alpha-synuclein pathology: hypotheses, discoveries, and yet unresolved questions from experimental and human brain studies. Acta Neuropathol. 2016;131(1):49–73. doi: 10.1007/s00401-015-1485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Putten H, Wiederhold KH, Probst A, Barbieri S, Mistl C, Danner S, Kauffmann S, Hofele K, Spooren WP, Ruegg MA, Lin S, Caroni P, Sommer B, Tolnay M, Bilbe G. Neuropathology in mice expressing human alpha-synuclein. J Neurosci. 2000;20(16):6021–6029. doi: 10.1523/JNEUROSCI.20-16-06021.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma D, Sen D. Role of the unfolded protein response in the pathogenesis of Parkinson's disease. Acta Neurobiol Exp (Wars) 2015;75(1):1–26. [PubMed] [Google Scholar]
- Wahlsten D. Standardizing tests of mouse behavior: reasons, recommendations, and reality. Physiology & behavior. 2001;73(5):695–704. doi: 10.1016/s0031-9384(01)00527-3. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Metten P, Phillips TJ, Boehm SL, 2nd, BurkhartKasch S, Dorow J, Doerksen S, Downing C, Fogarty J, RoddHenricks K, Hen R, McKinnon CS, Merrill CM, Nolte C, Schalomon M, Schlumbohm JP, Sibert JR, Wenger CD, Dudek BC, Crabbe JC. Different data from different labs: lessons from studies of gene-environment interaction. J Neurobiol. 2003;54(1):283–311. doi: 10.1002/neu.10173. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Bachmanov A, Finn DA, Crabbe JC. Stability of inbred mouse strain differences in behavior and brain size between laboratories and across decades. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(44):16364–16369. doi: 10.1073/pnas.0605342103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Tang BS, Guo JF. Parkinson's Disease and Cognitive Impairment. Parkinsons Dis. 2016;2016:6734678. doi: 10.1155/2016/6734678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao I, Takao K, Miyakawa T, Ito S, Setou M. Synaptic E3 ligase SCRAPPER in contextual fear conditioning: extensive behavioral phenotyping of Scrapper heterozygote and overexpressing mutant mice. PLoS One. 2011;6(2):e17317. doi: 10.1371/journal.pone.0017317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang TM, Yu SY, Guo P, Du Y, Hu Y, Piao YS, Zuo LJ, Lian TH, Wang RD, Yu QJ, Jin Z, Zhang W. Nonmotor symptoms in patients with Parkinson disease: A cross-sectional observational study. Medicine (Baltimore) 2016;95(50):e5400. doi: 10.1097/MD.0000000000005400. [DOI] [PMC free article] [PubMed] [Google Scholar]