Abstract
The autonomic nervous system (ANS), comprised of two primary branches, sympathetic and parasympathetic nervous system, plays an essential role in the regulation of vascular wall contractility and tension. The sympathetic and parasympathetic nerves work together to balance the functions of autonomic effector organs. The neurotransmitters released from the varicosities in the ANS can regulate the vascular tone. Norepinephrine (NE), adenosine triphosphate (ATP) and Neuropeptide Y (NPY) function as vasoconstrictors, whereas acetylcholine (Ach) and calcitonin gene-related peptide (CGRP) can mediate vasodilation. On the other hand, vascular factors, such as endothelium-derived relaxing factor nitric oxide (NO), and constriction factor endothelin, play an important role in the autonomic nervous system in physiologic conditions. Endothelial dysfunction and inflammation are associated with the sympathetic nerve activity in the pathological conditions, such as hypertension, heart failure, and diabetes mellitus. The dysfunction of the autonomic nervous system could be a risk factor for vascular diseases and the overactive sympathetic nerve is detrimental to the blood vessel. In this review, we summarize findings concerning the crosstalk between ANS and blood vessels in both physiological and pathological conditions and hope to provide insight into the development of therapeutic interventions of vascular diseases.
Keywords: The autonomic nervous system, blood vessels, neurotransmitters, endothelial dysfunction, vascular diseases
Introduction
The vascular system is a tubular structure throughout the body, which has a complex network and contains multiple components. The function of the blood vessel is to nourish the living body and maintain homeostasis. The functional integrity of the endothelial cells is a vital factor in vascular homeostasis. The dysfunction of endothelium is a fundamental element in the progression of atherosclerosis [1]. Risk factors such as hypertension, heart failure and diabetes mellitus impair endothelial function. In addition, the factors outside of vessels can also influence the vascular system. The autonomic nervous system (ANS) is involved in mediating the behavior of the endothelial function. In the anatomical view of ANS, endothelial cells (ECs) do not receive direct SNS innervation due to the long distances [2]. However, Neurotransmitters released from varicosities in the perivascular plexus via autonomic neuroeffector junctions can reach endothelial receptors and regulate endothelial function [3]. In the development of vascular diseases, the co-existence of ANS abnormality and endothelial dysfunction suggests the complex interactions between them.
The autonomic nervous system
The autonomic nervous system, including sympathetic and parasympathetic nervous systems (SNS and PSNS), plays an essential role in maintaining physiological homeostasis and regulating the responses of the acute stress. The SNS and PSNS work antagonistically, synergistically, or independently to balance the functions of autonomic effector organs [4].
The sympathetic nervous system
The sympathetic nerve enables organisms to respond in a proper manner to destabilizations in either internal or external circumstances. Sympathetic regulation is a critical component of vascular tone and blood pressure, and plays a pivotal role in maintaining cardiovascular homeostasis and regular physiological functions. Extrinsic stimuli, such as stress, trauma, hemorrhage and pain, can elevate the sympathetic nerve activity, which can directly increase vascular resistance. Major arteries and precapillary arterioles are innervated by sympathetic nerves, but other vessels, such as venules, capillaries and collecting veins are rarely innervated [5].
The SNS can go through mass activation during different kinds of emotions to allow individuals to respond to stress, threats and danger [6]. The basal level activity of sympathetic nerve can maintain the arteriole tone, as sympathetic ganglionic blockade induces a fall in arterial pressure [7]. Abnormal activation of the sympathetic nerves leads to decreases in blood flow and remarkable vasoconstriction which is mediated by α-adrenoreceptors [8]. The renal sympathetic nerve is a major contributor to the complex pathogenesis of hypertension in both clinical studies and experimental investigation. In a meta-analysis study, plasma norepinephrine, an indirect marker of sympathetic tone, is significantly elevated in hypertensive patients compared with the age-matched normotensive subjects [9]. The renal ablation approach has been recently developed to control blood pressure in hypertension patients [10]. The SNS can also contribute to inflammation, leukocyte activation, oxidative stress, and increased level of chemokines and cytokines, by which the SNS can exert vital function in regulating the physiological and pathological state [8].
The parasympathetic nervous system
Similar to SNS, the parasympathetic nervous system (PSNS) innervates multiple organ systems and plays a critical role in a diverse array of physiological processes, such as inflammation, immune response, heart rate, gastrointestinal peristalsis and digestion. Vagus nerve (VN), extending throughout the body, is the largest nerve and main parasympathetic division of the autonomic nervous system. It starts from the medulla oblongata of brainstem and exits the cranium through the jugular foramen. The bilateral vagus nerve accompanied with carotid artery has multiple branches in the neck, chest and abdomen, respectively joining into the pharyngeal plexus, cardiac plexus, pulmonary plexus, esophageal plexus, hepatic plexus, and abdominal plexus to regulate the functions of vital organs. The right and left vagal nerves innervate the sinoatrial node and the atrioventricular node, respectively, to regulate the heart rate [11]. Vagus nerve involves both afferent neurons and efferent neurons. The sensory afferent neurons, accounting for 70-80% vagus nerve fibers [12], send organ status to the brain, while the motor efferent nerve integrates information delivered to the central nervous system and controls the peripheral effectors. The vagus nerve not only regulates gut physiology, but also controls the cardiovascular, respiratory, immune and endocrine systems [13]. In addition, the vagus nerve mediates cholinergic anti-inflammatory pathway, the inflammatory reflex that controls immune function, and pro-inflammatory responses during infection and injury [14-16]. The sensory afferent vagus nerve fibers detect peripheral inflammatory mediators, such as cytokines, released by activated macrophages and other immune cells, and conveys signals to the brainstem nuclei, which integrates the visceral sensory information and coordinates the autonomic function and visceral activity [17,18].
Peripheral vagal afferents can be activated by responding directly to endotoxin, bacterial lipopolysaccharide (LPS) and cytokines, such as interleukin-1 (IL-1), tumor necrosis factor (TNF), interleukin-6 (IL-6) and interferon-gamma (IFN-γ). IL-1 receptors are expressed on vagal afferents and glomus cells (chemosensory) adjacent to the vagus nerve endings which can be activated by inflammatory stimulation to regulate the immune responses [18,19]. Activating vagus nerve efferents can significantly suppress systemic pro-inflammatory cytokine levels in rodents with endotoxemia as the acetylcholine (Ach) released from the nerve terminals binds to the α-7 nicotinic acetylcholine receptor (α7nAChR) expressed on macrophages to modulate the immune system response [20]. α7nAChR, expressed in the nervous and immune systems, is important for mediating anti-inflammatory signaling by inhibiting NF-κB nuclear translocation and activating the JAK2/STAT3 pathway [21-23]. In addition to the role of vagus nervous system (VNS) in regulating systemic inflammation, it also plays an important role in ischemia/reperfusion, sepsis, epilepsy, hemorrhagic shock, migraine, etc. [24,25]. Both animal and human studies have suggested that the vagus nerve stimulation has a potential protective role in various diseases.
Innervation of blood vessels by the neurotransmitters from ANS
Complex interactions exist between the ANS and blood vessels, and the perivascular ANS can regulate the vascular wall contractility and tension. The nerve terminal varicosities release neurotransmitters which diffuse to and interact with the vascular cells, not in the manner of forming synapses with target cells. The varicosities are mobile and has no special post-junction structure. The neurotransmitters affect the cells at close junctions [3]. Stimulation of the perivascular ANS can induce the release of vasoactive mediators, such as norepinephrine (NE), adenosine triphosphate (ATP) and Neuropeptide Y (NPY) that cause vasoconstriction, and acetylcholine (Ach) and calcitonin gene-related peptide (CGRP), which cause vasodilation (Figure 1).
Figure 1.
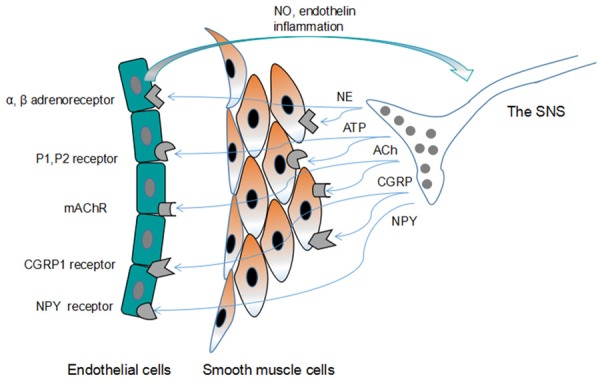
Schematic illustration of the innervation of blood vessels by the neurotransmitters from the autonomic nerve. The autonomic nerve terminal varicosities contain various neurotransmitters that are released and diffused to the effector cells (endothelial cells and smooth muscle cells). The effector cells express a variety of receptors that specifically interact with vasoactive factors from the nerve terminal. The endothelial cells also produce vasoactive factors NO and endothelin to influence the extent to which the ANS affects vascular tone. NE: norepinephrine; ATP: adenosine triphosphate; Ach: acetylcholine; CGRP: calcitonin gene-related peptide; NPY: neuropeptide Y; NO: nitric oxide.
NE
NE is a predominant endogenous neurotransmitter released mainly from the nerve terminals of sympathetic nerve and acts on different populations of adrenergic receptors [26]. Adrenoceptors includes α1, α2, β1, and β2 adrenoceptors. The α1 receptor mediates increase in intracellular calcium level, while α2 receptor inhibits intracellular cAMP by downregulating adenylate cyclase. Both β1 and β2 adrenoceptors can activate adenylate cyclase and increase the cAMP level [27]. NE released from the synaptic vesicle in response to stimulation can raise blood vessel pressure by the vasoconstrictive effects [28]. Activation of smooth muscle α1-adrenergic receptors contributes to smooth muscle cells contraction, resulting in vasoconstriction, while activation of endothelial α2-adrenergic receptors results in vasodilation by releasing NO [29-31]. The α2-adrenergic receptor-induced endothelial-dependent vasodilation can counteract the α1-adrenergic receptor-induced vasoconstriction on the smooth muscle cells [32,33]. Early studies showed that β-adrenergic receptors play a role in controlling the cardiac output and heart rate, but that they have little influence on the vascular resistance. Stimulation of the adrenergic nerve can blunt the cardiac functions with the deficiency of β1- and β2-adrenergic receptors in animal models [34]. But the other report showed that the β-adrenergic receptor activation on vascular smooth muscle cells contributes to vasodilation [30]. In addition, the blockade of β-adrenergic receptors may protect the endothelial cells from the elevation of sympathetic nerve activity in animal experiments [35]. A recent study showed that the β1-adrenergic-receptor antagonist metoprolol reduces reperfusion injury by targeting the haematopoietic compartment in acute myocardial infarction (AMI) patients [36].
ATP
It is well established that ATP is a major intracellular energy source. As an excitatory cotransmitter in autonomic nerves, however, ATP acts at the purinergic receptor (P2X and P2Y) to medicate the extracellular actions. The subtypes of purinergic receptors are P1 and P2 receptors. There are four P1 receptors (A1, A2A, A2B and A3) all binding with G proteins, and they are expressed on vascular endothelium and smooth muscle to mediate the vascular tone and smooth muscle proliferation. P2 receptors, based on signal transduction mechanisms and different molecular structure, are divided into ionotropic P2X receptors and G protein-coupled P2Y receptors [37]. To date, there are seven P2X receptor proteins (P2X1-P2X7) [38], and eight P2Y receptor proteins (P2Y1,2,4,6,11,12,13,14). The main receptors expressed on vascular smooth muscle cells are P2Y1, P2Y2, P2Y4 and P2Y6. Binding of ATP to these receptors activates phospholipase C by coupling to Gq/11 proteins and leads to elevation of intracellular calcium levels. Endothelial cells mainly express functional P2Y receptors, P2Y1 and P2Y2, and some vessels may also express P2Y4 and P2Y6 receptors [39,40].
NE and ATP are stored together in the same sympathetic vesicles. The earliest evidence showed that ATP was co-stored with NE in the perivascular sympathetic synaptic vesicles [41], and that co-release of ATP leads to vasoconstriction through P2X receptors, predominantly the P2X1 receptors, on smooth muscle cells [42]. Neurally released ATP, can produce a change in membrane potential, known as excitatory junction potentials (EJPs), mediates a rapid, transient depolarization of the smooth muscle cells via P2X receptors, and augments the influx of Ca2+ through L-type Ca2+ channels, leading to vasoconstriction [43]. Draid et al. reported that neurotransmitter ATP can be released from the terminal of perivascular nerve and acts on P2Y1 receptors of the endothelium, mediating hyperpolarization of the smooth muscle cells in chicken mesenteric artery [44]. The effect of the relative contribution of ATP and NE to artery appears to depend on the artery diameter. ATP via P2X1 receptors dominates in sympathetic control of vascular contractions in rat small mesenteric arteries, while NE via α1-adrenoceptors almost entirely mediates the response of large mesenteric arteries [45]. In addition, the contribution of ATP to vasoconstriction increases at higher pressure via purinergic activation of the vascular smooth muscle cells [43]. Above all, the contribution of ATP may be influenced by other factors to medicate the neurogenic vasoconstriction.
Ach
Ach, the first identified neurotransmitter, is released from parasympathetic nerves and induces smooth muscle cells contraction. The nicotinic acetylcholine receptors (nAChRs) have been divided into five subtypes: α, β, γ, δ and ε. The α class is composed of ten subunits (α1-α10), while the β class is composed of four subunits (β1-β4). Five subunits must be assembled to form a nAChR, whereas α7-α9 can form functional nAChRs as homooligomers [46]. The muscarinic acetylcholine receptors (mAChRs) are comprised of five related G protein-coupled receptors [47]. The vagus nerve may mediate cholinergic anti-inflammatory pathway and the inflammatory response, and the pro-inflammatory cytokines production can be modulated via nAChRs [16]. The mAChR family consists of five classes: M1 to M5. M1, M3 and M5 preferentially signal through the Gq/11 family of G proteins, whereas M2 and M4 are coupled to the G proteins of Gi/o family [47]. The mAChRs play an essential role in regulating heart rate, smooth muscle cells contraction and other fundamental physiological functions [48].
The cholinergic nerve endings innervate the muscular and endothelial layers of blood vessels [49]. It has been shown that the M3 AChRs on the endothelium mediate vasorelaxation by regulating the release of NO in arteries. In contrast, the activation of M2 and M3 receptors on smooth muscle cells by acetylcholine released from cholinergic nerve induces vascular contraction by inhibiting the NO production [50]. Another study showed that when M3 AChRs are inhibited by atropine on the endothelial cells, activation of nicotine receptors by Ach contributes to the endothelium-dependent relaxation through the PI3K/AKT pathway in the aorta of hypertensive animals [51]. Activation of the primary PSNS neurotransmitter acetylcholine receptors on endothelial cells induces vascular relaxation in human coronary arteries [52]. The cholinergic nerve innervation of vascular tone is a complicated process and needs to be further studied.
CGRP
CGRP, a 37-amino acid neuropeptide, is widely distributed in both central and peripheral nervous systems. It is usually found in the perivascular nerve, and often coexists with other peptides, such as substance P and neurokinin A [53]. It has been reported that CGRP is located in the perivascular region of human coronary veins and arteries to induce the vasodilation [54,55]. CGRP is the transcript alternatively spliced from the calcitonin/CGRP gene in a specific manner. CGRP mainly exists in two forms (α and β) acting on an uncommon receptor and the two forms possess similarly potent biological activity to induce vascular dilation [55]. The α-form is the widespread form of CGRP and β-form is synthesized in the enteric nerves. The structural similarity between β-form and α-form only differs in three amino acids [56]. Pharmacological analysis classifies the CGRP receptors into two subtypes, CGRP1 and CGRP2. CGRP1 is predominantly distributed in the cardiovascular system and has its own selective antagonist, while the ligands for CGRP2 receptors are lack of selectivity and the relevance of ligands to their receptors at molecular level is poorly understood [57]. There are three components for a functional CGRP receptor, calcitonin receptor-like receptor (CRLR) protein, a chaperone known as a single membrane spanning receptor activity modifying protein (RAMP1), and other receptor component protein [58]. The endothelium has been shown to express CGRP receptors [59], and the recent study showed that the endothelium expresses the functional CGRP receptors, the CRLR and RAMP1 protein components [60].
It was first reported that CGRP is released in the perivascular nerve terminal in rat mesenteric resistance arteries [61] and acts as a potent vasodilator neurotransmitter to induce vasodilation by binding to its receptor CGRP1 on the endothelium [59]. It had been already reported that, in the coronary and internal mammary arteries [62], thoracic aorta [63] and pulmonary arteries [64], CGRP can induce a potent vasodilatory action related to NO synthesis, an endothelium-derived relaxing factors. In addition, CGRP can also mediate an endothelium-independent vascular dilatation, which is involved in a cyclic adenosine monophosphate (cAMP)-dependent manner with the opening of potassium ATP (KATP) or large conductance Ca2+-activated potassium channels on SMCs [65,66]. Whereas Brain & Grant reported that CGRP does not play an essential role in the regulation of blood pressure at physiological condition, the level of CGRP changes in vascular disorders, such as migraine and Raynaud’s disease [57]. Together these findings indicated that CGRP is a potential neurotransmitter with vasodilator activity through its action on the cells in the vessel wall.
NPY
NPY is a 36 amino-acid neuropeptide widely distributed in the central nerves and the peripheral nerves. It is a sympathetic cotransmitter colocalized and released with NE in the SNS innervating the cardiovascular system [67,68]. NPY was initially shown to have a mild direct constriction effect on smooth muscle cells in the cerebral vasculature [69] but potentiated the NE-induced vasoconstriction powerfully in the isolated rabbit blood vessels [67]. The release of NPY results in endothelium-dependent constriction and proliferation of smooth muscle cells. NPY interacts with its receptors expressed on the endothelium and regulates angiogenesis by an autocrine signaling mechanism during tissue development [70]. NPY also plays a potential role in increasing leukocyte adhesion to endothelial cells [71]. The NPY antagonist PP56 has been shown to have an anti-inflammatory effects on both acute and chronic arthritis [72].
The effects of blood vessels on ANS
The endothelial cells line in the interior surface of blood vessels and play an important role in modulating structural and functional integrity of blood vessel. The main regulatory role of endothelium is to maintain the vascular tone, and the endothelium can also influence the extent to which the ANS affects vascular tone by vasoactive factors. NO, an important endothelium-derived relaxing factor, is generated from L-arginine by endothelial NO synthase, depending on cofactors such as tetra-hydrobiopterin [73]. Endothelin, a constriction factor released by endothelium, produces extremely powerful contraction in mammalian blood vessels in vitro [74]. Endothelial dysfunction e.g. inability of appropriate vasodilation, is closely related to the development of atherosclerosis. Before structural lesions, impaired endothelial responses can be observed in the early course of atherogenesis [75]. In pathological conditions, endothelial dysfunction may influence the ANS activity by modulating neurotransmitter release, reuptake, or receptor sensitivity. The inflammation accompanied with atherosclerosis is also associated with the dysfunction of sympathetic nerve.
The effect of NO and endothelin on ANS
In a healthy state, the endothelium and ANS work together to regulate vessel tone. The vasodilator and vasoconstrictor substances released from endothelial cells can maintain the vascular tone properly via affecting the activity of ANS. An earlier study reported that the release of NO by endothelium can blunt vasoconstrictive responses induced by α2-adrenergic nerve [31]. It has been shown that the endothelium inhibits neurogenic NE induced vasoconstriction in the presence of shear stress in isolated perfused rabbit carotid arteries, but the inhibition decreases by a guanylate cyclase inhibitor [76]. That suggested that endothelium under shear stress can modulate adrenergic vasoconstriction likely due to the production of NO [76]. A subsequent study reported that neurogenic vasoconstriction by NE is modulated by endothelial factor NO in the rat tail artery [77]. The endothelium may also control the metabolism of NE and provide a physical barrier of neurotransmitter diffusion into the blood vessel lumen [78]. In addition, NO not only inhibits the central but also the peripheral cardiac and vascular sympathetic activity [79]. ANS activity can also be influenced by endothelial constriction factors. The levels of endothelin can evoke different responses. Higher levels of endothelin increase the sensitivity of smooth muscle cells to NE and induce higher arterial pressure through enhanced vasoconstriction, while lower endothelin inhibits the sympathetic activity and results in slight decreases in arterial pressure through a suppression in vasoconstriction [80,81].
The effect of endothelial dysfunction on ANS
Emerging evidence implies an inability of endothelium on ANS in pathological conditions. It was reported that endothelial dysfunction damages the sympathetic activity and decreases the reuptake of NE into the sympathetic nerves to counteract the vasoconstriction factors in rabbit aorta [82]. Angiography induced coronary endothelial dysfunction can enhance the α-adrenergic agonist phenylephrine-dependent sympathetic constriction [83]. A clinical research reported that the elevated plasma von Willebrand Factor (vWF), representing the damage of endothelial cells, can predict the autonomic nerve deterioration. In addition, in young diabetic patients, the inability of endothelium may be responsible for the peripheral neuropathy [84]. The endothelial dysfunction can also enhance inflammatory responses to aggravate the damage of the ANS. Oxidative stress, as a factor linked with both endothelial and ANS function, causes sympathoexcitation via angiotensin II type 1 receptor (AT1R), and oral administration of AT1R blockers to reduce the level of oxidative stress can inhibit the sympathoexcitation in hypertensive rats [85]. Fructose-induced bioactive molecules involved in the increased oxidative stress and inflammatory parameters impair the cardiovascular autonomic modulation in hypertensive ovariectomized rats [86]. In the blood-brain barrier perivascular macrophages, the activation of cyclooxygenase 2 (COX-2), a blood-borne pro-inflammatory cytokine, generates prostaglandin E (2) that enters the brain to activate the sympathetic nerve [87]. In LPS-induced central inflammation, the production of cytokines and other inflammatory mediators was reported to elevate the renal sympathetic activity and blood pressure [88]. These studies suggest an important link between the SNS and inflammation in the abnormal condition.
The ANS and the vascular diseases
Hypertension
The sympathetic nervous system plays an important role in the pathogenesis of hypertension. Autonomic cardiovascular control is impaired in hypertension, leading to a reduction in the parasympathetic tone and an increase in the sympathetic influences to the heart and peripheral vessels. Cardiac output and systemic vascular resistance are the major effector components of neural blood pressure regulation [89].
There are several mechanisms that can explain individuals overdriven of sympathetic nerve with essential hypertension. First, an excessive adrenergic response, due to the environmental stimuli, initially lead to blood pressure variability followed by a sustained hypertensive state [90]. Second, hypoxia-induced chemoreceptor stimulation increases the influence of afferent sympathetic nerve fibers [9,91]. Additionally, a mutual excitatory influence between the sympathetic nervous system and the metabolic system is involved in cardiovascular dysregulation [92]. Early treatments for hypertension were ganglionic blocking agents and reserpine that can inhibit the uptake of catecholamines into the sympathetic synaptic vesicles [93]. Followed by other sympatholytic drugs, α- and β-adrenergic receptor antagonists were introduced to the clinic, but have disconcerting side effects. Clinical methods to decrease sympathetic nerve activity are bilateral renal nerve ablation by a catheter-based high-frequency current or ultrasound, which has been found to reduce hypertension [94]. In hypertension models, continuous electrical stimulation of the carotid sinus baroreceptors reduces sympathetic outflow and lowers blood pressure. An implantable carotid sinus stimulator (CVRx, Minneapolis, MN, USA) has been studied with resistant hypertension, but the primary efficacy was not reached [95]. Therefore, the future of this technology needs to be determined by an ongoing clinical trial.
Heart failure
The clinical manifestations of heart failure are characterized by autonomic imbalance with activation of sympathetic nerve and inhibition of the vagal nerve. The pathophysiology of heart failure is presumably a consequence of hemodynamic abnormalities related to the alteration in cardiac function and structure. The cardiac autonomic nervous system consists of sympathetic and vagus nerve, releasing different neurotransmitters and exert opposite, stimulatory and inhibitory effects on the cardiac tissue by adrenergic and muscarinic receptors. The sympathetic nerve originates mainly in the stellate traveling along the heart, and vagus nerve carries right and left vagus nerve extending to the heart and merging with sympathetic neurons to form cardiac plexus [96]. Overactivation of the SNS can lead to myocardial injury and alterations in cardiac loading and result in the increase of NE at the adrenergic nerve endings. Chronic sympathetic stimulation can lead to the increase in myocardial mass and enlargement of the left ventricular (LV) chamber by myocyte enlargement, interstitial growth and remodeling [97,98]. β-receptor blockade inhibiting the cardiac sympathetic drive has become a standard component of heart failure therapy through left ventricle dilation [99-101].
Diabetes mellitus
Diabetes mellitus is associated with both vascular and the ANS dysfunction, and the two may progress simultaneously. Type 2 diabetes mellitus (T2DM) is characterized by abnormal metabolic profiles. Abnormal ANS, which regulates energy balance, is implicated in the pathogenesis of T2DM [102]. The exaggerated sympathetic flow can downregulate β-adrenergic receptors in the peripheral nerve [103] and induce the SNS dysfunction to enhance energy consumption [104]. In addition, overactive sympathetic nerve may result in increased vascular resistance, heart rate and sodium retention [105]. Early community study focusing on the ANS and T2DM shows that the dysfunction of ANS may be associated with the T2DM development in healthy adults, which can also increase the risk of atherosclerosis [106]. It still remains unclear about the relationship between the activity of SNS and mortality in T2DM, but reducing the sympathetic activation may be a potential therapeutic strategy for T2DM.
Conclusion
This overview provides detailed information on the innervation of blood vessels by neurotransmitters from ANS and the effects of blood vessels on ANS. The neurotransmitters released from varicosities diffuse to the effectors mediating the function of the blood vessel. Perivascular ANS neurotransmitters, such as NE, ATP and NPY function as vasoactive mediators causing vascular contraction; while Ach and CGRP work antagonistically to induce vasodilatation. On the other hand, the vasodilator and vasoconstrictor substances NO and endothelin, released from blood vessel, can regulate the ANS activity in physiological conditions. Inflammatory factors derived from the blood vessel induce the imbalance of ANS in the pathological conditions. The abnormal activities of ANS are associated with several vascular diseases including hypertension, heart failure and diabetes mellitus. Although current studies suggest a complex interaction between the ANS and vascular system, the detailed mechanism of the interaction between them remains to be elucidated. Understanding the relationship between the ANS and vascular system may provide new and effective therapeutic strategies for vascular diseases.
Acknowledgements
This work was supported by Natural Science Foundation of China [grant 81620108001 and 91739302 to L.Z.] and the Priority Academic Program Development of Jiangsu Higher Education Institutions of China [to L.Z.].
Disclosure of conflict of interest
None.
References
- 1.Barton M, Haudenschild CC. Endothelium and atherogenesis: endothelial therapy revisited. J Cardiovasc Pharmacol. 2001;38(Suppl 2):S23–5. doi: 10.1097/00005344-200111002-00007. [DOI] [PubMed] [Google Scholar]
- 2.Burnstock G. Local mechanisms of blood flow control by perivascular nerves and endothelium. J Hypertens Suppl. 1990;8:S95–106. [PubMed] [Google Scholar]
- 3.Burnstock G. Non-synaptic transmission at autonomic neuroeffector junctions. Neurochem Int. 2008;52:14–25. doi: 10.1016/j.neuint.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Kenney MJ, Ganta CK. Autonomic nervous system and immune system interactions. Compr Physiol. 2014;4:1177–200. doi: 10.1002/cphy.c130051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruffolo RR Jr, Nichols AJ, Stadel JM, Hieble JP. Structure and function of alpha-adrenoceptors. Pharmacol Rev. 1991;43:475–505. [PubMed] [Google Scholar]
- 6.Wehrwein EA, Orer HS, Barman SM. Overview of the anatomy, physiology, and pharmacology of the autonomic nervous system. Compr Physiol. 2016;6:1239–78. doi: 10.1002/cphy.c150037. [DOI] [PubMed] [Google Scholar]
- 7.King AJ, Osborn JW, Fink GD. Splanchnic circulation is a critical neural target in angiotensin II salt hypertension in rats. Hypertension. 2007;50:547–56. doi: 10.1161/HYPERTENSIONAHA.107.090696. [DOI] [PubMed] [Google Scholar]
- 8.Navar LG. Physiology: hemodynamics, endothelial function, renin-angiotensin-aldosterone system, sympathetic nervous system. J Am Soc Hypertens. 2014;8:519–24. doi: 10.1016/j.jash.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malliani A, Pagani M, Pizzinelli P, Furlan R, Guzzetti S. Cardiovascular reflexes mediated by sympathetic afferent fibers. J Auton Nerv Syst. 1983;7:295–301. doi: 10.1016/0165-1838(83)90082-6. [DOI] [PubMed] [Google Scholar]
- 10.Tikkanen I, Lappalainen K. [Renal denervation in the treatment of hypertension] . Duodecim. 2013;129:825–32. [PubMed] [Google Scholar]
- 11.Puledda F, Goadsby PJ. Current approaches to neuromodulation in primary headaches: Focus on vagal nerve and sphenopalatine ganglion stimulation. Curr Pain Headache Rep. 2016;20:47. doi: 10.1007/s11916-016-0577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher JT, Brundage KL, Waldron MA, Connelly BJ. Vagal cholinergic innervation of the airways in newborn cat and dog. J Appl Physiol (1985) 1990;69:1525–31. doi: 10.1152/jappl.1990.69.4.1525. [DOI] [PubMed] [Google Scholar]
- 13.Browning KN, Verheijden S, Boeckxstaens GE. The vagus nerve in appetite regulation, mood, and intestinal inflammation. Gastroenterology. 2017;152:730–744. doi: 10.1053/j.gastro.2016.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–9. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 15.Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9:418–28. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pavlov VA, Tracey KJ. The vagus nerve and the inflammatory reflex--linking immunity and metabolism. Nat Rev Endocrinol. 2012;8:743–54. doi: 10.1038/nrendo.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goehler LE, Gaykema RP, Hansen MK, Anderson K, Maier SF, Watkins LR. Vagal immune-to-brain communication: a visceral chemosensory pathway. Auton Neurosci. 2000;85:49–59. doi: 10.1016/S1566-0702(00)00219-8. [DOI] [PubMed] [Google Scholar]
- 18.Marquette C, Linard C, Galonnier M, Van Uye A, Mathieu J, Gourmelon P, Clarençon D. IL-1beta, TNFalpha and IL-6 induction in the rat brain after partial-body irradiation: role of vagal afferents. Int J Radiat Biol. 2003;79:777–85. doi: 10.1080/09553000310001610998. [DOI] [PubMed] [Google Scholar]
- 19.Hansen MK, O’Connor KA, Goehler LE, Watkins LR, Maier SF. The contribution of the vagus nerve in interleukin-1beta-induced fever is dependent on dose. Am J Physiol Regul Integr Comp Physiol. 2001;280:R929–34. doi: 10.1152/ajpregu.2001.280.4.R929. [DOI] [PubMed] [Google Scholar]
- 20.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–62. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, Al-Abed Y, Wang H, Metz C, Miller EJ, Tracey KJ, Ulloa L. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10:1216–21. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 22.de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, Berthoud HR, Uematsu S, Akira S, van den Wijngaard RM, Boeckxstaens GE. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6:844–51. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 23.Pena G, Cai B, Liu J, van der Zanden EP, Deitch EA, de Jonge WJ, Ulloa L. Unphosphorylated STAT3 modulates alpha 7 nicotinic receptor signaling and cytokine production in sepsis. Eur J Immunol. 2010;40:2580–9. doi: 10.1002/eji.201040540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tecoma ES, Iragui VJ. Vagus nerve stimulation use and effect in epilepsy: what have we learned? Epilepsy Behav. 2006;8:127–36. doi: 10.1016/j.yebeh.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117:289–96. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Insel PA. Seminars in medicine of the Beth Israel Hospital, Boston. Adrenergic receptors--evolving concepts and clinical implications. N Engl J Med. 1996;334:580–5. doi: 10.1056/NEJM199602293340907. [DOI] [PubMed] [Google Scholar]
- 27.Schuller HM. Neurotransmitter receptor-mediated signaling pathways as modulators of carcinogenesis. Prog Exp Tumor Res. 2007;39:45–63. doi: 10.1159/000100045. [DOI] [PubMed] [Google Scholar]
- 28.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–46. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 29.Tesfamariam B, Weisbrod RM, Cohen RA. Cyclic GMP modulators on vascular adrenergic neurotransmission. J Vasc Res. 1992;29:396–404. doi: 10.1159/000158956. [DOI] [PubMed] [Google Scholar]
- 30.Guimaraes S, Moura D. Vascular adrenoceptors: an update. Pharmacol Rev. 2001;53:319–56. [PubMed] [Google Scholar]
- 31.Vanhoutte PM, Miller VM. Alpha 2-adrenoceptors and endothelium-derived relaxing factor. Am J Med. 1989;87:1S–5S. doi: 10.1016/0002-9343(89)90496-8. [DOI] [PubMed] [Google Scholar]
- 32.Cohen RA, Zitnay KM, Weisbrod RM, Tesfamariam B. Influence of the endothelium on tone and the response of isolated pig coronary artery to norepinephrine. J Pharmacol Exp Ther. 1988;244:550–5. [PubMed] [Google Scholar]
- 33.Cocks TM, Angus JA. Endothelium-dependent relaxation of coronary arteries by noradrenaline and serotonin. Nature. 1983;305:627–30. doi: 10.1038/305627a0. [DOI] [PubMed] [Google Scholar]
- 34.Chruscinski AJ, Rohrer DK, Schauble E, Desai KH, Bernstein D, Kobilka BK. Targeted disruption of the beta2 adrenergic receptor gene. J Biol Chem. 1999;274:16694–700. doi: 10.1074/jbc.274.24.16694. [DOI] [PubMed] [Google Scholar]
- 35.Skantze HB, Kaplan J, Pettersson K, Manuck S, Blomqvist N, Kyes R, Williams K, Bondjers G. Psychosocial stress causes endothelial injury in cynomolgus monkeys via beta1-adrenoceptor activation. Atherosclerosis. 1998;136:153–61. doi: 10.1016/s0021-9150(97)00202-5. [DOI] [PubMed] [Google Scholar]
- 36.Garcia-Prieto J, Villena-Gutiérrez R, Gómez M, Bernardo E, Pun-García A, García-Lunar I, Crainiciuc G, Fernández-Jiménez R, Sreeramkumar V, Bourio-Martínez R, García-Ruiz JM, Del Valle AS, Sanz-Rosa D, Pizarro G, Fernández-Ortiz A, Hidalgo A, Fuster V, Ibanez B. Neutrophil stunning by metoprolol reduces infarct size. Nat Commun. 2017;8:14780. doi: 10.1038/ncomms14780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–92. [PubMed] [Google Scholar]
- 38.Li M, Kawate T, Silberberg SD, Swartz KJ. Pore-opening mechanism in trimeric P2X receptor channels. Nat Commun. 2010;1:44. doi: 10.1038/ncomms1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burnstock G, Ralevic V. Purinergic signaling and blood vessels in health and disease. Pharmacol Rev. 2014;66:102–92. doi: 10.1124/pr.113.008029. [DOI] [PubMed] [Google Scholar]
- 40.Burnstock G. Purinergic regulation of vascular tone and remodelling. Auton Autacoid Pharmacol. 2009;29:63–72. doi: 10.1111/j.1474-8673.2009.00435.x. [DOI] [PubMed] [Google Scholar]
- 41.Burnstock G, Kennedy C. A dual function for adenosine 5’-triphosphate in the regulation of vascular tone. Excitatory cotransmitter with noradrenaline from perivascular nerves and locally released inhibitory intravascular agent. Circ Res. 1986;58:319–30. doi: 10.1161/01.res.58.3.319. [DOI] [PubMed] [Google Scholar]
- 42.Burnstock G. The fifth Heymans memorial lecture-Ghent, February 17, 1990. Co-transmission. Arch Int Pharmacodyn Ther. 1990;304:7–33. [PubMed] [Google Scholar]
- 43.Rummery NM, Brock JA, Pakdeechote P, Ralevic V, Dunn WR. ATP is the predominant sympathetic neurotransmitter in rat mesenteric arteries at high pressure. J Physiol. 2007;582:745–54. doi: 10.1113/jphysiol.2007.134825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Draid M, Shiina T, El-Mahmoudy A, Boudaka A, Shimizu Y, Takewaki T. Neurally released ATP mediates endothelium-dependent hyperpolarization in the circular smooth muscle cells of chicken anterior mesenteric artery. Br J Pharmacol. 2005;146:983–9. doi: 10.1038/sj.bjp.0706413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gitterman DP, Evans RJ. Nerve evoked P2X receptor contractions of rat mesenteric arteries; dependence on vessel size and lack of role of L-type calcium channels and calcium induced calcium release. Br J Pharmacol. 2001;132:1201–8. doi: 10.1038/sj.bjp.0703925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Graham A, Court JA, Martin-Ruiz CM, Jaros E, Perry R, Volsen SG, Bose S, Evans N, Ince P, Kuryatov A, Lindstrom J, Gotti C, Perry EK. Immunohistochemical localisation of nicotinic acetylcholine receptor subunits in human cerebellum. Neuroscience. 2002;113:493–507. doi: 10.1016/s0306-4522(02)00223-3. [DOI] [PubMed] [Google Scholar]
- 47.Fredriksson R, Lagerström MC, Lundin LG, Schiöth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63:1256–72. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- 48.Wess J, Eglen RM, Gautam D. Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat Rev Drug Discov. 2007;6:721–33. doi: 10.1038/nrd2379. [DOI] [PubMed] [Google Scholar]
- 49.Amiya E, Watanabe M, Komuro I. The relationship between vascular function and the autonomic nervous system. Ann Vasc Dis. 2014;7:109–19. doi: 10.3400/avd.ra.14-00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bolton TB, Lim SP. Action of acetylcholine on smooth muscle. Z Kardiol. 1991;80(Suppl 7):73–7. [PubMed] [Google Scholar]
- 51.Zou Q, Leung SW, Vanhoutte PM. Activation of nicotinic receptors can contribute to endothelium-dependent relaxations to acetylcholine in the rat aorta. J Pharmacol Exp Ther. 2012;341:756–63. doi: 10.1124/jpet.112.192229. [DOI] [PubMed] [Google Scholar]
- 52.van Zwieten PA, Hendriks MG, Pfaffendorf M, Bruning TA, Chang PC. The parasympathetic system and its muscarinic receptors in hypertensive disease. J Hypertens. 1995;13:1079–90. doi: 10.1097/00004872-199510000-00002. [DOI] [PubMed] [Google Scholar]
- 53.Lundberg JM, Franco-Cereceda A, Hua X, Hökfelt T, Fischer JA. Co-existence of substance P and calcitonin gene-related peptide-like immunoreactivities in sensory nerves in relation to cardiovascular and bronchoconstrictor effects of capsaicin. Eur J Pharmacol. 1985;108:315–9. doi: 10.1016/0014-2999(85)90456-x. [DOI] [PubMed] [Google Scholar]
- 54.Gulbenkian S, Saetrum Opgaard O, Ekman R, Costa Andrade N, Wharton J, Polak JM, Queiroz e Melo J, Edvinsson L. Peptidergic innervation of human epicardial coronary arteries. Circ Res. 1993;73:579–88. doi: 10.1161/01.res.73.3.579. [DOI] [PubMed] [Google Scholar]
- 55.Saetrum Opgaard O, Gulbenkian S, Bergdahl A, Barroso CP, Andrade NC, Polak JM, Queiroz e Melo JQ, Edvinsson L. Innervation of human epicardial coronary veins: immunohistochemistry and vasomotility. Cardiovasc Res. 1995;29:463–8. doi: 10.1016/0008-6363(96)88520-8. [DOI] [PubMed] [Google Scholar]
- 56.Rezaeian AH, Isokane T, Nishibori M, Chiba M, Hiraiwa N, Yoshizawa M, Yasue H. alphaCGRP and betaCGRP transcript amount in mouse tissues of various developmental stages and their tissue expression sites. Brain Dev. 2009;31:682–93. doi: 10.1016/j.braindev.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 57.Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev. 2004;84:903–34. doi: 10.1152/physrev.00037.2003. [DOI] [PubMed] [Google Scholar]
- 58.McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–9. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- 59.Hagner S, Haberberger R, Kummer W, Springer J, Fischer A, Böhm S, Göke B, McGregor GP. Immunohistochemical detection of calcitonin gene-related peptide receptor (CGRPR)-1 in the endothelium of human coronary artery and bronchial blood vessels. Neuropeptides. 2001;35:58–64. doi: 10.1054/npep.2000.0844. [DOI] [PubMed] [Google Scholar]
- 60.Boerman EM, Segal SS. Depressed perivascular sensory innervation of mouse mesenteric arteries with advanced age. J Physiol. 2016;594:2323–38. doi: 10.1113/JP270710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kawasaki H, Takasaki K, Saito A, Goto K. Calcitonin gene-related peptide acts as a novel vasodilator neurotransmitter in mesenteric resistance vessels of the rat. Nature. 1988;335:164–7. doi: 10.1038/335164a0. [DOI] [PubMed] [Google Scholar]
- 62.Raddino R, Pelà G, Manca C, Barbagallo M, D’Aloia A, Passeri M, Visioli O. Mechanism of action of human calcitonin gene-related peptide in rabbit heart and in human mammary arteries. J Cardiovasc Pharmacol. 1997;29:463–70. doi: 10.1097/00005344-199704000-00006. [DOI] [PubMed] [Google Scholar]
- 63.Gray DW, Marshall I. Human alpha-calcitonin gene-related peptide stimulates adenylate cyclase and guanylate cyclase and relaxes rat thoracic aorta by releasing nitric oxide. Br J Pharmacol. 1992;107:691–6. doi: 10.1111/j.1476-5381.1992.tb14508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wisskirchen FM, Burt RP, Marshall I. Pharmacological characterization of CGRP receptors mediating relaxation of the rat pulmonary artery and inhibition of twitch responses of the rat vas deferens. Br J Pharmacol. 1998;123:1673–83. doi: 10.1038/sj.bjp.0701783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nelson MT, Huang Y, Brayden JE, Hescheler J, Standen NB. Arterial dilations in response to calcitonin gene-related peptide involve activation of K+ channels. Nature. 1990;344:770–3. doi: 10.1038/344770a0. [DOI] [PubMed] [Google Scholar]
- 66.Standen NB, Quayle JM, Davies NW, Brayden JE, Huang Y, Nelson MT. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989;245:177–80. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- 67.Ekblad E, Edvinsson L, Wahlestedt C, Uddman R, Håkanson R, Sundler F. Neuropeptide Y co-exists and co-operates with noradrenaline in perivascular nerve fibers. Regul Pept. 1984;8:225–35. doi: 10.1016/0167-0115(84)90064-8. [DOI] [PubMed] [Google Scholar]
- 68.Hakanson R, Wahlestedt C, Ekblad E, Edvinsson L, Sundler F. Neuropeptide Y: coexistence with noradrenaline. Functional implications. Prog Brain Res. 1986;68:279–87. doi: 10.1016/s0079-6123(08)60244-7. [DOI] [PubMed] [Google Scholar]
- 69.Edvinsson L. Characterization of the contractile effect of neuropeptide Y in feline cerebral arteries. Acta Physiol Scand. 1985;125:33–41. doi: 10.1111/j.1748-1716.1985.tb07690.x. [DOI] [PubMed] [Google Scholar]
- 70.Taylor BK, Abhyankar SS, Vo NT, Kriedt CL, Churi SB, Urban JH. Neuropeptide Y acts at Y1 receptors in the rostral ventral medulla to inhibit neuropathic pain. Pain. 2007;131:83–95. doi: 10.1016/j.pain.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sung CP, Arleth AJ, Feuerstein GZ. Neuropeptide Y upregulates the adhesiveness of human endothelial cells for leukocytes. Circ Res. 1991;68:314–8. doi: 10.1161/01.res.68.1.314. [DOI] [PubMed] [Google Scholar]
- 72.Claxson A, Morris C, Blake D, Sirén M, Halliwell B, Gustafsson T, Löfkvist B, Bergelin I. The anti-inflammatory effects of D-myo-inositol-1.2. 6-trisphosphate (PP56) on animal models of inflammation. Agents Actions. 1990;29:68–70. doi: 10.1007/BF01964724. [DOI] [PubMed] [Google Scholar]
- 73.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–14. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 74.Davenport AP, Hyndman KA, Dhaun N, Southan C, Kohan DE, Pollock JS, Pollock DM, Webb DJ, Maguire JJ. Endothelin. Pharmacol Rev. 2016;68:357–418. doi: 10.1124/pr.115.011833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Neunteufl T, Katzenschlager R, Hassan A, Klaar U, Schwarzacher S, Glogar D, Bauer P, Weidinger F. Systemic endothelial dysfunction is related to the extent and severity of coronary artery disease. Atherosclerosis. 1997;129:111–8. doi: 10.1016/s0021-9150(96)06018-2. [DOI] [PubMed] [Google Scholar]
- 76.Tesfamariam B, Cohen RA. Inhibition of adrenergic vasoconstriction by endothelial cell shear stress. Circ Res. 1988;63:720–5. doi: 10.1161/01.res.63.4.720. [DOI] [PubMed] [Google Scholar]
- 77.Thorin E, Atkinson J. Modulation by the endothelium of sympathetic vasoconstriction in an in vitro preparation of the rat tail artery. Br J Pharmacol. 1994;111:351–7. doi: 10.1111/j.1476-5381.1994.tb14067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cohen RA, Weisbrod RM. Endothelium inhibits norepinephrine release from adrenergic nerves of rabbit carotid artery. Am J Physiol. 1988;254:H871–8. doi: 10.1152/ajpheart.1988.254.5.H871. [DOI] [PubMed] [Google Scholar]
- 79.Chowdhary S, Townend JN. Nitric oxide and hypertension: not just an endothelium derived relaxing factor! J Hum Hypertens. 2001;15:219–27. doi: 10.1038/sj.jhh.1001165. [DOI] [PubMed] [Google Scholar]
- 80.Nakamaru M, Tabuchi Y, Rakugi H, Nagano M, Ogihara T. Actions of endothelin on adrenergic neuroeffector junction. J Hypertens Suppl. 1989;7:S132–3. doi: 10.1097/00004872-198900076-00062. [DOI] [PubMed] [Google Scholar]
- 81.Tabuchi Y, Nakamaru M, Rakugi H, Nagano M, Higashimori K, Mikami H, Ogihara T. Effects of endothelin on neuroeffector junction in mesenteric arteries of hypertensive rats. Hypertension. 1990;15:739–43. doi: 10.1161/01.hyp.15.6.739. [DOI] [PubMed] [Google Scholar]
- 82.Sobey CG, Sozzi V, Woodman OL. Ischaemia/reperfusion enhances phenylephrine-induced contraction of rabbit aorta due to impairment of neuronal uptake. J Cardiovasc Pharmacol. 1994;23:562–8. doi: 10.1097/00005344-199404000-00007. [DOI] [PubMed] [Google Scholar]
- 83.Treasure CB, Manoukian SV, Klein JL, Vita JA, Nabel EG, Renwick GH, Selwyn AP, Alexander RW, Ganz P. Epicardial coronary artery responses to acetylcholine are impaired in hypertensive patients. Circ Res. 1992;71:776–81. doi: 10.1161/01.res.71.4.776. [DOI] [PubMed] [Google Scholar]
- 84.Plater ME, Ford I, Dent MT, Preston FE, Ward JD. Elevated von Willebrand factor antigen predicts deterioration in diabetic peripheral nerve function. Diabetologia. 1996;39:336–43. doi: 10.1007/BF00418350. [DOI] [PubMed] [Google Scholar]
- 85.Kishi T. Regulation of the sympathetic nervous system by nitric oxide and oxidative stress in the rostral ventrolateral medulla: 2012 Academic Conference Award from the Japanese Society of Hypertension. Hypertens Res. 2013;36:845–51. doi: 10.1038/hr.2013.73. [DOI] [PubMed] [Google Scholar]
- 86.Conti FF, Brito Jde O, Bernardes N, Dias Dda S, Sanches IC, Malfitano C, Llesuy SF, Irigoyen MC, De Angelis K. Cardiovascular autonomic dysfunction and oxidative stress induced by fructose overload in an experimental model of hypertension and menopause. BMC Cardiovasc Disord. 2014;14:185. doi: 10.1186/1471-2261-14-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu Y, Zhang ZH, Wei SG, Serrats J, Weiss RM, Felder RB. Brain perivascular macrophages and the sympathetic response to inflammation in rats after myocardial infarction. Hypertension. 2010;55:652–9. doi: 10.1161/HYPERTENSIONAHA.109.142836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang ZH, Yu Y, Wei SG, Felder RB. Centrally administered lipopolysaccharide elicits sympathetic excitation via NAD(P)H oxidase-dependent mitogen-activated protein kinase signaling. J Hypertens. 2010;28:806–16. doi: 10.1097/HJH.0b013e3283358b6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grassi G, Ram VS. Evidence for a critical role of the sympathetic nervous system in hypertension. J Am Soc Hypertens. 2016;10:457–66. doi: 10.1016/j.jash.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 90.Julius S, Sanchez R, Malayan S, Hamlin M, Elkins M, Brant D, Bohr DF. Sustained blood pressure elevation to lower body compression in pigs and dogs. Hypertension. 1982;4:782–8. doi: 10.1161/01.hyp.4.6.782. [DOI] [PubMed] [Google Scholar]
- 91.Trzebski A. Arterial chemoreceptor reflex and hypertension. Hypertension. 1992;19:562–6. doi: 10.1161/01.hyp.19.6.562. [DOI] [PubMed] [Google Scholar]
- 92.Mancia G, Grassi G, Giannattasio C, Seravalle G. Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension. 1999;34:724–8. doi: 10.1161/01.hyp.34.4.724. [DOI] [PubMed] [Google Scholar]
- 93.Sorota S. The sympathetic nervous system as a target for the treatment of hypertension and cardiometabolic diseases. J Cardiovasc Pharmacol. 2014;63:466–76. doi: 10.1097/FJC.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 94.Schmieder RE, Redon J, Grassi G, Kjeldsen SE, Mancia G, Narkiewicz K, Parati G, Ruilope L, van de Borne P, Tsioufis C. ESH position paper: renal denervation - an interventional therapy of resistant hypertension. J Hypertens. 2012;30:837–41. doi: 10.1097/HJH.0b013e328352ce78. [DOI] [PubMed] [Google Scholar]
- 95.Bisognano JD, Bakris G, Nadim MK, Sanchez L, Kroon AA, Schafer J, de Leeuw PW, Sica DA. Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension: results from the double-blind, randomized, placebo-controlled rheos pivotal trial. J Am Coll Cardiol. 2011;58:765–73. doi: 10.1016/j.jacc.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 96.Florea VG, Cohn JN. The autonomic nervous system and heart failure. Circ Res. 2014;114:1815–26. doi: 10.1161/CIRCRESAHA.114.302589. [DOI] [PubMed] [Google Scholar]
- 97.Babick A, Elimban V, Zieroth S, Dhalla NS. Reversal of cardiac dysfunction and subcellular alterations by metoprolol in heart failure due to myocardial infarction. J Cell Physiol. 2013;228:2063–70. doi: 10.1002/jcp.24373. [DOI] [PubMed] [Google Scholar]
- 98.Colucci WS. The effects of norepinephrine on myocardial biology: implications for the therapy of heart failure. Clin Cardiol. 1998;21(Suppl 1):I20–4. doi: 10.1002/clc.4960211305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cohn JN, Pfeffer MA, Rouleau J, Sharpe N, Swedberg K, Straub M, Wiltse C, Wright TJ MOXCON Investigators. Adverse mortality effect of central sympathetic inhibition with sustained-release moxonidine in patients with heart failure (MOXCON) Eur J Heart Fail. 2003;5:659–67. doi: 10.1016/s1388-9842(03)00163-6. [DOI] [PubMed] [Google Scholar]
- 100.Doherty NE 3rd, Seelos KC, Suzuki J, Caputo GR, O’Sullivan M, Sobol SM, Cavero P, Chatterjee K, Parmley WW, Higgins CB. Application of cine nuclear magnetic resonance imaging for sequential evaluation of response to angiotensin-converting enzyme inhibitor therapy in dilated cardiomyopathy. J Am Coll Cardiol. 1992;19:1294–302. doi: 10.1016/0735-1097(92)90337-m. [DOI] [PubMed] [Google Scholar]
- 101.Hall SA, Cigarroa CG, Marcoux L, Risser RC, Grayburn PA, Eichhorn EJ. Time course of improvement in left ventricular function, mass and geometry in patients with congestive heart failure treated with beta-adrenergic blockade. J Am Coll Cardiol. 1995;25:1154–61. doi: 10.1016/0735-1097(94)00543-y. [DOI] [PubMed] [Google Scholar]
- 102.Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–71. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 103.Bohm M, Flesch M, Schnabel P. Beta-adrenergic signal transduction in the failing and hypertrophied myocardium. J Mol Med (Berl) 1997;75:842–8. doi: 10.1007/s001090050175. [DOI] [PubMed] [Google Scholar]
- 104.Huggett RJ, Scott EM, Gilbey SG, Bannister J, Mackintosh AF, Mary DA. Disparity of autonomic control in type 2 diabetes mellitus. Diabetologia. 2005;48:172–9. doi: 10.1007/s00125-004-1601-6. [DOI] [PubMed] [Google Scholar]
- 105.Perin PC, Maule S, Quadri R. Sympathetic nervous system, diabetes, and hypertension. Clin Exp Hypertens. 2001;23:45–55. doi: 10.1081/ceh-100001196. [DOI] [PubMed] [Google Scholar]
- 106.Carnethon MR, Golden SH, Folsom AR, Haskell W, Liao D. Prospective investigation of autonomic nervous system function and the development of type 2 diabetes: the atherosclerosis risk in communities study, 1987-1998. Circulation. 2003;107:2190–5. doi: 10.1161/01.CIR.0000066324.74807.95. [DOI] [PubMed] [Google Scholar]


