Summary
CNS injury often severs axons. Scar tissue that forms locally at the lesion site is thought to block axonal regeneration, resulting in permanent functional deficits. We report that inhibiting the generation of progeny by a subclass of pericytes led to decreased fibrosis and extracellular matrix deposition after spinal cord injury in mice. Regeneration of raphespinal and corticospinal tract axons was enhanced and sensorimotor function recovery improved following spinal cord injury in animals with attenuated pericyte-derived scarring. Using optogenetic stimulation, we demonstrate that regenerated corticospinal tract axons integrated into the local spinal cord circuitry below the lesion site. The number of regenerated axons correlated with improved sensorimotor function recovery. In conclusion, attenuation of pericyte-derived fibrosis represents a promising therapeutic approach to facilitate recovery following CNS injury.
Keywords: spinal cord injury, scar, pericyte, fibrosis, axon regeneration, sensorimotor functional recovery, optogenetics
Graphical Abstract

Highlights
-
•
Inhibition of pericyte proliferation reduces fibrotic scar tissue following injury
-
•
Attenuated pericyte-derived scarring facilitates motor axon regeneration
-
•
Regenerated axons functionally re-integrate into the local spinal circuitry
-
•
Attenuated pericyte-derived scarring improves sensorimotor recovery
Attenuation of fibrotic tissue generation by a subset of pericytes promotes regeneration of serotonergic and corticospinal tract axons and improves functional recovery after spinal cord injury.
Introduction
Major obstacles blocking axonal regeneration after injury include scar formation, a long-lasting inflammatory response, proteoglycans, and myelin debris (Cregg et al., 2014, Silver et al., 2014, Yiu and He, 2006). Reactive astrocytes making up the glial scar have long been considered one of the main barriers for axonal regrowth (Cafferty et al., 2007, Xu et al., 2015, Yiu and He, 2006), although this is still debated (Anderson et al., 2016, Hara et al., 2017, Silver, 2016). Less attention has been given to the fibrotic, or stromal, compartment of the scar.
Fibrotic scar tissue formation by a subset of perivascular cells, termed type A pericytes, is required for regaining tissue integrity, but creates a scar core of fibroblast-like cells and dense extracellular matrix (Göritz et al., 2011). Previous attempts to remove or modify specific extracellular matrix molecules have shown potential to improve axonal regeneration (Brazda and Müller, 2009, Fitch and Silver, 2008). However, there are many different extracellular matrix proteins deposited in the fibrotic scar, and they often have complex, context-dependent functions and cannot easily be categorized as axon growth promoting or inhibiting (Condic and Lemons, 2002). The large number of inhibitory cues present in the scar makes molecule-specific targeting strategies impractical.
The identification of type A pericytes as the origin of fibroblast-like cells found in fibrotic scar tissue in the injured spinal cord (Göritz et al., 2011) enabled us in the current study to selectively reduce pericyte-derived scarring to assess its influence on axonal regeneration and functional recovery. We report that significantly more descending axons grow through the lesion site in the injured spinal cord when pericyte-derived fibrosis is moderately reduced, regenerating axons synaptically integrate below the lesion and functional recovery is improved. This identifies type A pericytes as a target for the development of therapies to promote axonal regeneration after CNS injury.
Results
Inhibiting the Generation of Progeny by Type A Pericytes Efficiently Reduces Extracellular Matrix Deposition and Alters Scar Composition in the Injured Spinal Cord
We used Glast-CreERT2 transgenic mice carrying a ROSA26-YFP reporter allele to genetically label type A pericytes and their progeny forming the fibrotic scar following spinal cord injury (Göritz et al., 2011). As previously established, following injury, inhibition of type A pericyte proliferation through cell-specific deletion of floxed KRas in mice with HRas and NRas null alleles by tamoxifen-induced genetic recombination with CreERT2 (we refer to these mice as Glast-Rasless) reduces fibrotic scar tissue formation (Figures 1A and S1A–S1L) in a recombination-dependent manner (Göritz et al., 2011).
Figure 1.
Attenuation of Pericyte-Derived Scarring Results in Reduced Fibrosis following Spinal Cord Injury
(A) Genetic strategy to block the generation of progeny by type A pericytes.
(B and C) Sagittal view of the lesion site in vehicle (B) and Tam (C) animals immunostained for PDGFRβ 2 wpi. Scale bars, 100 μm.
(D) Percentage of scar occupancy by PDGFRβ-expressing stromal cells in lesion sites of vehicle or tamoxifen animals 2 wpi. Dark gray and white circles represent Tam and Tam-def animals, respectively. Horizontal lines represent the mean.
(E) Top ten gene ontology terms significantly enriched in injury sites of vehicle versus Tam animals 2 wpi. Numbers on the right show differentially expressed genes falling into each term. Fold change >1.5, Padj < 0.05 by modified Fisher exact test (EASE score).
(F) Top ten canonical pathways differentially enriched in lesions of vehicle versus Tam animals 2 wpi. Fold change >1.5, p < 0.05 by right-tailed Fisher exact test.
(G) Heatmap of differentially expressed fibrosis-associated genes in the uninjured spinal cord and in lesion sites of vehicle and Tam animals 2 wpi. Unsupervised hierarchical clustering dendrogram based on Pearson correlations. Color code shows log10 (1 + fragments per kilobase of transcript sequence per million mapped fragments [FPKM]) values. Red and blue indicate low and high gene expression, respectively. Fold change >1.5, FDR adjusted p < 0.05.
n = 10 (vehicle), n = 12 (Tam) animals in (D) and n = 4 (Uninj., uninjured), n = 4 (vehicle), n = 4 (Tam) animals in (G). ∗∗∗∗p < 0.0001 by two-sided, unpaired Student’s t test.
See also Figures S1 and S2 and Tables S1 and S2.
Figure S1.
Genetic Strategy to Modulate the Generation of Type A Pericyte Progeny, Related to Figure 1
(A–D) Cross sections of the spinal cord of Glast-YFP (A, B) and Glast-Rasless-YFP (C, D) mice showing recombined (YFP+) type A pericytes (PDGFRβ+) tightly associated with the vasculature (podocalyxin+) under uninjured conditions (A, C) and type A pericyte-derived cells in the injured spinal cord 5 days after a dorsal funiculus incision (B, D). White arrowheads point at type A pericyte-derived cells that detached from the blood vessel wall.
(E–H) Cross sections of uninjured (E, G) and injured spinal cord 5 dpi (F, H) showing proliferation (EdU incorporation) of recombined pericytes and progeny in Glast-YFP (E, F) and Glast-Rasless-YFP (G, H) mice. White and yellow arrowheads in E, G depict cells single positive for EdU and YFP, respectively. White arrowheads in F, H show YFP+ cells that incorporated EdU.
(I) Density of recombined (Rec.) and non-recombined (Non rec.) PDGFRβ-expressing stromal cells in the uninjured and injured spinal cord 5 dpi of Glast-YFP and Glast-Rasless-YFP mice. Following injury, the number of recombined pericyte-derived stromal cells per area is greatly reduced in Glast-Rasless-YFP mice compared to Glast-YFP control mice.
(J) Proportion of PDGFRβ-expressing cells associated with (ON vessel) or detached from (OFF vessel) the blood vessel wall in the uninjured and injured spinal cord 5 dpi of Glast-Rasless-YFP and control Glast-YFP mice. Under homeostatic conditions all pericytes are associated with the vasculature in both Glast-YFP and Glast-Rasless-YFP animals. Upon injury, the percentage of PDGFRβ+ cells located in distance to the blood vessel wall is greatly reduced in Glast-Rasless-YFP mice paralleled by a higher percentage of cells remaining associated with the vessel compared to control Glast-YFP animals.
(K) Density of proliferating and non-proliferating PDGFRβ-expressing cells in the uninjured and injured spinal cord 5 dpi of Glast-Rasless-YFP and Glast-YFP control animals. In the uninjured spinal cord, PDGFRβ+ pericytes did not incorporate EdU in neither Glast-Rasless-YFP nor Glast-YFP mice. Injury-induced proliferation of PDGFRβ+ stromal cells is greatly decreased in Glast-Rasless-YFP mice compared to control Glast-YFP animals. The density of non-proliferating stromal cells is not significantly altered.
(L) Percentage of proliferating and non-proliferating recombined PDGFRβ-expressing cells in the uninjured and injured spinal cord 5 dpi of Glast-Rasless-YFP and Glast-YFP control animals. Under homeostatic conditions virtually no recombined cells incorporate EdU in Glast-Rasless-YFP or Glast-YFP mice. The proliferation of recombined PDGFRβ+ cells induced by the injury is dramatically decreased in Glast-Rasless-YFP animals compared to control Glast-YFP mice. This gives a relative increase in the proportion of non-proliferative recombined cells.
(M–R) Low power photographs and sagittal view of the spinal cord of Glast-Rasless-YFP mice showing dense (vehicle; M, P), reduced (intermediate recombination penetrance, Tam; N, Q) or low/no (full recombination penetrance, Tam-def; O, R) PDGFRβ+ fibrotic scarring at the injury site 2 weeks after a dorsal hemisection. Dashed circles mark the injured area and border a tissue defect (∗) in R. Images in P, Q are the same as in Figures 1B and 1C, respectively.
Scale bars represent 0.5 mm (M-O), 200 μm (R), 100 μm (P, Q) and 50 μm (A-H). Data in I-L shown as mean ± SEM. n = 3 (uninjured Glast-YFP), n = 4 (uninjured Glast-Rasless-YFP), n = 4-5 (Glast-YFP injury), n = 4 (Glast-Rasless-YFP injury) animals in I-L. ns, non-significant; ∗∗∗∗p < 0.0001 by One-Way ANOVA followed by Tukey’s post hoc test.
Two weeks after dorsal hemisection injuries, we found that 3/12 tamoxifen-injected Glast-Rasless mice showed highly efficient recombination levels, leading to complete block of fibrotic scar formation and failure to close the injury site. We refer to mice with this phenotype as Tam-tissue defect (Tam-def). The remaining 9/12 tamoxifen-injected Glast-Rasless mice displayed intermediate levels of recombination. We refer to mice with this phenotype as Tam. Tam animals had re-established tissue integrity but presented reduced fibrotic scar density in comparison with control mice of identical genotype injected with vehicle (hereafter referred to as vehicle animals). Vehicle animals presented dense PDGFRβ+ fibrotic scar cores (Figures 1B–1D and S1M–S1R).
We next characterized the gene expression associated with type A pericyte-derived scarring, by RNA sequencing. Using the Glast-Rasless line (Figure 1A), we compared whole lesion segments from vehicle- and tamoxifen-treated animals 2 weeks after spinal cord dorsal hemisection. We identified 1,099 genes that were differentially expressed (false discovery rate [FDR]-adjusted p < 0.05, fold change > ± 1.5) (Figure S2A; Tables S1 and S2). In line with the reduction of fibrotic scar tissue, gene ontology analyses revealed gene expression differences in categories such as extracellular matrix organization, collagen fibril organization, cell-matrix and cell-substrate adhesion, angiogenesis, and wound healing (Figures 1E, S2B, and S2C). Pathway analysis of differentially expressed genes revealed a notable similarity to hepatic fibrosis/stellate cell activation (Mederacke et al., 2013) (Figure 1F). We found a large number of fibrosis-associated genes, encoding for an array of extracellular matrix molecules and collagen-processing enzymes, displaying reduced levels of expression in Tam animals when compared to vehicle animals. Strikingly, the expression of fibrosis-associated genes in Tam animals clustered with the expression profile of uninjured animals, rather than vehicle animals, establishing that type A pericytes are required for the generation of fibrotic tissue and that reducing their generation of progeny blocks a broad range of injury-induced gene expression changes (Figure 1G). Examples of differentially expressed genes were validated by real-time qPCR and at the protein level (Figures S2D–S2J and S2L–S2P). Vehicle animals displayed increased expression of fibrillar collagens type I and III, basal lamina-associated collagen type IV, collagen type VI, and fibronectin at the injury site compared to Tam animals (Figures S2F–S2J and S2L–S2P).
Figure S2.
Attenuation of Injury-Induced Proliferation of Type A Pericytes Results in Reduced Fibrosis following Spinal Cord Injury, Related to Figure 1
(A) Heatmap of all differentially expressed genes between lesion sites of vehicle and Tam animals 2 wpi. Gene expression values of this cohort of genes in the uninjured spinal cord were included for comparison. Genes are clustered according to gene ontology (left margin). Color code shows TMM-normalized FPKM values. Red and blue indicate low and high gene expression, respectively; Fold change > 1.5; FDR adjusted p < 0.05.
(B and C) Top ten gene ontology cellular component (B) and molecular function (C) terms significantly enriched in injury sites of vehicle versus Tam animals 2 wpi. Numbers on the right show differentially expressed genes falling into each term. Fold change > 1.5, Padj < 0,05 by modified Fisher Exact test (EASE score).
(D) Relative expression level of fibrosis-associated genes 2 wpi compared with uninjured control mice determined by qRT-PCR.
(E) Western blot analyses of uninjured spinal cord tissue (Uninj.) and injury sites of vehicle (Veh) and Tam animals 2 wpi. YFP expression (recognized by anti-GFP antibody) is restricted to Glast-Rasless-YFP animals undergoing recombination of the reporter allele mediated by tamoxifen administration (Tam animals). Uninjured and injured control Glast-Rasless-YFP mice that received vehicle without tamoxifen (Veh animals) do not recombine and therefore show no expression of the YFP reporter protein. The housekeeping protein GAPDH was used as loading control.
(F–Q) Sagittal sections of the spinal cord of Glast-Rasless animals 2 wpi showing fibrotic (F-J, L-P) and glial (K, Q) scarring.
FPKM, fragments per kilobase of transcript sequence per million mapped fragments. Scale bar represents 200 μm (F-Q). Data in D shown as mean ± s.e.m. n = 4 (uninjured), n = 4 (vehicle), n = 4 (Tam) animals in A; n = 7-10 (vehicle), n = 11-12 (Tam) animals in D; n = 6 (vehicle), n = 6 (Tam) animals in E. ns, non-significant. ∗p < 0.05, ∗∗p < 0.01 by Mann-Whitney U-test.
Reduction of a specific cellular component of the scar can influence the contribution of other scar-forming cells (Anderson et al., 2016, Faulkner et al., 2004, Hara et al., 2017, Herrmann et al., 2008, Sabelström et al., 2013, Silver, 2016). Moreover, astrocytes and fibrotic cells interact to form a sharp lesion border (Bundesen et al., 2003). To assess the influence of reduced pericyte-derived scarring on other cell types, we analyzed changes in astrogliosis, inflammation, and oligodendrocyte precursor cell (OPC) numbers in Glast-Rasless mice 2 and 4 weeks after a dorsal hemisection (Figures S3A–S3R). The general inflammatory response, assessed as area covered by CD68+ macrophages/microglia, was not significantly changed between vehicle and Tam animals at 2 weeks post injury (wpi), while Tam-def animals showed an increased inflammatory response. During scar maturation/condensation at 4 wpi, Tam animals presented reduced inflammation compared to vehicle animals (Figures S3A–S3F and S3M). No changes were observed in the number of OPCs (non-vascular-associated NG2+/PDGFRα+ cells) (Assinck et al., 2017) flanking the lesion or in spared but reactive neural tissue across all three groups at 2 and 4 wpi (Figures S3N and S3O). Although no difference in general astrogliosis and glial scar state was detected at 2 wpi across groups, the glial process network immediately flanking the lesion core tended to present a less complex arrangement in Tam animals (Figures S2K, S2Q, S3P, and S3Q). At 4 wpi, differences among groups became more apparent with Tam animals exhibiting reduced general astrogliosis, decreased dense wall-like configuration of glial processes at the glial-fibrotic lesion border, and diminished astrocyte reactivity within the parenchyma when compared to vehicle animals (Figures S3G–S3L, S3P, and S3R).
Figure S3.
Reduction of Pericyte-Derived Scarring Influences the Response of Other Scar-Forming Cells, Related to Figure 2
(A–L) Sagittal views of the spinal cord 4 wpi showing CD68-expressing inflammatory cells (A-F) and GFAP-positive scar-forming astrocytes (B,D,F,G-L) in Glast-Rasless animals.
(M) Area occupied by CD68-positive cells spanning 500 μm rostral and caudal to the lesion center.
(N and O) Density of OPCs found within 100 μm rostral and caudal to the glial-fibrotic lesion border (scar-forming OPCs, N) and within a 100 μm wide strip of spared but reactive neural tissue 500 μm away from either side of the lesion core (O).
(P) Percentage of area occupied by GFAP-positive astrocytes within 500 μm rostral and caudal to the lesion center.
(Q and R) Glial scar score reflecting the complexity level of the glial network (from no astrocytic hypertrophy – score 0, to hypertrophied astrocytes with formation of glial limitans bordering the lesion core – score 3) at various distances rostral to the lesion site.
(S–V) BDA-traced CST axon tips intermingle and make contact with recombined (YFP+) type A pericyte-derived cells (S), CD68-expressing immune cells (T), reactive astrocytes (U) and OPCs (V) 2 wpi. White arrowheads point at CST axons contacting scar-forming cells.
(W–Z) Sagittal sections of the spinal cord at 4 wpi depicting regrowing CST axons in relation to GFAP-positive glial processes in vehicle (W), Tam (X, Y) and Tam-def animals (Z).
Dashed lines in E,F,K,L,Z border a tissue defect (∗). A,B; C,D and E,F denote paired images. Scale bars represent 25 μm (S-T), 100 μm (A-L) and 200 μm (W-Z). Data shown as mean ± SEM. 2 wpi, n = 10 (vehicle), n = 9 (Tam), n = 3 (Tam-def) animals; 4 wpi, n = 5 (vehicle), n = 5 (Tam), n = 2 (Tam-def) animals. ns, non-significant; ∗p < 0.05, ∗∗p < 0.01 by One-Way ANOVA followed by Tukey’s post hoc test in M-P and two-sided, unpaired Student’s t-test (corrected for multiple comparisons using Holm-Sidak method) in Q,R.
Attenuation of Pericyte-Derived Scarring Leads to Decreased Axonal Die Back
A spinal cord dorsal hemisection interrupts bilaterally descending motor pathways, including the serotonergic raphespinal tract (RST) and the corticospinal tract (CST) (Tuszynski and Steward, 2012). BDA-traced CST axons contacted glial and non-neural scar-forming cells 2 weeks following a dorsal hemisection (Figures 2A–2C and S3S–S3V). Because the injured spinal cord tissue rapidly is flanked by reactive astrocytes and OPCs, the glial scar represents the first barrier to regenerating axons (Fitch and Silver, 2008, Hackett and Lee, 2016). In line with these observations, we found that 46.9% ± 4.7% and 22.5% ± 4.2% of BDA+ CST axon tips make contact with GFAP+- reactive astrocytes and OPCs (Figures 2C, S3U, and S3V), respectively. Additionally, 16.1% ± 3.2% of the BDA+ axon tips contacted CD68+ cells, most likely reflecting axons undergoing macrophage-mediated axonal dieback (Busch et al., 2009) (Figures 2C and S3T). Interestingly, 6.6% ± 3.1% of BDA-traced axon tips were able to penetrate the glial scar and the glial-fibrotic interface and contacted YFP+ type A pericyte-derived stromal cells (Figures 2A–2C and S3S).
Figure 2.
Pericyte-Derived Scarring Influences Retraction Bulb Formation, Dieback, and Regeneration of CST Axons
(A and B) Sagittal view of the lesion site 2 wpi showing BDA+ CST axons stopping at the glial scar (GFAP+) and some axons reaching to the lesion core (A) and stopping at YFP+ pericyte-derived cells (B).
(C) Proportion of BDA+ CST axons contacting astrocytes, OPCs, immune cells and type A pericyte-derived stroma at 2 wpi.
(D–J and L) Sagittal view of the injured spinal cord showing BDA+ CST retraction bulbs (D–I) and quantification (J and L). White arrowheads point at axon tips. ∗Indicates the edge of a tissue defect (failure to close the injury site) in Tam-def animals.
(K and M) Mean distance of CST axon tips to the lesion margin.
(N–Q) Low-power images of BDA+ CST axons in sagittal sections of the injured spinal cord. Arrowheads indicate the lesion site.
D, V, R, C on the top right corner in (A) and (N) denote dorsal, ventral, rostral, and caudal to the injury, respectively. Scale bars represent 500 μm (N–Q), 200 μm (A and D–I), 50 μm (B), and 20 μm (insets, D–I). Data shown as mean ± SEM. n = 6 (type A stroma), n = 14–16 (astrocytes, OPCs, immune cells) in (C); 2 wpi, n = 10 (vehicle), n = 9 (Tam), n = 3 (Tam-def) animals in (J) and (K); 4 wpi, n = 5 (vehicle), n = 5 (Tam), n = 2 (Tam-def) animals in (L) and (M). ns, non-significant; ∗p < 0.05 by one-way ANOVA followed by Tukey’s post hoc test.
See also Figure S3.
To investigate the influence of reduced type A pericyte-derived scarring on axonal regeneration, we quantified BDA-traced CST axons in Glast-Rasless mice after a dorsal hemisection. BDA+ CST axons in vehicle animals showed extensive retraction bulb formation and axonal dieback at 2 and 4 wpi. In comparison to the vehicle group, Tam animals displayed fewer dystrophic end bulbs, and on average, BDA+ CST axons were found closer to the rostral lesion margin. In contrast to the Tam group, Tam-def animals showed no significant difference in CST retraction bulb formation and axonal dieback compared to vehicle animals at 2 or 4 wpi (Figures 2D–2M). No CST axon regeneration into or beyond the lesion site was detected in vehicle or Tam animals at 2 wpi (Figures 2N and 2O). At 4 wpi, we observed BDA+ CST axons reaching the vicinity of the lesion core in Tam animals, with axons growing into and beyond the scar in some instances. Vehicle animals showed no CST axon regeneration into or beyond the scar at 4 wpi (Figures 2P and 2Q). We noticed that while in vehicle and Tam-def animals, CST axons stopped proximal to the glial-fibrotic lesion border, regrowing CST axons aligned with astrocyte processes and extended into the margin and core of the lesion in Tam animals at 4 wpi (Figures S3W–S3Z).
Reducing Pericyte-Derived Scarring Enables Corticospinal and Raphespinal Tract Axon Regeneration
We next assessed CST axon regeneration in Glast-Rasless mice 18 weeks after a dorsal hemisection (Figures 3A–3W). Tamoxifen-induced genetic recombination with CreER is infrequently complete, as previously established (Göritz et al., 2011). The recombination efficiency in Glast-Rasless mice negatively correlated with fibrotic scar tissue generation and positively correlated with failure to close the injury site and the appearance of a tissue defect (Figures 3L–3N). Vehicle and Tam-def animals showed little or no CST axon regeneration into or beyond the lesion site. In contrast, 10.5% ± 2.1% of the traced CST axons (relative to traced CST axons found 3 mm proximal to the lesion in the same animals) extended into the lesion site in Tam animals (Figures 3A–3K, 3O, and 3P). This corresponded on average to 10.6 ± 2.6 traced CST axons reaching the lesion core per 10 sagittal sections. In 4 of the 10 Tam animals, CST fibers reached the scar core but did not grow beyond the lesion, while in the remaining 6 animals, CST axons extended caudally, reaching up to 4 mm beyond the lesion (Figures 3D–3F, 3H, and 3O–3R). Regenerated axons grew around, under, and through the lesion (Figures S4A and S4B). None of the analyzed animals had BDA labeled axons within the main dorsal CST caudal to the lesion (Figures S4C, S4E, S4H, and S4J), establishing that fibers found distal to the injury were not a result of sparing. Terminals of traced axons 4 mm caudal to the lesion site were found preferentially in the dorsal and intermediate gray matter and displayed synaptic markers (Figures 3R–3W, S4D, S4F, S4G, S4I, S4K, and S4L).
Figure 3.
Moderate Reduction of Pericyte-Derived Scarring Facilitates CST Axon Regeneration after Spinal Cord Injury
(A–H) Low-power images (A–F) and camera Lucida projections (G and H) of BDA+ CST axons in sagittal sections of the injured spinal cord. Arrowheads indicate the lesion site.
(I–K) Sagittal view of the injured spinal cord showing stalled BDA+ CST axons proximal to a tissue defect (∗) in a Tam-def animal. (I) and (J) denote paired images.
(L) Percentage of scar occupancy by PDGFRβ-expressing stromal cells in lesion sites of vehicle and tamoxifen animals assessed for axonal regrowth 18 wpi. Dark gray and white circles represent Tam and Tam-def animals, respectively. Horizontal lines represent the mean.
(M and N) Correlation between the recombination efficiency and percentage of scar core occupied by PDGFRβ+ stromal cells (M) or tissue defect volume (N) in Glast-Rasless mice. Matched animals are indicated with the same color in (M) and (N).
(O and P) Percentage of BDA+ CST axon regeneration (relative to the total number of traced CST axons present at 3 mm rostral to the lesion) at (O) the lesion core and (P) at different rostral (R) and caudal (C) distances from the injury site 18 wpi.
(Q) Cumulative percentage of BDA+ CST axon regeneration at 0.5 mm caudal to the injury.
(R) Distribution of BDA+ CST axon regeneration across different regions of the spinal cord assessed 4 mm caudal to the injury.
(S and T) Cross section of the spinal cord of a Tam animal showing regenerated vGlut1/2+ BDA-labeled CST axons 4 mm caudal to the lesion 18 wpi (T). Enlargement of box in (S).
(U–W) Three examples of BDA-labeled regenerated CST axons containing Synapsin I (Syn I)+ vesicles found in close proximity to postsynaptic GluR2/3 receptors, indicating synapses.
Scale bars represent 500 μm (A, D, I, and J), 250 μm (B and E), 200 μm (S), 100 μm (C, F, and K), 20 μm (T), and 2 μm (U–W). Data shown as mean ± SEM. n = 8 (vehicle), n = 10 (Tam), n = 2 (Tam-def) animals in (L)–(R). ns, non-significant; ∗∗∗∗p < 0.0001 by two-sided, unpaired Student’s t test in (L). ∗p < 0.05, ∗∗p < 0.01 by one-way ANOVA followed by Tukey’s post hoc test in (O). ∗∗∗p < 0.001 by Mann-Whitney U-test in (Q). Two-way RM ANOVA: main effect of group, F(1,16) = 5.649, p = 0.0303 in (P) and main effect of group, F(1,80) = 16.52, p = 0.0001 in (R). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001 by Fisher’s LSD post hoc test in (P) and (R).
See also Figure S4.
Figure S4.
Distribution of CST Axons Rostral and Caudal to Lesions with Dense or Attenuated Fibrotic Scarring, Related to Figure 3
(A and B) Composite projection images (top) and camera lucida projections (bottom) of serial sagittal sections of injured spinal cord demonstrating BDA+ CST axon growth through, around, and past the lesion site in Tam (B) but not vehicle (A) animals 18 wpi. Black color coded axons illustrate CST axon growth through the medial zone of the spinal cord (mostly dorsal and ventral column white matter and central canal), blue represents growth within the medial-lateral zone (mostly dorsal and ventral gray matter) and red depicts axons extending through the lateral zone (mostly white mater). Camera lucida projection images at the bottom were generated by stacking the medial, medial-lateral and lateral projections into one final projection image. White arrowheads and shaded gray area in composite projection images (top) and camera lucida projections (bottom) indicate the lesion site, respectively.
(C–L) Cross sections of the spinal cord showing the distribution of BDA-labeled CST axons 4 mm rostral (C, H) and caudal (D, I) to lesion sites of vehicle (C, D) or Tam (H, I) animals 18 wpi. E-G and J-L, Enlargement of boxes in (D) and (I), respectively. Dashed lines outline the ventral part of the dorsal column.
dCST, main dorsal corticospinal tract; dGM, dorsal gray matter; iGM, intermediate gray matter; vGM, ventral gray matter. Scale bars represent 500 μm (A, B), 100 μm (C, H), 50 μm (E-G, J-L) and 200 μm (D,I).
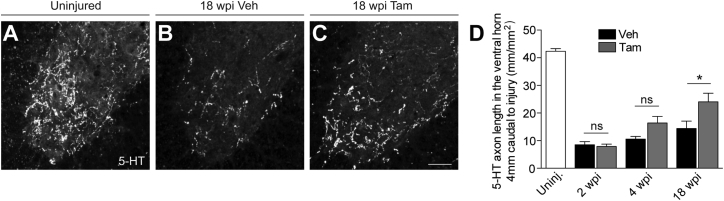
We then quantified RST axon density at 2, 4, and 18 wpi by immunohistochemistry for 5-hydroxytryptamine (5-HT) in the same vehicle and Tam animals used for CST analysis. The number of RST axons innervating the ventral horn 4 mm caudal to a dorsal hemisection increased over time, and the Tam group showed a significantly higher density of RST axons compared to vehicle animals at 18 wpi (Figure 4). The observed increase in RST axon density caudal to the lesion in Tam animals can be a result of true regeneration of transected RST axons and/or sprouting of nearby RST axon terminals that have been spared by the injury (Tuszynski and Steward, 2012).
Figure 4.
Moderate Reduction of Pericyte-Derived Scarring Facilitates RST Axon Regeneration after Spinal Cord Injury
(A–D) Coronal view of 5-HT-immunoreactive raphespinal fibers in the spinal cord ventral horn of Glast-Rasless-YFP mice 4 mm caudal to the lesion 18 wpi (A–C) and quantification (D).
Scale bars represent 50 μm (A–C). Data shown as mean ± SEM. n = 4 (Uninj.) animals; 2 wpi: n = 10 (vehicle), n = 12 (Tam); 4 wpi: n = 5 (vehicle), n = 7 (Tam); 18 wpi: n = 8 (vehicle), n = 12 (Tam) animals. ∗p < 0.05 by one-way ANOVA followed by Tukey’s post hoc test.
Optogenetic Analysis of CST Axon Signal Transmission
In the sensorimotor cortex, only layer V corticospinal neurons project directly to the spinal cord. CST axon terminals mainly target laminae III–VII and X, thus providing major excitatory input to dorsal horn neurons, (inter)segmental interneurons and premotor circuits in the mouse. To assess whether regenerated axons integrate into the local circuitry, we employed an optogenetic strategy (Jayaprakash et al., 2016, Jin et al., 2015), combining light-induced stimulation of regenerating CST axons with single-unit extracellular recordings in intermediate gray matter regions caudal to a dorsal hemisection.
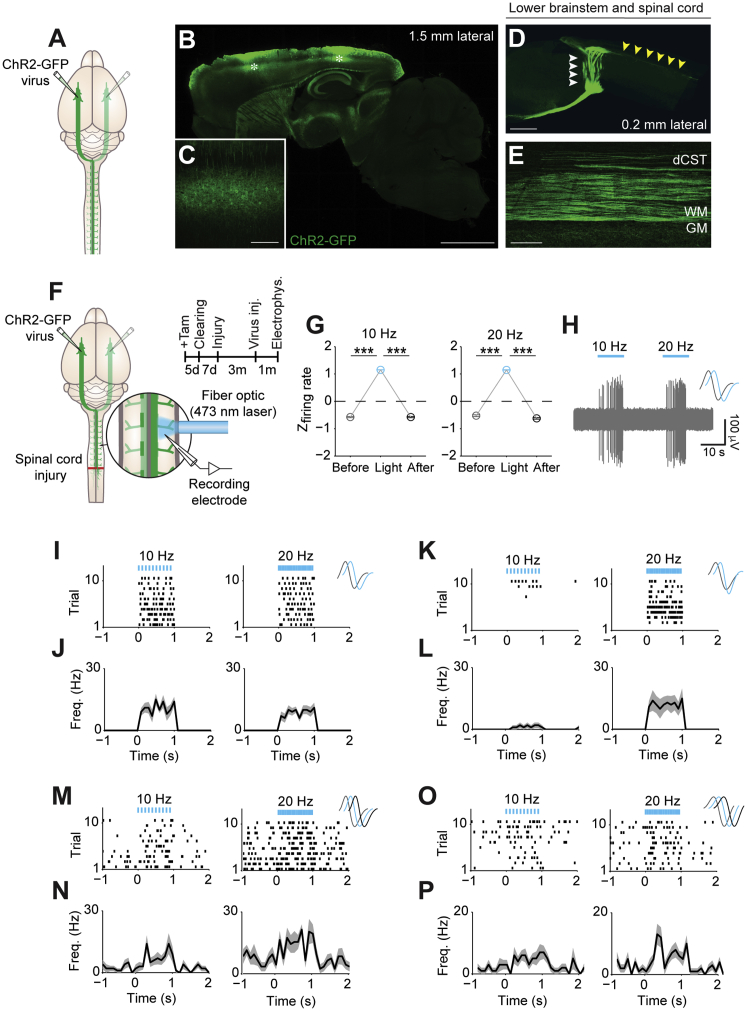
To trace CST axons, adeno-associated virus (AAV) expressing channelrhodopsin 2 (ChR2) fused to GFP (AAV9-CAG-ChR2-GFP) or only GFP (AAV9-CAG-GFP) was injected into layer V of the sensorimotor cortex in adult mice. ChR2-GFP and GFP labeling, respectively, could be detected throughout the neurospinal axis, with no labeling in dorsal root ganglia or peripheral nerve branches (Figures 5A–5E and S5).
Figure 5.
Local Photoactivation of ChR2-Expressing CST Axon Terminals Generates Postsynaptic Activity in Local Spinal Neurons
(A) Schematic depicting the strategy used to label the CST.
(B–E) Sagittal view of the brain (B–D) and spinal cord (D and E) 4 weeks after bilateral injection of AAV9-CAG-ChR2-GFP into layer V of the sensorimotor cortex demonstrating the effective expression of the opsin throughout the neurospinal axis. (C) Membrane-bound ChR2-GFP expression in pyramidal neurons. Asterisk in (B) depicts viral injection sites. White and yellow arrowheads in (D) point at the decussation of CST at the lower brainstem level and axons projecting down the spinal cord, respectively.
(F) Experimental outline illustrating the strategy employed to photoactivate ChR2-GFP+ CST axon terminals in the cervical spinal cord rostral to the lesion combined with recordings of local spinal neurons in anesthetized Glast-Rasless mice injected with AAV9-CAG-ChR2-GFP in the sensorimotor cortex.
(G) Average Z firing rate of spinal units in response to spinal illumination. Photoactivation of ChR2-GFP+ CST axon terminals elicits postsynaptic responses in spinal neurons rostral to the lesion.
(H) Representative spike trace from a spinal unit rostral to the lesion showing robust and time-locked recruitment upon photoactivation.
(I–L) Peri-event raster plots (I and K) and histograms (J and L) showing 2 examples of light-driven recruitment of new, previously silent, spinal units upon photoactivation.
(M–P) Peri-event raster plots (M and O) and histograms (N and P) of 2 light-driven spinal units that increased firing rate upon photoactivation.
For illustration of bilateral virus tracing in (A) and (F), injections into the left and right hemispheres of the brain are represented as bright and faded green color, respectively. Blue bars/lines represent 473 nm optical stimulation. Photostimulation in (G)–(P): 473 nm light, 5 mW, 3 ms pulses, 10 or 20 Hz. Insets in (H), (I), and (K) show spontaneous (gray) or light-evoked (blue) spike waveforms of recorded spinal neurons. Insets in (M) and (O) show spike waveforms of recorded spinal neurons before (gray), during (blue) or after (black) photostimulation. Before, 1 s before illumination; light, 1 s during illumination; after, 1 s post illumination. Scale bars, 2 mm (B), 1 mm (D), 200 μm (C and E). Data shown as mean ± SEM. n = 8 neurons in (G). ∗∗∗p < 0.001 by paired Student’s t test.
See also Figures S5 and S6.
Figure S5.
Viral Expression of ChR2 in the Mouse Sensorimotor Cortex, Related to Figures 5 and 6
(A–C) Cross section through the mouse sensorimotor cortex (A-C) showing expression of ChR2–GFP (C) in deep layer V neurons (A, B). Cux1 and Ctip2 label superficial and deep cortical layers, respectively. ∗ in C denotes involuntary infection of the brain surface.
(D–O) ChR2–GFP+ cells co-express the layer V specific marker ER81 (D-F), known CST projection neuron markers such as UCHL-1 (G-I), mu Crystallin (J-L) and the excitatory, glutamatergic neuron marker CaMKIIα (M-O). Yellow arrowheads point at cells single positive for ER81, UCHL-1, mu crystalline and CaMKIIα, respectively. White arrowheads denote ChR2-GFP+ cells co-expressing the marker.
(P) Percentage of ChR2-GFP+ neurons expressing (and not expressing) CaMKIIα in cortical layer V.
(Q–X) Cross sections through the dorsal root ganglion (Q-T) and peripheral nerve (U-X) showing the absence of ChR2-GFP labeling in NeuN-, neurofilament H-positive peripheral nervous system neurons and axons (Q-V), cholinergic spinal motor axons (ChAT-positive; S, T, W, X) and S100-, P0-positive satellite glial cells/ Schwann cells (Q, R, U-X).
A-C, D-F, G-I, J-L, M-O, Q, R; S, T; U, V and W, X denote paired images. ER81, also known as ETV1 (E twenty-six variant 1); UCHL-1, ubiquitin carboxyl-terminal hydrolase isozyme L1 (also known as PGP9.5); CaMKIIα, calcium/calmodulin-dependent protein kinase II alpha; ChAT, choline acetyltransferase. Data shown as mean ± SEM.; n = 3 animals in P. Scale bars represent 200 μm (A-C), 100 μm (D-O, Q, R, W, X) and 50 μm (S, T, U, V).
For validation of the optogenetic strategy, we first performed recordings of layer V sensorimotor cortex neurons in response to photoactivation in anesthetized animals transduced with ChR2-GFP. In isoflurane anesthetized animals, most cortical units appeared silent before photoactivation. Application of blue light at the cortical surface over sensorimotor cortex (473 nm, 10 Hz or 20 Hz, 3-ms pulse length) resulted in robust and time-locked spiking of layer V sensorimotor cortex pyramidal neurons (Figures S6A–S6G). We thereafter assessed cortico-spinal communication upon optogenetic orthodromic/antidromic activation of the CST in anesthetized animals. Unilateral photoactivation (473 nm, 10 Hz, 10-ms pulse length) of ChR2-expressing neurons in the sensorimotor cortex evoked orthodromic spiking in the contralateral cervical spinal cord (Figures S6H–S6M). The latencies to spike (∼10–20 ms) reflect the anatomical distance between the photostimulation and recording sites. Evoked action potentials in spinal neurons may be a direct result of CST input and/or upstream relay networks because the sensorimotor cortex also sends indirect projections, via motor centers in the brainstem, to the spinal cord. Conversely, unilateral photostimulation (473 nm, 10 Hz, 10-ms pulse length) of CST axon terminals in the cervical spinal cord elicited antidromic action potentials in CST neuronal cell bodies in the contralateral sensorimotor cortex (Figures S6N–S6S). Antidromic spikes had mean latencies of ∼10–15 ms, approximately matching the observed mean orthodromic spike latencies. Photoactivation (spinal and sensorimotor cortex, respectively) in animals transduced with AAV9-CAG-GFP did not result in light-evoked activity (Figures S6H–S6J and S6N–S6P).
Figure S6.
Optogenetic Activation of Pyramidal Neurons and Evaluation of Cortico-Spinal Communication by Orthodromic and Antidromic Activation of the CST, Related to Figures 5 and 6
(A) Schematic of optogenetic stimulation and recording paradigm in the sensorimotor cortex of anesthetized Glast-Rasless mice.
(B and C) Representative spike traces from a ChR2-expressing pyramidal neuron photostimulated at 10 (B) or 20 (C) Hz.
(D–G) Raster plots (D), peristimulus time histogram (PSTH; E), normalized change in firing rate (F) and spike probability (G) of a representative pyramidal neuron from the M1 cortex upon 10, 20, 30 and 40 Hz photoactivation.
(H and K) Schematic summary of orthodromic photostimulation of the CST paired with cervical spinal cord recordings in anesthetized Glast-Rasless mice transduced with control AAV9-CAG-GFP (H) or AAV-CAG-ChR2-GFP (K) in the sensorimotor cortex.
(I–M) Peri-event raster plots (I, L) and histograms (J, M) of representative units from the cervical spinal cord of animals transduced with AAV9-CAG-GFP (I, J) or AAV-CAG-ChR2-GFP (L, M) in the sensorimotor cortex upon 10 Hz cortical illumination.
(N and Q) Schematic summary depicting antidromic photostimulation of CST axon terminals in the cervical spinal cord combined with cortical recordings in anesthetized Glast-Rasless-YFP mice transduced with control AAV9-CAG-GFP (N) or AAV9-CAG-ChR2-GFP (Q) in the sensorimotor cortex.
(O–S) Peri-event raster plots (O, R) and histograms (P, S) of representative sensorimotor cortex layer V pyramidal neurons of animals transduced with AAV9-CAG-GFP (O, P) or AAV-CAG-ChR2-GFP (R, S) in the sensorimotor cortex upon 10 Hz spinal illumination.
Blue lines/bars represent 473 nm optical stimulation. Photostimulation: 5 mW, 3cms pulses, 10 or 20 or 30 or 40cHz in A-G and 10 mW, 10cms pulse, 10cHz in H-S. Inset in B, C shows spike waveforms of a typical pyramidal neuron before (gray), during (blue) or after (black) photoactivation and in J, M and P, S show spike waveforms of recorded spinal neurons and archetypal spike waveforms of pyramidal neurons, respectively. For illustration of bilateral virus transduction in schematics, injections into the left and right hemispheres of the brain are represented as bright and faded green color, respectively. Before, 1 s before illumination; Light, 1 s during illumination; After, 1 s post illumination. Data in F shown as mean ± SEM. ∗∗∗p < 0.001 by paired Student’s t test.
Regenerated CST Axons Functionally Integrate into the Spinal Circuitry Caudal to the Lesion
To validate that photostimulation of ChR2-expressing CST axon terminals drives spiking of spinal neurons, we performed local and restricted photoactivation in the spinal cord of anesthetized animals transduced with ChR2-GFP. Photoactivation (473 nm, 10 Hz or 20 Hz, 3 ms pulse length) targeted to the intact (non-lesioned) cervical spinal cord rostral to a dorsal hemisection in Glast-Rasless animals 18 wpi evoked postsynaptic responses in spinal neurons (Figures 5F–5P). Recruitment of silent spinal units (Figures 5I–5L) as well as increased firing were observed (Figures 5M–5P).
We next assessed the functionality of regenerated CST axon terminals caudal to the injury 18 wpi. Blue light (473 nm, 10 Hz or 20 Hz, 3 ms pulse length) was delivered 0.5–3 mm caudal to the injury site and single unit activity recorded at the same position (Figure 6). Activation of ChR2-GFP-expressing CST terminals caudal to the lesion site elicited clear spinal postsynaptic responses from the intermediate gray matter, establishing that regenerated CST axons form functional synapses with local spinal neurons in Tam animals (Figures 6H–6N). The short light-driven latencies to spike suggest monosynaptic activation of local spinal neurons by regenerated CST axons (Figure 6N). Photoactivation caudal to the injury in vehicle animals had no effect on postsynaptic responses (Figures 6A–6G). Taken together, the optogenetic experiments provide causal evidence for functional integration of regenerated CST axons in the spinal circuitry caudal to a dorsal hemisection in animals with reduced pericyte-derived scarring (Tam animals).
Figure 6.
Regenerated CST Axons Functionally Integrate into the Local Spinal Circuit Caudal to the Lesion
Optogenetic assessment of functional integration of regenerated CST axons in the injured spinal cord of vehicle (A–G) and Tam (H–N) animals, 18 wpi.
(A and H) Schematic and experimental outline of in vivo optogenetic stimulation and recording paradigm in the spinal cord caudal to the lesion.
(B and I) Average Z firing rate of spinal units in response to spinal illumination. Photostimulation does not modulate postsynaptic activity of local spinal neurons caudal to the lesion in vehicle animals (B) but drives postsynaptic activity in spinal neurons in Tam animals (I).
(C and J) Representative spike trace from a spinal unit recorded caudal to lesion showing no response in vehicle animals (C) and increased firing in response to illumination of regenerated ChR2-expressing CST axon terminals in Tam animals (J).
(D–G and K–N) Raster plots (D and K), peristimulus time histogram (PSTH) (E and L), normalized change in firing rate (F and M) and spike probability (G and N) of a representative spinal unit caudal to the lesion not responding (vehicle, D–G) and responding (Tam, K–N) to photoactivation.
Photostimulation in (B)–(G) and (I)–(N): 473 nm light, 5 mW, 3 ms pulses, 10 or 20 Hz. Data shown as mean ± SEM. n = 8 and n = 5 neurons in (B) and (I), respectively. ns, non-significant; ∗∗∗p < 0.001 by paired Student’s t test.
See also Figures S5 and S6.
Attenuated Pericyte-Derived Scarring Improves Sensorimotor Recovery
CST fibers mediate fine motor coordination, while serotonergic fibers modulate the activity of spinal motor systems (Tuszynski and Steward, 2012). Skilled locomotion, sensorimotor integration and limb placement can be evaluated using the horizontal ladder-walking test (Metz and Whishaw, 2002). When traversing the ladder, Tam animals showed an overall improved performance and a consistently reduced hind limb error rate compared to vehicle animals, from week 7 following spinal cord injury onward (Figure 7A). Similarly, the regularity index, reflecting inter-limb coordination, measured by automated gait analyses (Hamers et al., 2001, Neumann et al., 2009) stably improved in the Tam group from week 6 following injury onward, when compared to vehicle animals (Figure 7B). The number of regenerated CST axons found in the lesion core negatively correlated with the extent of fibrotic scarring and hindlimb error rate (Figures S7A and S7B).
Figure 7.
Attenuation of Pericyte-Derived Scarring Promotes Functional Recovery after Spinal Cord Injury
(A and B) Percentage of hind limb errors in the horizontal ladder test (A) and regularity index of step sequence using Catwalk automated gait analyses (B) after dorsal hemisection.
(C) Schematics and experimental outline for ChR2-assisted in vivo behavioral testing.
(D) Number of hind paw strokes in response to sensorimotor cortex photoactivation (3 × 10 s = 30 s in total).
(E and G) Percentage of ChR2-GFP+ CST (E) and 5-HT (G) axon density.
(F and H) Correlation between light-induced hind paw strokes and percentage of ChR2-GFP+ CST axons (F) or 5-HT axon density (H).
Data shown as mean ± SEM. n = 8 (vehicle), n = 10 (Tam) animals in (A) and (B) and n = 5 (Uninj., GFP), n = 4 (Uninj., ChR2-GFP), n = 7 (SCI vehicle, ChR2-GFP), n = 7 (SCI Tam, ChR2-GFP) animals in (D)–(H). ns, non-significant; ∗∗p < 0.01, ∗∗∗∗p < 0.0001 by two-sided, unpaired Student’s t test in (D), (E), and (G). Two-way RM ANOVA: main effect of group, F(1,16) = 9.546, p = 0.0070 in (A) and main effect of group, F(1,16) = 11.004, p = 0.0043 in (B). Holm-Sidak post hoc test, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 in (A) and (B).
Figure S7.
Attenuation of Pericyte-Derived Scarring Facilitates CST and RST Regeneration after Spinal Cord Injury, Related to Figure 7
(A and B) Correlation between the number of regenerated CST axons at the lesion core and the percentage of scar core occupied by PDGFRβ+ stromal cells (A) or percentage of hind limb errors in the horizontal ladder test (B), 18 wpi.
(C) Coronal view of the uninjured spinal cord at high thoracic level showing ChR2-GFP+ CST axon bundles running along the dorsal and dorsolateral funiculi and dense collateral innervation of the gray matter.
(D) Close up of the uninjured spinal cord depicting ChR2-GFP+ CST axon bundles exiting the main dCST and branching out to densely innervate the dorsal and intermediate gray matter.
(E and F) Cross sections of spinal cord from Glast-Rasless animals at 18 wpi illustrating the absence or presence of regenerated ChR2-GFP+ CST axons 0.5 mm caudal to lesions of vehicle (E) or Tam (F) animals, respectively.
(G–R) Co-immunostaining of 5-HT raphespinal fibers, ChAT-expressing motor neurons and synaptophysin-positive synaptic vesicles in coronal sections of the spinal cord ventral horn of uninjured (G-J) and injured vehicle (K-N) and Tam (O-R) animals 4 mm caudal to the lesion 18 wpi.
G-J; K-N and O-R depict paired images. dCST, dorsal corticospinal tract; dGM, dorsal gray matter; iGM, intermediate gray matter. n = 8 (vehicle), n = 10 (Tam) animals in A, B. Scale bars represent 500 μm (C), 100 μm (D-F), 50 μm (G, K, O) and 25 μm (H-J, L-N, P-R).
To further test the significance of improved axonal regeneration for the recovery of sensorimotor function we developed an optogenetic-based task in awake, freely moving animals (Figure 7C). Mice were bilaterally transduced with AAV9-CAG-ChR2-GFP or control AAV9-CAG-GFP virus in layer V of the sensorimotor cortex and bilaterally implanted with fiber optics 0.2 mm above the sites of virus injection. Sensorimotor cortex layer V output neurons project to reach spinal cord targets but also provide major excitatory input to the red nucleus and reticular formation, which project descending axons in the spinal cord. Unilateral optogenetic activation of the sensorimotor cortex (20 Hz, 5 ms pulse length) triggered vigorous limb movement (Cheng et al., 2014) in awake, freely moving uninjured animals transduced with AAV9-CAG-ChR2-GFP but failed to generate a similar behavioral output in uninjured AAV9-CAG-GFP transduced control animals (Figure 7D; Movie S1). Eighteen weeks following a dorsal hemisection, we observed an almost complete loss of light-evoked hind paw strokes in vehicle animals. However, Tam animals showed modest but significant increase in light-driven hind paw strokes compared to the vehicle group (Figure 7D; Movie S2). Post hoc analysis of the animals revealed that 10% ± 2% of the ChR2-GFP-expressing CST axons (relative to the total CST axon density found 4 mm proximal to the injury site) could be found 0.5 mm caudal to the lesion in dorsal and intermediate gray matter regions in Tam animals, confirming the findings from the earlier experiment (Figure 3). No ChR2-GFP-expressing axons could be found caudal to the lesion in animals with dense fibrotic scar (vehicle animals) (Figures S7C–S7F). We also assessed serotonergic axon density in the two groups of animals subjected to the functional experiments. The Tam animals showed increased density of 5-HT+ RST axons innervating the ventral horn 4 mm caudal to the lesion, compared to vehicle animals (Figures S7G–S7R). CST and RST axon densities 0.5 mm and 4 mm caudal to the lesion, respectively, correlated with light-driven hind paw strokes (Figures 7E–7H). Animals with improved motor performance presented the highest number of regenerated fibers caudal to the lesion, suggesting that the regenerated fibers may, at least in part, account for the observed behavioral improvement, most likely via indirect relay-circuits (Filli and Schwab, 2015, Hou, 2014).
Discussion
Scar formation by astrocytes (Anderson et al., 2016, Faulkner et al., 2004, Herrmann et al., 2008, Sabelström et al., 2013, Silver, 2016) and type A pericytes (Göritz et al., 2011) is crucial for sealing off the injured tissue and regaining tissue integrity after CNS lesions. At the same time, scar tissue is considered a major block for axonal regeneration. Most attention has been focused on astrocytes forming the glial component of the scar, and reactive astrocytes have long been thought to inhibit axonal regeneration (Cafferty et al., 2007, Xu et al., 2015, Yiu and He, 2006), although this notion has recently been questioned (Anderson et al., 2016, Silver, 2016). Pericytes give rise to fibroblast-like cells that constitute the fibrotic compartment of the scar and are required for the generation of fibrosis and extracellular matrix deposition. We demonstrate that pericyte-derived scarring represents a major barrier for axonal regrowth and that moderate inhibition of this process preserves intact wound healing and dampens inflammation and reactive astrogliosis while enabling axonal regrowth and improved functional recovery.
Using lineage tracing, we previously identified a small pericyte subset as the origin of scar-forming fibroblast-like cells following spinal cord injury (Göritz et al., 2011). Similarly, pericytes have also been implicated in dermal scarring and kidney fibrosis as the source of ECM-producing (myo)fibroblasts (Lin et al., 2008, Sundberg et al., 1996). However, a recent fate mapping study (Guimaraes-Camboa et al., 2017) targeting Tbx18-expressing pericytes and smooth muscle cells showed little proliferation and no contribution of this lineage to scar-forming cells in response to cortical stab wounds. There are two important points that may explain the discrepancy between the aforementioned studies. First, type A pericytes and Tbx18-expressing mural cells might represent non-overlapping populations. Our previous study established functional heterogeneity among pericytes, with scar formation being restricted to a small subset accounting for 10% of all pericytes (Göritz et al., 2011). Second, in contrast to spinal cord injuries, cortical stab lesions do not give rise to extensive fibrotic tissue, indicated by the absence of a substantial increase in Col1a1-GFP expressing cells post-injury (Guimaraes-Camboa et al., 2017).
Scar tissue is composed of different cell types with distinct cellular origin (Barnabé-Heider et al., 2010, Göritz et al., 2011, Meletis et al., 2008, Zhu et al., 2015a) and reduction of a specific cellular scar component will influence the contribution of other scar-forming cells. Prevention, deletion or attenuation of astrocyte scarring leads to increased neurotoxic inflammation and expansion of fibrotic scarring, compromising axon regeneration and worsening functional recovery (Anderson et al., 2016, Faulkner et al., 2004, Herrmann et al., 2008, Sabelström et al., 2013, Silver, 2016). Conversely, the decreased reactive astrogliosis and inflammation associated with reduced pericyte-derived scarring we describe here may contribute to the beneficial effect on axonal regeneration and functional recovery.
That fibrosis inhibits axonal regeneration is consistent with indirect observations in previous studies. For example, when promoting inherent neuronal regenerative capacity by experimentally reducing PTEN, CST axon regrowth is observed along glial bridges and in fibrotic tissue-free regions (Zukor et al., 2013). Moreover, microtubule stabilization was shown to reduce scarring and impact axon regeneration of serotonergic and growth-competent sensory neurons after spinal cord injury (Hellal et al., 2011, Ruschel et al., 2015). However, these studies did not distinguish between the effects of reduced scarring and axonal stabilization and targeted various cell types and cellular mechanisms. Using a cell-type-specific targeting strategy, we show that a moderate reduction of pericyte-derived scar tissue facilitates CST and RST axon regeneration and functional recovery.
CST axons, which show the greatest resistance to regeneration, mediate voluntary fine motor movement, a function that is much desired in spinal cord injury patients (Liu et al., 2011, Welniarz et al., 2017). As in most studies focusing on CST regeneration, we have used a dorsal hemisection model to ensure transection of CST projections. This lesion model spares main descending brainstem motor systems such as the vestibulospinal and reticulospinal tracts. Although not being directly responsible for most aspects of gross locomotor hind limb recovery, the CST/5-HT regenerated fibers could ultimately contribute via (inter)segmental, propriospinal, and other indirect relay-circuits to the observed improvement in functional recovery in Tam animals (Filli and Schwab, 2015, Flynn et al., 2017, Hou, 2014, Ueno et al., 2012).
While being more clinically relevant, severe injury models such as contusion lesions (commonly combined with BMS scoring to follow functional recovery), are not the preferred injury models to study CST regeneration, as not all the CST axons are destroyed by the lesion, making it difficult to distinguish between bona fide regeneration and sprouting of spared axons. Dense fibrotic scarring is seen following both dorsal hemisection and contusion injury (Zhu et al., 2015b) in rodents. Humans also form a fibrous extracellular matrix rich non-neural lesion core following traumatic spinal cord injury (Buss et al., 2007, Norenberg et al., 2004), suggesting that attenuation of pericyte-derived scarring may be explored as a therapeutic target to facilitate regeneration following CNS injury.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Goat anti 5-HT | Immunostar | 20079; RRID:AB_572262 |
| Rat anti 5-HT | Millipore | MAB352; RRID:AB_11213564 |
| Mouse anti CaMKIIα | Cell Signaling Technology | 50049; RRID:AB_2721906 |
| Rat anti CD31 | BD Biosciences | 550274; RRID:AB_393571 |
| Rat anti CD68 | AbD Serotec | MCA1957GA; RRID:AB_324217 |
| Goat anti ChAT | Millipore | AB144P; RRID:AB_207951 |
| Rabbit anti Collagen I | Abcam | ab21286; RRID:AB_446161 |
| Rabbit anti Collagen III | Abcam | ab7778; RRID:AB_306066 |
| Rabbit anti Collagen IV | AbD Serotec | 2150-1470; RRID:AB_2082660 |
| Goat anti Collagen IV | Southern Biotech | 1340-01; RRID:AB_2721907 |
| Goat anti Collagen VI | Southern Biotech | 1360-01; RRID:AB_2721908 |
| Rabbit anti Collagen VI | Abcam | ab6588; RRID:AB_305585 |
| Mouse anti mu Crystallin | Abcam | ab54669; RRID:AB_943673 |
| Rat anti Ctip2 | Abcam | ab18465; RRID:AB_2064130 |
| Rabbit anti Cux1 | Santa Cruz Biotechnology | sc-13024; RRID:AB_2261231 |
| Rabbit anti ER81 | Santa Cruz Biotechnology | sc-28681; RRID:AB_2100826 |
| Rabbit anti Fibronectin | Sigma-Aldrich | F3648; RRID:AB_476976 |
| Mouse anti GFAP (Cy3 conjugated) | Sigma-Aldrich | C9205; RRID:AB_476889 |
| Chicken anti GFAP | Millipore | AB5541; RRID:AB_177521 |
| Guinea pig anti GFAP | Synaptic Systems | 173 004; RRID:AB_10641162 |
| Goat anti GFP (FITC conjugated) | Abcam | ab6662; RRID:AB_305635 |
| Chicken anti GFP | Aves Labs | GFP-1020; RRID:AB_10000240 |
| Rabbit anti GluR2/3 | Millipore | AB1506; RRID:AB_90710 |
| Guinea pig anti vGlut1 | Millipore | AB5905; RRID:AB_2301751 |
| Guinea pig anti vGlut2 | Millipore | AB2251; RRID:AB_1587626 |
| Mouse anti NeuN | Millipore | MAB377; RRID:AB_2298772 |
| Guinea pig anti NeuN | Millipore | ABN90; RRID:AB_11205592 |
| Chicken anti NF-H | Millipore | AB5539; RRID:AB_177520 |
| Rabbit anti NG2 | Millipore | AB5320; RRID: AB_91789 |
| Rabbit anti P0 | Abcam | ab31851; RRID:AB_2144668 |
| Rabbit anti PDGFRα | Cell Signaling Technology | 3164S; RRID:AB_10694389 |
| Goat anti PDGFRα | R&D Systems | AF1062; RRID:AB_2236897 |
| Rabbit anti PDGFRβ | Abcam | ab32570; RRID:AB_777165 |
| Goat anti Podocalyxin | R&D Systems | AF1556; RRID:AB_354858 |
| Rabbit anti S100 | DAKO | Z0311; RRID:AB_10013383 |
| Mouse anti Synapsin I | Synaptic Systems | 106011; RRID:AB_2619772 |
| Rabbit anti Synapsin I | Millipore | AB1543P; RRID:AB_90757 |
| Mouse anti Synaptophysin | Abcam | ab8049; RRID:AB_2198854 |
| Rabbit anti UCHL1 | Abcam | ab10404; RRID:AB_297145 |
| Guinea pig anti UCHL1 | Abcam | ab10410; RRID:AB_297150 |
| Streptavidin Cy3 | Jackson Immunoresearch | 016-160-084; RRID:AB_2337244 |
| Streptavidin Alexa Fluor 594 | Jackson Immunoresearch | 016-580-084: RRID:AB_2337250 |
| Streptavidin Alexa Fluor 647 | Jackson Immunoresearch | 016-600-084; RRID:AB_2337251 |
| Donkey anti mouse biotin-SP (long spacer) | Jackson Immunoresearch | 715-066-151; RRID:AB_2340788 |
| Donkey anti rabbit biotin-SP (long spacer) | Jackson Immunoresearch | 711-066-152; RRID:AB_2340594 |
| Donkey anti rat Cy3 | Jackson Immunoresearch | 712-166-153; RRID:AB_2340669 |
| Donkey anti rat Alexa Fluor 594 | Jackson Immunoresearch | 712-586-153; RRID:AB_2340691 |
| Donkey anti rat Alexa Fluor 647 | Jackson Immunoresearch | 712-606-153; RRID:AB_2340696 |
| Donkey anti mouse Alexa Fluor 488 | Jackson Immunoresearch | 715-546-151; RRID:AB_2340850 |
| Donkey anti mouse Cy3 | Jackson Immunoresearch | 715-166-151; RRID:AB_2340817 |
| Donkey anti mouse Alexa Fluor 647 | Jackson Immunoresearch | 715-606-151; RRID:AB_2340866 |
| Donkey anti rabbit DyLight 405 | Jackson Immunoresearch | 711-476-152; RRID:AB_2632566 |
| Donkey anti rabbit Alexa Fluor 488 | Jackson Immunoresearch | 711-546-152; RRID:AB_2340619 |
| Donkey anti rabbit Cy3 | Jackson Immunoresearch | 711-166-152; RRID:AB_2313568 |
| Donkey anti rabbit Alexa Fluor 647 | Jackson Immunoresearch | 711-606-152; RRID:AB_2340625 |
| Donkey anti goat Alexa Fluor 488 | Jackson Immunoresearch | 705-546-147; RRID:AB_2340430 |
| Donkey anti goat Cy3 | Jackson Immunoresearch | 705-166-147; RRID:AB_2340413 |
| Donkey anti goat Alexa Fluor 647 | Jackson Immunoresearch | 705-606-147; RRID:AB_2340438 |
| Donkey anti chicken DyLight 405 | Jackson Immunoresearch | 703-476-155; RRID:AB_2632562 |
| Donkey anti chicken Alexa Fluor 488 | Jackson Immunoresearch | 703-546-155; RRID:AB_2340376 |
| Donkey anti chicken Cy3 | Jackson Immunoresearch | 703-166-155; RRID:AB_2340364 |
| Donkey anti chicken Alexa Fluor 647 | Jackson Immunoresearch | 703-606-155; RRID:AB_2340380 |
| Donkey anti chicken A488 | Jackson Immunoresearch | 712-546-153; RRID:AB_2340686 |
| Donkey anti chicken Cy3 | Jackson Immunoresearch | 712-166-153; RRID:AB_2340669 |
| Donkey anti guinea pig DyLight 405 | Jackson Immunoresearch | 706-476-148; RRID:AB_2632564 |
| Donkey anti guinea pig Alexa Fluor 488 | Jackson Immunoresearch | 706-546-148; RRID:AB_2340473 |
| Donkey anti guinea pig Cy3 | Jackson Immunoresearch | 706-166-148; RRID:AB_2340461 |
| Donkey anti guinea pig Alexa Fluor 647 | Jackson Immunoresearch | 706-606-148; RRID:AB_2340477 |
| Mouse anti GAPDH | Millipore | MAB374; RRID:AB_2107445 |
| Goat anti GFP | Abcam | ab6673; RRID:AB_305643 |
| Rabbit anti HSP47 | Acris | AP08508PU-N; RRID:AB_1954041 |
| Rabbit anti P4HB | Abnova | PAB12587; RRID:AB_10556016 |
| Rabbit anti PDGFRβ | Cell Signaling Technology | 3169; RRID:AB_2162497 |
| Rabbit anti Periostin | Abcam | ab14041; RRID:AB_2299859 |
| Rabbit anti S100A4 | Abcam | ab27957; RRID:AB_2183775 |
| Donkey anti mouse HRP | Jackson ImmunoResearch | 715-036-151; RRID:AB_2340774 |
| Donkey anti rabbit HRP | Jackson ImmunoResearch | 711-036-152; RRID:AB_2340590 |
| Donkey anti goat HRP | Jackson ImmunoResearch | 705-036-147; RRID:AB_2340392 |
| Bacterial and Virus Strains | ||
| AAV9-CAG-ChR2-GFP | University of North Carolina Viral Vector Core (Dr. Ed Boyden) | N/A |
| AAV9-CAG-GFP | University of North Carolina Viral Vector Core (Dr. Ed Boyden) | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| 4’6’-diamidino-2-phenylindole (DAPI) | Sigma-Aldrich | D9542 |
| Tamoxifen | Sigma-Aldrich | T5648 |
| Corn Oil | Sigma-Aldrich | C8267 |
| Dextran, biotin, 10000MW, lysine fixable (BDA-10000) | Invitrogen | D1956 |
| Vectashield antifade mounting medium | Vector Labs | H-1000 |
| 5-ethynyl-2′-deoxyuridine (EdU) | Molecular Probes | A10044 |
| PhosSTOP Phosphatase Inhibitor Cocktail Tablets | Sigma-Aldrich (Roche) | PHOSS-RO ROCHE |
| cOmplete Protease Inhibitor Tablets | Sigma-Aldrich (Roche) | CO-RO ROCHE |
| Critical Commercial Assays | ||
| Click-iT EdU A647 imaging kit | Molecular Probes | C10340 |
| A647 Antibody labeling kit | Molecular Probes | A20186 |
| Mouse on mouse (MOM) basic kit | Vector Labs | BMK-2202 |
| Pierce BCA protein assay kit | Thermo Scientific | 23225 |
| AllPrep DNA/RNA micro kit | QIAGEN | 80284 |
| SuperScript III first-strand synthesis system | Invitrogen | 18080051 |
| TaqMan universal master mix II | Applied Biosystems | 4440040 |
| Buffer RLT | QIAGEN | 79216 |
| AllPrep DNA/RNA/protein mini kit | QIAGEN | 80004 |
| Qubit RNA BR assay kit | Life Technologies | Q10210 |
| Agilent RNA 6000 nano kit | Agilent Technologies | 5067-1511 |
| Deposited Data | ||
| Raw data files for RNA-seq | This paper | NCBI GEO: GSE93976 |
| Mouse reference genome, mm9, NCBI Build 37 | Mouse Genome Sequencing Consortium / NCBI | https://www.ncbi.nlm.nih.gov/grc/mouse |
| Experimental Models: Organisms/Strains | ||
| Mouse: Tg(Glast-CreERT2) | Slezak et al., 2007 | N/A |
| Mouse: HRas–/–;NRas–/–; KRaslox/lox (Rasless) | Drosten et al., 2010 | N/A |
| Mouse: B6.129X1-Gt(Rosa)26Sortm1(EYFP)Cos/J (R26R-YFP Cre reporter line) | The Jackson Laboratory | JAX:006148 |
| Oligonucleotides | ||
| See Table S3 | N/A | N/A |
| Software and Algorithms | ||
| Catwalk XT | Noldus Information Technology | N/A |
| Prism version 6.0g for Mac | GraphPad Software, Inc | N/A |
| ImageJ/Fiji version 2.0.-rc-43/1.51 g for Mac | Schindelin et al., 2012 | N/A |
| QIAGEN’s Ingenuity Pathway Analysis (IPA) | QIAGEN | http://www.ingenuity.com |
| Database for Annotation, Visualization and Integrated Discovery (DAVID) | Huang et al., 2009a, Huang et al., 2009b | https://david.ncifcrf.gov/tools.jsp |
| TopHat version 1.3.3 | Trapnell et al., 2009 | N/A |
| Picard Tools version 1.29 | Broad Institute, Cambridge, MA, USA | http://broadinstitute.github.io/picard |
| Cufflinks version 1.3.0 | Trapnell et al., 2010 | N/A |
| HTSeq version 0.5.1 | Anders et al., 2015 | N/A |
| R/Bioconductor package DSeq | Anders and Huber, 2010 | N/A |
| MClust spike sorting toolbox version 1.0 for MATLAB | A David Redish | https://www.mathworks.com/matlabcentral/fileexchange/19311-mclust-spike-sorting-toolbox |
Contact for Reagent and Resource Sharing
Further information and requests for reagents should be directed to and will be fulfilled by the Lead Contact, Christian Göritz (christian.goeritz@ki.se).
Experimental Model and Subject Details
Animals
Glast-CreERT2 (Slezak et al., 2007) and Rasless (Drosten et al., 2010) mice were crossed to the R26R-yellow fluorescent protein (YFP) Cre-reporter line (obtained from the Jackson Laboratory, B6.129X1-Gt(Rosa)26Sortm1(EYFP)Cos/J, JAX: 006148, (Srinivas et al., 2001)) to generate Glast-Rasless-YFP mice. All Rasless mice used were homozygous for HRas and NRas null alleles and homozygous for floxed KRas alleles. For some experiments Glast-CreERT2 mice were crossed to the R26R-YFP Cre-reporter line only to generate Glast-YFP mice. All animals were on a C57BL/6J background and ≥ 8 weeks old at the onset of experiments and both male and female mice were used. Animals were housed in group in standardized cages with a 12:12 h light:dark cycle with unrestricted access to food and water. All experimental procedures were carried out in accordance to the Swedish and European Union guidelines and approved by the institutional ethical committee (Stockholms Norra Djurförsöksetiska Nämnd).
Method Details
Genetic labeling of transgenic mice
Recombination in Glast-Rasless-YFP animals was induced by a daily intra-peritoneal injection of 2 mg of tamoxifen (Sigma, 20 mg/ml in 1:9 ethanol:corn oil) for 5 consecutive days. Vehicle animals (matched mice with the same genotype) received the same number of injections of the solvent (1:9 ethanol:corn oil) without tamoxifen. Animals were randomly assigned to receive either vehicle alone or tamoxifen. Glast-YFP animals were recombined with tamoxifen using the same protocol aforementioned.
Spinal cord injury
Injuries were performed after a 7 days clearing period starting after the last tamoxifen injection. Tamoxifen and its active metabolite 4-hydroxytamoxifen have a half-life of 6–12 h in the mouse (Robinson et al., 1991). Analysis of CreERT2 distribution in the adult mouse spinal cord 6 days after the last tamoxifen administration has demonstrated that there is no CreERT2 in the nucleus of cells at this time, directly demonstrating that tamoxifen has been cleared at this time point (Meletis et al., 2008). Therefore, the chosen 7 days wash out period ensures that no tamoxifen is left at the time of injury or after, which could affect the response to injury. Moreover, it guarantees that all recombination occurs prior to the insult, so in case other cells than type A pericytes start expressing the Glast-CreERT2 transgene in response to the injury, it will not result in recombination.
Dorsal funiculus incision SCI model
Glast-CreERT2 x R26R-YFP (Glast-YFP; n = 4-5) and Glast-CreERT2 x R26R-YFP x Rasless (Glast-Rasless-YFP, tamoxifen injected; n = 4) mice were anesthetized with 4% isoflurane until unconscious followed by 2% isoflurane during surgery. Analgesia (Temgesic/Buprenorphine, Schering-Plough, 0.1 mg/kg body weight and Rimadyl/Carprofen, Pfizer, 5 mg/kg body weight; subcutaneous injection) was administered for postoperative pain relief and a uniform layer of Viscotears eye gel (2 mg/g, Novartis) was applied onto the eye ball to prevent drying. A laminectomy was performed at the mid-thoracic level to expose the dorsal portion of the spinal cord and the dorsal funiculus (located between the dorsal horns and the midline) and adjacent gray matter were cut transversely to a depth of 0.8mm. This incision was extended rostrally with microsurgical scissors to span one segment (adapted from (Meletis et al., 2008)). All animals received local anesthesia (Xylocaine/Lidocaine, AstraZeneca, 10 mg/ml, 2 drops on the spinal cord surface) 2-3 min prior to the lesion. For recovery from surgery animals were placed on a heating pad and only returned to their home cage once they were fully awake.
Following SCI, EdU was given twice by intraperitoneal injections (12 mg/ml, 100 μL per injection) at 6 hr interval, followed by EdU administration in the drinking water for up to 5 days (0.2 mg/ml and 1% sucrose, exchanged every 2-3 days and kept in dark) to label dividing cells. A group of Glast-YFP (n = 3) and Glast-Rasless-YFP (n = 4) mice underwent the same EdU regimen but received no injury.
Dorsal hemisection SCI model and CST tracing
Glast-YFP (n = 6, 2 wpi) and Glast-Rasless-YFP (n = 54; Vehicle: 2 wpi: n = 10, 4 wpi: n = 5, 18 wpi: n = 8, Tamoxifen: 2 wpi: n = 12, 4 wpi: n = 7, 18 wpi: n = 12) mice were anesthetized with 4% isoflurane until unconscious followed by 2% isoflurane during surgery. A laminectomy was performed at T1-T2 levels to expose the dorsal portion of the spinal cord. To minimize meningeal fibroblast invasion into the lesion core the dura mater was carefully cleared off from the exposed area. A bilateral dorsal hemisection lesion of the spinal cord was performed at T1 with a pair of microscissors to a depth of 0.8 mm to sever the CST. For recovery from surgery animals were placed on a heating pad and only returned to their home cage once they were fully awake. Surgeries were conducted in a blinded fashion. Two weeks before the termination of the experiments, mice were anesthetized with 4% isoflurane until unconscious followed by 2% isoflurane during surgery and received bilateral cortical injections of 10% biotin dextran amine (BDA, 10000 MW, Life Technologies) to anterogradely label the CST. Briefly, animals were head-fixed in a stereotaxic frame and burr holes were drilled over the sensorimotor cortex. Four microinjections (0.4 μL per injection) of BDA were targeted to layer V in the sensorimotor cortex (two injections / hemisphere; from bregma, AP ± 0.5 mm, LM 1 mm, DV 0.55 mm and AP ± 0.5 mm, LM −1 mm, DV 0.55 mm) at a rate of 0.1 μl/min using a 10 μL NanoFil syringe with a 36 g beveled needle tip (World Precision Instruments) and a microinjector (UltraMicroPump III and Micro4 microsyringe pump controller, World Precision Instruments). After injection, the needle was kept in place for an additional 5 min to allow virus diffusion and prevent backflow of the virus to the surface, and then slowly withdrawn. For recovery from surgery animals were placed on a heating pad and only returned to their home cage once they were fully awake. For animals sacrificed 2 weeks after dorsal hemisection lesion, BDA was injected on the day before the SCI surgery. For experiments terminated 4 and 18 weeks after dorsal hemisection lesion, mice were injected with BDA two and sixteen weeks after the SCI surgery, respectively.
For all surgeries, analgesia (Temgesic/Buprenorphine, Schering-Plough, 0.1 mg/kg body weight and Rimadyl/Carprofen, Pfizer, 5 mg/kg body weight; subcutaneous injection) was administered for postoperative pain relief and a uniform layer of Viscotears eye gel (2 mg/g, Novartis) was applied onto the eye ball to prevent drying.
All animals received local anesthesia (Xylocaine/Lidocaine, AstraZeneca; 10 mg/ml, 2 drops on the spinal cord surface) 2-3 min prior to spinal lesions or craniotomies.
In vivo electrophysiology and optogenetics
Uninjured Glast-Rasless-YFP animals (n = 8) and Glast-Rasless-YFP animals subjected to SCI injury 12-14 weeks earlier (n = 3 injected with vehicle, n = 5 injected with tamoxifen) were anesthetized with 4% isoflurane until unconscious followed by 2% isoflurane during surgery. Animals were placed in a stereotaxic frame and burr holes were drilled to expose the brain. Four microinjections (0.4 μL per injection) of an AAV expressing ChR2-GFP or GFP (AAV9-CAG-ChR2-GFP, titer 3x1012 virus molecules/mL; AAV9-CAG-GFP, titer 2x1012 virus molecules/mL, University of North Carolina Viral Vector Core) were targeted to layer V in the sensorimotor cortex (two injections / hemisphere; from bregma, AP 0.5 mm, LM 1 mm, DV 0.55 mm and AP −0.5 mm, LM 1 mm, DV 0.55 mm) at a rate of 0.1 μl/min using a 10 μL NanoFil syringe with a 36 g beveled needle tip (World Precision Instruments) and a microinjector (UltraMicroPump III and Micro4 microsyringe pump controller, World Precision Instruments). After injection, the needle was kept in place for an additional 5 min to allow virus diffusion and prevent backflow of the virus to the surface, and then slowly withdrawn. For recovery from surgery animals were placed on a heating pad and only returned to their home cage once they were fully awake. For all surgeries, analgesia (Temgesic/Buprenorphine, Schering-Plough, 0.1 mg/kg body weight and Rimadyl/Carprofen, Pfizer, 5 mg/kg body weight; subcutaneous injection) was administered for postoperative pain relief and Viscotears eye gel (2 mg/g, Novartis) was applied onto the eye ball to prevent drying.
All animals received local anesthesia (Xylocaine/Lidocaine, AstraZeneca; 10 mg/ml, 2 drops on the spinal cord surface) 2-3 min prior to craniotomies.
Four to six weeks after viral injections (i.e., 18 weeks post SCI for injured animals) in vivo single unit extracellular recordings from the sensorimotor cortex and spinal cord were performed in conjunction with optogenetics under anesthesia (1% isoflurane in O2). Animals were placed in a stereotaxic frame, the head was immobilized with ear bars and the cortical region previously injected with virus exposed. Viscotears eye gel (2mg/g; Novartis) was applied onto the eye ball to prevent drying. For uninjured animals the cervical spinal cord was exposed and stabilized with spinal cord clamps (Narishige, Japan). For injured animals the spinal cord segments encompassing the injury site plus 2-3 segments above and 2-3 segments below the injury were exposed and stabilized as aforementioned. The body temperature was maintained at 37°C using a feedback-controlled heating pad (Harvard Apparatus #507221F).
For the recordings, 32-channel silicon probes (4 shanks with 2 tetrodes, designed for extracellular somatic recordings, model number: A4x2-tet-5mm-150-200-312-A32, NeuroNexus Technologies) and a Digital Lynx 4SX acquisition system with Cheetah data acquisition software (Neuralynx) were used. A multimode optical fiber (200 μm, 0.22 NA, Thorlabs), held in place with a stereotaxic apparatus and positioned at the surface of the target area, was used for light delivery (447 or 473 nm). A patch cable (Doric Lenses) was connected from the optical fiber to a blue or violet blue laser (Cobolt MLD 473 nm, Cobolt or CNI/OEM diode laser 447 nm, Changchun New Industries Optoelectronics Technology, respectively) controlled by custom software written in LabView (National Instruments).
The electrode was stereotaxically lowered into the sensorimotor cortex or intermediate gray matter of the spinal cord. Baseline activity recordings of each unit were conducted for 30 s before light-evoked activity was measured. Both uninjured and injured animals were used for cortical soma recordings, orthodromic and antidromic activation of CST neurons. For cortical soma recordings, the silicone probe was lowered into layer V of the sensorimotor cortex previously injected with the virus and the ipsilateral brain surface was exposed to pulses of blue light (5 mW, 3-5 ms pulses, 10-40 Hz). For orthodromic activation of CST neurons, the brain surface over the sensorimotor cortex previously injected with the virus was photostimulated (10 mW, 10 ms pulse length, 10 Hz) and the electrode targeted to the contralateral spinal cord intermediate gray matter (i.e., cervical spinal cord for uninjured animals and 2-3 segments rostral to the injury site for lesioned animals). For antidromic activation of CST neurons, light (10 mW, 10 ms pulse length, 10 Hz) was delivered to one side of the spinal cord surface to activate CST axon terminals (i.e., cervical spinal cord for uninjured animals and 2-3 segments rostral to the injury site for lesioned animals) while recording action potentials in layer V neuronal cell bodies in the contralateral sensorimotor cortex previously injected with the virus.
To assess whether local photostimulation of ChR2-GFP+ CST axons terminals in the non-injured cervical portion of the spinal cord is able to generate post synaptic activity in neighboring local spinal neurons, we illuminated the spinal cord surface 2-3 segments rostral to the lesion area with pulsed blue light (5 mW, 3 ms pulses, 10 Hz or 20 Hz) and positioned the electrode in the ipsilateral intermediate spinal gray matter underneath the illuminated area. In order to investigate functional synaptic connectivity by regenerated ChR2-GFP+ CST axons in injured GLAST-Rasless-YFP mice blue light photostimulation (5 mW, 3 ms pulses, 10 Hz or 20 Hz) was delivered to the spinal cord surface 0.5-3 mm caudal to the injury site. Postsynaptic responses were recorded by targeting the electrode to the ipsilateral intermediate gray matter underneath the illuminated area.
Unit signals were amplified (x10,000), bandpass filtered (600-6,000 Hz), and sampled at 32 kHz. Single units were manually sorted and identified based on various spike waveform features using MClust spike sorting toolbox for MATLAB (A.D. Redish). Only well-isolated single units (isolation distance > 15, L-ratio < 0.2, and the spikes < 0.01% at ISI < 2 ms) were used in the data analysis (Schmitzer-Torbert et al., 2005). To examine the effects of optogenetic stimulation on cells modulated by CST fibers, we constructed spike raster plots and peri-stimulus time histograms (PSTHs) for each neuron.
After the recordings, the animals were euthanized by intraperitoneal injection of sodium pentobarbital and transcardially perfused with cold PBS followed by 4% formaldehyde in PBS. Animals presenting tissue defects/holes (Tam-def) were not used for recordings. Post hoc analyses confirmed that spinal cord injured animals used for recordings did not present CST spared fibers caudal to the injury.
Behavioral testing combined with optogenetics
For behavioral experiments in conjunction with optogenetic manipulations Glast-Rasless-YFP animals subjected to SCI 12-14 weeks earlier (n = 7 injected with vehicle, n = 7 injected with tamoxifen) received two microinjections of an AAV expressing ChR2-GFP (AAV9-CAG-ChR2-GFP) targeted to layer V in the sensorimotor cortex (one injection / hemisphere, 0.5 μL per injection; from bregma, AP 0 mm, ML 1 mm, DV 0.55 mm) at a rate of 0.1 μl/min using a 10 μL NanoFil syringe with a 36 g beveled needle tip (World Precision Instruments) and a microinjector (UltraMicroPump III and Micro4 microsyringe pump controller, World Precision Instruments). After injection, the needle was kept in place for an additional 5 min to allow virus diffusion and prevent backflow of the virus to the surface, and then slowly withdrawn. Uninjured Glast-Rasless-YFP control animals were injected with AAV9-CAG-GFP (n = 6) or AAV9-CAG-ChR2-GFP (n = 4).
Concurrent with viral injections fiber optic implants (made in house (Sparta et al., 2011); 200 μm multimode fiber optic, 0.22 NA, Thorlabs; 230 μm ferrules, Precision Fiber Products) were placed 0.2 mm above the sites of viral injection and permanently secured onto the skull with two anchor screws and light-cured dental adhesive cement (Tetric EvoFlow, Ivoclar Vivadent Corporate). The blue light from the laser penetrates approximately 0.5 mm from the tip of the fiber optic and stimulates the apical dendrites and soma of cortical layer V ChR2-expressing neurons (Arenkiel et al., 2007, Gibson et al., 2014).
Four to six weeks after viral injections (i.e., 18 weeks post SCI for injured animals) the animals were connected to a 200 mW 447 nm violet blue laser system (CNI/OEM diode laser 447 nm, Changchun New Industries Optoelectronics Technology) with a fiber optic rotary joint patch cable (Thor Labs), placed in the center of a transparent plexiglas box (20 × 20 × 20 cm) and allowed to freely explore the box for 5-10 min. All animals underwent 3 trials of photostimulation per hemisphere in which light was on for 10 s/trial (2.5 mW, 5 ms pulses, 20 Hz). Animals were videotaped during the task with a Sony Camcorder and analyses done offline at low speed by an observer blinded to the experimental animal groups. The number of light-induced paw strokes was counted for each hind limb (contralateral and ipsilateral triggered-movement) and plotted as number of light-induced hind paw strokes / 30 s. Animals presenting spared CST fibers and tissue defects/holes (Tam-def) were not included in the analyses.
Optogenetic stimulation triggered limb motor output (paw strokes) in animals injected with AAV9-CAG-ChR2-GFP, but not in animals injected with the control virus AAV9-CAG-EGFP (Movies S1 and S2).
Immunohistochemistry and microscopy
Animals were euthanized by intraperitoneal injection of sodium pentobarbital and transcardially perfused with cold PBS followed by 4% formaldehyde in PBS. Brains, and spinal cords and in some cases dorsal root ganglia (DRGs) and peripheral nerves (sciatic and brachial nerves) were dissected out and post fixed in formaldehyde overnight at 4°C. A Leica M165 C stereomicroscope equipped with a Leica IC80 HD camera was used to photograph the dissected spinal cords. Spinal cords, DRGs and peripheral nerves were then cryoprotected in 30% sucrose and coronal (20 μm) or sagittal (18 μm) cryosections were collected on alternating slides. Coronal and sagittal brain sections (30 μm) were obtained by vibratome tissue sectioning.
Sections were incubated with blocking solution (10% normal donkey serum in PBS, with 0.3% Triton X-100) for 1 hr at room temperature, then incubated at 4°C or room temperature in a humidified chamber overnight with primary antibodies diluted in 10% normal donkey serum. For antibodies raised in mouse, the M.O.M. kit (Vector Labs) was used following the manufacturer’s instructions. The following primary antibodies were used: CD31 (1:100, rat, BD Biosciences), CD68 (1:200, rat, AbD Serotec), Collagen I (1:200, rabbit, Abcam), Collagen III (1:100, rabbit, Abcam), Collagen IV (1:200, rabbit, AbD Serotec; 1:50, goat, Southern Biotech), Collagen VI (1:100, rabbit, Abcam; 1:50, goat, Southern Biotech), Fibronectin (1:400, rabbit, Sigma-Aldrich), GFAP (1:200, guinea pig, Synaptic Systems; 1:1000, mouse directly conjugated to Cy3, Sigma-Aldrich; 1:1000, chicken, Millipore), GFP (1:2000, goat directly conjugated to FITC, Abcam; 1:10000, chicken, Aves Labs), NG2 (1:200, rabbit, Millipore), PDGFRα (1:100, goat, R&D Systems; 1:100, rabbit, Cell Signaling Technology), PDGFRβ (1:200, rabbit, Abcam), Podocalyxin (1:200, goat, R&D Systems), 5-HT (1:10000, goat, Immunostar; 1:200, rat, Millipore), S100 (1:500, rabbit, DAKO), GluR 2/3 (1:200, rabbit, Millipore), Synapsin I (1:100, mouse, Synaptic Systems; 1:500, rabbit, Millipore), Synaptophysin (1:50, mouse, Abcam), NeuN (1:500, mouse directly conjugated to Alexa Fluor 647 in house, Millipore; 1:1000, guinea pig, Millipore), NF-H (1:1000, chicken, Millipore), vGlut1 (1:5000, guinea pig, Millipore), vGlut2 (1:5000, guinea pig, Millipore), ChAT (1:100, goat, Millipore), P0 (1:500, rabbit, Abcam), Cux1 (1:100, rabbit, Santa Cruz), Ctip2 (1:500, rat, Abcam), UCHL1 (1:1000, rabbit, Abcam; 1:200, guinea pig, Abcam), ER81 (1:200, rabbit, Santa Cruz), mu crystallin (1:100, mouse, Abcam), CaMKIIα (1:100, mouse, Cell Signaling Technology). The NeuN antibody from Millipore (clone A60) was directly conjugated to Alexa Fluor 647 in house using a Monoclonal Antibody Labeling kit following the manufacturer’s description (Life Technologies).
After washing, antibody staining was revealed using species-specific fluorophore-conjugated (1:500, DyLight 405, Alexa Fluor 488, Cy3, Alexa Fluor 594, Alexa Fluor 647 from Jackson Immunoresearch) or biotin-conjugated secondary antibodies (1:500, Jackson Immunoresearch). Biotinylated secondary antibodies were revealed with fluorophore-conjugated streptavidin (1:500, Cy3, Alexa Fluor 594 and Alexa Fluor 647 from Jackson Immunoresearch). To visualize traced BDA+ CST axons sections were incubated with Cy3-conjugated streptavidin (1:500, Jackson Immunoresearch). EdU was detected with the Click-iT EdU Alexa Fluor 647 imaging kit (Life Technologies) according to the manufacturer’s instructions. Control sections were stained with secondary antibody alone. Cell nuclei were stained with DAPI (4’6’-diamidino-2-phenylindole) (1 μg/ml, Sigma-Aldrich). Slides were mounted with Vectashield Mounting Medium (Vector Labs).
Images were acquired with a Zeiss Axioplan 2 upright epifluorescent microscope, Leica TCS SP8X or a Zeiss LSM510 META confocal microscope. Image processing and assembly were performed with ImageJ/Fiji (version 2.0.-rc-43/1.51 g), Adobe Photoshop CC 2015.1.2 and Illustrator CC 2015.1.2 for Mac.
Immunoblotting
Two weeks after a bilateral dorsal hemisection Glast-Rasless-YFP mice (n = 6, vehicle; n = 6, tamoxifen) were euthanized by intraperitoneal injection of sodium pentobarbital and transcardially perfused with ice-cold PBS to remove blood contaminants. Injured sites were quickly dissected out and pooled for protein extraction (2 injury sites were pooled as one biological replicate, n = 3). Tissue was homogenized in lysis buffer (50 mM Tris-HCl, pH 7.5, 1 mM EDTA, 1 mM EGTA, 1 mM sodium orthovanadate, 50 mM sodium fluoride, 1% Triton X-100 plus protease- and phosphatase-inhibiting cocktail [Roche]), sonicated and the supernatant was collected after centrifugation at 10,000 g for 30 min at 4°C. Uninjured spinal cord tissue from Glast-Rasless-YFP mice (n = 6) was used as a control and processed in the same way.
Proteins (50 μg) were separated on Novex NuPAGE 4%–12% Bis-Tris Gels (Invitrogen) and then transferred to nitrocellulose membranes (Trans Blot Turbo Transfer Pack Nitrocellulose, BioRad). Membranes were blocked for 1h with 5% skimmed milk (wt/vol) and probed with primary antibodies followed by incubation with the appropriate horseradish peroxidase-conjugated secondary antibodies (1:20000, Jackson Immunoresearch). Western blots were developed using an enhanced chemiluminescence substrate mixture (ECL Prime, Amersham). The following primary antibodies were used: GFP (1:2000, goat, Abcam), PDGFRβ (1:4000, rabbit, Cell Signaling Technology), Periostin (1:10000, rabbit, Abcam), S100A4 (1:1000, rabbit, Abcam), HSP47 (1:200, rabbit, Acris), P4HB (1:10000, rabbit, Abnova) and GAPDH (1:40000, mouse, Millipore).
Quantitative Reverse Transcription PCR
Two weeks after a bilateral dorsal hemisection Glast-Rasless-YFP mice (n = 7-10, vehicle; n = 11-12, tamoxifen) were euthanized by intraperitoneal injection of sodium pentobarbital and transcardially perfused with ice-cold PBS to remove blood contaminants. Injured and uninjured segments were quickly dissected out and snap frozen in liquid nitrogen.
Total RNA was extracted using the AllPrep DNA/RNA micro kit (QIAGEN). The RNA from each sample was reverse transcribed to cDNA using the SuperScript III First Strand kit (Invitrogen). cDNA (0.1 μg) from each sample was then amplified with TaqMan Universal PCR master mix II (Applied Biosystems) and TaqMan probe-based gene expression assays (Applied Biosystems). The following TaqMan gene expression assays were used: PDGFRB (Assay ID: Mm00435546), COL1A1 (Assay ID: Mm00801666), COL1A2 (Assay ID: Mm00483888), COL3A1 (Assay ID: Mm01254476), COL6A5 (Assay ID: Mm01231908), ACTA1 (Assay ID: Mm00808218), ELN (Assay ID: Mm00514670), FN1 (Assay ID: Mm01256744), TNN (Assay ID: Mm00625680), ASPN (Assay ID: Mm00445945), POSTN (Assay ID: Mm00450111), CD209A (Assay ID: Mm00460067), CD248 (Assay ID: Mm00547485), CCL21A (Assay ID: Mm03646971), MMP3 (Assay ID: Mm00440295), MMP13 (Assay ID: Mm00439491), CPZ (Assay ID: Mm00462216), FAP (Assay ID: Mm01329177), S100A4 (Assay ID: Mm00803372), LOX (Assay ID: Mm00495386), P4HB (Assay ID: Mm01243188) and HPRT (Assay ID: Mm01545399, housekeeping gene).
QRT-PCR was conducted with the ViiA 7 Real-Time PCR System (Applied Biosystems). Relative expression was calculated using the 2-ΔCt method. The fold change in mRNA levels between uninjured and injured conditions was calculated using ΔΔCt.
Quantitative analysis
All analyses and measurements were conducted in a blind fashion.
The scar core occupancy by stromal cells was determined based on PDGFRβ expression. Five alternate sagittal sections per animals spanning the lesion center and spaced 130 μm apart were immunostained for PDGFRβ, GFAP and DAPI. The lesion site was photographed using a Zeiss Axioplan 2 epifluorescent microscope and the scar core (GFAP negative area within the GFAP-defined borders) was manually outlined. Measurements were carried out using the ImageJ/Fiji software (version 2.0.-rc-43/1.51 g for Mac). The area occupied by PDGFRβ signal (scar occupancy) was thresholded and presented as a percentage of the total lesion core area. The same photographs were used to determine the volume of tissue defects. Tissue defects (areas devoid of cells bordered by GFAP) were manually outlined and determined using the ImageJ/Fiji software. Volume estimations were calculated using the formula: V = ΣA x T, in which V = volume, A = area associated with tissue defect, T = distance between each sampled region.
To determine the recombination efficiency, the total number of recombined (YFP-expressing) pericytes (type A pericytes) was counted on three 20 μm coronal cervical sections 600 μm rostral to the injury per animal. To calculate the relative recombination efficiency, values were normalized to the animal showing the highest number of recombined pericytes per section.
Proliferation of recombined (YFP-positive) and non-recombined (YFP-negative) pericytes (PDGFRβ-positive cells) was assessed in both intact spinal cords and 5 days after a dorsal funiculus incision SCI at mid-thoracic level. EdU, PDGFRβ and YFP expressing cells in uninjured segments and within the lesion core were counted in 4-5 coronal sections in 4-5 animals per group and expressed as number of cells per area or as a percentage.
The number of PDGFRβ-positive pericytes in direct contact with the podocalyxin-positive endothelium (ON vessel) or detached from the blood vessel (OFF vessel) within the lesion core and in uninjured spinal cord segments was quantified in 4-5 coronal sections in 4-5 animals per group and expressed as a percentage of total PDGFRβ-positive cells.
To determine the proportion of AAV9-CAG-ChR2-GFP infected cells that are CaMKIIα-positive excitatory pyramidal neurons in layer V of the sensorimotor cortex, the total number of GFP+/NeuN+ and CaMKIIα+ cells was counted across 5 coronal sections per animal (n = 3). The result was expressed as the percentage of ChR2-GFP neurons that are CaMKIIα-positive or CaMKIIα-negative over the total number of ChR2-GFP infected neurons.
To quantify regenerated CST fibers growing up to and past the lesion site in Glast-Rasless-YFP mice 18 weeks after dorsal hemisection, every third section of all the sagittal sections from each mouse (∼30 sections in total spaced ∼50 μm apart) was processed to visualize BDA+ CST axons. The sum of axons crossing perpendicular lines at 3, 1 and 0.5 mm rostral to the lesion and 0, 0.5, 1, 2 and 3 mm caudal to the lesion was recorded in all sections from each animal (Cafferty et al., 2010). Data were normalized to the number of CST axons at 3 mm rostral to the lesion and presented as percentage of CST regeneration. For correlation analyses, the average raw number of axons per 10 sagittal sections ± SEM. was plotted. Animals with spared CST fibers were excluded.
The density of BDA+ CST fibers 4 mm rostral and 4 mm caudal the lesion was measured using the ImageJ/Fiji software. Labeled fibers within the dorsal (laminae I-V), intermediate (laminae VI, VII and X) and ventral (laminae VIII and IX) spinal cord gray matter were selected by thresholding and the skeletonize function (Arganda-Carreras et al., 2010) was used to measure the average fiber length in 3 sections per mouse. Data were normalized to the density of CST axons at 4 mm rostral to the lesion and presented as percentage of CST regeneration. Animals presenting spared CST fibers and tissue defects/holes (Tam-def) were excluded from the analyses.
To assess the proportion of BDA-traced CST axon tips contacting glial and non-neural scar-forming cells 2 weeks after dorsal hemisection, 4-6 sagittal sections per animal were used for each cell type analyses. Glast-YFP mice were used to visualize type A pericyte-derived cells. To label immune cells and reactive astrocytes sections were immunostained for CD68 and GFAP, respectively. NG2 and PDGFRα are commonly used to label OPCs but these markers are also expressed by vessel associated pericytes (Assinck et al., 2017, Göritz et al., 2011, He et al., 2016) and, after injury, NG2 expression is additionally found in macrophages (Jones et al., 2002) and PDGFRα in stromal fibroblasts. For this reason, only PDGFRα/NG2 double positive cells non-associated with blood vessels (immunolabeled for podocalyxin) and localized outside the lesion core were categorized as OPCs (Assinck et al., 2017). The number of CST axon tips contacting each scar-forming cell type was expressed as a percentage of total CST axons.
To assess for differences in inflammation, astrogliosis and OPC numbers in Glast-Rasless-YFP animals 2 and 4 weeks after a dorsal hemisection, 4-6 sagittal sections per animal were immunostained for CD68, GFAP and NG2/PDGFRα. The area occupied by CD68-positive cells within 500 μm either side of the lesion center was thresholded and measured using ImageJ/Fiji software (version 2.0.-rc-43/1.51 g for Mac). Similarly, the area covered by GFAP-positive signal (astrogliosis) within 500 μm rostral and caudal to the lesion center was thresholded and presented as a percentage of the total area. The number of OPCs (non-vascular associated NG2/PDGFRα double positive cells) was counted within 100 μm rostral and caudal to the glial-fibrotic lesion border (scar-forming OPCs) and in a 100 μm wide strip of spared but reactive neural tissue 500 μm away from either side of the lesion core. The results were expressed as number of OPCs per area.
Scoring of the glial scar state in Glast-Rasless-YFP animals 2 and 4 weeks after dorsal hemisection was based on a semiquantitative method developed by Hsu et al., 2006, Hsu et al., 2008) for analyses of transverse sections and adapted to sagittal sections by Hellal et al. (2011). The complexity level of the glial network (from no astrocytic hypertrophy – score 0, to hypertrophied astrocytes with formation of glial limitans bordering the lesion core – score 3) was evaluated over 1 mm long spinal cord segments covering the rostral side of the lesion. For analyses, each segment was partitioned into 200 μm wide strips starting from the lesion border and extending rostrally. Four to six sagittal sections, spanning the medial, medial-lateral and lateral portions of the spinal cord, were immunostained for GFAP and scored per animal. Scores representing the complexity and/or extent of astrogliosis in each strip of injured spinal cord were averaged across sections and animals and presented as mean glial scar score.
The percentage of BDA+ CST axons presenting retraction bulbs and fiber retraction analyses 2 and 4 weeks after dorsal hemisection were quantified in sagittal sections from the medial portion of the spinal cord containing the main dCST (4-5 sections per animal). To determine the average distance of axons to the lesion margin, the gap between all BDA+ CST axonal tips and the proximal edge of the lesion (interface of glial and fibrotic scars) was measured using the ImageJ/Fiji software. Animals presenting CST spared fibers caudal to the injury were excluded from the analyses.
In Glast-Rasless-YFP animals injected with AAV9-CAG-ChR2-GFP, the density of GFP+ CST fibers found 4 mm rostral to lesion and 0.5 mm caudal to the injury site 18 weeks after dorsal hemisection was analyzed in a similar manner. Labeled fibers within the spinal cord gray matter were selected by thresholding and the skeletonize function was used to measure the average fiber length in 3 sections per mouse. Data were normalized to the density of CST axons at 4 mm rostral to the lesion and presented as percentage of CST regeneration. To determine the density of GFP+ CST fibers in uninjured Glast-Rasless-YFP animals injected with AAV9-CAG-GFP or AAV9-CAG-ChR2-GFP, matched segments of the spinal cord were used for quantifications. Labeled fibers within the spinal cord gray matter were selected by thresholding and the skeletonize function was used to measure the average fiber length in 3 sections per mouse. The density of CST fibers found at high thoracic level (corresponds roughly to the same spinal cord segment as 0.5 mm caudal to the injury) is approximately 70%–85% of the CST density at low cervical level (matches the same spinal cord segment as 4 mm rostral to the lesion), indicating that the thoracic spinal cord is less innervated by CST fibers than the cervical spinal cord. This is in agreement with the rostro-caudal decrease in size of the CST (Bareyre et al., 2005). Animals presenting spared CST fibers and tissue defects/holes (Tam-def) were excluded from the analyses.
The density of 5HT-immunoreactive raphespinal fibers within the spinal cord ventral horn in injured Glast-Rasless-YFP animals 2, 4 and 18 weeks after dorsal hemisection (4 mm caudal to the lesion) was determined using the ImageJ/Fiji software. To quantify the density of 5-HT+ fibers in uninjured Glast-Rasless-YFP animals, matched segments of the spinal cord were used for measurements. Labeled fibers were selected by thresholding and the skeletonize function was used to measure the average fiber length in 5 sections per animal. Results are presented as average length of 5-HT axons in the ventral horn per area.
Camera lucida reconstructions
Camera lucida reconstructions of CST axonal growth were generated by thresholding BDA+ CST axons from 10 sagittal sections in ImageJ and then superimposing the traces into a single image using Adobe Illustrator. Reconstruction images of sagittal sections representing 3 regions of the spinal cord were color coded and collapsed to render a final projection of CST axonal growth through the lesion site. The black illustrates CST axon growth through the medial zone (mostly dorsal and ventral column white matter and central canal), blue represents for the medial-lateral zone (mostly dorsal and ventral gray matter) and the red for the lateral zone (mostly white mater).
Behavioral testing
Horizontal ladder-walking test
Sensorimotor impairment due to spinal cord lesions is reflected by errors when a mouse crosses a horizontal ladder. Skilled locomotion, sensorimotor integration and limb placement can be evaluated using the horizontal ladder-walking test (Metz and Whishaw, 2002).
The horizontal ladder-walking test apparatus consisted of clear Plexiglas side walls (1 m in length) and unevenly spaced metal rungs (to prevent habituation) elevated approximately 15 cm from the ground.
Glast-Rasless-YFP mice (n = 8 vehicle; n = 12 tamoxifen) were pre-trained on the horizontal ladder for one week prior to injury and then assessed on the day before the surgery to provide baseline data. After bilateral dorsal hemisection SCI, animals were weekly tested for up to 9 weeks and then every second week until 15 weeks after injury. Each experiment was performed in triplicate and recorded using a digital camera for offline analyses. The average number of hind limb misplacements while the mice walked through the 1 m long horizontal ladder was manually scored by an observer blinded to the treatment. A complete miss, slip, or replacement of the paw during placement was considered as an error. The average number of errors per trial was normalized to the average total number of steps per trial and the results presented as a percentage of error. Animals presenting spared CST fibers and tissue defects/holes (Tam-def) were excluded from the analyses.
Catwalk automated gait analysis
The Catwalk XT video-based analysis system (Noldus Information Technology, Wageningen, the Netherlands) was used to assess gait and locomotion in voluntarily walking mice. When the animals traverse the Catwalk glass runaway, their footprints are captured and allow subsequent calculation of multiple parameters related to print dimensions and the time and distance relationships between footprints. CatWalk XT gait analysis has been validated in multiple neurological disorders and lesion models including SCI (Hamers et al., 2001, Neumann et al., 2009).
During one week preceding surgery, Glast-Rasless-YFP mice (n = 8 vehicle; n = 12 tamoxifen) were daily trained on the Catwalk runaway to make consecutive uninterrupted runs and then assessed on the day before the surgery to provide baseline data. After bilateral dorsal hemisection SCI, animals were weekly tested for up to 9 weeks and then every second week until 15 weeks after injury. Recorded footprints were analyzed with CatWalk software version 9.1 by an observer blinded to the treatment. Three runs per animal and time point with a minimum of four step cycles each were analyzed per animal. The regularity index (% index) is presented as an overall measure of the degree of inter-limb coordination during gait, as measured by the number of normal step sequence patterns (NSSP), multiplied by the number of paws and divided by the number of paw placements; RI = 100% × (NSSP × 4) / number of paw placements. Animals presenting spared CST fibers and tissue defects (Tam-def) were excluded from the analyses.
RNA-seq: Isolation, sequencing and analysis
Two weeks after bilateral dorsal hemisection, spinal cords of Glast-Rasless-YFP mice (Vehicle, n = 4; Tamoxifen, n = 4) were dissected out and injured segments (parenchymal segments spanning 1 mm from each side of the lesion) were rapidly snap frozen in liquid nitrogen. Uninjured Glast-Rasless-YFP mice with identical genotype received vehicle and were used as controls (n = 4).
RNA extraction and quality control
The tissue was disrupted and homogenized in Buffer RLT (QIAGEN) and using FastPrep Cell Disrupter (Thermo Electron). The tissue was placed in the FastPrep tubes together with 350 μl RLT buffer and run for 4 s at setting 6, followed by centrifugation at 4°C for 5 min at 12 000 rpm. The water phase were carefully collected and transferred to the AllPrep spin column (QIAGEN) and centrifuged for 30 s at 10 000 rpm. The elute were transferred to a new tube (Eppendorf) and the protocol for AllPrep DNA/RNA/protein for tissue samples were resumed from step 6 according to manufacture’s instructions for RNA extraction (QIAGEN). The concentration of the extracted RNA was determined using Qubit RNA BR Assay Kit (Life Technologies) and quality of the total RNA was determined using the RNA 6000 Nano chip on the 2100 Bioanalyzer automated electrophoresis system (Agilent Technologies).
Library preparation and sequencing
Total RNA was used for library preparation of each sample, which was subsequently bar-coded and prepared according to manufacturer’s instructions (Illumina). The libraries were clustered on a cBot cluster-generation system using an Illumina HiSeq paired-end cluster-generation kit and sequenced as paired-end, 2x100 bp on the Illumina HiSeq 2000. All lanes were spiked with 1% phiX control library. The sequencing run was performed according to the manufacturer’s instructions and generated a total of 487 million read pairs with a median of 17 million read pairs per sample.
Sequence read alignment and analysis
All sequences were aligned to the mouse genome reference mm9 with TopHat (Trapnell et al., 2009) version 1.3.3 and duplicates were removed using Picard Tools (http://broadinstitute.github.io/picard) version 1.29. Fragments per kilobase of exon per million mapped fragments (FPKM) values for features were calculated by Cufflinks (Trapnell et al., 2010) version 1.3.0 and the raw read counts were generated using HTSeq (Anders et al., 2015) version 0.5.1. The R/Bioconductor package DESeq (Anders and Huber, 2010) was used to call differential gene expression on counts generated by HTSeq. To identify transcripts that were differentially expressed between vehicle and tamoxifen groups, we defined a criteria of a 1.5-fold and greater difference plus a significant (p < 0.05) false discovery rate (FDR).
Pathway analysis was carried out using the QIAGEN’s Ingenuity Pathway Analysis (IPA, QIAGEN Redwood City, https://www.qiagenbioinformatics.com/products/ingenuity-pathway-analysis). The top ten canonical pathways differentially enriched (fold change > 1.5, p < 0.05) in lesions from vehicle versus tamoxifen animals 2 weeks after injury are presented.
Gene ontology analysis was performed using The Database for Annotation, Visualization and Integrated Discovery (DAVID (Huang et al., 2009a, Huang et al., 2009b) bioinformatics tools. The top ten gene ontology terms (biological process, molecular function and cellular component) significantly enriched in injury sites from vehicle versus tamoxifen animals 2 weeks after injury are presented (fold change > 1.5, p < 0.05).
Quantification and Statistical Analysis
Sample sizes were determined based on previous experience. No statistical methods have been used to predetermine sample size. Data are presented as mean ± standard error of the mean (SEM). For some graphs individual data points are plotted. Sample sizes (n) of animals, number of biological repeats of experiments and statistical methods used are indicated in the corresponding figure legends. All datasets were tested for Gaussian distribution using the Shapiro–Wilk test. In some instances sample groups were too small to test for Gaussian distribution (< 8 samples) and parametric tests were preferred because nonparametric tests lack suitable power to detect differences in small sample groups. p values were calculated using two-tailed unpaired or paired Student’s t test, Mann-Whitney U-test and one way- or two-way ANOVA. Repeated-measures were applied when appropriate. Post hoc correction tests were employed following significance with an ANOVA and described in the legend of the respective figure. For correlation analyses, Pearson correlation coefficients (Pearson r) and p values are reported.
Differences were considered statistically significant at p values below 0.05. All data were analyzed using GraphPad Prism version 6.0g software for Mac.
Data and Software Availability
The accession number for the RNA-seq data reported in this paper is NCBI GEO: GSE93976.
Acknowledgments
We thank Göritz and Frisén lab members for valuable comments on the manuscript, M. Barbacid for providing Ras mutant mice, and F. W. Pfrieger for providing Glast-CreERT2 transgenic mice. The authors acknowledge support from Science for Life Laboratory, the Knut and Alice Wallenberg Foundation, Karolinska Institutet, the National Genomics Infrastructure funded by the Swedish Research Council, and Uppsala Multidisciplinary Center for Advanced Computational Science for assistance with massively parallel sequencing (alternatively genotyping) and access to the UPPMAX computational infrastructure. D.O.D. was supported by the Foundation for Science and Technology from the Portuguese government (SFRH/BD/63164/2009). C.G. is a Hållsten Academy and a Knut and Alice Wallenberg Academy Fellow. Research in the C.G. lab was supported by the European Union’s Seventh Framework Programme (FP7)/ERC-2012-StG 310938 PERICYTESCAR, the Swedish Research Council, SFO StratRegen, Hjärnfonden, Ming Wai Lau Centre, the Swedish Cancer Foundation, JPND DACAPO-AD, and Wings for Life Foundation. Research in the J.F. lab was supported by the Swedish Research Council, the Swedish Cancer Foundation, Tobias Stiftelsen, SSF, and the Knut och Alice Wallenbergs Stiftelse and Torsten Söderberg Foundation.
Author Contributions
D.O.D. performed axonal regeneration and behavioral experiments and analyses. B.W.S. performed RNA-seq and analyses. D.H. performed validation of RNA-seq and contributed to experiments and analyses. H.K. and D.O.D. performed optogenetics and electrophysiological experiments. H.K. performed analyses. C.G. and J.F. designed and supervised the study. D.O.D., M.C., and J.L. designed experiments. D.O.D., J.F., and C.G. wrote the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: March 1, 2018
Footnotes
Supplemental Information includes seven figures, three tables, and two movies and can be found with this article online at https://doi.org/10.1016/j.cell.2018.02.004.
Contributor Information
Christian Göritz, Email: christian.goeritz@ki.se.
Jonas Frisén, Email: jonas.frisen@ki.se.
Supplemental Information
Complete list of FPKM expression values for uninjured and injured Veh and Tam animals 2 weeks after spinal cord injury.
Differential expression analysis of RNA-seq data between injured Veh and Tam animals 2 weeks after spinal cord injury. Fold change cut-off ≥ ±1.5; FDR adjusted P ≤0.05. Inf and –Inf represent genes with infinite and –infinite fold change expression, respectively.
Video clip of unilateral photoactivation of the layer V sensorimotor cortex (447 nm light, 10 mW, 20 Hz, 5 ms pulse length) in an awake, freely moving uninjured Glast-Rasless animal transduced with AAV9-CAG-ChR2-GFP, showing vigorous lightdriven paw strokes. Blue light stimulation failed to generate a similar behavioral output in a control uninjured animal transduced with AAV9-CAG-GFP.
Video clip of unilateral photoactivation of the layer V sensorimotor cortex (447 nm light, 10 mW, 20 Hz, 5 ms pulse length) in awake, freely moving injured Glast-Rasless animals transduced with AAV9-CAG-ChR2-GFP. Eighteen weeks after spinal cord injury, the example vehicle animal shows an almost complete loss of lightevoked hind paw strokes. In contrast, the example Tam animal regained modest but increased hind paw strokes in response to blue light stimulation.
References
- Anders S., Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S., Pyl P.T., Huber W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M.A., Burda J.E., Ren Y., Ao Y., O’Shea T.M., Kawaguchi R., Coppola G., Khakh B.S., Deming T.J., Sofroniew M.V. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532:195–200. doi: 10.1038/nature17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenkiel B.R., Peca J., Davison I.G., Feliciano C., Deisseroth K., Augustine G.J., Ehlers M.D., Feng G. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron. 2007;54:205–218. doi: 10.1016/j.neuron.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arganda-Carreras I., Fernández-González R., Muñoz-Barrutia A., Ortiz-De-Solorzano C. 3D reconstruction of histological sections: Application to mammary gland tissue. Microsc. Res. Tech. 2010;73:1019–1029. doi: 10.1002/jemt.20829. [DOI] [PubMed] [Google Scholar]
- Assinck P., Duncan G.J., Plemel J.R., Lee M.J., Stratton J.A., Manesh S.B., Liu J., Ramer L.M., Kang S.H., Bergles D.E. Myelinogenic plasticity of oligodendrocyte precursor cells following spinal cord contusion injury. J. Neurosci. 2017;37:8635–8654. doi: 10.1523/JNEUROSCI.2409-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bareyre F.M., Kerschensteiner M., Misgeld T., Sanes J.R. Transgenic labeling of the corticospinal tract for monitoring axonal responses to spinal cord injury. Nat. Med. 2005;11:1355–1360. doi: 10.1038/nm1331. [DOI] [PubMed] [Google Scholar]
- Barnabé-Heider F., Göritz C., Sabelström H., Takebayashi H., Pfrieger F.W., Meletis K., Frisén J. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell. 2010;7:470–482. doi: 10.1016/j.stem.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Brazda N., Müller H.W. Pharmacological modification of the extracellular matrix to promote regeneration of the injured brain and spinal cord. Prog. Brain Res. 2009;175:269–281. doi: 10.1016/S0079-6123(09)17518-0. [DOI] [PubMed] [Google Scholar]
- Bundesen L.Q., Scheel T.A., Bregman B.S., Kromer L.F. Ephrin-B2 and EphB2 regulation of astrocyte-meningeal fibroblast interactions in response to spinal cord lesions in adult rats. J. Neurosci. 2003;23:7789–7800. doi: 10.1523/JNEUROSCI.23-21-07789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch S.A., Horn K.P., Silver D.J., Silver J. Overcoming macrophage-mediated axonal dieback following CNS injury. J. Neurosci. 2009;29:9967–9976. doi: 10.1523/JNEUROSCI.1151-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss A., Pech K., Kakulas B.A., Martin D., Schoenen J., Noth J., Brook G.A. Growth-modulating molecules are associated with invading Schwann cells and not astrocytes in human traumatic spinal cord injury. Brain. 2007;130:940–953. doi: 10.1093/brain/awl374. [DOI] [PubMed] [Google Scholar]
- Cafferty W.B., Yang S.H., Duffy P.J., Li S., Strittmatter S.M. Functional axonal regeneration through astrocytic scar genetically modified to digest chondroitin sulfate proteoglycans. J. Neurosci. 2007;27:2176–2185. doi: 10.1523/JNEUROSCI.5176-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferty W.B., Duffy P., Huebner E., Strittmatter S.M. MAG and OMgp synergize with Nogo-A to restrict axonal growth and neurological recovery after spinal cord trauma. J. Neurosci. 2010;30:6825–6837. doi: 10.1523/JNEUROSCI.6239-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M.Y., Wang E.H., Woodson W.J., Wang S., Sun G., Lee A.G., Arac A., Fenno L.E., Deisseroth K., Steinberg G.K. Optogenetic neuronal stimulation promotes functional recovery after stroke. Proc. Natl. Acad. Sci. USA. 2014;111:12913–12918. doi: 10.1073/pnas.1404109111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condic M.L., Lemons M.L. Extracellular matrix in spinal cord regeneration: getting beyond attraction and inhibition. Neuroreport. 2002;13:A37–A48. doi: 10.1097/00001756-200203040-00002. [DOI] [PubMed] [Google Scholar]
- Cregg J.M., DePaul M.A., Filous A.R., Lang B.T., Tran A., Silver J. Functional regeneration beyond the glial scar. Exp. Neurol. 2014;253:197–207. doi: 10.1016/j.expneurol.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten M., Dhawahir A., Sum E.Y., Urosevic J., Lechuga C.G., Esteban L.M., Castellano E., Guerra C., Santos E., Barbacid M. Genetic analysis of Ras signalling pathways in cell proliferation, migration and survival. EMBO J. 2010;29:1091–1104. doi: 10.1038/emboj.2010.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner J.R., Herrmann J.E., Woo M.J., Tansey K.E., Doan N.B., Sofroniew M.V. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filli L., Schwab M.E. Structural and functional reorganization of propriospinal connections promotes functional recovery after spinal cord injury. Neural Regen. Res. 2015;10:509–513. doi: 10.4103/1673-5374.155425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch M.T., Silver J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp. Neurol. 2008;209:294–301. doi: 10.1016/j.expneurol.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn J.R., Conn V.L., Boyle K.A., Hughes D.I., Watanabe M., Velasquez T., Goulding M.D., Callister R.J., Graham B.A. Anatomical and Molecular Properties of Long Descending Propriospinal Neurons in Mice. Front. Neuroanat. 2017;11:5. doi: 10.3389/fnana.2017.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson E.M., Purger D., Mount C.W., Goldstein A.K., Lin G.L., Wood L.S., Inema I., Miller S.E., Bieri G., Zuchero J.B. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344:1252304. doi: 10.1126/science.1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göritz C., Dias D.O., Tomilin N., Barbacid M., Shupliakov O., Frisén J. A pericyte origin of spinal cord scar tissue. Science. 2011;333:238–242. doi: 10.1126/science.1203165. [DOI] [PubMed] [Google Scholar]
- Guimaraes-Camboa N., Cattaneo P., Sun Y., Moore-Morris T., Gu Y., Dalton N.D., Rockenstein E., Masliah E., Peterson K.L., Stallcup W.B. Pericytes of multiple organs do not behave as mesenchymal stem cells in vivo. Cell Stem Cell. 2017;20:345–359. doi: 10.1016/j.stem.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett A.R., Lee J.K. Understanding the NG2 glial scar after spinal cord injury. Front. Neurol. 2016;7:199. doi: 10.3389/fneur.2016.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamers F.P., Lankhorst A.J., van Laar T.J., Veldhuis W.B., Gispen W.H. Automated quantitative gait analysis during overground locomotion in the rat: its application to spinal cord contusion and transection injuries. J. Neurotrauma. 2001;18:187–201. doi: 10.1089/08977150150502613. [DOI] [PubMed] [Google Scholar]
- Hara M., Kobayakawa K., Ohkawa Y., Kumamaru H., Yokota K., Saito T., Kijima K., Yoshizaki S., Harimaya K., Nakashima Y., Okada S. Interaction of reactive astrocytes with type I collagen induces astrocytic scar formation through the integrin-N-cadherin pathway after spinal cord injury. Nat. Med. 2017;23:818–828. doi: 10.1038/nm.4354. [DOI] [PubMed] [Google Scholar]
- He L., Vanlandewijck M., Raschperger E., Andaloussi Mäe M., Jung B., Lebouvier T., Ando K., Hofmann J., Keller A., Betsholtz C. Analysis of the brain mural cell transcriptome. Sci. Rep. 2016;6:35108. doi: 10.1038/srep35108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellal F., Hurtado A., Ruschel J., Flynn K.C., Laskowski C.J., Umlauf M., Kapitein L.C., Strikis D., Lemmon V., Bixby J. Microtubule stabilization reduces scarring and causes axon regeneration after spinal cord injury. Science. 2011;331:928–931. doi: 10.1126/science.1201148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann J.E., Imura T., Song B., Qi J., Ao Y., Nguyen T.K., Korsak R.A., Takeda K., Akira S., Sofroniew M.V. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J. Neurosci. 2008;28:7231–7243. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S. Relay strategies combined with axon regeneration: a promising approach to restore spinal cord injury. Neural Regen. Res. 2014;9:1177–1179. doi: 10.4103/1673-5374.135322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu J.Y., McKeon R., Goussev S., Werb Z., Lee J.U., Trivedi A., Noble-Haeusslein L.J. Matrix metalloproteinase-2 facilitates wound healing events that promote functional recovery after spinal cord injury. J. Neurosci. 2006;26:9841–9850. doi: 10.1523/JNEUROSCI.1993-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu J.Y., Bourguignon L.Y., Adams C.M., Peyrollier K., Zhang H., Fandel T., Cun C.L., Werb Z., Noble-Haeusslein L.J. Matrix metalloproteinase-9 facilitates glial scar formation in the injured spinal cord. J. Neurosci. 2008;28:13467–13477. doi: 10.1523/JNEUROSCI.2287-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang da W., Sherman B.T., Lempicki R.A. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang da W., Sherman B.T., Lempicki R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Jayaprakash N., Wang Z., Hoeynck B., Krueger N., Kramer A., Balle E., Wheeler D.S., Wheeler R.A., Blackmore M.G. Optogenetic interrogation of functional synapse formation by corticospinal tract axons in the injured spinal cord. J. Neurosci. 2016;36:5877–5890. doi: 10.1523/JNEUROSCI.4203-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D., Liu Y., Sun F., Wang X., Liu X., He Z. Restoration of skilled locomotion by sprouting corticospinal axons induced by co-deletion of PTEN and SOCS3. Nat. Commun. 2015;6:8074. doi: 10.1038/ncomms9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L.L., Yamaguchi Y., Stallcup W.B., Tuszynski M.H. NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors. J. Neurosci. 2002;22:2792–2803. doi: 10.1523/JNEUROSCI.22-07-02792.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S.L., Kisseleva T., Brenner D.A., Duffield J.S. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am. J. Pathol. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Tedeschi A., Park K.K., He Z. Neuronal intrinsic mechanisms of axon regeneration. Annu. Rev. Neurosci. 2011;34:131–152. doi: 10.1146/annurev-neuro-061010-113723. [DOI] [PubMed] [Google Scholar]
- Mederacke I., Hsu C.C., Troeger J.S., Huebener P., Mu X., Dapito D.H., Pradere J.P., Schwabe R.F. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat. Commun. 2013;4:2823. doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meletis K., Barnabé-Heider F., Carlén M., Evergren E., Tomilin N., Shupliakov O., Frisén J. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 2008;6:e182. doi: 10.1371/journal.pbio.0060182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz G.A., Whishaw I.Q. Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: a new task to evaluate fore- and hindlimb stepping, placing, and co-ordination. J. Neurosci. Methods. 2002;115:169–179. doi: 10.1016/s0165-0270(02)00012-2. [DOI] [PubMed] [Google Scholar]
- Neumann M., Wang Y., Kim S., Hong S.M., Jeng L., Bilgen M., Liu J. Assessing gait impairment following experimental traumatic brain injury in mice. J. Neurosci. Methods. 2009;176:34–44. doi: 10.1016/j.jneumeth.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norenberg M.D., Smith J., Marcillo A. The pathology of human spinal cord injury: defining the problems. J. Neurotrauma. 2004;21:429–440. doi: 10.1089/089771504323004575. [DOI] [PubMed] [Google Scholar]
- Robinson S.P., Langan-Fahey S.M., Johnson D.A., Jordan V.C. Metabolites, pharmacodynamics, and pharmacokinetics of tamoxifen in rats and mice compared to the breast cancer patient. Drug Metab. Dispos. 1991;19:36–43. [PubMed] [Google Scholar]
- Ruschel J., Hellal F., Flynn K.C., Dupraz S., Elliott D.A., Tedeschi A., Bates M., Sliwinski C., Brook G., Dobrindt K. Axonal regeneration. Systemic administration of epothilone B promotes axon regeneration after spinal cord injury. Science. 2015;348:347–352. doi: 10.1126/science.aaa2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabelström H., Stenudd M., Réu P., Dias D.O., Elfineh M., Zdunek S., Damberg P., Göritz C., Frisén J. Resident neural stem cells restrict tissue damage and neuronal loss after spinal cord injury in mice. Science. 2013;342:637–640. doi: 10.1126/science.1242576. [DOI] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitzer-Torbert N., Jackson J., Henze D., Harris K., Redish A.D. Quantitative measures of cluster quality for use in extracellular recordings. Neuroscience. 2005;131:1–11. doi: 10.1016/j.neuroscience.2004.09.066. [DOI] [PubMed] [Google Scholar]
- Silver J. The glial scar is more than just astrocytes. Exp. Neurol. 2016;286:147–149. doi: 10.1016/j.expneurol.2016.06.018. [DOI] [PubMed] [Google Scholar]
- Silver J., Schwab M.E., Popovich P.G. Central nervous system regenerative failure: role of oligodendrocytes, astrocytes, and microglia. Cold Spring Harb. Perspect. Biol. 2014;7:a020602. doi: 10.1101/cshperspect.a020602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slezak M., Göritz C., Niemiec A., Frisén J., Chambon P., Metzger D., Pfrieger F.W. Transgenic mice for conditional gene manipulation in astroglial cells. Glia. 2007;55:1565–1576. doi: 10.1002/glia.20570. [DOI] [PubMed] [Google Scholar]
- Sparta D.R., Stamatakis A.M., Phillips J.L., Hovelsø N., van Zessen R., Stuber G.D. Construction of implantable optical fibers for long-term optogenetic manipulation of neural circuits. Nat. Protoc. 2011;7:12–23. doi: 10.1038/nprot.2011.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S., Watanabe T., Lin C.S., William C.M., Tanabe Y., Jessell T.M., Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg C., Ivarsson M., Gerdin B., Rubin K. Pericytes as collagen-producing cells in excessive dermal scarring. Lab. Invest. 1996;74:452–466. [PubMed] [Google Scholar]
- Trapnell C., Pachter L., Salzberg S.L. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C., Williams B.A., Pertea G., Mortazavi A., Kwan G., van Baren M.J., Salzberg S.L., Wold B.J., Pachter L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010;28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski M.H., Steward O. Concepts and methods for the study of axonal regeneration in the CNS. Neuron. 2012;74:777–791. doi: 10.1016/j.neuron.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno M., Hayano Y., Nakagawa H., Yamashita T. Intraspinal rewiring of the corticospinal tract requires target-derived brain-derived neurotrophic factor and compensates lost function after brain injury. Brain. 2012;135:1253–1267. doi: 10.1093/brain/aws053. [DOI] [PubMed] [Google Scholar]
- Welniarz Q., Dusart I., Roze E. The corticospinal tract: Evolution, development, and human disorders. Dev. Neurobiol. 2017;77:810–829. doi: 10.1002/dneu.22455. [DOI] [PubMed] [Google Scholar]
- Xu B., Park D., Ohtake Y., Li H., Hayat U., Liu J., Selzer M.E., Longo F.M., Li S. Role of CSPG receptor LAR phosphatase in restricting axon regeneration after CNS injury. Neurobiol. Dis. 2015;73:36–48. doi: 10.1016/j.nbd.2014.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu G., He Z. Glial inhibition of CNS axon regeneration. Nat. Rev. Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Soderblom C., Krishnan V., Ashbaugh J., Bethea J.R., Lee J.K. Hematogenous macrophage depletion reduces the fibrotic scar and increases axonal growth after spinal cord injury. Neurobiol. Dis. 2015;74:114–125. doi: 10.1016/j.nbd.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Soderblom C., Trojanowsky M., Lee D.H., Lee J.K. Fibronectin matrix assembly after spinal cord injury. J. Neurotrauma. 2015;32:1158–1167. doi: 10.1089/neu.2014.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukor K., Belin S., Wang C., Keelan N., Wang X., He Z. Short hairpin RNA against PTEN enhances regenerative growth of corticospinal tract axons after spinal cord injury. J. Neurosci. 2013;33:15350–15361. doi: 10.1523/JNEUROSCI.2510-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Complete list of FPKM expression values for uninjured and injured Veh and Tam animals 2 weeks after spinal cord injury.
Differential expression analysis of RNA-seq data between injured Veh and Tam animals 2 weeks after spinal cord injury. Fold change cut-off ≥ ±1.5; FDR adjusted P ≤0.05. Inf and –Inf represent genes with infinite and –infinite fold change expression, respectively.
Video clip of unilateral photoactivation of the layer V sensorimotor cortex (447 nm light, 10 mW, 20 Hz, 5 ms pulse length) in an awake, freely moving uninjured Glast-Rasless animal transduced with AAV9-CAG-ChR2-GFP, showing vigorous lightdriven paw strokes. Blue light stimulation failed to generate a similar behavioral output in a control uninjured animal transduced with AAV9-CAG-GFP.
Video clip of unilateral photoactivation of the layer V sensorimotor cortex (447 nm light, 10 mW, 20 Hz, 5 ms pulse length) in awake, freely moving injured Glast-Rasless animals transduced with AAV9-CAG-ChR2-GFP. Eighteen weeks after spinal cord injury, the example vehicle animal shows an almost complete loss of lightevoked hind paw strokes. In contrast, the example Tam animal regained modest but increased hind paw strokes in response to blue light stimulation.