Abstract
Rationale
The paraventricular nucleus of the thalamus (PVT) has been shown to mediate cue-motivated behaviors, such as sign- and goal-tracking, as well as reinstatement of drug-seeking behavior. However, the role of the PVT in mediating individual variation in cue-induced drug-seeking behavior remains unknown.
Objectives
To determine if inactivation of the PVT differentially mediates cue-induced drug-seeking behavior in sign-trackers and goal-trackers.
Methods
Rats were characterized as sign-trackers (STs) or goal-trackers (GTs) based on their Pavlovian conditioned approach behavior. Rats were then exposed to 15 days of cocaine self-administration, followed by a 2-week forced abstinence period and then extinction training. Rats then underwent tests for cue-induced reinstatement and general locomotor activity, prior to which they received an infusion of either saline (control) or baclofen/muscimol (B/M) to inactivate the PVT.
Results
Relative to control animals of the same phenotype, GTs show a robust increase in cue-induced drug-seeking behavior following PVT inactivation, whereas the behavior of STs was not affected. PVT inactivation did not affect locomotor activity in either phenotype.
Conclusion
In GTs, the PVT appears to inhibit the expression of drug seeking, presumably by attenuating the incentive value of the drug cue. Thus, inactivation of the PVT releases this inhibition in GTs, resulting in an increase in cue-induced drug-seeking behavior. PVT inactivation did not affect cue-induced drug-seeking behavior in STs, suggesting that the role of the PVT in encoding the incentive motivational value of drug cues differs between STs and GTs.
Keywords: paraventricular nucleus of the thalamus, reinstatement, individual variation, sign-tracker, goal-tracker, cocaine, relapse, reward cue
Introduction
For addicted individuals, relapse often results from exposure to cues (e.g. people, places, paraphernalia) that have been associated with the drug-taking experience (for review see Shaham et al. 2003, Tomie et al. 2008). Exposure to these cues alone can cause intense feelings of craving (Childress et al. 1988, Childress et al. 1993), which can, in turn, elicit drug-seeking behaviors (see Shaham et al. 2003). These cue-reward associations are, in part, mediated by Pavlovian learning processes. During Pavlovian learning, a cue that reliably precedes the delivery of reward acquires predictive value. That is, the cue becomes a predictor, signaling the availability of reward. However, predictive cues can also acquire incentive motivational value, rendering them into powerful motivators and making them desirable in-and-of themselves (Stewart et al. 1984, Robinson et al. 1993). This process, known as incentive salience attribution, transforms predictive stimuli into “motivational magnets” (Berridge et al. 2009), allowing these stimuli to gain inordinate control and elicit maladaptive behaviors, such as compulsive drug seeking. Importantly, only for some individuals do reward cues acquire both predictive and incentive properties.
Using a Pavlovian conditioned approach (PCA) paradigm, we have shown that rats can be classified as goal-trackers (GTs), those that attribute reward-cues primarily with predictive value, or sign-trackers (STs), those that attribute both predictive and incentive value to reward-cues. In this paradigm, the presentation of a lever (conditioned stimulus, CS) always precedes the delivery of a food reward (unconditioned stimulus, US). That is, food delivery is non-contingent upon an instrumental response. While both GTs and STs learn the relationship between the lever-CS and food-US, the nature of their Pavlovian conditioned approach response differs. Upon lever-CS presentation, rats classified as GTs attend to the location of impending food delivery; while STs approach and manipulate the lever-CS itself. Relative to GTs, STs also respond more avidly for presentation of the lever-CS during a test of conditioned reinforcement (Robinson et al. 2009). The ability of the lever-CS to bias attention and elicit approach behavior, and to acquire reinforcing properties (Robinson and Flagel 2009), indicates that the reward-cue has become imbued with incentive value for STs, to a greater extent than GTs. This enhanced propensity to attribute incentive salience to food-cues has been associated with a number of other addiction-related behaviors. For example, rats that sign-track to food-associated cues do the same to cues associated with drugs of abuse, including cocaine and opioids (Yager et al. 2013, Yager et al. 2015). In addition, relative to GTs, STs are more impulsive (Flagel et al. 2010, Lovic et al. 2011), have higher cocaine break-points (Saunders et al. 2011), and are more susceptible to cue-induced reinstatement of drug-seeking behavior (Saunders et al. 2010, Saunders et al. 2013, see also Kawa et al. 2016). Thus, the sign-tracker/goal-tracker animal model supports the long-standing notion that Pavlovian incentive learning processes are critical to drug-motivated behaviors (Bolles 1972, Bindra 1978, Toates 1981, Stewart et al. 1984, Robinson and Berridge 1993).
The sign-tracker/goal-tracker animal model has provided a novel foundation to dissociate the neural mechanisms underlying predictive vs. incentive learning (Flagel et al. 2017). Indeed, using this model, it has been shown that food- and drug-associated cues engage different circuitry in STs vs. GTs (Flagel et al. 2011a, Yager et al. 2015, Haight et al. 2017). Relative to GTs, STs show greater engagement of the so-called “motive circuit” (Kalivas et al. 2005), suggesting that this circuit encodes the incentive properties of reward cues (Flagel et al. 2011a, Haight et al. 2014). One brain region showing robust ST/GT differences in cue-induced neuronal activation is the paraventricular nucleus of the thalamus (PVT) (Flagel et al. 2011a, Yager et al. 2015). The PVT is a midline thalamic structure that acts as an interface between cortical, limbic and motor circuits, relaying information regarding arousal and reward, among other functions, to the striatum (Kelley et al. 2005). Thus, it is not surprising that this nucleus has been implicated in reward learning (Flagel et al. 2011a, Haight et al. 2015, Yager et al. 2015, Do-Monte et al. 2017, Haight et al. 2017, Ong et al. 2017, Otis et al. 2017) as well as a number of other complex behaviors, including fear learning (Li et al. 2014, Do-Monte et al. 2015, Penzo et al. 2015) and anxiety-related behaviors (Li et al. 2010, Barson et al. 2015). Work from our laboratory suggests that the PVT acts as a central node via the hypothalamic-thalamic-striatal axis to regulate the attribution of incentive salience to reward cues and the expression of the resultant behaviors (Haight et al. 2017). Using excitotoxic lesions, we have shown that taking the PVT “offline” causes an increase in sign-tracking behavior to a food-paired cue in rats with an inherent tendency to goal-track (Haight et al. 2015). Thus, the PVT appears to act as a “brake” on incentive motivational processes, and releasing this brake allows for the attribution of incentive salience to reward cues and/or expression of corresponding cue-motivated behaviors, at least in goal-trackers.
In recent years, the PVT has been increasingly acknowledged for its role in addiction-related behaviors (Deutch et al. 1995, Deutch et al. 1998, Young et al. 1998, Stephenson et al. 1999, James et al. 2013, Browning et al. 2014, Haight and Flagel 2014, Yeoh et al. 2014, Neumann et al. 2016, Zhu et al. 2016, Matzeu et al. 2017), with a particular emphasis on reinstatement of drug-seeking behavior (Hamlin et al. 2009, James et al. 2010, Matzeu et al. 2015, Matzeu et al. 2016). However, these prior studies were not designed to examine individual differences in the role of the PVT in cue-motivated behaviors (Flagel et al. 2011a, Haight and Flagel 2014, Yager et al. 2015, Haight et al. 2017). In the current study, we assessed whether the role of the PVT in cue-induced drug-seeking behavior differs depending on inherent individual differences in cue-reward learning. To do so, rats were first exposed to Pavlovian conditioning and characterized as STs or GTs, and subsequently underwent 15 days of cocaine self-administration followed by 2 weeks of forced abstinence. Following extinction training, rats were tested for cue-induced reinstatement of drug-seeking behavior, prior to which rats received an infusion of either saline or a cocktail of baclofen and muscimol (GABAB and GABAA agonists, respectively) to transiently inactivate the PVT. Based on our prior work demonstrating that a lesion to the PVT enhances the incentive motivational value of a reward cue selectively in GTs (Haight et al. 2015), we hypothesized that inactivating the PVT would result in an increase in cue-induced cocaine-seeking behavior in GTs, rendering them comparable to STs. That is, removal of the PVT “brake” in GTs would result in the expression of incentive value of the cocaine-cue and thereby enhance cue-induced cocaine-seeking behavior selectively in this phenotype.
Methods
Subjects
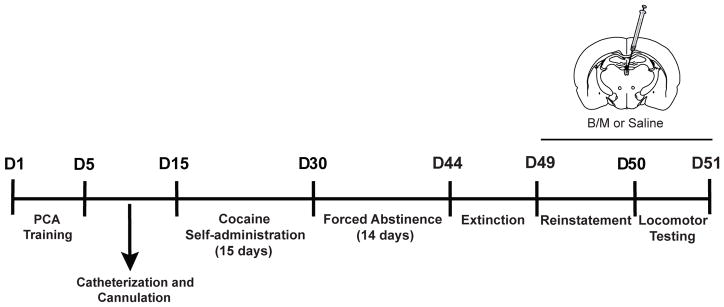
A total of 252 male Sprague-Dawley rats weighing between 200–250 g upon arrival from Charles River (Saint-Constant, Canada and Raleigh, NC, USA) were initially screened for use in this study. Upon arrival, rats were pair-housed in a climate-controlled room with a 12-hour light: dark cycle (lights on at 06:00 h or 07:00 h depending on daylight savings time). Rats had ad libitum access to water and food throughout the entire study. Rats were allowed to acclimate to the new environment for seven days before the experiment began. After surgeries, all rats were single housed for the remainder of the study to decrease the chance of damage to the surgical implants. All behavioral testing occurred during the light cycle, between 08:00 h to 19:00 h. Testing times for specific procedures are included below. The experimental timeline is shown in Fig. 1, with details of each procedure in the following sections. All experimental procedures conformed to the standards in The Guide for the Care and Use of Laboratory Animals: Eight Edition, revised in 2011, published by the National Academy of Sciences, and approved by the University of Michigan Institutional Animal Care and Use Committee.
Fig. 1. Experimental timeline.
Rats underwent Pavlovian conditioned approach (PCA) training and were then implanted with indwelling jugular catheters and double cannula into the anterior and posterior PVT. Cocaine self-administration (15 days), forced abstinence (14 days) and extinction training (18 sessions) followed. Rats were given an injection of either of baclofen/muscimol (B/M, 6/0.6 pmol/nl) or saline into the PVT prior to cue-induced reinstatement and the locomotor test. The total duration of the study was approximately 50 days
Pavlovian conditioned approach (PCA) training
After the 7-day acclimation period, rats were handled for three days and given 45-mg banana-flavored grain pellets (about 30 pellets per cage; Bio-Serv, Flemington, NJ, USA) in their home cage. This allowed the rats to habituate to the experimenters as well as the food reward used during Pavlovian conditioned approach (PCA) training. PCA training occurred in standard behavioral testing chambers (MED Associates, St. Albans, VT, USA; 20.5 × 24.1 cm floor area, 29.2 cm high) housed within sound-attenuating boxes equipped with a ventilation fan to provide air circulation and constant background noise. In the center of one of the walls of the testing chamber was a food magazine located 6-cm above the grid floor and attached to a pellet dispenser. The food magazine was equipped with an infrared photobeam that, when broken, recorded “contact” with the food magazine. To the right or the left of the food magazine, and at the same height, was a retractable lever that was illuminated upon presentation. A minimum of 10-g of force was necessary to deflect the lever and be registered as a lever “contact”. In the middle of the opposite wall, 1-cm from the top of the chamber, there was a white house light that was illuminated for the duration of each training session.
Rats underwent one day of pre-training in which the food magazine was initially baited with two 45-mg banana-flavored pellets to direct the rats’ attention to the site of reward delivery. The house light was turned on after a 5-min acclimation period to the testing chamber, and upon illumination of the house light the pre-training session began and lasted approximately 12.5 minutes. Pre-training sessions consisted of 25 trials, during which the lever remained retracted, but food pellets were randomly delivered into the food magazine, with one pellet delivered per trial on a variable interval 30-second schedule (range 0–60 seconds), for a total of 25 pellets. Following pre-training, rats underwent PCA training sessions with 25 trials per session. Illumination of the house light again signaled session “start”. During each trial an illuminated lever (conditioned stimulus, CS) was presented in the chamber for 8 seconds, and immediately upon its retraction a food pellet (unconditioned stimulus, US) was delivered to the adjacent food magazine. These 25 lever-CS/food-US pairings occurred on a variable interval 90-second schedule (range 30–150 seconds), and each session lasted approximately 40 minutes. Rats underwent one training session per day for 5 days, between the hours of 10:00 h and 14:00 h.
Med Associates software recorded the following information: (1) magazine contacts during lever-CS presentation, (2) latency to the first magazine contact during lever-CS presentation, (3) number of lever-CS contacts, (4) latency to the first lever-CS contact during presentation, and (5) the number of magazine contacts between lever-CS presentations (i.e. during the inter-trial interval). These measures allowed for the quantification of the PCA index, which is used to characterize the behavioral phenotype of each rat based on the conditioned response (CR). Information from session 4 and 5 of training were averaged and used to compute the PCA index as previously described (Meyer et al. 2012). This index incorporates response bias, latency and vigor of each response and ranges from −1 to 1. A score of −1 indicates an extreme goal-tracker (GT) with a CR always directed toward the food magazine upon lever-CS presentation. A score of 1 indicates an extreme sign-tracker (ST) with a CR always directed toward the lever-CS upon presentation. For this study, GTs had scores between −1 to −0.3, STs between 0.3 and 1, and intermediate responders, those that vacillate between contacting the lever or the food magazine during lever-CS presentation, a score between −0.29 to 0.29. Intermediate responders (n=56) were subsequently excluded as this behavioral phenotype was not pertinent to the current goals; but these rats were used for other studies.
Surgical procedures
Following PCA training, all STs and GTs underwent catheterization surgery to place indwelling catheters into the jugular vein for cocaine self-administration, and stereotaxic surgery immediately followed to place cannulas into the anterior and posterior PVT for localized pharmacological inactivation. For catheterization surgery rats were anesthetized using ketamine (90 mg/kg i.p.) and xylazine (10 mg/kg i.p.) and implanted with indwelling jugular vein catheters as previously described (Crombag et al. 2000, Flagel et al. 2003). Ketamine and xylazine were used for this surgery to ensure the rats remained properly anesthetized for the duration of the surgery, and to allow the surgeons to quickly and efficiently implant the catheter. After catheterization surgery rats were given an injection of saline (5 ml, s.c.) to minimize dehydration before undergoing stereotaxic surgery. Once rats were fully ambulatory, they were anesthetized with 5% isoflurane and maintained under anesthesia using 2% isoflurane. Isoflurane was used for this surgery as there was higher risk of the time it takes to complete this surgery going beyond the time limit that ketamine and xylazine can safely anesthetize a rat. Additionally, the rats recover from isoflurane anesthesia at a faster rate compared to ketamine and xylazine, thus providing a safer means of anesthesia for the second surgery in one day. Rats were fitted into the ear bars of the stereotaxic apparatus (David Kopf Instruments, Tujunga, CA) that was outfitted with a digital manipulator arm (Stoelting, Wood Dale, IL). The scalp was cleaned with ethanol and Betadine solution (Purdue Products, Stamford, CT), and then an incision was made to expose the skull. The skull was then leveled within +/− 0.1 mm of the bregma and lambda coordinates. Chronic guide cannulas (26 gauge, stainless steel; PlasticsOne) were inserted 1 mm above the anterior (relative to bregma: AP −2.0, ML 1.0, DV −4.5) and posterior (relative to bregma: AP −3.0, ML 1.0, DV −4.5) PVT at a 10° angle to the midline to circumvent the superior sagittal sinus and prevent unnecessary bleeding. Due to an initially low success rate of correct injector placement, a subset of rats included in this study had different DV coordinates (relative to bregma: anterior DV −4.6; posterior DV: −4.6), but all other coordinates remained the same. Cannulas were secured to the skull using screws and acrylic dental cement (Ortho-Jet, Lans Dental Manufacturing, Wheeling, IL). A double cannula steel stylet (PlasticsOne) the same length as the guide cannula was inserted into the guide cannula to prevent occlusion. A screw top was put on top of the guide cannula to prevent the rats from removing the stylets.
Rats received an injection of Flunixin (2.5 mg/kg s.c.) and an infusion of gentamicin sulfate (1 mg/ml i.v., 0.2 ml) on the day of surgery and the day following surgery. Rats also received an i.v. infusion of heparin (100 units/ml, 0.05 ml) and gentamicin sulfate (1 mg/ml, 0.05 ml) daily to maintain catheter patency and decrease the chance of infection throughout the cocaine self-administration paradigm. Following surgeries, rats were allowed to recover for a minimum of 10 days, and all sutures and surgical staples were removed during this time. Prior to the start of the cocaine self-administration paradigm, and before advancing to each subsequent infusion criterion, catheters were checked for patency using methohexital sodium diluted in sterile saline (10 mg/ml i.v., 0.1 ml). If the rat did not exhibit ataxia within 10 seconds of methohexital sodium administration they were removed from the study for loss of catheter patency.
Cocaine self-administration
Cocaine self-administration occurred in the same chambers as PCA training. However, chambers were reconfigured to contain just two nose ports located 4-cm from the grid floor. One nose port was designated “inactive” and one “active”. The active port was on the opposite side of the wall as the lever-CS was during PCA training to minimize side bias. One minute after the program was initiated, the house light was illuminated along with a discrete cue light located in the active port. The discrete cue light in the active port remained on for 20 seconds at the start of each session to direct the rat’s attention to the port. During this time and for the remainder of the session, pokes were recorded in both ports, but only those in the active port resulted in drug infusion (i.e. pokes into the inactive port were without consequence). Reinforcement occurred on a fixed-ratio 1 (FR1) schedule, such that one entry into the active port resulted in a 0.5 mg/kg infusion of cocaine (Mallinckrodt, St. Louis, MO) diluted in 0.9% sterile saline, delivered in 25 μl over 1.6 seconds. Simultaneous with the cocaine infusion, the discrete cue light in the active port was illuminated and stayed on for a total of 20 seconds, during which head entries into the active port are recorded, but without consequence. Infusion criteria (IC) were used to ensure that all rats received the same number of cocaine infusions, and cocaine cue-light pairings (Saunders and Robinson 2010, Saunders and Robinson 2011, Saunders et al. 2013, Flagel et al. 2016). An IC refers to the number of cocaine infusions the rat had to receive to terminate the session (Saunders and Robinson 2010), and thus the number of cocaine cue-light pairings each rat received (i.e. IC5 means the rat would receive 5 cocaine infusions, and 5 cocaine cue-light pairings, during the session). Once rats met the IC, or after 5 hours, sessions were terminated. Self-administration training occurred once per day between the hours of 8:00 h and 19:00 h for 15 consecutive days using the following schedule: four days at IC5, three days at IC10, three days at IC20 and five days at IC45. In order to move to the next IC rats had to successfully meet each IC for at least 2 consecutive sessions and maintain catheter patency. If these contingencies were not met, the rat was excluded from the study (loss of catheter patency, n=15 (ST: 8, GT: 7); did not meet IC, n=51 (ST: 28, GT: 23)). At IC45, the dose of cocaine was decreased to 0.2 mg/kg/infusion to promote a higher response rate and to encourage rats to reach criterion before the session time limit (Saunders and Robinson 2010). After self-administration training, rats then underwent 14 days of forced abstinence during which they were left undisturbed in the colony room. This time period was chosen as it has been shown to result in an increase in cue-induced drug-seeking behavior compared to shorter periods of abstinence (Grimm et al. 2001).
Extinction training
Extinction training commenced after the 14-day abstinence period. Testing chambers remained in the same configuration as cocaine self-administration, and entries into the active and the inactive port were recorded but without consequence. Thus, head entries into the active port did not result in cocaine delivery nor the presentation of the cue-light. Extinction sessions lasted for 45 minutes and occurred three times a day for six days between the hours of 9:00 h and 17:00 h, for a total of 18 sessions. The last three extinction sessions occurred the same day as the test for cue-induced reinstatement. In order to undergo the test for cue-induced reinstatement rats must have completed the 18 extinction sessions and have fewer than 10 entries into the active port during each of the last two sessions, which all rats included in final analysis accomplished. Before the last extinction training session (session 18) the cannula “dust” cap and stylet were removed, the injector was inserted into the cannula and removed, and then the stylet and cap were put back into place to habituate the rats to the injection procedure that would occur prior to the test for cue-induced reinstatement.
Cue-induced reinstatement test
The cue-induced reinstatement test occurred immediately following the last extinction training session (e.g. session 18). Rats were counterbalanced into two different drug treatment groups based first on PCA score. Within each group, rats were further counterbalanced based on the number of port entries during self-administration sessions and behavior during the extinction sessions. Treatment groups received either a mixed cocktail of agonists to the GABA-B (baclofen) and GABA-A receptors (muscimol; Sigma-Aldrich, St. Louis, MO), or a saline injection (control group). Baclofen/muscimol (B/M) was given at a dose of 6 pmol/nl and 0.6 pmol/nl respectively, as infusion of this dose into the PVT has previously been shown to affect cocaine-seeking behavior (Browning et al. 2014, Matzeu et al. 2015). Injections occurred in a room adjacent to the testing room and were administered using a standard dual infusion pump (Pump 11 Elite, Harvard Apparatus) with P50 tubing connecting the two 1-μl syringes (Hamilton) to the injector (33 gauge with a 1-mm projection; Plastics One). Injections occurred at a rate of 100 nl/min for two minutes (total of 200 nl volume), and the injector was left in place for an additional two minutes to allow the drug to diffuse away from the injector and throughout the PVT (Browning et al. 2014). Following the injection, the stylet and cap were replaced, and the rat was brought into the testing room and placed into the Med Associates testing chamber. The house light came on one minute after program initiation, and head entries into the active and inactive port were recorded for the duration of the session. During the cue-induced reinstatement test, head entries into the active port resulted in the presentation of the cue-light for 20 seconds (same as in self-administration training), but no cocaine infusion. That is, presentation of the cue-light previously associated with drug delivery acted as a conditioned reinforcer, and entries into the active port were used as a measure of cocaine-seeking behavior. Entries into the inactive port were recorded, but without consequence. Sessions terminated after 45 minutes, and testing occurred between the hours of 15:00 h and 17:00 h.
Locomotor testing
A subset of rats (9 STs, 13 GTs) were assessed for the effects of PVT inactivation on general locomotor activity. The day after the cue-induced reinstatement test rats were put into a locomotor testing chamber (43 × 21.5 cm floor area, 25.5 cm high) outfitted with infrared beams mounted 2.3 and 6.5 cm above the grid floor to track lateral and rearing movements, respectively. All testing occurred under red light between the hours of 12:00 and 16:00. Rats underwent a 45-minute habituation period for which they were placed into the locomotor testing chamber and left undisturbed, but activity was recorded. Following the conclusion of habituation, rats were removed from the test chamber and given the same drug infusions (i.e. B/M or saline) they received prior to the reinstatement test on the preceding day. All infusion procedures were identical to those for the cue-induced reinstatement test, with injections occurring at a rate of 100 nl/min for 2 minutes (total of 200 nl volume) and the injector left in place for an additional 2 minutes (Browning et al. 2014). Rats were then placed back into the locomotor testing chamber and underwent a 45-minute test session. For both the habituation and test session, lateral and rearing locomotor movements were recorded in 5-minute increments and cumulative locomotor activity was calculated based on the sum of these movements across the 45-min session. Once the session was complete for all of the rats, rats were removed from the test chambers and placed back into their home cages in the colony room.
Histology
After all testing was complete, rats were anesthetized with ketamine (90 mg/kg i.p.) and xylazine (10 mg/kg i.p.) and subsequently received an infusion of 2% Chicago Sky Blue dye (200 nl total at a rate of 100 nl/min; Sigma-Aldrich, St. Louis, MO) into the PVT in order to identify the injection site. Rats then underwent transcardial perfusion with 0.9% saline followed by 4% formaldehyde at 4°C (pH= 7.4) with an injector still inserted into the guide cannula. Brains were extracted and remained in formaldehyde for 24 hours at 4°C. Brains were then cryoprotected for 24 hours in graduated sucrose solutions (10%, 20% then 30% sucrose in phosphate buffer, pH= 7.4) at 4°C over the course of 3 days. Brains were encased in Tissue-Plus O.C.T. (Fisher HealthCare, Houston, TX), frozen using dry ice and sectioned coronally on a cryostat at a thickness of 40 μm. After sectioning, brains were mounted and stained using Eosin-Y (Sigma-Aldrich, St. Louis, MO), dehydrated with ethanol solutions, exposed to three xylene washes and then coverslipped with Permount (Fisher Scientific, Fair Lawns, NJ). Verification of injection sites was done using a Leica DM1000 light microscope (Buffalo Grove, IL). Two experimenters, blind to group assignments, scored the injector sites as being within or outside of the boundaries of the PVT for both the anterior (relative to bregma: AP: −1.8 to −2.28) and posterior (relative to bregma: AP: −2.76 to −3.24) PVT sites with the guidance of a rat brain stereotaxic atlas (Paxinos G 2007). Only rats in which both scorers agreed on having correct injector placement within the PVT boundaries were included in the final analyses as indicated below.
Statistical analysis
All PCA training, cocaine self-administration and extinction training sessions were analyzed using a linear mixed-effects model with SPSS Statistics Program (Statistical Package for the Social Sciences), version 22 (IBM, Armok, NY, USA). The best covariance structure was selected using the lowest Akaike’s information criterion for each dataset. Behavior during the cue-induced reinstatement test was analyzed using a three-way ANOVA. To compare behavior during the last extinction session to that during the cue-induced reinstatement test, a repeated-measures ANOVA was used. A repeated measures ANOVA was also used to analyze differences in locomotor activity between the habituation and test session for the locomotor activity test. All ANOVAs were performed using StatView, version 5.0 (SAS Institute Inc., Cary, NC, USA). To determine if there was a significant relationship between the rate of extinction and cue-induced reinstatement, a quadratic regression model was fit to each rat’s extinction training curve. The intercept, linear and quadratic term were then regressed onto the number of pokes into the active port during the reinstatement test. Importantly, this analysis accounts for differences in extinction behavior that may otherwise confound behavior during the reinstatement test. These analyses were carried out using SPSS, version 22. Statistical significance was set at p<0.05 for all tests. When significant main effects or interactions were detected post-hoc analyses were conducted using Bonferroni tests to correct for multiple comparisons.
Results
Histology
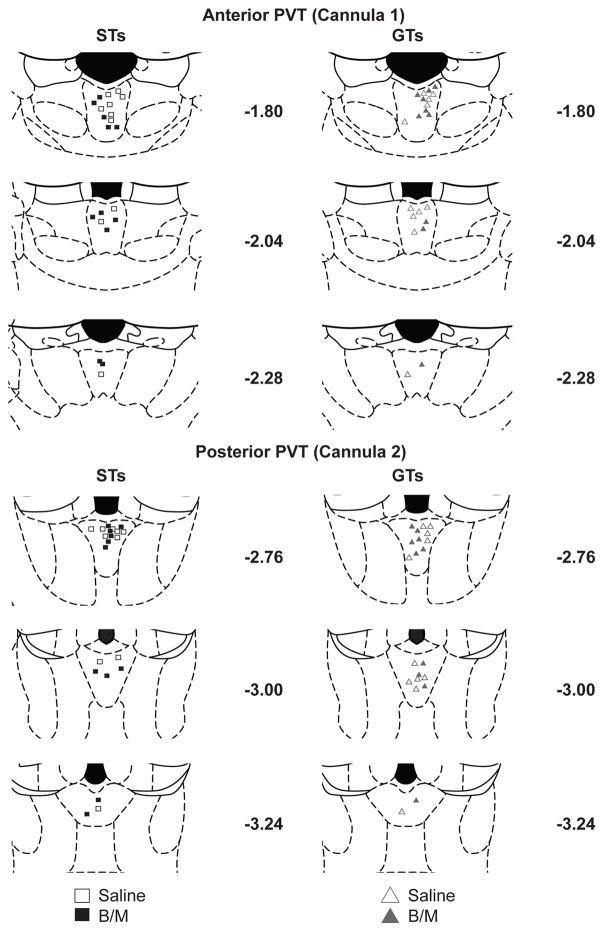
Fig. 2 shows a map of the cannula placements of the rats with accurate placements in the anterior and posterior PVT. Of those rats that successfully completed the behavioral portion of the study, only those with correct cannula placement in both the anterior and posterior PVT were included in final analysis (ST Saline, n=10; ST B/M, n=11; GT Saline, n=11; GT B/M, n=10).
Fig. 2. Representation of double cannula placements in the anterior (cannula 1) and posterior (cannula 2) portions of the PVT with respect to bregma.
Only those rats considered to have successful cannula placements are included and shown separated by phenotype (STs, left; GTs, right) and treatment group (open symbols saline; closed symbols baclofen/muscimol). (ST Saline, n=10; ST B/M, n=11; GT Saline, n=11; GT B/M, n=10)
Pavlovian conditioned approach (PCA) training
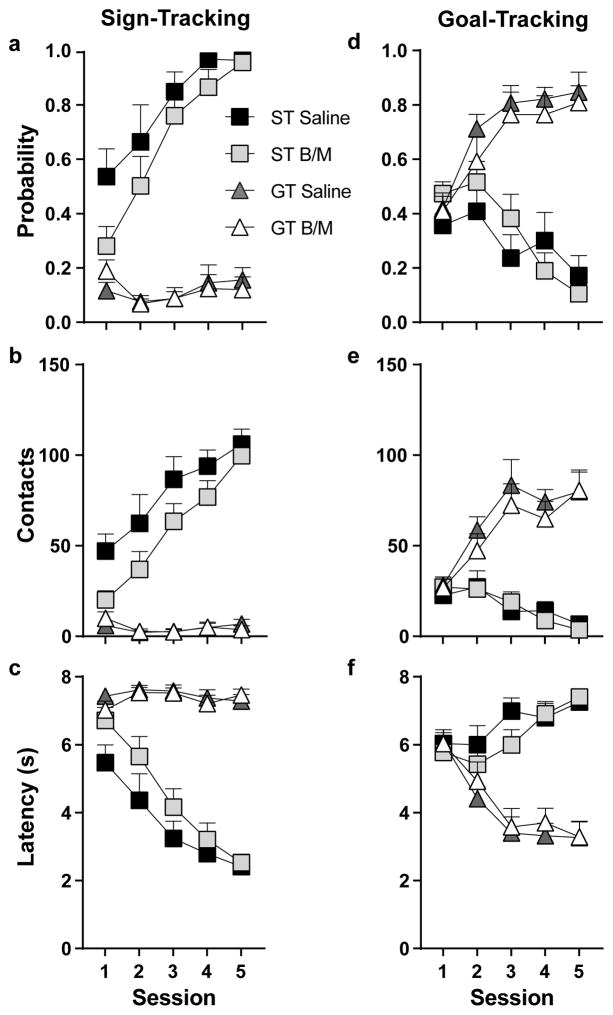
PCA behavior was analyzed across training sessions using the following dependent variables: probability to contact the lever or magazine, the number of lever or magazine contacts, and latency to contact the lever or magazine. Phenotype (ST or GT), Treatment (B/M or saline) and Session were used as the independent variables. For all measures (see Fig. 3) there was a significant Effect of Phenotype, Effect of Session and a Phenotype x Session interaction (p<0.05). Relative to GTs, rats characterized as STs showed a greater probability to contact the lever (F1,43 = 172.19, p<0.001), a greater number of contacts with the lever (F1,38 = 122.78, p<0.001), and a lower latency to contact the lever (F1,42 = 136.61, p<0.001) (Fig. 3a–c). Post-hoc analyses revealed a significant difference between phenotypes on all five sessions for these measures (p<0.001). In contrast, GTs showed a greater probability to contact the food magazine (F1,42 = 48.02, p<0.001), a greater number of contacts with the food magazine (F1,41 = 56.97, p<0.001), and a lower latency to contact the food magazine (F1,38 = 46.20, p<0.001) compared to STs (Fig. 3d–f). Post-hoc analyses revealed a significant difference between phenotypes for the probability to contact the food magazine and the number of magazine contacts during sessions two through five (p<0.05), and differences in the latency to contact the food magazine during sessions three through five (p<0.001). There were no significant differences between Treatment groups, nor were there significant interactions with this variable, even when phenotypes were analyzed separately. This is to be expected as groups were balanced based on their PCA behavior, and treatment did not occur during this phase of the experimental design (see Fig. 1).
Fig. 3. Individual variation in the acquisition of Pavlovian conditioned approach training.
Mean + SEM for (a) probability to contact the lever, (b) lever contacts, (c) latency to contact the lever, (d) probability to contact the food magazine, (e) food magazine contacts, and (f) latency to contact the food magazine across 5 Pavlovian conditioning sessions. Rats with a conditioned response directed toward the lever were classified as STs (Saline, n=10; B/M, n=11), and rats with a conditioned response directed toward the food magazine were classified as GTs (Saline, n=11; B/M, n=10). Rats are separated by their test day treatment (saline or B/M (baclofen/muscimol)), but did not receive treatment prior to or during Pavlovian conditioning
STs and GTs do not differ in the acquisition of cocaine self-administration
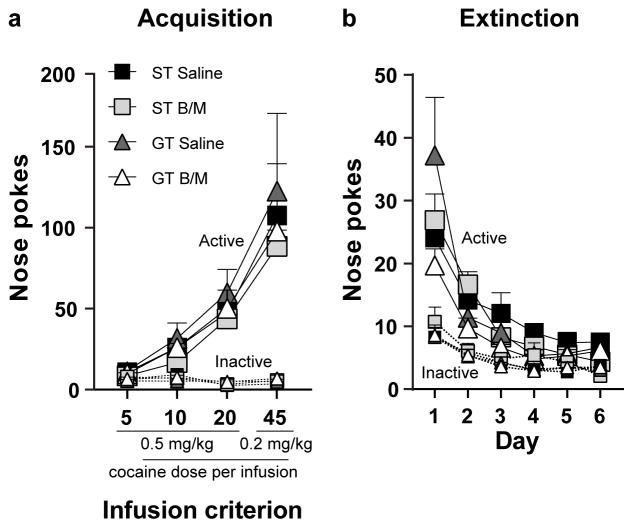
Cocaine self-administration behavior was analyzed across IC using nose pokes as the dependent variable, and Phenotype (ST or GT), Treatment (B/M or saline), and Port (active or inactive) as the independent variables. As shown in Fig. 4a, all rats discriminated between the active and inactive port (Effect of Port, F1,76 = 175.62, p<0.001) and increased their responding into the ports at each successive IC (Effect of IC, F3,76 = 60.48, p<0.001). There was also a significant IC x Port interaction (F3,76 = 61.12, p<0.001), indicating that responses into the active port increased across IC (F3,76=121.07, p<0.001), while responses into the inactive ports did not change across IC, as to be expected. Indeed, rats successfully differentiated between the two ports at every stage of training (Effect of Port, IC5: p=0.002; IC10: p<0.001; IC20: p<0.001; IC45: p<0.001). There were no significant Effects of Treatment (F1,76 = 1.90, p=0.17) nor Phenotype (F1,76 = 1.46, p=0.23), and no significant interactions with these variables. These data are consistent with those reported in previous studies showing that STs and GTs do not differ from one another in the acquisition of cocaine self-administration using these doses of cocaine and the IC paradigm (Saunders and Robinson 2010, see also Beckmann et al. 2011).
Fig. 4. Acquisition and extinction of cocaine self-administration.
(a) Mean + SEM for nose pokes into the active and inactive ports across four infusion criterion (IC) in STs (Saline, n=10; B/M, n=11) and GTs (Saline, n=11; B/M, n=10). All rats differentiated between the active and inactive port (p<0.0001) across each IC (p<0.0001), and there were no significant differences between phenotype or treatment groups (saline or B/M). The cocaine dose at IC5, IC10 and IC20 was 0.5 mg/kg/infusion, and at IC45 it was 0.2 mg/kg/infusion. (b) Mean + SEM for nose pokes into the active and inactive ports for STs (Saline, n=10; B/M, n=11) and GTs (Saline, n=11; B/M, n=10) across extinction training days (3 sessions per day). Rats decreased cocaine-seeking behavior throughout extinction training (p<0.0001), regardless of phenotype or assigned treatment group for the subsequent reinstatement test (saline or B/M). A Day x Phenotype interaction (p=0.20) was present, however when behavior is analyzed per session, and not grouping sessions into a day, this relationship is no longer present
STs and GTs do not differ in the rate of extinction
Extinction behavior was analyzed across training days using nose pokes (average of the 3 sessions per day) as the dependent variable, and Phenotype (ST or GT), Treatment (B/M or saline), and Port (active or inactive) as the independent variables. Cocaine-seeking behavior decreased with repeated extinction training days (Effect of Day, F5,76 = 23.85, p<0.001) (Fig. 4b). A significant Effect of Port (F1,86 = 55.63, p<0.001) showed that rats differentiated between the active and inactive port (Fig. 4b). However, a significant Day x Port interaction (F5,76 = 7.44, p<0.001) revealed that as extinction training progressed rats stopped preferring the active port over the inactive port; this was especially evident later in training as nose pokes into the active port decreased (Fig. 4b). There was not a significant Effect of Treatment (F1,86 = 1.26, p=0.27), nor was there a significant Effect of Phenotype (F1,86 = 1.11, p=0.30). There was, however, a significant interaction between Phenotype and Day (F5,76= 2.88, p=0.02). Post-hoc analyses revealed that STs and GTs differ from one another in extinction behavior during the second (p=0.03) and fourth (p=0.01) training days. Yet, when each extinction session was included in the analysis (rather than averaging across the three sessions per day) there was not a significant Effect of Phenotype (F1,86 = 1.49, p=0.23), nor any significant interactions with this variable. These findings are in agreement with those reporting that STs and GTs do not differ in their rate of extinction of instrumental drug-taking behavior (Saunders and Robinson 2011, Ahrens et al. 2016).
Inactivation of the PVT affected cue-induced cocaine-seeking behavior selectively in GTs
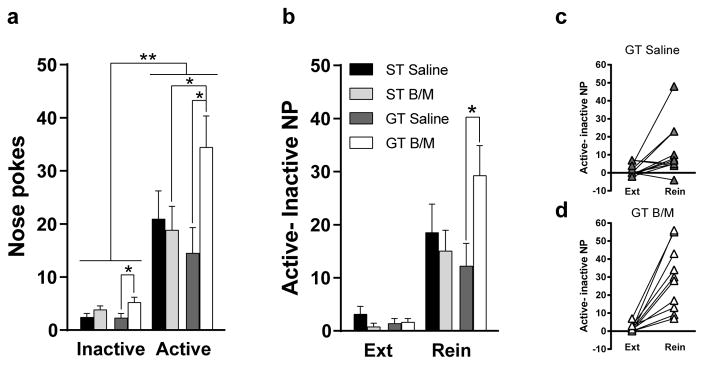
Drug-seeking behavior during the cue-induced reinstatement test was analyzed using nose pokes as the dependent variable, and Phenotype (ST or GT), Treatment (B/M or saline), and Port (active or inactive) as the independent variables. Rats differentiated between the active and inactive port during cue-induced reinstatement (Effect of Port, F1, 76 = 51.48, p<0.001), with all groups showing a preference for the active port compared to the inactive port (active vs. inactive for each group, p<0.03) (Fig. 5a). There was an overall Effect of Treatment (F1,76 = 4.53, p=0.04), and a significant Phenotype x Treatment interaction (F1,76 = 5.09, p=0.03), suggesting that PVT inactivation differentially affected the responding of STs and GTs at both ports. In GTs, PVT inactivation resulted in a greater number of nose pokes into the active (p=0.02) and inactive port (p=0.04) compared to GT controls. Inactivation of the PVT in STs had no effect on responses in either port compared to ST controls; but responding in the active port was significantly different between STs and GTs following PVT inactivation (p<0.05; Fig. 5a). This latter effect is due to the significant increase in drug-seeking behavior in GTs following B/M. It should be noted, however, that, in contrast to previous studies (Saunders and Robinson 2010, Saunders et al. 2013), the ST control group did not show significantly greater cocaine seeking compared to GT controls (p=0.38). Nonetheless, these data highlight a role for the PVT in mediating cue-induced drug-seeking behavior in GTs.
Fig. 5. Effects of transient inactivation of the PVT on cue-induced reinstatement of drug-seeking behavior.
(a) Mean + SEM of nose pokes into the active and inactive port during cue-induced reinstatement. There was a significant Effect of Port (p<0.001), Effect of Treatment (p=0.04) and a significant Phenotype x Treatment interaction (p=0.03). PVT inactivation resulted in greater drug-seeking behavior in GTs compared to GT controls (p=0.02), and a significant difference in drug-seeking behavior between STs and GTs (p<0.05). (b) Mean + SEM active-inactive nose pokes (NP) during the last extinction session (Ext) and cue-induced reinstatement (Rein). PVT inactivation resulted in greater drug-seeking behavior in GTs compared to GT controls when accounting for an increase in pokes into the inactive port (p=0.03). Mean + SEM active-inactive NP during the last extinction session (Ext) and cue-induced reinstatement (Rein) for (c) each GT rat in the saline group and (d) each GT rat in the B/M group. (ST Saline, n=10; ST B/M, n=11; GT Saline, n=11; GT B/M, n=10). *p<0.05, **p<0.01
To account for the differences in responding in the inactive port in GTs that received B/M relative to those that received saline, we subtracted the number of responses in the inactive port from those in the active port as an index of drug-seeking behavior during the last extinction session and during the cue-induced reinstatement test. This index was then analyzed across sessions (i.e. extinction vs. reinstatement) with Phenotype (ST or GT) and Treatment (B/M or saline) as the independent variables. This analysis revealed that all groups showed enhanced cocaine-seeking behavior during the reinstatement test relative to behavior during the last extinction training session (Effect of Session, F1,38 = 51.42, p<0.0001) (Fig. 5b). A significant Phenotype x Treatment interaction (F1,38 = 5.12, p=0.03) after “correcting” for differences in pokes into the inactive port, indicates enhanced cue-induced cocaine-seeking behavior in GTs following PVT inactivation (p=0.03; Fig. 5b). These findings are also illustrated in Fig. 5c and 5d, which show individual differences in responding during extinction and reinstatement for GTs treated with saline (Fig. 5c) relative to those treated with B/M (Fig. 5d). Taken together, these data demonstrate a key role for the PVT in mediating the propensity for cue-induced drug-seeking behavior in this phenotype.
Rate of extinction predicts cue-induced reinstatement of cocaine-seeking behavior in control groups
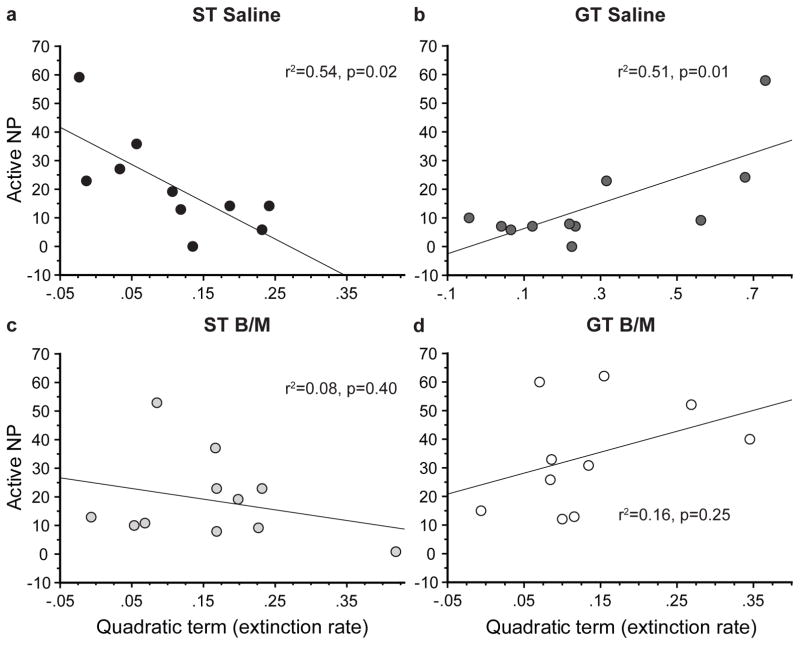
We found that the rate of decrease in responses in the active port during extinction training (i.e. extinction rate) predicted the number of responses into the active port during cue-induced reinstatement (Fig. 6). Specifically, for STs, a faster decrease in pokes into the active port during extinction training resulted in a lower number of pokes into the active port during reinstatement (F1,8 = 9.215, p=0.02; quadratic term= −129.94; Fig. 6a). In contrast, for GTs, a faster extinction rate resulted in a greater number of pokes into the active port during reinstatement (F1,8 = 9.176, p=0.01; quadratic term= 43.79; Fig. 6b). Importantly, the significant relationship between the rate of extinction and cue-induced drug-seeking behavior was only present in the control groups. That is, PVT inactivation obscured the significant relationship between these variables for both STs (F1,8 = 0.78, p=0.40; Fig. 6c) and GTs (F1,8 = 1.52, p=0.25; Fig. 6d). These data further highlight the notion that GTs and STs capture different forms of reward learning, both of which may be relevant to addiction liability (Saunders and Robinson 2010, Saunders et al. 2013, Saunders et al. 2014, Kawa et al. 2016, Pitchers et al. 2017), and both of which appear to be mediated by the PVT (Haight and Flagel 2014, Haight et al. 2015, Haight et al. 2017).
Fig. 6. Rate of extinction predicts cue-induced reinstatement of drug-seeking behavior in control groups.
Scatterplots showing the relationship between the extinction rate and the number of pokes into the active port during cue-induced reinstatement of rats for each group (STs: Saline, n=10; B/M, n=11; GTs: Saline, n=11; B/M, n=10). (a) A faster extinction rate in STs resulted in a lower number of pokes made into the active port during reinstatement (p=0.02; r2=0.54), (b) while a faster extinction rate in GTs resulted in a greater number of pokes into the active port during reinstatement (p=0.01; r2=0.51). There were no significant relationships between extinction rate and pokes into the active port during reinstatement in (c) STs (p=0.40, r2=0.08) or (d) GTs (p=0.25, r2=0.16) with PVT inactivation
Inactivation of the PVT does not affect general locomotor activity
To assess whether PVT inactivation had any effects on general locomotor activity, rats were first allowed to habituate to the locomotor testing chamber and then received either saline or B/M (same treatment as that prior to the reinstatement test) before being placed back into the chamber. Locomotor activity was analyzed across sessions (habituation or test) with Phenotype (ST or GT) and Treatment (B/M or saline) as the independent variables. There was not a significant effect of Phenotype (F1,18=0.63, p=0.44), nor a significant effect of Treatment (F1,18=0.028, p=0.87). There was, however, a significant effect of Session (Effect of Session, F1,18 = 35.15, p<0.0001; Fig. 7). As evident in Fig. 7, there was an overall decrease in locomotor activity during the test session relative to the habituation session. This is likely due to an attenuation in novelty-induced locomotion after habituation to the testing chamber. There was also a significant Session x Treatment interaction (F1,18 = 8.38, p=0.01) suggesting that the effects of treatment differed between habituation and test sessions, but not between phenotypes. Post-hoc comparisons did not reveal any additional significant effects. Thus, transient inactivation of PVT does not appear to affect general locomotor activity.
Fig. 7. Effects of PVT inactivation on locomotor activity.
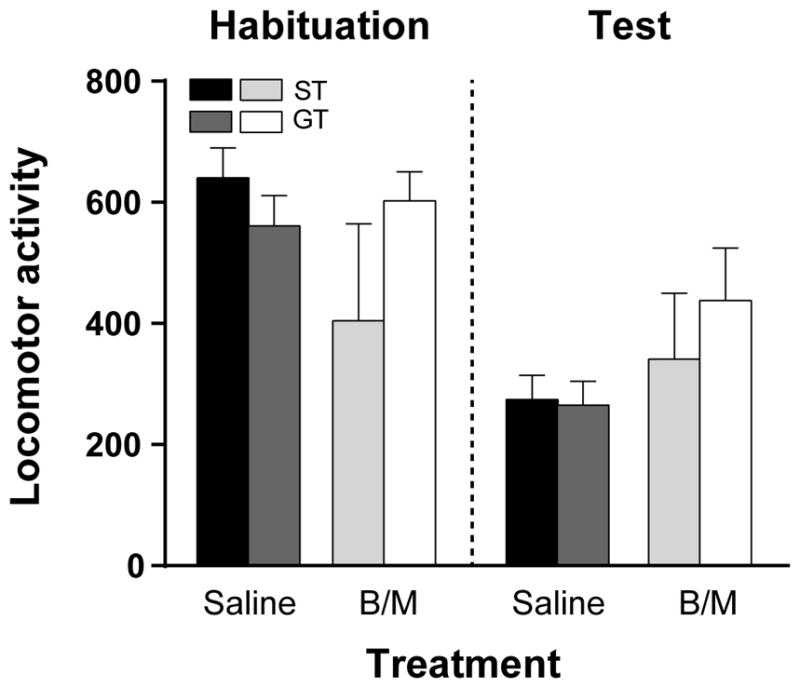
Mean + SEM for locomotor activity during habituation, followed by a test session before which rats received either saline or B/M into the PVT. There were no significant effects of phenotype or treatment for this measure, but all rats tended to decrease locomotor activity during the test session relative to habituation (p<0.0001). (ST Saline, n=5; ST B/M, n=4; GT Saline, n=6; GT B/M, n=7)
Discussion
In the current study, we assessed the role of the PVT in cue-induced reinstatement of cocaine-seeking behavior using an animal model that captures individual variation in the propensity to attribute incentive salience to reward-cues. It is well-established (Robinson and Flagel 2009, Robinson et al. 2014) that both goal-tracker and sign-tracker rats attribute predictive value to reward-cues, but sign-trackers also attribute enhanced incentive motivational value to these cues, which relies on different neural mechanisms (Flagel et al. 2011a, Flagel et al. 2011b, Yager et al. 2015, Haight et al. 2017). The PVT has been identified as a central node that may mediate both predictive and incentive learning via its multiple interconnected neural networks (Flagel et al. 2011a, Haight et al. 2017). In addition, this nucleus has been implicated in response to drugs of abuse and in the reinstatement of drug-seeking behavior (Deutch et al. 1995, Deutch et al. 1998, Stephenson et al. 1999, Hamlin et al. 2009, James et al. 2010, James et al. 2011, Browning et al. 2014, Yeoh et al. 2014, Matzeu et al. 2015, Matzeu et al. 2016, Matzeu et al. 2017). The role of the PVT in encoding the motivational value of a cue light previously associated with cocaine delivery was assessed here in STs and GTs. During the cue-induced reinstatement test, responses into the port that previously resulted in drug delivery, now resulted in presentation of the drug-cue-light. Inactivation of the PVT resulted in a robust increase in cocaine-seeking behavior during this test, but selectively in GTs compared to controls of the same phenotype. Importantly, this effect held true in GTs even after accounting for differences in responding in the inactive port following PVT inactivation, and these differences do not appear to be due to gross changes in locomotor activity. Although PVT inactivation did not significantly affect cue-induced drug-seeking behavior in STs compared to controls of the same phenotype, this manipulation did result in a difference between the phenotypes. That is, following PVT inactivation, STs show attenuated responding relative to GT, but this effect is primarily due to the significant increase in responding following PVT inactivation in GTs. These and other findings (Haight et al. 2015, Haight et al. 2017) suggest that, for GTs, the PVT may act as a “brake” on the incentive motivational properties of reward cues and removal of this “brake” unmasks the incentive value of such cues, thereby evoking maladaptive cue-driven behaviors.
The design of this experiment was such that it minimized the likelihood of any prior behavioral testing affecting the outcomes of PVT inactivation on cue-induced reinstatement. STs and GTs did not differ from one another in cocaine self-administration behavior. These data are consistent with previous results using this schedule of training (Saunders and Robinson 2010, Saunders and Robinson 2011, Saunders et al. 2013, Flagel et al. 2016), which ensured that all rats received the same number of drug-cue pairings during self-administration. In addition, there were no significant differences between phenotypes in the rate of extinction of drug-seeking behavior (when session was considered as the repeated variable), which is also consistent with previous studies (Ahrens et al. 2016). However, an additional analysis revealed that the rate of extinction did affect responding during the cue-induced reinstatement test, and did so differentially for GTs and STs. For GTs, the faster the rats decreased responding into the active port during extinction, the greater the number of pokes into the active port during the reinstatement test. In contrast, for STs, a faster decrease in responding during extinction resulted in fewer pokes into the active port during reinstatement. This differential relationship between the rate of extinction and subsequent cue-induced drug-seeking behavior in GTs and STs has not been previously reported, but further highlights the distinct learning mechanisms that may underlie different forms of addiction liability in these two phenotypes (Saunders and Robinson 2010, Saunders et al. 2013, Saunders et al. 2014, Kawa et al. 2016, Pitchers et al. 2017). Moreover, the fact that these relationships were obscured in both phenotypes following inactivation of the PVT suggests that this nucleus is important for linking prior experiences with subsequent behavior, and does so via its differential role in the learning mechanisms underlying individual variation in cue-motivated behaviors (Haight and Flagel 2014, Haight et al. 2015, Haight et al. 2017).
Prior studies have reported that STs show greater cue-induced reinstatement of cocaine-seeking behavior compared to GTs (Saunders and Robinson 2010, Saunders et al. 2013), but this finding was not replicated in the current study, perhaps due to methodological differences. Here we enforced a two-week abstinence period during which the rats remained undisturbed, whereas prior studies using the ST/GT model used a one-month abstinence period. Although the two-week period has been shown to result in robust drug-seeking behavior compared to shorter time periods (Grimm et al. 2002), the one-month abstinence period is known to even further enhance cue-induced drug-seeking behavior (Grimm et al. 2001). Indeed, it appears that longer abstinence periods that permit robust “incubation of craving” effects (Grimm et al. 2001) are required to reveal enhanced cue-induced drug-seeking behavior in STs relative to GTs (Saunders and Robinson 2010). Although we did not observe ST/GT differences in reinstatement behavior in the current study, we did find that a two-week abstinence period was sufficient to elicit cue-induced drug-seeking behavior, and, importantly, to capture the effects of PVT inactivation on individual differences in this behavior. It should also be noted that those studies previously reporting differences in cue-induced drug-seeking behavior between STs and GTs implemented extinction training prior to the abstinence period (Saunders and Robinson 2010, Saunders et al. 2013). Conversely, in the current study, extinction occurred after abstinence, and immediately preceding the test for reinstatement. This is especially noteworthy given the differential relationship revealed between the rate of extinction and cue-induced drug-seeking behavior in control STs and GTs in the current study. It will be important for future studies to further investigate this relationship and to systematically examine individual variation in cue-induced drug-seeking behavior following various extinction training procedures and forced abstinence periods.
Decreasing neuronal transmission in the PVT has previously been shown to result in a robust decrease in drug-seeking behavior following cue- (Matzeu et al. 2015), drug- (James et al. 2010) or context-induced (Hamlin et al. 2009) reinstatement. In contrast, here we report an increase in cue-induced drug-seeking behavior following PVT inactivation, but selectively in GTs. These seemingly discrepant findings are likely due to a combination of factors, including the type of reinstatement models that were used and the incorporation of individual differences in the current experimental design. Indeed, it is well-established that different forms of reinstatement recruit different neural circuits (Shaham et al. 2003, Kalivas and Volkow 2005, Crombag et al. 2008, Khoo et al. 2017) and that drug-associated stimuli engage brain regions, including the PVT, to a different degree in STs and GTs (Yager et al., 2015). Furthermore, relative to STs, GTs are more prone to reinstatement elicited by contextual cues (Saunders et al. 2014), and forebrain cholinergic activity appears to mediate this vulnerability (Pitchers et al. 2017). Thus, it is conceivable that the PVT plays a role in both cue- and context-induced reinstatement (Hamlin et al. 2009, Matzeu et al. 2015), but the form of the reinstatement “trigger” and inherent differences in the propensity to attribute incentive motivational value to said “triggers” determine its exact role, which is dependent upon the circuitry involved. We postulate that projections from the prelimbic cortex to the PVT are particularly important in mediating the attribution of incentive salience to reward cues (Flagel et al. 2011a, Paolone et al. 2013, Haight et al. 2017, Pitchers et al. 2017), and ongoing studies are investigating the role of this circuit in cue- vs. context-induced reinstatement in STs and GTs.
Another methodological detail that likely contributed to the present findings is the fact that both the anterior and posterior regions of the PVT were simultaneously inactivated in the current study; whereas some of the prior studies examining the role of the PVT in drug-seeking behavior targeted just one of these sub-regions (Hamlin et al. 2009, Matzeu et al. 2015, Matzeu et al. 2016). Importantly, these two sub-regions are known to differ in their afferent and efferent connections (Li et al. 2008, Li et al. 2012, Hsu et al. 2014, Kirouac 2015, Dong et al. 2017). While both sub-regions project to the NAc, the anterior PVT (aPVT) sends a denser projection to the NAc shell (Dong et al. 2017). When Hamlin and colleagues (Hamlin et al. 2009) demonstrated a role for the PVT in context-induced reinstatement, they also showed that this renewal of drug-seeking behavior engaged the PVT-NAc shell pathway, which included the entire rostrocaudal extent of the PVT. Additionally, the PVT-NAc pathway is involved in the acquisition of cocaine self-administration (Neumann et al. 2016), as well as mediating symptoms during drug withdrawal (Zhu et al. 2016). Recently, however, it was shown that pharmacological inactivation of the anterior, but not the posterior, PVT increases sucrose-seeking behavior upon reward omission, and that this behavior is specifically mediated by aPVT projections to the NAc shell (Do-Monte et al. 2017). In contrast, differences in food-cue-induced neuronal activity between STs and GTs seems to be restricted to cells projecting from the posterior PVT (pPVT) to the “shore” (area bordering the core/shell) of the nucleus accumbens (Haight et al., 2017). The pPVT receives dense orexinergic projections from the lateral hypothalamus (LH) (Kirouac et al. 2005), and antagonism of orexin 2 receptors in this subregion decreases drug-seeking behavior (Matzeu et al., 2016). Relative to GTs, STs show enhanced food-cue-induced neuronal activity in cells projecting from the LH to the PVT (Haight et al. 2017), and orexin receptor antagonism in the PVT appears to decrease the incentive value of reward cues (Haight 2016). Taken together, these data support the notion that distinct neuronal networks within the PVT, presumably related to rostrocaudal subdivisions and corresponding circuitry, differentially mediate appetitive and addiction-related behaviors (for review and further discussion see Millan et al. 2017). Thus, it is conceivable that selective inactivation of either the aPVT or pPVT would have different effects on cue-induced drug-seeking behavior in STs and GTs than those in the current study, for which the entire PVT was targeted. Based on the findings described above, we hypothesize that selective inactivation of the posterior PVT would attenuate cue-induced drug-seeking behavior in STs relative to controls of the same phenotype, an effect that was not observed here. Given the complex and heterogeneous circuitry of the PVT, ongoing studies are exploiting chemogenetic tools to better elucidate the role of specific circuits and cell types within this nucleus in drug-seeking behavior.
The sign-tracker/goal-tracker animal model has allowed us to parse the incentive from the predictive value of reward cues and to begin to identify the neural networks underlying these distinct forms of learning (Flagel et al. 2011a, Yager et al. 2015, Flagel and Robinson 2017, Haight et al. 2017). Most studies to-date that have exploited this model of individual variation to study the underlying brain mechanisms have focused on neuronal responses to food-cues that were attributed with incentive or predictive value following classical Pavlovian conditioning paradigms (Flagel et al. 2010, Flagel et al. 2011b, Haight and Flagel 2014, Haight et al. 2017). In the current study, however, we targeted a specific nucleus that had been identified as a key player in these Pavlovian learning processes (Flagel et al. 2011a, Haight and Flagel 2014, Haight et al. 2015, Yager et al. 2015, Haight et al. 2017), to determine whether the same nucleus acts to encode the incentive value of a cue that was previously paired with operant drug delivery. While it is known that the neural circuitry mediating Pavlovian conditioning can differ from that mediating instrumental behavior (Ostlund et al. 2007, Yin et al. 2008, Wassum et al. 2011), the paraventricular nucleus of the thalamus appears to be involved in both (Hamlin et al. 2009, James et al. 2010, Browning et al. 2014, Haight et al. 2015, Matzeu et al. 2015, Neumann et al. 2016, Do-Monte et al. 2017, Matzeu et al. 2017, Otis et al. 2017). The current findings support a role for this nucleus in the attribution of incentive value to reward cues and suggest that, in a subset of individuals, the PVT acts to suppress the learned incentive value of such cues. That is, there is likely a mechanism in place for all individuals to attribute incentive motivational value to reward cues, but only for some individuals is this incentive value revealed. In our model, the PVT appears to “mask” the incentive value for GTs and encode the incentive value for STs (Haight et al. 2015, Haight et al. 2017). The exact mechanism by which this occurs is not yet known, but prior and ongoing studies in our lab suggest that projections from the prelimbic cortex to the PVT may act to inhibit the incentive value of reward cues in GTs, likely via downstream effects on PVT-NAc shell projections. In contrast, in STs, the subcortical hypothalamic-PVT-striatal pathway presumably overrides any “top-down” cortical inhibition, allowing for the encoding of incentive value during learning and subsequent expression of resultant behaviors.
In conclusion, the results of the current study further support the notion that the PVT acts as a central node that differentially regulates cue-motivated behaviors in STs and GTs. These findings extend prior work, demonstrating a role for this nucleus in mediating the incentive value of drug-paired cues following an instrumental paradigm. Inactivation of the PVT enhances cue-induced drug-seeking behavior, but only in GTs relative to controls of the same phenotype. Thus, in GTs, the PVT appears to inhibit the expression of the incentive motivational value of a cocaine-associated cue-light, resulting in suppression of drug-seeking behavior during cue-induced reinstatement. The fact that inactivation of the PVT results in a difference in cue-induced drug-seeking behavior between STs and GTs, suggests that this nucleus also plays a role in encoding the incentive value of drug-cues for STs, albeit to a different degree and likely via a different neural circuit. Future studies are warranted to better elucidate the neural circuits underlying individual variation in cue-motivated and addiction-related behaviors, and the role of the PVT within these circuits.
Acknowledgments
Funding
Funding for this work was provided by the National Institute on Drug Abuse branch of the National Institutes of Health (RO1DA038599) awarded to SBF, and training grants T32DA007821 (BNK) and T32DA007268 (IRC).
We would like to thank Drs. Jonathan Morrow, Aram Parsegian and Joshua Haight for their feedback on a previous version of this manuscript. We would also like to thank Drs. Brady West and Corey Powell from the Consulting for Statistics, Computing and Analytics Research team at the University of Michigan for their helpful input on statistical modeling for portions of the data. The experiments were designed by BNK and SBF, and conducted by BNK, MSK, IRC and PC. Data was analyzed by BNK, and BNK and SBF wrote the manuscript.
Footnotes
Conflict of Interest
The authors declare that the research was conducted in the absence of any financial or commercial relationships that could be construed as a potential conflict of interest.
References
- Ahrens AM, Singer BF, Fitzpatrick CJ, Morrow JD, Robinson TE. Rats that sign-track are resistant to Pavlovian but not instrumental extinction. Behav Brain Res. 2016;296:418–430. doi: 10.1016/j.bbr.2015.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Leibowitz SF. GABA-induced inactivation of dorsal midline thalamic subregions has distinct effects on emotional behaviors. Neurosci Lett. 2015;609:92–96. doi: 10.1016/j.neulet.2015.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann JS, Marusich JA, Gipson CD, Bardo MT. Novelty seeking, incentive salience and acquisition of cocaine self-administration in the rat. Behav Brain Res. 2011;216(1):159–165. doi: 10.1016/j.bbr.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr Opin Pharmacol. 2009;9(1):65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindra D. How adaptive behavior is produced: a perceptual motivation alternative to response reinforcement. Behavior and Brain Sciences. 1978;(1):41–91. [Google Scholar]
- Bolles R. Reinforcement, expectancy, and learning. Psychological Review. 1972;79:394–409. doi: 10.1037/h0033120. [DOI] [Google Scholar]
- Browning JR, Jansen HT, Sorg BA. Inactivation of the paraventricular thalamus abolishes the expression of cocaine conditioned place preference in rats. Drug Alcohol Depend. 2014;134:387–390. doi: 10.1016/j.drugalcdep.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress A, Ehrman R, McLellan AT, O’Brien C. Conditioned craving and arousal in cocaine addiction: a preliminary report. NIDA Res Monogr. 1988;81:74–80. [PubMed] [Google Scholar]
- Childress A, Ehrman R, McLellan AT, O’Brien CP. Classically conditioned factors in drug dependence. In: Lowinson J, Millman RP, editors. Comprehensive Textbook of Substance Abuse. Baltimore: Williams and Wilkins; 1993. pp. 56–69. [Google Scholar]
- Crombag HS, Badiani A, Maren S, Robinson TE. The role of contextual versus discrete drug-associated cues in promoting the induction of psychomotor sensitization to intravenous amphetamine. Behav Brain Res. 2000;116(1):1–22. doi: 10.1016/s0166-4328(00)00243-6. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, Shaham Y. Review. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutch AY, Bubser M, Young CD. Psychostimulant-induced Fos protein expression in the thalamic paraventricular nucleus. J Neurosci. 1998;18(24):10680–10687. doi: 10.1523/JNEUROSCI.18-24-10680.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutch AY, Ongur D, Duman RS. Antipsychotic drugs induce Fos protein in the thalamic paraventricular nucleus: a novel locus of antipsychotic drug action. Neuroscience. 1995;66(2):337–346. doi: 10.1016/0306-4522(94)00571-l. [DOI] [PubMed] [Google Scholar]
- Do-Monte FH, Minier-Toribio A, Quinones-Laracuente K, Medina-Colon EM, Quirk GJ. Thalamic Regulation of Sucrose Seeking during Unexpected Reward Omission. Neuron. 2017;94(2):388–400. e384. doi: 10.1016/j.neuron.2017.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do-Monte FH, Quinones-Laracuente K, Quirk GJ. A temporal shift in the circuits mediating retrieval of fear memory. Nature. 2015;519(7544):460–463. doi: 10.1038/nature14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Li S, Kirouac GJ. Collateralization of projections from the paraventricular nucleus of the thalamus to the nucleus accumbens, bed nucleus of the stria terminalis, and central nucleus of the amygdala. Brain Struct Funct. 2017 doi: 10.1007/s00429-017-1445-8. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Cameron CM, Pickup KN, Watson SJ, Akil H, Robinson TE. A food predictive cue must be attributed with incentive salience for it to induce c-fos mRNA expression in cortico-striatal-thalamic brain regions. Neuroscience. 2011a;196:80–96. doi: 10.1016/j.neuroscience.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Chaudhury S, Waselus M, Kelly R, Sewani S, Clinton SM, Thompson RC, Watson SJ, Jr, Akil H. Genetic background and epigenetic modifications in the core of the nucleus accumbens predict addiction-like behavior in a rat model. Proc Natl Acad Sci U S A. 2016;113(20):E2861–2870. doi: 10.1073/pnas.1520491113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PE, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011b;469(7328):53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE. Neurobiological Basis of Individual Variation in Stimulus-Reward Learning. Curr Opin Behav Sci. 2017;13:178–185. doi: 10.1016/j.cobeha.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, Phillips PE, Akil H. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacology. 2010;35(2):388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Vazquez DM, Robinson TE. Manipulations during the second, but not the first, week of life increase susceptibility to cocaine self-administration in female rats. Neuropsychopharmacology. 2003;28(10):1741–1751. doi: 10.1038/sj.npp.1300228. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412(6843):141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Shaham Y, Hope BT. Effect of cocaine and sucrose withdrawal period on extinction behavior, cue-induced reinstatement, and protein levels of the dopamine transporter and tyrosine hydroxylase in limbic and cortical areas in rats. Behav Pharmacol. 2002;13(5–6):379–388. doi: 10.1097/00008877-200209000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haight JL. Dissertation. University of Michigan; 2016. Elucidating the role of the paraventricular nucleus of the thalamus in cue-motivated behavior. [Google Scholar]
- Haight JL, Flagel SB. A potential role for the paraventricular nucleus of the thalamus in mediating individual variation in Pavlovian conditioned responses. Front Behav Neurosci. 2014;8:79. doi: 10.3389/fnbeh.2014.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haight JL, Fraser KM, Akil H, Flagel SB. Lesions of the paraventricular nucleus of the thalamus differentially affect sign- and goal-tracking conditioned responses. Eur J Neurosci. 2015;42(7):2478–2488. doi: 10.1111/ejn.13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haight JL, Fuller ZL, Fraser KM, Flagel SB. A food-predictive cue attributed with incentive salience engages subcortical afferents and efferents of the paraventricular nucleus of the thalamus. Neuroscience. 2017;340:135–152. doi: 10.1016/j.neuroscience.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, Choi EA, McNally GP. Paraventricular thalamus mediates context-induced reinstatement (renewal) of extinguished reward seeking. Eur J Neurosci. 2009;29(4):802–812. doi: 10.1111/j.1460-9568.2009.06623.x. [DOI] [PubMed] [Google Scholar]
- Hsu DT, Kirouac GJ, Zubieta JK, Bhatnagar S. Contributions of the paraventricular thalamic nucleus in the regulation of stress, motivation, and mood. Front Behav Neurosci. 2014;8:73. doi: 10.3389/fnbeh.2014.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Charnley JL, Flynn JR, Smith DW, Dayas CV. Propensity to ‘relapse’ following exposure to cocaine cues is associated with the recruitment of specific thalamic and epithalamic nuclei. Neuroscience. 2011;199:235–242. doi: 10.1016/j.neuroscience.2011.09.047. [DOI] [PubMed] [Google Scholar]
- James MH, Charnley JL, Jones E, Levi EM, Yeoh JW, Flynn JR, Smith DW, Dayas CV. Cocaine- and amphetamine-regulated transcript (CART) signaling within the paraventricular thalamus modulates cocaine-seeking behaviour. PLoS One. 2010;5(9):e12980. doi: 10.1371/journal.pone.0012980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Dayas CV. What about me…? The PVT: a role for the paraventricular thalamus (PVT) in drug-seeking behavior. Front Behav Neurosci. 2013;7:18. doi: 10.3389/fnbeh.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kawa AB, Bentzley BS, Robinson TE. Less is more: prolonged intermittent access cocaine self-administration produces incentive-sensitization and addiction-like behavior. Psychopharmacology (Berl) 2016;233(19–20):3587–3602. doi: 10.1007/s00213-016-4393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86(5):773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Khoo SY, Gibson GD, Prasad AA, McNally GP. How contexts promote and prevent relapse to drug seeking. Genes Brain Behav. 2017;16(1):185–204. doi: 10.1111/gbb.12328. [DOI] [PubMed] [Google Scholar]
- Kirouac GJ. Placing the paraventricular nucleus of the thalamus within the brain circuits that control behavior. Neurosci Biobehav Rev. 2015;56:315–329. doi: 10.1016/j.neubiorev.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Kirouac GJ, Parsons MP, Li S. Orexin (hypocretin) innervation of the paraventricular nucleus of the thalamus. Brain Res. 2005;1059(2):179–188. doi: 10.1016/j.brainres.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Li S, Kirouac GJ. Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. J Comp Neurol. 2008;506(2):263–287. doi: 10.1002/cne.21502. [DOI] [PubMed] [Google Scholar]
- Li S, Kirouac GJ. Sources of inputs to the anterior and posterior aspects of the paraventricular nucleus of the thalamus. Brain Struct Funct. 2012;217(2):257–273. doi: 10.1007/s00429-011-0360-7. [DOI] [PubMed] [Google Scholar]
- Li Y, Dong X, Li S, Kirouac GJ. Lesions of the posterior paraventricular nucleus of the thalamus attenuate fear expression. Front Behav Neurosci. 2014;8:94. doi: 10.3389/fnbeh.2014.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li S, Wei C, Wang H, Sui N, Kirouac GJ. Orexins in the paraventricular nucleus of the thalamus mediate anxiety-like responses in rats. Psychopharmacology (Berl) 2010;212(2):251–265. doi: 10.1007/s00213-010-1948-y. [DOI] [PubMed] [Google Scholar]
- Lovic V, Saunders BT, Yager LM, Robinson TE. Rats prone to attribute incentive salience to reward cues are also prone to impulsive action. Behav Brain Res. 2011;223(2):255–261. doi: 10.1016/j.bbr.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzeu A, Cauvi G, Kerr TM, Weiss F, Martin-Fardon R. The paraventricular nucleus of the thalamus is differentially recruited by stimuli conditioned to the availability of cocaine versus palatable food. Addict Biol. 2017;22(1):70–77. doi: 10.1111/adb.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzeu A, Kerr TM, Weiss F, Martin-Fardon R. Orexin-A/Hypocretin-1 Mediates Cocaine-Seeking Behavior in the Posterior Paraventricular Nucleus of the Thalamus via Orexin/Hypocretin Receptor-2. J Pharmacol Exp Ther. 2016;359(2):273–279. doi: 10.1124/jpet.116.235945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzeu A, Weiss F, Martin-Fardon R. Transient inactivation of the posterior paraventricular nucleus of the thalamus blocks cocaine-seeking behavior. Neurosci Lett. 2015;608:34–39. doi: 10.1016/j.neulet.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD, Robinson TE. Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PLoS One. 2012;7(6):e38987. doi: 10.1371/journal.pone.0038987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan EZ, Ong Z, McNally GP. Paraventricular thalamus: Gateway to feeding, appetitive motivation, and drug addiction. Prog Brain Res. 2017;235:113–137. doi: 10.1016/bs.pbr.2017.07.006. [DOI] [PubMed] [Google Scholar]
- Neumann PA, Wang Y, Yan Y, Wang Y, Ishikawa M, Cui R, Huang YH, Sesack SR, Schluter OM, Dong Y. Cocaine-Induced Synaptic Alterations in Thalamus to Nucleus Accumbens Projection. Neuropsychopharmacology. 2016;41(9):2399–2410. doi: 10.1038/npp.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong ZY, Liu JJ, Pang ZP, Grill HJ. Paraventricular Thalamic Control of Food Intake and Reward: Role of Glucagon-Like Peptide-1 Receptor Signaling. Neuropsychopharmacology. 2017;42(12):2387–2397. doi: 10.1038/npp.2017.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. Orbitofrontal cortex mediates outcome encoding in Pavlovian but not instrumental conditioning. J Neurosci. 2007;27(18):4819–4825. doi: 10.1523/JNEUROSCI.5443-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis JM, Namboodiri VM, Matan AM, Voets ES, Mohorn EP, Kosyk O, McHenry JA, Robinson JE, Resendez SL, Rossi MA, Stuber GD. Prefrontal cortex output circuits guide reward seeking through divergent cue encoding. Nature. 2017;543(7643):103–107. doi: 10.1038/nature21376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolone G, Angelakos CC, Meyer PJ, Robinson TE, Sarter M. Cholinergic control over attention in rats prone to attribute incentive salience to reward cues. J Neurosci. 2013;33(19):8321–8335. doi: 10.1523/JNEUROSCI.0709-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos GWC. The rat brain in stereotaxic coordinates. Burlington, MA: Academic Press; 2007. [Google Scholar]
- Penzo MA, Robert V, Tucciarone J, De Bundel D, Wang M, Van Aelst L, Darvas M, Parada LF, Palmiter RD, He M, Huang ZJ, Li B. The paraventricular thalamus controls a central amygdala fear circuit. Nature. 2015;519(7544):455–459. doi: 10.1038/nature13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchers KK, Phillips KB, Jones JL, Robinson TE, Sarter M. Diverse roads to relapse: A discriminative cue signaling cocaine availability is more effective in renewing cocaine-seeking in goal-trackers than sign-trackers, and depends on basal forebrain cholinergic activity. J Neurosci. 2017 doi: 10.1523/JNEUROSCI.0990-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Flagel SB. Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biol Psychiatry. 2009;65(10):869–873. doi: 10.1016/j.biopsych.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Yager LM, Cogan ES, Saunders BT. On the motivational properties of reward cues: Individual differences. Neuropharmacology. 2014;76(Pt B):450–459. doi: 10.1016/j.neuropharm.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, O’Donnell EG, Aurbach EL, Robinson TE. A cocaine context renews drug seeking preferentially in a subset of individuals. Neuropsychopharmacology. 2014;39(12):2816–2823. doi: 10.1038/npp.2014.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. A cocaine cue acts as an incentive stimulus in some but not others: implications for addiction. Biol Psychiatry. 2010;67(8):730–736. doi: 10.1016/j.biopsych.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. Individual variation in the motivational properties of cocaine. Neuropsychopharmacology. 2011;36(8):1668–1676. doi: 10.1038/npp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Yager LM, Robinson TE. Cue-evoked cocaine “craving”: role of dopamine in the accumbens core. J Neurosci. 2013;33(35):13989–14000. doi: 10.1523/JNEUROSCI.0450-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168(1–2):3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Stephenson CP, Hunt GE, Topple AN, McGregor IS. The distribution of 3,4-methylenedioxymethamphetamine “Ecstasy”-induced c-fos expression in rat brain. Neuroscience. 1999;92(3):1011–1023. doi: 10.1016/s0306-4522(99)00049-4. [DOI] [PubMed] [Google Scholar]
- Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev. 1984;91(2):251–268. [PubMed] [Google Scholar]
- Toates FM. The control of ingestive behaviour by internal and external stimuli--a theoretical review. Appetite. 1981;2(1):35–50. doi: 10.1016/s0195-6663(81)80035-9. [DOI] [PubMed] [Google Scholar]
- Tomie A, Grimes KL, Pohorecky LA. Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain Res Rev. 2008;58(1):121–135. doi: 10.1016/j.brainresrev.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Ostlund SB, Balleine BW, Maidment NT. Differential dependence of Pavlovian incentive motivation and instrumental incentive learning processes on dopamine signaling. Learn Mem. 2011;18(7):475–483. doi: 10.1101/lm.2229311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager LM, Pitchers KK, Flagel SB, Robinson TE. Individual variation in the motivational and neurobiological effects of an opioid cue. Neuropsychopharmacology. 2015;40(5):1269–1277. doi: 10.1038/npp.2014.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager LM, Robinson TE. A classically conditioned cocaine cue acquires greater control over motivated behavior in rats prone to attribute incentive salience to a food cue. Psychopharmacology (Berl) 2013;226(2):217–228. doi: 10.1007/s00213-012-2890-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoh JW, James MH, Graham BA, Dayas CV. Electrophysiological characteristics of paraventricular thalamic (PVT) neurons in response to cocaine and cocaine- and amphetamine-regulated transcript (CART) Front Behav Neurosci. 2014;8:280. doi: 10.3389/fnbeh.2014.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Balleine BW. Reward-guided learning beyond dopamine in the nucleus accumbens: the integrative functions of cortico-basal ganglia networks. Eur J Neurosci. 2008;28(8):1437–1448. doi: 10.1111/j.1460-9568.2008.06422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CD, Deutch AY. The effects of thalamic paraventricular nucleus lesions on cocaine-induced locomotor activity and sensitization. Pharmacol Biochem Behav. 1998;60(3):753–758. doi: 10.1016/s0091-3057(98)00051-3. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Wienecke CF, Nachtrab G, Chen X. A thalamic input to the nucleus accumbens mediates opiate dependence. Nature. 2016;530(7589):219–222. doi: 10.1038/nature16954. [DOI] [PMC free article] [PubMed] [Google Scholar]