Abstract
Activation of inflammatory signaling pathways is of central importance in the pathogenesis of alcoholic liver disease (ALD) and nonalcoholic steatohepatitis (NASH). Nod-like receptors (NLRs) are intracellular innate immune sensors of microbes and danger signals that control multiple aspects of inflammatory responses. Recent studies demonstrated that NLRs are expressed and activated in innate immune cells as well as in parenchymal cells in the liver. For example, NLRP3 signaling is involved in liver ischemia-reperfusion (I/R) injury and silencing of NLRP3 can protect the liver from I/R injury. In this article, we review the evidence that highlights the critical importance of NLRs in the prevalent liver diseases. The significance of NLR-induced intracellular signaling pathways and cytokine production is also evaluated.
Keywords: Nod-like receptors (NLRs), liver diseases, NLRP3
Introduction
The liver is the first organ exposed to orally administered xenobiotics after absorption from the intestine, and it is a major site of biotransformation and metabolism [1,2]. Meanwhile, the liver is a sentinel organ in a unique position to monitor pathogen-associated molecules in the portal and systemic circulations [3]. It is increasingly recognized that not only immune cells but also the parenchymal cells of the liver, including hepatocytes and liver endothelial cells, play important roles in the immune response to a wide range of liver problems, from alcoholic liver disease (ALD) to acetaminophen toxicity to liver ischaemia-reperfusion (I/R) injury [4-6].
Liver diseases represent a significant cause of morbidity and mortality worldwide [7,8]. Up to 2% of all deaths are attributable to liver-related etiology in industrialized countries [9]. Overall, liver diseases rank the ninth as cause of death. Among all gastrointestinal diseases, liver disease is the second leading cause of death after colorectal cancer [10]. A majority of chronic and acute liver diseases share a component of liver inflammation and injury mediated by the innate immune response [11]. These conditions comprise prevalent liver diseases such as ALD [12], nonalcoholic steatohepatitis (NASH) [13], Non-alcoholic fatty liver disease (NAFLD) [14], Acetaminophen (N-acetyl-para-aminophenol) hepatotoxicity [15], viral hepatitis, primary biliary cirrhosis, sclerosing cholangitis, paracetamol-induced liver injury, and autoimmune hepatitis [16]. Innate immunity is the first line of defense against microbial invasion and includes physical and chemical barriers, humoral factors, phagocytic cells, and a group of pattern-recognition receptors that identify pathogenassociated molecular patterns (PAMPs) expressed on invading pathogens [17,18]. The examples of pattern-recognition receptors include a group of Toll-like receptors (TLRs), helicase receptors, and NLRs [19]. The liver, and its multiple cell types, including hepatocytes, are therefore central components that initiate and regulate innate immune pathways [20]. Here we will discuss the expression and function of NLRs in liver diseases.
Many researches in recent years have focused on characterizing the roles and signaling pathways of NLR family members in regulating the immune response [21,22]. To date, NLR proteins include an N-terminal caspase recruitment domain (CARD) or pyrin domain, a central nucleotidebinding, oligomerization domain, and a C-terminal leucine-rich repeat (LRR) domain. The NLR family, containing 23 members in human and 34 in mice, is generally thought to sense microbial products or danger signals for inflammasome activation [23-25]. The NLRs are divided into two major subfamilies: NODs (also referred to as NLRCs) and NLRPs (previously called NALPs) [26]. NLRC 1-5 contain a CARD protein, NLRP 1-14 contain pyrin, and NAIPs contain a BIR protein. In healthy catfish tissues, all NOD genes are found to be ubiquitously expressed. After infection with Edwardsiella tarda, Aeromonas hydrophila, Streptococcus iniae, or channel catfish hemorrhage reovirus (CCRV), expression of NOD1, NOD2, NLRC3, and NLRC5 show a significant up-regulation in the liver [27]. Importantly, recent evidence suggested that hepatocytes express functional NOD1, NOD2, and NLRP3, while NLRP1, NLRP3 and NLRC4/NALP4 are detected in hepatic stellate cells (HSC) [28,29].
More studies in this field indicate hepatocyte NLRs as important components of the innate immune system in the liver. Thus, here we review the recent advances in our understanding of the mechanistic basis of NLR activation with a specific focus on the three NLRs (NOD1, NOD2 and NLRP3), which play significant roles in liver diseases. The importance of NLRs activation in liver diseases is also discussed in this review.
New advances of NLRs in the liver diseases
Recently, new roles for pattern recognition receptors (PRRs) NLRs in the liver have been proposed. Melanie J. Scott et al. has shown that hepatocytes express functional NOD1 and NOD2 [30]. The demonstration that invalidation of NOD1 in mice protects against polymorphonuclear neutrophil (PMN) induced liver injury in the I/R model indicates that inhibition of NOD1 may represent an attractive target for optimizing therapeutic techniques in liver transplantation [31]. Interestingly, Melanie J. Scott et al. also found that NOD2 activation forms part of a collective immune response to pathogens and organ injury [30]. Furthermore, Mathilde Body-Malapel et al. showed that NOD2 is involved in liver injury model induced by concanavalin A-driven lymphocyte activation [32]. Therefore, NOD1 and NOD2 may play important regulatory roles in many inflammatory processes involving the liver. Notably, a recent study showed that NLRP1 and NLRP3 are prominently expressed in Kupffer cells (KC) and liver sinusoidal endothelial cells, moderately expressed in periportal myofibroblasts and HSC, but virtually absent in primary cultured hepatocytes. In addition, challenge with the lipopolysaccharides (LPS) resulted in a time- and concentration-dependent expression of the NLR family members (NOD1, NOD2, NLRP1, NLRP3 and NLRC4/NALP4) in cultured HSC and a strong transcriptional activation of NLRP3 in hepatocytes, Moreover, LPS stimulation could induce the components of the NLRP3 inflammasome in the liver, which is associated with increased IL-1β production [33], and decreased mRNA abundance of liver TLR4, MyD88, IRAK1, TRAF6, NOD1, NOD2, RIPK2, and NF-κB p65, but to the LPS-challenged pigs, Aspartate (Asp) supplementation can increased this [34]. Furthermore, it has been recently demonstrated that the NLR family members NLRP6/NALP6 and NLRP3 in conjunction with IL-18 negatively regulate the progression of non-alcoholic fatty liver disease. The aberrant gut microbiota in NLRP6 inflammasome-deficient mice induces colonic inflammation through epithelial induction of CCL5 (RANTES) secretion [14]. Interestingly, Amina A. Negash showed that hepatitis C virus (HCV) induces the maturation of pro-IL-1β in macrophages through activation of the NLRP3 inflammasome [35] (Table 1).
Table 1.
The role of NOD1, NOD2 and NLRP3 in liver disease, with focus on the experimental model and regulation mechanism
| NLR | Liver disease | Experimental models | Role in liver disease | Reference |
|---|---|---|---|---|
| NOD1 | liver inflammation and infection | C57BL/6 mice | Stimulate hepatocytes with NOD1 ligand (C12-iEDAP) inducing NF-κB activation, activate MAP kinases, express chemokines CCL5 (RANTES) and CXCL1 (KC) | [30] |
| LPS-induced liver injury | pigs | NOD1 and its adaptor molecule (RIPK2) were reduced, and the level of liver TNF-α was decreased simultaneously in the pigs fed a fish oil diet after LPS challenge. | [118] | |
| polymorphonuclear neutrophils (PMN)-induced liver injury. | mice | invalidation of NOD1 protects against PMN induced liver injury in the I/R model | [31] | |
| NOD2 | LPS-induced liver injury | pigs | NOD2 and its adaptor molecule (RIPK2) were reduced in the pigs fed a fish oil diet | [118] |
| concanavalin A-induced liver injury | C57BL/6 mice | a regulatory mechanism affecting immune cells infiltrating the liver and hepatocytes | [32] | |
| NLRP3 | Liver ischemia-reperfusion (I/R) injury | Male C57BL/6J mice/liver nonparenchymal cells (NPCs) | Silencing NLRP3 ameliorated I/R-induced hepatocellular injury and reduced IL-1β, IL-18, HMGB1, IL-6, and TNFα release via inhibition of caspase-1 and NF-κB activity | [83] |
| acetaminophen-induced liver injury | mice | NLRP3 inflammasome pathway play the important role in generating mature IL-1β and IL-18 | [15] | |
| Endotoxin-induced Liver Injury | mice | TLR4/NLRP3-mediated caspase-1 activation process | [33] | |
| alcoholic hepatitis (AH) liver disease | liver biopsies | NRLP3 was not upregulate but rather NAIP was upregulated | [26] | |
| Non-alcoholic fatty liver disease (NAFLD) | mice | NlRP3-/- mice developed exacerbated NASH compared to wt mice | [14] | |
| Chronic hepatitis C virus (HCV) infection | THP-1 cells | NLRP3 inflammasome stimulates IL-1β production to drive proinflammatory cytokine, chemokine, and immune-regulatory gene expression networks linked with HCV disease severity | [34] | |
| liver fibrosis | LX-2 cells, Primary HSCs, Mice | Mice lacking the inflammasome-sensing and adaptor molecules, NLRP3 and apoptosis-associated speck-like protein containing CARD, reduced CCl4 and TAA-induced liver fibrosis. | [29] | |
| LPS-induced liver damage | mice | activate the NLRP3 inflammasome and caspase-1, secrete IL-1β and IL-18 | [33] |
NOD1
The NLR family member NOD1 (NLRC1) is a 108-kDa protein, which consists of three major domains: a CARD, a nucleotide binding domain (NBD, also known as NACHT), and 10 LRRs [36]. It is well known that NOD1 is prototypically activated by γ-tri-DAP, a component of peptidoglycans (PGNs) found in bacterial cell walls [37,38]. Additionally, NOD1 is stimulated mainly by bacterial cell wall components from Gram-negative bacteria, which make up a large part of gut flora [39]. In the hepatocytes, chemokine expression was likely stimulated by gut pathogens in response to NOD1 activation, therefore forming an important part of the mechanism involved in attracting immune cells to the liver to defend the host. After been activated, NOD1 transduces signals leading to induction of protein kinases that drive the activation of nuclear factor-κB (NF-κB), IRF family transcription factors, and AP-1/c-Jun [40,41]. Insertion/deletion polymorphisms in the NOD1 gene have been associated with varieties of disorders, including sarcoidosis, Crohn’s disease, Rheumatoid Arthritis and inflammatory bowel disease, making this protein as a key target for drug discovery [42-44].
NOD1 in liver inflammation and infection
The liver is ideally placed to initiate and regulate immune responses to pathogens released from the gut and transported in the hepatic portal vein (e.g. after changes in gut permeability following hemorrhagic shock) or detected in the systemic blood stream [45]. Liver inflammation is a common trigger of liver disease, and is considered as the main driver of hepatic tissue damage leading to fibrogenesis and hepatocellular carcinoma (HCC) [46,47]. In Western countries, most of the chronic liver diseases (CLD) are ascribed to chronic hepatitis B and hepatitis C infection, alcohol consumption, metabolic disease, drug/toxin-induced liver injury, and/or autoimmune causes. It is well known that the inflammatory phenotype during CLD can be led by the innate immune system [48]. Liver innate immune cells, including KC, monocytes, neutrophils, natural killer (NK) cells, dendritic cells (DCs) and NKT cells, could initiate and maintain hepatic inflammation through inducing cytokine production [49]. It has been demonstrated that a dysregulated expression of cytokine after liver injury can result in excessive cell death of hepatocytes in liver diseases [50]. In addition, cytokines can activate effector functions of immune cells and hepatocytic intracellular signaling pathways, thus playing crucial roles in the interplay between intrahepatic immune cells and hepatocytes.
Specifically, Melanie J. Scott et al. have clearly shown that NOD1 is activated by NOD1 specific ligands. This activation likely contributes to systemic and local immune responses to pathogens [30]. Furthermore, NOD1 was highly expressed in liver, especially in hepatocytes. RIP2, the main signaling partner for NODs, is also expressed. Stimulation of hepatocytes with NOD1 ligand (C12-iEDAP) initiates NF-κB signaling and MAP kinases, therefore inducing the expression of chemokines CXCL1 (KC) and CCL5, which are lymphocyte chemoattractants lead to augmentation of the adaptive immune response. As CCL5 also contributes to hepatic wound healing and hepatic fibrosis, hepatocyte NOD1 activation is a main pathway for CCL5 production [51]. It is also well accepted that NOD1 stimulation in hepatocytes induces chemokine production and it can synergize with cytokines to increase NO and iNOS production.
NOD1 in polymorphonuclear neutrophil (PMN) induced liver injury
PMNs play an important role in the innate immune response to infection and tissue trauma. Due to their high mobility and capability in releasing potent cytotoxic mediators, the aim of PMN recruitment is to eliminate invading microorganisms and/or remove dying cells from sites of inflammation. However, in pathologic contexts such as ischemia-reperfusion, endotoxemia, obstructive cholestasis, or alcoholic liver disease, excessive activation of PMNs leads to additional tissue damage [52,53]. Sebastien Dharancy et al. demonstrated that infiltration of PMN in necrotic areas of livers in NOD1-/- mice is decreased compared with those of NOD1+/+ mice after CCl4 exposure. Moreover, PMNs isolated from NOD1-/- mice display an obvious decreased migration capacity compared with NOD1+/+ PMNs, whereas FK 565, a potent NOD1 ligand, increases PMN migration [31]. In addition, knockout of NOD1 significantly reduces FK 565-induced activation of mitogen-activated protein kinase and NF-κB. In an I/R model of PMN-induced liver injury, FK 565 increases lesions, whereas NOD1-/- mice are protected. Moreover, Sébastien Dharancy et al. found that NOD1-/- mice display fewer severe lesions in the I/R model. Although a NOD1 activator exacerbates phenotypes induced by lipopolysaccharide and thioglycolate. Sébastien Dharancy et al. detected that in the thioglycolate peritonitis model the phenotype of NOD1-/- mice is less pronounced, suggesting that the central role of NOD1 in PMNs is of greater impact in the liver than in other organs. Furthermore, Genetic and pharmacologic studies indicate NOD1 as a modulator of PMN function and migration in liver [31]. Therefore, NOD1 may represent a new therapeutic target in PMN dependent liver diseases (Figure 1).
Figure 1.

The role of NOD1 in hepatocytes. Recognized by NOD1, C12-iEDAP can initiate NF-κB signaling and MAP kinases in hepatocytes, therefore inducing the expression of chemokines CXCL1 and CCL5, leading to augmentation of the adaptive immune response.
NOD2
NOD2 (NLRC2, also termed as CARD15; BLAU; IBD1; PSORAS1; CLR16. 3), a member of the NLR family of leucine rich repeat proteins, is encoded by the CARD15 gene and plays an important role in innate immune response [54]. NOD2 is detectable in antigen-presenting cells (APCs), epithelial cells, macrophages, monocytes, T lymphocytes, Paneth cells, and DC. It senses both Gram-positive and Gram-negative bacteria through PGN and muramyl dipeptide (MDP) as an innate pathogen sensor [55-57]. Recent data has shown that NOD2 is of interest in human disease. Du P et al. found that NOD2 was upregulated in kidney biopsies from diabetic patients and high-fat diet/streptozotocin-induced diabetic mice [58]. Further, NOD2 deficiency ameliorates renal injury in diabetic mice. In addition, the function of NOD2 may be due to its role in the regulation of innate immune responses to pathogens as well as to constituents of the normal microbiota. It is noticeable that mutations in NOD2 are associated with Blau syndrome, early onset sarcoidosis and Crohn’s disease (CD). For instance, recent study well demonstrated that single nucleotide polymorphisms (SNPs) of the NOD2 gene displayed the strongest correlation with the progression of CD which is regarded as a chronic intestinal inflammatory disorder led by the interaction of numerous genetic and environmental factors [59,60].
NOD2 in concanavalin A-induced liver injury
Liver injury is associated with raised bacterial translocation related to an intestinal phenomenon designated “leaky gut”, which contributes to a rise in bacterial cell wall products, also called PAMP [61]. Notably, PAMP may enhance inflammatory processes within the liver through their interaction with pathogen recognition receptors. NOD2 is a new identified cytosolic pathogen recognition receptors, which functions at the crossroads of innate and adaptive immune responses [62,63]. Melanie J. Scott. et al. demonstrated that NOD2 activation forms part of a collective immune response to pathogens and organ injury, therefore it is interesting to speculate that hepatocyte NOD2 may play an important regulatory role in many inflammatory processes involving the liver [30].
The induction of NOD2 expression linked to cytokine production in different models of liver inflammation highlights the similarities between intestinal cells and hepatocytes, indicating common regulatory mechanisms involved in the innate immune response and its subsequent inflammation process in these two organs [32]. Indeed, Rosenstiel et al. had previously demonstrated that TNF-α and IFN-γ synergistically cooperate to induce NOD2 expresion in intestinal epithelial cells [64]. Mathilde Body-Malapel et al. then confirmed the same phenomenon at the cellular level using a hepatocyte cell line [65]. Moreover, they showed the first evidence that NOD2 expression is up-regulated during liver injury. In addition, its expression was detected in isolated human hepatocytes and at comparable levels in immune mononuclear cells, as evident by the double staining of NOD2 in CD68 positive cells. Recent data revealed that NOD2-/- mice are resistant to ConA-induced hepatitis, supporting the regulative role of NOD2 in hepatic injury through modulating IFN-γ production by immune cells [66]. Furthermore, the role of NOD2 acting through its ligand is confirmed by the exacerbation of ConA-induced liver injury after treatment with MDP and the absence of exacerbation of liver injury in NOD2-/- mice that received MDP.
Importantly it was shown that NOD2 is the specific sensor for MDP, a frequently described immunostimulatory peptidoglycan motif common to all bacteria. MDP recognition by NOD2 leads to activation of NF-κB and induces proinflammatory cytokine production by immune cells through a Rip-like interactive clarp kinase-dependent signaling pathway [67,68]. In addition, MDP is a potent inducer of cytokines in freshly isolated peripheral blood mononuclear cells (PBMC), splenocytes and hepatocytes [32]. Interestingly, NOD2 regulates the inflammatory process in the digestive tract.
Taken together, NOD2 is involved in regulation of liver injury and associated with multiple human pathologies including inflammatory bowel disease, indicating it may act as a new therapeutic target for liver diseases (Figure 2).
Figure 2.
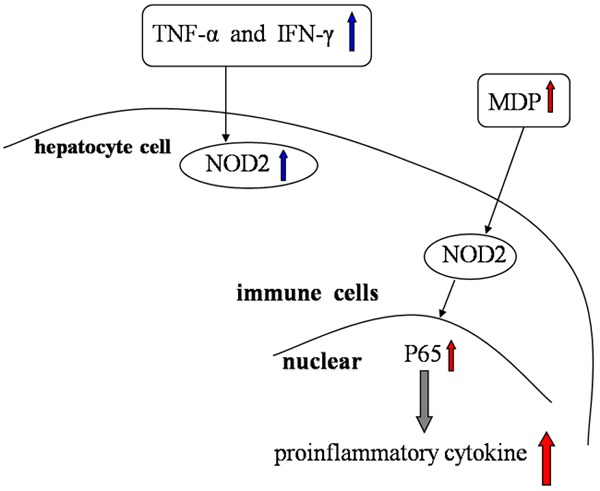
The role of NOD2 in immune cells. TNF-α and IFN-γ synergistically cooperate to induce NOD2 expression in hepatocyte cells. MDP recognition by NOD2 leads to activation of NF-κB and induces proinflammatory cytokine production by immune cells.
NLRP3
The prominent NLR, NLRP3 (also known as cryopyrin, NALP3 or PYPAF1) contains typical domains for an NLR; an N-terminal pyrin domain (PYD) followed by a central NBD and C-terminal LRR. NLRP3 (NACHT, LRR, and PYD domains-containing protein 3, cryoporin) was first described by Hoffman et al., who discovered four single mutations in the NLRP3 gene, in families with familial cold autoinflammatory syndrome and Muckle-Wells syndrome, which lead to increased IL-1β production [68-70]. Later, Agostini et al. reported that NLRP3 forms an IL-1β-processing inflammasome complex [71]. The inflammasomes are pattern recognition receptors that have recently been identified to recognize a diverse range of conserved molecular motifs unique to microorganisms. NLRP3 along with apoptosis-associated speck-like protein (ASC) and caspase-1 form NLRP3 inflammasomes [72]. To date, the NLRP3 inflammasomes which called that the trigger caspase 1-dependent maturation of the precursors of IL-1β and IL-18 cytokines, is one of the most extensively studied inflammasomes and is capable of sensing a wide variety of alarm signals. As we all known, the apoptosis-associated speck-like protein containing CARD(ASC) and the mitochondrial antiviral signaling protein (MAVS) are important for NLRP3-dependent inflammasome function [73]. Since NLRP3 does not contain a CARD domain, the presence of the adaptor molecule is necessary for the complex formation [74,75].
Expression of the NLRP3 inflammasome proteins can be detected in a variety of immune and non-immune cells, including monocytes/macrophages, T cells, myofibroblasts/fibroblasts, keratinocytes, KC and liver sinusoidal endothelial cells (LSEC) and HSC [76,77]. Generally it is understood that endogenous signals able to activate NLRP3 contain MSU crystals and ATP [78]. Additionally, it has been shown that the direct positional involvement of the endoplasmic reticulum (ER) and mitochondria is critical in this signaling [79]. Moreover, A. Phillip West et al. have summarized the role of the mitochondria in the innate immune response [80]. Recently novel molecular pathways required for immune cells in respond to tissue injury and death were identified. These pathways are initiated by the activation of one of a family of cytosolic sensory molecule termed NLRs [76,81]. The signals that activate these sensory molecules are varied, and include PAMPs as well as products of cellular apoptosis. A large number of stimuli have been identified as inducing inflammasome activation. For example, inflammasome activation by gram-negative bacteria requires NLRC4, and in contrast inflammasome activation by noninfectious signals including many endogenous signals requires NLRP3 [29].
NLRP3 in liver ischemia reperfusion (I/R) injury
I/R injury is a phenomenon whereby hypoxic organ damage is accentuated after return of blood flow and oxygen delivery [82]. Hepatic ischemia followed by reperfusion is a crucial clinical problem during partial hepatectomy, hypovolemic shock, and trauma, characterized by apoptosis and necrosis of hepatocytes [83]. Liver I/R injury is a multifactorial process. Furthermore, the process of liver I/R injury is a cascade of inflammatory events involving multiple interconnected factors, including hepatic sinusoidal endothelial cell injury and disturbances of microvascular circulation, activation of KC, production and release of reactive oxygen species (ROS) and inflammatory mediators [54,82,84].
Clearly, warm I/R is clinically relevant in liver resections when hepatic inflow occlusion and total vascular exclusion are used to reduce blood loss [85]. Thus, it is characterized as a cascade of prominent inflammatory events including excessive production of proinflammatory mediators such as ROS, IL-1β, IL-18, IL-6, TNF-α, and HMGB1, which could activate the NLRP3 inflammasome [86]. NLRP3 is involved in the recognition of numerous exogenous and host ligands, including bacterial RNA, ATP, and uric acid crystals, and is also triggered by low concentrations of intracellular potassium (K+ efflux) and increased levels of reactive oxygen species [87,88]. Consistently, Ping Zhu et al. also found that NLRP3 expression in liver nonparenchymal cells (NPCs) is increased during liver I/R. To date, the characteristics of I/R are hepatocyte death, release of damage-associated molecular patterns (DAMPs), inflammatory cell infiltration, KC activation, ROS production, and disruption of LSEC that can all lead to inflammasome activation. Importantly, silencing NLRP3 ameliorates I/R-induced hepatocellular injury and reduces IL-1β, IL-18, HMGB1, IL-6, and TNF-α release via inhibition of Caspase-1 and NF-κB activity [86]. Therefore, NLRP3 signaling may play an important certain role in liver I/R.
Recently, Ping Zhu et al. showed that hydrodynamic injection of pNLRP3-shRNA plasmid via the tail vein can be achieved easily. Moreover, they demonstrated that NLRP3 expression was effectively inhibited by pNLRP3-shRNA plasmid transfection both in vitro and in vivo, which protects against warm hepatic injury, and this protective effect is associated with less inflammatory cells infilatration, reduced production of proinflammatory cytokines and HMGB1 [86]. Overall, this may provide a new strategy for treatment of liver I/R in the clinic.
NLRP3 in APAP (N-acetyl-para-aminophenol) hepatotoxicity and drug-induced liver injury (DILI)
Drug-induced liver injury (DILI) is a significant public health problem, accounting for over half of all cases of acute liver failure. APAP is one of the most widely used nonprescription drugs for its analgesic and antipyretic activities [89,90]. Moreover, APAP hepatotoxicity is the most common cause of death due to acute liver failure and is increasingly recognized as a significant public health problem. The initial outcome in APAP-induced hepatotoxicity is a toxic-metabolic injury leading to hepatocyte death by necrosis and apoptosis, which leads to the secondary activation of the innate immune response involving upregulation of inflammatory cytokines released from NK cells, NKT cells, and neutrophils [91,92]. The molecular pathways for innate immune activation after hepatocyte death are very interesting, as they are likely common to sterile inflammation. Interestingly, Avlin B. Imaeda et al. have shown that acetaminophen treatment results in hepatocyte death and that free DNA released from apoptotic hepatocytes activates TLR9, and therefore triggering a signaling cascade that increases transcription of the genes encoding pro-IL-1β and pro-IL-18 in sinusoidal endothelial cells [15].
APAP-induced liver injury remains the leading cause of DILI [93]. In APAP-induced liver injury, release of DAMPs from necrotic hepatocytes and sinusoidal endothelial cells triggers sterile inflammation via pattern recognition receptors including TLRs and NLRs [94,95]. Avlin B. Imaeda et al. found that APAP liver injury is attenuated in mice lacking components of NLRP3 inflammasome, suggesting a role for NLRP3 inflammasome in APAP-induced liver injury. They have identified the involvement of NLRP3 signal in amplification of APAP-induced liver toxicity. The NLRP3 inflammasome provides the signal for cleavage and activation of these pro-cytokines. By activating caspase-1, the enzyme responsible for generating mature IL-1β and IL-18 from pro-IL-1β and pro-IL-18, respectively, the NLRP3 inflammasome plays a crucial role in the step of proinflammatory cytokine activation following acetaminophen-induced liver injury [15]. In addition, total liver pro-IL-1β levels is increased in NLRP3-/- mice to a degree comparable to that in NLRP3+/+ mice. However, there was no increase in serum IL-1β in NLRP3-/- mice, consistent with a role of NLRP3 in Caspase-1 activation. Collectively, recent studies indicated the critical roles of NLRP3 inflammasome pathway for IL-1β and IL-18 in APAP-induced liver injury.
NLRP3 in non-alcoholic fatty liver disease (NAFLD)
Non-alcoholic fatty liver disease (NAFLD) is a set of syndromes that ranging from hepatic steatosis to others, such as non-alcoholic steatohepatitis (NASH) and cirrhosis, which may induce hepatocellular carcinoma (HCC). It has been reported that 10-35% of general population suffers from NAFLD [96-98]. In addition, the prevalence of the disease has increased dramatically during the previous decade probably because of both, the changes of life-style (decreased physical activity and alterations in dietary habits) and the increased detection rate [96]. While most patients with NAFLD remain asymptomatic, 20% of them progress to develop NASH, which in turn leads to cirrhosis, portal hypertension, hepatocellular carcinoma [99-101]. Despite its high prevalence, factors leading to progression from NAFLD to NASH remain poorly understood and no treatment has been proved effective.
Generally a “two hit” mechanism is proposed to drive NAFLD/NASH pathogenesis. Hepatic steatosis is the first hit, which is closely associated with lipotoxicity-induced mitochondrial abnormalities that sensitize the liver to additional pro-inflammatory insults. Then the second hit contains enhanced lipid peroxidation and increased generation of ROS [100]. Inflammasomes are sensors of endogenous or exogenous PAMPs or DAMPs that govern cleavage of effector proinflammatory cytokines such as pro-IL-1β and pro-IL-18. Most of DAMPs trigger the generation of ROS, which are known to activate the NLRP3 inflammasome [101].
IR and inflammatory responses which mediate NF-κB-dependent signaling pathways through a number of cytokines such as TNF and IL-6 is the major pathological changes in NASH [72]. To evaluate the role of the NLRP3 inflammasome in NASH progression, singly-housed NLRP3-/- and +/+ animals were fed with methionine-choline deficient diet (MCDD) and evaluated disease progression. Interestingly, NLRP3-/- mice developed exacerbated NASH compared to WT mice as judged by increased levels of serum ALT and AST, in addition to NAFLD activity inflammation scores. Moreover, Jorge Henao-Mejia et al. discovered that inflammasomes act as steady-state sensors and regulators of the colonic microbiota, and that a deficiency in components of the inflammasome, NLRP3 involve IL-18 but not IL-1R, results in the development of an altered transmissible, colitogenic intestinal microbial community. In addition, this microbiota is associated with increased representation of members of Bacteroidetes (Prevotellaceae) and the bacterial phylum TM7, and reductions in representation of members of the genus Lactobacillus in the Firmicutes phylum. Futhermore, electron microscopy (EM) studies disclose aberrant colonization of crypts of lieberkuhn with bacteria with morphologic features of prevotellaceae. Strikingly, co-housing of Asc-/- and IL-18-/- mice with WT animals, prior to induction of NASH with MCDD result in significant exacerbation of NASH in the WT cagemates, as compared to singly-housed, age- and gender-matched WT controls. In co-housed WT mice, disease severity reaches comparable levels to that of co-housed Asc-/- and IL-18-/- mice. Moreover, significantly increased numbers of multiple inflammatory cell types are detected in the liver of WT (Asc-/-) compared to WT mice. Similar findings are obtained in WT mice co-housed with caspase-1-/-, NLRP3-/- mice. To exclude the possibility that aberrant microbiota represented in all mice maintained in the vivarium, Jorge Henao-Mejia et al. co-housed WT mice with other strains of NLR-deficient mice that were either obtained from the same source as Asc-/- and NLRP3-/- mice. None of these strains featured a similar phenotype [14]. Finally, these observations suggested that the transmissible colitogenic microbiota present in inflammasome-deficient mice is a key contributor to the enhanced NASH.
Yu-Gang Wang et al. Find that the expression levels of NLRP3, caspase-1, and ASC mRNA greatly increase with time, and the corresponding protein expression levels are significantly higher in the high-fat diet(HF)+LPS group than in the HF and control groups [72]. All this indicating that NLRP3 inflammasomes influence NASH development. As we all know, IL-1β which is regulated by NLRP3 inflammasomes can induce the expression of adhesion molecules, proinflammatory cytokines, and chemokines such as TNF-α IL-6, and IL-8 [102]. IL-1β gene knockout inhibited the progression of simple steatosis to steatohepatitis in a diet-induced mouse NASH model. Studies have shown that NLRP3 inflammasomes/IL-1β could be a new target for treating NASH. Injection of IL-1 receptor blockers or IL-Trap, a newgeneration IL-1β antagonist [103], can inhibit excessive IL-1β secretion caused by NLRP3 imbalance, and may thus be used treat related genetic or acquired diseases [104].
NLRP3 in liver fibrosis
Liver fibrosis, irrespective of aetiology, is a dynamic and highly integrated molecular, tissue and cellular process that leads to progressive accumulation of extracellular matrix (ECM) components in an attempt to limit hepatic damage in chronic liver diseases [105]. Liver fibrosis results from persistent liver jury, including viral hepatitis, alcohol abuse, metabolic diseases, autoimmune diseases, and cholestatic liver diseases [106]. The terminal outcome of liver fibrosis is liver cirrhosis, a condition characterized by distortion of the normal architecture, septae and nodule formation, altered blood flow, portal hypertension, hepatocellular carcinoma and ultimately liver failure [107]. The HSC is the main fibrogenic cell type orchestrating the deposition of ECM in the injured liver and has been identified as a primary effector in liver inflammation [108,109].
It is well known that HSCs, located in the spaces of disse, are resident perisinusoidal cells in the subendothelial space between hepatocytes and sinusoidal endothelial cells. Notably, HSCs are in close contact with hepatocytes, sinusoidal endothelial cells, and autonomic nerve fibers. Clearly, a irreplaceable role of activated HSCs in collagen and ECM production has been previously observed [110]. The ability of HSCs to respond to tissue injury and death in their immediate vicinity is shared by plenty of other cells including cells of the immune system such as DC [111,112].
In addition, Azuma Watanabe, et al. initially demonstrated expression of NLRP3 and the adaptor protein ASC in the human LX-2 line and freshly isolated primary mouse HSC. Activation of the NLRP3 inflammasome by MSU crystals induces upregulation of TGF-β and collagen1 in LX-2 cells and in primary HSC, which occurred within activation of the NLRP3 inflammasome and does not occur in primary HSC from ASC-deficient mice, and thus demonstrating that the NLRP3 inflammasome is functional in HSC, and its activation induces upregulation of genes associated with HSC matrix deposition [29].
Notably a recent study confirmed the importance of inflammasome activation in HSC biology and liver fibrosis in vivo [113]. Moreover, Azuma Watanabe, et al. proved that mice lacking NLRP3 have significantly reduced liver collagen after 8 wk of CCl4, consistent with the results generated from TAA-induced fibrotic model. Thus, these results were in concert with that NLRP3 inflammasome components are present and function in HSC while they are required for the development of liver fibrosis [29]. Additionally, another study by Gieling et al. suggested that IL-1 mediates the progression of liver fibrosis [114]. They demonstrated that IL-1α and IL-1β peak on day 1 are followed by a peak of type I collagen on day 3 in liver injury with thioacetamide.
NLRP3 in LPS-induced liver damage
The endotoxin LPS, a component of Gram-negative bacteria, plays an crucial role in acute liver injury as well as chronic liver diseases including fatty liver associated with either alcohol consumption or metabolic syndrome and obesity. Elevated LPS levels are detected in the portal and systemic blood of patients with alcoholic and nonalcoholic fatty liver disease. In addition, LPS has also been implicated in insulin resistance as well as in steatohepatitis in non-alcoholic fatty liver disease [115]. Increasing evidence suggested that gut-derived LPS through the gut-liver axis affects the extent of liver damage in many different types of inflammatory liver diseases [116]. It is well known that LPS is a prototypical ligand for the PRR, TLR4 [117]. TLR4 induces downstream signaling via the MyD88 adapter molecule and induces production of proinflammatory cytokines through activation of the regulatory factor NF-κB [116]. In the liver, TLR4 is expressed in both parenchymal and immune cells, thereby providing potential for LPS-induced activation [118]. Novel studies suggested that that the NLRP3 inflammasome is activated by both PAMPs, including LPS and bacterial RNA, and DAMPs [88,119]. In response to stimulation by either DAMPs or PAMPs, NLRP3 interacts with pro-caspase-1 through the adaptor molecule to form the inflammasome, which leads to activation of Caspase-1 [120]. In addition, active Caspase-1 has been shown to cleave other substances, such as Caspase-7 and sterol regulatory element-binding proteins, therefore playing a pivotal role in cell death and survival [121]. By the way, mRNA levels of IL-1β have been shown to increase in the liver in response to LPS stimulation. Michal Ganz, et al. demonstrated that there was a significant increase in liver mRNA of NLRP3 after > 4 h LPS stimulation, with a peak at 6 h, both at the mRNA and protein levels and this is associated with increased IL-1β production [36].
NLRP3 in hepatitis C virus (HCV)-mediated liver damage
Chronic inflammation is a major contributor to disease and is the basis of HCV-mediated liver damage [122]. HCV is a hepatotropic, enveloped virus that carries a single-stranded positive-sense RNA genome, and chronically infects nearly 3% of the world’s population. HCV productively infects hepatocytes to induce liver inflammation and progressive tissue damage leading to fibrosis and cirrhosis. These processes underlie liver dysfunction and are thought to drive the onset of liver cancer [123,124]. However, the molecular mechanisms by which HCV stimulates hepatic inflammation are not defined. IL-1β is a central component of the cytokine milieu that accompanies both acute and chronic inflammation and viral disease [125]. During microbial infection, IL-1β production is induced by cellular sensing of PAMP motifs within microbial macromolecules and/or by metabolic products that are accumulated from infection [126]. Production of active IL-1β requires two signals, “signal one” for activating NF-κB in stimulated cells and inducing IL-1β mRNA expression, and “signal two” for activating a NLR to promote downstream Caspase-1 cleavage and processing of pro-IL-1β into a biologically active, secreted cytokine.
Amina A. Negash et al. defined that the hepatic macrophage/HCV interface and NLRP3 inflammasome-dependant production of IL-1β as critical features underlying liver disease in chronic HCV infection [36]. In this study, they reveal that exposure of macrophages to HCV induces IL-1β expression, maturation and secretion through a process of infection-independent phagocytic virus uptake that activates MyD88/TLR7 and NLRP3 inflammasome pathways. It also show that viral induce these signaling pathways causing an inflammatory response in patients with Chronic Hepatitis C Virus (HCV). Concomitantly, it induces a potassium efflux which activates the NLRP3 inflammasome for IL-1β processing and secretion. Thus, suppress NLRP3 or IL-1β activity could offer a therapeutic action to mitigate hepatic inflammation (Figure 3).
Figure 3.
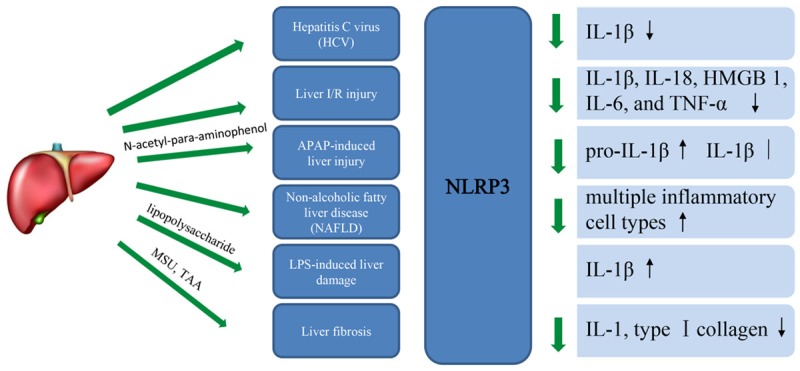
The function of NLRP3 in liver disease, with focus on cytokines.
Conclusions
In conclusion, there is clearly a very preliminary list of NLRs that regulate pathology in a series of complex liver diseases. Needless to say this list is likely to increase, as is the number of liver diseases themselves, where we are yet to discover the importance of inflammasome derived cytokines. Furthermore, there has been little discussion about the role of multiple NLRs in the complex interplay of infectious diseases.
Moreover, novel data provide insight into the mechanisms of cell-specific induction of inflammatory cytokines and of the interplay between proinflammatory and anti-inflammatory cytokines mediating NLR induced cytotoxicity. Further studies are needed to evaluate the cross talk between liver parenchymal and nonparenchymal cells. Understanding the cell-specific role of NLR signaling in ALD and NAFLD will further provide new insights into the pathogenesis of these liver diseases. Increasing knowledge about the NLRs activated in liver diseases will surely develop hand-in-hand with the efforts of the pharmaceutical industry, to create molecules that can intervene in this way.
Acknowledgements
This project was supported by the Chinese National Natural Science Foundation Project (81100302), the Provincial Natural Science Research Project of Colleges and Universities of Anhui Province (No. KJ2016A348) and the fund of Anhui medical university doctoral start research (No. 0601067101), Anhui Medical University early contact research of clinical medicine (2015-ZQKY-47).
Disclosure of conflict of interest
None.
Abbreviations
- AH
alcoholic hepatitis
- ALD
alcoholic liver disease
- ASC
apoptosis-associated speck-like protein
- APAP
N-acetyl-para-aminophenol
- APC
antigen-presenting cell
- CARD
caspase recruitment domain
- CCRV
channel catfish hemorrhage reovirus
- CD
Crohn’s disease
- CLD
chronic liver diseases
- DC
dendritic cell
- DILI
Drug-induced liver injury
- ECM
extracellular matrix
- EM
electron microscopy
- ER
endoplasmic reticulum
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HSC
hepatic stellate cell
- I/R
ischaemia/reperfusion
- KC
Kupffer cell
- LPS
lipopolysaccharide
- LRR
leucine-rich repeat
- LSEC
liver sinusoidal endothelial cell
- MAVS
mitochondrial antiviral signaling protein
- MCDD
methionine-choline deficient diet
- MDP
muramyl dipeptide
- MyD88
myeloid differentiation factor 88
- NASH
nonalcoholic steatohepatitis
- NBD
nucleotide binding domain
- NAFLD
Non-alcoholic fatty liver disease
- NF-κB
nuclear factor-κB
- NLR
NOD-nucleotide binding oligomerization domain like receptor
- NK
natural killer
- NOD
nucleotide binding oligomerization domain
- NPC
nonparenchymal cell
- PAMP
pathogenassociated molecular pattern
- PBMC
peripheral blood mononuclear cell
- PGN
peptidoglycan
- PMN
polymorphonuclear neutrophil
- PYD
pyrin domain
- ROS
reactive oxygen species
- SNP
single nucleotide polymorphism
- TLR
Toll-like receptor
References
- 1.Ni HM, Williams JA, Yang H, Shi YH, Fan J, Ding WX. Targeting autophagy for the treatment of liver diseases. Pharmacol Res. 2012;66:463–474. doi: 10.1016/j.phrs.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guicciardi ME, Malhi H, Mott JL, Gores GJ. Apoptosis and necrosis in the liver. Compr Physiol. 2013;3:977–1010. doi: 10.1002/cphy.c120020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kern M, Popov A, Scholz K, Schumak B, Djandji D, Limmer A, Eggle D, Sacher T, Zawatzky R, Holtappels R, Reddehase MJ, Hartmann G, Debey-Pascher S, Diehl L, Kalinke U, Koszinowski U, Schultze J, Knolle PA. Virally infected mouse liver endothelial cells trigger CD8+ T-cell immunity. Gastroenterology. 2010;138:336–346. doi: 10.1053/j.gastro.2009.08.057. [DOI] [PubMed] [Google Scholar]
- 4.Knolle PA, Gerken G. Local control of the immune response in the liver. Immunol Rev. 2000;174:21–34. doi: 10.1034/j.1600-0528.2002.017408.x. [DOI] [PubMed] [Google Scholar]
- 5.Knolle PA, Limmer A. Neighborhood politics: the immunoregulatory function of organ-resident liver endothelial cells. Trends Immunol. 2001;22:432–437. doi: 10.1016/s1471-4906(01)01957-3. [DOI] [PubMed] [Google Scholar]
- 6.Li Z, Diehl AM. Innate immunity in the liver. Curr Opin Gastroenterol. 2003;19:565–571. doi: 10.1097/00001574-200311000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL. Centers for disease control and prevention 2000 growth charts for the United States: improvements to the 1977 national center for health statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 8.Burroughs A, McNamara D. Liver disease in Europe. Aliment Pharmacol Ther. 2003;18(Suppl 3):54–59. doi: 10.1046/j.0953-0673.2003.01729.x. [DOI] [PubMed] [Google Scholar]
- 9.Kim WR, Brown RS Jr, Terrault NA, El-Serag H. Burden of liver disease in the United States: summary of a workshop. Hepatology. 2002;36:227–242. doi: 10.1053/jhep.2002.34734. [DOI] [PubMed] [Google Scholar]
- 10.Petrasek J, Csak T, Szabo G. Toll-like receptors in liver disease. Adv Clin Chem. 2013;59:155–201. doi: 10.1016/b978-0-12-405211-6.00006-1. [DOI] [PubMed] [Google Scholar]
- 11.Petrasek J, Csak T, Ganz M, Szabo G. Differences in innate immune signaling between alcoholic and non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2013;28(Suppl 1):93–98. doi: 10.1111/jgh.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petrasek J, Mandrekar P, Szabo G. Toll-like receptors in the pathogenesis of alcoholic liver disease. Gastroenterol Res Pract. 2010;2010 doi: 10.1155/2010/710381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szabo G, Mandrekar P. A recent perspective on alcohol, immunity, and host defense. Alcohol Clin Exp Res. 2009;33:220–232. doi: 10.1111/j.1530-0277.2008.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, Camporez JP, Shulman GI, Gordon JI, Hoffman HM, Flavell RA. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, Flavell RA, Mehal WZ. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mozer-Lisewska I, Kowala-Piaskowska A, Mania A, Jenek R, Samara H, Kaczmarek E, Sikora J, Sluzewski W, Zeromski J. Expression of pattern recognition receptors in liver biopsy specimens of children chronically infected with HBV and HCV. Folia Histochem Cytobiol. 2011;49:410–416. doi: 10.5603/fhc.2011.0058. [DOI] [PubMed] [Google Scholar]
- 17.Schroder K, Deretic V. Innate immunity, the constant gardener of antimicrobial defense. Curr Opin Microbiol. 2013;16:293–295. doi: 10.1016/j.mib.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22:240–273. doi: 10.1128/CMR.00046-08. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei XQ, Guo YW, Liu JJ, Wen ZF, Yang SJ, Yao JL. The significance of Toll-like receptor 4 (TLR4) expression in patients with chronic hepatitis B. Clin Invest Med. 2008;31:E123–130. doi: 10.25011/cim.v31i3.3469. [DOI] [PubMed] [Google Scholar]
- 20.Mozer-Lisewska I, Sluzewski W, Kaczmarek M, Jenek R, Szczepanski M, Figlerowicz M, Kowala-Piaskowska A, Zeromski J. Tissue localization of Toll-like receptors in biopsy specimens of liver from children infected with hepatitis C virus. Scand J Immunol. 2005;62:407–412. doi: 10.1111/j.1365-3083.2005.01670.x. [DOI] [PubMed] [Google Scholar]
- 21.Mansson Kvarnhammar A, Tengroth L, Adner M, Cardell LO. Innate immune receptors in human airway smooth muscle cells: activation by TLR1/2, TLR3, TLR4, TLR7 and NOD1 agonists. PLoS One. 2013;8:e68701. doi: 10.1371/journal.pone.0068701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miao EA, Warren SE. Innate immune detection of bacterial virulence factors via the NLRC4 inflammasome. J Clin Immunol. 2010;30:502–506. doi: 10.1007/s10875-010-9386-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–559. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Ting JP, Lovering RC, Alnemri ES, Bertin J, Boss JM, Davis BK, Flavell RA, Girardin SE, Godzik A, Harton JA, Hoffman HM, Hugot JP, Inohara N, Mackenzie A, Maltais LJ, Nunez G, Ogura Y, Otten LA, Philpott D, Reed JC, Reith W, Schreiber S, Steimle V, Ward PA. The NLR gene family: a standard nomenclature. Immunity. 2008;28:285–287. doi: 10.1016/j.immuni.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sha Z, Abernathy JW, Wang S, Li P, Kucuktas H, Liu H, Peatman E, Liu Z. NOD-like subfamily of the nucleotide-binding domain and leucine-rich repeat containing family receptors and their expression in channel catfish. Dev Comp Immunol. 2009;33:991–999. doi: 10.1016/j.dci.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Rosenzweig HL, Planck SR, Rosenbaum JT. NLRs in immune privileged sites. Curr Opin Pharmacol. 2011;11:423–428. doi: 10.1016/j.coph.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robertson SJ, Girardin SE. Nod-like receptors in intestinal host defense: controlling pathogens, the microbiota, or both? Curr Opin Gastroenterol. 2013;29:15–22. doi: 10.1097/MOG.0b013e32835a68ea. [DOI] [PubMed] [Google Scholar]
- 28.Boaru SG, Borkham-Kamphorst E, Tihaa L, Haas U, Weiskirchen R. Expression analysis of inflammasomes in experimental models of inflammatory and fibrotic liver disease. J Inflamm (Lond) 2012;9:49. doi: 10.1186/1476-9255-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe A, Sohail MA, Gomes DA, Hashmi A, Nagata J, Sutterwala FS, Mahmood S, Jhandier MN, Shi Y, Flavell RA, Mehal WZ. Inflammasome-mediated regulation of hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1248–1257. doi: 10.1152/ajpgi.90223.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scott MJ, Chen C, Sun Q, Billiar TR. Hepatocytes express functional NOD1 and NOD2 receptors: a role for NOD1 in hepatocyte CC and CXC chemokine production. J Hepatol. 2010;53:693–701. doi: 10.1016/j.jhep.2010.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dharancy S, Body-Malapel M, Louvet A, Berrebi D, Gantier E, Gosset P, Viala J, Hollebecque A, Moreno C, Philpott DJ, Girardin SE, Sansonetti PJ, Desreumaux P, Mathurin P, Dubuquoy L. Neutrophil migration during liver injury is under nucleotide-binding oligomerization domain 1 control. Gastroenterology. 2010;138:1546–1556. 1556.e1–5. doi: 10.1053/j.gastro.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Body-Malapel M, Dharancy S, Berrebi D, Louvet A, Hugot JP, Philpott DJ, Giovannini M, Chareyre F, Pages G, Gantier E, Girardin SE, Garcia I, Hudault S, Conti F, Sansonetti PJ, Chamaillard M, Desreumaux P, Dubuquoy L, Mathurin P. NOD2: a potential target for regulating liver injury. Lab Invest. 2008;88:318–327. doi: 10.1038/labinvest.3700716. [DOI] [PubMed] [Google Scholar]
- 33.Ganz M, Csak T, Nath B, Szabo G. Lipopolysaccharide induces and activates the Nalp3 inflammasome in the liver. World J Gastroenterol. 2011;17:4772–4778. doi: 10.3748/wjg.v17.i43.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leng W, Liu Y, Shi H, Li S, Zhu H, Pi D, Hou Y, Gong J. Aspartate alleviates liver injury and regulates mRNA expressions of TLR4 and NOD signaling-related genes in weaned pigs after lipopolysaccharide challenge. J Nutr Biochem. 2014;25:592–599. doi: 10.1016/j.jnutbio.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 35.Negash AA, Ramos HJ, Crochet N, Lau DT, Doehle B, Papic N, Delker DA, Jo J, Bertoletti A, Hagedorn CH, Gale M Jr. IL-1beta production through the NLRP3 inflammasome by hepatic macrophages links hepatitis C virus infection with liver inflammation and disease. PLoS Pathog. 2013;9:e1003330. doi: 10.1371/journal.ppat.1003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chamaillard M, Girardin SE, Viala J, Philpott DJ. Nods, Nalps and Naip: intracellular regulators of bacterial-induced inflammation. Cell Microbiol. 2003;5:581–592. doi: 10.1046/j.1462-5822.2003.00304.x. [DOI] [PubMed] [Google Scholar]
- 37.Shaw PJ, Barr MJ, Lukens JR, McGargill MA, Chi H, Mak TW, Kanneganti TD. Signaling via the RIP2 adaptor protein in central nervous system-infiltrating dendritic cells promotes inflammation and autoimmunity. Immunity. 2011;34:75–84. doi: 10.1016/j.immuni.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strober W, Murray PJ, Kitani A, Watanabe T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol. 2006;6:9–20. doi: 10.1038/nri1747. [DOI] [PubMed] [Google Scholar]
- 39.Correa RG, Milutinovic S, Reed JC. Roles of NOD1 (NLRC1) and NOD2 (NLRC2) in innate immunity and inflammatory diseases. Biosci Rep. 2012;32:597–608. doi: 10.1042/BSR20120055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carneiro LA, Magalhaes JG, Tattoli I, Philpott DJ, Travassos LH. Nod-like proteins in inflammation and disease. J Pathol. 2008;214:136–148. doi: 10.1002/path.2271. [DOI] [PubMed] [Google Scholar]
- 41.Askari N, Correa RG, Zhai D, Reed JC. Expression, purification, and characterization of recombinant NOD1 (NLRC1): a NLR family member. J Biotechnol. 2012;157:75–81. doi: 10.1016/j.jbiotec.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plantinga TS, Fransen J, Knevel R, Netea MG, Zwerina J, Helsen MM, van der Meer JW, van Riel PL, Schett G, van der Helm-van Mil AH, van den Berg WB, Joosten LA. Role of NOD1 polymorphism in susceptibility and clinical progression of rheumatoid arthritis. Rheumatology (Oxford) 2013;52:806–814. doi: 10.1093/rheumatology/kes404. [DOI] [PubMed] [Google Scholar]
- 43.Lu WG, Zou YF, Feng XL, Yuan FL, Gu YL, Li X, Li CW, Jin C, Li JP. Association of NOD1 (CARD4) insertion/deletion polymorphism with susceptibility to IBD: a meta-analysis. World J Gastroenterol. 2010;16:4348–4356. doi: 10.3748/wjg.v16.i34.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vasseur F, Sendid B, Jouault T, Standaert-Vitse A, Dubuquoy L, Francois N, Gower-Rousseau C, Desreumaux P, Broly F, Vermeire S, Colombel JF, Poulain D. Variants of NOD1 and NOD2 genes display opposite associations with familial risk of Crohn's disease and anti-saccharomyces cerevisiae antibody levels. Inflamm Bowel Dis. 2012;18:430–438. doi: 10.1002/ibd.21817. [DOI] [PubMed] [Google Scholar]
- 45.Nagaki M, Moriwaki H. Implication of cytokines: Roles of tumor necrosis factor-alpha in liver injury. Hepatol Res. 2008;38(Suppl 1):S19–28. doi: 10.1111/j.1872-034X.2008.00422.x. [DOI] [PubMed] [Google Scholar]
- 46.Berasain C, Castillo J, Perugorria MJ, Latasa MU, Prieto J, Avila MA. Inflammation and liver cancer: new molecular links. Ann N Y Acad Sci. 2009;1155:206–221. doi: 10.1111/j.1749-6632.2009.03704.x. [DOI] [PubMed] [Google Scholar]
- 47.Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Czaja AJ, Manns MP. Advances in the diagnosis, pathogenesis, and management of autoimmune hepatitis. Gastroenterology. 2010;139:58–72. e54. doi: 10.1053/j.gastro.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 49.Liaskou E, Wilson DV, Oo YH. Innate immune cells in liver inflammation. Mediators Inflamm. 2012;2012:949157. doi: 10.1155/2012/949157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schattenberg JM, Schuchmann M, Galle PR. Cell death and hepatocarcinogenesis: dysregulation of apoptosis signaling pathways. J Gastroenterol Hepatol. 2011;26(Suppl 1):213–219. doi: 10.1111/j.1440-1746.2010.06582.x. [DOI] [PubMed] [Google Scholar]
- 51.Seki E, De Minicis S, Gwak GY, Kluwe J, Inokuchi S, Bursill CA, Llovet JM, Brenner DA, Schwabe RF. CCR1 and CCR5 promote hepatic fibrosis in mice. J Clin Invest. 2009;119:1858–1870. doi: 10.1172/JCI37444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bautista AP. Neutrophilic infiltration in alcoholic hepatitis. Alcohol. 2002;27:17–21. doi: 10.1016/s0741-8329(02)00206-9. [DOI] [PubMed] [Google Scholar]
- 53.Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15–26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- 54.Proell M, Riedl SJ, Fritz JH, Rojas AM, Schwarzenbacher R. The Nod-like receptor (NLR) family: a tale of similarities and differences. PLoS One. 2008;3:e2119. doi: 10.1371/journal.pone.0002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosenzweig HL, Galster KT, Planck SR, Rosenbaum JT. NOD1 expression in the eye and functional contribution to IL-1beta-dependent ocular inflammation in mice. Invest Ophthalmol Vis Sci. 2009;50:1746–1753. doi: 10.1167/iovs.08-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hisamatsu T, Suzuki M, Reinecker HC, Nadeau WJ, McCormick BA, Podolsky DK. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology. 2003;124:993–1000. doi: 10.1053/gast.2003.50153. [DOI] [PubMed] [Google Scholar]
- 57.Gutierrez O, Pipaon C, Inohara N, Fontalba A, Ogura Y, Prosper F, Nunez G, Fernandez-Luna JL. Induction of Nod2 in myelomonocytic and intestinal epithelial cells via nuclear factor-kappa B activation. J Biol Chem. 2002;277:41701–41705. doi: 10.1074/jbc.M206473200. [DOI] [PubMed] [Google Scholar]
- 58.Du P, Fan B, Han H, Zhen J, Shang J, Wang X, Li X, Shi W, Tang W, Bao C, Wang Z, Zhang Y, Zhang B, Wei X, Yi F. NOD2 promotes renal injury by exacerbating inflammation and podocyte insulin resistance in diabetic nephropathy. Kidney Int. 2013;84:265–276. doi: 10.1038/ki.2013.113. [DOI] [PubMed] [Google Scholar]
- 59.Seiderer J, Schnitzler F, Brand S, Staudinger T, Pfennig S, Herrmann K, Hofbauer K, Dambacher J, Tillack C, Sackmann M, Goke B, Lohse P, Ochsenkuhn T. Homozygosity for the CARD15 frameshift mutation 1007fs is predictive of early onset of Crohn’s disease with ileal stenosis, entero-enteral fistulas, and frequent need for surgical intervention with high risk of re-stenosis. Scand J Gastroenterol. 2006;41:1421–1432. doi: 10.1080/00365520600703900. [DOI] [PubMed] [Google Scholar]
- 60.Schaffler H, Schneider N, Hsieh CJ, Reiner J, Nadalin S, Witte M, Konigsrainer A, Blumenstock G, Lamprecht G. NOD2 mutations are associated with the development of intestinal failure in the absence of Crohn’s disease. Clin Nutr. 2013;32:1029–1035. doi: 10.1016/j.clnu.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 61.Inamura T, Miura S, Tsuzuki Y, Hara Y, Hokari R, Ogawa T, Teramoto K, Watanabe C, Kobayashi H, Nagata H, Ishii H. Alteration of intestinal intraepithelial lymphocytes and increased bacterial translocation in a murine model of cirrhosis. Immunol Lett. 2003;90:3–11. doi: 10.1016/j.imlet.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 62.Suzuki M, Cela R, Bertin TK, Sule G, Cerullo V, Rodgers JR, Lee B. NOD2 signaling contributes to the innate immune response against helper-dependent adenovirus vectors independently of MyD88 in vivo. Hum Gene Ther. 2011;22:1071–1082. doi: 10.1089/hum.2011.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lecat A, Piette J, Legrand-Poels S. The protein Nod2: an innate receptor more complex than previously assumed. Biochem Pharmacol. 2010;80:2021–2031. doi: 10.1016/j.bcp.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 64.Rosenstiel P, Fantini M, Brautigam K, Kuhbacher T, Waetzig GH, Seegert D, Schreiber S. TNF-alpha and IFN-gamma regulate the expression of the NOD2 (CARD15) gene in human intestinal epithelial cells. Gastroenterology. 2003;124:1001–1009. doi: 10.1053/gast.2003.50157. [DOI] [PubMed] [Google Scholar]
- 65.Nicoletti F, Zaccone P, Xiang M, Magro G, Di Mauro M, Di Marco R, Garotta G, Meroni P. Essential pathogenetic role for interferon (IFN-)gamma in concanavalin A-induced T cell-dependent hepatitis: exacerbation by exogenous IFN-gamma and prevention by IFN-gamma receptor-immunoglobulin fusion protein. Cytokine. 2000;12:315–323. doi: 10.1006/cyto.1999.0561. [DOI] [PubMed] [Google Scholar]
- 66.Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nunez G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem. 2001;276:4812–4818. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- 67.Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nunez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 68.Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29:301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 70.Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011;29:707–735. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 72.Wang YG, Fang WL, Wei J, Wang T, Wang N, Ma JL, Shi M. The involvement of NLRX1 and NLRP3 in the development of nonalcoholic steatohepatitis in mice. J Chin Med Assoc. 2013;76:686–692. doi: 10.1016/j.jcma.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 73.Peng Y, French BA, Tillman B, Morgan TR, French SW. The inflammasome in alcoholic hepatitis: Its relationship with Mallory-Denk body formation. Exp Mol Pathol. 2014;97:305–313. doi: 10.1016/j.yexmp.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 75.Inoue M, Shinohara ML. NLRP3 Inflammasome and MS/EAE. Autoimmune Dis. 2013;2013:859145. doi: 10.1155/2013/859145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Satoh T, Kambe N, Matsue H. NLRP3 activation induces ASC-dependent programmed necrotic cell death, which leads to neutrophilic inflammation. Cell Death Dis. 2013;4:e644. doi: 10.1038/cddis.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang H, Mao L, Meng G. The NLRP3 inflammasome activation in human or mouse cells, sensitivity causes puzzle. Protein Cell. 2013;4:565–568. doi: 10.1007/s13238-013-3905-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dinarello CA. Mutations in cryopyrin: bypassing roadblocks in the caspase 1 inflammasome for interleukin-1beta secretion and disease activity. Arthritis Rheum. 2007;56:2817–2822. doi: 10.1002/art.22841. [DOI] [PubMed] [Google Scholar]
- 79.Lerner AG, Upton JP, Praveen PV, Ghosh R, Nakagawa Y, Igbaria A, Shen S, Nguyen V, Backes BJ, Heiman M, Heintz N, Greengard P, Hui S, Tang Q, Trusina A, Oakes SA, Papa FR. IRE1alpha induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab. 2012;16:250–264. doi: 10.1016/j.cmet.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.West AP, Shadel GS, Ghosh S. Mitochondria in innate immune responses. Nat Rev Immunol. 2011;11:389–402. doi: 10.1038/nri2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 82.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, Billiar TR. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bamboat ZM, Balachandran VP, Ocuin LM, Obaid H, Plitas G, DeMatteo RP. Toll-like receptor 9 inhibition confers protection from liver ischemia-reperfusion injury. Hepatology. 2010;51:621–632. doi: 10.1002/hep.23365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Takeuchi D, Yoshidome H, Kato A, Ito H, Kimura F, Shimizu H, Ohtsuka M, Morita Y, Miyazaki M. Interleukin 18 causes hepatic ischemia/reperfusion injury by suppressing anti-inflammatory cytokine expression in mice. Hepatology. 2004;39:699–710. doi: 10.1002/hep.20117. [DOI] [PubMed] [Google Scholar]
- 85.Cai C, Shi X, Korff S, Zhang J, Loughran PA, Ruan X, Zhang Y, Liu L, Billiar TR. CD14 contributes to warm hepatic ischemia-reperfusion injury in mice. Shock. 2013;40:115–121. doi: 10.1097/SHK.0b013e318299d1a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu P, Duan L, Chen J, Xiong A, Xu Q, Zhang H, Zheng F, Tan Z, Gong F, Fang M. Gene silencing of NALP3 protects against liver ischemia-reperfusion injury in mice. Hum Gene Ther. 2011;22:853–864. doi: 10.1089/hum.2010.145. [DOI] [PubMed] [Google Scholar]
- 87.Petrilli V, Dostert C, Muruve DA, Tschopp J. The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol. 2007;19:615–622. doi: 10.1016/j.coi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 88.Martinon F. Signaling by ROS drives inflammasome activation. Eur J Immunol. 2010;40:616–619. doi: 10.1002/eji.200940168. [DOI] [PubMed] [Google Scholar]
- 89.Lee WM. Acetaminophen toxicity: changing perceptions on a social/medical issue. Hepatology. 2007;46:966–970. doi: 10.1002/hep.21926. [DOI] [PubMed] [Google Scholar]
- 90.Kaplowitz N. Acetaminophen hepatoxicity: what do we know, what don’t we know, and what do we do next? Hepatology. 2004;40:23–26. doi: 10.1002/hep.20312. [DOI] [PubMed] [Google Scholar]
- 91.Liu ZX, Han D, Gunawan B, Kaplowitz N. Neutrophil depletion protects against murine acetaminophen hepatotoxicity. Hepatology. 2006;43:1220–1230. doi: 10.1002/hep.21175. [DOI] [PubMed] [Google Scholar]
- 92.Liu ZX, Govindarajan S, Kaplowitz N. Innate immune system plays a critical role in determining the progression and severity of acetaminophen hepatotoxicity. Gastroenterology. 2004;127:1760–1774. doi: 10.1053/j.gastro.2004.08.053. [DOI] [PubMed] [Google Scholar]
- 93.Han D, Shinohara M, Ybanez MD, Saberi B, Kaplowitz N. Signal transduction pathways involved in drug-induced liver injury. Handb Exp Pharmacol. 2010:267–310. doi: 10.1007/978-3-642-00663-0_10. [DOI] [PubMed] [Google Scholar]
- 94.Martin-Murphy BV, Holt MP, Ju C. The role of damage associated molecular pattern molecules in acetaminophen-induced liver injury in mice. Toxicol Lett. 2010;192:387–394. doi: 10.1016/j.toxlet.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cavassani KA, Moreira AP, Habiel D, Ito T, Coelho AL, Allen RM, Hu B, Raphelson J, Carson WFt, Schaller MA, Lukacs NW, Omary MB, Hogaboam CM, Kunkel SL. Toll like receptor 3 plays a critical role in the progression and severity of acetaminophen-induced hepatotoxicity. PLoS One. 2013;8:e65899. doi: 10.1371/journal.pone.0065899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 97.Kojima S, Watanabe N, Numata M, Ogawa T, Matsuzaki S. Increase in the prevalence of fatty liver in Japan over the past 12 years: analysis of clinical background. J Gastroenterol. 2003;38:954–961. doi: 10.1007/s00535-003-1178-8. [DOI] [PubMed] [Google Scholar]
- 98.Zhou YJ, Li YY, Nie YQ, Ma JX, Lu LG, Shi SL, Chen MH, Hu PJ. Prevalence of fatty liver disease and its risk factors in the population of South China. World J Gastroenterol. 2007;13:6419–6424. doi: 10.3748/wjg.v13.i47.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shimada M, Hashimoto E, Taniai M, Hasegawa K, Okuda H, Hayashi N, Takasaki K, Ludwig J. Hepatocellular carcinoma in patients with non-alcoholic steatohepatitis. J Hepatol. 2002;37:154–160. doi: 10.1016/s0168-8278(02)00099-5. [DOI] [PubMed] [Google Scholar]
- 100.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 101.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 102.Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- 103.Kalliolias GD, Liossis SN. The future of the IL-1 receptor antagonist anakinra: from rheumatoid arthritis to adult-onset Still’s disease and systemic-onset juvenile idiopathic arthritis. Expert Opin Investig Drugs. 2008;17:349–359. doi: 10.1517/13543784.17.3.349. [DOI] [PubMed] [Google Scholar]
- 104.Verma D, Lerm M, Blomgran Julinder R, Eriksson P, Soderkvist P, Sarndahl E. Gene polymorphisms in the NALP3 inflammasome are associated with interleukin-1 production and severe inflammation: relation to common inflammatory diseases? Arthritis Rheum. 2008;58:888–894. doi: 10.1002/art.23286. [DOI] [PubMed] [Google Scholar]
- 105.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tacke F, Weiskirchen R. Update on hepatic stellate cells: pathogenic role in liver fibrosis and novel isolation techniques. Expert Rev Gastroenterol Hepatol. 2012;6:67–80. doi: 10.1586/egh.11.92. [DOI] [PubMed] [Google Scholar]
- 109.Holt AP, Salmon M, Buckley CD, Adams DH. Immune interactions in hepatic fibrosis. Clin Liver Dis. 2008;12:861–882. x. doi: 10.1016/j.cld.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Karlmark KR, Wasmuth HE, Trautwein C, Tacke F. Chemokine-directed immune cell infiltration in acute and chronic liver disease. Expert Rev Gastroenterol Hepatol. 2008;2:233–242. doi: 10.1586/17474124.2.2.233. [DOI] [PubMed] [Google Scholar]
- 112.Chai NL, Fu Q, Shi H, Cai CH, Wan J, Xu SP, Wu BY. Oxymatrine liposome attenuates hepatic fibrosis via targeting hepatic stellate cells. World J Gastroenterol. 2012;18:4199–4206. doi: 10.3748/wjg.v18.i31.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wree A, Eguchi A, McGeough MD, Pena CA, Johnson CD, Canbay A, Hoffman HM, Feldstein AE. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology. 2014;59:898–910. doi: 10.1002/hep.26592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gieling RG, Wallace K, Han YP. Interleukin-1 participates in the progression from liver injury to fibrosis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1324–1331. doi: 10.1152/ajpgi.90564.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ghanim H, Abuaysheh S, Sia CL, Korzeniewski K, Chaudhuri A, Fernandez-Real JM, Dandona P. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diabetes Care. 2009;32:2281–2287. doi: 10.2337/dc09-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Szabo G, Bala S. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol. 2010;16:1321–1329. doi: 10.3748/wjg.v16.i11.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen F, Liu Y, Zhu H, Hong Y, Wu Z, Hou Y, Li Q, Ding B, Yi D, Chen H. Fish oil attenuates liver injury caused by LPS in weaned pigs associated with inhibition of TLR4 and nucleotide-binding oligomerization domain protein signaling pathways. Innate Immun. 2013;19:504–515. doi: 10.1177/1753425912472003. [DOI] [PubMed] [Google Scholar]
- 118.Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. 2008;48:322–335. doi: 10.1002/hep.22306. [DOI] [PubMed] [Google Scholar]
- 119.Martinon F. Detection of immune danger signals by NALP3. J Leukoc Biol. 2008;83:507–511. doi: 10.1189/jlb.0607362. [DOI] [PubMed] [Google Scholar]
- 120.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 121.Yu HB, Finlay BB. The caspase-1 inflammasome: a pilot of innate immune responses. Cell Host Microbe. 2008;4:198–208. doi: 10.1016/j.chom.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 122.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 123.Tang H, Grise H. Cellular and molecular biology of HCV infection and hepatitis. Clin Sci (Lond) 2009;117:49–65. doi: 10.1042/CS20080631. [DOI] [PubMed] [Google Scholar]
- 124.Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol. 2006;1:23–61. doi: 10.1146/annurev.pathol.1.110304.100230. [DOI] [PubMed] [Google Scholar]
- 125.Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JP. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kanneganti TD. Central roles of NLRs and inflammasomes in viral infection. Nat Rev Immunol. 2010;10:688–698. doi: 10.1038/nri2851. [DOI] [PMC free article] [PubMed] [Google Scholar]


