Version Changes
Revised. Amendments from Version 1
We have tried to clarify some queries of reviewers and added few points. 1. Regarding types of arrhythmias on fulminant myocarditis: Cardiac arrhythmias are varied in presentation, ranging from Sinus arrest, AV block, Ventricular tachycardia and Ventricular fibrillation during acute phase18. 2. Uses of medicines: High dose of vitamin C is used to reduce the risk of myocardial injury; Coenzyme Q 10 is used for myocardial preservation, fructose diphosphate to improve cardiac metabolism and mannitol to reduce cerebral edema. 3. Regarding prognosis: Acute fulminant myocarditis, if properly and aggressively treated, has excellent long-term survival even if the patient may present with severe hemodynamic compromise 19. Complete heart block on initial ECG may also have an excellent prognosis, although mechanical assistance may be warranted as shown in the study by Lee E Y et. al 1. 4. We have not done any viral marker, autoimmune marker or endomyocardial biopsy.
Abstract
Fulminant myocarditis is a life-threatening clinical condition. It is the inflammation of myocardium leading to acute heart failure, cardiogenic shock and cardiac arrhythmias. Incidence of fulminant myocarditis is low and mortality is high. Most grievous complications of fulminant myocarditis is mainly cardiac arrhythmias; if there is delay on active management of the patient, it may be fatal. Here, we describe a case of III° atrioventricular block due to fulminant myocarditis that was managed with non-invasive transcutaneous cardiac pacing in the absence of ECMO. The non-invasive transcutaneous pacemaker is a safe, effective and convenient device to revert arrhythmias.
Keywords: III° A-V block, Fulminant Myocarditis, non-invasive transcutaneous cardiac pacing, ECMO
Abbreviations
A-V block- Atrioventricular block; ECG- Electrocardiogram; ECMO- Extracorporeal Membrane Oxygenation; LVEF- Left ventricular ejection fraction
Introduction
Fulminant myocarditis is a life-threatening clinical condition. It is inflammation of myocardium leading to acute heart failure and cardiac conduction abnormalities with rapid deterioration. There are about 10–38% cases of fulminant myocarditis among all cases of acute myocarditis 1. Causes of fulminant myocarditis may be of viral, bacterial or non-infectious origin 1– 3. Diagnosis of fulminant myocarditis is very difficult because of non-specific symptoms and diagnostic tools. There may be signs of acute heart failure, cardiogenic shock, or life-threatening cardiac arrhythmias. Cardiac arrhythmias are varied in presentation, ranging from Sinus arrest, AV block, Ventricular tachycardia and Ventricular fibrillation during acute phase 4. Here we present a case of fulminant myocarditis presenting with different clinical features and IIIº A-V block, which was successfully managed with non-invasive transcutaneous pacing.
Case report
A 3 ½ year old female child having a productive cough and 5–6 episodes of the passage of loose stool for 2 days was taken to local hospital after she had sudden convulsion for about 2 minutes. At the hospital, she again had convulsion for 1–2 minutes and her heart rate dropped to 30 bpm. She was given atropine, dexamethasone, dextrose and per rectal choral hydrate at the local hospital (doses not known) and immediately referred to Renmin Hospital. She had no significant past medical history, drug sensitivity or allergies. On arrival she was conscious but lethargic and dyspnoeic. Both pupils were round, 4mm and reactive to light. Her vitals were T36.4 °C, HR 62 bpm, RR 65/min, BP 90/41mmHg and SPO2 80%. Table 1 shows the results of routine laboratory measurements. Her lips were cyanosed and she had pale and cold extremities. Neck and throat examination was normal. Chest examination showed b/l crackles and irregular heart rate with no murmurs. The abdomen was soft and non-tender. Electrocardiogram (ECG) showed- III° atrio-ventricular (A-V) block; left anterior fascicular block, ST-T changes ( Figure 1). She had cardiac arrest three times in the emergency department at 15–20 minutes interval. She was resuscitated with chest compression along with Isoproterenol and adrenaline. Subsequently, her heart rate was maintained in between 70–90 beats/min. Provisional diagnosis was acute fulminant myocarditis with bronchial pneumonia.
Table 1. Laboratory investigations from day of admission to discharge.
| Blood Investigations | 0
DOA |
1
DOA |
2
DOA |
3
DOA |
4
DOA |
7
DOA |
12
DOA |
19
DOA |
27
DOA |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC (4–10×10^9/L) | 19.86 | ↑ | 20.02 | 17.16 | ↑ | 18.42 | ↑ | 15.61 | ↑ | 12.51 | ↑ | 10.52 | ↑ | 10.01 | ↑ | 8.43 | ||
| Neutrophils (50–75%) | 68.7 | 70.8 | 85.4 | ↑ | 80.47 | ↑ | 47.6 | ↓ | 55.8 | 69.3 | 57.6 | 57.8 | ||||||
| Lymphocytes (20–40%) | 23.9 | 22.4 | 10.4 | ↓ | 14.4 | ↓ | 45.9 | ↑ | 37 | 27 | 35.9 | 35 | ||||||
| Monocytes (3–8%) | 7.3 | 6.2 | 4.1 | 4.1 | 5.6 | 5 | 3.1 | 5.6 | 5 | |||||||||
| Eosinophils (0.5–5%) | 0 | ↓ | 0.5 | 0 | ↓ | 0 | ↓ | 0.6 | 1.6 | 0.4 | ↓ | 0.6 | 1.6 | |||||
| Basophils (0–1%) | 0.1 | 0.1 | 0.1 | 0.1 | 0.3 | 0.6 | 0.2 | 0.3 | 0.6 | |||||||||
| ANC (2–7.5×10^9/L) | 13.65 | ↑ | 14.1 | 14.67 | ↑ | 13.67 | ↑ | 7.42 | 3.89 | 7.83 | ↑ | 4.42 | 3.89 | |||||
| ALC (0.8–4×10^9/L) | 4.74 | ↑ | 2.22 | 1.78 | 1.88 | 7.16 | ↑ | 5.86 | ↑ | 1.68 | 3.16 | 2.86 | ||||||
| Hemoglobin (110–170 g/L) | 110 | ↓ | 107 | 95 | ↓ | 97 | ↓ | 108 | ↓ | 110 | ↓ | 116 | 122 | 129 | ||||
| Platelets count(100–
300×10^9/L) |
203 | 200 | 143 | 132 | 403 | ↑ | 257 | 311 | ↑ | 403 | ↑ | 257 | ||||||
| ESR (0–15 mm in 1 hr) | 1 | |||||||||||||||||
| Blood Glucose (3.89–6.11
mmol/L) |
11.2 | ↑ | 6.1 | 3.8 | ↓ | 4.6 | 4.2 | 7.2 | ↑ | 5.3 | ||||||||
| Potassium (K) (3.5–5.4
mmol/L) |
4.3 | ↓ | 5.18 | 4.46 | 3.5 | 3.52 | 4.13 | 4.4 | ||||||||||
| Sodium (Na) (135–148
mmol/L) |
131 | ↓ | 138 | 129 | ↓ | 133.8 | ↓ | 134.6 | ↓ | 141 | 142 | |||||||
| Calcium (Ca) (2.05–2.55
mmol/L) |
1.58 | ↓ | 1.6 | ↓ | 1.62 | ↓ | 2.27 | 2.23 | 2.38 | |||||||||
| Blood Urea (1.8–7.1
mmol/L) |
21.35 | ↑ | 21.45 | ↑ | 21.79 | ↑ | 6.88 | 4.98 | 5.29 | 4.1 | ||||||||
| creatinine (44–106 umol/L) | 158.5 | ↑ | 167 | ↑ | 211.6 | ↑ | 63.5 | 57.5 | 49.6 | 50 | ||||||||
| Uric Acid (129–417 umol/L) | 977 | ↑ | 980 | ↑ | 935 | ↑ | 274 | 253 | 169 | |||||||||
| ALT (8–40 U/L) | 8526 | ↑ | 6589 | ↑ | 1406 | ↑ | 552 | ↑ | 52 | ↑ | 27 | |||||||
| AST (5–40 U/L) | 4724 | ↑ | 3245 | ↑ | 2319 | ↑ | 309 | ↑ | 309 | ↑ | 31 | |||||||
| α HBDH ( 72–182 IU/L) | 3895 | ↑ | 2145 | ↑ | 1061 | ↑ | 631 | ↑ | 489 | ↑ | 192 | ↑ | ||||||
| ALP (40–150 IU/L) | 151 | ↑ | 144 | 140 | ↑ | 127 | 126 | 141 | ||||||||||
| γGGT (7–54 U/L) | 26 | 28 | 28 | 58 | ↑ | 53 | 48 | |||||||||||
| LDH (100–300 IU/L) | 10140 | ↑ | 9876 | ↑ | 2155 | ↑ | 746 | ↑ | 456 | ↑ | 223 | 145 | ||||||
| Total Protein (60–85 g/L) | 56.8 | ↓ | 60.4 | 63.6 | 66.8 | 72.8 | 72.7 | |||||||||||
| Albumin (35–55 g/L) | 33.5 | ↓ | 33 | ↓ | 32 | ↓ | 39 | 43 | 41.1 | |||||||||
| Globulin ( 20–35 g/L) | 23.3 | 26.9 | 31.6 | 34 | 34 | 31.6 | ||||||||||||
| Creatine Kinase (25–200
IU/L) |
1170 | ↑ | 1245 | ↑ | 1679 | ↑ | 109 | 89 | 39 | 35 | ||||||||
| CKMB (0–25 U/L) | 247 | ↑ | 187 | ↑ | 104 | ↑ | 48 | ↑ | 48 | ↑ | 10 | 13 | ||||||
| Troponin T (0–0.08 ng/ml) | 0.361 | ↑ | 0.024 | 0.018 | ||||||||||||||
| ASO Titre (0–166 IU/ml) | 7 | 24 | ||||||||||||||||
| CRP (0–10 mg/L) | 0.9 | 0.1 | ||||||||||||||||
| Atrial Blood Gas | ||||||||||||||||||
| PH (7.35 – 7.45) | 7.34 | ↓ | 7.39 | ANC: Absolute Neutrophil count
ALC: Absolute Lymphocytes cont |
||||||||||||||
| PaO2 (80–100 mmHg) | 82.1 | 88 | ||||||||||||||||
| PaCO2 (35–45 mmHg) | 23 | ↓ | 36 | |||||||||||||||
| HCO3 (22–26 mEq/L) | 13.3 | ↓ | 24.2 | |||||||||||||||
| Anion Gap (10–15 mEq/L) | 31 | ↑ | 15.2 | |||||||||||||||
| Stool Routine | Normal | |||||||||||||||||
| Urine routine | Normal | |||||||||||||||||
| Mycoplasma titer | Negative | |||||||||||||||||
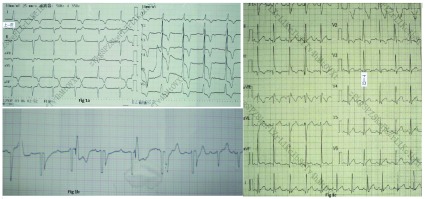
Figure 1.
( A) Electrocardiogram (ECG) at emergency showing- III°atrio-ventricular block; left anterior fascicular block, ST-T changes; ( B) ECG recording during transcutaneous pacing; ( C) ECG at the time of discharge, which is normal.
The patient was admitted to hospital after explaining the disease condition and prognosis to parents. She was on continuous oxygen, dopamine, diazepam, Immunoglobulin, ceftriaxone, IV fluids on a maintenance dose and nebulization with Ipratropium bromide (250mcg/ nebulization) along with a high dose of vitamin C (to reduces the risk of myocardial injury), Coenzyme Q 10 (for myocardial protection), fructose diphosphate (to improve cardiac metabolism) and mannitol (to reduce cerebral edema). However, she again had a cardiac arrest. In addition, ECG showed sinus P wave and no QRS with heart rate dropped from 70 to 20bpm. The patient was resuscitated with chest compression, atropine, and adrenaline. Isoproterenol was started at 1.5mcg/kg/min and increased up to 2mcg/kg/min. Subsequently, her heart rate was maintained at 60–70 bpm. Her heart rate again decreased to 30bpm when isoproterenol was discontinued. As there was no extracorporeal membrane oxygenation (ECMO) machine in our hospital and transfer was not possible, the patient was prepped for non-invasive transcutaneous cardiac pacing.
Cardiac pacing was adjusted to 16 mA and rate 90 bpm. The patient’s heart rate was controlled at 80–100 bpm. Her complexion gradually became reddish, cyanosis gradually improved but she had developed eyelid edema. She had passed urine about 130ml twice in 12 hours. Dopamine was increased to 7mcg/kg/min and she was started on furosemide.
At first 24 hours after cardiac pacing, the patient was conscious. She had passed urine 4 times about 700ml. But facial puffiness was still present. Her heart rate was maintained at the rate of 110–130bpm and SPO2 was 96% with oxygen. Chest pacing was reduced to 14 mA, and the frequency was changed to 70 bpm. After 48hours of pacing, the heart rate was improved to 100–110bpm with few ventricular premature beats. Then pacing was reduced to 12 mA, frequency changed to 60bpm. The pacing current and frequency were gradually slowed down and discontinued. Then, sinus rhythm was established with the heart rate of 100–110bpm with ECG monitoring. The heart rate fluctuated at 80–100bpm with frequent ventricular premature beats. Echocardiography showed left ventricular myocardial wall thickening and thickening of endocardium with left ventricular ejection fraction (LVEF 50%), suggestive of endocardial fibroelastosis (EF). Chest radiograph showed increased lung texture and enlarged cardiac shadow ( Figure 2). Captopril, hydrochlorothiazide, and spironolactone were started to reduce cardiac remodeling and to protect heart function. Furosemide was continued. Mannitol was stopped after the patient’s MRI scan revealed normal findings.
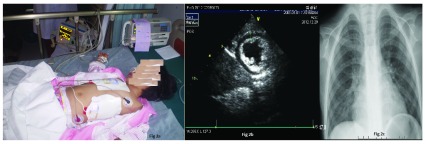
Figure 2.
( A) Patient on non-invasive transcutaneous pacing; ( B) Echocardiography after 48 hours of admission showing left ventricular myocardial wall thickening and thickening of endocardium; ( C) Chest X ray showing increased lung texture and enlarged cardiac shadow.
The patient’s HR was in between 80–100 bpm, with blood pressure was increasing gradually. Dopamine was tapered and stopped at 72 hours, after her BP reached 110/78 mmHg. The chest became gradually clear and her heart sounds were also normal. ECG monitoring also showed improvement with decreased numbers of premature beats and gradual change of S-T segments towards normal.
The patient was discharged on the 28th day after admission after her all routine investigations returned to normal ( Table 1). ECG showed sinus rhythm with heart rate 102 bpm. Echocardiography showed normal cardiac chambers, normal wall motion with EF 60%. Her final diagnosis was fulminant myocarditis with III° A-V block and bronchial pneumonia. On discharge, the patient was advised to continue captopril 6.25mg bid, metoprolol succinate 6.25mg bid, prednisone 1mg / kg orally for six months.
At six month follow up the patient’s echocardiography had returned to normal with LVEF 65%, and prednisone was reduced to 0.5mg/kg orally for 15 days and with a tapering dose for the next 15 days. After one year follow-up, she had no complaints and no significant abnormalities noted on echocardiography.
All doses of medications can be seen in Table 2.
Table 2. List of medications, including doses and duration, given to the patient during hospital admission.
| Doses of medicine and duration | ||
|---|---|---|
| Medicine | Doses | Route/duration |
| Atropine | 0.25mg | IV When reqired |
| Adrenaline | 0.2mg | IV When reqired |
| Isoproterenol | 0.2mcg | IV- bolus at ER |
| 0.15mcg/kg/min | IV in 50ml of 5% glucose | |
| 0.2mcg/kg/min | IV in 50ml of 5% glucose | |
| Dopamine | 3–5mcg/kg/min | IV in 50ml of 5% glucose |
| Diazepam | 0.5mg/kg | IV when reqired |
| Phenobarbital | 2mg/kg | IV when reqired |
| Mannitol | 42 ml | IV 6 hourly for 2 days from DOA |
| 42 ml | IV 8 hourly for next 2 days | |
| 42ml | IV 12 hourly for next 2 days then stop | |
| Fructose diphosphate | 3.4g /OD | IV for 10 days |
| Ceftriaxone | 100mg/kg/day | IV 12 hourly from DOA for 10 days |
| Piperacillin tazobactam | 1.125gm/day | IV 12 hourly from 3 DOA for 10 days |
| Immunoglobulin | 5 gm | IV daily for 5 days |
| Methylprednisolone | 1.5mg/kg/day | IV for 5 days |
| prednisone | 10 mg / OD | PO from 6 DOA and on discharge also |
| Captopril | 6.25mg / BID | PO from 3 DOA and on discharge also |
| Spironolactone | 10mg/OD | PO from 5 DOA and on discharge also |
| Furosemide | 10mg | IV 12hourly from 2nd DOA to 5DOA |
| Hydrochlorothiazide | 10mg | PO 12 hourly from 5 DOA till discharge |
| Coenzyme Q10 | 5 mg | PO 8hourly from 2nd DOA till discharge |
| Vitamin C | 3 gm | IV 12 hourly from 2nd DOA till discharge |
Discussion
In children, sometimes myocarditis is self-limiting. However, if it progresses there is the risk of acute cardiac failure, hemodynamic disturbances, and arrhythmias leading to significant morbidity and mortality. Mortality due to myocarditis for infants is more than 75%, whereas for children it is more than 25% 1, 5– 9. There is no any specific clinical course and investigations to diagnose fulminant myocarditis. Initially, they present with flu-like symptoms and later develop sudden onset of cardiac symptoms that rapidly deteriorate 2. Neonates may present with fever, poor feeding, and listlessness and sometimes with danger signs like apnea, episodic cyanosis, and diaphoresis. Older children present with respiratory or gastrointestinal symptoms. Among them only a few present with chest pain 10. Diagnosis is mainly done on the basis of: Clinical presentation, blood profile, including CBC, electrolytes, creatinine kinase, creatine kinase MB isoenzyme, C-reactive protein, Troponin T, Troponin I, antistreptolysin O titer, polymerase chain reaction to detect viral antigens, autoantibodies marker, liver enzymes, ECG, Echocardiography, ultrasonography and even Cardiac MRI 3 which are mostly supportive. If echocardiography shows low LVEF in children with fulminant myocarditis, the prognosis is poor 11. Mortality in fulminant myocarditis is mainly due to cardiac arrhythmias among which structural changes, parameters of ventricular dynamics and vascular changes are responsible for the increased incidence 12. Acute fulminant myocarditis, if properly and aggressively treated, has excellent long-term survival even if the patient may present with severe hemodynamic compromise 13. Complete heart block on initial ECG may also have an excellent prognosis, although mechanical assistance may be warranted as shown in the study by Lee E Y et al. 1. This can be managed with percutaneous cardiopulmonary support, ECMO, intra-aortic balloon pumping, or the ventricular assisted device 14, 15. ECMO remains an effective approach in children for the management of acute fulminant myocarditis 16. In addition, intravenous immunoglobulin and high dose steroids help to reduce inflammation 17. Patients managed with immunoglobulin, steroids or mechanical support for fulminant myocarditis may have higher survival rate compared to those not receiving these therapies 14.
In the present case, we tried to manage initially with Isoproterenol but were unsuccessful. So we applied the non-invasive transcutaneous pacemaker to revert the A-V block. Pads or electrodes detachment, patient non-cooperation and skin-burn due to high voltage electric current are its limitations. In contrast, intraventricular cardiac pacing is time-consuming; much more risky and surgical site wound infection is common. Beland et al, Kelly et al in their articles note that non-invasive transcutaneous pacemaker is the safe, effective and suitable equipment for children 18, 19.
Conclusion
Acute fulminant myocarditis is a grievous condition with high morbidity and mortality. No delay should be had on starting immunoglobulin and steroids if suspected. If there is the arrhythmia, the patient should be immediately started on ECMO, percutaneous cardiopulmonary support or ventricular assisted device. If these are not available, then non-invasive transcutaneous cardiac pacing must be started, which is a safe, convenient and cost-effective device to revert arrhythmias caused by myocarditis.
Consent
We have taken written informed consent from the child`s legal guardian (her father) to use and publish his child`s medical case history and any accompanying images.
Data availability
All data underlying the results are available as part of the article and no additional source data are required.
Acknowledgements
We wish to thank Dr. Wu Bing, (Resident Cardiology Department II, Renmin Hospital), entire doctor and nursing staffs Department of Pediatrics I, Renmin Hospital and to the child parents for the consent.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 2; referees: 2 approved]
References
- 1. Lee EY, Lee HL, Kim HT, et al. : Clinical features and short-term outcomes of pediatric acute fulminant myocarditis in a single center. Korean J Pediatr. 2014;57(11):489–495. 10.3345/kjp.2014.57.11.489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lobo ML, Taguchi Â, Gaspar HA, et al. : Fulminant myocarditis associated with the H1N1 influenza virus: case report and literature review. Rev Bras Ter Intensiva. 2014;26(3):321–326. 10.5935/0103-507X.20140046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vashist S, Singh GK: Acute myocarditis in children: current concepts and management. Curr Treat Options Cardiovasc Med. 2009;11(5):383–91. 10.1007/s11936-009-0039-z [DOI] [PubMed] [Google Scholar]
- 4. Ichikawa R, Sumitomo N, Komori A, et al. : The follow-up evaluation of electrocardiogram and arrhythmias in children with fulminant myocarditis. Circ J. 2011;75(4):932–8. 10.1253/circj.CJ-10-0918 [DOI] [PubMed] [Google Scholar]
- 5. Forcada P, Beigelman R, Milei J: Inapparent myocarditis and sudden death in pediatrics. Diagnosis by immunohistochemical staining. Int J Cardiol. 1996;56(1):93–7. 10.1016/S0167-5273(96)02752-0 [DOI] [PubMed] [Google Scholar]
- 6. Maron BJ, Gohman TE, Aeppli D: Prevalence of sudden cardiac death during competitive sports activities in Minnesota high school athletes. J Am Coll Cardiol. 1998;32(7):1881–4. 10.1016/S0735-1097(98)00491-4 [DOI] [PubMed] [Google Scholar]
- 7. Neuspiel DR, Kuller LH: Sudden and unexpected natural death in childhood and adolescence. JAMA. 1985;254(10):1321–5. 10.1001/jama.1985.03360100071016 [DOI] [PubMed] [Google Scholar]
- 8. Topaz O, Edwards JE: Pathologic features of sudden death in children, adolescents, and young adults. Chest. 1985;87(4):476–82. 10.1378/chest.87.4.476 [DOI] [PubMed] [Google Scholar]
- 9. Niimura I, Maki T: Sudden cardiac death in childhood. Jpn Circ J. 1989;53(12):1571–80. 10.1253/jcj.53.1571 [DOI] [PubMed] [Google Scholar]
- 10. Levine MC, Klugman D, Teach SJ: Update on myocarditis in children. Curr Opin Pediatr. 2010;22(3):278–83. 10.1097/MOP.0b013e32833924d2 [DOI] [PubMed] [Google Scholar]
- 11. Pei L, Yang N, Yang YH, et al. : Clinical features and prognostic factors in children with fulminant myocarditis. Zhongguo Dang Dai Er Ke Za Zhi. 2015;17(11):1232–6. [PubMed] [Google Scholar]
- 12. Klein RM, Vester EG, Brehm MU, et al. : [Inflammation of the myocardium as an arrhythmia trigger]. Z Kardiol. 2000;89 Suppl 3:24–35. [PubMed] [Google Scholar]
- 13. McCarthy RE, 3rd, Boehmer JP, Hruban RH, et al. : Long-term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. N Engl J Med. 2000;342(10):690–5. 10.1056/NEJM200003093421003 [DOI] [PubMed] [Google Scholar]
- 14. Saji T, Matsuura H, Hasegawa K, et al. : Comparison of the Clinical Presentation, Treatment, and Outcome of Fulminant and Acute Myocarditis in Children. Circ J. 2012;76(5):1222–1228. 10.1253/circj.CJ-11-1032 [DOI] [PubMed] [Google Scholar]
- 15. Taoka M, Shiono M, Hata M, et al. : Child with fulminant myocarditis survived by ECMO Support--report of a child case. Ann Thorac Cardiovasc Surg. 2007;13(1):60–4. [PubMed] [Google Scholar]
- 16. Ning B, Zhang C, Lin R, et al. : Local experience with extracorporeal membrane oxygenation in children with acute fulminant myocarditis. PLoS One. 2013;8(12):e82258. 10.1371/journal.pone.0082258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tucker CE, Fernandez MJ, Morrison CC: Successful Treatment of Fulminant Myocarditis. J Clin Case Rep. 2013;3:256 10.4172/2165-7920.1000256 [DOI] [Google Scholar]
- 18. Béland MJ, Hesslein PS, Finlay CD, et al. : Noninvasive transcutaneous cardiac pacing in children. Pacing Clin Electrophysiol. 1987;10(6):1262–1270. 10.1111/j.1540-8159.1987.tb04962.x [DOI] [PubMed] [Google Scholar]
- 19. Kelly JS, Royster RL, Angert KC, et al. : Efficacy of noninvasive transcutaneous cardiac pacing patients undergoing cardiac surgery. Anesthesiology. 1989;70(5):747–51. 10.1097/00000542-198905000-00006 [DOI] [PubMed] [Google Scholar]