Abstract
Objective
To analyze associations of suprapatellar fat pad (SPFP) hyperintense signal alterations and mass effect with progression of patellofemoral osteoarthritis (OA) and clinical symptoms over 48 months.
Materials and Methods
Subjects from the Osteoarthritis Initiative (n=426; 51.8±3.8 years; 49.8% women) without radiographic tibiofemoral OA underwent 3T-MRI of their right knees and clinical evaluation using the Knee Injury and Osteoarthritis Outcome Score at baseline and 48 months. Elevated SPFP signal was assessed on intermediate-weighted fat-saturated TSE images. Mass effect was defined as convex posterior contour. Patellofemoral cartilage, bone marrow lesions (BML), and subchondral cysts were assessed with the Whole-Organ Magnetic Resonance Imaging Score (WORMS). Associations of SPFP imaging findings with MRI and clinical progression were assessed using general linear models and logistic regressions.
Results
Baseline SPFP signal alterations were found in 51% of the subjects (n=217), of which 11% (n=23) additionally had a mass effect. Progression of cartilage lesions was significantly higher in subjects with signal alteration versus without (adjusted mean increases, 95% CI; patella: 0.29 [−0.07, 0.64] vs. −0.04 [−0.40, 0.31]; p<0.001; trochlea: 0.47 [0.16, 0.77] vs. 0.31 [0.01, 0.61]; p=0.007). BML progression was also more likely in subjects with signal alteration (OR 1.75, 95% CI [1.09, 2.82]; p=0.021). Mass effect was not associated with joint degeneration and SPFP findings were not associated with clinical worsening (p>0.18 for all).
Conclusion
Patellofemoral joint degeneration over 48 months was significantly increased in subjects with SPFP signal alteration, suggesting an association between SPFP abnormalities and the progression of patellofemoral OA.
Introduction
The role of the three intra-capsular, extra-synovial fat pads of the knee joint in joint degeneration and the development of knee osteoarthritis (OA) is poorly understood. Most studies have focused on the infrapatellar or Hoffa’s fat pad, considering it to be relevant for the progression of knee osteoarthritis [1–3]: it has been suggested to contribute to joint lubrication, and to absorb loading forces [4–7]. Associations of infrapatellar fat pad imaging findings and joint degeneration have been inconsistent, with an increase in the volume of Hoffa’s fat pad either being associated with accelerated [8] or slowed cartilage degeneration [9], and signal intensity alterations associated with worsening of clinical symptoms and knee OA imaged with MRI [3].
Less is known about the role of the prefemoral and the suprapatellar fat pads (SPFP) in the evolution of joint degeneration and clinical symptoms. The latter, also known as quadriceps fat pad, is located within the joint capsule superior to the patella. It fills the gap between the quadriceps tendon, the retropatellar cartilage and the suprapatellar joint recess, which separates it from the prefemoral fat pad [10–13]. Analogous to the impingement syndrome of the infrapatellar fat pad, also known as Hoffa’s disease [4, 5, 14–16], similar abnormalities may be found in the SPFP, which have typical MRI findings and may be associated with clinical symptoms [10, 11, 17, 18].
However, data on the relevance of SPFP MR imaging findings in OA is scarce: Previous cross-sectional studies found possible correlations between SPFP enlargement and signal alterations in fluid-sensitive MRI sequences [10], interpreted as edema, and meniscal tears [11] and anterior knee pain [10, 11], while a third study found no significant association with knee pain, patellofemoral malalignment or patellofemoral OA [17]. A fourth cross-sectional study reported an association between SPFP signal alterations and mass effect with knee pain as well as signal alteration and bone marrow lesions, but not degenerative changes such as cartilage defects [18]. To our best knowledge, associations of SPFP imaging findings with patellofemoral joint degeneration and clinical worsening have not been assessed yet in a longitudinal analysis.
Therefore, the aims of this study were: (i) to analyze the association of SPFP MR imaging findings with progression of patellofemoral OA, as assessed by the modified Whole-Organ Magnetic Resonance Imaging Score (WORMS) [19, 20] over 48 months and (ii) to evaluate associations between SPFP abnormalities and clinical outcome over 48 months.
Methods
Database and Subjects
We included subjects from the Osteoarthritis Initiative (OAI; oai.ucsf.edu), an ongoing, longitudinal, prospective, multi-center cohort study. The OAI is sponsored by the U.S. National Institutes of Health (NIH) for investigation of diagnosis, treatment and prevention of OA. For this analysis, subjects from the incidence (with risk factors for developing symptomatic knee OA) and the normal control cohort (no knee pain or OA risk factors) were eligible. Pertinent OA risk factors were overweight, previous knee injury or surgery, Heberden’s nodes, frequent knee bending activity or a family history of total knee replacement.
To focus on a relatively young population with none or early degenerative changes in the tibiofemoral joint, only subjects younger than 60 years and a baseline Kellgren-Lawrence (KL) score of 0 or 1 in the right knee (n=995) were eligible [21]. Complete baseline and follow-up patellofemoral WORMS readings were available for the right knees of a sample of 443 of these potentially eligible subjects previously obtained by our group for several NIH-funded studies (Fig 1) [22–28]. In order to minimize any potential influence caused by abnormalities of the extensor mechanism and especially the quadriceps tendon as well as inflammatory processes leading to effusion-synovitis, subjects showing these findings at baseline (extensor mechanism, n=7; effusion-synovitis, n=10) were excluded.
Fig. 1.
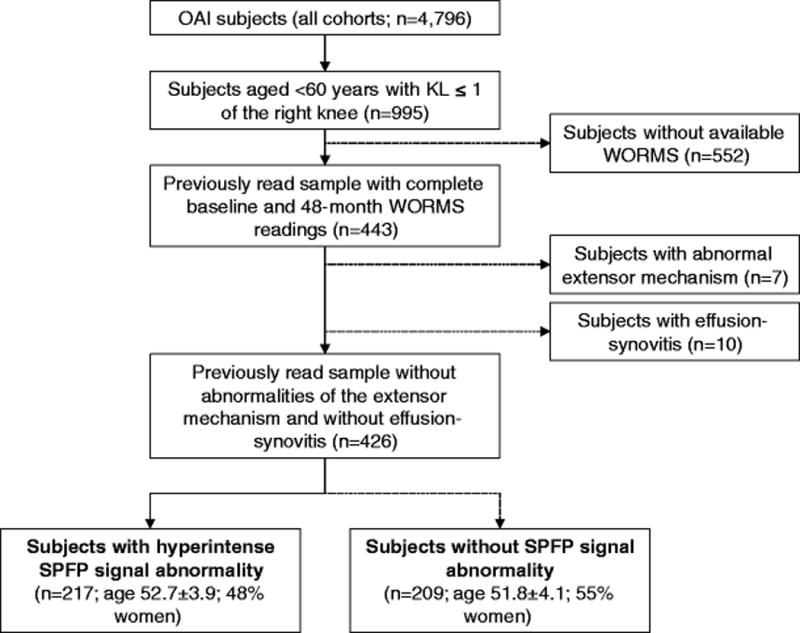
Flowchart illustrating patient selection from the OAI database.
Baseline characteristics of the remaining subjects (N=426) did not differ significantly from the potentially eligible subjects (n=552) in the OAI cohort that were not included in this analysis (p>0.10 for all outcome variables).
Informed written consent was obtained from all subjects; the study was HIPAA-compliant and approved by the local institutional review boards of all participating centers.
MR Imaging and Analysis
MR images were acquired using four identical 3.0T scanners (Siemens Trio; Siemens Healthcare, Erlangen, Germany) and quadrature transmit-receive coils (USA Instruments, Aurora, OH) at four sites (University of Maryland, School of Medicine, Baltimore, MD; University of Pittsburgh, Pittsburgh, PA; Memorial Hospital of Rhode Island, Pawtucket, RI and The Ohio State University, Columbus, OH). SPFP imaging characteristics were assessed primarily in a sagittal intermediate-weighted (IW) fat-saturated 2D turbo spin-echo (TSE) sequence (sequence parameters, see Table 1). A coronal IW 2D TSE sequence, a sagittal T2-weighted 3D dual-echo in steady state (DESS) sequence and its axial reformations were also used to assess morphologic cartilage changes and other knee joint structures. Further details about the image acquisition are available in the OAI MR protocol [29].
Table 1.
Knee MR acquisition parameters of sequences assessed for our study according to the OAI study protocol (adapted from Peterfy CG et al.[29]). Coil: quadrature transmit-receive coil (USA Instruments, Aurora, OH)
| Scan | COR IW 2D TSE | SAG 3D DESS WE1 | COR T1W 3D FLASH WE | SAG IW 2D TSE FS2 |
|---|---|---|---|---|
| Plane | Coronal | Sagittal | Coronal | Sagittal |
| Fat suppression | No | WE | WE | FS |
| Matrix (phase/frequency) | 307/384 | 307/384 | 512/512 | 313/448 |
| No. of slices | 35 | 160 | 80 | 37 |
| FOV (mm) | 140 | 140 | 160 | 160 |
| Slice thickness/ gap (mm) | 3/0 | 0.7/0 | 1.5/0 | 3/0 |
| Flip angle (°) | 180 | 25 | 12 | 180 |
| TE/TR (ms) | 29/3700 | 4.7/16.3 | 7.57/20 | 30/3200 |
| Bandwidth (Hz/pixel) | 352 | 185 | 130 | 248 |
| No. of excitations (averaged) | 1 | 1 | 1 | 1 |
| Echo-train length | 7 | 1 | 1 | 5 |
| Acquisition time (min) | 3.4 | 10.6 | 8.6 | 4.7 |
COR = coronal; DESS = dual-echo in steady state; FLASH = fast low-angle shot; FS = fat suppression; TSE = turbo spin-echo; FOV = field of view; IW = intermediate-weighted; SAG = sagittal; TE = echo time; TR = repetition time; WE = water excitation.
also used for axial and coronal multi-planar reformations (MPR); slice thickness, 1.5mm each.
used for the assessment of SPFP imaging characteristics.
The UCSF-modified WORMS grading [19, 20] was used to semi-quantitatively assess articular cartilage, bone marrow lesions (BML) and subchondral cysts of the patellofemoral joint at baseline and 48-month follow-up. Cartilage was graded on an incremental scale from 0 (normal cartilage thickness and signal) to 6 (diffuse full thickness loss in more than 75% of the region) and BML and subchondral cysts were graded on an incremental scale from 0 to 3 according to their size. A single score each for each feature was assigned to the patella and the trochlea. The total patellofemoral WORMS represents the sum of all subscores for cartilage, BML and subchondral cysts both in the patella and trochlea. The evolution of subscores was expressed by delta values, describing the difference between values at 48 months and baseline.
In order to minimize any potential influence caused by abnormalities of the extensor mechanism and especially the quadriceps tendon, all baseline MR examinations were reviewed for partial or complete tears or abnormal swellings of the tendon. Furthermore, the suprapatellar bursa was evaluated for the presence of effusion-synovitis, consisting of synovial thickening and joint effusion, as described before [30, 31]. Subjects showing these signs were excluded from the analysis (see Database and Subjects; Fig 1).
Since patellar malalignment has been shown to be associated with knee pain and patellofemoral OA progression [32], baseline MRI examinations of all subjects were also assessed for the following abnormal imaging findings by a board-certified radiologist with four years of experience in musculoskeletal radiology (L.F.): A patellar bisect offset of more than 65%, a patellar tilt of more than 9° [33], a patella alta expressed by a modified Insall-Salvati ratio of more than 2 [34], and a sulcus angle of more than 145° [35, 36].
Evaluation of SPFP signal alteration and mass effect
SPFP findings at baseline and 48 months were evaluated by a board-certified radiologist with four years of experience in musculoskeletal radiology (J.M.W.) and a second radiologist with four years of experience in musculoskeletal radiology (B.J.S.) independently in all MR examinations, while evaluations of two time points in the same patient were separated by at least four weeks. In case of disagreement, a consensus reading was performed with a third board-certified musculoskeletal radiologist (T.M.L., 23 years of experience). Radiologists were blinded to morphological readings and SPFP findings at the other time point, respectively, as well as to demographic or clinical information.
SPFP signal characteristics were assessed on the IW fat-saturated 2D TSE sequence in direct comparison to signal levels of the prefemoral fat pad as a standard of reference (Fig. 2), similar to the previously described method [10, 11, 18]. Signal level was considered normal if SPFP signal intensity was comparable to or lower than the prefemoral fat pad signal, and abnormal, if the relative SPFP signal intensity was higher compared to the prefemoral fat pad. Other fatty structures, such as subcutaneous fat, were evaluated to ensure homogeneous fat-saturation throughout the field-of-view.
Fig. 2.
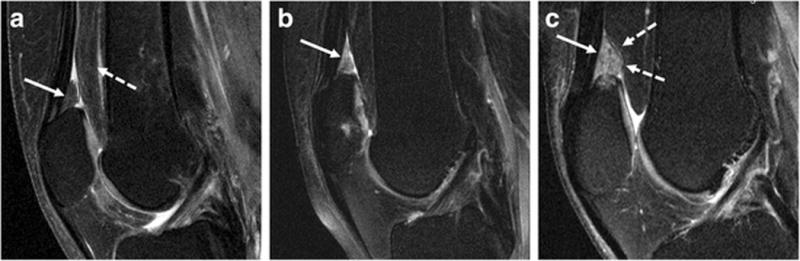
Representative sagittal fat-saturated intermediate-weighted fast spin-echo sequences (FOV, 160mm; slice thickness, 3mm; gap, 0mm; flip angle, 180°; TE/TR, 30/3200ms; acquisition time, 4.7mins) of the right knee in three subjects: A—Normal appearance of the SPFP with isointense signal of the SPFP (arrow) compared to the posterior suprapatellar (prefemoral) fat pad (dashed arrow) and without mass effect; B—hyperintense signal alteration of the SPFP (arrow); C—Hyperintense signal alteration (arrow) and mass effect with convex posterior border of the SPFP (dashed arrows).
The configuration of the posterior border of the SPFP was assessed in the mid-sagittal section. Mass effect was defined as the presence of a convex posterior border contour in at least two continuous slices in contrast to a normal SPFP morphology, i.e. a triangular shape with a linear or almost linear delineation of the posterior border, as previously described (Fig. 2) [10, 17, 18].
Clinical findings
Clinical knee findings at baseline and follow-up were assessed using four subscales of the Knee injury and Osteoarthritis Outcome Score (KOOS) [37], measuring knee pain, any symptoms other than pain (such as stiffness, swelling, or limited range of movement), sport and recreation function, and knee-related quality of life (QOL). Subscales range from 0 to 100 with the best value of KOOS being 100. Change of any of the subscales over 48 months was expressed as the difference between the absolute 48-months and baseline scores with a negative value indicating clinical worsening. A KOOS subscale worsening was considered to reliably represent a clinically relevant progression if the difference between 48-months and baseline exceeded the minimal detectable change (MDC) values as previously described for the osteoarthritic knee [38]: Pain ≥13; symptoms other than pain≥16; sport and recreation function ≥20 and QOL ≥21.
Statistical Analysis
To compare subject characteristics, Fisher’s exact tests were used for categorical data and Student’s t-tests for numerical and approximately normally distributed data. Descriptive statistics were used to assess the prevalence of MRI findings.
To assess differences in baseline WORMS subscores in the patellofemoral joint as well as changes in these WORMS subscores over 48 months in subjects with versus without SPFP signal abnormalities and in subjects with versus without SPFP mass effect (each as separate independent variable in all models) at baseline, general linear models adjusted for the presence of patellofemoral malalignment parameters at baseline (as described above), age, sex, baseline BMI and Kellgren-Lawrence (KL) score as well as OAI cohort affiliation were used with the baseline value or the delta of the respective WORMS subscore as dependent variable, respectively. Logistic regression models adjusted for the same parameters were used to test the association between the presence of a SPFP signal alteration or mass effect at baseline (both as separate independent variable in all models) and progression of WORMS subscores (indicating any difference between a 48-month and baseline score larger than 0) separately for each compartment.
General linear models (for baseline values and delta values for outcomes) and logistic regressions (for the presence of a MDC) were also used to assess associations between SPFP signal characteristics and KOOS subscales. Intra- and inter-reader reproducibility for WORMS subscores and the assessment of signal alteration and mass effect was assessed using intra-class correlation coefficients (ICC).
We conducted a subgroup analysis in subjects with a normal SPFP signal at baseline to evaluate associations between incident SPFP imaging findings (between baseline and 48 months) and progression of patellofemoral joint degeneration using the same analysis methods as in the primary analyses.
Statistical analyses were performed with SPSS 23 (IBM, Armonk, New York), using a two-sided 0.05 level of significance.
Reproducibility
Reproducibility results for WORMS readings have been described previously by our group [39–41]. ICCs range between 0.92 and 0.99 for intrareader agreement and 0.91 and 0.98 for interreader agreement. For grading of SPFP imaging findings intra-observer reproducibility was calculated in 60 randomly selected baseline or 48-months studies by a single radiologist (J.M.W.), with intrareader ICCs of 0.97 for the presence of a signal abnormality and 0.88 for the presence of mass effect. Interreader ICCs, assessed in all subjects (J.M.W. and B.J.S.), were 0.94 for signal abnormality, and 0.85 for mass effect. A consensus reading was necessary in 16 (3.7%) of the cases for SPFP signal abnormality and 19 (4.3%) for mass effect.
Results
Prevalence of SPFP abnormalities and association with baseline MR imaging findings
In this sample of n=426 subjects (mean age, 51.8±3.8; BMI, 27.7±4.1; 49.8% female; 75.0% KL score of 0), the prevalence of a hyperintense signal alteration was 50.9%, (n=217). Of these subjects, 10.6% (n=23) showed a SPFP mass effect in addition to the signal alteration. A mass effect of the SPFP was not seen in any of the subjects without SPFP signal alteration.
Subjects with a SPFP signal alteration were significantly older than controls (52.7±3.9 vs. 51.8±4.1 years, p=0.021), while no other significant differences were found between the groups regarding their demographic parameters (Table 2) or KOOS subscales (p>0.48 for all outcome variables; from general linear models).
Table 2.
Baseline characteristics of subjects with versus without SPFP signal alteration at baseline
| Parameter | Subjects with SPFP signal alteration (n=217) | Controls without SPFP signal alteration (n=209) | P value |
|---|---|---|---|
| Female sex (n; %) | 103 (47.5%) | 115 (55.0%) | 0.119 |
| Age (years; mean ± SD) | 52.7 ± 3.9 | 51.8 ± 4.1 | 0.021 |
| BMI (kg/m2; mean ± SD) | 26.4 ± 4.0 | 27.0 ± 4.4 | 0.166 |
| Baseline Kellgren-Lawrence (KL; n; %) | KL 0: 157 (72.4%) KL 1: 60 (27.6%) |
KL 0: 161 (77.0%) KL 1: 48 (23.0%) |
0.267 |
At baseline, no significant differences in any of the patellofemoral WORMS subscores were found between subjects with and without SPFP signal alterations (Table 3; p>0.06 for all outcome variables; from general linear models). In addition, no significant differences were found between subjects with and without SPFP mass effect (p>0.10 for all outcome variables).
Table 3.
Adjusted mean values of patellofemoral WORMS subscores at baseline and their change over 48-month as well as prevalence and progression rates of structural abnormalities in subjects with versus without SPFP signal alteration at baseline
| Time point | Parameter | Subjects with SPFP signal alteration* | Controls without SPFP signal alteration* | P value*; odds ratio (OR [95% confidence intervals] from logistic regressions) |
|---|---|---|---|---|
| Baseline | WORMS patellar cartilage (mean, 95% confidence interval [CI]) | 1.99 [1.26, 2.72] | 1.72 [1.08, 2.36] | 0.092 |
| WORMS trochlea cartilage (mean, 95% CI) | 1.11 [0.54, 1.67] | 0.98 [0.41, 1.53] | 0.236 | |
| WORMS patellar BML (mean, 95% CI) | 0.54 [0.09, 0.98] | 0.52 [0.08, 0.97] | 0.871 | |
| WORMS trochlea BML (mean, 95% CI) | 0.27 [−0.07, 0.62] | 0.16 [−0.19, 0.50] | 0.080 | |
| WORMS patellar subchondral cysts (mean, 95% CI) | 0.24 [−0.01, 0.49] | 0.20 [−0.05, 0.45] | 0.361 | |
| WORMS trochlea subchondral cysts (mean, 95% CI) | 0.14 [−0.08, 0.35] | 0.09 [−0.16, 0.34] | 0.063 | |
| WORMS patellofemoral joint1 (mean, 95% CI) | 3.81 [1.69, 5.93] | 3.42 [1.78, 5.05] | 0.060 | |
| Partial-thickness cartilage defect present (WORMS 2, 3, 4; n; % of subgroup) | 114 (52.5%) | 97 (46.4%) | 0.215 | |
| Full-thickness cartilage defect present (WORMS 2.5, 5, 6; n; % of subgroup) | 16 (7.4%) | 14 (6.7%) | 0.795 | |
| Change over 48 months | Delta WORMS patellar cartilage (mean, 95% CI) | 0.29 [−0.07, 0.64] | −0.04 [−0.40, 0.31] | <0.001 |
| Delta WORMS trochlea cartilage (mean, 95% CI) | 0.47 [0.16, 0.77] | 0.31 [0.01, 0.61] | 0.007 | |
| Delta WORMS patellar BML (mean, 95% CI) | 0.11 [−0.24, 0.46] | 0.01 [−0.34, 0.36] | 0.135 | |
| Delta WORMS trochlea BML (mean, 95% CI) | 0.18 [−0.11, 0.47] | 0.13 [−0.15, 0.42] | 0.416 | |
| Delta WORMS patellar subchondral cysts (mean, 95% CI) | −0.04 [−0.29, 0.22] | −0.06 [−0.31, 0.20] | 0.708 | |
| Delta WORMS trochlea subchondral cysts (mean, 95% CI) | 0.21 [−0.02, 0.43] | 0.18 [−0.04, 0.40] | 0.556 | |
| Delta total WORMS patellofemoral joint (mean, 95% CI) | 1.21 [0.34, 2.08] | 0.53 [−0.34, 1.40] | <0.001 | |
| Progression of WORMS cartilage1 (n; %) | 98 (45.2%) | 44 (21.1%) | <0.001 (OR 3.71 [2.33, 5.90]) | |
| Progression of WORMS BML1 (n; %) | 62 (28.6%) | 38 (18.2%) | 0.021 (OR 1.75 [1.09, 2.82]) | |
| Progression of total WORMS patellofemoral joint1,2 (n; %) | 121 (55.8%) | 64 (30.6%) | <0.001 (OR 3.58 [2.30, 5.56]) |
Estimated marginal means for numerical outcome variables and P-values from general linear models; odds ratios for binary outcome variables and P-values from logistic regressions. All models adjusted for presence of mass effect and patella malalignment parameters, age, sex, BMI, KL and OAI cohort affiliation at baseline.
Progression indicating any difference of WORMS subscores larger than 0 between 48 months and baseline either in the patella or trochlea, or both.
Composed of subscores for cartilage, BML, and subchondral cysts in the patella and trochlea.
In total, 37 subjects (8.7%) showed one or several signs of patellar malalignment, consisting of 6 subjects (1.4%) with patella alta, 13 (3.1%) with abnormal patellar bisect offset, 29 with abnormal patellar tilt (6.8%), and 18 (4.2%) with trochlear dysplasia. None of those findings was significantly associated with the presence of a SPFP signal alteration or mass effect (p>0.12 for all).
Longitudinal change in SPFP imaging findings over 48 months
In a subgroup analysis of the 209 subjects (49.1%) with a previously normal SPFP signal, a hyperintense signal alteration at 48-month follow-up was found in 32 subjects (15.3%; Fig 3). When comparing subjects with versus without newly developed SPFP signal abnormality, no significant associations with any of the baseline parameters (presence of mass effect or patellofemoral malalignment, age, sex, BMI, KL, OAI cohort affiliation; p>0.05 for all outcome variables, from adjusted logistic regression) were found. The change in cartilage WORMS of the patella was significantly higher in subjects that developed a SPFP signal alteration over 48 months compared to subjects with normal signal at both time points (adjusted mean changes [95% CI]; 0.22 [−0.11–0.56] vs. −0.01 [−0.28–0.25]; p=0.023 from general linear model), while no significant differences were found in any of the other WORMS subscores (p>0.30 for all other outcome variables).
Fig. 3.
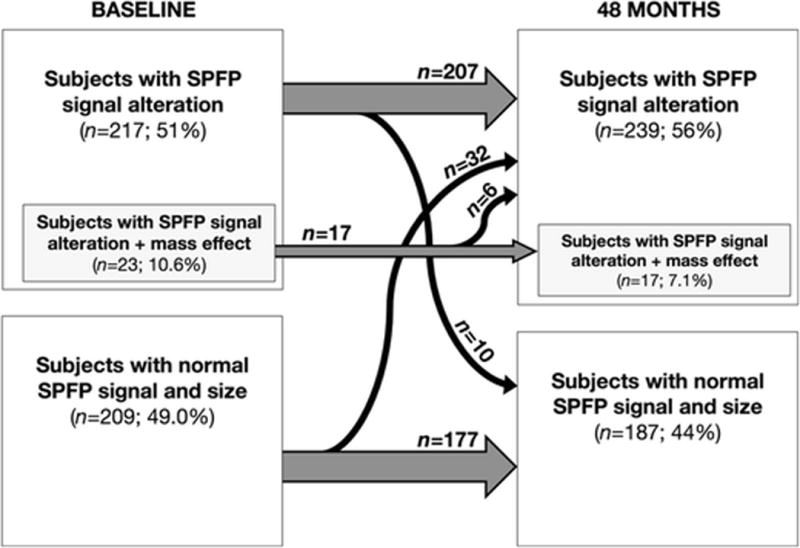
Evolution of SPFP signal alterations and mass effect over time. Grey arrows indicate numbers of subjects with unchanging SPFP imaging findings, while slim black arrows indicate number of subjects changing from one group to the other over 48 months.
In six of the 23 patients with a SPFP mass effect at baseline, this finding resolved over 48 months, and none of the subjects showed a new mass effect (Fig 3). In 10 of 217 subjects (4.6%) with a signal abnormality at baseline, the SPFP showed a normal signal after 48 months, whereas 207 of these subjects (95.4%) showed identical signal characteristics compared to baseline (Fig 3).
Evolution of degenerative changes and association with baseline SPFP imaging characteristics
Progression of patellofemoral cartilage degenerative disease over 48 months, as expressed by the change in WORMS subscore for cartilage, was significantly higher in subjects with a SPFP signal alteration at baseline compared to subjects with normal SPFP signal (adjusted mean changes, 95% confidence interval; patella: 0.29 [−0.07, 0.64] vs. −0.04 [−0.40, 0.31]; p<0.001; trochlea: 0.47 [0.16, 0.77] vs. 0.31 [0.01, 0.61]; p=0.007), as was the evolution of total WORMS for the patellofemoral joint (1.21 [0.34, 2.08] vs. 0.53 [−0.34, 1.40]; p<0.001; all from general linear models; Table 3 and Fig. 4). No significant differences in any of the WORMS subscores were found between the subjects with and without a SPFP mass effect at baseline (p>0.18 for all outcome variables).
Fig. 4.
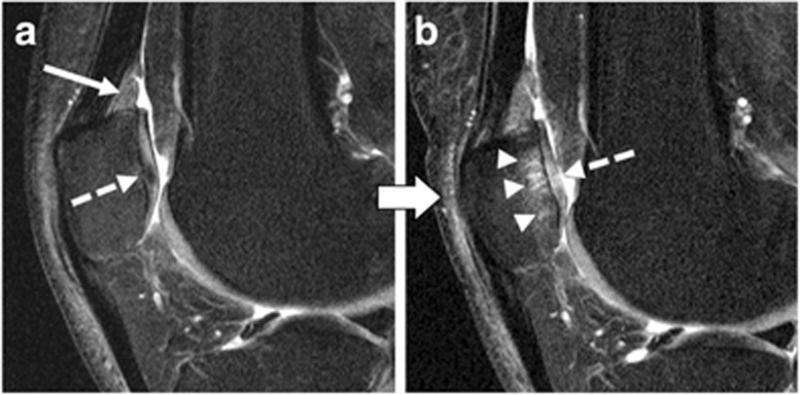
Right knee of the same subject at baseline (A) and after 48 months (B) as shown by sagittal intermediate-weighted fat-saturated turbo spin-echo sequences (FOV, 160mm; slice thickness, 3mm; gap, 0mm; flip angle, 180°; TE/TR, 30/3200ms; acquisition time, 4.7mins). A—Hyperintense signal alteration of the SPFP (arrow) and early degenerative changes of the patellar cartilage shown as hypointense signal inhomogeneity (dashed arrow). B—The same subject showing patellofemoral cartilage degeneration after 48 months including an extensive subchondral bone marrow lesion (arrowheads) and a full thickness cartilage fissure in the patella (dashed arrow).
After dichotomizing subjects in groups with versus without progression of WORMS subscores, subjects with a SPFP signal alteration at baseline were found to be at a significantly higher risk for the progression of BML in the patella (odds ratio (OR), 95% confidence interval; 1.72 [1.01, 2.90]; p=0.044) and overall in the patellofemoral joint (OR 1.75 [1.09, 2.82]; p=0.021; from logistic regressions). Similarly, subjects with a SPFP signal alteration were significantly more likely to show a cartilage progression (OR 3.71 [2.33, 5.90]; p<0.001) and a progression of the total patellofemoral WORMS over 48 months (OR 3.58 [2.30, 5.56]; p<0.001). Again, no significant associations between the presence of a SPFP mass effect at baseline and the progression of any of the WORMS subscores were found (p>0.05 for all outcome variables).
Evolution of clinical scores and association with baseline and follow-up SPFP imaging characteristics
Over 48 months, subjects showed only minimal changes regarding their clinical performance as assessed by KOOS (delta pain, mean −0.07±13.02; delta symptoms other than pain, −0.59±10.17; delta sport and recreation function −0.04±16.17; delta knee-related QOL, 2.27±15.40). Evolution of any of the KOOS subscales was not significantly associated with the presence of a SPFP signal alteration at baseline (p>0.17 for all outcome variables, from general linear models). Similarly, the presence of a minimal detectable change (MDC) was not associated with the presence of a SPFP signal alteration at baseline for any of the KOOS subscales (p>0.18 for all outcome variables, from adjusted logistic regressions).
In a subgroup analysis of subjects with a new SPFP signal alteration at follow-up (N=32), no significant differences were found in the evolution of KOOS subscales compared to subjects with a normal signal at both time points (N=177; p>0.05 for all outcome variables, from general linear models).
Discussion
In our study, the presence of a hyperintense signal alteration of the SPFP at baseline was significantly associated with increased degeneration of the patellofemoral cartilage and progression of patella BML over 48 months. Interestingly, no such associations were found between the presence of a SPFP mass effect and the degeneration of any structure in the patellofemoral joint. A SPFP signal alteration at baseline was not associated with any clinical worsening; nor was the development of a new SPFP signal alteration over 48 months.
To our best knowledge, this is the first longitudinal study assessing the associations of SPFP MR imaging findings with patellofemoral joint degeneration and clinical outcomes of subjects at risk for or with early tibiofemoral OA. Few studies have previously addressed the significance of SPFP findings cross-sectionally: Roth et al. reported a prevalence of SPFP signal alteration of 54% and of SPFP mass effect of 12% in 84 subjects without history of knee surgery [10], which corresponds well with the numbers found by Wang et al. [18], Shabshin et al. [11] and Tsavalas et al. [17] as well as our results, even though prevalence of SPFP mass effect in our population was slightly lower.
While Wang et al. found a significant association between the presence of a SPFP signal intensity alteration as detected by 1.5-T MRI and bone marrow lesions, they did not find any associations with other MRI findings, nor between SPFP mass effect and any other MRI findings [18]. Similarly, the other available cross-sectional studies did not find any significant associations between the presence of SPFP findings and degenerative change in the knee joint such as cartilage abnormalities either [10, 11, 17]. This corresponds with our finding that baseline WORMS subscores did not differ significantly between subjects with versus without SPFP signal alterations.
In this context, our finding that SPFP signal alteration was associated with joint degeneration over 48 months, but not baseline pathologies, suggests that pathologies associated with signal alterations may promote structural damage in other joint structures.
The presence of a SPFP signal alteration was not associated with clinical progression as measured by the KOOS subscales. Three other cross-sectional studies did not identify an association between signal alterations or mass effect and clinical performance either [10, 11, 17]. Of note, their models did not adjust for possible other causes of pain, and questionnaires and examinations were not standardized. In contrast, cartilage loss and BML in the patellofemoral joint—which were significantly associated with SPFP signal alterations in our study—have been reported to be associated with knee pain [42]. Also, Wang et al. found the presence of SPFP signal intensity alteration to be significantly associated with knee pain in their cross-sectional analysis [18]. However, in their study subjects were substantially older than in our study, and associations between SPFP imaging characteristics and both, knee pain and bone marrow lesions were more evident in subjects with radiographic OA. In contrast, we included only subjects with a KL score of 0 or 1 in order to focus our analysis on a relatively healthy population and to reduce bias by other joint pathologies. However, this may be the reason why only minimal clinical progression over 48 months was detected, and may have reduced the sensitivity of our study for clinical worsening. Overall, SPFP signal abnormalities were associated with structural changes—i.e., cartilage loss and bone marrow lesions—which themselves may become clinically relevant at a later time point, but subjects with signal abnormalities did not suffer from a significantly higher worsening of clinical symptoms. Therefore, to further assess the clinical relevance of SPFP pathologies, future studies may investigate older subjects or those with more advanced clinical and/or radiographic degenerative disease.
SPFP mass effect was found in about eleven percent of the subjects with SPFP signal alteration, but none of the subjects with normal SPFP signal. Mass effect was not associated with patellofemoral joint degeneration, or any of the clinical parameters. Therefore, in our study, the relevance of a SPFP mass effect for early OA changes to the patellofemoral joint was considered negligible, and no further conclusions may be drawn regarding its relevance for knee OA.
Articular fat pads are structurally similar to subcutaneous tissue [43], and it is assumed they contribute to joint lubrication, stability, and absorption of forces generated in the moving joint [4–7]. For the infrapatellar fat pad, it has been shown that acute or repetitive trauma or surgery can induce hemorrhage and inflammation [6, 44], subsequently leading to hypertrophy and impingement [16]. It has been shown that an increase in signal intensity on fluid-sensitive fat-saturated sequences, representing an increase in tissue fluid referred to as edema, is sensitive in the acute phase of the disease [4, 5, 45]. More recently, it has been suggested that the infrapatellar fat pad also plays a relevant role in the development of OA [1–3]. OA is a disease with multifactorial pathophysiology, including inflammatory processes, modulated by mediators, some of which ultimately accelerate cartilage degeneration [46–52]. The infrapatellar fat pad, as the other intracapsular articular fat pads, consists mostly of adipocytes, but also contains various immune cells and mesenchymal stem cells, all of which are able to interact with other joint tissues, making it likely that inflammation of the infrapatellar fat pad may accelerate degenerative changes [1, 2]. Less is known about the SPFP, but it may be assumed that pathophysiology is similar to that observed in the infrapatellar fat pad. Hence, an abnormal SPFP signal may represent ongoing inflammatory processes eventually leading to the degeneration of other joint structures. Since we focused on a relatively young and healthy population to minimize a possible bias by other advanced joint pathologies, it is an intrinsic limitation that no histological samples of the SPFP or other joint tissues were available.
This study has some other limitations. We only analyzed a subset of eligible subjects, who had WORMS readings from previous studies. Our statistical evaluation showed that our sample had sufficient power to provide significant results and therefore we considered this as large enough. Note that baseline, demographic characteristics of the analyzed sample and the entire sample of eligible subjects were not significantly different.
An assessment of the infrapatellar or Hoffa’s fat pad was not part of our analysis, since we focused on the SPFP and assessment of knee joint structures using WORMS. Therefore, pathologies in the infrapatellar fat pad may possibly have confounded our results. Also, our patient selection was based on the KL score evaluated in a.p. radiographs. Per OAI imaging protocol, no lateral radiographs were acquired, which reduces the sensitivity to degenerative changes in the patellofemoral joint. However, we used this parameter as selection criterion in order to exclude subjects with substantial tibiofemoral OA. As a consequence, our findings apply to a range of baseline patellofemoral OA severity and not solely to early patellofemoral disease. At baseline, though, subjects had relatively mild degenerative changes of their patellofemoral cartilage and subchondral bone, and WORMS baseline parameters did not significantly differ between subjects with and without SPFP signal alterations.
In this study, we focused specifically on the SPFP. As stated before, the infrapatellar or Hoffa’s fat pad is currently a topic of avid research with a number of recent publications [3, 8, 53]. In contrast, the SPFP fat pat has been less well studied, which is why this longitudinal analysis adds important insights to the role of fat pads in knee joint degeneration.
Finally, our outcome measures for knee pain, other symptoms, and function were relatively unspecific for the patellofemoral joint.
Per OAI protocol, the Knee injury and Osteoarthritis Outcome Score (KOOS) was used as primary clinical and functional outcome parameter [37]. KOOS is well established as an outcome parameter both in subjects with osteoarthritis and younger subjects with knee injuries or post-injury arthritis, however, knee pain and symptoms are only assessed globally for the whole knee joint, which diminishes its specificity for patellofemoral pathologies. In future prospective analyses of the clinical impact of fat pad abnormalities, a specific metric for anterior knee pain may be beneficial.
Of note, analogous to Wang et al. [18], Shabshin et al. [11], and Roth et al. [10], we chose to use a binary parameter to confirm or reject the presence of a SPFP signal intensity alteration. Tsavalas et al. used a numeric parameter to describe the SPFP signal intensity (i.e., a relative signal intensity index equaling mean signal intensity difference between the suprapatellar and prefemoral fat pad divided by background noise standard deviation) [17], which significantly correlated with SPFP mass effect, however, SPFP mass effect was not associated with symptoms or patellofemoral degeneration in this previous study. In addition, sagittal spectral selective fat-saturated intermediate-weighted fast spin-echo sequences as used in our analysis do not allow for accurate quantitative measurements due to field inhomogeneities. While a numeric parameter as used by Tsavalas et al. may suggest a quantitative measurement, a likely bias is introduced e.g. by magnetic field inhomogeneities and other examination conditions.
In summary, hyperintense SPFP signal alterations—indicating SPFP edema—were a frequent finding in middle-aged subjects with and without risk factors for OA but no radiographic tibiofemoral OA. Our longitudinal analysis showed that subjects with SPFP signal alterations have more severe progression of patellofemoral cartilage defects and patella BML over 48 months compared to subjects with normal SPFP signal. On the other hand, SPFP mass effect was not associated with a higher patellofemoral cartilage progression, and no imaging feature was associated with clinical worsening. Overall our findings suggest that SPFP signal abnormalities may lead to progressive degenerative changes and eventually to patellofemoral OA.
Acknowledgments
Funding
The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Pfizer, Inc.; Novartis Pharmaceuticals Corporation; Merck Research Laboratories; and GlaxoSmithKline. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript received the approval of the OAI Publications Committee based on a review of its scientific content and data interpretation. The analyses in this study were funded through the NIH (National Institute of Arthritis and Musculoskeletal and Skin Diseases grants P50-AR060752 and R01-AR064771).
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Ioan-Facsinay A, Kloppenburg M. An emerging player in knee osteoarthritis: the infrapatellar fat pad. Arthritis Res Ther. 2013;15(6):225. doi: 10.1186/ar4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clockaerts S, Bastiaansen-Jenniskens YM, Runhaar J, Van Osch GJ, Van Offel JF, Verhaar JA, et al. The infrapatellar fat pad should be considered as an active osteoarthritic joint tissue: a narrative review. Osteoarthritis Cartilage. 2010;18(7):876–882. doi: 10.1016/j.joca.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 3.Han W, Aitken D, Zhu Z, Halliday A, Wang X, Antony B, et al. Signal intensity alteration in the infrapatellar fat pad at baseline for the prediction of knee symptoms and structure in older adults: a cohort study. Ann Rheum Dis. 2016;75(10):1783–1788. doi: 10.1136/annrheumdis-2015-208360. [DOI] [PubMed] [Google Scholar]
- 4.Saddik D, McNally EG, Richardson M. MRI of Hoffa’s fat pad. Skeletal Radiol. 2004;33(8):433–444. doi: 10.1007/s00256-003-0724-z. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson JA, Lenchik L, Ruhoy MK, Schweitzer ME, Resnick D. MR imaging of the infrapatellar fat pad of Hoffa. Radiographics. 1997;17(3):675–691. doi: 10.1148/radiographics.17.3.9153705. [DOI] [PubMed] [Google Scholar]
- 6.Gallagher J, Tierney P, Murray P, O’Brien M. The infrapatellar fat pad: anatomy and clinical correlations. Knee Surg Sports Traumatol Arthrosc. 2005;13(4):268–272. doi: 10.1007/s00167-004-0592-7. [DOI] [PubMed] [Google Scholar]
- 7.Pan F, Han W, Wang X, Liu Z, Jin X, Antony B, et al. A longitudinal study of the association between infrapatellar fat pad maximal area and changes in knee symptoms and structure in older adults. Ann Rheum Dis. 2015;74(10):1818–1824. doi: 10.1136/annrheumdis-2013-205108. [DOI] [PubMed] [Google Scholar]
- 8.Cowan SM, Hart HF, Warden SJ, Crossley KM. Infrapatellar fat pad volume is greater in individuals with patellofemoral joint osteoarthritis and associated with pain. Rheumatol Int. 2015;35(8):1439–1442. doi: 10.1007/s00296-015-3250-0. [DOI] [PubMed] [Google Scholar]
- 9.Cai J, Xu J, Wang K, Zheng S, He F, Huan S, et al. Association Between Infrapatellar Fat Pad Volume and Knee Structural Changes in Patients with Knee Osteoarthritis. J Rheumatol. 2015;42(10):1878–1884. doi: 10.3899/jrheum.150175. [DOI] [PubMed] [Google Scholar]
- 10.Roth C, Jacobson J, Jamadar D, Caoili E, Morag Y, Housner J. Quadriceps fat pad signal intensity and enlargement on MRI: prevalence and associated findings. AJR Am J Roentgenol. 2004;182(6):1383–1387. doi: 10.2214/ajr.182.6.1821383. [DOI] [PubMed] [Google Scholar]
- 11.Shabshin N, Schweitzer ME, Morrison WB. Quadriceps fat pad edema: significance on magnetic resonance images of the knee. Skeletal Radiol. 2006;35(5):269–274. doi: 10.1007/s00256-005-0043-7. [DOI] [PubMed] [Google Scholar]
- 12.Staeubli HU, Bollmann C, Kreutz R, Becker W, Rauschning W. Quantification of intact quadriceps tendon, quadriceps tendon insertion, and suprapatellar fat pad: MR arthrography, anatomy, and cryosections in the sagittal plane. AJR Am J Roentgenol. 1999;173(3):691–698. doi: 10.2214/ajr.173.3.10470905. [DOI] [PubMed] [Google Scholar]
- 13.Schweitzer ME, Falk A, Pathria M, Brahme S, Hodler J, Resnick D. MR imaging of the knee: can changes in the intracapsular fat pads be used as a sign of synovial proliferation in the presence of an effusion? AJR Am J Roentgenol. 1993;160(4):823–826. doi: 10.2214/ajr.160.4.8456672. [DOI] [PubMed] [Google Scholar]
- 14.Hoffa A. Influence of adipose tissue with regard to the pathology of the knee joint. JAMA. 1904;43:795–796. [Google Scholar]
- 15.Metheny JA, Mayor MB. Hoffa disease: chronic impingement of the infrapatellar fat pad. Am J Knee Surg. 1988;1:134–139. [Google Scholar]
- 16.Kumar D, Alvand A, Beacon JP. Impingement of infrapatellar fat pad (Hoffa’s disease): results of high-portal arthroscopic resection. Arthroscopy. 2007;23(11):1180–1186 e1181. doi: 10.1016/j.arthro.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Tsavalas N, Karantanas AH. Suprapatellar fat-pad mass effect: MRI findings and correlation with anterior knee pain. AJR Am J Roentgenol. 2013;200(3):W291–296. doi: 10.2214/AJR.12.8821. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Han W, Wang X, Pan F, Liu Z, Halliday A, et al. Mass effect and signal intensity alteration in the suprapatellar fat pad: associations with knee symptoms and structure. Osteoarthritis Cartilage. 2014;22(10):1619–1626. doi: 10.1016/j.joca.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 19.Stehling C, Lane NE, Nevitt MC, Lynch J, McCulloch CE, Link TM. Subjects with higher physical activity levels have more severe focal knee lesions diagnosed with 3T MRI: analysis of a non-symptomatic cohort of the osteoarthritis initiative. Osteoarthritis Cartilage. 2010;18(6):776–786. doi: 10.1016/j.joca.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stehling C, Liebl H, Krug R, Lane NE, Nevitt MC, Lynch J, et al. Patellar cartilage: T2 values and morphologic abnormalities at 3.0-T MR imaging in relation to physical activity in asymptomatic subjects from the osteoarthritis initiative. Radiology. 2010;254(2):509–520. doi: 10.1148/radiol.09090596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bucknor MD, Nardo L, Joseph GB, Alizai H, Srikhum W, Nevitt MC, et al. Association of cartilage degeneration with four year weight gain–3T MRI data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2015;23(4):525–531. doi: 10.1016/j.joca.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gersing AS, Solka M, Joseph GB, Schwaiger BJ, Heilmeier U, Feuerriegel G, et al. Progression of cartilage degeneration and clinical symptoms in obese and overweight individuals is dependent on the amount of weight loss: 48-month data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2016;24(7):1126–1134. doi: 10.1016/j.joca.2016.01.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joseph GB, Hou SW, Nardo L, Heilmeier U, Nevitt MC, McCulloch CE, et al. MRI findings associated with development of incident knee pain over 48 months: data from the osteoarthritis initiative. Skeletal Radiol. 2016;45(5):653–660. doi: 10.1007/s00256-016-2343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jungmann PM, Nevitt MC, Baum T, Liebl H, Nardo L, Liu F, et al. Relationship of unilateral total hip arthroplasty (THA) to contralateral and ipsilateral knee joint degeneration – a longitudinal 3T MRI study from the Osteoarthritis Initiative (OAI) Osteoarthritis Cartilage. 2015;23(7):1144–1153. doi: 10.1016/j.joca.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kretzschmar M, Heilmeier U, Yu A, Joseph GB, Liu F, Solka M, et al. Longitudinal analysis of cartilage T2 relaxation times and joint degeneration in African American and Caucasian American women over an observation period of 6 years – data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2016;24(8):1384–1391. doi: 10.1016/j.joca.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kretzschmar M, Lin W, Nardo L, Joseph GB, Dunlop DD, Heilmeier U, et al. Association of Physical Activity Measured by Accelerometer, Knee Joint Abnormalities, and Cartilage T2 Measurements Obtained From 3T Magnetic Resonance Imaging: Data From the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2015;67(9):1272–1280. doi: 10.1002/acr.22586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin W, Alizai H, Joseph GB, Srikhum W, Nevitt MC, Lynch JA, et al. Physical activity in relation to knee cartilage T2 progression measured with 3 T MRI over a period of 4 years: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2013;21(10):1558–1566. doi: 10.1016/j.joca.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16(12):1433–1441. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12(3):177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Hunter DJ, Guermazi A, Lo GH, Grainger AJ, Conaghan PG, Boudreau RM, et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score) Osteoarthritis Cartilage. 2011;19(8):990–1002. doi: 10.1016/j.joca.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunter DJ, Zhang YQ, Niu JB, Felson DT, Kwoh K, Newman A, et al. Patella malalignment, pain and patellofemoral progression: the Health ABC Study. Osteoarthritis Cartilage. 2007;15(10):1120–1127. doi: 10.1016/j.joca.2007.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Draper CE, Besier TF, Santos JM, Jennings F, Fredericson M, Gold GE, et al. Using real-time MRI to quantify altered joint kinematics in subjects with patellofemoral pain and to evaluate the effects of a patellar brace or sleeve on joint motion. J Orthop Res. 2009;27(5):571–577. doi: 10.1002/jor.20790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grelsamer RP, Meadows S. The modified Insall-Salvati ratio for assessment of patellar height. Clin Orthop Relat Res. 1992;(282):170–176. [PubMed] [Google Scholar]
- 35.van Huyssteen AL, Hendrix MR, Barnett AJ, Wakeley CJ, Eldridge JD. Cartilage-bone mismatch in the dysplastic trochlea. An MRI study. J Bone Joint Surg Br. 2006;88(5):688–691. doi: 10.1302/0301-620X.88B5.16866. [DOI] [PubMed] [Google Scholar]
- 36.Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19–26. doi: 10.1007/BF01552649. [DOI] [PubMed] [Google Scholar]
- 37.Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)–development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88–96. doi: 10.2519/jospt.1998.28.2.88. [DOI] [PubMed] [Google Scholar]
- 38.Collins NJ, Misra D, Felson DT, Crossley KM, Roos EM. Measures of knee function: International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Injury and Osteoarthritis Outcome Score Physical Function Short Form (KOOS-PS), Knee Outcome Survey Activities of Daily Living Scale (KOS-ADL), Lysholm Knee Scoring Scale, Oxford Knee Score (OKS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Activity Rating Scale (ARS), and Tegner Activity Score (TAS) Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S208–228. doi: 10.1002/acr.20632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baum T, Joseph GB, Arulanandan A, Nardo L, Virayavanich W, Carballido-Gamio J, et al. Association of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with knee pain: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2012;64(2):248–255. doi: 10.1002/acr.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baum T, Stehling C, Joseph GB, Carballido-Gamio J, Schwaiger BJ, Muller-Hocker C, et al. Changes in knee cartilage T2 values over 24 months in subjects with and without risk factors for knee osteoarthritis and their association with focal knee lesions at baseline: data from the osteoarthritis initiative. J Magn Reson Imaging. 2012;35(2):370–378. doi: 10.1002/jmri.22834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwaiger BJ, Gersing AS, Mbapte Wamba J, Nevitt MC, McCulloch CE, Link TM. Can Signal Abnormalities Detected with MR Imaging in Knee Articular Cartilage Be Used to Predict Development of Morphologic Cartilage Defects? 48-Month Data from the Osteoarthritis Initiative. Radiology. 2016;281(1):158–167. doi: 10.1148/radiol.2016152308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stefanik JJ, Gross KD, Guermazi A, Felson DT, Roemer FW, Zhang Y, et al. The relation of MRI-detected structural damage in the medial and lateral patellofemoral joint to knee pain: the Multicenter and Framingham Osteoarthritis Studies. Osteoarthritis Cartilage. 2015;23(4):565–570. doi: 10.1016/j.joca.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vahlensieck M, Linneborn G, Schild H, Schmidt HM. Hoffa’s recess: incidence, morphology and differential diagnosis of the globular-shaped cleft in the infrapatellar fat pad of the knee on MRI and cadaver dissections. Eur Radiol. 2002;12(1):90–93. doi: 10.1007/s003300100982. [DOI] [PubMed] [Google Scholar]
- 44.Duri ZA, Aichroth PM, Dowd G. The fat pad. Clinical observations. Am J Knee Surg. 1996;9(2):55–66. [PubMed] [Google Scholar]
- 45.Morini G, Chiodi E, Centanni F, Gattazzo D. Hoffa’s disease of the adipose pad: magnetic resonance versus surgical findings. Radiol Med. 1998;95(4):278–285. [PubMed] [Google Scholar]
- 46.Chowdhury TT, Salter DM, Bader DL, Lee DA. Signal transduction pathways involving p38 MAPK, JNK, NFkappaB and AP-1 influences the response of chondrocytes cultured in agarose constructs to IL-1beta and dynamic compression. Inflamm Res. 2008;57(7):306–313. doi: 10.1007/s00011-007-7126-y. [DOI] [PubMed] [Google Scholar]
- 47.Gosset M, Berenbaum F, Levy A, Pigenet A, Thirion S, Saffar JL, et al. Prostaglandin E2 synthesis in cartilage explants under compression: mPGES-1 is a mechanosensitive gene. Arthritis Res Ther. 2006;8(4):R135. doi: 10.1186/ar2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213(3):626–634. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 49.Guilak F, Fermor B, Keefe FJ, Kraus VB, Olson SA, Pisetsky DS, et al. The role of biomechanics and inflammation in cartilage injury and repair. Clin Orthop Relat Res. 2004;(423):17–26. doi: 10.1097/01.blo.0000131233.83640.91. [DOI] [PubMed] [Google Scholar]
- 50.Goldring MB, Berenbaum F. The regulation of chondrocyte function by proinflammatory mediators: prostaglandins and nitric oxide. Clin Orthop Relat Res. 2004;(427 Suppl):S37–46. doi: 10.1097/01.blo.0000144484.69656.e4. [DOI] [PubMed] [Google Scholar]
- 51.Goldring MB. Osteoarthritis and cartilage: the role of cytokines. Curr Rheumatol Rep. 2000;2(6):459–465. doi: 10.1007/s11926-000-0021-y. [DOI] [PubMed] [Google Scholar]
- 52.Stannus OP, Jones G, Blizzard L, Cicuttini FM, Ding C. Associations between serum levels of inflammatory markers and change in knee pain over 5 years in older adults: a prospective cohort study. Ann Rheum Dis. 2013;72(4):535–540. doi: 10.1136/annrheumdis-2011-201047. [DOI] [PubMed] [Google Scholar]
- 53.Roemer FW, Jarraya M, Felson DT, Hayashi D, Crema MD, Loeuille D, et al. Magnetic resonance imaging of Hoffa’s fat pad and relevance for osteoarthritis research: a narrative review. Osteoarthritis Cartilage. 2016;24(3):383–397. doi: 10.1016/j.joca.2015.09.018. [DOI] [PubMed] [Google Scholar]


