Abstract
AIM
To assess the performance of BALAD, BALAD-2 and their component biomarkers in predicting outcome of hepatocellular carcinoma (HCC) patients after liver transplant.
METHODS
BALAD score and BALAD-2 class are derived from bilirubin, albumin, alpha-fetoprotein (AFP), Lens culinaris agglutinin-reactive AFP (AFP-L3), and des-gamma-carboxyprothrombin (DCP). Pre-transplant AFP, AFP-L3 and DCP were measured in 113 patients transplanted for HCC from 2000 to 2008. Hazard ratios (HR) for recurrence and death were calculated. Univariate and multivariate regression analyses were conducted. C-statistics were used to compare biomarker-based to predictive models.
RESULTS
During a median follow-up of 12.2 years, 38 patients recurred and 87 died. The HRs for recurrence in patients with elevated AFP, AFP-L3, and DCP defined by BALAD cut-off values were 2.42 (1.18-5.00), 1.86 (0.98-3.52), and 2.83 (1.42-5.61), respectively. For BALAD, the HRs for recurrence and death per unit increased score were 1.48 (1.15-1.91) and 1.59 (1.28-1.97). For BALAD-2, the HRs for recurrence and death per unit increased class were 1.45 (1.06-1.98) and 1.38 (1.09-1.76). For recurrence prediction, the combination of three biomarkers had the highest c-statistic of 0.66 vs. 0.64, 0.61, 0.53, and 0.53 for BALAD, BALAD-2, Milan, and UCSF, respectively. Similarly, for death prediction, the combination of three biomarkers had the highest c-statistic of 0.66 vs 0.65, 0.61, 0.52, and 0.50 for BALAD, BALAD-2, Milan, and UCSF. A new model combining biomarkers with tumor size at the time of transplant (S-LAD) demonstrated the highest predictive capability with c-statistics of 0.71 and 0.69 for recurrence and death.
CONCLUSION
BALAD and BALAD-2 are valid in transplant HCC patients, but less predictive than the three biomarkers in combination or the three biomarkers in combination with maximal tumor diameter (S-LAD).
Keywords: Alpha-fetoprotein, AFP-L3, Des-gamma-carboxyprothrombin, BALAD, BALAD-2, Hepatocellular carcinoma, Liver transplant, Recurrence, Outcome
Core tip: BALAD score and BALAD-2 class incorporating alpha-fetoprotein (AFP), AFP-L3, and des-gamma-carboxyprothrombin are used to predict survival of patients with hepatocellular carcinoma. However, there were limited numbers of patients who received liver transplant in previous cohorts in which performance of the BALAD was studied. Our study showed that pre-transplant BALAD score and BALAD-2 class are useful for predicting outcome of hepatocellular carcinoma patients receiving liver transplant. However, a more predictive model uses the combination of all three biomarkers using the cut-offs from the BALAD score along with maximum tumor size at the time of transplant.
INTRODUCTION
The incidence of hepatocellular carcinoma (HCC) in the United States has increased 3-fold in the last 30 years[1]. Currently, liver cancer has also become the second leading cause of cancer-related deaths worldwide[2]. Liver transplant is one of the few curative treatments that can achieve a 5-year survival rate of 70% for some HCC patients. However, to be eligible for a liver transplant, patients with HCC have to meet a rigorous set of criteria. Despite these selection criteria, recurrence of cancer is seen in up to 20% of HCC patients that undergo liver transplantation[3]. This high proportion of recurrences calls into question the liver transplant guidelines used for patients with cancer. For patients with HCC, the Milan and UCSF criteria have been used as standards to determine the eligibility for liver transplant[4,5]. Although adherence to the Milan criteria has been associated with relatively lower recurrence rates after transplantation, it is still considered suboptimal because it relies primarily on tumor morphologic characteristics[6]. Other liver transplant guidelines have been proposed, but similar to the Milan and UCSF criteria, they fail to incorporate the biological behavior of the tumor[6,7].
To achieve more objective models for selection of HCC patients for liver transplant, several serum tumor biomarkers have been evaluated to assess the biological aggressiveness of HCC. Multiple studies suggest that high pre-transplant alpha fetoprotein (AFP), a widely known HCC biomarker, is associated with poor post-transplant outcomes[8] and the AFP model, combining alpha-fetoprotein (AFP) with the tumor number and tumor size, has been proposed and validated to predict HCC recurrence[9]. The BALAD score, a model that incorporates the use of 5 serum biomarkers, has been successful in predicting the survival and recurrence of patients with HCC[10]. In addition to assessing the remnant liver function via the Bilirubin and Albumin levels, the BALAD score incorporates 3 additional serum tumor biomarkers, namely AFP, Lens culinaris agglutinin-reactive AFP (AFP-L3), and des-gamma-carboxyprothrombin (DCP). However, previous studies, including a validation study, have only included a limited number of liver transplant patients[11-14].
The aim of this study was to assess the performance of the discontinuous BALAD and continuous BALAD-2 scores in patients who underwent liver transplant for HCC. In addition, we aimed to assess the utility of each component of the BALAD in predicting outcomes and to develop a more effective model for liver transplant patients.
MATERIALS AND METHODS
Study population and data abstraction
There were 299 patients with HCC who underwent liver transplant between January 2000 and December 2008. Of the 299 patients, 113 had available results of all five biomarkers within two days before the liver transplant. The HCC diagnosis criteria included (1) explanted liver pathology; or (2) a new liver mass with largest diameter of > 1 cm, arterial enhancement and portal venous washout on computed tomography or magnetic resonance imaging. Patients with warfarin use and congenital biliary disorder which could alter the bilirubin level, such as Gilbert disease, were excluded. The transplant selection criteria for the HCC patients during the study period were primarily based on the Milan criteria. Staging within the extended UCSF criteria was accepted in 17 patients based on provider selection and organ availability at the time of transplant. Most patients with intermediate stage disease beyond Milan criteria received locoregional treatment with transarterial chemoembolization prior to liver transplantation. For surveillance for post-transplant HCC recurrence, patients underwent CT scan of the abdomen and chest along with serum AFP at 4, 8, 12, 18, and 24 mo post-transplant.
Patient age, sex, race, etiology of liver disease, date of HCC diagnosis, date of liver transplant, baseline tumor characteristics at the time of diagnosis, and at the time of imaging closest to the transplant (diameter of the largest tumor, tumor number, macrovascular invasion), biomarker results, recurrence date, death date and last follow-up date were abstracted. The Child-Turcotte-Pugh (CTP) class and MELD score were calculated at the time closest to liver transplant in every patient regardless of cirrhosis status. Tumor size and tumor number were also determined from the most recent imaging study prior to the transplant. The Milan and UCSF criteria were also determined from the imaging prior to and closest to the transplant date. HCC recurrence was defined by the presence of new malignant masses seen on imaging, either intrahepatic or extrahepatic metastases, as assessed by the radiologist. The tumor response to treatment was assessed according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST), version 1.0. The survival of patients who were lost to follow-up was obtained using the Accurint system.
BALAD score and BALAD-2 class were calculated based on five biomarkers including total bilirubin, albumin, AFP, AFP-L3, and DCP measured within the two days prior to transplant (Tables 1 and 2). The GALAD and GALAD-z scores were also calculated based on gender, age, and biomarkers within the same period (Table 3).
Table 1.
BALAD score calculation
| 0 point | 1 point | 2 points | 3 points | |
| Bilirubin (mg/dL) | < 1.0 | 1.0-2.0 | > 2.0 | |
| Albumin (g/dL) | > 3.5 | 2.8-3.5 | < 2.8 | |
| Summation of these 2 points, then classified as A (0-1), B (2-3), C (4) | ||||
| Albumin-Bilirubin | A | B | C | - |
| No. of elevated markers1 | 0 | 1 | 2 | 3 |
| Summation of these 2 points for BALAD score (0-5) | ||||
Defined by AFP > 400 ng/mL, AFP-L3 > 15%, and DCP > 100 ng/mL.
Table 2.
BALAD-2 class calculation
| Linear predictor = 0.02 × (AFP - 2.57) + 0.012 × [(AFP-L3) - 14.19] + 0.19 × [ln(DCP) - 1.93] + 0.17 × [(bilirubin)1/2- 4.50] - 0.09 × (albumin - 35.11) |
| AFP capped at 50000 units. AFP and DCP modeled as /1000 units. |
| Units: Bilirubin (μmol/L), albumin (g/L), AFP and DCP (ng/mL), AFP-L3 (%). |
| class 1 (≤ -1.74), class 2 (> -1.74 to -0.91), class 3 (> -0.91 to 0.24), class 4 (> 0.24) |
Table 3.
GALAD-z and GALAD score calculation
| GALAD-z = -10.08 + 0.09 × (Age) + 1.67 × (sex) + 2.34 × log(AFP) + 0.04 × (AFP-L3) + 1.33 × log(DCP) |
| GALAD score = exp (GALAD-z)/[1 + exp(GALAD-z)] |
Sex = 1 for male and 0 for female.
Measurement of biomarkers
Serum samples were collected and stored at -80 °C. AFP, AFP-L3, and DCP were measured simultaneously using a liquid-phase binding assay on the µTASWako i30 instrument (Wako Life Sciences Inc., Mountain View, CA, United States). Details of the sample processing and biomarker results were previously published[15].
Statistical analysis
Baseline characteristics were reported as mean ± standard deviation (SD) or median and interquartile range for continuous variables, and percentage for categorical variables. Hazard ratios (HRs) for time to recurrence and death were calculated for each variable and each BALAD score and BALAD-2 class grouping. HRs were presented as HR (95%CI, P value). C statistics were used to compare different scores. All analyses were performed using SAS 9 (SAS Institute, Cary, NC, United States). P < 0.05 was considered as statistically significant.
RESULTS
Demographic characteristics
Of the 113 included patients, the majority were male (n = 86, 76%), with viral hepatitis C as the most common liver disease etiology (n = 66, 58%) as shown in Table 4. There were 104 (92%) patients with cirrhosis of whom 13 (12%), 76 (67%), and 24 (21%) patients had CTP class A, B, and C cirrhosis, respectively. There were 1 (1%), 39 (35%), 7 (6%), 40 (35%), and 26 (23%) patients with BCLC stage 0, A, B, C, and D HCC, respectively. There were no patients with portal or nodal invasion. BCLC stages C and D were assigned because of poor ECOG performance status and/or CTP class C cirrhosis. The median (range) of total bilirubin and albumin at the time of transplant were 2.3 (0.2-29.5) mg/dL and 3.2 (2.1-5.2) g/dL. For the tumor biomarkers, the median (range) of AFP, AFP-L3, and DCP were 25.3 (0.8-27800) ng/dL, 12 (1-86.5)%, and 1.2 (0.2-1480) ng/mL, respectively. The median waiting time for the included patients was 2.8 (range 0-20) mo.
Table 4.
Baseline characteristics of 113 hepatocellular carcinoma patients who underwent liver transplant with available biomarker results n (%)
| Variables | Value |
| Age, yr, mean ± SD | 58.2 ± 8.3 |
| Male sex | 86 (76) |
| Race | |
| White | 91 (80) |
| Asian | 11 (10) |
| Others | 7 (6) |
| Unknown | 4 (4) |
| Etiology | |
| Hepatitis virus C | 66 (58) |
| Hepatitis virus B | 11 (10) |
| Alcohol | 14 (12) |
| Non-alcoholic fatty liver disease or cryptogenic | 14 (12) |
| Others | 8 (7) |
| Cirrhosis | 104 (92) |
| CTP class | |
| A | 13 (12) |
| B | 76 (67) |
| C | 24 (21) |
| MELD score, median (range) | 14.2 (6.4–38.6) |
| ECOG status | |
| 0 | 57 (50) |
| 1 | 34 (30) |
| 2 | 19 (17) |
| 3 | 3 (3) |
| Diameter of the largest tumor at the time of transplant by imaging, cm, mean ± SD | 2.7 ± 1.6 |
| Tumor number at the time of transplant | |
| 1 | 73 (64.6) |
| 2 | 26 (23.0) |
| 3 | 7 (6.2) |
| ≥ 4 | 7 (6.2) |
| BCLC staging | |
| Stage 0 | 1 (1) |
| Stage A | 39 (35) |
| Stage B | 7 (6) |
| Stage C | 40 (35) |
| Stage D | 26 (23) |
| Within Milan criteria at diagnosis | 87 (77) |
| Within UCSF criteria at diagnosis | 96 (85) |
| Within Milan criteria at transplant | 88 (78) |
| Within UCSF criteria at transplant | 105 (93) |
| AFP model score > 2 | 26 (23) |
| Total bilirubin, mg/dL, median (range) | 2.3 (0.2-29.5) |
| Albumin, g/dL, median (range) | 3.2 (2.1-5.2) |
| AFP, ng/mL, median (range) | 25.3 (0.8-27800) |
| AFP > 400 ng/mL | 18 (16) |
| AFP-L3, %, median (range) | 12 (1-86.5) |
| AFP-L3 > 15% | 45 (40) |
| DCP, ng/mL, median (range) | 1.2 (0.2-1480) |
| DCP > 1.2 ng/mL | 56 (50) |
AFP: Alpha-fetoprotein; AFP-L3: Lens culinaris agglutinin-reactive alpha-fetoprotein; CTP: Child-Turcotte-Pugh; DCP: Des-gamma-carboxyprothrombin.
Of the 113 included patients, 87 (77%) and 96 (85%) were within Milan and UCSF criteria at the time of diagnosis; and 88 (78%) and 105 (93%) were within Milan and UCSF criteria at the time of transplant, respectively. The AFP level was not included in the transplant selection criteria during the study period. Of the 113 patients, 111 patients received TACE, 1 received RFA and 1 received both TACE and RFA prior to liver transplant. Thirty-nine patients (35%) had available imaging for evaluating the locoregional therapy response. Sixty-nine patients had baseline imaging at the time of HCC diagnosis but did not have follow-up imaging after locoregional therapy as most of these patients underwent transplantation shortly after TACE. Another 5 patients had radiology reports in the medical record but did not have the images available for review as the imaging was performed outside Mayo Clinic. Of the 39 patients with imaging available for assessing the treatment response, 29 (74%) were responders (13 complete response and 16 partial response) and 10 (26%) were non-responders (8 stable disease and 2 progressive disease) according to the mRECIST criteria.
According to the explant pathology reports, there were 19, 53, 16, and 2 patients with well-, moderately-, poorly-, and undifferentiated tumors, respectively. There were 23 patients with no report of tumor differentiation. The correlations of the number of elevated tumor biomarkers according to the BALAD score cut-off with the BALAD score are shown in Supplementary Figure 1. There was no correlation between number of elevated tumor biomarkers (P = 0.34), or BALAD score (P = 0.28) with tumor differentiation.
Figure 1.
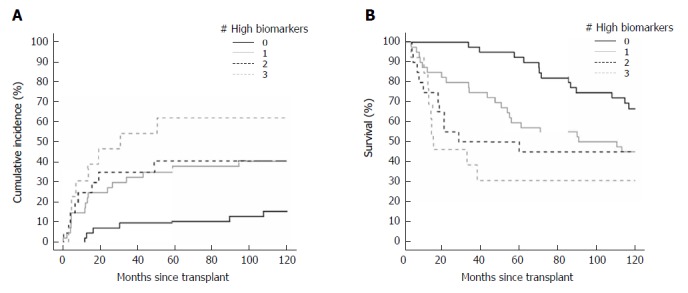
Cumulative incidence of recurrence curve (A) and Kaplan-Meyer survival curve (B) by number of elevated tumor biomarkers.
Factors associated with HCC recurrence and death after liver transplant
During a median follow-up of 12.2 years, 38 patients had recurrence and 87 died. The median survival was 10.2 years. The 3-year and 5-year survivals were 74.3% (95%CI: 66.7%-82.8%) and 66.3% (95%CI: 58.1%-75.6%).
By Cox proportional hazard ratio, the diameter of the largest tumor at the time of transplant was associated with both transplant outcomes with HRs per centimeter of 1.27 (1.04-1.56, P = 0.02) for recurrence and 1.21 (1.03-1.41, P = 0.02) for death. A neutrophil-lymphocyte ratio of more than 4 also correlated with outcomes, with HRs of 2.24 (1.17-4.26, P = 0.04) for recurrence, and 1.66 (1.004-2.73, P = 0.048) for death. We did not find any significant increases in risk of recurrence or death for either tumor number or hypothyroidism (Table 5).
Table 5.
Univariate models for recurrence and death outcome
| Variable |
Hazard ratio for recurrence |
Hazard ratio for death |
||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| MELD score (per point) | 1.03 (0.98-1.09) | 0.26 | 1.05 (1.003-1.09) | 0.04a |
| Diameter of the largest tumor at time of transplant (per cm) | 1.27 (1.04-1.56) | 0.02a | 1.21 (1.03-1.41) | 0.02 |
| Tumor number at time of transplant | 1.001 (0.73-1.37) | 1.00 | 0.93 (0.72-1.20) | 0.57 |
| Neutrophil lymphocyte ratio > 4 | 2.24 (1.17-4.26) | 0.02a | 1.66 (1.004-2.73) | 0.048a |
| Hypothyroidism | 1.26 (0.55-2.85) | 0.59 | 1.54 (0.82-2.90) | 0.18 |
| BALAD components | ||||
| Albumin (per g/dL) | 0.75 (0.41-1.38) | 0.36 | 0.69 (0.43-1.13) | 0.14 |
| Bilirubin (per mg/dL) | 1.03 (0.98-1.09) | 0.21 | 1.04 (0.995-1.08) | 0.08 |
| AFP: > 400 ng/mL | 2.42 (1.18-5.00) | 0.02a | 3.27 (1.84-5.80) | < 0.001b |
| AFP-L3 > 15% | 1.86 (0.98-3.52) | 0.056 | 1.88 (1.14-3.09) | 0.01a |
| DCP > 1.2 ng/mL | 2.83 (1.42-5.61) | 0.003b | 2.40 (1.43-4.04) | < 0001b |
| BALAD Score | ||||
| 0 | Reference | Reference | ||
| 1 | 0.70 (0.20-2.47) | 0.58 | 1.14 (0.40-3.23) | 0.81 |
| 2 | 1.18 (0.37-3.75) | 0.78 | 2.01 (0.75-5.38) | 0.17 |
| 3 | 1.99 (0.62-6.36) | 0.24 | 2.73 (0.99-7.51) | 0.052 |
| 4 | 2.97 (0.84-10.58) | 0.09 | 4.68 (1.52-14.36) | 0.007b |
| 5 | 5.02 (0.92-27.54) | 0.06 | 17.40 (3.81-79.47) | < 0.001b |
| BALAD Score (per increase of 1) | 1.48 (1.15-1.91) | 0.002b | 1.59 (1.28-1.97) | < 0.001b |
| BALAD-2 Score | ||||
| 1 | Reference | Reference | ||
| 2 | 0.41 (0.12-1.32) | 0.13 | 1.07 (0.50-2.28) | 0.86 |
| 3 | 1.53 (0.66-3.54) | 0.32 | 1.76 (0.87-3.54) | 0.11 |
| 4 | 2.17 (0.90-5.25) | 0.09 | 2.45 (1.16-5.17) | 0.02a |
| BALAD-2 Score (per increase of 1) | 1.45 (1.06-1.98) | 0.02a | 1.38 (1.09-1.76) | 0.008b |
| Within Milan criteria at diagnosis | 1.69 (0.84-3.41) | 0.14 | 2.17 (1.25-3.78) | 0.006b |
| Within UCSF criteria at diagnosis | 1.85 (0.85-4.05) | 0.12 | 3.19 (1.75-5.84) | < 0.001b |
| Within Milan criteria at transplant | 1.24 (0.59-2.62) | 0.57 | 1.06 (0.57-1.95) | 0.86 |
| Within UCSF criteria at transplant | 0.33 (0.05-2.43) | 0.28 | 0.68 (0.21-2.17) | 0.51 |
| z-GALAD | 1.12 (1.03-1.21) | 0.006b | 1.12 (1.06-1.19) | < 0.001b |
| GALAD score | 3.01 (1.14-7.91) | 0.03a | 3.22 (1.48-7.00) | 0.003b |
| AFP model cutoff > 2 (explant) | 2.82 (1.47-5.41) | 0.002b | 2.83 (1.67-4.82) | < 0.001b |
| AFP model (per increase of 1, explant) | 1.42 (1.20-1.68) | < 0.001b | 1.34 (1.16-1.54) | < 0.001b |
P < 0.05,
P < 0.01, statistical difference. AFP: Alpha-fetoprotein; AFP-L3: Lens culinaris agglutinin-reactive alpha-fetoprotein; DCP: Des-gamma-carboxyprothrombin.
Levels of all three tumor biomarkers that exceeded the BALAD score cut-off were associated with increased recurrence and death outcomes in the transplant cohort, whereas albumin and bilirubin, the other components of the BALAD score, were not associated with either outcome. The HRs for recurrence of elevated AFP, AFP-L3, and DCP according to the BALAD score cut-off were 2.42 (1.18-5.00, P = 0.02), 1.86 (0.98-3.52, P = 0.056), and 2.83 (1.42-5.61, P = 0.003), respectively. Similarly, the HRs for death were 3.27 (1.84-5.80, P < 0.001), 1.88 (1.14-3.09, P = 0.01), and 2.40 (1.43-4.04, P < 0.001), respectively. The cumulative incidence of recurrence curve and Kaplan-Meyer survival curve by number of elevated biomarkers are shown in Figure 1A and B, respectively.
BALAD score and BALAD-2 class and risk of HCC recurrence and death
When classified by the BALAD score, there were 14, 31, 33, 23, 9, and 3 patients with BALAD scores of 0 to 5, respectively. By BALAD-2 class there were 29, 30, 34, and 20 patients in BALAD-2 classes 1 to 4, respectively.
For BALAD scores of 1, 2, 3, 4, and 5 vs 0, the HRs for recurrence were 0.70 (0.20-2.47), 1.18 (0.37-3.75), 1.99 (0.62-6.36), 2.97 (0.84-10.58), and 5.02 (0.92-27.54); and HRs for death were 1.14 (0.40-3.23), 2.01 (0.75-5.38), 2.73 (0.99-7.51), 4.68 (1.52-14.36), and 17.40 (3.81-79.47), respectively (Figure 2A and B). The HRs per each unit increase in BALAD score for recurrence and death were 1.48 (1.15-1.91) and 1.59 (1.28-1.97). For BALAD-2 classes 2, 3, and 4 vs 1, the HRs for recurrence were 0.41 (0.12-1.32), 1.53 (0.66-3.54), and 2.17 (0.90-5.25); and HRs for death were 1.07 (0.50-2.28), 1.76 (0.87-3.54), and 2.45 (1.16-5.17) (Figure 3A and B). The HRs per each unit increase in BALAD-2 class for recurrence and death were 1.45 (1.06-1.98) and 1.38 (1.09-1.76), respectively. A multivariate model of diameter of the largest tumor with BALAD and BALAD-2 was created (Tables 6 and 7). The risk of recurrence was 1.53 (1.17-2.01) per increase of 1 in the BALAD score and 1.42 (1.05-2.03) per increase of one BALAD-2 class. The risk of death was 1.57 (1.27-1.96) per increase of 1 in the BALAD score and 1.37 (1.07-1.76) per increase of 1 BALAD-2 class.
Figure 2.
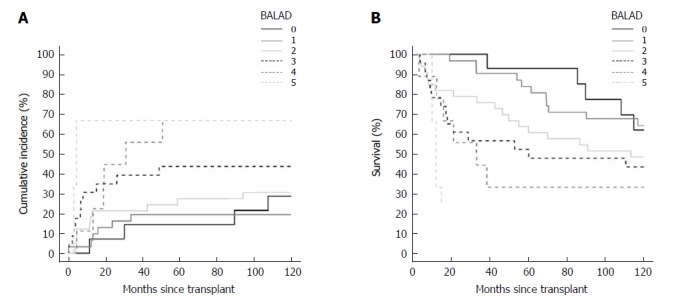
Cumulative incidence of recurrence curve (A) and Kaplan-Meyer survival curve (B) by BALAD score.
Figure 3.
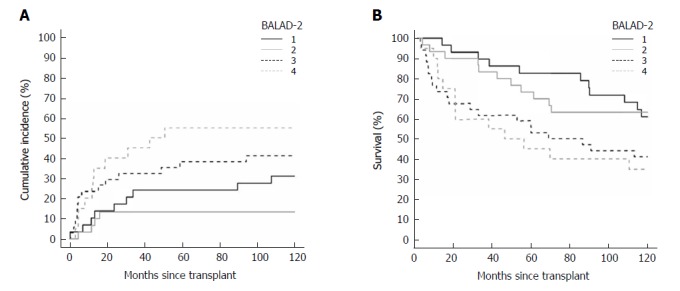
Cumulative incidence of recurrence curve (A) and Kaplan-Meyer survival curve (B) by BALAD-2 class.
Table 6.
Multivariate model for recurrence outcome with BALAD and BALAD-2
| Variable |
Hazard ratio with BALAD |
Hazard ratio with BALAD-2 |
||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Diameter of the largest tumor at time of transplant (per cm) | 1.33 (1.07-1.66) | 0.02b | 1.30 (1.05-1.59) | 0.014a |
| Neutrophil-lymphocyte ratio | 1.55 (0.78-3.14) | 0.21 | 1.76 (0.90-3.49) | 0.10 |
| BALAD (per increase of 1) | 1.53 (1.17-2.01) | 0.002b | - | - |
| BALAD-2 (per increase of 1) | - | - | 1.45 (1.05-2.03) | 0.02a |
P < 0.05,
P < 0.01, statistical difference.
Table 7.
Multivariate model for death outcome with BALAD and BALAD-2
| Variable | Hazard ratio with BALAD | Hazard ratio with BALAD-2 | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Diameter of the largest tumor at time of transplant (per cm) | 1.24 (1.04-1.48) | 0.016a | 1.20 (1.02-1.42) | 0.03a |
| Neutrophil-lymphocyte ratio | 1.13 (0.67-1.92) | 0.64 | 1.31 (0.78-2.19) | 0.31 |
| BALAD (per increase of 1) | 1.57 (1.27-1.96) | < 0.0001 | - | - |
| BALAD-2 (per increase of 1) | - | - | 1.37 (1.07-1.76) | 0.013a |
P < 0.05, bP < 0.01, statistical difference.
In addition, the HRs for early recurrence were also calculated. Early recurrence was defined as recurrence occurring within 36 mo after transplant. Of the 38 patients with any recurrence, 31 had early recurrence. The BALAD score had better performance for early than overall recurrence with a HR of 1.66 (1.24-2.22) per each unit increase of BALAD score, whereas the BALAD-2 class had similar performance for both recurrence outcomes with a HR of 1.46 (1.04-2.07) per increase of 1 class (Supplementary Table 1).
Multivariate model of elevated tumor biomarkers combination with tumor size
Based on the results of the univariate analysis, we combined the elevated tumor biomarkers including AFP, AFP-L3, and DCP with diameter of the largest tumor per centimeter increase in diameter (Table 8). In this multivariate model, diameter of the largest tumor and elevated DCP remained significantly associated with recurrence and death, whereas elevated AFP was only associated with death but not with recurrence. AFP-L3 did not relate to either recurrence or death in this multivariate model. The c-statistics for the combined models were 0.71 (0.62-0.81) and 0.69 (0.61-0.77) for recurrence and death, respectively.
Table 8.
Multivariate model of biomarkers and tumor size at time of transplant (S-LAD)
| Variable |
Hazard ratio for recurrence |
Hazard ratio for death |
||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Diameter of the largest tumor at time of transplant (per cm) | 1.30 (1.05-1.61) | 0.02a | 1.29 (1.08-1.55) | 0.006b |
| AFP: > 400 ng/mL | 1.63 (0.70-3.83) | 0.26 | 2.40 (1.19-4.83) | 0.02a |
| AFP-L3 > 15% | 0.995 (0.46-2.18) | 0.99 | 1.01 (0.54-1.88) | 0.98 |
| DCP > 1.2 ng/mL | 2.69 (1.28-5.64) | 0.009b | 2.33 (1.31-4.13) | 0.004b |
| c-statistic (95%CI) | 0.71 (0.62-0.81) | 0.69 (0.61-0.77) | ||
P < 0.05,
P < 0.01, statistical difference. AFP: Alpha-fetoprotein; AFP-L3: Lens culinaris agglutinin-reactive alpha-fetoprotein; DCP: Des-gamma-carboxyprothrombin.
Comparisons to the currently used models
The c-statistic was used to compare models which predict outcome of liver transplant patients. A combination of elevated tumor biomarkers based on the BALAD score cut-offs demonstrated the highest c-statistic for prediction of both recurrence and death, with values of 0.66 (0.57-0.75) and 0.66 (0.59-0.73), respectively. For the outcome of recurrence, BALAD and BALAD-2 (per increase of 1 score/class) showed c-statistics of 0.64 (0.55-0.73) and 0.61 (0.52-0.70), respectively. For the outcome of death, BALAD and BALAD-2 showed c-statistics of 0.65 (0.58-0.73) and 0.61 (0.54-0.68). The c-statistics for the Milan and UCSF criteria at the time of diagnosis and prior to transplant, the GALAD, and AFP explant models are shown in Table 9.
Table 9.
Comparison of models to predict outcome of liver transplant patients
| Variable |
c-statistic (95%CI) |
|
| For recurrence | For death | |
| Number of elevated biomarkers | 0.66 (0.57-0.75) | 0.66 (0.59-0.73) |
| BALAD Score (per increase of 1) | 0.64 (0.55-0.73) | 0.65 (0.58-0.73) |
| BALAD-2 Score (per increase of 1) | 0.61 (0.52-0.70) | 0.61 (0.54-0.68) |
| Within Milan criteria at diagnosis | 0.56 (0.49-0.62) | 0.58 (0.54-0.63) |
| Within UCSF criteria at diagnosis | 0.55 (0.49-0.60) | 0.59 (0.55-0.63) |
| Within Milan criteria at transplant | 0.53 (0.46-0.59) | 0.52 (0.47-0.57) |
| Within UCSF criteria at transplant | 0.53 (0.48-0.58) | 0.50 (0.47-0.54) |
| z-GALAD | 0.63 (0.53-0.72) | 0.64 (0.56-0.72) |
| GALAD score | 0.63 (0.53-0.72) | 0.64 (0.56-0.72) |
| AFP model (explant model) | 0.59 (0.51-0.67) | 0.58 (0.51-0.65) |
DISCUSSION
The pre-transplant BALAD score and BALAD-2 class had a moderate capability to predict both recurrence and death in liver transplant HCC patients. The most predictive model was the combination of three tumor biomarkers using the cut-offs for the BALAD score. In addition, our study showed that large tumor size, high neutrophil-lymphocyte ratio, and elevated individual tumor biomarkers were associated with recurrence and mortality of patients with HCC who underwent transplant.
Tumor size was found to be significantly related to the outcomes in our cohort with HRs per centimeter of 1.27 for recurrence and 1.21 for death. This supports the use of the Milan and UCSF criteria which are based on tumor size, tumor number and vascular invasion[4,5]. The correlation of increased tumor size and elevated tumor biomarkers with outcomes has been shown in previous cohorts[16,17]. Accordingly, the biomarkers can potentially be used as more convenient predictors of patient outcome.
BALAD and BALAD-2 score contain two major components; the bilirubin-albumin score representing liver functional reserve and the three biomarkers representing tumor biology that independently reflect different characteristics of HCC progression[10]. In our study, by using the cut-off of the tumor markers according to the BALAD score, the three tumor biomarkers individually were predictive for recurrence and mortality. This is concordant with many previous studies of HCC patients receiving transplants[8,18]. High biomarker levels can reflect a poor prognosis, as a high DCP level is related to tumor vascular invasion and portal vein thrombosis[19], whereas a high AFP-L3 level has also been found to be related to vascular invasion and infiltrative growth[20].
The differences between the previous cohorts in which the predictive capability of the BALAD score was shown and our current study is the treatment received and the time of biomarker measurement. The nationwide study of HCC in the Japanese population found that the BALAD score was effective, regardless of the treatment[13]. However, this was concluded with a limited proportion of patients in the cohort receiving liver transplant as a treatment. In contrast to the previous studies of the BALAD score, we found that the c-statistic of the combination of the three biomarkers was the highest among all the tested models, including BALAD and BALAD-2. This finding could be explained by the almost immediate restoration of normal functioning of the liver after liver transplant, and thus consequently the less significant roles of bilirubin and albumin as predictors of outcomes after transplant[21].
By combining the three tumor biomarkers with tumor size, we created a model that is more predictive of both recurrence and survival (S-LAD model). A previous study from our group combined each of the biomarkers with the Milan criteria and found a significant improvement in the ability of the Milan criteria to predict recurrence[15]. In addition to this previous study, as HCC is considered a highly heterogeneous disease[22], the combination of the three biomarkers could further improve the predictive model. The GALAD score is another model that uses the combination of biomarkers with sex and age and which was originally developed for predicting risk of HCC in patients with cirrhosis[23]. Interestingly, the GALAD score also showed good performance in predicting both outcomes in our study. However, age and sex were not found to have any correlation with liver transplant outcomes in our study.
It is important to note that the proportion of recurrences after liver transplant in this study is higher than in previous studies in tertiary care centers[3]. Thirty-eight of the 113 patients (33.6%) with available serum had recurrence. However, when considering all HCC patients who underwent liver transplant during the same period, 43 of 299 patients (14.4%) had recurrence. Per report from the Mayo Clinic Transplant Biorepository, serum samples from patients with non-recurrent HCC were more frequently requested, which led to an unequal availability of the samples from patients with and without HCC recurrence. To control for the effect of the difference in sample availability on this study, we compared the characteristics and survival outcomes of non-recurrent patients without samples to those of patients with samples, finding no substantial differences in their baseline characteristics (Supplementary Table 2).
A major strength of this study is that we were able to assess the performance of BALAD, BALAD-2, and their component tumor biomarkers, and included the largest number of transplant HCC patients evaluated thus far. However, there are several limitations to our study. For most of the patients we did not have biomarker results at the time of diagnosis, as was used in the model development and most of the validation cohorts. Thus, the BALAD score and BALAD-2 class at the time of diagnosis were not available for our study. In addition, with the relatively small number of patients, further validation with a larger cohort is needed.
In conclusion, the combination of the three biomarkers used in the BALAD score along with maximal tumor diameter (S-LAD) was the most predictive model for recurrence and death outcomes for HCC patients receiving liver transplants. However, validation of this new S-LAD model is warranted. Unlike the performance for other HCC treatment modalities, the BALAD score and BALAD-2 class are less predictive for recurrence and death in HCC patients with liver transplant, presumably because liver function is restored after liver transplantation.
ARTICLE HIGHLIGHTS
Research background
Liver transplant is one of the curative treatments for hepatocellular carcinoma (HCC). However, with the limited availability of donor organs, it is essential to select patients who will derive the most benefit from transplant. The alpha-fetoprotein (AFP) model has been widely used for this purpose. In the development cohort of the BALAD model by Toyoda et al, liver transplant patients were excluded. In the validation cohort in four countries by Chan et al, there were only 21 transplant patients included, and in the Japan Nationwide study from Toyoda et al, an unknown number of transplant patients were classified in the other treatment group. There is therefore very limited data on the utility of the BALAD model in patients with liver transplant.
Research motivation
The BALAD model has been shown to be a promising predictor of outcome in hepatocellular carcinoma patients receiving most treatment modalities, but there is very limited data on its performance in hepatocellular carcinoma patients receiving liver transplants. The BALAD model incorporates three tumor biomarkers which represent the underlying biology of hepatocellular carcinomas, as well as the serum bilirubin and albumin, which reflect the extent of the underlying liver dysfunction in patients with chronic liver disease. Individually, the AFP, AFP-L3, and des-gamma-carboxyprothrombin (DCP) have been shown to predict the recurrence and survival of hepatocellular carcinoma patients receiving liver transplants. However, presumably due to replacement of the diseased liver during transplantation, it has been shown that the serum bilirubin and albumin are not predictive of patient outcomes post liver transplant.
Research objectives
We aimed to assess the performance of the discontinuous BALAD and continuous BALAD-2 scores in patients who underwent liver transplant for HCC. Further, we assessed the performance of each component of the BALAD in predicting outcomes and propose a more effective model for liver transplant patients.
Research methods
We included patients with hepatocellular carcinoma receiving liver transplants between 2000 and 2008 for whom blood samples were available to allow testing and calculation of the BALAD scores. Patient characteristics, the components of the BALAD model, BALAD score, and BALAD-2 class were analyzed to calculate hazard ratios for recurrence and death. Currently used predictive models including the Milan and UCSF criteria, GALAD score, and AFP model were compared with the BALAD models using c-statistics. A new multivariate model incorporating the three tumor markers and largest tumor diameter was created from these statistically significant variables. The long follow-up period allows assessment of the long term outcomes of the liver transplant patients.
Research results
113 patients were included in the study. The diameter of the largest tumor at the time of transplant, neutrophil-lymphocyte ratio of more than 4, elevated AFP, AFP-L3, and DCP by BALAD score cut-off were associated with both recurrence and death. The HRs per each unit increase in BALAD score for recurrence and death were 1.48 (1.15-1.91) and 1.59 (1.28-1.97). The HRs per each unit increase in BALAD class for recurrence and death were 1.45 (1.06-1.98) and 1.38 (1.09-1.76), respectively. By c-statistics, a model based on the combination of AFP, AFP-L3, and DCP using the BALAD score cut-off had a higher predictive performance than any of the prior models (0.66 for both recurrence and death). Further, a multivariate model incorporating the three biomarkers and the largest diameter of the tumor, designated the S-LAD model, showed a higher c-statistic than all other models (0.71 for recurrence and 0.69 for death). The main limitation of this study is the need for validation of the S-LAD model.
Research conclusions
BALAD and BALAD-2 are valid in transplant HCC patients, but less predictive than the three biomarkers in combination or the three biomarkers in combination with largest tumor diameter (S-LAD).
Research perspectives
Due to the limited number of patients included, further cohort studies to assess the performance of the BALAD and S-LAD models in hepatocellular carcinoma patients receiving liver transplant are warranted.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Supported by Mayo Clinic Center for Clinical and Translational Science (CCATS), No. NCATS 1UL1TR002377-01; Mayo Clinic Center for Cell Signaling in Gastroenterology, No. NIDDK P30DK084567-09; and Wako Life Sciences, Inc.
Institutional review board statement: This study was approved by Mayo Clinic Institutional Review Board (IRB 17-001912).
Informed consent statement: The IRB waived the requirement written informed consent for this minimal risk retrospective study.
Conflict-of-interest statement: Roberts LR has received grant funding from BTG, Gilead Sciences and Wako Life Sciences; Yamada H is an employee of Wako Life Sciences. There are no other potential conflicts of interest for the rest of the authors.
Peer-review started: December 4, 2017
First decision: December 13, 2017
Article in press: March 18, 2018
P- Reviewer: Johnson P, Rodriguez-Peralvarez ML, Sugawara Y S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
Contributor Information
Nicha Wongjarupong, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN 55905, United States; Department of Medicine, Faculty of Medicine, Chulalongkorn University and King Chulalongkorn Memorial Hospital, Thai Red Cross Society, Bangkok 10400, Thailand.
Gabriela M Negron-Ocasio, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN 55905, United States; University of Puerto Rico Medical Sciences Campus, San Juan 00921, Puerto Rico.
Roongruedee Chaiteerakij, Department of Medicine, Faculty of Medicine, Chulalongkorn University and King Chulalongkorn Memorial Hospital, Thai Red Cross Society, Bangkok 10400, Thailand.
Benyam D Addissie, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN 55905, United States.
Essa A Mohamed, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN 55905, United States.
Kristin C Mara, Division of Biomedical Statistics and Informatics, Mayo Clinic, Rochester, MN 55905, United States.
William S Harmsen, Division of Biomedical Statistics and Informatics, Mayo Clinic, Rochester, MN 55905, United States.
J Paul Theobald, Division of Laboratory Medicine, Department of Laboratory Medicine and Pathology, Mayo Clinic College of Medicine, Rochester, MN 55905, United States.
Brian E Peters, Division of Laboratory Medicine, Department of Laboratory Medicine and Pathology, Mayo Clinic College of Medicine, Rochester, MN 55905, United States.
Joseph G Balsanek, Division of Laboratory Medicine, Department of Laboratory Medicine and Pathology, Mayo Clinic College of Medicine, Rochester, MN 55905, United States.
Melissa M Ward, Division of Laboratory Medicine, Department of Laboratory Medicine and Pathology, Mayo Clinic College of Medicine, Rochester, MN 55905, United States.
Nasra H Giama, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN 55905, United States.
Sudhakar K Venkatesh, Department of Radiology, Mayo Clinic College of Medicine, Rochester, MN 55905, United States.
Denise M Harnois, Department of Transplantation, Mayo Clinic Florida, Jacksonville, FL 32224, United States.
Michael R Charlton, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN 55905, United States.
Hiroyuki Yamada, Wako Life Sciences, Incorporated, Mountain View, CA 94043, United States.
Alicia Algeciras-Schimnich, Division of Laboratory Medicine, Department of Laboratory Medicine and Pathology, Mayo Clinic College of Medicine, Rochester, MN 55905, United States.
Melissa R Snyder, Division of Laboratory Medicine, Department of Laboratory Medicine and Pathology, Mayo Clinic College of Medicine, Rochester, MN 55905, United States.
Terry M Therneau, Division of Biomedical Statistics and Informatics, Mayo Clinic, Rochester, MN 55905, United States.
Lewis R Roberts, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN 55905, United States. roberts.lewis@mayo.edu.
References
- 1.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimmerman MA, Ghobrial RM, Tong MJ, Hiatt JR, Cameron AM, Hong J, Busuttil RW. Recurrence of hepatocellular carcinoma following liver transplantation: a review of preoperative and postoperative prognostic indicators. Arch Surg. 2008;143:182–8; discussion 188. doi: 10.1001/archsurg.2007.39. [DOI] [PubMed] [Google Scholar]
- 4.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 5.Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 6.Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A; OLT for HCC Consensus Group. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11–e22. doi: 10.1016/S1470-2045(11)70175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazzaferro V, Bhoori S, Sposito C, Bongini M, Langer M, Miceli R, Mariani L. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl. 2011;17 Suppl 2:S44–S57. doi: 10.1002/lt.22365. [DOI] [PubMed] [Google Scholar]
- 8.Hakeem AR, Young RS, Marangoni G, Lodge JP, Prasad KR. Systematic review: the prognostic role of alpha-fetoprotein following liver transplantation for hepatocellular carcinoma. Aliment Pharmacol Ther. 2012;35:987–999. doi: 10.1111/j.1365-2036.2012.05060.x. [DOI] [PubMed] [Google Scholar]
- 9.Duvoux C, Roudot-Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, Francoz C, Compagnon P, Vanlemmens C, Dumortier J, et al. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012;143:986–94.e3; quiz e14-5. doi: 10.1053/j.gastro.2012.05.052. [DOI] [PubMed] [Google Scholar]
- 10.Toyoda H, Kumada T, Osaki Y, Oka H, Urano F, Kudo M, Matsunaga T. Staging hepatocellular carcinoma by a novel scoring system (BALAD score) based on serum markers. Clin Gastroenterol Hepatol. 2006;4:1528–1536. doi: 10.1016/j.cgh.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 11.Kitai S, Kudo M, Minami Y, Haji S, Osaki Y, Oka H, Seki T, Kasugai H, Sasaki Y, Matsunaga T. Validation of a new prognostic staging system for hepatocellular carcinoma: a comparison of the biomarker-combined Japan Integrated Staging Score, the conventional Japan Integrated Staging Score and the BALAD Score. Oncology. 2008;75 Suppl 1:83–90. doi: 10.1159/000173428. [DOI] [PubMed] [Google Scholar]
- 12.Chan SL, Mo F, Johnson P, Li L, Tang N, Loong H, Chan AW, Koh J, Chan AT, Yeo W. Applicability of BALAD score in prognostication of hepatitis B-related hepatocellular carcinoma. J Gastroenterol Hepatol. 2015;30:1529–1535. doi: 10.1111/jgh.13005. [DOI] [PubMed] [Google Scholar]
- 13.Toyoda H, Tada T, Johnson PJ, Izumi N, Kadoya M, Kaneko S, Kokudo N, Ku Y, Kubo S, Kumada T, et al. Validation of serological models for staging and prognostication of HCC in patients from a Japanese nationwide survey. J Gastroenterol. 2017;52:1112–1121. doi: 10.1007/s00535-017-1321-6. [DOI] [PubMed] [Google Scholar]
- 14.Berhane S, Toyoda H, Tada T, Kumada T, Kagebayashi C, Satomura S, Schweitzer N, Vogel A, Manns MP, Benckert J, et al. Role of the GALAD and BALAD-2 Serologic Models in Diagnosis of Hepatocellular Carcinoma and Prediction of Survival in Patients. Clin Gastroenterol Hepatol. 2016;14:875–886.e6. doi: 10.1016/j.cgh.2015.12.042. [DOI] [PubMed] [Google Scholar]
- 15.Chaiteerakij R, Zhang X, Addissie BD, Mohamed EA, Harmsen WS, Theobald PJ, Peters BE, Balsanek JG, Ward MM, Giama NH, et al. Combinations of biomarkers and Milan criteria for predicting hepatocellular carcinoma recurrence after liver transplantation. Liver Transpl. 2015;21:599–606. doi: 10.1002/lt.24117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toyoda H, Kumada T, Tada T, Sone Y, Kaneoka Y, Maeda A. Tumor Markers for Hepatocellular Carcinoma: Simple and Significant Predictors of Outcome in Patients with HCC. Liver Cancer. 2015;4:126–136. doi: 10.1159/000367735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura S, Nouso K, Sakaguchi K, Ito YM, Ohashi Y, Kobayashi Y, Toshikuni N, Tanaka H, Miyake Y, Matsumoto E, et al. Sensitivity and specificity of des-gamma-carboxy prothrombin for diagnosis of patients with hepatocellular carcinomas varies according to tumor size. Am J Gastroenterol. 2006;101:2038–2043. doi: 10.1111/j.1572-0241.2006.00681.x. [DOI] [PubMed] [Google Scholar]
- 18.Taketomi A, Sanefuji K, Soejima Y, Yoshizumi T, Uhciyama H, Ikegami T, Harada N, Yamashita Y, Sugimachi K, Kayashima H, et al. Impact of des-gamma-carboxy prothrombin and tumor size on the recurrence of hepatocellular carcinoma after living donor liver transplantation. Transplantation. 2009;87:531–537. doi: 10.1097/TP.0b013e3181943bee. [DOI] [PubMed] [Google Scholar]
- 19.Koike Y, Shiratori Y, Sato S, Obi S, Teratani T, Imamura M, Yoshida H, Shiina S, Omata M. Des-gamma-carboxy prothrombin as a useful predisposing factor for the development of portal venous invasion in patients with hepatocellular carcinoma: a prospective analysis of 227 patients. Cancer. 2001;91:561–569. doi: 10.1002/1097-0142(20010201)91:3<561::aid-cncr1035>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 20.Tada T, Kumada T, Toyoda H, Kiriyama S, Sone Y, Tanikawa M, Hisanaga Y, Kitabatake S, Kuzuya T, Nonogaki K, et al. Relationship between Lens culinaris agglutinin-reactive alpha-fetoprotein and pathologic features of hepatocellular carcinoma. Liver Int. 2005;25:848–853. doi: 10.1111/j.1478-3231.2005.01111.x. [DOI] [PubMed] [Google Scholar]
- 21.Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O’Beirne J, Fox R, Skowronska A, Palmer D, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550–558. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeng KS, Chang CF, Jeng WJ, Sheen IS, Jeng CJ. Heterogeneity of hepatocellular carcinoma contributes to cancer progression. Crit Rev Oncol Hematol. 2015;94:337–347. doi: 10.1016/j.critrevonc.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Johnson PJ, Pirrie SJ, Cox TF, Berhane S, Teng M, Palmer D, Morse J, Hull D, Patman G, Kagebayashi C, et al. The detection of hepatocellular carcinoma using a prospectively developed and validated model based on serological biomarkers. Cancer Epidemiol Biomarkers Prev. 2014;23:144–153. doi: 10.1158/1055-9965.EPI-13-0870. [DOI] [PubMed] [Google Scholar]


