Abstract
Background and Purpose
The evidence supporting the deleterious cardiovascular health effects of living near a major roadway is growing, although this association is not universal. In primary analyses, we hypothesized that residential proximity to a major roadway would be associated with incident ischemic stroke, and that cardiovascular risk factors would modify that association.
Methods
The Northern Manhattan Study (NOMAS) is an ongoing, population-based cohort study designed to measure cardiovascular risk factors, stroke incidence, and other outcomes in a multiethnic urban population. Recruitment occurred from 1993–2001 and participants are followed-up annually by telephone. Residential addresses at baseline were geocoded and Euclidean distance to nearest major roadway was estimated and categorized as in prior studies. We used Cox proportional hazard models to calculate hazard ratios (HRs) and 95% confidence intervals (95%CIs) for the association of this distance to incidence of stroke and other outcomes, adjusting for sociodemographic and cardiovascular risk factors, year at baseline, and neighborhood socioeconomic status. We assessed whether these associations varied by age, sex, smoking status, diabetes, and hypertension.
Results
During a median follow-up period of 15 years (n=3,287), 11% of participants were diagnosed with ischemic stroke. Participants living <100m from a roadway had a 42% (95%CI 1.01–2.02) higher rate of ischemic stroke versus those living >400m away. This association was more pronounced among non-current smokers (HR 1.54; 95%CI 1.05–2.26) and not evident among smokers (HR 0.69; 95%CI 0.23–2.06). There was no clear pattern of association between proximity to major roadways and other cardiovascular events including myocardial infarction, all-cause death, or vascular death.
Conclusions
In this urban multiethnic cohort we found evidence supporting that within-city variation in residential proximity to major roadway is associated with higher risk of ischemic stroke. An individual’s smoking history modified this association, with the association remaining only among participants not currently smokers.
Background
Despite recent advances in treatment and prevention, stroke remains the leading cause of serious long-term disability and heart disease is the leading cause of death in the United States.1–3 There are approximately 795,000 strokes and 735,000 myocardial infarctions (MI) each year.1–3 While the mortality associated with stroke and MI has decreased, the morbidity associated with these diseases remains high, with a huge cost burden of approximately $17.5 billion per year for direct stroke costs and $11.3 billion for direct MI costs.1,3 Conventional risk factors such as hypertension, diabetes, sedentary behavior, and smoking do not account for all of the variation in stroke risk.4–6 Identifying novel modifiable risk factors is therefore of great importance.
The evidence supporting the deleterious effects of residential proximity to a major roadway is growing; however studies looking the effect of living near a major roadway on stroke are limited.7,8 Therefore, we set out to evaluate the relationship between proximity to roadways and incident ischemic stroke in an urban setting within the context of the multi-ethnic Northern Manhattan Study (NOMAS). We hypothesized that the incidence of stroke events would be greater among individuals living closer to a major roadway.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
NOMAS is an ongoing, prospective, population-based cohort study designed to measure cardiovascular risk factors and outcomes in a stroke-free multi-ethnic urban population in Northern Manhattan. Cohort recruitment occurred from 1993 to 2001 and participants are followed-up annually by telephone. Eligibility included age ≥ 40 years, residency in one of Northern Manhattan’s 5 zip codes, living in a home with a telephone, and no history of clinical stroke. Detailed methods of participant recruitment, baseline evaluation, and follow-up have been described previously.9
All activities pertaining to NOMAS were approved by the Institutional Review Boards at Columbia University Medical Center and the University of Miami. Written consent was provided by each participant at enrollment.
Residential Proximity to Major Roadway
Participants’ residential addresses were collected at baseline examination and geocoded using Geosupport Batch Address Translator Desktop Edition (NYC Department of City Planning, New York, NY). Participants with primary addresses in New York City were included in the study (99.7%, n=3,287). Eleven individuals were excluded due to insufficient address information.
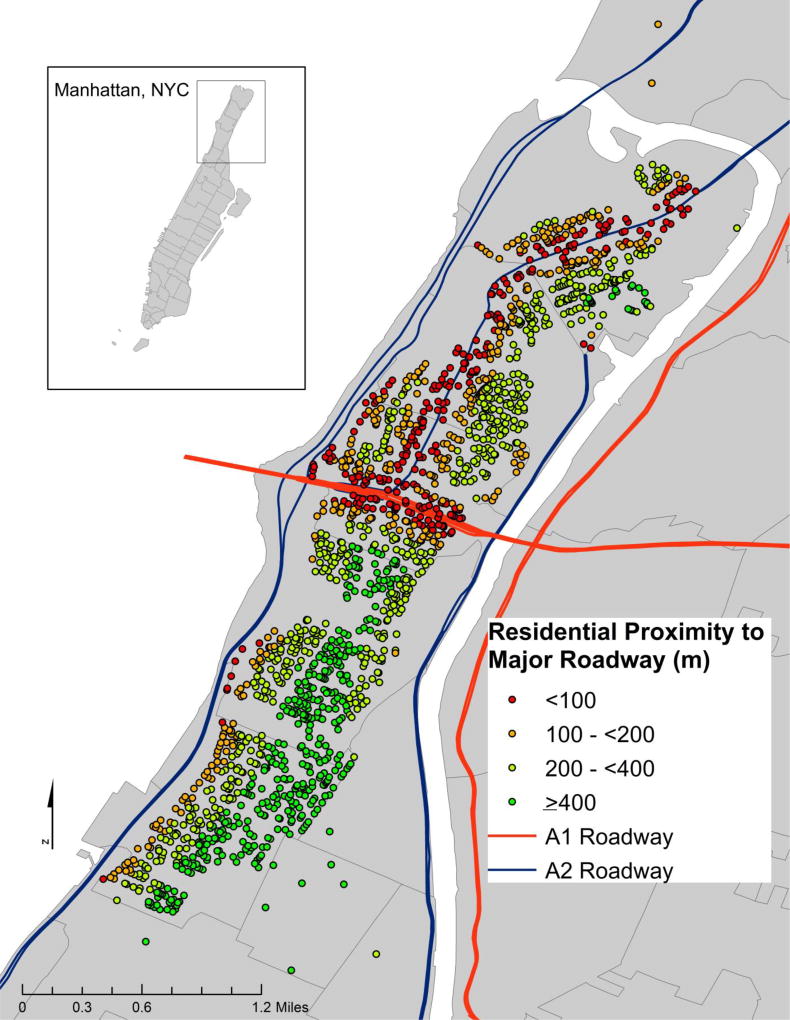
We used ArcGIS (version 10.3.1, ESRI, Inc., Redlands, CA) to calculate the Euclidean distance from residence to nearest major roadway, defined as US Census Features Class A1 (primary highway with limited access) and A2 roadways (nationally and regionally important highways that do not have limited access), which include most federal and interstate highways and some larger state and county highways. Distance to roadway was modeled as a log-transformed continuous variable (per interquartile range (IQR)) based on prior studies10,11 and also categorized into <100, 100–<200, 200– <400, and ≥ 400 meters (Figure 1).
Figure 1.
Distribution of Exposure throughout Northern Manhattan, with US census defined A1 and A2 roadways
Outcome ascertainment
The primary outcome for this analysis was incident ischemic stroke. Secondary outcomes included incident MI, all cause death, and vascular death. All subjects were followed annually via telephone to detect any new neurological or cardiac symptoms, interval hospitalizations, or death. Medical records were reviewed to identify events and verify details of suspected events. Suspected strokes were adjudicated independently by two neurologists. Any disagreements were adjudicated by the principal investigators (R.L.S. and M.E.). Incident MI was defined using criteria adapted from the Cardiac Arrhythmia Suppression Trial and the Lipid Research Clinics Coronary Primary Prevention Trial. The presence of an MI was adjudicated by cardiologists independently after review of all the clinical data. Cause of death was obtained through discussions with the participant’s family, review of medical records, and if possible, a copy of the death certificate. Vascular death was defined as a death due to cardiac causes, underlying heart disease or stroke.12
Risk Factor Evaluation
At enrollment, participants underwent in-person interviews in their primary language conducted by trained interviewers to assess baseline health status and risk factors using validated data collection instruments, physical, and neurological examinations. Race-ethnicity was collected through self-identification. Cardiovascular risk factors (smoking, alcohol use, hypertension (HTN), diabetes, body mass index (BMI), and high-density lipoprotein levels (HDL)) were assessed using standardized questions and clinical data obtained at the time of exam, as previously described.13,14 Smoking status was dichotomized into ‘non-current smokers’, defined as individuals reporting being former or never smokers, and ‘current smokers’. Moderate alcohol use was defined as current drinking of >1 drink per month and ≤ 2 drinks per day. Physical activity was defined as engaging in one or more leisure-time activities during the 10 days prior to enrollment. Cardiac disease was defined as having at least one of the following: coronary heart disease, angina, vascular heart disease, bypass surgery or angioplasty, history of atrial fibrillation, heart attack, or medication for any cardiac condition. A summary z-score for neighborhood socioeconomic status (SES) was derived at the census tract level as a neighborhood measure of wealth, education, and occupation (Supplemental Table 1).15
Statistical Methods
Distributions of sociodemographic characteristics and cardiovascular risk factors at baseline were calculated as means for continuous variables and proportions for categorical variables. Cox proportional hazard models were used to evaluate the association between residential proximity to roadway and the four outcomes, expressed as hazard ratios (HR) and 95% confidence intervals (95% CI) (Model details provided in the online-only Data Supplement). We present results from unadjusted models (Model 1) and models adjusted for sociodemographic characteristics (age at baseline, sex, race-ethnicity, education, insurance status, year of enrollment, and neighborhood SES; Model 2). Model 3 further adjusted for cardiovascular risk factors ascertained at baseline (smoking, alcohol use, physical activity, HTN, BMI, HDL, diabetes, cardiac disease). To assess effect modification, we tested for multiplicative interaction between risk factors and residential distance to roadway. In sensitivity analyses, we first fitted sub-distribution Cox proportional hazard models with mortality as a competing risk for incident stroke or MI. We then ran a series of sub-analyses removing any participants with history of MI or cardiac disease.
We evaluated possible interaction by age (<70 vs. ≥70 years), sex, smoking status (current smokers vs. non-current smokers), diabetes, and hypertension. Interaction terms of potential effect modifiers and continuous exposures were each included independently in a series of fully adjusted models for each outcome of interest. Interaction terms with a p-value<0.10 were considered potentially statistically significant and models were stratified to look at differences between groups.
All analyses were performed in SAS version 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
Characteristics for the cohort at baseline (n= 3,287) are outlined in Table 1. Median age [IQR] at time of enrollment was 69 years [14]. Of the cohort, 37% were men, 53% were Hispanic, 24% were non-Hispanic black, and 21% were non-Hispanic white. On average, participants had lived in Northern Manhattan for 28 years [23]. Participants lived a median distance [IQR; 25th and 75th percentiles] from a major roadway of 248.1 [253.7; 136.1, 389.8] meters, and 17% of the cohort lived less than 100 meters from a major roadway (Figure 1, Supplementary Table 2).
Table 1.
Cohort Characteristics (n=3,287)
| Sociodemographic Characteristics | Mean [SD] or n (%) | |
|---|---|---|
| Age at baseline, y | 69 [14] | |
| Men | 1,222 (37.2) | |
| Race-ethnicity | ||
| White non-Hispanic | 688 (20.9) | |
| Black non-Hispanic | 797 (24.3) | |
| Hispanic | 1,725 (52.5) | |
| Other | 77 (2.3) | |
| Completed High School | 1,502 (45.7) | |
| Medicaid or Uninsured | 1,434 (43.9) | |
| Cardiovascular Risk Factors | ||
| Current Smoker | 559 (17) | |
| Any Physical Activity | 1,901 (57.8) | |
| Moderate Alcohol Intake* | 1,080 (32.9) | |
| Hypertension† | 2,420 (73.6) | |
| Diabetes‡ | 716 (21.8) | |
| Any Cardiac Disease | 789 (24.0) | |
| Mean Body Mass Index§ | 27.1 [6.2] | |
| Mean High-Density Lipoprotein Cholesterol§ | 44.0 [19] | |
| Outcomes | ||
| Incident Ischemic Stroke | 361 (11.0) | |
| Incident Myocardial Infarction | 368 (11.2) | |
| All-cause death | 1854 (56.4) | |
| Vascular Death | 803 (24.4) | |
IQR:interquartile range.
Moderate alcohol use ≤ 2 servings/day,
Hypertension = systolic blood pressure >140 mm/Hg, diastolic blood pressure recording >90 mm/Hg (average of two measurements), physician diagnosis, self-report,
Diabetes=fasting blood glucose ≥126 mg/dL, self-report, insulin, hypoglycemic use,
Standardized
During a median follow-up period of 15 years (range 0.2 – 23.1), 11.0 % of individuals (n=361) were diagnosed with ischemic stroke, and 11.2% (n=368) experienced a MI. Overall, 56.4% (n=1854) of the participants had died by the end of the follow-up period, 24.4% (n=803) due to vascular causes.
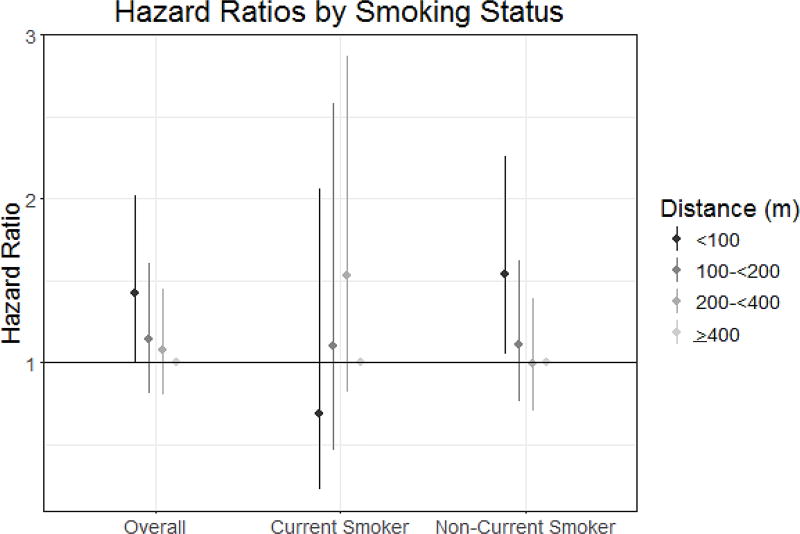
Living < 100 meters from a major roadway was associated with a 42% higher rate of incident ischemic stroke (HR: 1.42, 95% CI 1.01, 2.02) when compared to those living > 400m away, in models fully adjusted for sociodemographic and cardiovascular risk factors. A similar pattern was seen with a continuous measure of distance to major roadway; an IQR difference in residential proximity to a major roadway was associated with a 13% higher rate of ischemic stroke (HR: 1.13, 95% CI: 1.02, 1.25) (Table 2; Figure 2). There was no clear pattern of association between residential proximity to major roadways and MI, all-cause death, or vascular death (Table 2).
Table 2.
Associations between Residential Proximity to Major Roadway and Outcomes
| Outcome | Exposure | Model 1* | Model 2† | Model 3‡ | |||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | ||
| Ischemic Stroke | <100 m | 1.08 | 0.80, 1.45 | 1.35 | 0.96, 1.91 | 1.42 | 1.01, 2.02 |
| 100– <200 m | 0.92 | 0.69, 1.24 | 1.16 | 0.83, 1.61 | 1.14 | 0.81, 1.60 | |
| 200– <400 m | 1.00 | 0.77, 1.31 | 1.10 | 0.83, 1.48 | 1.08 | 0.80, 1.45 | |
| ≥400 m | --- | --- | --- | --- | --- | --- | |
| Log(distance)§ | 1.04 | 0.93, 1.15 | 1.11 | 1.01, 1.23 | 1.13 | 1.02, 1.25 | |
| Myocardial Infarction | <100 m | 1.15 | 0.85, 1.56 | 1.01 | 0.71, 1.45 | 1.00 | 0.69, 1.44 |
| 100– <200 m | 1.03 | 0.77, 1.37 | 0.89 | 0.63, 1.27 | 0.89 | 0.63, 1.26 | |
| 200– <400 m | 1.08 | 0.82, 1.40 | 1.09 | 0.81, 1.47 | 0.98 | 0.72, 1.33 | |
| ≥400 m | --- | --- | --- | --- | --- | --- | |
| Log(distance)§ | 1.05 | 0.95, 1.16 | 1.00 | 0.89, 1.12 | 1.00 | 0.89, 1.14 | |
| All-cause Mortality | <100 m | 0.85 | 0.74, 0.98 | 0.95 | 0.81, 1.12 | 0.95 | 0.81, 1.13 |
| 100– <200 m | 0.92 | 0.81, 1.04 | 1.06 | 0.91, 1.23 | 1.04 | 0.90, 1.21 | |
| 200– <400 m | 0.90 | 0.80, 1.01 | 1.07 | 0.94, 1.21 | 1.04 | 0.91, 1.18 | |
| ≥400 m | --- | --- | --- | --- | --- | --- | |
| Log(distance)§ | 0.95 | 0.91, 1.00 | 0.99 | 0.94, 1.04 | 1.00 | 0.95, 1.06 | |
| Vascular Mortality | <100 m | 0.86 | 0.70, 1.07 | 0.92 | 0.72, 1.17 | 0.92 | 0.71, 1.19 |
| 100– <200 m | 0.90 | 0.74, 1.09 | 0.92 | 0.78, 1.23 | 0.94 | 0.74, 1.19 | |
| 200– <400 m | 0.90 | 0.76, 1.08 | 1.04 | 0.85, 1.26 | 1.00 | 0.82, 1.23 | |
| ≥400 m | --- | --- | --- | --- | --- | --- | |
| Log(distance)§ | 0.94 | 0.87, 1.02 | 0.97 | 0.90, 1.05 | 0.98 | 0.89, 1.07 | |
HR:hazard ratio, CI:Confidence interval. Scaled to interquartile range change
unadjusted model
adjusted for age at baseline, sex, race-ethnicity, education, insurance status, year of baseline, neighborhood socioeconomic status
adjusted for variables in Model 2 plus smoking, alcohol use, physical activity, body mass index, hypertension, high density lipoprotein levels, diabetes, any cardiac disease
Figure 2.
Hazard ratios and 95% confidence intervals of the association between residential distance to major roadway and incident ischemic stroke, overall and stratified by smoking status. All HRs fully adjusted for sociodemographic and cardiovascular risk factors.
We found evidence that the association between proximity to major roadway and ischemic stroke varied among subgroups defined by smoking history (p=0.05). Specifically, the association between proximity to roadway and ischemic stroke was stronger among participants who were non-current smokers versus current smokers living less than 100m from a roadway (HR: 1.54, 95% CI 1.05, 2.26 and HR: 0.69 95% CI 0.23, 2.06, respectively; Figure 2). A similar pattern was observed with the continuous measure of proximity to roadway (data not shown). We observed no statistically significant interactions by age, sex, diabetes, or hypertension.
In sensitivity analyses analyzing mortality as a competing risk, the magnitude of association between proximity to roadway and ischemic stroke and MI remained unchanged, both in overall models and in models stratified by smoking status. In addition, there were no substantial differences in effects when participants with a history of MI or cardiac disease were removed from analyses (Data not shown).
Discussion
In this urban, population-based cohort in Northern Manhattan, we found evidence that residential proximity to a major roadway was associated with a higher risk of incident ischemic stroke. Individuals living closest were particularly at risk; living less than 100 meters away from a major road was associated with a 42% higher risk of ischemic stroke as compared to those living more than 400 meters away. There also appeared to be a positive relationship between those living 100–400 meters from a major roadway and rate of ischemic stroke, although not statistically significant. In addition, we found evidence of interaction by smoking status. Among non-current smokers, the effect of living within 100 meters of a major roadway on incident ischemic stroke was significantly higher than that of individuals who currently smoke. Contrary to our hypothesis, however, we did not observe evidence of an association between proximity to major roadway and the risk of other cardiovascular events such as MI, vascular death, or all cause death.
The evidence supporting the deleterious health effects of living near a major roadway is growing. Individuals living in close proximity to a major roadway have been shown to be at increased risk for stroke,7,8 CVD,16,17 hypertension,18 and post-stroke mortality.11 Overall, the key exposures believed to be related to living near a major roadway, traffic-related air pollution and noise pollution, have been found to be associated with increased rates of cardio- and cerebrovascular diseases, though results have been inconsistent. Long-term exposure to air pollution has been shown to be associated with increased risk of morbidity and mortality due to CVD,19 but several studies have reported contrasting results.20,21 The pattern between long-term exposure and neurological disorders is similar. Higher levels of outdoor air pollution have generally been associated with a higher risk of stroke events,22 though several studies have reported non-significant associations.23,24 Traffic-related noise pollution, another byproduct of living near a major roadway, has been associated with many of the same adverse health effects including CVD,25–27 stroke,28,29 and all-cause and cardiovascular mortality.16,29
There are several hypothesized mechanisms for how air pollution might affect the brain and cerebral vasculature. A series of experimental animal studies indicate that ambient particles may enter the central nervous system either through the circulatory system or intra-nasally by direct translocation through the olfactory bulb.30,31 Once inside pollutant particles activate a series of systemic inflammatory pathways leading to vascular inflammation,32,33 impaired microvascular reactivity,34 and changes in cerebral hemodynamics.35
It has also been suggested that the association between traffic pollution and cerebrovascular disease may be mediated through cardiovascular mechanisms, as there is substantial evidence of an association between long-term exposure to air pollution and several cardiovascular risk factors including diabetes36–38, total cholesterol and triglycerides39,and blood pressure.39–41 In addition, exposure to pollution has been linked to greater carotid atherosclerotic burden.42,43 In this study, however, adjustment for cardiovascular risk factors strengthened the independent association between residential distance to roadway and ischemic stroke, indicating that in this aging population cardiovascular risk factors were not acting as mediators between the relationship between pollution and ischemic stroke.
Results of the current analysis provide evidence of statistically and clinically significant associations between residential proximity to roadway and incident ischemic stroke, but not MI or mortality. While these cardiovascular diseases share many of the same risk factors, there are some important differences that may be contributing to the results of our study based on their distributions in this cohort.44 Some risk factors differ in the magnitude of their effect on stroke compared with heart disease; for example, while hypertension and hyperlipidemia are both important risk factors for both conditions, hypertension tends to have a greater effect on stroke while dyslipidemia tends to have a larger effect on cardiac disease. Some risk factors, like atrial fibrillation, are important for stroke but probably do not play a major role for heart disease. Stroke is also more heterogeneous than coronary atherosclerosis; only about 20% of stroke is due to atherosclerosis, while other mechanisms such as embolism and small vessel disease may play important roles. Given the older age of our cohort and the fact that in primary analyses we didn’t exclude people with history of cardiac disease at baseline, some of the cohort may be undergoing treatment for heart disease, hypertension, or dyslipidemia, leading to attenuated estimates of incident cardiovascular disease. In sensitivity analyses where individuals with prior history of MI or cardiac disease were removed, however, we did not see significant differences in effect sizes (data not shown).
Our study had several limitations, first of which is specific to the NOMAS study sample. In an urban study area with a limited geographic extent such as Northern Manhattan, there may have been limited spatial variability in exposure levels. In addition, much of the residential land use is similar throughout the study area with the majority of residents living in multifamily mid-rise apartment buildings, further limiting variability in traffic pollution exposures. However, this is also a strength of the study since this is one of the few studies to focus primarily on intra-urban variation in traffic pollution, potentially eliminating many confounders that may have existed in prior studies that compared participants living in different urban and/or rural areas. In addition, although we adjusted for individual-level measures of SES in our analyses (race-ethnicity, education, and insurance status), we included a validated census derived SES z-score to adjust further for variations in neighborhood level SES throughout the study area. Another limitation was that our pollution estimates were estimated based on residential location at one time point at baseline during late life. Because of the older age of our participants, and the high percentage of them retired at time of study, we did not have data on lifetime workplace pollution exposures or time spent in locations outside of the home. Given the observational study design, the associations observed in the current study should not be viewed as causal relationships.
On the other hand, there were several strengths to our study. We were able to estimate the influence of individual-level estimates of residential distance to roadways on the cardiovascular health of a stroke-free aging urban population with geographic stability. We utilized the heterogeneous NOMAS population with a well-documented study methodology and ascertainment of clinical risk factors. In addition, all outcomes were identified and adjudicated by trained research associates and neurologists. In addition, this analysis had a larger sample size and a longer prospective follow up period than many prior studies, allowing for long-term ascertainment of cardiovascular events and death.
Conclusions
We found evidence suggesting that within-city variation in residential proximity to a major roadway is associated with higher risk of incident ischemic stroke in an urban, population-based sample of stroke-free participants. Further, an individual’s smoking status modified this association, with the association remaining only among non-current smokers. These finding speak to the need for further studies to confirm these findings using measured levels of air pollutants.
Supplementary Material
Acknowledgments
Sources of Funding
Funding for this project was provided by the National Institute of Health R01-NS029993-24 and R01-ES020871 grants. The content is the responsibility of the authors and does not necessarily represent the official views of the sponsoring institutions.
Footnotes
Disclosures
All authors have no disclosures to report.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart Disease and Stroke Statistics—2017 Update: A Report From the American Heart Association. Circulation. 2017;135 doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stroke 10:Fast facts on stroke. National Stroke Association; [Accessed July, 8, 2017]. http://www.stroke.org/sites/default/files/resources/NSA_FactSheet_Stroke_101_2014.pdf. [Google Scholar]
- 3.Ovbiagele B, Goldstein LB, Higashida RT, Howard VJ, Johnston SC, Khavjou OA, et al. Forecasting the Future of Stroke in the United States. Stroke. 2013;44 doi: 10.1161/STR.0b013e31829734f2. [DOI] [PubMed] [Google Scholar]
- 4.Feigin VL, Roth GA, Naghavi M, Parmar P, Krishnamurthi R, Chugh S, et al. Global burden of stroke and risk factors in 188 countries, during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016;15:913–924. doi: 10.1016/S1474-4422(16)30073-4. [DOI] [PubMed] [Google Scholar]
- 5.O’Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388:761–75. doi: 10.1016/S0140-6736(16)30506-2. [DOI] [PubMed] [Google Scholar]
- 6.Dufouil C, Beiser A, McLure LA, Wolf PA, Tzourio C, Howard VJ, et al. Revised Framingham Stroke Risk Profile to Reflect Temporal Trends. Circulation. 2017;135:1145–1159. doi: 10.1161/CIRCULATIONAHA.115.021275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finkelstein MM, Jerrett M, Sears MR. Environmental inequality and circulatory disease mortality gradients. J Epidemiol Community Heal. 2005;59:481–487. doi: 10.1136/jech.2004.026203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maheswaran R, Elliott P. Stroke mortality associated with living near main roads in England and wales:a geographical study. Stroke. 2003;34:2776–80. doi: 10.1161/01.STR.0000101750.77547.11. [DOI] [PubMed] [Google Scholar]
- 9.Sacco RL, Boden-Albala B, Abel G, Lin I-F, Elkind M, Hauser Wa, et al. Race-Ethnic Disparities in the Impact of Stroke Risk Factors: The Northern Manhattan Stroke Study. Stroke. 2001;32:1725–1731. doi: 10.1161/01.str.32.8.1725. [DOI] [PubMed] [Google Scholar]
- 10.Kulick ER, Wellenius GA, Kaufman JD, DeRosa JT, Kinney PL, Cheung YK, et al. Long-Term Exposure to Ambient Air Pollution and Subclinical Cerebrovascular Disease in NOMAS (the Northern Manhattan Study) Stroke. 2017;48 doi: 10.1161/STROKEAHA.117.016672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilker EH, Mostofsky E, Lue S-H, Gold D, Schwartz J, Wellenius GA, et al. Residential proximity to high-traffic roadways and poststroke mortality. J. Stroke Cerebrovasc. Dis. 2013;22:e366–72. doi: 10.1016/j.jstrokecerebrovasdis.2013.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willey JZ, Rodriguez CJ, Moon YP, Paik MC, Di Tullio MR, Homma S, et al. Coronary death and myocardial infarction among Hispanics in the Northern Manhattan Study: exploring the Hispanic paradox. Ann. Epidemiol. 2012;22:303–9. doi: 10.1016/j.annepidem.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kulick ER, Moon YP, Cheung K, Willey JZ, Sacco RL, Elkind MS. Racial-ethnic disparities in the association between risk factors and diabetes: The Northern Manhattan Study. Prev. Med. (Baltim) 2016;83:31–6. doi: 10.1016/j.ypmed.2015.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kargman D, Sacco R, Boden-Albala B, Malic M, Hauser W, Shea S. Validity of telephone interview data for vascular disease risk factors in a racially mixed urban community: the Northern Manhattan Stroke Study. Neuroepidemiology. 1999;18:174–184. doi: 10.1159/000026209. [DOI] [PubMed] [Google Scholar]
- 15.Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, et al. Neighborhood of residence and incidence of coronary heart disease. N. Engl. J. Med. 2001;345:99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 16.Gan WQ, Davies HW, Koehoorn M, Brauer M. Association of Long-term Exposure to Community Noise and Traffic-related Air Pollution With Coronary Heart Disease Mortality. Am. J. Epidemiol. 2012;175:898–906. doi: 10.1093/aje/kwr424. [DOI] [PubMed] [Google Scholar]
- 17.Tonne C, Melly S, Mittleman M, Coull B, Goldberg R, Schwartz J. A case-control analysis of exposure to traffic and acute myocardial infarction. Environ. Heal. Perspect. 2007;115 doi: 10.1289/ehp.9587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kingsley SL, Eliot MN, Whitsel EA, Wang Y, Coull BA, Hou L, et al. Residential proximity to major roadways and incident hypertension in post-menopausal women. Environ. Res. 2015;142:522–528. doi: 10.1016/j.envres.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brook RD, Rajagopalan S, Pope CA, Brook JR, Bhatnagar A, Diez-Roux AV, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–78. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 20.Beelen R, Raaschou-Nielsen O, Stafoggia M, Andersen ZJ, Weinmayr G, Hoffmann B, et al. Effects of long-term exposure to air pollution on natural-cause mortality: An analysis of 22 European cohorts within the multicentre ESCAPE project. Lancet. 2014;383 doi: 10.1016/S0140-6736(13)62158-3. [DOI] [PubMed] [Google Scholar]
- 21.Zhang P, Dong G, Sun B, Zhang L, Chen X, Ma N, et al. Long-term exposure to ambient air pollution and mortality due to cardiovascular disease and cerebrovascular disease in Shenyang, China. PLoS One. 2011;6:e20827. doi: 10.1371/journal.pone.0020827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheers H, Jacobs L, Casas L, Nemery B, Nawrot TS. Long-Term Exposure to Particulate Matter Air Pollution Is a Risk Factor for Stroke: Meta-Analytical Evidence. Stroke. 2015;46:3058–3066. doi: 10.1161/STROKEAHA.115.009913. [DOI] [PubMed] [Google Scholar]
- 23.Barnett AG, Williams GM, Schwartz J, Best TL, Neller AH, Petroeschevsky AL, et al. The effects of air pollution on hospitalizations for cardiovascular disease in elderly people in Australian and New Zealand cities. Environ. Health Perspect. 2006;114:1018–23. doi: 10.1289/ehp.8674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wing JJ, Adar SD, Sánchez BN, Morgenstern LB, Smith MA, Lisabeth LD. Ethnic differences in ambient air pollution and risk of acute ischemic stroke. Environ. Res. 2015;143:62–7. doi: 10.1016/j.envres.2015.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Babisch W. Transportation noise and cardiovascular risk: updated review and synthesis of epidemiological studies indicate that the evidence has increased. Noise Health. 2006;8:1–29. doi: 10.4103/1463-1741.32464. [DOI] [PubMed] [Google Scholar]
- 26.Selander J, Nilsson ME, Bluhm G, Rosenlund M, Lindqvist M, Nise G, et al. Long-Term Exposure to Road Traffic Noise and Myocardial Infarction. Epidemiology. 2009;20:272–279. doi: 10.1097/EDE.0b013e31819463bd. [DOI] [PubMed] [Google Scholar]
- 27.Van Kempen EM, Kruize H, Boshuizen HC, Ameling CB, Staatsen BAM, de Hollander AEM. The association between noise exposure and blood pressure and ischemic heart disease: a meta-analysis. Environ. Health Perspect. 2002;110:307–17. doi: 10.1289/ehp.02110307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sørensen M, Lühdorf P, Ketzel M, Andersen ZJ, Tjønneland A, Overvad K, et al. Combined effects of road traffic noise and ambient air pollution in relation to risk for stroke? Environ. Res. 2014;133C:49–55. doi: 10.1016/j.envres.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Halonen JI, Hansell AL, Gulliver J, Morley D, Blangiardo M, Fecht D, et al. Road traffic noise is associated with increased cardiovascular morbidity and mortality and all-cause mortality in London. Eur. Heart J. 2015;36:2653–61. doi: 10.1093/eurheartj/ehv216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, et al. Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol. 2004;16:437–45. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- 31.Peters A, Veronesi B, Calderón-Garcidueñas L, Gehr P, Chen LCLL, Geiser M, et al. Translocation and potential neurological effects of fine and ultrafine particles a critical update. Part. Fibre Toxicol. 2006;3:13. doi: 10.1186/1743-8977-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell A, Oldham M, Becaria A, Bondy SC, Meacher D, Sioutas C, et al. Particulate matter in polluted air may increase biomarkers of inflammation in mouse brain. Neurotoxicology. 2005;26:133–40. doi: 10.1016/j.neuro.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Levesque S, Taetzsch T, Lull ME, Kodavanti U, Stadler K, Wagner A, et al. Diesel exhaust activates and primes microglia: air pollution, neuroinflammation, and regulation of dopaminergic neurotoxicity. Environ. Health Perspect. 2011;119:1149–55. doi: 10.1289/ehp.1002986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adar SD, Klein R, Klein BEK, Szpiro AA, Cotch MF, Wong TY, et al. Air Pollution and the microvasculature: a cross-sectional assessment of in vivo retinal images in the population-based multi-ethnic study of atherosclerosis (MESA) PLoS Med. 2010;7:e1000372. doi: 10.1371/journal.pmed.1000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wellenius GA, Boyle LD, Wilker EH, Sorond FA, Coull BA, Koutrakis P, et al. Ambient fine particulate matter alters cerebral hemodynamics in the elderly. Stroke. 2013;44:1532–6. doi: 10.1161/STROKEAHA.111.000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park SK, Adar SD, O’Neill MS, Auchincloss AH, Szpiro A, Bertoni AG, et al. Long-term exposure to air pollution and type 2 diabetes mellitus in a multiethnic cohort. Am. J. Epidemiol. 2015;181:327–336. doi: 10.1093/aje/kwu280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eze IC, Hemkens LG, Bucher HC, Hoffmann B, Schindler C, Künzli N, et al. Association between ambient air pollution and diabetes mellitus in Europe and North America: systematic review and meta-analysis. Environ. Health Perspect. 2015;123:381–9. doi: 10.1289/ehp.1307823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rajagopalan S, Brook RD. Air pollution and type 2 diabetes: mechanistic insights. Diabetes. 2012;61:3037–45. doi: 10.2337/db12-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shanley RP, Hayes RB, Cromar KR, Ito K, Gordon T, Ahn J. “Particulate Air Pollution and Clinical Cardiovascular Disease Risk Factors”. Epidemiology. 2015 doi: 10.1097/EDE.0000000000000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foraster M, Künzli N, Aguilera I, Rivera M, Agis D, Vila J, et al. High blood pressure and long-term exposure to indoor noise and air pollution from road traffic. Environ. Health Perspect. 2014;122:1193–200. doi: 10.1289/ehp.1307156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Honda T, Eliot MN, Eaton CB, Whitsel E, Stewart JD, Mu L, et al. Long-term exposure to residential ambient fine and coarse particulate matter and incident hypertension in post-menopausal women. Environ. Int. 2017;105:79–85. doi: 10.1016/j.envint.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adar SD, Sheppard L, Vedal S, Polak JF, Sampson PD, Diez Roux AV, et al. Fine particulate air pollution and the progression of carotid intima-medial thickness: a prospective cohort study from the multi-ethnic study of atherosclerosis and air pollution. PLoS Med. 2013;10:e1001430. doi: 10.1371/journal.pmed.1001430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaufman JD, Adar SD, Allen RW, Barr RG, Budoff MJ, Burke GL, et al. Prospective study of particulate air pollution exposures, subclinical atherosclerosis, and clinical cardiovascular disease. Am. J. Epidemiol. 2012;176:825–837. doi: 10.1093/aje/kws169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Plassman BL, Williams JW, Burke JR, Holsinger T, Benjamin S. Systematic Review: Factors Associated With Risk for and Possible Prevention of Cognitive Decline in Later Life. Ann. Intern. Med. 2010;153:182. doi: 10.7326/0003-4819-153-3-201008030-00258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.