Abstract
The aim of the present study is to assess the comparative efficacy of different dietary approaches on glycaemic control in patients with type 2 diabetes mellitus using a systematic review of the literature. Electronic and hand searches were performed until July 2017. The inclusion criteria were defined as follows: (1) randomized trial with a dietary approach; (2) adults with type 2 diabetes mellitus; (3) outcome either HbA1c (%) and/or fasting glucose (mmol/l); (4) minimum intervention period of 12 weeks. For each outcome measure, random effects network meta-analysis was performed in order to determine the pooled effect of each intervention relative to each of the other interventions. A total of 56 trials comparing nine dietary approaches (low-fat, Vegetarian, Mediterranean, high-protein, moderate-carbohydrate, low-carbohydrate, control, low GI/GL, Palaeolithic) enrolling 4937 participants were included. For reducing HbA1c, the low-carbohydrate diet was ranked as the best dietary approach (SUCRA: 84%), followed by the Mediterranean diet (80%) and Palaeolithic diet (76%) compared to a control diet. For reducing fasting glucose, the Mediterranean diet (88%) was ranked as the best approach, followed by Palaeolithic diet (71%) and Vegetarian diet (63%). The network analysis also revealed that all dietary approaches significantly reduce HbA1c (− 0.82 to − 0.47% reduction) and fasting glucose (− 1.61 to − 1.00 mmol/l reduction) compared to a control diet. According to the network meta-analysis the Mediterranean diet is the most effective and efficacious dietary approach to improve glycaemic control in type 2 diabetes patients.
Electronic supplementary material
The online version of this article (10.1007/s10654-017-0352-x) contains supplementary material, which is available to authorized users.
Keywords: Systematic review, Diet, Type 2 diabetes mellitus, Network meta-analysis, Evidence synthesis
Background
According to the most recent data by the International Diabetes Federation and the World Health Organization, type 2 diabetes (T2D) represents one of the most important health problems, causing enormous costs, with an estimated prevalence of 350–400 million cases worldwide [1, 2].
To prevent onset of T2D, high-quality diets have been recognized to play a critical role [3–5]. Nutrition therapy plays an integral role in the management of T2D, particularly after initial clinical diagnosis, in order to reduce or delay diabetes associated complications. One major approach is the loss of weight by a hypocaloric diet [6]. However, there is limited evidence on the optimal dietary approaches to control hyperglycaemia in T2D patients [7] and uncertainty regarding the optimal proportion of energy coming from carbohydrates, protein, and fat for patients with T2D [8].
Meta-analyses showed that some dietary approaches such as a low-carbohydrate, low-glycaemic index/load, high protein-, Vegetarian-, and Mediterranean dietary approaches were effective in reducing HbA1c [9, 10]. Nevertheless, other meta-analyses reported conflicting results [7, 11, 12].
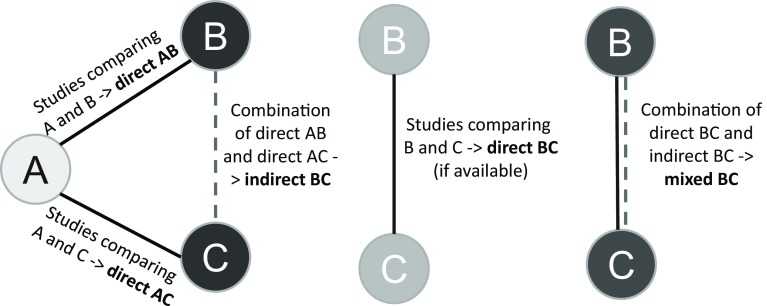
One of the most important questions that remain to be answered is which dietary approach offers the greatest benefits. For answering this question, a promising method is network meta-analysis (NMA), which is an extension of pairwise meta-analysis that enables a simultaneous comparison of multiple interventions. NMA combines direct (i.e., from trials comparing directly two interventions) and indirect (i.e., from a connected root via one more intermediate comparators) evidence in a network of trials (Fig. 1). In this way, it enables inference about every possible comparison between a pair of intervention in the network even when some comparisons have never been evaluated in a trial. A fundamental assumption of NMA, often called the transitivity assumption, is that trials comparing different sets of interventions (e.g., AB and AC trials) should be similar enough in all characteristics that may affect the outcome. For more details on the methodology of NMA we directed the readers to relevant tutorials [13–15].
Fig. 1.
Example of direct, indirect and mixed relative effects in a hypothetical triangle comparing three interventions
To our knowledge, no study has been conducted that compared simultaneously different dietary approaches in the management of T2D. Therefore, our aim is to compare the efficacy of different dietary approaches in clinical trials on glycaemic control in patients with T2D using the novel method of NMA.
Methods
The review was registered in PROSPERO International Prospective Register of Systematic Reviews https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=47464 and our strategy for the systematic review and NMA was pre-defined in a published protocol [16]. The present systematic review was planned, conducted, and reported in adherence to standards of quality for reporting systematic reviews and NMA [17, 18].
Search strategy
The literature search was performed using the electronic databases PubMed, Cochrane Central Register of Controlled Trials (CENTRAL), and Google Scholar until July 2017 with no restriction to language and calendar date using a pre-defined search strategy (Supplementary Appendix S1).
Furthermore, systematic reviews, and the reference lists from the retrieved articles were screened to search for additional relevant studies. Searches were conducted by two authors with disagreements being resolved by involvement of another reviewer.
Eligibility criteria
Studies were included in the review if they met all of the following criteria:
-
(i)Randomized comparison study design (parallel or cross-over) between different dietary approaches (energy restricted diets, iso-caloric, or ad libitum diets):
- Low carbohydrate (LC) diet (< 25% carbohydrates of total energy intake; high intake of animal and/or plant protein; often high intake of fat) [19];
- Moderate-carbohydrate diet (25–45% carbohydrates of total energy intake; 10–20% protein intake) [19];
- High protein (HP) diet (> 20% protein intake of total energy intake; high intake of animal and/or plant protein; < 35% fat) [20];
- Vegetarian/Vegan diet (no meat and fish/no animal products) [23];
- Palaeolithic diet [29];
- Control diet: no intervention or minimal intervention [30];
-
(ii)
Minimum intervention period of 12 weeks;
-
(iii)
Patients with a mean age ≥ 18 years, following the diagnosis criteria of the American Diabetes Association or according to the internationally recognized standards for patients with T2D [31];
-
(iv)
The primary outcome is glycosylated haemoglobin HbA1c (%) and the secondary outcome was defined fasting glucose (mmol/l).
The following studies were excluded:
-
(i)
Randomized trials including pregnant women, children, and adolescents, patients with abnormal glucose metabolism;
-
(ii)
Intervention studies solely based on dietary supplements or single foods;
-
(iii)
Intervention studies using dietary supplements as placebo;
-
(iv)
Studies with an exercise/medication [32, 33] co-intervention that was not applied in all the intervention/control groups;
-
(v)
Interventions based on very low energy diets (i.e., < 600 kcal/day).
Data extraction
After determination of the study selection, two reviewers extracted the following characteristics: name of first author, year of publication, study origin (country), study design (RCT: parallel or cross-over), sample size, mean baseline age, mean baseline BMI, mean baseline HbA1c, study duration, sex, description of the different dietary intervention arms, specification of the control group, type of diet (energy restricted, ad libitum, iso-caloric), drop outs, presence of comorbidities, hypoglycaemic drugs, antihypertensive medication, lipid lowering medication. Outcome data include: post-intervention values with corresponding standard deviations for glycosylated haemoglobin and fasting plasma glucose.
Risk of bias assessment
Full copies of the studies were assessed by two authors for methodological quality using the risk of bias assessment tool from the Cochrane Collaboration [34]. The following sources of bias were assessed: selection bias (random sequence generation and allocation concealment), performance bias (blinding of participants and personnel), attrition bias (incomplete outcome data), and reporting bias (selective reporting).
Studies were classified as being at low risk of bias (if at least three out of a maximum of five items were rated as low risk; and maximum one item rated with a high risk of bias), high risk of bias (if at least two out of a maximum of five items were rated as high risk), and moderate/unclear risk (all other studies) using the risk of bias assessment tool from the Cochrane Collaboration.
Dealing with missing data
We contacted authors to receive missing outcome data (3 authors sent additional data, see acknowledgements). If the post-intervention values with the corresponding standard deviations were not available, the change scores with the corresponding standard deviations were used, according to the guidelines of the Cochrane Handbook [35].
Evaluation of synthesis assumptions
Data synthesis
Description of the available data
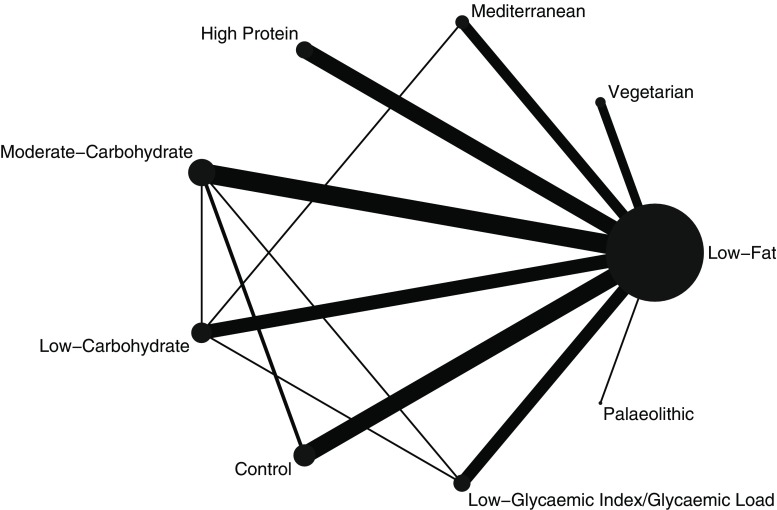
We present for all included trials study and population characteristics describing the available data and important variables (e.g., age, length of follow-up, outcome relevant baseline risk factors, etc.). We illustrate the available direct comparisons between different dietary interventions and control group using a network diagram for each outcome [36]. The size of the nodes is proportional to the sample size of each dietary intervention and the thickness of the lines proportional to the number of studies available.
Assessment of transitivity
Transitivity is the fundamental assumption of indirect comparisons and NMA, and its violation threatens the validity of the findings obtained from a network of studies. To evaluate the assumption of transitivity we compared the distribution of the potential effect modifiers across the available direct comparisons. We considered the following effect modifiers: body weight, duration of diabetes, mean baseline age, and study duration.
Statistical analysis
For each outcome measure of interest, we performed random effects NMA in order to determine the pooled relative effect of each dietary intervention against every other intervention in terms of the post-intervention values. NMA was used to synthesize the direct and indirect effects. The method of NMA is an extension of the standard pairwise meta-analysis that enables a simultaneous comparison of multiple interventions, forming a connected network while preserving the internal randomization of individual trials. We ran random effects NMA for each outcome to estimate all possible pairwise relative effects and to obtain a clinically meaningful relative ranking of the different dietary interventions. We present the summary mean differences with their 95% CI in a league table. We estimated the relative ranking of the different diets for each outcome using the distribution of the ranking probabilities and the surface under the cumulative ranking curves (SUCRA) [37]. For each outcome we assumed a common network-specific heterogeneity parameter and estimated the predictive intervals to assess how much this heterogeneity affects the relative effects with respect to the additional uncertainty anticipated in future studies [38]. We fitted all analyses described in a frequentist framework using Stata [39] (network package [40]) and produced presentation tools with the network graphs package [41].
Assessment of inconsistency
To evaluate the presence of statistical inconsistency (i.e., disagreement between the different sources of evidence) in the data, we employed both local and global approaches [42]. Specifically, we used the loop-specific approach [43] to detect loops of evidence that might present important inconsistency as well as the side-splitting approach [44] to detect comparisons for which direct estimates disagree with indirect evidence from the entire network. Global methods investigate the presence of inconsistency jointly from all possible sources in the entire network simultaneously. For this purpose, we used the design-by-treatment interaction model [45, 46].
Subgroup and sensitivity analyses
For comparability reasons, we performed subgroup analyses in accordance to previous pairwise meta-analyses investigating the effects of dietary interventions, by taking into account study duration (≥ 12 vs. < 12 months) [19, 47], sample size (≥ 100 vs. < 100) [48], and age (≥ 60 vs. < 60 years) [48]. We also conducted sensitivity analyses by analysing only studies considered being at low risk of bias, and by excluding risk of bias trials. We ran also a meta-regression analysis to investigate the association between the primary outcome (HbA1c) and mean differences in weight change.
Small study effects and publication bias
We drew inference on the risk for publication bias based primarily on non-statistical considerations; hence by considering how likely it is that studies may have been conducted but not published based on the expertise of the investigators in the field. We also produced the comparison-adjusted funnel plot [36] and fit a network meta-regression model to assess the magnitude of funnel plot asymmetry for the primary outcome.
Credibility of the evidence
To make inferences about the credibility of evidence from the NMA we used the GRADE system extended for NMA following the approach suggested by Salanti et al. (see the Supplementary Appendix S2 for details) [42].
Results
Out of 3852 records identified by the literature search, 115 full text articles were assessed in detail as they reported on one or more of dietary approaches and T2D in the title/abstract (Supplementary Figure S1). Of these, 59 were excluded, with the reasons for exclusion summarized in Supplementary Table S1.
Overall, 56 trials [29, 49–103] met the eligibility criteria and provided sufficient data to be included in the meta-analysis. The included studies were published between 1978 and 2016 and had enrolled a total of 4937 T2D patients. Eighteen trials were conducted in North America, 14 trials in Europe, 8 trials in Asia, and 16 trials in Australia and New Zealand. The study duration ranged between 3 and 48 months; the patients’ mean age was between 44 and 67 years, and their BMI between 25 (Asian population) and 43 kg/m2. The general and specific study characteristics are summarized in Supplementary Table S2 and S3.
Twenty-one trials were judged to be low risk–, seven trials to be high risk of bias, and 28 trials were classified as moderate/unclear risk of bias studies. With regard to the specific items of the risk of bias assessment tool by the Cochrane Collaboration, 56% of the included studies indicate a low risk of bias for random-sequence generation, 23% for allocation concealment, 0% for blinding, 63% for incomplete data outcome, and 79% for selective reporting (Supplementary Figure S2).
The studies applied heterogeneous definitions for the different intervention diets. The fat intake varied across the different LF trials by ~ 10–15% of total energy intake, and also the intervention protocols varied among the trials (i.e., group meeting, dietary counselling, and intensity). Moreover, hypocaloric, iso-caloric, and ad libitum diets were included in the NMA. Moreover the definition of a control diet showed some difference across the included trials. Four out of the ten trials were based on “no intervention”, whereas the other six trials were based on minimal intervention (standard dietary advice). We thus had to harmonize the single studies and formed classes of dietary approaches.
Figure 2 shows the network diagrams of direct comparison for HbA1c with the number of studies reflected by the size of the edges, and the number of patients reflected by the size of the nodes. The highest number of trials include moderate-carbohydrate diet compared to LF diets [68–80] (n = 13), LF diet compared to control diets [72, 73, 88–95] (n = 10), HP diet compared to LF diets [60–67] (n = 8), and LC diet compared to LF diets [57, 81–87] (n = 8).
Fig. 2.
Network diagram for HbA1c: The size of the nodes is proportional to the total number of participants allocated to each dietary approach and the thickness of the lines proportional to the number of studies evaluating each direct comparison
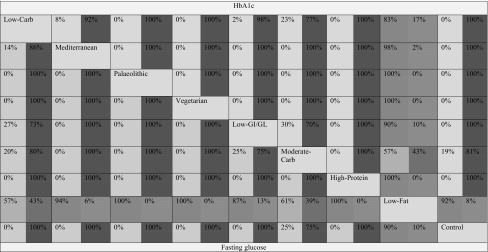
NMA simultaneously analyse both direct comparisons of interventions within trials and indirect comparisons across trials based on a common comparator. Since none of the studies have compared B (Vegetarian) and C (Mediterranean), but each has been compared with a common comparator A (LF), then we assume an indirect comparison of B and C on the direct comparison of B and A and the direct comparison of C and A [104]. Table 1 shows the percentage of statistical contribution coming from direct and indirect comparisons for each dietary approach compared to each other. It was shown that most of the contribution to the study effects came from indirect comparisons. Direct comparisons dominated the comparisons of Vegetarian/Mediterranean/HP/moderate-carbohydrate/LC/LGI/GL/Palaeolithic/control diet with a LF diet for both outcomes. In general, there are no important differences in the examined effect modifiers across comparisons apart from the duration of diabetes which does not seem to be distributed similarly across the different comparisons. For some comparisons such as LC versus LGI/GL, LC versus moderate carbohydrate, LGI/GL versus moderate carbohydrate, and Palaeolithic versus LF, we do not have enough studies and we could not test transitivity appropriately (Supplementary Figure S3–6).
Table 1.
Percentage contribution of each direct estimate derived from direct (blue) and indirect (red) comparisons (the colour corresponds to the percentage of contribution)
The values above the dietary approaches correspond to the percentage contribution of direct and indirect comparisons between the row and columns for HbA1c (e.g., the percentage contribution of direct comparisons for HbA1c between Low-Carb and Low-Fat diet is 83%, and 17% for the indirect comparisons). The values below the dietary approaches correspond to the percentage contribution of direct and indirect comparisons between the column and the rows for fasting glucose (e.g., the percentage contribution of direct comparisons for fasting glucose between Low-Carb and Low-Fat diet is 57%, and 43% for the indirect comparisons). GI/GL, glycaemic index/load. (Color table online)
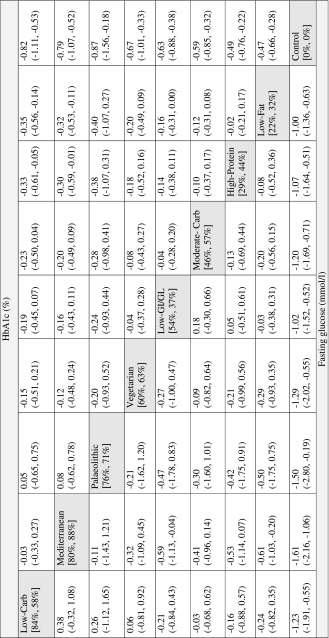
The effect size estimates for the comparison of every dietary approach compared with each other dietary approach on HbA1c and fasting glucose outcomes are given in Table 2. All dietary approaches were more effective in reducing HbA1c (− 0.82 to − 0.47% reduction) and fasting glucose (− 1.61 to − 1.00 mmol/l reduction) compared to a control diet. The Mediterranean (MD: − 0.32, 95% − 0.53, − 0.11) and the LC diet (MD: − 0.35, 95% − 0.56, − 0.14) were more effective in reducing HbA1c compared to a LF diet. Moreover, the LC diet was also more effective in HbA1c reduction compared to a HP diet (MD: − 0.33, 95% − 0.61, − 0.05). The Mediterranean diet was more effective in reducing fasting glucose compared to a LF- (MD: − 0.61 mmol/l, 95% − 1.03, − 0.20) and LGI/GL diet (MD: − 0.59 mmol/l, 95% − 1.13, − 0.04) (Supplementary Figure S7 and S8, Table 2). In addition, the LGI/GL diet was associated with a trend for a reduction in HbA1c compared to the LF diet (MD: − 0.16, 95% − 0.31, − 0.00). The LC diet had the highest SUCRA value (84%), followed by the Mediterranean diet (80%), and Palaeolithic diet (76%) for HbA1c, whereas the Mediterranean diet (88%) had the highest SUCRA value for fasting glucose, followed by Palaeolithic diet (71%) and Vegetarian diet (63%) (Table 2). The rankograms did not imply the presence of important uncertainty in ranking for HbA1c; more uncertain appeared to be the relative ranking for fasting glucose though (Supplementary Figure S9 and S10).
Table 2.
League table showing the results of the network meta-analysis comparing the effects (mean difference: MD) of all dietary approaches and 95% confidence intervals (95% CI)
The values above the dietary approaches correspond to the MD and 95% CI in HbA1c (%) between the row and columns (e.g., the MD in HAb1c between Low-Carb and Low-Fat diet is −0.35%). The value below the dietary approaches correspond to the MD in fasting glucose (mmol/l) between the column and the row (e.g., the MD fasting glucose between Low-Carb and Low-Fat diet is −0.24 mmol/l). The values in square brackets represent the SUCRA for HbA1c and fasting glucose (e.g., the LC diet was ranked as the best dietary approach for reducing HbA1c, SUCRA: 84%; the Mediterranean diet was ranked as the best dietary approach for reducing fasting glucose, SUCRA: 88%). GI/GL, glycaemic index/load
The side-splitting approach suggested important inconsistency for HbA1c in the comparisons of LF versus LC, LF versus control diet, and moderate-carbohydrate versus LC diet (Supplementary Table S4 and S5). For fasting glucose, significant inconsistency was observed for LF versus LC diet and moderate-carbohydrate versus LC diet. The loop-specific approach showed important inconsistency in the loop formed by the aforementioned diets for both outcomes (Supplementary Figure S11 and S12). The design-by-treatment model showed also significant inconsistency for HbA1c (p = 0.03), but not for FG (p = 0.32).
This apparent inconsistency might reflect the low contribution of direct comparisons to the total estimate. The important inconsistency in the loop for LF versus moderate-carbohydrate and LC might be explained by several differences across LF dietary approaches (hypocaloric if compared to a control diet; often iso-caloric if compared to other dietary approaches), differences in ratio of fat to carbohydrate intake, and differences in fatty acid composition among moderate carbohydrate approaches and LC dietary approaches (larger weight loss compared to other interventions).
In the subgroup analyses for study duration, sample size, and age we could show that LC diets were more effective in reducing HbA1c in the shorter-term (< 12 months), in smaller size studies, and including patients ≥ 60 years. Mediterranean, moderate-carbohydrate and LGI/GL, HP, and LF diets were more effective in reducing HbA1c in the longer-term, in larger size studies, and in studies including patients < 60 years (Supplementary Table S6–11). Although the power was very small for several comparisons, these characteristics may partly explain the presence of inconsistency. Furthermore, in the low-risk of bias sensitivity analysis the results of the primary analysis were generally confirmed. Hence, both the Mediterranean diet and the LC diet were more effective to decrease HbA1c compared to a LF diet, whereas the results for FG were not significant (Supplementary Table S12). All the results of the main analysis were confirmed in the sensitivity analysis excluding high risk of bias trials (Supplementary Table S13). In univariate meta-regression analysis we could show that mean reduction in HbA1c was significantly (p = 0.04) related to mean difference in weight change between dietary approaches (Supplementary Figure S11).
The comparison-adjusted funnel plots for both outcomes appear slightly asymmetric when LF dietary approaches were compared to all other dietary approaches. However, the network meta-regression model that accounted for differences in study variance did not yield a statistically significant coefficient (Supplementary Figure S14 and S15).
The credibility of evidence was rated very low for the comparisons Mediterranean versus LF; LC versus LF; LGI versus LF, moderate-carbohydrate versus LC, Mediterranean versus HP, and LC versus HP. The very low credibility was driven by significant inconsistency. For the other comparisons the credibility of evidence was rated low, and for three comparisons the quality of evidence was rated moderate (LF vs. Palaeolithic, Mediterranean vs. Palaeolithic, LGI/GL vs. Palaeolithic) (Supplementary Figure S16, Supplementary Appendix S2).
Discussion
By applying NMA, we ranked 9 different dietary approaches (Vegetarian, Mediterranean, HP, moderate-carbohydrate, LC, LGI/GL, Palaeolithic, LF and control diet) regarding their comparative efficacy for glycaemic control in patients with T2D. The ranking according to SUCRA showed the highest value for the LC diet, followed by the Mediterranean diet, and Palaeolithic diet for HbA1c, whereas the Mediterranean diet had the highest SUCRA value for fasting glucose, followed by Palaeolithic diet and Vegetarian diet. However, the credibility of evidence was rated very low for the LC, as well as for some comparison with the Mediterranean diet. The NMA also revealed that all dietary approaches significantly reduce HbA1c (− 0.47 to − 0.82% reduction) and fasting glucose (− 1.00 to − 1.61 mmol/l reduction) compared to a control diet.
In line with our observations, pairwise meta-analyses have shown that LC diets were more effective in HbA1c and body weight reduction in the short-term compared to other diets, whereas no superiority was observed in the long-term [105, 106]. Weight loss as an important effect modifier for HbA1c and fasting glucose reduction may potentially explain the observed inconsistency between LC and the other dietary approaches. Despite the moderate quality of evidence grading in the NMA, the findings for Palaeolithic diet should be interpreted with caution since only one trial was available. Finally, it is important to note that LC diets were more effective in reducing HbA1c in patients ≥ 60 years, whereas the Mediterranean, moderate-carbohydrate, LGI/GL, HP, and LF diets were more effective in HbA1c reduction in patients < 60 years, compared to patients ≥ 60 years. Irrespective of the age of the study participants, HbA1c reductions have been reported to be of similar degree following either LC or LF dietary regimens [87]. In contrast, other studies demonstrated stronger decreases in HbA1c in individuals subjected to a LC approach [73]. It remains speculative whether these differences might be due to an age-dependency of LC effectiveness as shown in the present subgroup analysis. Given the fact that various authorities have proposed specific guidelines for glycemic control in older adults [107, 108] to minimize the risk of hypoglycemia, these observations need to be confirmed by larger RCTs mainly in patients ≥ 60 years.
In the past, with traditional pairwise meta-analysis, Ajala and co-workers compared various diets modifying macronutrient intake on glycaemic control and weight loss in patients with T2D [10]. In 2003, Brand-Miller et al. [109] could show a beneficial effect specifically of LGI foods as compared to regular or high GI diets on HbA1c and fructosamine in subjects with type 1 or type 2 diabetes, however, this study included mostly randomized trials with a duration time of less than 12 weeks, whereas we included only trials with a minimum intervention period of 12 weeks. The duration of time is an important factor in dietary- and overall lifestyle intervention trials, since participants adherence declines over time, and improvements in risk factors are often larger in the short term, compared to the longer term [57, 110].
Comparable effects were shown for carbohydrate-restricted diets by Kirk et al. [111]. In a meta-analysis of RCTs by Huo et al. [112], a Mediterranean diet did result in significantly more pronounced decreases in parameters of glycaemic control and weight loss as compared to control diets. In addition, Dong et al. [113] observed improvements in HbA1c but not in fasting plasma glucose following a meta-analytical synthesis of data from randomized trials comparing HP with low-protein diets. In another meta-analysis, Yokoyama et al. [9] demonstrated favourable effects of Vegetarian diets on glycaemic control in patients with T2D. The results of our NMA extend the current knowledge from previous pairwise meta-analyses, since we were the first to rank 9 different dietary approaches regarding their comparative efficacy by analysing simultaneously both direct and indirect effects. We could show that a plant-based diet such as the Mediterranean diet is the most effective dietary approach to improve glycaemic control in T2D patients. This will affect evidence-based decision-making with respect to dietary regimens by providing a reliable basis for dietary recommendations in the management of T2D.
With respect to mechanisms of action, the effects of LC diets, Mediterranean diets or Palaeolithic diets on HbA1c might be mediated by their higher amounts of food groups such as fruits, vegetables, or whole grains providing antioxidants or fibre, known to improve insulin sensitivity or to directly inhibit production of advanced glycosylated end products [114–116]. The additional benefit of a Mediterranean diet on fasting plasma glucose might be exerted via dietary polyphenols (e.g., flavonoids, phenolic acids, resveratrol, lignans) provided by key components of the Mediterranean diet such as olive oil, nuts, red wine, legumes, fruits, and vegetables [48, 117–119]. Moreover, the meta-regression analysis showed that HbA1c reduction was significantly related to mean differences in weight change, indicating that weight loss is another important mechanism to improve glycaemic control.
Both HbA1c and fasting plasma glucose are considered to be clinical tools for the assessment of glycaemic control. However, these parameters might not accurately determine short-term fluctuations in glycaemia within a day or long-term variations within several months. Glycaemic variability is supposed to be an independent predictor of diabetic complications [120].
Optimal control of glycaemic parameters in T2D subjects is an essential step to reduce the risk of long-term health damages associated with the disease. According to the Asian Pacific Study, attenuations in fasting glucose levels of 1 mmol/l are associated with a 23% lower risk of CVD [121]. Moreover, the authors of the United Kingdom Prevention Study considered hyperglycaemia to be a more relevant predictor of coronary events in the course of T2D when compared with increased insulin levels [122]. In a retrospective study by Currie et al. [123] investigating 47,970 patients with T2D, HbA1c values higher than 6.5% were associated with an increased mortality rate. In the EPIC-Norfolk study, an increase in HbA1c of 1 percentage point was associated with a 20–30% increase in mortality or in risk of cardiovascular events [124]. Likewise, an HbA1c increase of 1 percentage point was associated with a relative risk for death from any cause of 1.24 in men and 1.28 in women [124]. This underlines the validity of HbA1c and fasting plasma glucose in monitoring the management of T2D.
Strength and limitations
This systematic review includes the application of novel NMA methods, which simultaneously combine direct and indirect evidence. Additional strengths are the high number of included trials, the comprehensive literature search, the a priori published systematic review protocol, identification of inconsistency, and the credibility of evidence assessment.
A limitation of this review lies within the number and qualities of the studies available. Overall, 7 of 56 trials were at high risk of bias mostly due to lack of allocation concealment, and blinding. However, the sensitivity analysis excluding the high risk of bias trials confirmed all the results of main NMA. Another important limitation is that analyses were based on the original intended randomized design, not by adherence to the actual dietary approach and/or macronutrient composition and caloric intake consumed. This means that although patients were randomized to various diets or controls, details on their actual adherence to the dietary program were not accounted for in the analyses. The heterogeneous definition for the different dietary approaches and the overlap between some dietary approaches is another limitation. In some cases a LGI/GL or a HP diet would also fulfil the criteria of a LF diet, whereas on the contrary a LF would never fulfil the criteria of other dietary approaches. The observed statistical inconsistency, which was also reflected in the GRADE assessments, is another important limitation of the ranking and lowers the confidence in the effect estimates being used in the analysis. As shown in the subgroup analyses we observed significant differences between LC compared to other dietary approaches for study duration, sample size, and patients’ age.
Conclusion
According to the NMA, the Mediterranean diet seems to be the most effective and efficacious dietary approach to improve glycaemic control in T2D patients. These findings need to be seen under the light of very low to moderate credibility of evidence. However, the findings could nevertheless influence dietary recommendations in the management of T2D.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Iris Shai, Ph.D.; Thomas Wolever, Ph.D.; and Miguel Martinez-Gonzalez, Ph.D. for sending us additional data for the corresponding meta-analysis.
Authors’ contributions
LS, AC, HB, GH contributed to the conception and design of the systematic review and meta-analysis. LS, AC, CS, HB, were involved in the acquisition and analysis of the data. LS, AC, CS, HB, interpreted the results. LS, AC, GH, CS, HB, drafted this manuscript. All authors provided critical revisions of the protocol and approved submission of the final manuscript.
Compliance with ethical standards
Competing interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s10654-017-0352-x) contains supplementary material, which is available to authorized users.
Contributor Information
Lukas Schwingshackl, Phone: +49 (0)33200 88-2712, Email: lukas.schwingshackl@dife.de.
Anna Chaimani, Email: anna.chaimani@parisdescartes.fr.
Georg Hoffmann, Email: georg.hoffmann@univie.ac.at.
Carolina Schwedhelm, Email: carolina.schwedhelm@dife.de.
Heiner Boeing, Email: boeing@dife.de.
References
- 1.WHO . Fact sheet no. 312: Diabetes. Geneva: WHO; 2013. [Google Scholar]
- 2.International Diabetes Federation. 2013; Sixth edition. https://www.idf.org/sites/default/files/EN_6E_Atlas_Full_0.pdf. Accessed 19 Feb 2016.
- 3.Schwingshackl L, Bogensberger B, Hoffmann G. Diet quality as assessed by the Healthy Eating Index, Alternate Healthy Eating Index, dietary approaches to stop hypertension score, and health outcomes: an updated systematic review and meta-analysis of cohort studies. J Acad Nutr Diet. 2018;118(1):74–100. doi: 10.1016/j.jand.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 4.Schwingshackl L, Hoffmann G, Lampousi AM, et al. Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol. 2017;32(5):363–375. doi: 10.1007/s10654-017-0246-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwingshackl L, Missbach B, Konig J, Hoffmann G. Adherence to a Mediterranean diet and risk of diabetes: a systematic review and meta-analysis. Public Health Nutr. 2015;18(7):1292–1299. doi: 10.1017/S1368980014001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Diabetes Association 3. Foundations of care and comprehensive medical evaluation. Diabetes Care. 2016;39(Suppl 1):S23–S35. doi: 10.2337/dc16-S006. [DOI] [PubMed] [Google Scholar]
- 7.Schwingshackl L, Hoffmann G. Comparison of the long-term effects of high-fat v. low-fat diet consumption on cardiometabolic risk factors in subjects with abnormal glucose metabolism: a systematic review and meta-analysis. Br J Nutr. 2014;111(12):2047–2058. doi: 10.1017/S0007114514000464. [DOI] [PubMed] [Google Scholar]
- 8.Evert AB, Boucher JL, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2014;37(Suppl 1):S120–S143. doi: 10.2337/dc14-S120. [DOI] [PubMed] [Google Scholar]
- 9.Yokoyama Y, Barnard ND, Levin SM, Watanabe M. Vegetarian diets and glycemic control in diabetes: a systematic review and meta-analysis. Cardiovasc Diagn Ther. 2014;4(5):373–382. doi: 10.3978/j.issn.2223-3652.2014.10.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ajala O, English P, Pinkney J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am J Clin Nutr. 2013;97(3):505–516. doi: 10.3945/ajcn.112.042457. [DOI] [PubMed] [Google Scholar]
- 11.Carter P, Achana F, Troughton J, Gray LJ, Khunti K, Davies MJ. A Mediterranean diet improves HbA1c but not fasting blood glucose compared to alternative dietary strategies: a network meta-analysis. J Hum Nutr Diet. 2014;27(3):280–297. doi: 10.1111/jhn.12138. [DOI] [PubMed] [Google Scholar]
- 12.Emadian A, Andrews RC, England CY, Wallace V, Thompson JL. The effect of macronutrients on glycaemic control: a systematic review of dietary randomised controlled trials in overweight and obese adults with type 2 diabetes in which there was no difference in weight loss between treatment groups. Br J Nutr. 2015;114(10):1656–1666. doi: 10.1017/S0007114515003475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods. 2012;3(2):80–97. doi: 10.1002/jrsm.1037. [DOI] [PubMed] [Google Scholar]
- 14.Leucht S, Chaimani A, Cipriani AS, Davis JM, Furukawa TA, Salanti G. Network meta-analyses should be the highest level of evidence in treatment guidelines. Eur Arch Psychiatry Clin Neurosci. 2016;266(6):477–480. doi: 10.1007/s00406-016-0715-4. [DOI] [PubMed] [Google Scholar]
- 15.Mavridis D, Giannatsi M, Cipriani A, Salanti G. A primer on network meta-analysis with emphasis on mental health. Evid Based Men Health. 2015;18(2):40–46. doi: 10.1136/eb-2015-102088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwingshackl L, Chaimani A, Hoffmann G, Schwedhelm C, Boeing H. Impact of different dietary approaches on glycemic control and cardiovascular risk factors in patients with type 2 diabetes: a protocol for a systematic review and network meta-analysis. Syst Rev. 2017;6(1):57. doi: 10.1186/s13643-017-0455-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 18.Chaimani A, Caldwell DM, Li T, Higgins JP, Salanti G. Additional considerations are required when preparing a protocol for a systematic review with multiple interventions. J Clin Epidemiol. 2017 doi: 10.1016/j.jclinepi.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Schwingshackl L, Hoffmann G. Comparison of effects of long-term low-fat vs high-fat diets on blood lipid levels in overweight or obese patients: a systematic review and meta-analysis. J Acad Nutr Diet. 2013;113(12):1640–1661. doi: 10.1016/j.jand.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Schwingshackl L, Hoffmann G. Long-term effects of low-fat diets either low or high in protein on cardiovascular and metabolic risk factors: a systematic review and meta-analysis. Nutr J. 2013;12:48. doi: 10.1186/1475-2891-12-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwingshackl L, Hoffmann G. Long-term effects of low glycemic index/load vs. high glycemic index/load diets on parameters of obesity and obesity-associated risks: a systematic review and meta-analysis. Nutr Metab Cardiovasc Dis. 2013;23(8):699–706. doi: 10.1016/j.numecd.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Schwingshackl L, Hobl LP, Hoffmann G. Effects of low glycaemic index/low glycaemic load vs. high glycaemic index/high glycaemic load diets on overweight/obesity and associated risk factors in children and adolescents: a systematic review and meta-analysis. Nutr J. 2015;14:87. doi: 10.1186/s12937-015-0077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haider LM, Schwingshackl L, Hoffmann G, Ekmekcioglu C. The effect of vegetarian diets on iron status in adults: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2017 doi: 10.1080/10408398.2016.1259210. [DOI] [PubMed] [Google Scholar]
- 24.Schwingshackl L, Hoffmann G. Mediterranean dietary pattern, inflammation and endothelial function: a systematic review and meta-analysis of intervention trials. Nutr Metab Cardiovasc Dis. 2014;24(9):929–939. doi: 10.1016/j.numecd.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Schwingshackl L, Hoffmann G. Adherence to Mediterranean diet and risk of cancer: a systematic review and meta-analysis of observational studies. Int J Cancer. 2014;135(8):1884–1897. doi: 10.1002/ijc.28824. [DOI] [PubMed] [Google Scholar]
- 26.Schwingshackl L, Hoffmann G. Does a Mediterranean-type diet reduce cancer risk? Curr Nutr Rep. 2016;5:9–17. doi: 10.1007/s13668-015-0141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwingshackl L, Hoffmann G. Adherence to Mediterranean diet and risk of cancer: an updated systematic review and meta-analysis of observational studies. Cancer Med. 2015;4(12):1933–1947. doi: 10.1002/cam4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwingshackl L, Schwedhelm C, Galbete C, Hoffmann G. Adherence to Mediterranean diet and risk of cancer: an updated systematic review and meta-analysis. Nutrients. 2017;9(10):1063. doi: 10.3390/nu9101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jonsson T, Granfeldt Y, Ahren B, et al. Beneficial effects of a Paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study. Cardiovasc Diabetol. 2009;8:35. doi: 10.1186/1475-2840-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rees K, Hartley L, Flowers N, et al. ‘Mediterranean’ dietary pattern for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;8:Cd009825. doi: 10.1002/14651858.CD009825.pub2. [DOI] [PubMed] [Google Scholar]
- 31.American Diabetes Association (2) Classification and diagnosis of diabetes. Diabetes Care. 2015;38(Suppl):S8–S16. doi: 10.2337/dc15-S005. [DOI] [PubMed] [Google Scholar]
- 32.Schwingshackl L, Missbach B, Dias S, Konig J, Hoffmann G. Impact of different training modalities on glycaemic control and blood lipids in patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetologia. 2014;57(9):1789–1797. doi: 10.1007/s00125-014-3303-z. [DOI] [PubMed] [Google Scholar]
- 33.Schwingshackl L, Dias S, Strasser B, Hoffmann G. Impact of different training modalities on anthropometric and metabolic characteristics in overweight/obese subjects: a systematic review and network meta-analysis. PLoS ONE. 2013;8(12):e82853. doi: 10.1371/journal.pone.0082853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane-handbook.org. Accessed 10 May 2017.
- 36.Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS ONE. 2013;8(10):e76654. doi: 10.1371/journal.pone.0076654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163–171. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 38.Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. doi: 10.1136/bmj.d549. [DOI] [PubMed] [Google Scholar]
- 39.StataCorp . Stata statistical software: release 14. College Station: StataCorp LP; 2015. [Google Scholar]
- 40.White IR. Network meta-analysis. Stata J. 2015;15:951–985. [Google Scholar]
- 41.Chaimani A, Salanti G. Visualizing assumptions and results in network meta-analysis: the network graphs package. Stata J. 2015;15:905–950. [Google Scholar]
- 42.Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE. 2014;9(7):e99682. doi: 10.1371/journal.pone.0099682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683–691. doi: 10.1016/s0895-4356(97)00049-8. [DOI] [PubMed] [Google Scholar]
- 44.Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29(7–8):932–944. doi: 10.1002/sim.3767. [DOI] [PubMed] [Google Scholar]
- 45.Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. 2012;3(2):98–110. doi: 10.1002/jrsm.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jackson D, Barrett JK, Rice S, White IR, Higgins JP. A design-by-treatment interaction model for network meta-analysis with random inconsistency effects. Stat Med. 2014;33(21):3639–3654. doi: 10.1002/sim.6188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meng Y, Bai H, Wang S, Li Z, Wang Q, Chen L. Efficacy of low carbohydrate diet for type 2 diabetes mellitus management: a systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2017;131:124–131. doi: 10.1016/j.diabres.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 48.Schwingshackl L, Lampousi AM, Portillo MP, Romaguera D, Hoffmann G, Boeing H. Olive oil in the prevention and management of type 2 diabetes mellitus: a systematic review and meta-analysis of cohort studies and intervention trials. Nutr Diabetes. 2017;7:e262. doi: 10.1038/nutd.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barnard ND, Cohen J, Jenkins DJ, et al. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74-wk clinical trial. Am J Clin Nutr. 2009;89(5):1588s–1596s. doi: 10.3945/ajcn.2009.26736H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mishra S, Xu J, Agarwal U, Gonzales J, Levin S, Barnard ND. A multicenter randomized controlled trial of a plant-based nutrition program to reduce body weight and cardiovascular risk in the corporate setting: the GEICO study. Eur J Clin Nutr. 2013;67(7):718–724. doi: 10.1038/ejcn.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nicholson AS, Sklar M, Barnard ND, Gore S, Sullivan R, Browning S. Toward improved management of NIDDM: a randomized, controlled, pilot intervention using a lowfat, vegetarian diet. Prev Med. 1999;29(2):87–91. doi: 10.1006/pmed.1999.0529. [DOI] [PubMed] [Google Scholar]
- 52.Kahleova H, Matoulek M, Malinska H, et al. Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with Type 2 diabetes. Diabet Med. 2011;28(5):549–559. doi: 10.1111/j.1464-5491.2010.03209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee YM, Kim SA, Lee IK, et al. Effect of a brown rice based vegan diet and conventional diabetic diet on glycemic control of patients with type 2 diabetes: a 12-week randomized clinical trial. PLoS ONE. 2016;11(6):e0155918. doi: 10.1371/journal.pone.0155918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ceriello A, Esposito K, La Sala L, et al. The protective effect of the Mediterranean diet on endothelial resistance to GLP-1 in type 2 diabetes: a preliminary report. Cardiovasc Diabetol. 2014;13:140. doi: 10.1186/s12933-014-0140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Esposito K, Maiorino MI, Ciotola M, et al. Effects of a Mediterranean-style diet on the need for antihyperglycemic drug therapy in patients with newly diagnosed type 2 diabetes: a randomized trial. Ann Intern Med. 2009;151(5):306–314. doi: 10.7326/0003-4819-151-5-200909010-00004. [DOI] [PubMed] [Google Scholar]
- 56.Lasa A, Miranda J, Bullo M, et al. Comparative effect of two Mediterranean diets versus a low-fat diet on glycaemic control in individuals with type 2 diabetes. Eur J Clin Nutr. 2014;68(7):767–772. doi: 10.1038/ejcn.2014.1. [DOI] [PubMed] [Google Scholar]
- 57.Shai I, Schwarzfuchs D, Henkin Y, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359(3):229–241. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- 58.Elhayany A, Lustman A, Abel R, Attal-Singer J, Vinker S. A low carbohydrate Mediterranean diet improves cardiovascular risk factors and diabetes control among overweight patients with type 2 diabetes mellitus: a 1-year prospective randomized intervention study. Diabetes Obes Metab. 2010;12(3):204–209. doi: 10.1111/j.1463-1326.2009.01151.x. [DOI] [PubMed] [Google Scholar]
- 59.Itsiopoulos C, Brazionis L, Kaimakamis M, et al. Can the Mediterranean diet lower HbA1c in type 2 diabetes? Results from a randomized cross-over study. Nutr Metab Cardiovasc Dis. 2011;21(9):740–747. doi: 10.1016/j.numecd.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 60.Brinkworth GD, Noakes M, Parker B, Foster P, Clifton PM. Long-term effects of advice to consume a high-protein, low-fat diet, rather than a conventional weight-loss diet, in obese adults with type 2 diabetes: one-year follow-up of a randomised trial. Diabetologia. 2004;47(10):1677–1686. doi: 10.1007/s00125-004-1511-7. [DOI] [PubMed] [Google Scholar]
- 61.Krebs JD, Elley CR, Parry-Strong A, et al. The Diabetes Excess Weight Loss (DEWL) Trial: a randomised controlled trial of high-protein versus high-carbohydrate diets over 2 years in type 2 diabetes. Diabetologia. 2012;55(4):905–914. doi: 10.1007/s00125-012-2461-0. [DOI] [PubMed] [Google Scholar]
- 62.Larsen RN, Mann NJ, Maclean E, Shaw JE. The effect of high-protein, low-carbohydrate diets in the treatment of type 2 diabetes: a 12 month randomised controlled trial. Diabetologia. 2011;54(4):731–740. doi: 10.1007/s00125-010-2027-y. [DOI] [PubMed] [Google Scholar]
- 63.Luger M, Holstein B, Schindler K, Kruschitz R, Ludvik B. Feasibility and efficacy of an isocaloric high-protein vs. standard diet on insulin requirement, body weight and metabolic parameters in patients with type 2 diabetes on insulin therapy. Exp Clin Endocrinol Diabetes. 2013;121(5):286–294. doi: 10.1055/s-0033-1341472. [DOI] [PubMed] [Google Scholar]
- 64.Parker B, Noakes M, Luscombe N, Clifton P. Effect of a high-protein, high-monounsaturated fat weight loss diet on glycemic control and lipid levels in type 2 diabetes. Diabetes Care. 2002;25(3):425–430. doi: 10.2337/diacare.25.3.425. [DOI] [PubMed] [Google Scholar]
- 65.Pedersen E, Jesudason DR, Clifton PM. High protein weight loss diets in obese subjects with type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis. 2014;24(5):554–562. doi: 10.1016/j.numecd.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 66.Watson N, Dyer K, Buckley J, et al. Effects of low-fat diets differing in protein and carbohydrate content on cardiometabolic risk factors during weight loss and weight maintenance in obese adults with type 2 diabetes. Nutrients. 2016;8(5):289. doi: 10.3390/nu8050289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wycherley TP, Noakes M, Clifton PM, Cleanthous X, Keogh JB, Brinkworth GD. A high-protein diet with resistance exercise training improves weight loss and body composition in overweight and obese patients with type 2 diabetes. Diabetes Care. 2010;33(5):969–976. doi: 10.2337/dc09-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brehm BJ, Lattin BL, Summer SS, et al. One-year comparison of a high-monounsaturated fat diet with a high-carbohydrate diet in type 2 diabetes. Diabetes Care. 2009;32(2):215–220. doi: 10.2337/dc08-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brunerova L, Smejkalova V, Potockova J, Andel M. A comparison of the influence of a high-fat diet enriched in monounsaturated fatty acids and conventional diet on weight loss and metabolic parameters in obese non-diabetic and type 2 diabetic patients. Diabet Med. 2007;24(5):533–540. doi: 10.1111/j.1464-5491.2007.02104.x. [DOI] [PubMed] [Google Scholar]
- 70.de Bont AJ, Baker IA, St Leger AS, et al. A randomised controlled trial of the effect of low fat diet advice on dietary response in insulin independent diabetic women. Diabetologia. 1981;21(6):529–533. doi: 10.1007/BF00281543. [DOI] [PubMed] [Google Scholar]
- 71.Heilbronn LK, Noakes M, Clifton PM. Effect of energy restriction, weight loss, and diet composition on plasma lipids and glucose in patients with type 2 diabetes. Diabetes Care. 1999;22(6):889–895. doi: 10.2337/diacare.22.6.889. [DOI] [PubMed] [Google Scholar]
- 72.Milne RM, Mann JI, Chisholm AW, Williams SM. Long-term comparison of three dietary prescriptions in the treatment of NIDDM. Diabetes Care. 1994;17(1):74–80. doi: 10.2337/diacare.17.1.74. [DOI] [PubMed] [Google Scholar]
- 73.Rock CL, Flatt SW, Pakiz B, et al. Weight loss, glycemic control, and cardiovascular disease risk factors in response to differential diet composition in a weight loss program in type 2 diabetes: a randomized controlled trial. Diabetes Care. 2014;37(6):1573–1580. doi: 10.2337/dc13-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sato J, Kanazawa A, Makita S, et al. A randomized controlled trial of 130 g/day low-carbohydrate diet in type 2 diabetes with poor glycemic control. Clin Nutr. 2016 doi: 10.1016/j.clnu.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 75.Shige H, Nestel P, Sviridov D, Noakes M, Clifton P. Effect of weight reduction on the distribution of apolipoprotein A-I in high-density lipoprotein subfractions in obese non-insulin-dependent diabetic subjects. Metabolism. 2000;49(11):1453–1459. doi: 10.1053/meta.2000.17668. [DOI] [PubMed] [Google Scholar]
- 76.Walker KZ, O’Dea K, Nicholson GC, Muir JG. Dietary composition, body weight, and NIDDM. Comparison of high-fiber, high-carbohydrate, and modified-fat diets. Diabetes Care. 1995;18(3):401–403. doi: 10.2337/diacare.18.3.401. [DOI] [PubMed] [Google Scholar]
- 77.Wolever TM, Gibbs AL, Mehling C, et al. The Canadian Trial of Carbohydrates in Diabetes (CCD), a 1-y controlled trial of low-glycemic-index dietary carbohydrate in type 2 diabetes: no effect on glycated hemoglobin but reduction in C-reactive protein. Am J Clin Nutr. 2008;87(1):114–125. doi: 10.1093/ajcn/87.1.114. [DOI] [PubMed] [Google Scholar]
- 78.Yamada Y, Uchida J, Izumi H, et al. A non-calorie-restricted low-carbohydrate diet is effective as an alternative therapy for patients with type 2 diabetes. Int Med. 2014;53(1):13–19. doi: 10.2169/internalmedicine.53.0861. [DOI] [PubMed] [Google Scholar]
- 79.Hockaday TD, Hockaday JM, Mann JI, Turner RC. Prospective comparison of modified fat-high-carbohydrate with standard low-carbohydrate dietary advice in the treatment of diabetes: one year follow-up study. Br J Nutr. 1978;39(2):357–362. doi: 10.1079/bjn19780045. [DOI] [PubMed] [Google Scholar]
- 80.McLaughlin T, Carter S, Lamendola C, et al. Clinical efficacy of two hypocaloric diets that vary in overweight patients with type 2 diabetes: comparison of moderate fat versus carbohydrate reductions. Diabetes Care. 2007;30(7):1877–1879. doi: 10.2337/dc07-0301. [DOI] [PubMed] [Google Scholar]
- 81.Daly ME, Paisey R, Paisey R, et al. Short-term effects of severe dietary carbohydrate-restriction advice in Type 2 diabetes–a randomized controlled trial. Diabet Med. 2006;23(1):15–20. doi: 10.1111/j.1464-5491.2005.01760.x. [DOI] [PubMed] [Google Scholar]
- 82.Davis NJ, Tomuta N, Schechter C, et al. Comparative study of the effects of a 1-year dietary intervention of a low-carbohydrate diet versus a low-fat diet on weight and glycemic control in type 2 diabetes. Diabetes Care. 2009;32(7):1147–1152. doi: 10.2337/dc08-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dyson PA, Beatty S, Matthews DR. A low-carbohydrate diet is more effective in reducing body weight than healthy eating in both diabetic and non-diabetic subjects. Diabet Med. 2007;24(12):1430–1435. doi: 10.1111/j.1464-5491.2007.02290.x. [DOI] [PubMed] [Google Scholar]
- 84.Guldbrand H, Dizdar B, Bunjaku B, et al. In type 2 diabetes, randomisation to advice to follow a low-carbohydrate diet transiently improves glycaemic control compared with advice to follow a low-fat diet producing a similar weight loss. Diabetologia. 2012;55(8):2118–2127. doi: 10.1007/s00125-012-2567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iqbal N, Vetter ML, Moore RH, et al. Effects of a low-intensity intervention that prescribed a low-carbohydrate vs. a low-fat diet in obese, diabetic participants. Obesity. 2010;18(9):1733–1738. doi: 10.1038/oby.2009.460. [DOI] [PubMed] [Google Scholar]
- 86.Stern L, Iqbal N, Seshadri P, et al. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: one-year follow-up of a randomized trial. Ann Intern Med. 2004;140(10):778–785. doi: 10.7326/0003-4819-140-10-200405180-00007. [DOI] [PubMed] [Google Scholar]
- 87.Tay J, Luscombe-Marsh ND, Thompson CH, et al. Comparison of low- and high-carbohydrate diets for type 2 diabetes management: a randomized trial. Am J Clin Nutr. 2015;102(4):780–790. doi: 10.3945/ajcn.115.112581. [DOI] [PubMed] [Google Scholar]
- 88.Andrews RC, Cooper AR, Montgomery AA, et al. Diet or diet plus physical activity versus usual care in patients with newly diagnosed type 2 diabetes: the Early ACTID randomised controlled trial. Lancet. 2011;378(9786):129–139. doi: 10.1016/S0140-6736(11)60442-X. [DOI] [PubMed] [Google Scholar]
- 89.Coppell KJ, Kataoka M, Williams SM, Chisholm AW, Vorgers SM, Mann JI. Nutritional intervention in patients with type 2 diabetes who are hyperglycaemic despite optimised drug treatment–Lifestyle Over and Above Drugs in Diabetes (LOADD) study: randomised controlled trial. BMJ. 2010;341:c3337. doi: 10.1136/bmj.c3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang MC, Hsu CC, Wang HS, Shin SJ. Prospective randomized controlled trial to evaluate effectiveness of registered dietitian-led diabetes management on glycemic and diet control in a primary care setting in Taiwan. Diabetes Care. 2010;33(2):233–239. doi: 10.2337/dc09-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kaplan RM, Hartwell SL, Wilson DK, Wallace JP. Effects of diet and exercise interventions on control and quality of life in non-insulin-dependent diabetes mellitus. J Gen Intern Med. 1987;2(4):220–228. doi: 10.1007/BF02596443. [DOI] [PubMed] [Google Scholar]
- 92.Li X, Cai X, Ma X, et al. Short- and long-term effects of wholegrain oat intake on weight management and glucolipid metabolism in overweight type-2 diabetics: a randomized control trial. Nutrients. 2016;8(9):549. doi: 10.3390/nu8090549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pritchard DA, Hyndman J, Taba F. Nutritional counselling in general practice: a cost effective analysis. J Epidemiol Community Health. 1999;53(5):311–316. doi: 10.1136/jech.53.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Uusitupa M, Laitinen J, Siitonen O, Vanninen E, Pyorala K. The maintenance of improved metabolic control after intensified diet therapy in recent type 2 diabetes. Diabetes Res Clin Pract. 1993;19(3):227–238. doi: 10.1016/0168-8227(93)90118-o. [DOI] [PubMed] [Google Scholar]
- 95.Liu H, Zhang M, Wu X, Wang C, Li Z. Effectiveness of a public dietitian-led diabetes nutrition intervention on glycemic control in a community setting in China. Asia Pac J Clin Nutr. 2015;24(3):525–532. doi: 10.6133/apjcn.2015.24.3.07. [DOI] [PubMed] [Google Scholar]
- 96.Brand JC, Colagiuri S, Crossman S, Allen A, Roberts DC, Truswell AS. Low-glycemic index foods improve long-term glycemic control in NIDDM. Diabetes Care. 1991;14(2):95–101. doi: 10.2337/diacare.14.2.95. [DOI] [PubMed] [Google Scholar]
- 97.Fabricatore AN, Wadden TA, Ebbeling CB, et al. Targeting dietary fat or glycemic load in the treatment of obesity and type 2 diabetes: a randomized controlled trial. Diabetes Res Clin Pract. 2011;92(1):37–45. doi: 10.1016/j.diabres.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jenkins DJ, Kendall CW, Augustin LS, et al. Effect of legumes as part of a low glycemic index diet on glycemic control and cardiovascular risk factors in type 2 diabetes mellitus: a randomized controlled trial. Arch Intern Med. 2012;172(21):1653–1660. doi: 10.1001/2013.jamainternmed.70. [DOI] [PubMed] [Google Scholar]
- 99.Jenkins DJ, Kendall CW, McKeown-Eyssen G, et al. Effect of a low-glycemic index or a high-cereal fiber diet on type 2 diabetes: a randomized trial. JAMA. 2008;300(23):2742–2753. doi: 10.1001/jama.2008.808. [DOI] [PubMed] [Google Scholar]
- 100.Jenkins DJ, Kendall CW, Vuksan V, et al. Effect of lowering the glycemic load with canola oil on glycemic control and cardiovascular risk factors: a randomized controlled trial. Diabetes Care. 2014;37(7):1806–1814. doi: 10.2337/dc13-2990. [DOI] [PubMed] [Google Scholar]
- 101.Ma Y, Olendzki BC, Merriam PA, et al. A randomized clinical trial comparing low-glycemic index versus ADA dietary education among individuals with type 2 diabetes. Nutrition. 2008;24(1):45–56. doi: 10.1016/j.nut.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Westman EC, Yancy WS, Jr, Mavropoulos JC, Marquart M, McDuffie JR. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutrit Metabol. 2008;5:36. doi: 10.1186/1743-7075-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Saslow LR, Kim S, Daubenmier JJ, et al. A randomized pilot trial of a moderate carbohydrate diet compared to a very low carbohydrate diet in overweight or obese individuals with type 2 diabetes mellitus or prediabetes. PLoS ONE. 2014;9(4):e91027. doi: 10.1371/journal.pone.0091027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hoaglin DC, Hawkins N, Jansen JP, et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2. Value Health. 2011;14(4):429–437. doi: 10.1016/j.jval.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 105.Snorgaard O, Poulsen GM, Andersen HK, Astrup A. Systematic review and meta-analysis of dietary carbohydrate restriction in patients with type 2 diabetes. BMJ Open Diabetes Res Care. 2017;5(1):e000354. doi: 10.1136/bmjdrc-2016-000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hashimoto Y, Fukuda T, Oyabu C, et al. Impact of low-carbohydrate diet on body composition: meta-analysis of randomized controlled studies. Obes Rev. 2016;17(6):499–509. doi: 10.1111/obr.12405. [DOI] [PubMed] [Google Scholar]
- 107.American Diabetes Association Older adults. Diabetes Care. 2017;40(Suppl 1):S99–s104. doi: 10.2337/dc17-S014. [DOI] [PubMed] [Google Scholar]
- 108.International Diabetes Federation-Global guidelines: managing older people with type 2 diabetes. 2017. https://www.idf.org/e-library/guidelines/78-global-guideline-for-managing-older-people-with-type-2-diabetes.html. Accessed 05 Nov 2017.
- 109.Brand-Miller J, Hayne S, Petocz P, Colagiuri S. Low-glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care. 2003;26(8):2261–2267. doi: 10.2337/diacare.26.8.2261. [DOI] [PubMed] [Google Scholar]
- 110.Group TLAR Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369(2):145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kirk JK, Graves DE, Craven TE, Lipkin EW, Austin M, Margolis KL. Restricted-carbohydrate diets in patients with type 2 diabetes: a meta-analysis. J Am Diet Assoc. 2008;108(1):91–100. doi: 10.1016/j.jada.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 112.Huo R, Du T, Xu Y, et al. Effects of Mediterranean-style diet on glycemic control, weight loss and cardiovascular risk factors among type 2 diabetes individuals: a meta-analysis. Eur J Clin Nutr. 2015;69(11):1200–1208. doi: 10.1038/ejcn.2014.243. [DOI] [PubMed] [Google Scholar]
- 113.Dong JY, Zhang ZL, Wang PY, Qin LQ. Effects of high-protein diets on body weight, glycaemic control, blood lipids and blood pressure in type 2 diabetes: meta-analysis of randomised controlled trials. Br J Nutr. 2013;110(5):781–789. doi: 10.1017/S0007114513002055. [DOI] [PubMed] [Google Scholar]
- 114.Liese AD, Roach AK, Sparks KC, Marquart L, D’Agostino RB, Jr, Mayer-Davis EJ. Whole-grain intake and insulin sensitivity: the Insulin Resistance Atherosclerosis Study. Am J Clin Nutr. 2003;78(5):965–971. doi: 10.1093/ajcn/78.5.965. [DOI] [PubMed] [Google Scholar]
- 115.Xiao JB, Hogger P. Dietary polyphenols and type 2 diabetes: current insights and future perspectives. Curr Med Chem. 2015;22(1):23–38. doi: 10.2174/0929867321666140706130807. [DOI] [PubMed] [Google Scholar]
- 116.Schwingshackl L, Strasser B, Hoffmann G. Effects of monounsaturated fatty acids on glycaemic control in patients with abnormal glucose metabolism: a systematic review and meta-analysis. Ann Nutr Metab. 2011;58(4):290–296. doi: 10.1159/000331214. [DOI] [PubMed] [Google Scholar]
- 117.Medina-Remon A, Tresserra-Rimbau A, Pons A, et al. Effects of total dietary polyphenols on plasma nitric oxide and blood pressure in a high cardiovascular risk cohort. The PREDIMED randomized trial. Nutr Metab Cardiovasc Dis. 2015;25(1):60–67. doi: 10.1016/j.numecd.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 118.Wainstein J, Ganz T, Boaz M, et al. Olive leaf extract as a hypoglycemic agent in both human diabetic subjects and in rats. J Med Food. 2012;15(7):605–610. doi: 10.1089/jmf.2011.0243. [DOI] [PubMed] [Google Scholar]
- 119.Guasch-Ferre M, Merino J, Sun Q, Fito M, Salas-Salvado J. Dietary polyphenols, Mediterranean diet, prediabetes, and type 2 diabetes: a narrative review of the evidence. Oxid Med Cell Longev. 2017;2017:6723931. doi: 10.1155/2017/6723931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gorst C, Kwok CS, Aslam S, et al. Long-term glycemic variability and risk of adverse outcomes: a systematic review and meta-analysis. Diabetes Care. 2015;38(12):2354–2369. doi: 10.2337/dc15-1188. [DOI] [PubMed] [Google Scholar]
- 121.Lawes CM, Parag V, Bennett DA, et al. Blood glucose and risk of cardiovascular disease in the Asia Pacific region. Diabetes Care. 2004;27(12):2836–2842. doi: 10.2337/diacare.27.12.2836. [DOI] [PubMed] [Google Scholar]
- 122.Turner RC, Millns H, Neil HA, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23) BMJ. 1998;316(7134):823–828. doi: 10.1136/bmj.316.7134.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Currie CJ, Peters JR, Tynan A, et al. Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet. 2010;375(9713):481–489. doi: 10.1016/S0140-6736(09)61969-3. [DOI] [PubMed] [Google Scholar]
- 124.Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med. 2004;141(6):413–420. doi: 10.7326/0003-4819-141-6-200409210-00006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.