Abstract
The Notch signaling pathway plays a crucial role in neurodevelopment and in adult brain homeostasis. We aimed to further investigate Notch pathway activity in bipolar disorder (BD) and schizophrenia (SCZ) by conducting a pathway analysis. We measured plasma levels of Notch ligands (DLL1 and DLK1) using enzyme immunoassays in a large sample of patients (SCZ n = 551, BD n = 246) and healthy controls (HC n = 639). We also determined Notch pathway related gene expression levels by microarray analyses from whole blood in a subsample (SCZ n = 338, BD n = 241 and HC n = 263). We found significantly elevated Notch ligand levels in plasma in both SCZ and BD compared to HC. Significant gene expression findings included increased levels of RFNG and KAT2B (p < 0.001), and decreased levels of PSEN1 and CREBBP in both patient groups (p < 0.001). RBPJ was significantly lower in SCZ vs HC (p < 0.001), and patients using lithium had higher levels of RBPJ (p < 0.001). We provide evidence of altered Notch signaling in both SCZ and BD compared to HC, and suggest that Notch signaling pathway may be disturbed in these disorders. Lithium may ameliorate aberrant Notch signaling. We propose that drugs targeting Notch pathway could be relevant in the treatment of psychotic disorders.
Introduction
Schizophrenia (SCZ) and bipolar disorder (BD) are severe mental disorders that have prompted extensive research as they are among the leading causes of worldwide disability1,2. The neurodevelopmental hypothesis for SCZ suggests that the neuroanatomical defects associated with SCZ are caused by dysregulation of brain development3. In BD, brain morphological alterations are more subtle, but these together with behavioral changes prior to onset of illness support neurodevelopmental abnormalities also for BD4. In addition to developmental abnormalities, accelerated grey matter decline, aberrant brain connectivity and biochemical changes in the adult brain may indicate neurodegenerative processes in SCZ5,6; this may also be present in BD, albeit substantially less prominent7.
Notch signaling is well known as a master regulator of neural stem cells and neural development, and orchestrates nervous system development and patterning by regulating neurogenesis, axonal growth, synaptogenesis and predisposing neurons to apoptosis also in the adult brain8. These facts make it a pertinent candidate for exploration in psychotic disorders. The Notch signaling pathway was first associated with SCZ through genetic findings linking the NOTCH4 gene to SCZ in British parent-offspring trios9, and later confirmed by larger genome wide association studies10. Initial studies investigating Notch in BD were inconclusive11,12, however, in 2012 we found increased gene expression of NOTCH4 in BD13.
In addition to being important in regulating neural cell proliferation, differentiation, and neural cellular growth8, Notch is a crucial contributor in adaptive and innate immune responses14. Notch and its ligands (e.g. delta-like 1, DLL1) have also been implicated in endothelial cell dysregulation and vascular inflammation15,16 as well as macrophage activation17, and is involved in the interaction between immune cells and the brain during ischemic stroke. This may be relevant also in relation to severe psychotic disorders as immune-pathogenic mechanisms have been implicated in SCZ and BD, partly based on the demonstration of low-grade systemic inflammation including T cell activation in these patients18.
Based on the emerging significance of neuro-inflammation and immunogenetics in SCZ and BD, we hypothesized that Notch signaling components in inflammatory cells, both as a proxy for CNS tissues, but also representing important cells that could modulate different neural processes, would be dysregulated. Thus, we aimed to characterize the Notch signaling pathway in patients with SCZ, BD and healthy controls (HC) by conducting a pathway analysis on the mRNA level in whole blood, as well as investigating plasma levels of secreted Notch ligands. Our secondary aim was to investigate whether potential alterations in Notch pathway mRNA expression or secreted ligands are associated with the use of psychotropic medication.
Results
Demographics and clinical characteristics
Socio-demographic and clinical characteristics of the participants are shown in Table 1. The plasma protein cohort and the whole blood mRNA cohort showed the same differences between SCZ, BD and HC groups with the exception of one clinical characteristic: SCZ patients had higher CDSS scores in whole blood cohort, but not in the plasma protein cohort. The main differences between patients and HC were age, ethnicity and sex.
Table 1.
Demographic and clinical characteristics of participants.
| Parameters | Plasma (Notch ligand) cohort | Whole blood (mRNA) cohort | ||||||
|---|---|---|---|---|---|---|---|---|
| SCZ (N = 551) | BD (N = 246) | HC (N = 639) | Post Hoc Analysis |
SCZ (N = 338) | BD (N = 241) | HC (N = 263) | Post Hoc Analysis |
|
| Male sex, N (%) | 334 (60.6) | 97 (39.4) | 364 (57.0) | SCZ > HC > BD | 275 (61.5) | 90 (39.5) | 144 (54.8) | SCZ > HC > BD |
| Ethnicity (Cauc. %) | 444 (80.6) | 213 (86.6) | 629 (98.4) | HC > BD > SCZ | 228 (90.1) | 140 (94.6) | 208 (100) | HC > BD > SCZ |
| Medication N(%): | ||||||||
| Antipsychotics | 510 (84.6) | 167 (66.0) | — | SCZ > BD | 234 (92.5) | 114 (77.0) | — | SCZ > BD |
| Lithium | 12 (2.0) | 51 (20.2) | — | BD > SCZ | 3 (1.2) | 32 (21.6) | — | BD > SCZ |
| Antidepressants | 179 (31.5) | 95 (38.8) | — | BD > SCZ | 35 (13.8) | 66 (44.6) | — | BD > SCZ |
| Mood stabilizers | 56 (9.3) | 87 (34.4) | — | BD > SCZ | 78 (30.8) | 57 (38.5) | — | BD > SCZ |
| Age (years) | 27 (13) | 29 (18) | 31 (13) | BD, HC > SCZ | 29 (14) | 35 (20) | 31.0 (13) | BD, HC > SCZ |
| DOI (years) | 4 (8) | 4 (10) | — | BD > SCZ | 4 (9) | 5 (13) | — | BD > SCZ |
| PANSS total score | 62 (22) | 44 (13) | — | SCZ > BD | 64 (24) | 45 (14) | — | SCZ > BD |
| YMRS total score | 3 (9) | 2 (5) | — | SCZ > BD | 3 (9) | 2 (6) | — | SCZ > BD |
| IDS total score | 17 (19) | 17 (16) | — | NS | 18 (18) | 15 (16) | — | NS |
| CDSS total score | 5 (8) | 4 (6) | — | NS | 5 (7) | 3 (7) | — | SCZ > BD |
| GAF-S | 40 (15) | 57 (16) | — | BD > SCZ | 40 (14) | 54 (17) | — | BD > SCZ |
| GAF-F | 42 (14) | 51 (19) | — | BD > SCZ | 42 (15) | 50 (17) | — | BD > SCZ |
Abbreviations: SCZ = Schizophrenia; BD = Bipolar Disorder; HC = Healthy Controls; Cauc. = Caucasians; NS = Non-Significant; DOI = Duration of illness; PANSS = Positive and Negative Syndrome Scale; YMRS = Young Mania Rating Scale; IDS = Inventory of Depressive Symptoms; CDSS = Calgary Depression Scale for Schizophrenia; GAF-S = Global Assessment of Functioning - Symptom Scale; GAF-F = Global Assessment of Functioning - Function Scale.
Categorical data are given as percent in brackets, while continuous data are given as median with interquartile range. Post hoc analysis is performed using Pearson Chi-square for categorical data, and Mann-Whitney U tests for continuous data. Differences between groups are significant when p < 0.05.
Plasma levels of Notch ligands
The plasma levels of the secreted Notch ligands and group comparisons are summarized in Table 2. Patients had significantly higher plasma levels of DLL1 compared to HCs, with SCZ having significantly higher levels than BD after controlling for age and gender. DLK1 levels showed nominally significant increase in both patient groups compared to HC with no differences between BD and SCZ.
Table 2.
Differences between groups for plasma markers of Notch pathway after controlling for age and gender.
| plasma ligands | M(IQR) | SCZ vs. HC | BD vs. HC | SCZ vs. BD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SCZ | BD | HC | df | t | F | df | t | F | df | t | F | |
| DLL1 | 5 (1.8) | 4.7 (1.6) | 4.5 (1.3) | 1232 | 8.8*** | 32.01*** | 889 | 3.26** | 10.81*** | 848 | 3.00** | 9.13*** |
| DLK1 | 186 (170) | 188 (164) | 180 (149) | 1243 | 2.23* | 10.62*** | 893 | 2.00* | 11.38*** | 854 | 0.08 | 9.06*** |
*p < 0.05 **p < 0.03 ***p < 0.001
Abbreviations: M = median; IQR = interquartile range; SCZ = schizophrenia; BD = bipolar disorder; HC = healthy controls; DLL1 = Delta-like protein 1; DLK1 = Delta Like Non-Canonical Notch Ligand 1.
ANCOVA using linear regression models. Results are significant if p < 0.03, and nominally significant if 0.03 < p < 0.05 (Bonferroni correction).
Gene expression in whole blood
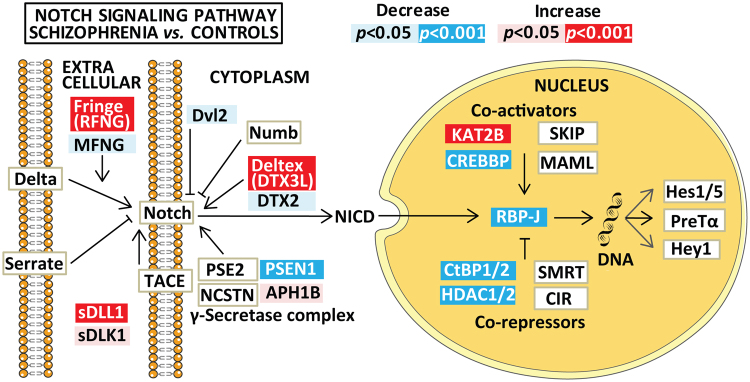
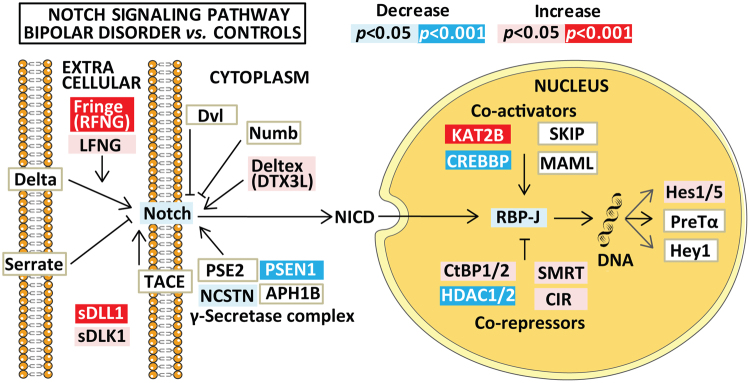
The mRNA expression of Notch pathway genes are summarized in Figs 1 and 2 and Table 3, and nominally significant findings (0.001 < p < 0.05) in Supplementary Table 1. Effect size estimates were small in general with the highest value on 0.16 for KAT2B. Compared to HC, both SCZ and BD had increased levels of RFNG and KAT2B, and decreased levels of PSEN1 and CREBBP mRNA expression. In addition, the SCZ group had increased DTX3L and decreased RBPJ, CTBP and HDAC mRNA expression compared to HC (Fig. 1). The main significant differences between SCZ and BD were lower LFNG and increased NOTCH2 in SCZ vs BD (Table 3 and Supplementary Figure 1).
Figure 1.
Summary of significant and nominally significant findings in Notch pathway mRNA expression between the schizophrenia and the healthy control group after controlling for age, gender and Bmal1. Results are given as p-values, adjusted for multiple testing, where significant results are indicated in red/dark blue (for increased/decreased mRNA expression) and nominally significant results (0.001 < p < 0.05) are shown as pink/light blue (for increased/decreased mRNA expression). Non-significant results are depicted as boxes with white background. The figure is based on the Notch signaling pathway in the KEGG database (hsa04330, version date 5/9/17). Hey1 is included by the authors due to its known role as a target gene for Notch signaling30.
Figure 2.
Summary of significant and nominally significant findings in Notch pathway mRNA expression between the bipolar disorder and the healthy control group after controlling for age, gender and Bmal1. Results are given as p-values, adjusted for multiple testing, where significant results are indicated in red/dark blue (for increased/decreased mRNA expression) and nominally significant results (0.001 < p < 0.05) are shown as pink/light blue (for increased/decreased mRNA expression). Non-significant results are depicted as boxes with white background. The figure is based on the Notch signaling pathway in the KEGG database (hsa04330, version date 5/9/17). Hey1 is included by the authors due to its known role as a target gene for Notch signaling30.
Table 3.
Significant differences between patients and controls in Notch signaling pathway gene mRNA expression after controlling for age and gender.
| Genes | specificity | SCZ vs. HC | BD vs. HC | SCZ vs. BD | |
|---|---|---|---|---|---|
| B | B | B | |||
| Receptor and Cytoplasm | LFNG | +++ | −0.01 | 0.07** | −0.09*** |
| RFNG | +++ | 0.11*** | 0.07*** | 0.03 | |
| NOTCH2 | +++ | 0.01 | −0.04** | 0.06*** | |
| DTX3L | +++ | 0.05*** | 0.00 | 0.05** | |
| PSEN1 | ++ | −0.03*** | −0.02*** | −0.01 | |
| Nucleus | KAT2B | ++ | 0.16*** | 0.13*** | 0.03 |
| CREBBP | + | −0.10*** | −0.09*** | −0.01 | |
| CTBP1 | ++ | −0.03*** | −0.01 | −0.02* | |
| CTBP2 | ++ | −0.03*** | −0.01 | −0.02** | |
| HDAC1 | ++ | −0.08*** | −0.04* | −0.04* | |
| HDAC2 | ++ | −0.06*** | −0.05* | −0.01 | |
| RBPJ | + | −0.07*** | −0.01 | −0.06** |
*p < 0.05 **p < 0.01 ***p < 0.001
Specificity: + (unspecific, involved in many pathways), ++ (involved in up to 3 additional pathways e.g. Wnt, NF-kappa), +++ (exclusively Notch pathway related gene).
Abbreviations: SCZ = Schizophrenia; BD = Bipolar disorder; HC = Healthy controls; B = Unstandardized regression coefficient.
Gene names are listed according to the HUGO Gene Nomenclature Committee.
Results are given as effect size estimates from the linear regression analysis after correction for age, sex and BMAL1 expression. Results are significant if p < 0.001, and nominally significant if 0.001 < p < 0.05 (Bonferroni correction).
Role of medication
We found that patients using lithium (n = 35) had significantly higher RBPJ expression compared to patients not taking lithium (n = 366, p < 0.001) (Supplementary Table 2). As the BD group had higher levels of RBPJ mRNA we also controlled for diagnosis. Our result remained significant after controlling for diagnosis (t = 3.35 p = 0.001 F = 5.63 n = 400) and when investigating lithium in the BD group alone (t = 3.51 p = 0.001 F = 3.42 n = 148). This association does not seem to be dosage dependent, as we observed no correlation between serum levels of lithium and RBPJ expression. We found nominally significant effects of antipsychotics and mood stabilizers on DLK1 levels, and a weak dose dependent association between antidepressants and DLL1 levels (Supplementary Table 2).
Discussion
In the present study we evaluated circulating levels of secreted Notch ligands from plasma and mRNA expression of Notch family members and transcripts that may influence Notch signaling in whole blood in a large population of patients with SCZ and BD as well as in heathy controls. We found significant differences in the concerted regulation of Notch mRNA species between patients and controls, in addition to an increase in circulating levels of secreted Notch ligands. Our main findings include (i) raised levels of DLL1, which is a soluble Notch ligand with potential inhibiting effect on Notch signaling, (ii) down-regulation of PSEN1 and RBPJ (SCZ), potentially impairing intracellular Notch signaling, and (iii) up-regulation of RFNG mRNA, which may inhibit ligand-receptor interactions, further impairing Notch signaling.
DLL1 and DLK1 are soluble Notch ligands that are cleaved from their respective transmembrane forms by ADAM proteins, and likely inhibit Notch signaling19,20. Thus, the increased plasma levels of DLL1 and potentially DLK1 in SCZ and BD could reflect attenuated Notch activity in these disorders. We have previously identified elevated ADAM17 mRNA levels in BD, suggesting that ADAM17 may be involved in the heightened shedding of Notch ligands in our patient group21. Fringe proteins modulate Notch signaling by sensitizing notch receptors to delta ligands and attenuating notch-serrate interactions22. Radical fringe (RFNG) is abundantly expressed in the rat brain, and has been found to negatively modulate Notch signaling23. Both SCZ and BD patients had significantly elevated RFNG expression compared to HC. This further supports a possible impediment in Notch signaling in patients vs. controls. PSEN1- controlled γ-secretase activity is essential for Notch signaling as it releases the Notch intracellular domain (NICD) that subsequently enters the nucleus to regulate gene activity. Thus, decreased PSEN1 expression in patients also favors attenuated Notch signaling, but may also have multiple functions outside of the γ-secretase complex24. The implications of enhanced expression of DTX3L are less clear. DTX3L belongs to the deltex family, which can facilitate intracellular trafficking of the NICD from the membrane to the nucleus. However, several proteins interact with deltex, and together they destabilize Notch receptors, acting as Notch signaling inhibitors25.
In the nucleus, the RBP-J protein enables NICD to bind to DNA and regulate the expression of notch target genes (HES and HEY). SCZ patients had decreased RBPJ mRNA expression compared to HC. RBPJ deletion in mouse embryonic brain causes neural stem/progenitor cells to prematurely differentiate into neurons thus depleting the neuronal stem cell population26. There is also evidence indicating that RBP-J is necessary for hippocampal-dependent learning and memory in mice27. Thus, a shift towards downregulation could hamper proliferation in the brain and interfere with hippocampal functions. The downregulated co-repressors (i.e. CTBPs and HDACs) and upregulated co-activator KAT2B could imply a shift in favor of increased target gene transcription especially in SCZ vs controls. However, as the mRNA expression of RBPJ is downregulated, the significance of these alterations is unclear, possibly reflecting compensatory mechanisms. Furthermore, these transcriptional regulators are not restricted to Notch signaling and could be related to other signaling pathways28. In contrast to our finding of decreased HDAC1/2 in SCZ, a recent smaller study demonstrated increased HDAC1 mRNA in blood leukocytes and in the hippocampus and prefrontal cortex of patients with SCZ who were subjected to early life stress29.
Activation of the Notch signaling pathway induces transcription of target genes (HES and HEY). Hes and hey proteins predominantly act as transcriptional repressors30 and, depending on cellular context31, impede cell differentiation, and prompt cell proliferation also in the adult brain26. We did not observe altered expression of target genes in this study, thus our finding of an attenuated pattern of Notch signaling pathway should be interpreted with some caution.
We show for the first time in a naturalistic study that patients taking lithium had higher levels of RBPJ expression compared to patients not medicated with lithium but using other psychotropic medication. This finding is in line with a previous study that proposed that lithium may activate the Notch pathway through the inhibition of glycogen synthase kinase-3β32.
Our finding of a possibly hampered Notch signaling may seem at odds with the enhanced T cell response and chronic low grade systemic inflammation in SCZ and BD. However, the role of the adaptive immune system and T cell-mediated immune responses in these patients are still unclear, with some studies favoring a TH2 shift at least in SCZ33,34. Notch has emerged as an important regulator of TH-cell differentiation35 and stimulation with Notch ligands, including DLL1, promotes a TH1 phenotype which may be inhibited by soluble DLL136. Thus, our finding of increased plasma DLL1 and hampered Notch signaling as reflected by decreased RBPJ, is compatible with an altered T cell function potentially favoring development of Th2 cells. A recent meta-analysis suggested a shift towards a TH2 phenotype in SCZ based on circulating levels of typical TH1 and TH2 cytokines while in vitro studies favored a TH1 response in the patients. Thus, the role of Notch on T-cell mediated immune responses in the psychiatric disorders needs further study.
There are some limitations to the current study. Firstly, using whole blood as proxy for the brain has limitations, especially when we take into consideration that the Notch signaling pathway is almost exclusively dependent on cell-cell interaction. However, current technology does not yet enable us to investigate gene expression in vivo in the brain. We are thus dependent on either post-mortem studies which are limited by artificial changes in gene expression, or alternatively, evaluation of easily accessible tissues that may reflect processes in the brain to a certain degree37. Secondly, the use of whole blood does not necessarily reflect the levels in various leukocyte subsets such as T cells and monocytes. Third, due to the naturalistic nature of our study most patients were using a combination of psychotropic medication which prevented exploration of medication in monotherapy. Fourth, the patients were not completely matched with controls in relation to age, ethnicity and sex, but these factors were adjusted for in the statistical analyses.
In conclusion, we demonstrate altered Notch signaling in whole blood in SCZ compared to HC. Although less clear, our results also indicate altered Notch signaling in BD compared to HC. Further, we show an association between the use of lithium and Notch pathway activation. The demonstrated pattern of Notch molecules suggest that the Notch signaling pathway may be compromised in patients compared to controls. There is emerging evidence from rodent studies that Notch signaling is important in adult brain, and partakes in the regulation of adult neural stem cell migration, morphology, synaptic plasticity and survival of neurons8. Future studies should be aimed at further exploring the Notch signaling pathway in severe mental disorders, and investigate if therapy targeting this system could be relevant in psychotic disorders.
Methods
The present study is part of the Norwegian Centre for Mental Disorders Research, University of Oslo and Oslo University Hospital, and collaborating Norwegian hospitals13. The study is approved by the Regional Committee for Medical Research Ethics and the Norwegian Data Inspectorate and all research was performed in accordance with these guidelines and regulations. We obtained written informed consent from all participants. The biobank is approved by the Norwegian Directorate of Health.
Participants
We included patients in the study if they had DSM-IV diagnoses of schizophrenia spectrum disorders or bipolar spectrum disorders, IQ >70, and age between 18 and 65 years (for details see ref.13). We randomly selected healthy controls without a prior history of severe psychiatric disorders (or in any of their first-degree relatives), or substance/alcohol abuse/dependency from the same catchment area from the National Population Registry (www.ssb.no) (for details see ref.13). For the present analyses, we included patients and controls if they had no coexisting autoimmune or inflammatory disease, cancer or ongoing infections, were not using anti-inflammatory drugs, and had C-reactive Protein (CRP) levels below 20 mg/L.
Clinical Assessments
The clinical assessment and diagnostic interviews were carried out by a team of psychologists and physicians who were trained until satisfactory inter-rater reliability was obtained38,39. We used the Structured Clinical Interview for DSM-IV Axis I Disorders40 to obtain diagnoses. We evaluated clinical symptoms using the Young Mania Rating Scale (YMRS)41, Inventory of Depressive Symptoms (IDS)42, Calgary Depression Scale for Schizophrenia (CDSS)43 and Positive and Negative Syndrome Scale (PANSS)44. Global functioning and symptom load was measured with the Global Assessment of Functioning (GAF), split version. The scale has two measures reflecting functioning (GAF-F) and symptom load (GAF-S)45.
Plasma protein assessment
DLL1 and DLK1 were measured in duplicate using commercially available antibodies (R&D Systems, Abingdon, UK) in a 384 format using a combination of a SELMA (Jena, Germany) pipetting robot and a BioTek (Winooski, VT, USA) dispenser/washer. Absorption was read at 450 nm with wavelength correction set to 540 nm using an ELISA plate reader (Bio-Rad, Hercules, CA, USA). Intra- and inter-assay coefficients of variation were 3.9% and 3.7% for DLL1 and 5.0% and 8.4% for DLK1, respectively. We observed no diurnal variation for DLL1 and DLK1: in a smaller group of HC (n = 13) blood was collected at 4 time-points within 24 hours (mean intra-individual CV ± SD was 5.8 ± 5.2%, p = 0.27 for DLL1; and CV ± SD = 5.8 ± 6.4%, p = 0.86 for DLK1). Further, we found no postprandial variation for DLL1 and DLK1 (n = 13, fasting vs. non-fasting; mean intra-individual CV ± SD was 11.1 ± 7.6%, p = 0.15 for DLL1 and CV ± SD = 9.8 ± 11.0%, p = 0.84 for DLK1). Detection limits were 10 pg/mL and 25 pg/mL for DLL1 and DLK1, respectively, as defined as 3xSD of assay buffer (n = 10).
RNA isolation and microarray analysis
RNA isolation
Blood samples were collected using Tempus Blood RNA Tubes. Total RNA was extracted with ABI PRISM 6100 Nucleic Acid PrepStation and TEMPUS 12-port RNA Isolation Kit according to manufacturer’s protocol. High-Capacity cDNA Reverse Transcription Kit was used for reverse transcription of 1 µg RNA.
Global Transcriptomics Analyses
We selected 49 Notch pathway related genes using the Kyoto Encyclopedia of Genes and Genomes database (http://www.genome.jp/kegg/pathway.html, hsa04330, version date 5/9/17). In addition, we included HEY1 as it is a well-recognized Notch signaling pathway target gene30. For each sample 200 ng of total RNA was biotin labelled and amplified using the Illumina TotalPrep-96 RNA Amplification Kit (Thermo Fisher, Waltham, MA, USA). Global analysis of gene expression was performed with Illumina HumanHT-12 v4 Bead Chip (Illumina, San Diego, CA, USA) consisting of more than 47 000 probes (ie. transcripts). For this purpose, 842 samples (263 HC, 338 SCZ and 241 BD) passed labeling and scanning. Raw microarray scan files were exported using the Illumina GenomeStudio software and loaded into R for downstream analysis using specific packages provided by BioConductor46. Lumi was used to detect outliers47. ComBat from the SVA R package was used to correct for technical batch effects, like RNA extraction batch, RNA extraction method, DNase treatment batch, cRNA labelling batch and chip hybridization48. Further quality control, quantile-normalization and log2-transformation were done using Limma49.
Association between medication and protein/mRNA
We used the defined daily dose (DDD) of psychotropic medications, which is the assumed average maintenance dose per day for a drug used for its main indication in adults, to investigate associations between medication and proteins/mRNA. We calculated the dose relative to DDD for antipsychotics, mood stabilizers and antidepressants according to the guidelines from the World Health Organization Collaborating Center for Drug Statistics Methodology (https://www.whocc.no/atcdd), and used serum concentration levels for lithium. We selected key Notch signaling pathway related genes whose expression was significantly altered in our analyses (PSEN1, RBPJ and RFNG) in addition to plasma proteins.
Statistical analysis
Plasma proteins
We used the SPSS software package for Windows, version 24.0 for the statistical analyses of demographic data and plasma proteins. We assessed data normality using the Kolmogorov-Smirnov and Shapiro-Wilk tests. As distributions were skewed, we used the Kruskal-Wallis test, the Mann-Whitney U test and the chi-square test to investigate differences in demographic data and plasma proteins between groups. Associations between medication and proteins/mRNA were investigated by Spearman’s Rank correlation test. We controlled for age and sex in linear regression models. We inspected the histogram and the normal P-P plot of the regression standard residuals. In case of skewed distribution of the residuals we repeated our analyses using ln transformation of the dependent variable, and then by removing outliers defined as above −3 and below 3 studentized deleted residuals saved from the regression analyses.
Global Transcriptomics
Analysis was performed on batch-adjusted log2-transformed values. We used the R software environment to investigate group differences in mRNA expression. We fitted a linear model using age, sex and Bmal1 expression as covariates. Bmal1 is a critical component of the molecular clock50, and we used its expression level to adjust for differences in time of blood sampling and circadian rhythm between patients and HC.
Medication
We used ANCOVA to explore the effect of medication while controlling for age, sex and other medication groups.
Correction for multiple testing
We corrected for multiple testing according to the Bonferroni method. Alpha was set at p < 0.001 for our main microarray analyses (investigating 49 genes), and at p < 0.03 for the two plasma proteins. Our secondary analyses investigating the effects of medication were explorative in nature, and we also applied Bonferroni to correct for these analyses separately from the main analyses. Alpha for the secondary analyses was thus set at p < 0.003 (correcting for 20 tests).
Data availability
The datasets generated and analyzed during the current study are not publicly available due to Institutional Review Board restrictions but are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
The authors thank the participants of the study, and Thomas D. Bjella, Eivind Bakken and Line Gundersen for their skillful research administrative assistance. Funded by KG Jebsen Stiftelsen, Research Council of Norway (223273, 248778), South East Norway Health Authority (2017-112). We also acknowledge the technical support and service from the Genomics Core Facility at the Department of Clinical Science, the University of Bergen.
Author Contributions
E.Z.H., O.A.A., T.U. designed the study and wrote first version of manuscript. E.Z.H., I.D., R.H.M., S.H., E.S.G., I.M., V.M.S., P.A. and N.E.S. provided data. H.R.B., F.K. and E.Z.H. did data analyses. E.Z.H., I.M., O.A.A., S.D., V.M.S., P.A. and T.U. interpreted the results. All authors critically revised this manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-23703-w.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferrari AJ, et al. The prevalence and burden of bipolar disorder: findings from the Global Burden of Disease Study 2013. Bipolar disorders. 2016;18:440–450. doi: 10.1111/bdi.12423. [DOI] [PubMed] [Google Scholar]
- 2.Chong HY, et al. Global economic burden of schizophrenia: a systematic review. Neuropsychiatric disease and treatment. 2016;12:357–373. doi: 10.2147/NDT.S96649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Archives of general psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 4.O’Shea KS, McInnis MG. Neurodevelopmental origins of bipolar disorder: iPSC models. Molecular and cellular neurosciences. 2016;73:63–83. doi: 10.1016/j.mcn.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Pino O, et al. Neurodevelopment or neurodegeneration: review of theories of schizophrenia. Actas espanolas de psiquiatria. 2014;42:185–195. [PubMed] [Google Scholar]
- 6.Bartholomeusz CF, et al. Structural neuroimaging across early-stage psychosis: Aberrations in neurobiological trajectories and implications for the staging model. The Australian and New Zealand journal of psychiatry. 2017;51:455–476. doi: 10.1177/0004867416670522. [DOI] [PubMed] [Google Scholar]
- 7.Savitz JB, Price JL, Drevets WC. Neuropathological and neuromorphometric abnormalities in bipolar disorder: view from the medial prefrontal cortical network. Neuroscience and biobehavioral reviews. 2014;42:132–147. doi: 10.1016/j.neubiorev.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Ables JL, Breunig JJ, Eisch AJ, Rakic P. Not(ch) just development: Notch signalling in the adult brain. Nature reviews. Neuroscience. 2011;12:269–283. doi: 10.1038/nrn3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei J, Hemmings GP. The NOTCH4 locus is associated with susceptibility to schizophrenia. Nature genetics. 2000;25:376–377. doi: 10.1038/78044. [DOI] [PubMed] [Google Scholar]
- 10.Stefansson H, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–747. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahearn EP, et al. Investigation of Notch3 as a candidate gene for bipolar disorder using brain hyperintensities as an endophenotype. American journal of medical genetics. 2002;114:652–658. doi: 10.1002/ajmg.10512. [DOI] [PubMed] [Google Scholar]
- 12.Prathikanti S, et al. Neither single-marker nor haplotype analyses support an association between genetic variation near NOTCH4 and bipolar disorder. American journal of medical genetics. Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2004;131b:10–15. doi: 10.1002/ajmg.b.20123. [DOI] [PubMed] [Google Scholar]
- 13.Dieset I, et al. Up-regulation of NOTCH4 gene expression in bipolar disorder. The American journal of psychiatry. 2012;169:1292–1300. doi: 10.1176/appi.ajp.2012.11091431. [DOI] [PubMed] [Google Scholar]
- 14.Radtke F, Fasnacht N, Macdonald HR. Notch signaling in the immune system. Immunity. 2010;32:14–27. doi: 10.1016/j.immuni.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Butko E, Pouget C, Traver D. Complex regulation of HSC emergence by the Notch signaling pathway. Developmental biology. 2016;409:129–138. doi: 10.1016/j.ydbio.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quillard T, Charreau B. Impact of notch signaling on inflammatory responses in cardiovascular disorders. International journal of molecular sciences. 2013;14:6863–6888. doi: 10.3390/ijms14046863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shang Y, Smith S, Hu X. Role of Notch signaling in regulating innate immunity and inflammation in health and disease. Protein & cell. 2016;7:159–174. doi: 10.1007/s13238-016-0250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergink V, Gibney SM, Drexhage HA. Autoimmunity, inflammation, and psychosis: a search for peripheral markers. Biological psychiatry. 2014;75:324–331. doi: 10.1016/j.biopsych.2013.09.037. [DOI] [PubMed] [Google Scholar]
- 19.Mishra-Gorur K, Rand MD, Perez-Villamil B, Artavanis-Tsakonas S. Down-regulation of Delta by proteolytic processing. The Journal of cell biology. 2002;159:313–324. doi: 10.1083/jcb.200203117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falix FA, Aronson DC, Lamers WH, Gaemers IC. Possible roles of DLK1 in the Notch pathway during development and disease. Biochimica et biophysica acta. 2012;1822:988–995. doi: 10.1016/j.bbadis.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Hoseth EZ, et al. A Study of TNF Pathway Activation in Schizophrenia and Bipolar Disorder in Plasma and Brain Tissue. Schizophrenia bulletin. 2017 doi: 10.1093/schbul/sbw183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeuchi H, Haltiwanger RS. Significance of glycosylation in Notch signaling. Biochemical and biophysical research communications. 2014;453:235–242. doi: 10.1016/j.bbrc.2014.05.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mikami T, Ohnaka Y, Nakamura A, Kurosaka A, Itoh N. Radical fringe negatively modulates Notch signaling in postmitotic neurons of the rat brain. Brain research. Molecular brain research. 2001;86:138–144. doi: 10.1016/S0169-328X(00)00278-3. [DOI] [PubMed] [Google Scholar]
- 24.Duggan SP, McCarthy JV. Beyond gamma-secretase activity: The multifunctional nature of presenilins in cell signalling pathways. Cellular signalling. 2016;28:1–11. doi: 10.1016/j.cellsig.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Hori K, Sen A, Kirchhausen T, Artavanis-Tsakonas S. Regulation of ligand-independent Notch signal through intracellular trafficking. Communicative & integrative biology. 2012;5:374–376. doi: 10.4161/cib.19995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imayoshi I, Sakamoto M, Yamaguchi M, Mori K, Kageyama R. Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:3489–3498. doi: 10.1523/JNEUROSCI.4987-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu S, Wang Y, Worley PF, Mattson MP, Gaiano N. The canonical Notch pathway effector RBP-J regulates neuronal plasticity and expression of GABA transporters in hippocampal networks. Hippocampus. 2015;25:670–678. doi: 10.1002/hipo.22402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stankiewicz TR, Gray JJ, Winter AN, Linseman DA. C-terminal binding proteins: central players in development and disease. Biomolecular concepts. 2014;5:489–511. doi: 10.1515/bmc-2014-0027. [DOI] [PubMed] [Google Scholar]
- 29.Bahari-Javan S, et al. HDAC1 links early life stress to schizophrenia-like phenotypes. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:E4686–e4694. doi: 10.1073/pnas.1613842114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fischer A, Gessler M. Delta-Notch–and then? Protein interactions and proposed modes of repression by Hes and Hey bHLH factors. Nucleic acids research. 2007;35:4583–4596. doi: 10.1093/nar/gkm477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aster JC. In brief: Notch signalling in health and disease. The Journal of pathology. 2014;232:1–3. doi: 10.1002/path.4291. [DOI] [PubMed] [Google Scholar]
- 32.Espinosa L, Ingles-Esteve J, Aguilera C, Bigas A. Phosphorylation by glycogen synthase kinase-3 beta down-regulates Notch activity, a link for Notch and Wnt pathways. The Journal of biological chemistry. 2003;278:32227–32235. doi: 10.1074/jbc.M304001200. [DOI] [PubMed] [Google Scholar]
- 33.Debnath M. Adaptive Immunity in Schizophrenia: Functional Implications of T Cells in the Etiology, Course and Treatment. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2015;10:610–619. doi: 10.1007/s11481-015-9626-9. [DOI] [PubMed] [Google Scholar]
- 34.Brambilla P, et al. Increased M1/decreased M2 signature and signs of Th1/Th2 shift in chronic patients with bipolar disorder, but not in those with schizophrenia. Translational psychiatry. 2014;4:e406. doi: 10.1038/tp.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amsen D, Antov A, Flavell RA. The different faces of Notch in T-helper-cell differentiation. Nature reviews. Immunology. 2009;9:116–124. doi: 10.1038/nri2488. [DOI] [PubMed] [Google Scholar]
- 36.Maekawa Y, et al. Delta1-Notch3 interactions bias the functional differentiation of activated CD4+ T cells. Immunity. 2003;19:549–559. doi: 10.1016/S1074-7613(03)00270-X. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan PF, Fan C, Perou CM. Evaluating the comparability of gene expression in blood and brain. American journal of medical genetics. Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2006;141b:261–268. doi: 10.1002/ajmg.b.30272. [DOI] [PubMed] [Google Scholar]
- 38.Lagerberg TV, et al. Indications of a dose-response relationship between cannabis use and age at onset in bipolar disorder. Psychiatry research. 2014;215:101–104. doi: 10.1016/j.psychres.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 39.Ventura J, Liberman RP, Green MF, Shaner A, Mintz J. Training and quality assurance with the Structured Clinical Interview for DSM-IV (SCID-I/P) Psychiatry research. 1998;79:163–173. doi: 10.1016/S0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- 40.First, M. B., Spitzer, R. L., Goibbon, M. & Williams, J. B. W. Structured Clinical Interview for DSM-IV Axis I Disorders: Patient Edition (SCID-P), Version 2. Vol. Biometrics Research (New York State Psychiatric Institute 1995).
- 41.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. The British journal of psychiatry: the journal of mental science. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 42.Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26:477–486. doi: 10.1017/S0033291700035558. [DOI] [PubMed] [Google Scholar]
- 43.Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophrenia research. 1990;3:247–251. doi: 10.1016/0920-9964(90)90005-R. [DOI] [PubMed] [Google Scholar]
- 44.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 45.Pedersen G, Hagtvet KA, Karterud S. Generalizability studies of the Global Assessment of Functioning-Split version. Comprehensive psychiatry. 2007;48:88–94. doi: 10.1016/j.comppsych.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 46.Ritchie ME, Dunning MJ, Smith ML, Shi W, Lynch AG. BeadArray expression analysis using bioconductor. PLoS computational biology. 2011;7:e1002276. doi: 10.1371/journal.pcbi.1002276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Du P, Kibbe WA, Lin S. M. lumi: a pipeline for processing Illumina microarray. Bioinformatics (Oxford, England) 2008;24:1547–1548. doi: 10.1093/bioinformatics/btn224. [DOI] [PubMed] [Google Scholar]
- 48.Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics (Oxford, England) 2012;28:882–883. doi: 10.1093/bioinformatics/bts034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ritchie ME, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic acids research. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ko, C. H. & Takahashi, J. S. Molecular components of the mammalian circadian clock. Human molecular genetics15 Spec No2, R271–277, 10.1093/hmg/ddl207 (2006). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available due to Institutional Review Board restrictions but are available from the corresponding author on reasonable request.