Highlights
-
•
We conducted a review and meta-analysis of esophageal cancer sex ratios in mainland Africa using data from 197 populations in 36 countries.
-
•
We observed a consistent male excess in incidence rates overall and in the high-risk Eastern and Southern African regions.
-
•
A male excess was evident in 30–39 year olds in high-risk Eastern and Southern African regions.
-
•
Our findings suggest that a substantial fraction of the African EC burden could be avoided by targeting gender-specific exposures.
Abbreviations: AC, adenocarcinoma; AFCRN, African Cancer Registry Network; ASR, age-standardised rate; CI, confidence interval; CI5, Cancer Incidence in Five Continents (publication series/data source); CIA, Cancer in Africa (publication/data source); CISSA, Cancer in Sub-Saharan Africa (publication/data source); EC, esophageal cancer; ESCC, esophageal squamous cell carcinoma; IARC, International Agency for Research on Cancer; IRR, incidence rate ratio; M:F, male-to-female; PAH, polycyclic aromatic hydrocarbon; PBCR, population-based cancer registry; UN, United Nations
Keywords: Esophageal cancer, Sex ratios, Africa, Meta-analysis, Systematic review
Abstract
Esophageal squamous cell carcinoma (ESCC) remains the predominant histological subtype of esophageal cancer (EC) in many transitioning countries, with an enigmatic and geographically distinct etiology, and consistently elevated incidence rates in many Eastern and Southern African countries. To gain epidemiological insights into ESCC patterns across the continent, we conducted a systematic review and meta-analysis of male-to-female (M:F) sex ratios of EC age-standardised (world) incidence rates in Africa according to geography, time and age at diagnosis. Data from 197 populations in 36 countries were included in the analysis, based on data from cancer registries included in IARC’s Cancer Incidence in Five Continents, Cancer in Africa and Cancer in Sub-Saharan Africa reports, alongside a systematic search of peer-reviewed literature. A consistent male excess in incidence rates overall (1.7; 95% CI: 1.4, 2.0), and in the high-risk Eastern (1.6; 95% CI: 1.4, 1.8) and Southern (1.8; 95% CI: 1.5, 2.0) African regions was observed. Within the latter two regions, there was a male excess evident in 30–39 year olds that was not observed in low-risk regions. Despite possible referral biases affecting the interpretability of the M:F ratios in place and time, the high degree of heterogeneity in ESCC incidence implies a large fraction of the disease is preventable, and directs research enquiries to elucidate early-age exposures among young men in Africa.
1. Introduction
Esophageal cancer (EC) is the 8th most common cancer worldwide and is ranked 6th for cancer-related mortality [1]. The dominant histological subtype of EC worldwide is esophageal squamous cell carcinoma (ESCC), followed by adenocarcinoma (AC) [2]. Incidence rates of ESCC display marked geographic variation worldwide, with notably high incidence occurring in certain regions within countries in South America (e.g. Brazil), Asia (e.g. China and Iran) and Southern and Eastern Africa – the focus of this paper – where incidence rates are 20-fold higher than in West Africa [3]. In this African EC corridor of high risk, ESCC accounts for 94% of EC cases [2] and is the 2nd most common cancer in some populations e.g. in Malawian males [4], where age-standardised (world) incidence rates (ASR) are over 30 per 100,000. The diagnosis of ESCC often occurs at late-stage following the onset of dysphagia. Prognosis is extremely poor, thus the identification of risk factors and primary prevention strategies are a priority for reducing the burden [5].
Few etiologic studies of ESCC have been conducted in Africa relative to other high incidence regions worldwide, and as a consequence, the epidemiology of the disease in endemic regions remains poorly understood. Nevertheless, several well-established ESCC carcinogens including tobacco and alcohol consumption are likely to partially explain the elevated incidence [[5], [6]] and studies thereof are underway. Etiological clues can be additionally gained from descriptive studies of ESCC in Africa, including investigating differences in incidence between the sexes, as has been conducted for other cancers [[7], [8], [9]]. Establishing the presence or absence of a sex differential in different African settings could provide clues as to the nature of contributing risk factors, i.e. whether gender-associated behaviours and exposures are likely to be implicated. While gender differences in certain exposures are unclear in sub-Saharan Africa (e.g. gender-specific patterns of dietary intake are not well described), it is evident that several potential contributors, e.g. tobacco and alcohol use, are more prevalent among males [10]. Further, a stand-out feature of the East African ESCC burden is the unusually high number of young (aged < 40 years old) patients [11]. Investigating whether a sex difference is evident in these young age groups, and at what age it manifests, will be of particular value in pointing to potential contributions of early life exposures and inherited susceptibility.
We therefore aimed in this study to examine sex differentials in incidence rates of EC across Africa. Using data from cancer registries included in IARC’s Cancer Incidence in Five Continents (CI5), Cancer in Africa (CIA) and Cancer in Sub-Saharan Africa (CISSA) reports, alongside a systematic search of peer-reviewed literature, we explored variations by country, region, over time and by age, with a focus on high-risk regions in Eastern and Southern Africa.
2. Material and methods
We undertook a systematic review and meta-analysis of published data, reports and studies from which sex ratios (male-to-female) for EC incidence in Africa could be estimated for individual study ‘populations’ unique in place, calendar year and population group (e.g. ethnicity). Note: we refer to the ratio of male to female incidence rates as sex ratios as per the reporting of incidence data by biological sex, but use them as a tool to investigate gender-associated behaviours and exposures, rather than sex-linked biological risk factors – see [12].
2.1. Data sources and inclusion criteria
EC incidence sex ratios were estimated for populations satisfying the following criteria: (i) located in mainland countries within the United Nations (UN) demarcation of Africa; (ii) adult populations aged 20 years and over; (iii) indigenous African populations (predominantly or exclusively). Initially, no restrictions on year of diagnosis were applied.
Three data sources were used. Age and sex-specific EC (ICD-10 C15) incidence data were extracted from the CI5 series of volumes [13] a compendium of high quality cancer incidence based on submissions from population-based cancer registries (PBCR) at the national or subnational level, that is, in volumes for which African data were compiled.
Additional age- and sex-specific incidence data were sourced from the Cancer in Africa (CIA) [14] and Cancer in Sub-Saharan Africa (CISAA) (in-press), which captures incidence data from population-based cancer registries in Africa who are part of the African Cancer Registry Network (AFCRN). Finally, a systematic review of peer-reviewed literature was conducted using the following databases: Medline via Ovid, Google Scholar, Embase, Global Health, African Journals Online and BHOI-INCTR Cancer Control Library using the following terms: esophag* OR oesophag*, cancer OR neopl* OR tumour OR carcin* OR malig*, Africa OR Sub-Saharan OR country name (listed individually, including historical names e.g. Rhodesia). No lower date restrictions were imposed and studies published before August 2017 were included. Manuscripts were excluded if they did not include sex-specific incidence counts, were occupational cohorts or used sampling designs that were suggestive of deliberately modifying sex distributions. To avoid duplication due to the many journal articles presenting registry data, studies that overlapped with CI5 or the CIA/CISSA data, based on population group, place and date of diagnosis ranges, were excluded and CI5/CIA/CISSA data were retained.
Most data sources either did not report on EC histological types separately (ESCC vs AC) or the percentage of morphological verification was low, which prevented us from performing analyses on histologically verified ESCC. Estimates from a previous study [2] which reported the global incidence of EC by histological subtype were examined and the mean ESCC proportion for Africa was 93%. Therefore, we deemed this a negligible limitation.
2.2. Data extraction
From each source and for each population, we extracted the following data: country; location; derived UN geographic region in 2017; source or study design (population-based cancer registry (PBCR), other registry, case series, cases from case-control studies or cohorts); male, female and total number of EC cases; male, female and combined ASR; person-years or population size; calendar year and age group of diagnosis.
2.3. Statistical analysis
For each population, estimated sex incidence rate ratios (IRRs) of EC, defined as ratios of EC cancer incidence rates between sexes (male-to-female), were calculated by fitting Poisson regression models on EC counts, with a log offset of person-years if available or population data from censuses if not, with covariates included for sex and, if available, age (20–29 years, 30–39, 40–49, 50–59, 60–69, and 70+). The adjusted IRR was then estimated as exp(βsex). Studies that had no population data available were modelled in a similar fashion, but without a log offset, to produce a count ratio as an estimate of the sex IRR. To examine the comparability of sex count ratios and of sex IRRs, we also calculated count ratios for the latter and compared these ratios to the known IRR. The mean ratio of count ratio to IRR was 1.02 and the correlation was strong (Spearman’s rank correlation coefficient ρ = 0.91, Fig. S1 in Supporting information) suggesting that count ratios could be used as good proxy of IRRs when population size is missing. Throughout, populations with no EC cases for both males and females did not contribute. If there were zero cases in either sex, they were also excluded if the total number of cases was less than five, and if five or more, zero events were censored with the value of 0.5.
Country-specific sex ratios were estimated by performing a random effects meta-analysis of each country’s population-specific estimates (derived as above) and presented on a forest plot. Heterogeneity across studies/populations was tested using the I-squared (I2) statistic, which estimates the percentage (0 to 100%) of total variation across studies that is due to heterogeneity rather than chance. A value of 0% indicates no heterogeneity, and larger values show increasing heterogeneity. Meta-analyses were presented according to broad region and overall EC incidence: high incidence (Eastern Africa); high incidence (Southern Africa) and low incidence. “High incidence” refers to those countries where either male or female ASRs were greater than their respective world mean (male:9.0; female:3.1) according to GLOBOCAN 2012 estimates. While GLOBOCAN estimates don’t permit a robust demarcation of a high incidence region, based on the above criteria, the following countries were categorised as high incidence: Malawi, Uganda, Kenya, South Africa, Tanzania, Mozambique, Zimbabwe, Zambia, Swaziland, Ethiopia and Sudan. The other countries that meet the criteria, but did not contribute to this analysis are Lesotho, Burundi, Botswana, Comoros, South Sudan, Somalia, Madagascar, Rwanda, Angola, Djibouti and Eritrea.
Age group-specific EC sex IRRs were also estimated using a Poisson regression stratified by age group (with the same age groups as above) and were then used in a meta-analysis by regions based on geography and incidence. In the event that a provided age group overlapped two age groups, the upper age category was assumed, e.g. age stratum 25–34 were categorised in the 30–39 as older EC patients are more common.
To interpret results in the context of the world EC scenario, African sex IRRs were compared with those estimated from CI5 volume X registries, ensuring no overlapping populations. Sex IRRs and absolute male excesses were plotted against female EC ASRs, taken from the present analysis where available and GLOBCAN 2012 estimates were substituted in their absence.
Statistical analyses were performed using R, version 3.3.1, [15] and p-values lower than .05 were considered statistically significant.
3. Results
3.1. Studies and data included
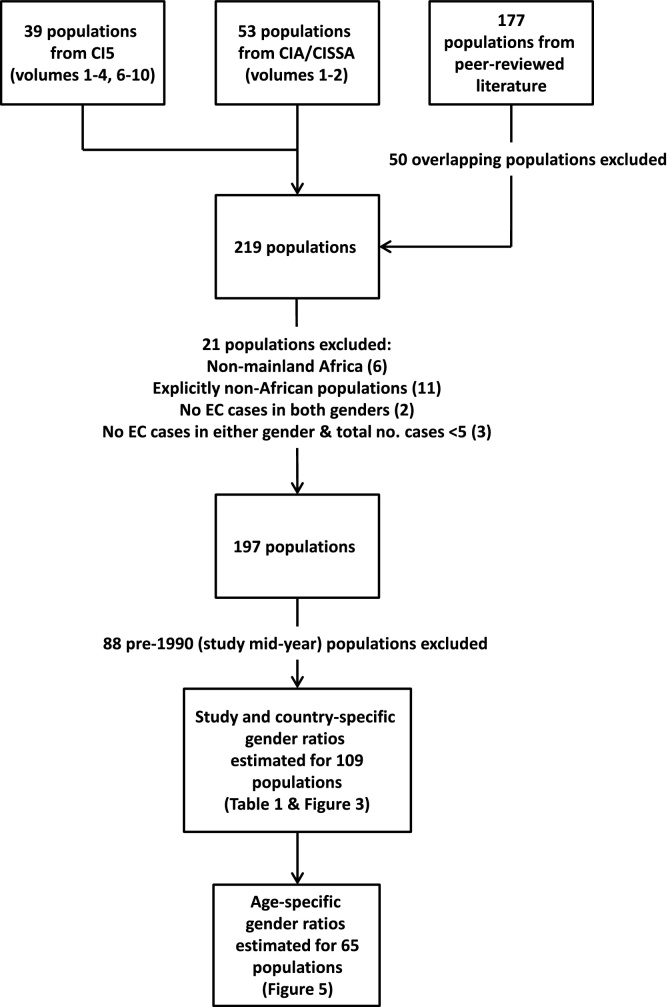
A flow chart of data inclusion in the different analytical components is presented in Fig. 1.
Fig. 1.
Flow diagram of population inclusion in the different analytical components of the study. Population refers to a group of individuals sharing a common location, period of diagnosis and ethnicity.
After exclusions, EC incidence data for 35 populations from 13 African countries were included from CI5 (4791 EC cases), including 4 from the EC high incidence corridor (i.e. countries with a male or female ASR greater than its global average): Malawi, South Africa, Uganda and Zimbabwe. CIA/CISSA data contributed a further 41 populations from 22 countries (15,669 EC cases), including from 12 countries not reported in CI5. A systematic review of peer-reviewed literature yielded 121 eligible populations from 25 countries (25,035 EC cases), including data from 11 additional countries to CI5 or CIA/CISSA. Fifty (28%) literature studies initially identified (177 populations) were excluded due to duplication. From all sources, a total of 197 populations from 36 of the 49 UN mainland African member states met the inclusion criteria and were retained for analysis – resulting in a sample size of 45,495 EC cases: 29,828 in men and 15,667 in women. The vast majority of cases were from countries in the Eastern (27%) and Southern African (61%) regions.
3.2. Temporal variation in EC sex ratios
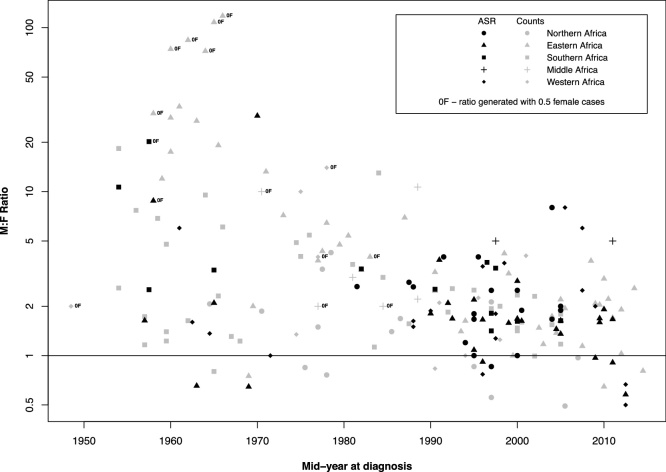
Overall, 11% of M:F ratios were <1, 36% were between 1 to <2, 27% were between 2 to <4 and 26% were 4% or over. Plotting ratios by time, a strong negative trend was observed (Fig. 2). In particular, pre-1990 ratios from Eastern and Southern Africa were much higher and have substantially declined. Some estimates [16] had over 30 men, whilst no female EC cases were present. Due to this temporal trend, all pre-1990s data were excluded from further analyses (88 populations). Studies reporting data from before 1990 and the ratios calculated using them are presented in Table S1 for reference (Supporting information).
Fig. 2.
Male-to-female esophageal cancer incidence ratios (y-axis displayed in log scale) plotted against the mid-year of diagnosis, estimated by IRRs (black points) or count ratios (grey points).
3.3. Geographic variation in EC sex ratios
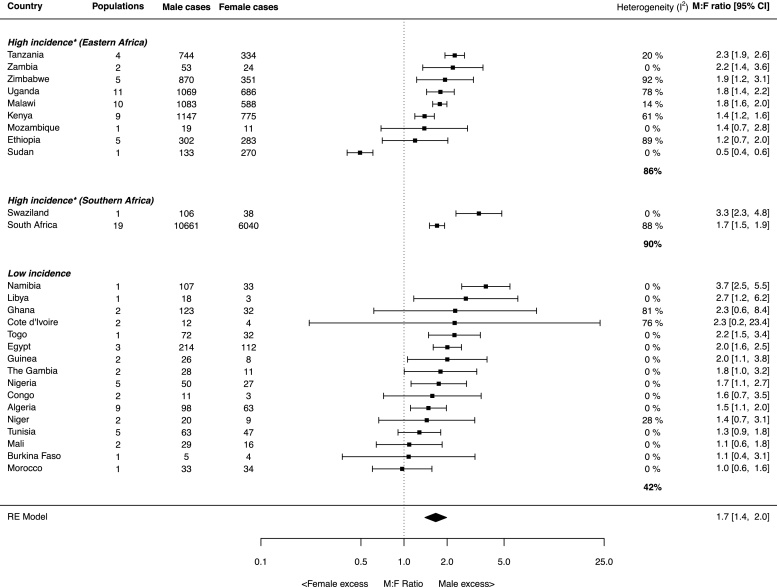
The sex ratios, calculated for each individual study, that were used to generate country-specific estimates are presented in Table 1 and are organised by UN geographic region and country. Country and region-specific (revised regions based on EC incidence) sex ratios for 27 countries are displayed in the forest plot in Fig. 3. All estimates are age-adjusted, except for four countries (Morocco, Sudan, Zambia and Togo).
Table 1.
Study/population-specific sex ratios estimated using Poisson regression. *Study designs: 1 = registry data; 2 = case series; 3 = case-control study.
| First author (year) | Data source | Location | Year at diagnosis | Study design* | M:F ratio | Male cases (ASR, where available) | Female cases (ASR, where available) | Total cases |
|---|---|---|---|---|---|---|---|---|
| Northern Africa | ||||||||
| Algeria | ||||||||
| IARC (1997) | CI5 (vol. VII) | Setif | 1990–1993 | 1 | 2.03 | 9 (1.2) | 4 (0.3) | 13 |
| Meguenni (1998) [14] | Literature | Tlemcen | 1994–1996 | 2 | 0.86 | 6 | 7 | 13 |
| IARC (2002) | CI5 (vol. VIII) | Algiers | 1993–1997 | 1 | 1.77 | 34 (0.9) | 19 (0.5) | 53 |
| IARC (2003) | CIA | Batna | 1995–1999 | 1 | 0.70 | 5 | 9 | 14 |
| IARC (2003) | CIA | Constantine | 1994–1997 | 1 | 1.50 | 3 (0.4) | 1 (0.1) | 4 |
| IARC (2003) | CIA | Oran | 1996–1998 | 1 | 2.09 | 17 (1.5) | 8 (0.6) | 25 |
| IARC (2003) | CIA | Setif | 1993–1997 | 1 | 1.73 | 9 (0.5) | 5 (0.3) | 14 |
| IARC (2007) | CI5 (vol. IX) | Setif | 1998–2002 | 1 | 0.84 | 3 (0.2) | 4 (0.2) | 7 |
| IARC (2013) | CI5 (vol. X) | Setif | 2003–2007 | 1 | 1.64 | 12 (0.4) | 6 (0.2) | 18 |
| Egypt | ||||||||
| Bahnassy (2005) [17] | Literature | Cairo | 1996–1998 | 2 | 2.13 | 34 | 16 | 50 |
| IARC (2007) | CI5 (vol. IX) | Gharbiah | 1999–2002 | 1 | 1.99 | 70 (1.7) | 40 (0.9) | 110 |
| IARC (2013) | CI5 (vol. X) | Gharbiah | 2003–2007 | 1 | 1.99 | 110 (1.7) | 56 (0.9) | 166 |
| Libya | ||||||||
| IARC (2013) | CI5 (vol. X) | Benghazi | 2003–2005 | 1 | 2.70 | 18 (1.6) | 3 (0.2) | 21 |
| Morocco | ||||||||
| Chbani (2012) [18] | Literature | Fez | 2004–2010 | 2 | 0.97 | 33 | 34 | 67 |
| Sudan | ||||||||
| Gasmelseed (2015) [19] | Literature | Gezira state | 1999–2012 | 2 | 0.49 | 133 | 270 | 403 |
| Tunisia | ||||||||
| IARC (2003) | CIA | Sousse | 1993–1997 | 1 | 1.02 | 3 (0.4) | 3 (0.4) | 6 |
| IARC (2003) | CIA | Tunis | 1994 | 1 | 1.10 | 18 (1.2) | 16 (1.0) | 34 |
| IARC (2003) | CIA | Sfax | 1997 | 1 | 1.10 | 2 (0.6) | 2 (0.7) | 4 |
| IARC (2007) | CI5 (vol. IX) | Sousse | 1998–2002 | 1 | 1.61 | 5 (0.5) | 2 (0.2) | 7 |
| IARC (2013) | CI5 (vol. X) | North | 2003–2005 | 1 | 1.40 | 35 (0.5) | 24 (0.3) | 59 |
| Total cases (Northern Africa) | 559 | 529 | 1088 | |||||
| Eastern Africa | ||||||||
| Ethiopia | ||||||||
| Ali (1998) [20] | Literature | Addia Ababa | 1992–1996 | 2 | 1.63 | 88 | 54 | 142 |
| Ashine & Lemma (1999) [21] | Literature | Sidama | 1986–1995 | 2 | 3.23 | 42 | 13 | 55 |
| Shewaye & Seme (2016) [22] | Literature | Addis Ababa | 2014–2015 | 3 | 0.81 | 96 | 119 | 215 |
| Leon (2017) [23] | Literature | Addis Ababa | 2012–2012 | 3 | 1.03 | 37 | 36 | 73 |
| IARC (In press) | CIA | Addis Ababa | 2012–2013 | 1 | 0.65 | 39 (2.2) | 61 (3.8) | 100 |
| Kenya | ||||||||
| White (2002) [24] | Literature | Tenwek | 1989–1998 | 2 | 1.40 | 160 | 114 | 274 |
| IARC (2003) | CIA | Eldoret | 1998–2000 | 1 | 1.62 | 89 (24.5) | 51 (15.5) | 140 |
| Lodenyo (2005) [25] | Literature | Nairobi | 1998–2001 | 2 | 1.00 | 34 | 34 | 68 |
| Ogendo (2007) [26] | Literature | Nairobi | 2001–2005 | 2 | 1.17 | 32 | 27 | 59 |
| Dawsey (2010) [11] | Literature | Tenwek (<30 year olds) | 1996–2009 | 2 | 1.48 | 65 | 44 | 109 |
| Parker (2011) [27] | Literature | Tenwek | 1999–2009 | 3 | 1.74 | 177 | 102 | 279 |
| Patel (2013) [28] | Literature | Eldoret | 2003–2006 | 3 | 1.37 | 92 | 67 | 159 |
| IARC (In press) | CIA | Eldoret | 2008–2011 | 1 | 1.79 | 220 (28.5) | 122 (16.9) | 342 |
| IARC (In press) | CIA | Nairobi | 2007–2011 | 1 | 1.00 | 278 (12.1) | 214 (12.5) | 492 |
| Malawi | ||||||||
| Banda (2001) [29] | Literature | Blantyre district | 1994–1998 | 1 | 2.11 | 148 (15.4) | 70 (9.3) | 218 |
| IARC (2003) | CIA | Blantyre | 2000–2001 | 1 | 1.80 | 65 (17.4) | 31 (10.7) | 96 |
| Crofts (2008) [30] | Literature | Blantyre | 2007–2008 | 2 | 1.14 | 24 | 21 | 45 |
| Mothes (2009) [31] | Literature | Zomba | 2004–2006 | 2 | 2.20 | 83 | 38 | 121 |
| Gyorki (2012) [32] | Literature | Lilongwe | 2005–2006 | 2 | 1.94 | 33 | 17 | 50 |
| Wolf (2012) [33] | Literature | Lilongwe | 2008–2010 | 2 | 2.08 | 183 | 88 | 271 |
| IARC (2013) | CI5 (vol. X) | Blantyre | 2003–2007 | 1 | 1.61 | 320 (37.6) | 182 (23) | 502 |
| Mtonga (2013) [34] | Literature | Blantyre | 2010 | 2 | 0.65 | 11 | 17 | 28 |
| Mlombe (2015) [35] | Literature | Lilongwe & Blantyre | 2011–2013 | 3 | 1.91 | 63 | 33 | 96 |
| IARC (In press) | CIA | Blantyre | 2009–2010 | 1 | 1.66 | 153 (30.8) | 91 (19.3) | 244 |
| Mozambique | ||||||||
| IARC (In press) | CIA | Beira | 2009–2013 | 1 | 1.39 | 19 (5.5) | 11 (3.3) | 30 |
| Tanzania | ||||||||
| IARC (2003) | CIA | Dar es Salaam | 1990–1991 | 1 | 2.63 | 60 | 23 | 83 |
| IARC (2003) | CIA | Kilimanjaro | 1998–2000 | 1 | 3.33 | 76 | 22 | 98 |
| Mchembe (2013) [36] | Literature | Mwanza & Dar es Salaam | 2008–2013 | 2 | 2.22 | 226 | 102 | 328 |
| Gabel (2016) [37] | Literature | Dar es Salaam | 2006–2013 | 2 | 2.04 | 382 | 187 | 569 |
| Uganda | ||||||||
| Wabinga (1993) | Literature | Kyadondo county | 1989–1991 | 1 | 1.65 | 43 (13.0) | 26 (7.2) | 69 |
| IARC (1997) | CI5 (vol. VII) | Kampala | 1991–1993 | 1 | 1.84 | 63 (18.2) | 36 (8.7) | 99 |
| Wabinga (2000) | Literature | Kyadondo county | 1991–1994 | 1 | 1.51 | 83 (15.8) | 55 (9.4) | 138 |
| Wabinga (2000) | Literature | Kyadondo county | 1995–1997 | 1 | 1.08 | 68 (13.0) | 63 (14.2) | 131 |
| IARC (2002) | CI5 (vol. VIII) | Kampala | 1993–1997 | 1 | 1.16 | 104 (13.2) | 91 (12.2) | 195 |
| IARC (2003) | CIA | Mbarara | 1997–2000 | 1 | 4.25 | 42 | 9 | 51 |
| IARC (2007) | CI5 (vol. IX) | Kampala | 1998–2002 | 1 | 1.76 | 121 (14.1) | 77 (8.4) | 198 |
| IARC (2013) | CI5 (vol. X) | Kyadondo county | 2003–2007 | 1 | 1.47 | 157 (15.6) | 125 (11.5) | 282 |
| Alema & Iva (2014) [38] | Literature | Northern Uganda | 2009–2011 | 2 | 2.86 | 53 | 18 | 71 |
| Okello (2016) [39] | Literature | Mbarara | 2003–2014 | 3 | 3.79 | 53 | 14 | 67 |
| IARC (In press) | CIA | Kyadondo county | 2008–2012 | 1 | 1.92 | 282 (22.6) | 172 (11.8) | 454 |
| Zambia | ||||||||
| Kayamba (2015) [40] | Literature | Lusaka | 2013–2014 | 3 | 2.57 | 36 | 14 | 50 |
| Asombang (2016) [41] | Literature | Lusaka | 2010–2012 | 3 | 1.70 | 17 | 10 | 27 |
| Zimbabwe | ||||||||
| IARC (1997) | CI5 (vol. VII) | Harare | 1990–1992 | 1 | 3.68 | 153 (29.5) | 22 (7.7) | 175 |
| IARC (2002) | CI5 (vol. VIII) | Harare | 1993–1997 | 1 | 2.23 | 220 (19.3) | 65 (8.8) | 285 |
| IARC (2007) | CI5 (vol. IX) | Harare | 1998–2002 | 1 | 2.57 | 191 (15.1) | 57 (5.3) | 248 |
| IARC (2013) | CI5 (vol. X) | Harare | 2003–2006 | 1 | 1.49 | 181 (22.2) | 99 (15.3) | 280 |
| IARC (In press) | CIA | Harare | 2010–2012 | 1 | 0.93 | 125 (14.6) | 108 (16.1) | 233 |
| Total cases (Eastern Africa) | 5287 | 3052 | 8339 | |||||
| Southern Africa | ||||||||
| Namibia | ||||||||
| IARC (2003) | CIA | 1995–1998 | 1 | 3.73 | 107 (6.3) | 33 (1.7) | 140 | |
| South Africa | ||||||||
| Levy (1998) [42] | Literature | Soweto | 1998 | 3 | 2.00 | 26 | 13 | 39 |
| Dietzsch (2002) [43] | Literature | Cape Town | 2002 | 3 | 2.30 | 23 | 10 | 33 |
| Pacella–Norman (2002) [44] | Literature | Johannesburg | 1995–1999 | 3 | 1.93 | 267 | 138 | 405 |
| IARC (2003) | CIA | 1989–1992 | 1 | 2.49 | 6592 (23.1) | 2990 (9.1) | 9582 | |
| IARC (2003) | CIA | Elim | 1991–1994 | 1 | 2.41 | 41 | 16 | 57 |
| IARC (2003) | CIA | Transkei region | 1996–1998 | 1 | 1.34 | 134 (37.5) | 173 (26.5) | 307 |
| IARC (2003) | CIA | Transkei region | 1996–1998 | 1 | 1.64 | 117 (62.5) | 97 (34.5) | 214 |
| Dlamini (2005) [45] | Literature | Kwazulu Natal | 2005 | 2 | 1.17 | 47 | 40 | 87 |
| Dandara (2006) [46] | Literature | Western Cape province | 1997–2003 | 3 | 1.42 | 85 | 60 | 145 |
| Matsha (2006) [47] | Literature | Transkei region | 2003–2005 | 2 | 1.54 | 142 | 92 | 234 |
| Li (2008) [48] | Literature | Cape Town | 1997–2003 | 4 | 1.47 | 84 | 57 | 141 |
| Van der Merwe (2010) [49] | Literature | Free State province | 1995 | 2 | 2.51 | 158 | 63 | 221 |
| Van der Merwe (2010) [49] | Literature | Free State province | 2000 | 2 | 2.34 | 103 | 44 | 147 |
| Van der Merwe (2010) [49] | Literature | Free State province | 2005 | 2 | 1.79 | 68 | 38 | 106 |
| Somdyala (2010) [50] | Literature | Eastern Cape province | 1998–2002 | 1 | 0.93 | 444 (32.7) | 479 (20.2) | 923 |
| Sitas (2012) [51] | Literature | Johannesburg | 1995–2006 | 2 | 1.60 | 429 | 268 | 697 |
| IARC (2013) | CI5 (vol. X) | Eastern Cape province | 2003–2007 | 1 | 1.62 | 475 (32.0) | 533 (19.6) | 1008 |
| Sewram (2014) [52] | Literature | Eastern Cape province | 2001–2003 | 3 | 0.99 | 334 | 336 | 670 |
| Dandara (2015) [53] | Literature | Cape Town | 1977–2007 | 2 | 1.84 | 1092 | 593 | 1685 |
| Swaziland | ||||||||
| IARC (2003) | CIA | Manzini | 1996–1999 | 1 | 3.33 | 106 (14.0) | 38 (4.1) | 144 |
| Total cases (Southern Africa) | 10874 | 6111 | 16985 | |||||
| Middle Africa | ||||||||
| Congo | ||||||||
| IARC (2003) | CIA | Brazzaville | 1996–1999 | 1 | 1.57 | 4 (0.5) | 1 (0.1) | 5 |
| IARC (In press) | CIA | Brazzaville | 2009–2013 | 1 | 1.58 | 7 (0.5) | 2 (0.1) | 9 |
| Total cases (Middle Africa) | 11 | 3 | 14 | |||||
| Western Africa | ||||||||
| Burkina Faso | ||||||||
| IARC (2003) | CIA | Ouagadougou | 1998 | 1 | 1.08 | 5 | 4 | 9 |
| Cote d'Ivoire | ||||||||
| Echimane (2000) [54] | Literature | Abidjan | 1995–1997 | 1 | 9.00 | 9 (0.7) | 1 (0.2) | 10 |
| IARC (In press) | CIA | Abidjan | 2012–2013 | 1 | 0.81 | 3 (0.2) | 3 (0.3) | 6 |
| Ghana | ||||||||
| Tettey (2012) [55] | Literature | Accra | 1992–2010 | 2 | 4.07 | 122 | 30 | 152 |
| IARC (In press) | CIA | Kumasi | 2012–2013 | 1 | 1.06 | 1 (0.1) | 2 (0.2) | 3 |
| Guinea | ||||||||
| IARC (2003) | CIA | Conakry | 1996–1999 | 1 | 2.10 | 13 (0.9) | 5 (0.5) | 18 |
| IARC (In press) | CIA | Conakry | 2001–2010 | 1 | 1.92 | 13 (0.8) | 3 (0.1) | 16 |
| Mali | ||||||||
| IARC (1997) | CI5 (vol. VII) | Bamako | 1988–1992 | 1 | 0.99 | 12 (1.5) | 7 (0.8) | 19 |
| IARC (2002) | CI5 (vol. VIII) | Bamako | 1994–1996 | 1 | 1.17 | 17 (3.0) | 9 (1.7) | 26 |
| Niger | ||||||||
| IARC (2003) | CIA | Niamey | 1993–1999 | 1 | 0.94 | 7 (1.0) | 7 (1.3) | 14 |
| IARC (In press) | CIA | Niamey | 2006–2009 | 1 | 2.08 | 13 (1.2) | 2 (0.2) | 15 |
| Nigeria | ||||||||
| Awojobi (1996) | Literature | Eruwa, Oyo state | 1986–1995 | 1 | 0.83 | 5 | 6 | 11 |
| Ojo (1996) | Literature | Ife-Ijesha | 1993–1995 | 1 | 1.00 | 2 | 2 | 4 |
| Adegboye (2002) [56] | Literature | Ibadan | 1986–1996 | 2 | 2.10 | 21 | 10 | 31 |
| IARC (2003) | CIA | Ibadan | 1998–1999 | 1 | 2.31 | 7 (1.1) | 2 (0.3) | 9 |
| IARC (In press) | CIA | Ibadan | 2006–2009 | 1 | 1.93 | 15 (1.0) | 7 (0.4) | 22 |
| The Gambia | ||||||||
| IARC (2002) | CI5 (vol. VIII) | 1997–1998 | 1 | 1.27 | 6 (1.4) | 3 (1.1) | 9 | |
| IARC (In press) | CIA | 2007–2011 | 1 | 2.15 | 22 (1.0) | 8 (0.5) | 30 | |
| Togo | ||||||||
| Amegbor (2008) [57] | Literature | Lome | 1986–2005 | 2 | 2.25 | 72 | 32 | 104 |
| Total cases (Western Africa) | 365 | 143 | 508 | |||||
| Total cases | 17096 | 9838 | 26934 | |||||
Fig. 3.
Forest plot of country and region-specific sex ratios estimated using random effects models. Point estimates and their corresponding populations and diagnosis periods are shown in Table 1. *High incidence denotes countries for which the male or female EC ASR exceeds the global EC incidence rate in GLOBOCAN.
A male excess of EC was observed for the majority of countries investigated, including the high incidence regions of Eastern and Southern Africa, where a significant male excess predominated. Estimated regional ratios were 1.6 (95% CI: 1.4, 1.8) and 1.8 (95% CI: 1.5, 2.0) for Eastern and Southern Africa, respectively. The countries with the highest male excesses were Tanzania in Eastern Africa (2.3; 95% CI: 1.9, 2.6) and Swaziland in Southern Africa (3.3; 95% CI: 2.3, 4.8), which was based on 1996–1999 incidence data from the Swaziland Cancer Registry published in Cancer in Africa [14]. In contrast, a significant female excess was only observed in Sudan (0.5; 95% CI: 0.4, 0.6) and is based solely on regional (not country-wide) case series data from a single study [19]. The absence of a significant male excess was observed for two other Eastern African countries: Mozambique (1.4; 95% CI: 0.7, 2.8) and Ethiopia (1.2; 95% CI: 0.7, 2.0). Estimates for several countries, mostly in the low incidence region, were based on a limited number of cases. This was particularly the case for Burkina Faso (9 cases), Congo (14 cases) and Cote d’Ivoire (16 cases).
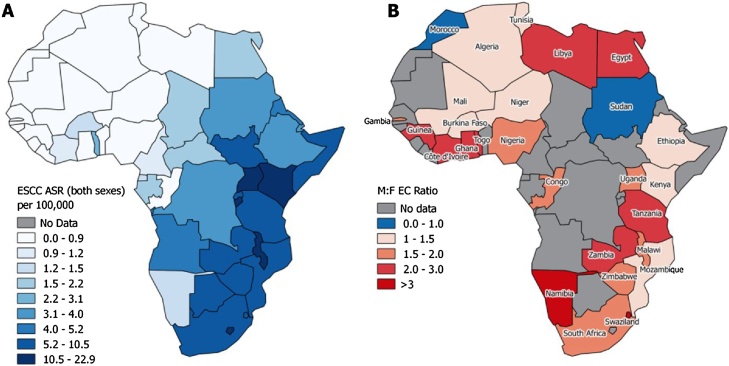
The above country-specific summaries mask a large degree of heterogeneity, demonstrated by the I2 estimates in Fig. 3, particularly for Ethiopia, Kenya, Uganda, Zimbabwe, South Africa, Ghana and Cote d’Ivoire. Country-specific estimates are also mapped in Fig. 4 and visual examination of the map shows no obvious spatial trends.
Fig. 4.
(A) Country-specific estimated age-standardised incidence rates of esophageal squamous cell carcinoma (Arnold et al. [2]) and (B) country-specific sex ratios of esophageal cancer estimated in the present study.
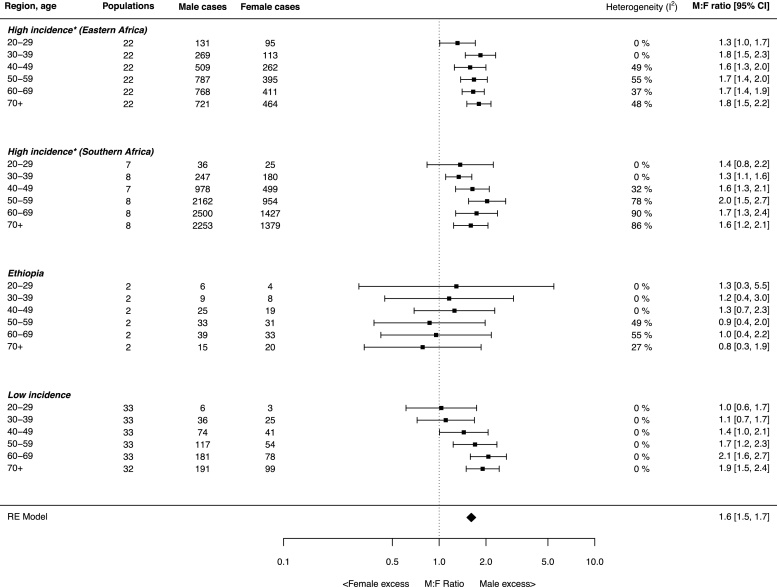
3.4. Age-specific EC sex ratios
Age-specific sex ratios are shown in Fig. 5 and were grouped by region, with the exception of Ethiopia, which was displayed separately due to the lack of evidence of a male excess found for this country. For the Eastern and Southern African high incidence regions, there was evidence (although not significant) of a male excess (M:F 1.3 and 1.4) as early as age 20–29 years in both regions. The 20–29 year age group estimate for Eastern Africa has a large contribution from a focussed study of <30 year olds (n = 109, M:F = 1.48) in West Kenya [11]. The excess was lower and non-significant when this study was omitted (1.2; 95% CI: 0.8, 1.7), but was already present in Eastern Africa (M:F = 1.8) in 30–39 year olds. A greater degree of heterogeneity was observed between estimates for older age groups in the Eastern and Southern African high incidence regions and Ethiopia. Conversely, there was no heterogeneity in the low incidence region, a male excess was only apparent in ages 40 and over.
Fig. 5.
Forest plot of age-specific sex ratios grouped by region. Estimates were made using random effects models. *High incidence denotes countries for which the male or female EC ASR exceeds the global mean in GLOBOCAN.
3.5. Sources of heterogeneity
To investigate whether data source differences explained the heterogeneity within country-specific sex ratios, country-specific estimates were calculated using registry (both CI5 and CIA) and literature data separately. Countries with notably high (I-squared > 60%) heterogeneities, from which sufficient data were available from both registry and literature sources (Uganda, Kenya, Ethiopia, South Africa) did not show a marked reduction in heterogeneity. For example, in Uganda, the sex ratio estimates were 1.7 and 1.9 for registry and literature data, respectively (1.8 combined) with I-squared values of 74% and 80% (78% combined).
Upon further investigation by location within country (estimates provided here and not in tables), although Kenya had a smaller relative male excess (M:F 1.4) than other high incidence East African countries (M:F of 1.8–2.3), heterogeneity was explained by location: estimates from the capital Nairobi were nearer 1 (M:F 1.0 (0.9, 1.2), I2 = 0%), whilst the combined ratio elsewhere, largely studies in Bomet and West Kenya, was 1.6 (1.4, 1.8), I2 = 0%. Similarly in Uganda, the capital had a smaller relative male excess (M:F 1.5 (1.3–1.8) I2 = 53%) than other areas of the country (M:F 3.5 (2.4, 4.9), I2 = 0%). In South Africa, national data provided the highest ratio (M:F 2.5) and the Eastern Cape/Transkei area the lowest (1.3 (1.0–1.6)). Furthermore, when restricted to count ratios alone, estimates may have been more susceptible to spatial variation if population sex imbalances were present. We were able to observe this for one study [53], reporting on a rural population in South Africa with a dominant female population above age 20. Age-specific EC counts were reported without corresponding population denominators, but an overall ASR was also reported. The age-adjusted sex ratio generated using EC counts was 0.93, but increased to 1.62 when the ratio was calculated using the ratio of ASRs.
4. Discussion
This study is the first to systematically review and report sex ratios of EC incidence in Africa and does so for 27 countries. The principal findings were (i) a decrease in male-to-female ratios over time; (ii) an overall male excess in EC incidence across Africa of between 1.4 and 2 fold; (iii) a high degree of heterogeneity both between and within country-specific estimates and (iv) in high incidence countries where young cases occur, a male excess already evident in 30–39 year olds.
The study was hindered by a comparatively low quantity of high quality cancer incidence data in African countries, namely due to the lack of established, population based cancer registries on the continent. Sex differentials in EC incidence rates can be estimated using GLOBOCAN, but their validity is undermined by the fact that many incidence rates, in the absence of high quality population based registry data, are based on those from neighbouring countries [1]; only 2% of the continent’s population was included in the last volume of CI5 [58]. Many of the country-specific estimates reported in this paper are based on data from a single site or region and do not represent the country as a whole – e.g. Zimbabwe, for which data were exclusively from the capital city of Harare. However, the situation is improving, and the wealth of new data from the CISSA series, in addition to the few registries already reported in CI5 and CIA, enabled a meaningful analysis that was sufficient to investigate EC sex ratio by time, geography and age. In many instances, we relied on count ratios to estimate rate ratios of EC. While we demonstrated that count ratios generally agreed with rate ratios (Fig. S1), exceptions may occur in populations with an uneven underlying sex distribution. The two most extreme examples that we observed were an almost two-fold increase in a male excess (3.68–6.62) in the predominantly male population of Harare, Zimbabwe and the disappearance of a male excess (1.62–0.89) in the predominantly female population of the Eastern Cape province of South Africa. These are the farthest points above and below the line in Fig. S1, respectively.
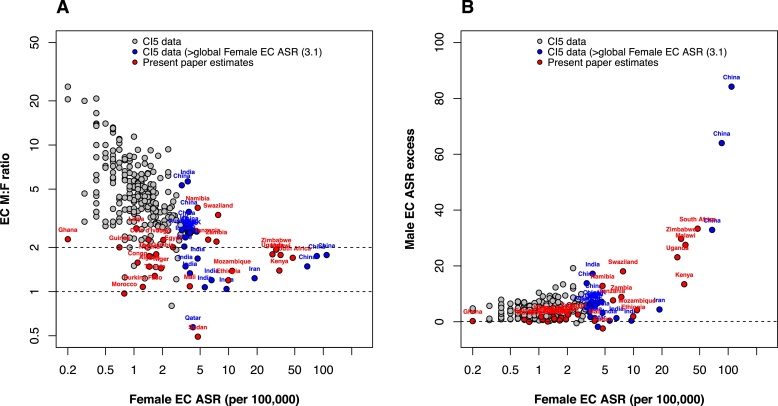
Comparisons between estimates derived in the present paper and worldwide estimates are shown in Fig. 6. Fig. 6(A) shows a decrease in sex ratios with increasing incidence in women (used here as an indicator of underlying incidence in the general population). For populations with very high EC incidence (ASRF > 20), sex ratios in Africa (1.5–2) are comparable to those from Chinese registries, and these exceed those from Iran (1.23). For populations with medium EC incidence (ASRF 5–20), sex ratios in a few African countries (e.g. Tanzania, Zambia) are higher than ratios from other registries, mostly in India, with comparable rates. Fig. 6(B) demonstrates that, although the male excess is much lower in higher incidence countries on a relative scale, the absolute difference in rates between men and women increases sharply with increasing underlying incidence.
Fig. 6.
Male-to-female EC ratios plotted against EC ASR in women. Global data (excluding Africa) from CI5 is plotted in comparison to African estimates from the present study. CI5 data are plotted by individual registry and labelled by country.
The substantial decline that we observed in sex ratios over time could be attributed to a number of factors. While etiological insights are of primary interest, gender inequalities in healthcare access giving rise to referral biases, may also affect sex-differentials in incidence in the African setting [[58], [59]]. There is some indication of this in sub-Saharan Africa, with present-day male referral bias having been reported in children [60] and middle-aged women [61] as well as historical reports of barriers faced by elderly women in rural areas [62]. Such referral biases would be expected to affect other cancer sites too, but we are not aware of any studies examining this issue. Exposure profiles may also have changed with time. Whatever the underlying reason for much higher earlier EC male excesses, it was necessary to exclude earlier data as they did not represent the present day scenario. While there were no obvious spatial trends in sex ratios, the large degree of heterogeneity among country-specific estimates, which persisted after stratifying by data source, suggests that risk profiles differ on a short spatial scale within countries. This is not unexpected given the large spatial variation in EC rates, both in Africa and other high incidence areas.
In respect of the putative risk factors for ESCC – tobacco, alcohol, hot beverages, environmental chemical exposures (PAHs, nitrosamines and mycotoxins) and micronutrient deficiencies – tobacco and alcohol consumption are both more prevalent among African males [[63], [64]], although the under-reporting of illicit home brew consumption [65] limits the interpretive value of alcohol statistics. It is likely that PAH exposures are higher for females due to time spent cooking indoors with poor ventilation [66]. Elucidating the gender differences between specific risk factors will form a component of ongoing etiologic research. While a male excess was evident, an abnormally high incidence rates among females exists in African high incidence countries. This generates two possible hypotheses to be tested in future: (1) that male-patterned exposures are exacerbating the effects of an underlying, more ubiquitous factor or, alternatively (2) that independent, female-specific exposures are prevalent, but are less potent than those found in men. Countries, such as Sudan and Ethiopia, where a statistically significant male excess was not observed, may offer unique opportunities to test these hypotheses.
Further, the presence of the male excess from early ages (30s) particularly in Eastern Africa, is intriguing. When compared to other upper aerodigestive or respiratory cancers in selected populations (US SEER white and black, Scotland, Calvados), although extremely rare, M:F lung cancer IRRs are 1.2 in US Whites in the 30s, and 2 in Scotland. In the 30s, EC almost never occurs, but for oral cavity and pharynx, male excesses are already present (1.5 Scotland, 1.6 US White, 2.0 US Black, 8.3 Calvados). Thus, observing such a young male excess in East Africa may not be implausible, though the drivers of any young ECs are unknown. A male excess, if not due to gender referral biases, must at least partially be driven by environmental agents acting from early in the life-course, or particularly potent ones, in addition to inherited susceptibility. For the latter, family history of EC has been reported as a risk factor with a high prevalence in some studies [11] but not all.
The presence of a non-negligible male excess in many of the high EC incidence countries of Southern and Eastern Africa points towards a potentially large preventable burden of disease. First, epidemiologic studies should be able to identify factors that contribute to the male excess, as exposure heterogeneity must be present. At M:F ratios of 1.5 and 2 respectively, if male incidence rates could be reduced to those in females, 20% and 33% of all EC cases, and 33% and 50% of male EC cases would be prevented. Whatever the root causes of high incidence rates of EC in Eastern and Southern Africa, these findings suggest that a large fraction of the disease burden could be prevented by targeting male patterned, modifiable risk factors.
Author contributions
VAM and JS conceived the study. DRSM, LB and RH performed reviews, data compilation and statistical analyses. All authors made substantial contributions to the intellectual content of the manuscript via data interpretation, review and authorship of the text.
Funding
The work reported was undertaken during the tenure of a Postdoctoral Fellowship from the International agency for Research on cancer, partially supported by the European Commission FP7 Marie Curie Actions – People – Co-funding of regional, national and international programmes (COFUND).
Conflict of interest
None.
Acknowledgement
The authors are grateful to Dr Max Parkin for sharing advice regarding the manuscript.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.canep.2018.01.020.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Ferlay J. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J. Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M., Soerjomataram I., Ferlay J., Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64:387. doi: 10.1136/gutjnl-2014-308124. [DOI] [PubMed] [Google Scholar]
- 3.Schaafsma T. Africa's Oesophageal Cancer Corridor: geographic variations in incidence correlate with certain micronutrient deficiencies. PLoS One. 2015;10(11):e0142648. doi: 10.1371/journal.pone.0140107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chasimpha S.J., Parkin D.M., Masamba L., Dzamalala C.P. Three year cancer incidence in Blantyre, Malawi (2008–2010) Int. J. Cancer. 2017;141(4):694–700. doi: 10.1002/ijc.30777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCormack V.A. Informing etiologic research priorities for squamous cell esophageal cancer in Africa: a review of setting-specific exposures to known and putative risk factors. Int. J. Cancer. 2016;140(2):259–271. doi: 10.1002/ijc.30292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamangar F., Chow W.H., Abnet C.C., Dawsey S.M. Environmental causes of esophageal cancer. Gastroenterol. Clin. North Am. 2009;38:27–57. doi: 10.1016/j.gtc.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ly D., Forman D., Ferlay J., Brinton L.A., Cook M.B. An international comparison of male and female breast cancer incidence rates. Int. J. Cancer. 2013;132:1918–1926. doi: 10.1002/ijc.27841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devesa S.S., Bray F., Vizcaino A.P., Parkin D.M. International lung cancer trends by histologic type: male: female differences diminishing and adenocarcinoma rates rising. Int. J. Cancer. 2005;117:294–299. doi: 10.1002/ijc.21183. [DOI] [PubMed] [Google Scholar]
- 9.Rahbari R., Zhang L., Kebebew E. Thyroid cancer gender disparity. Future Oncol. 2010;6:1771–1779. doi: 10.2217/fon.10.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pampel F. Tobacco use in sub-Sahara Africa: estimates from the demographic health surveys. Soc. Sci. Med. 2008;66:1772–1783. doi: 10.1016/j.socscimed.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawsey S.P. Esophageal cancer in young people: a case series of 109 cases and review of the literature. PLoS One. 2010;5:e14080. doi: 10.1371/journal.pone.0014080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips S.P. Defining and measuring gender: a social determinant of health whose time has come. Int. J. Equity Health. 2005;4:11. doi: 10.1186/1475-9276-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.IARC. Cancer Incidence in Five Continents. Volumes I-X. Online Resource. Available: http://ci5.iarc.fr/Default.aspx. (2017).
- 14.Parkin D. Epidemiology and Prevention. IARC Press; Lyon: 2003. Cancer in Africa. [Google Scholar]
- 15.RCoreTeam. R. A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. (2016).
- 16.Gelfand M. Health care for Africans in Rhodesia. Cent. Afr. J. Med. 1976;22:252–257. [PubMed] [Google Scholar]
- 17.Bahnassy A.A., Zekri A.R.N., Abdallah S., El-Shehaby A.M., Sherif G.M. Human papillomavirus infection in Egyptian esophageal carcinoma: correlation with p53, p21waf, mdm2, C-erbB2 and impact on survival. Pathol. Int. 2005;55:53–62. doi: 10.1111/j.1440-1827.2005.01804.x. [DOI] [PubMed] [Google Scholar]
- 18.Chbani L., Hafid I., Berraho M., Nejjari C., Amarti A. Digestive cancers in Morocco: Fez-Boulemane region. Pan Afr. Med. J. 2012;13 [PMC free article] [PubMed] [Google Scholar]
- 19.Gasmelseed N. Patterns of esophageal cancer in the national cancer institute at the university of Gezira, in Gezira State, Sudan, in 1999–2012. Asian Pac. J. Cancer Prev. 2015;16:6481–6490. doi: 10.7314/apjcp.2015.16.15.6481. [DOI] [PubMed] [Google Scholar]
- 20.Ali A., Ersumo T., Johnson O. Oesophageal carcinoma in Tikur Anbessa Hospital, Addis Ababa. East Afr. Med. J. 1998;75:590–593. [PubMed] [Google Scholar]
- 21.Ashine S., Lemma B. Malignant tumours at Yirga Alem Hospital. Ethiop. Med. J. 1999;37:163–172. [PubMed] [Google Scholar]
- 22.Shewaye A., Seme A. Risk factors associated with oesophageal malignancy among Ethiopian patients: a case control study. East Central Afr. J. Surg. 2016;21:33–39. [Google Scholar]
- 23.Leon M.E. Qat use and esophageal cancer in Ethiopia: a pilot case-control study. PLoS One. 2017;12:e0178911. doi: 10.1371/journal.pone.0178911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White R.E., Abnet C.C., Mungatana C.K., Dawsey S.M. Oesophageal cancer: a common malignancy in young people of Bomet District, Kenya. Lancet. 2002;360:462–463. doi: 10.1016/S0140-6736(02)09639-3. [DOI] [PubMed] [Google Scholar]
- 25.Lodenyo H. Patterns of upper gastrointestinal diseases based on endoscopy in the period 1998–2001. Afr. J. Health Sci. 2005;12:49–54. doi: 10.4314/ajhs.v12i1.30800. [DOI] [PubMed] [Google Scholar]
- 26.Ogendo S. Weight change post oesophagectomy for carcinoma of oesophagus. East Afr. Med. J. 2007;84:271–278. doi: 10.4314/eamj.v84i6.9536. [DOI] [PubMed] [Google Scholar]
- 27.Parker R.K. Stents for proximal esophageal cancer: a case-control study. Gastrointest. Endosc. 2011;73:1098–1105. doi: 10.1016/j.gie.2010.11.036. [DOI] [PubMed] [Google Scholar]
- 28.Patel K., Wakhisi J., Mining S., Mwangi A., Patel R. Esophageal cancer, the topmost cancer at MTRH in the Rift Valley, Kenya, and its potential risk factors. ISRN Oncol. 2013;2013:503249. doi: 10.1155/2013/503249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banda L.T., Parkin D.M., Dzamalala C.P., Liomba N.G. Cancer incidence in Blantyre, Malawi 1994–1998. Trop. Med. Int. Health. 2001;6:296–304. doi: 10.1046/j.1365-3156.2001.00707.x. [DOI] [PubMed] [Google Scholar]
- 30.Crofts T. A tale of two cities?oesophageal cancer in Malawi and Scotland. Malawi Med. J. 2008;20:135–139. doi: 10.4314/mmj.v20i4.10974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mothes H. Do patients in rural Malawi benefit from upper gastrointestinal endoscopy? Trop. Dr. 2009;39:73–76. doi: 10.1258/td.2008.080142. [DOI] [PubMed] [Google Scholar]
- 32.Gyorki D.E., Muyco A., Kushner A.L., Brennan M.F., Kingham T.P. Cancer surgery in low-income countries: an unmet need. Arch. Surg. 2012;147:1135–1140. doi: 10.1001/archsurg.2012.1265. [DOI] [PubMed] [Google Scholar]
- 33.Wolf L.L. Esophagogastroduodenoscopy in a public referral hospital in Lilongwe, Malawi: spectrum of disease and associated risk factors. World J. Surg. 2012;36:1074–1082. doi: 10.1007/s00268-012-1490-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mtonga P. Biopsy case mix and diagnostic yield at a Malawian central hospital. Malawi Med. J. 2013;25:62–64. [PMC free article] [PubMed] [Google Scholar]
- 35.Mlombe Y.B. Environmental risk factors for oesophageal cancer in Malawi: a case-control study. Malawi Med. J. 2015;27:88–92. doi: 10.4314/mmj.v27i3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mchembe M.D. Endoscopic and clinicopathological patterns of esophageal cancer in Tanzania: experiences from two tertiary health institutions. World J. Surg. Oncol. 2013;11:257. doi: 10.1186/1477-7819-11-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gabel J.V. Clinical and epidemiologic variations of esophageal cancer in Tanzania. World J. Gastrointest. Oncol. 2016;8:314. doi: 10.4251/wjgo.v8.i3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alema O.N., Iva B. Cancer of the esophagus: histopathological sub-types in northern Uganda. Afr. Health Sci. 2014;14:17–21. doi: 10.4314/ahs.v14i1.4. jAFHS.v14.i1.pg17[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okello S. Population attributable fraction of Esophageal squamous cell carcinoma due to smoking and alcohol in Uganda. BMC Cancer. 2016;16:446. doi: 10.1186/s12885-016-2492-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kayamba V. HIV infection and domestic smoke exposure, but not human papillomavirus, are risk factors for esophageal squamous cell carcinoma in Zambia: a case-control study. Cancer Med. 2015;4:588–595. doi: 10.1002/cam4.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asombang A.W. Esophageal squamous cell cancer in a highly endemic region. World J. Gastroenterol. 2016;22:2811. doi: 10.3748/wjg.v22.i9.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levy R., Oosthuizen M., Degiannis E., Lambrechts H. Elevated reversible and irreversible lipid peroxidation in human oesophageal cancer. Anticancer Res. 1998;18:1325–1328. [PubMed] [Google Scholar]
- 43.Dietzsch E., Parker M.I. Infrequent somatic deletion of the 5'region of the COL1A2 gene in oesophageal squamous cell cancer patients. Clin. Chem. Lab. Med. 2002;40:941–945. doi: 10.1515/CCLM.2002.165. [DOI] [PubMed] [Google Scholar]
- 44.Pacella-Norman R. Risk factors for oesophageal, lung, oral and laryngeal cancers in black South Africans. Br. J. Cancer. 2002;86:1751–1756. doi: 10.1038/sj.bjc.6600338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dlamini Z., Bhoola K. Esophageal cancer in African blacks of Kwazulu Natal, South Africa: an epidemiological brief. Ethn. Dis. 2005;15:786–789. [PubMed] [Google Scholar]
- 46.Dandara C., Li D.P., Walther G., Parker M.I. Gene-environment interaction: the role of SULT1A1 and CYP3A5 polymorphisms as risk modifiers for squamous cell carcinoma of the oesophagus. Carcinogenesis. 2006;27:791–797. doi: 10.1093/carcin/bgi257. [DOI] [PubMed] [Google Scholar]
- 47.Matsha T. Traditional home-brewed beer consumption and iron status in patients with esophageal cancer and healthy control subjects from Transkei, South Africa. Nutr. Cancer. 2006;56:67–73. doi: 10.1207/s15327914nc5601_9. [DOI] [PubMed] [Google Scholar]
- 48.Li D.-P., Dandara C., Walther G., Parker M.I. Genetic polymorphisms of alcohol metabolising enzymes: their role in susceptibility to oesophageal cancer. Clin. Chem. Lab. Med. 2008;46:323–328. doi: 10.1515/CCLM.2008.073. [DOI] [PubMed] [Google Scholar]
- 49.Van der Merwe T. Epidemiological changes in oesophageal cancer at National Hospital, Bloemfontein: 1995, 2000 and 2005. Afr. J. Primary Health Care Family Med. 2010;2:1–5. [Google Scholar]
- 50.Somdyala N.I., Bradshaw D., Gelderblom W.C., Parkin D.M. Cancer incidence in a rural population of South Africa, 1998–2002. Int. J. Cancer. 2010;127:2420–2429. doi: 10.1002/ijc.25246. [DOI] [PubMed] [Google Scholar]
- 51.Sitas F. The spectrum of HIV-1 related cancers in South Africa. Int J. Cancer. 2000;88:489–492. doi: 10.1002/1097-0215(20001101)88:3<489::aid-ijc25>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 52.Sewram V., Sitas F., O'Connell D., Myers J. Diet and esophageal cancer risk in the Eastern Cape Province of South Africa. Nutr. Cancer. 2014;66:791–799. doi: 10.1080/01635581.2014.916321. [DOI] [PubMed] [Google Scholar]
- 53.Dandara C., Robertson B., Dzobo K., Moodley L., Parker M.I. Patient and tumour characteristics as prognostic markers for oesophageal cancer: a retrospective analysis of a cohort of patients at Groote Schuur Hospital. Eur. J. Cardiothorac. Surg. 2015;49:629–634. doi: 10.1093/ejcts/ezv135. [DOI] [PubMed] [Google Scholar]
- 54.Echimane A.K. Cancer incidence in Abidjan, Ivory Coast. Cancer. 2000;89:653–663. doi: 10.1002/1097-0142(20000801)89:3<653::aid-cncr22>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 55.Tettey M. The changing epidemiology of esophageal cancer in sub-Saharan Africa–the case of Ghana. Pan Afr. Med. J. 2012;13 [PMC free article] [PubMed] [Google Scholar]
- 56.Adegboye V., Obajimi M., Ogunseyinde A., Brimmo I., Adebo A. Trans-hiatal oesophagectomy as a palliative treatment for carcinoma of the oesophagus. East Afr. Med. J. 2002;79:311–316. doi: 10.4314/eamj.v79i6.8851. [DOI] [PubMed] [Google Scholar]
- 57.Amegbor K., Napo-Koura G., Songne-Gnamkoulamba B., Redah D., Tekou A. Epidemiological and pathological aspects of gastrointestinal tumors in Togo. Gastroentérologie Clinique et Biologique. 2008;32:430–434. doi: 10.1016/j.gcb.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 58.Klasen S. Nutrition health and mortality in sub-Saharan Africa: is there a gender bias? J. Dev. Stud. 1996;32(6):913–932. [Google Scholar]
- 59.Chirowa F., Atwood S., Van der Putten M. Gender inequality, health expenditure and maternal mortality in sub-Saharan Africa: a secondary data analysis. Afr. J. Primary Health Care Family Med. 2013;5:1–5. [Google Scholar]
- 60.Rockers P.C., McConnell M. Child gender and parental reporting of illness symptoms in sub-Saharan Africa. Am. J. Trop. Med. Hyg. 2017;96:994–1000. doi: 10.4269/ajtmh.16-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ibanez-Gonzalez D.L., Norris S.A. Chronic non-communicable disease and healthcare access in middle-aged and older women living in Soweto South Africa. PLoS One. 2013;8:e78800. doi: 10.1371/journal.pone.0078800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Skinner M.E. Malignant disease of the gastrointestinal tract in the Rhodesian African, with special reference to the urban population of Bulawayo. A preliminary report. Natl. Cancer Inst. Monogr. 1967;25:57–71. [PubMed] [Google Scholar]
- 63.Sreeramareddy C.T., Pradhan P.M., Sin S. Prevalence, distribution, and social determinants of tobacco use in 30 sub-Saharan African countries. BMC Med. 2014;12:243. doi: 10.1186/s12916-014-0243-x. s12916-014-0243-x[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.(GISAH), G. I. S. o. A. a. H. Global Health Observatory Data Repository, World Health Organization 2014 (2014). http://apps.who.int/gho/data/node.main.A1036?lang=en&showonly=GISAH.
- 65.Papas R.K. Estimating alcohol content of traditional brew in Western Kenya using culturally relevant methods: the case for cost over volume. AIDS Behav. 2010;14:836–844. doi: 10.1007/s10461-008-9492-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Titcombe M.E., Simcik M. Personal and indoor exposure to PM(2).(5) and polycyclic aromatic hydrocarbons in the southern highlands of Tanzania: a pilot-scale study. Environ. Monit. Assess. 2011;180:461–476. doi: 10.1007/s10661-010-1799-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.