Abstract
Background: The effect of the concomitant use of sodium benzoate (NaB) and ascorbic acid on human health remains controversial. Therefore, the current study is designed to investigate the effect of NaB and ascorbic acid on the testicular function of adult Wistar rats. Methods: Adult Wistar rats were randomly allotted into Control (vehicle; received 1 ml of distilled water), NaB-treated (SB-treated; received 100 mg/kg body weight; b.w), ascorbic acid-treated (AA-treated; received 150 mg/kg b.w) and NaB+ ascorbic acid-treated (SB+AA-treated) groups. The treatment lasted for 28 days and the administration was given orally. The body weight change was monitored. Semen analysis, biochemical assay and histological examination were performed. Results: Treatment with NaB significantly altered the cytoarchitecture of testicular tissue, sperm quality, testicular endocrine function and oxidative stress status without any alteration in body weight gain compared to control. In addition, treatment with NaB+ ascorbic acid exacerbated testicular tissue disruption, impaired sperm quality and testicular endocrine impairment with significant reduction in oxidative stress and unaltered body weight gain when compared with NaB-treated group. Conclusion: This study suggests that ascorbic acid and NaB synergistically aggravates testicular dysfunction. This is independent of oxidative stress status.
Keywords: Antioxidant, ascorbic acid, oxidative stress, sodium benzoate, testicular function
Introduction
Ascorbic acid (AA) or vitamin C is a water soluble vitamin that can be found as a white or slightly yellow crystal or powder with a minor acidic taste [1,2]. In humans its deficiency has been associated with a wide spectrum of clinical manifestations such as scurvy, which is a lethal condition unless appropriately treated [3]. The antioxidant role of AA in maintaining motor abilities has been well documented [4]. It has been shown that AA elicits its antioxidant effect through donation of electrons and protection of other compounds from oxidation. In this process, vitamin C itself is oxidized but unreactive, and therefore ascorbic acid has been described as a good free radical scavenger [5]. Several studies in human have demonstrated the effects of vitamin C on intestinal iron absorption, reduction of harmful oxidants in the stomach and vascular responsiveness. These effects have been reported to be mediated by the antioxidant actions of vitamin C [6], which may play a role in iron absorption, gastric cancer prevention, vascular disease and hypertension [7]. Vitamin C can also protect and stimulate biosynthesis of endothelial nitric oxide (NO), which is important for vascular relaxation [8,9].
Vitamin C appears to have beneficial effects on erectile and testicular functions in healthy and unhealthy subjects with reproductive problems [10]. Some evidence suggests that erectile and testicular dysfunction is associated with low plasma vitamin C concentrations [11]. Previous study has also shown that vitamin C present in fruits and vegetables may protect NO from oxidation and ameliorate erectile and testicular dysfunctions [12]. This accounts for protective role of fruits and vegetables on reproductive organs [13]. It has been demonstrated that vitamin C restored acute hyperglycemia-induced testicular dysfunction in healthy subjects [14,15]. In addition, a number of studies suggests vitamin C as a powerful antioxidant in biological systems in vitro, and protects organism against external and internal oxidants [3,16]. In fact, ascorbic acid remains essential for the structural and functional integrity of androgen-dependent reproductive organs [15,17].
One of the most widely used preservatives and antimicrobial agents in a variety of products, such as salads, pickles, carbonated drinks, jams, fruit juices and sauces is sodium benzoate (NaB) [18]. NaB is the salt form of benzoic acid that is used as preservative agent in medications, cosmetic compounds and shampoos [18]. NaB, at 250 mg/kg body weight (i.v) is used as part of the treatment of hyperammonaemia that occurs as an inborn error in the urea cycle. It is also considered effective in reducing plasma-glycine in non-ketotic hyperglycemia, although it may not be effective in preventing mental retardation [19]. Also, a recent study shows that NaB may be beneficial as an add-on therapy (1 gm/day) in schizophrenia [20]. However, its concentration as preservative has been limited by Food and Drug administration (FDA) to 0.1% by weight [21,22] and is allowed as an animal food additive up to 0.1% according to the World Health Organization (WHO) official publication [23]. It has been shown non-toxic in its organic form but its synthetic form at a chronic dose is toxic to living organisms [24].
Available evidence shows that exposure of NaB to AA may form benzene, a known carcinogen [25,26], but most of the beverages that tested higher for benzene have been reformulated and subsequently tested below the safety limit [26]. However, heat, light and shelf life can increase the rate at which benzene is formed [25]. Benzene has been reported to cause anaemia, severe depression of the immune system which produces generalized allergy symptoms, leukemia, various other blood cancers, pre-cancerous blood conditions and may interfere with reproductive function [25]. NaB has been reported to decrease plasma level of testosterone, gonadotrophins, thyroid hormones; T3 and T4 [27]. Also, recent study shows that co-treatment with NaB and AA leads to alteration of the structure of cerebellum [28]. However, the effect of NaB exposure to AA on testicular function is not known. Therefore, this study is designed to elucidate the effect of co-treatment with NaB and AA on testicular function in adult Wistar rats.
Materials and methods
Chemicals
The entire chemicals and reagents were of AR grade, which were obtained from Sigma Chemical, St. Louis, MO, USA.
Animals, grouping and protocol
Thirty adult male Wistar rats weighing 190-220 g were obtained from the animal house, College of Medicine and Health Sciences, University of Ilorin, Ilorin, Kwara State, Nigeria. The rats were housed in cages and maintained in a ventilated room at 25±2°C, on a 12-h light/12-h dark cycle. Unrestricted access to standard rat chow and water were ensured. After two week of acclimatization, the rats were allotted into groups (n=5 each); Control (vehicle; received 1 ml of distilled water), NaB-treated (SB-treated; received 100 mg/kg b.w), ascorbic acid-treated (AA-treated; received 150 mg/kg b.w) and NaB+ ascorbic acid-treated (SB+AA-treated). The treatment lasted for 28 days and the administration was given orally. The procedures were approved by the institutional review ethical committee of University of Ilorin, in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Initial and final body weights were monitored using animal weighing balance (Olympia SCL66110 model, Kent Scientific Corporation, Torrington, CT06790, USA) and the body weight gain was estimated.
Sample preparation and biochemical analysis
After the treatment, the rats were anesthetized using pentobarbital sodium (50 mg/kg, i.p). Blood was collected from the apex of the heart into heparinized bottle and centrifuged at 3000 rpm for 15 minutes using a bench centrifuge and the plasma was extracted for analysis. Biochemical analysis of plasma malondialdehyde (MDA) and superoxide dismutase (SOD) were measured by standardized enzymatic colorimetric methods using assay kit from Randox Laboratory Ltd. (Co. Antrim, UK) and hypophyseal-gonadotropic hormones (Follicle stimulating hormone; FSH, Luteinizing hormone; LH and testosterone; TT) were measured with enzyme-linked immunosorbent assay (ELISA) kits from Fortress diagnostics Ltd (Antrim, UK).
Measurement of sperm concentration
The epididymis was removed and minced in 5 ml of normal saline, placed in a rocker for 10 minutes and allowed to incubate for 2 minutes at room temperature. The supernatant was diluted after incubation at 1:100 with a solution containing sodium bicarbonate and 1 ml formalin (35%). The new improved Neuber’s counting chamber (hematocytometer) was used in counting the total number of spermatozoa. About 10 µl of the diluted sperm suspension was transferred to each counting chamber of the hematocytometer and observed under a light microscope.
Measurement of sperm motility
The fluid from the caudal epididymis was diluted with Tris buffer solution [29] to 0.5 ml. An aliquot of the solution was then observed under the light microscope. The mean motility estimation was reported as the final score for each sample. The score was done by calculating motile spermatozoa per unit area and was expressed as percentage motility.
Sperm morphology
The morphology of the spermatozoa was determined using the original dilution for motility, diluted 1:20 with 10% neutral buffered formalin. The sperm cells were categorized based on the presence of one or more abnormal features such as tail defects (short, irregular coiled or multiple tail), neck and middle piece defects (distended, irregular, bent or abnormally thin middle piece), and head defects (small, large, double or detached head). Findings were expressed as percentage of morphologically normal sperm [30].
Sperm progressivity
The sperm progressivity was determined by subjecting grading system as described by world health organization (2005) (Grade): A; excellent forward directional movement, B; good forward directional movement, C; fair forward directional movement, D; poor forward directional movement.
Histology
Testes were excised, blotted and fixed in 10% buffered formaldehyde overnight, dehydrated and embedded in paraffin. The paraffin-embedded samples were sectioned at 5 μm thickness. Haematoxylin and eosin (H&E) staining technique was used and the slides were examined using light microscopy [31].
Morphometric analysis
The prepared testicular tissues were used for histomorphometric analysis and cross section, lumen and germinal epithelia diameters were quantified using a computer software Image-J (National Institute of Health, USA).
Statistical analysis
Data were expressed as means ± SEM. Group analysis was performed with SPSS, version 22 of statistical software. One-way analysis of variance (ANOVA) was used to compare the means of variables among the groups. Bonferroni’s test was used to identify the significance of pair wise comparison of mean values and significant differences were accepted at P<0.05.
Results
Ascorbic acid does not alter body weight in rats treated with sodium benzoate
There was no significant change in body weight gain of SB-treated group, but body weight gain significantly increased in AA-treated group when compared with control group. However, concomitant treatment with ascorbic acid during administration of sodium benzoate did not alter body weight gain when compared with control and SB-treated groups (Figure 1).
Figure 1.
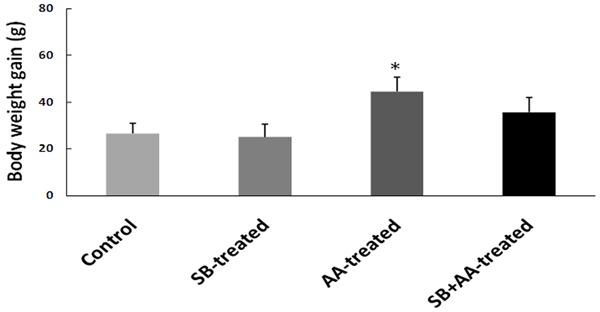
Effect of ascorbic acid and sodium benzoate treatment on body weight of adult Wistar rats. Data are expressed as mean ± S.E.M. n=5. Data were analysed by one-way ANOVA followed by Bonferroni post hoc test. (*P<0.05 vs Control, #P<0.05 vs SB-treated).
Ascorbic acid exacerbates testicular tissue disruption in rats treated with sodium benzoate
Histological changes in the testes have been reported to alter sperm quality and function [32]. The photomicrographs of a section of testis showed deleterious basement membrane, distorted spermatogenic cell and seminiferous tubule with lumen vacuolation in SB-treated group (Figure 2B), showed normal testicular tissue with hyperplasia of sertoli cells in AA-treated group (Figure 2C) and while deleterious lumen, disrupted basement membrane, hyperplasia of sertoli cells, disruption of spermatogenic cells and degeneration of interstitium were observed in SB+AA treated group (Figure 2D). Histomorphometric parameters also showed significant increase in lumen diameter, germinal epithelia diameter and significant reduction in cross section diameter in SB+AA-treated rats compared to control and SB-treated rats (Figure 3).
Figure 2.
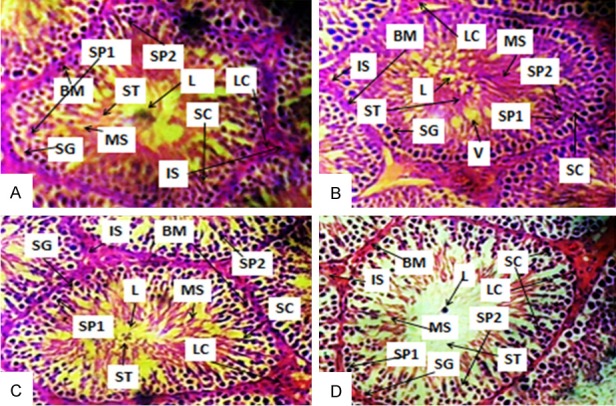
Photomicrographs of a section of testis of Control (A), BS- (B), AA- (C) and BS+AA-treated rats (D). SG-Spermatogonium, L-Lumen, SP1-Primary spermatocyte, SP2-Secondary spermatocyte, BM-Basement membrane, MS-Mature spermatocyte, LC-Leydig cells, SC-Sertoli cells, ST-Spermatids and IS-Interstitium, V-Vacuole. (A) Showed normal testicular tissue; (B) Showed deleterious basement membrane, distorted spermatogenic cell and seminiferous tubule with lumen vacuolation; (C) Showed normal testicular tissue with hyperplasia of sertoli cells and (D) showed deleterious lumen, disrupted basement membrane, hyperplasia of sertoli cells, disruption of spermatogenic cells and degeneration of interstitium. (H&E paraffin stain; ×400, transverse section).
Figure 3.

Effect of ascorbic acid and sodium benzoate treatment on testicular histomorphometry parameters: Cross section diameter (A), Lumen diameter (B), Germinal epithelia diameter (C) in adult Wistar rats. Data are expressed as mean ± S.E.M. n=5. Data were analysed by one-way ANOVA followed by Bonferroni post hoc test. (*P<0.05 vs Control, #P<0.05 vs SB-treated).
Ascorbic acid worsens sperm quality in rats treated with sodium benzoate
As indicated in Table 1, treatment with sodium benzoate significantly decreased sperm count and viability ratio when compared with control group. In addition, concomitant treatment with ascorbic acid worsened sperm count and viability ratio when compared with control and SB-treated group. Also, sperm motility and progressivity were not significantly altered in SB-treated group when compared with control. However, treatment with ascorbic acid and sodium benzoate significantly impaired sperm motility (P<0.05) and progressivity (C) when compared with control and SB-treated rats.
Table 1.
Effect of ascorbic acid and sodium benzoate treatment on sperm count, motility, morphology, progressivity and viability ratio of spermatozoa
| Group | Control | SB-treated | AA-treated | SB+AA-treated |
|---|---|---|---|---|
| Sperm count (×106/mL) | 82.05±4.50 | 60.43±4.10* | 85.80±9.20 | 46.30±3.09*,# |
| Sperm motility (%) | 89.30±5.00 | 70.24±7.50 | 90.70±4.50 | 54.64±6.05*,# |
| Sperm morphology (% Normal) | 79.98±6.09 | 62.51±09.50 | 82.75±8.20 | 58.50±3.20* |
| Sperm progressivity (Grade) | A | B | A | C |
| Viability ratio (%) | 83.34±5.20 | 64.74±4.00* | 97.52±6.40 | 50.60±5.30*,# |
Data are expressed as mean ± S.E.M. n=5. Data were analysed by one-way ANOVA followed by Bonferroni post hoc test.
P<0.05 vs Control;
P<0.05 vs SB-treated.
For sperm progressivity (Grade): A; excellent forward directional movement, B; good forward directional movement, C; fair forward directional movement, D; poor forward directional movement.
Ascorbic acid impairs testicular endocrine function in rats treated with sodium benzoate
Administration of sodium benzoate led to a significant decrease in the circulating levels of FSH and LH with no alteration in the level of testosterone (TT) compared with control group. However, circulating levels of FSH, LH and TT were significantly reduced in SB+AA-treated group when compared with control and SB-treated groups (Figure 4).
Figure 4.
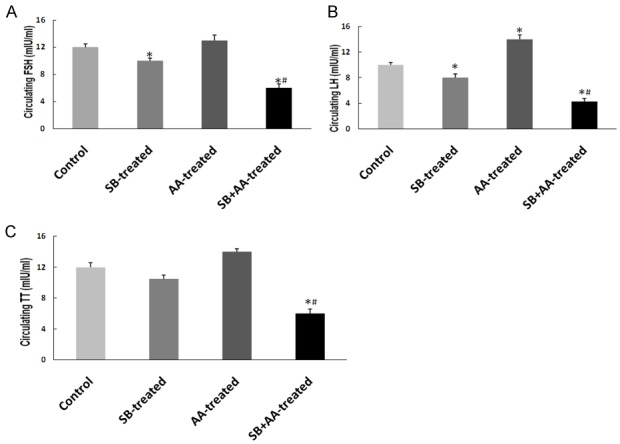
Effect of ascorbic acid and sodium benzoate treatment on circulating FSH (A), LH (B) and TT (C) in adult Wistar rats. Data are expressed as mean ± S.E.M. n=5. Data were analysed by one-way ANOVA followed by Bonferroni post hoc test. (*P<0.05 vs Control, #P<0.05 vs SB-treated).
Ascorbic acid reduces oxidative stress in rats treated with sodium benzoate
Plasma malondialdehyde (MDA) and superoxide dismutase (SOD) are biomarkers of oxidative stress. Administration of sodium benzoate led to a significant increase in plasma MDA and significant decrease in plasma SOD when compared with control group. However, the level of plasma MDA was significantly reduced while the level of plasma SOD was unaltered in SB+AA-treated group when compared with SB-treated group (Figure 5).
Figure 5.
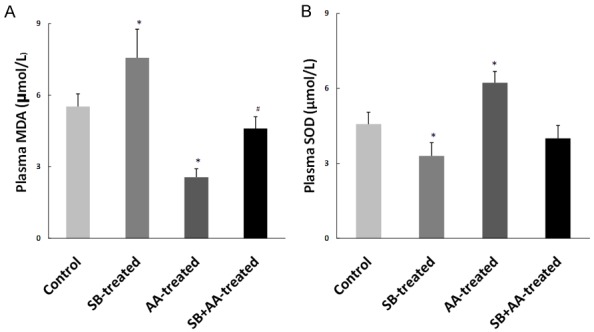
Effect of ascorbic acid and sodium benzoate treatment on circulating level of plasma MDA and SOD in adult Wistar rats. Data are expressed as mean ± S.E.M. n=5. Data were analysed by one-way ANOVA followed by Bonferroni post hoc test. (*P<0.05 vs Control, #P<0.05 vs SB-treated).
Discussion
The present study revealed that administration of NaB significantly altered testicular spermatogenic and endocrine functions, which was associated with increased plasma MDA and decreased plasma SOD without significantly altered body weight gain when compared with control group. In addition, concomitant treatment with ascorbic acid significantly exacerbated testicular spermatogenic and endocrine dysfunctions, which was associated with significant reduction in plasma MDA and unaltered level of plasma SOD when compared with SB-treated group. However, there were no significant changes in body weight gain and plasma MDA and SOD in SB+AA-treated group when compared with control group.
The present finding that treatment with NaB did not alter the body weight gain when compared with control is consistent with a previous study [27], but contrary to recent study by Priya et al., who observed a dose and time related significant changes in the body weight gain in rats treated with NaB compared to control group [33]. We found that the body weight gain was significantly increased in AA-treated rats (Figure 1), which is consistent with previous study that administration of ascorbic acid significantly increased body weight [34]. However, concomitant treatment with ascorbic acid and NaB did not alter the body weight gain when compared with control and SB-treated groups. This implies that the alteration of testicular spermatogenic and endocrine functions is independent of body weight change.
Previous studies have shown that NaB altered the structural architecture of the testis which was evident in disruption of seminiferous tubule and spermatogenic process [27]. The photomicrographs of a section of testis in the present study corroborated earlier observation by showing deleterious basement membrane, distorted spermatogenic cell and seminiferous tubule with lumen vacuolation (Figure 2B), which accounts for significant reduction in sperm counts, viability and progressivity when compared with control group (Table 1), where as the cytoarchitecture of testicular histology was preserved in AA-treated group (Figure 2C). However, co-treatment with ascorbic acid and NaB revealed deleterious lumen, disrupted basement membrane, hyperplasia of sertoli cells, disruption of spermatogenic cells and degeneration of interstitium (Figure 2D), which was associated with significant increase in lumen diameter, germinal epithelia diameter and significant reduction in cross section diameter (Figure 3). These worsened the sperm quality by showing significant reduction in sperm counts, viability ratio and progressivity when compared with control and SB-treated groups. Histological changes of testicular tissue have been documented to reveal impaired sperm quality and function [32]. The present finding implies that co-treatment with ascorbic acid and NaB aggravates testicular spermatogenic dysfunction. This finding provides first evidence to the effect of ascorbic acid and NaB exposure on testicular function.
The importance of pituitary-testicular axis in male reproductive functions cannot be overstated. Testosterone which is regulated by LH from anterior pituitary gland plays a vital role in final maturation of spermatozoon and while FSH is needed for the maintenances of the gametogenic function of the testis [35]. The results of this study showed significant reduction in FSH, LH and testosterone in rats that were exposed to NaB (Figure 4), which is consistent with previous observations [27]. The possibility of the low levels of plasma FSH and LH concentration following NaB exposure in this study may be due to increased oxidative stress. Oxidative stress may suppress the sensitivity of the gonadotrophic cells to gonadotropin-releasing hormone and, therefore, may prevent gonadotropin secretion [36]. Inhibition of FSH and LH by NaB may be a result of its negative effect on central nervous system that can inhibit the neural stimulus essential for the release of pituitary gonadotrophins [37], leading to a lack of pituitary gonadotrophins essential for initiating and completing spermatogenesis and steroidogenesis in the testis [38]. In this regard Sohrabi et al., reported many alterations attributed to the direct cytotoxic effects of NaB leading to decrease testosterone synthesis [27]. The results of this study revealed that ascorbic acid significantly worsened the plasma levels of FSH, LH and testosterone in SB+AA-treated group. The significant reduction in FSH, LH and testosterone levels observed in SB+AA-treated rats when compared with control and SB-treated groups is not associated with oxidative stress status. Ascorbic acid, a potent antioxidant did not ameliorate NaB-induced testicular dysfunction. Rather it aggravates testicular dysfunction which is possibly not mediated by the oxidative stress. This implies that ascorbic acid with its anti-oxidant property [3] did not prevent the depressive effects of NaB on the hypothalamus that secrete gonadotrophin which controls pituitary gonadotrophes. These results were contrary to Fernandes et al.; Obianime and Roberts [39,40] who earlier reported that ascorbic acid supplement reverted FSH, LH and testosterone secretion in hyperglycemic and cadmium-induced toxicity in male rats. Hence, the present result suggests that concomitant treatment with ascorbic acid and NaB aggravates testicular spermatogenic and endocrine dysfunctions.
Conclusion
The present study suggests that ascorbic acid and NaB synergistically aggravates testicular dysfunction. This is independent of oxidative stress status. Future studies on the effect of ascorbic acid on NaB exposure should be targeted on the possible mechanism of action.
Disclosure of conflict of interest
None.
References
- 1.Nishikimi M, Yagi K. Biochemistry and molecular biology of ascorbic acid biosynthesis. Subcell Biochem. 1996;25:17–39. doi: 10.1007/978-1-4613-0325-1_2. [DOI] [PubMed] [Google Scholar]
- 2.Lachapelle MY, Drouin G. Inactivation dates of the human and guinea pig vitamin C genes. Genetica. 2010;139:199–207. doi: 10.1007/s10709-010-9537-x. [DOI] [PubMed] [Google Scholar]
- 3.Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, Chen S, Corpe C, Dutta A, Dutta SK, Levine M. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr. 2003;22:18–35. doi: 10.1080/07315724.2003.10719272. [DOI] [PubMed] [Google Scholar]
- 4.Harrison FE, Yu SS, Van Den Bossche KL, Li L, May JM, McDonald MP. Elevated oxidative stress and sensorimotor deficits but normal cognition in mice that cannot synthesize ascorbic acid. J Neurochem. 2008;106:1198–1208. doi: 10.1111/j.1471-4159.2008.05469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bielski BH, Richter HW, Chan PC. Some properties of the ascorbate free radical. Ann N Y Acad Sci. 1975;258:231–237. doi: 10.1111/j.1749-6632.1975.tb29283.x. [DOI] [PubMed] [Google Scholar]
- 6.Halliwell B. Vitamin C: poison, prophylactic or panacea? Trends Biochem Sci. 1999;24:255–259. doi: 10.1016/s0968-0004(99)01418-8. [DOI] [PubMed] [Google Scholar]
- 7.Hallberg L, Brune M, Rossander-Hulthen L. Is there a physiological role of vitamin C in iron absorption? Ann N Y Acad Sci. 1987;498:324–332. doi: 10.1111/j.1749-6632.1987.tb23771.x. [DOI] [PubMed] [Google Scholar]
- 8.Duffy SJ, Gokce N, Holbrook M, Huang A, Frei B, Keaney JF, Vita JA. Treatment of hypertension with ascorbic acid. Lancet. 1999;354:2048–2049. doi: 10.1016/s0140-6736(99)04410-4. [DOI] [PubMed] [Google Scholar]
- 9.Heller R, Unbehaun A, Schellenberg B, Mayer B, Werner-Felmayer G, Werner ER. L-ascorbic acid potentiates endothelial nitric oxide synthesis via a chemical stabilization of tetrahydrobiopterin. J Biol Chem. 2001;276:40–47. doi: 10.1074/jbc.M004392200. [DOI] [PubMed] [Google Scholar]
- 10.Sonmez M, Demirci E. The effect of intramuscular vitamin C administration on semen quality in rams. J Firat Univ Health Vet Sci. 2003;17:195–201. [Google Scholar]
- 11.Fazeli P, Zamiri MJ, Farshad A, Khalili B. Effects of vitamin C on testicular and seminal characteristics of Markhoz goats. Iran J Vet Res. 2010;11:267–274. [Google Scholar]
- 12.Sing B, Chand D, Sing P, Yadran N. Effect of vitamin C addition to the diluent on the quality of deep frozen Murrah buffalo bull (Bubalus bubalis) semen. Int J Anim Sci. 1996;11:131–132. [Google Scholar]
- 13.Luck MR, Jeyaseelan I, Scholes RA. Ascorbic acid and fertility (Minireview) Biol Reprod. 1995;52:262–266. doi: 10.1095/biolreprod52.2.262. [DOI] [PubMed] [Google Scholar]
- 14.Wilson L. Sperm agglutination in human serum and blood. Proc Soc Exp Biol Med. 1954;85:652–655. doi: 10.3181/00379727-85-20982. [DOI] [PubMed] [Google Scholar]
- 15.Mangoli E, Pourentezari M, Anvari M, Talebi AR, Nahangi H. The improvement of sperm parameters and chromatin quality by vitamin C. Researcher. 2012;4:43–49. [Google Scholar]
- 16.Mitchell AF. Le mercure: un tueur du 21eme siècle. NUTRANEWS Science, Nutrition, Prevention, et Sante 2001; 2eme partie. [Google Scholar]
- 17.Chinoy MR, Sharma JD, Sanjeevan AG, Chinoy NJ. Structural changes in male reproductive organs and spermatozoa of scorbutic guinea-pigs. Proc Ind Natr Sci Acad. 1983;49:628–635. [Google Scholar]
- 18.Chen Q, Huang NN, Huang JT, Chen S, Fan J, Li C, Xie FK. Sodium benzoate exposure downregulates the expression of tyrosine hydroxylase and dopamine transporter in dopaminergic neurons in developing zebrafish. Birth Defects Res B Dev Reprod Toxicol. 2009;86:85–91. doi: 10.1002/bdrb.20187. [DOI] [PubMed] [Google Scholar]
- 19.McCormick GC, Speaker TJ. Comparison of the acute toxicity, distribution, fate and some pharmacological properties of the non-benzenoid aromatic compound acid with those of benzoid and naphtoic acids. Toxicol Appl Pharmacol. 1973;25:478. [Google Scholar]
- 20.Mental Health Research Institute Staff Publications, University of Michigan. Mental Health Research Institute. 2013 [Google Scholar]
- 21.Pahan K. Immunomodulation of experimental allergic encephalomyelitis by cinnamon metabolite sodium benzoate. Immunopharmacol Immunotoxicol. 2011;33:586–593. doi: 10.3109/08923973.2011.561861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Häberle J, Boddaert N, Burlina A, Chakrapani A, Dixon M, Huemer M, Karall D, Martinelli D, Crespo PS, Santer R, Servais A, Valayannopoulos V, Lindner M, Rubio V, Dionisi-Vici C. Suggested guidelines for the diagnosis and management of urea cycle disorders. Orphanet J Rare Dis. 2012;7:32. doi: 10.1186/1750-1172-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. Evaluation of certain food additives and contaminants. World Health Organ Tech Rep Ser. 2013:1–75. [PubMed] [Google Scholar]
- 24.Wells SD. Cancer health risks of sodium benzoate. The Health Cancer Journal. 2011:11–13. [Google Scholar]
- 25.Corriher SC. Sodium benzoate and ascorbic acid. The Health Wyze Report Journal. 2007:21–24. [Google Scholar]
- 26.United States Food and Drug Administration. Data on benzene in soft drinks and other beverages. United States Food and Drug Administration 16 May 2007. Retrieved 7 November 2013. [Google Scholar]
- 27.Sohrabi D, Alipour M, Reza M. The effect of sodium benzoate on testicular gonadotropins and thyroid hormones level in adult (Balb/c) mice. Feyz. 2008;12:7–11. [Google Scholar]
- 28.Noorafshan A, Mahboobeh E, Saied K. Stereological studies of the effects of sodium benzoate or ascorbic acid on rats’ cerebellum. Saudi Med J. 2014;35:1494–1500. [PMC free article] [PubMed] [Google Scholar]
- 29.Sonmez M, Turk G, Yuce A. The effect of ascorbic acid supplementation on sperm quality, lipid peroxidation and testosterone levels of male Wister rats. Theriogenology. 2005;63:2063–2072. doi: 10.1016/j.theriogenology.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Oyewopo AO, Olaniyi SK, Oyewopo CI, Jimoh AT. Radiofrequency electromagnetic radiation from cell phone causes defective testicular function in male Wistar rats. Andrologia. 2017;49 doi: 10.1111/and.12772. [DOI] [PubMed] [Google Scholar]
- 31.Oyewopo AO, Saalu LC, Osinubu AA, Imosemi IO, Omotosho GO, Adefolaju GA. The attenuating effects of zinc on propoxur-induced oxidative stress, impaired spermatogenesis and deranged steroidogenesis in Wistar rat. Journal of Medicine and Medical Sciences. 2010;5:178–184. [Google Scholar]
- 32.Elzeinová F, Pěknicová J, Děd L, Kubátová A, Margaryan H, Dorosh A, Makovický P, Rajmon R. Adverse effect of tetracycline and doxycycline on testicular tissue and sperm parameters in CD1 outbred mice. Exp Toxicol Pathol. 2013;65:911–917. doi: 10.1016/j.etp.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Priya RJ, Sridhar R, Balachandran C, Manohar BM. Effect of sodium benzoate treatment: on Body weight of wistar rats. Indian Vet J. 2010;87:303–304. [Google Scholar]
- 34.Schorah CJ, Tormey WP, Brooks GH, Robertshaw AM, Young GA, Talukder R, Kelly JF. The effect of vitamin C supplements on body weight, serum proteins, and general health of an elderly population. Am J Clin Nutr. 1981;34:871–6. doi: 10.1093/ajcn/34.5.871. [DOI] [PubMed] [Google Scholar]
- 35.Barrett KE, Barman SM, Boitano S, Brooks HL. Ganong’s review of medical physiology. 23rd edition. Tata Mc Graw Hill Education Private Limited; 2011. p. 404. [Google Scholar]
- 36.Kamel FA, Kubajak CL. Modulation of gonadotropin secretion by corticosterone interaction with gonadal steroids and mechanism of action. Endocrinol. 1987;121:561–565. doi: 10.1210/endo-121-2-561. [DOI] [PubMed] [Google Scholar]
- 37.Reddy A, Sood A, Rust PF, Busby JE, Varn E, Mathur RS. The effect of nicotine on invitro sperm motion characteristics. J Assist Repord Genet. 1995;12:217–223. doi: 10.1007/BF02211802. [DOI] [PubMed] [Google Scholar]
- 38.Aydos K, Guven MC, Can B, Ergun A. Nicotine toxicity to the ultra-structure of the testis in rats. BJU Int. 2001;88:622–626. doi: 10.1046/j.1464-4096.2001.02384.x. [DOI] [PubMed] [Google Scholar]
- 39.Fernandes GS, Fernandez CD, Campos KE, Damasceno DC, Anselmo-Franci JA, Kempinas WD. Vitamin C partially attenuates male reproductive deficits in hyperglycemic rats. Reprod Biol Endocrinol. 2011;9:100. doi: 10.1186/1477-7827-9-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oyeyemi WA, Kolawole TA, Shittu ST, Ajah R, Oyeyemi BF. Effects of ascorbic acid on reproductive functions of male Wistar rats exposed to nicotine. J Afr Ass Physiol Sci. 2014;2:110–116. [Google Scholar]


