Abstract
Currently there is a lack of accurate biomarkers for diagnosis and prognosis in advanced liver diseases. Either the occurrence of first decompensation, or diagnosis of acute on chronic liver failure, severe alcoholic hepatitis, or hepatocellular carcinoma (HCC), none of the available biomarkers are satisfactory. Metabolomics is the newest of omics, being much closer than the others to the actual phenotype and pathologic changes that characterizes a certain condition. It deals with a much wider spectrum of low molecular weight bio-compounds providing a powerful platform for discovering novel biomarkers and biochemical pathways to improve diagnostic, prognostication and therapy. Until now metabolomics was applied in a wide spectrum of liver conditions, but the findings were contradictory. This review proposes a synthesis of the existing evidences of metabolomics use in advanced chronic liver diseases, decompensated liver cirrhosis, severe alcoholic hepatitis and HCC.
Keywords: Metabolomics, Biomarker, Prediction, Advanced chronic liver disease, Decompensation, Alcoholic hepatitis, Hepatocellular carcinoma
Core tip: Currently there is a lack of accurate biomarkers for diagnosis and prognosis in advanced liver diseases. Either the occurrence of first decompensation, or diagnosis of acute on chronic liver failure, severe alcoholic hepatitis, or hepatocellular carcinoma (HCC), none of the available biomarkers are satisfactory. This review proposes a synthesis of the existing evidences of metabolomics use in advanced chronic liver diseases, decompensated liver cirrhosis, severe alcoholic hepatitis and HCC.
INTRODUCTION
Hepatic fibrosis is a dynamic process that may progress to liver cirrhosis in the context of an active etiological factor. In compensated stages, physical exam by itself cannot distinguish between severe fibrosis and constituted liver cirrhosis. This is why in recent years the term compensated advanced chronic liver disease (cACLD) was introduced[1]. In this stage it is essential to establish the risk of decompensation and the best method to do it is by measuring hepatic venous pressure gradient (HVPG)[2]. However, HVPG measurement is not widely available and it is considered invasive[3]. Therefore, in the last years huge efforts were done to find new biomarkers or non-invasive methods to assess prognosis.
The occurrence of decompensation, with its various manifestations (ascites and variceal bleeding most often) is in direct relation with the increase in portal pressure, namely clinically significant portal hypertension, which represent an HVPG > 10 mmHg[4]. The life expectancy of these patients without liver transplantation is significantly lower than in compensated stages[5].
Acute decompensation associated with organ failures and increased short-term mortality is defined by the concept of acute on chronic liver failure (ACLF) syndrome, which was recently defined[6]. The most frequent precipitating factors for ACLF are bacterial infections, acute flares in viral B advanced liver disease and alcohol consumption but the clinical features are identical regardless the precipitating factor[7]. There are some clinical situations where making a therapeutic decision based on the available non-invasive diagnostic tools proves to be difficult. Thus, without liver biopsy it is impossible to differentiate between severe alcoholic hepatitis and decompensated cirrhosis, which is essential for the indication of cortisone treatment[8]. Moreover, around one third of decompensated patients are infected at presentation[9,10] and without routine cultures the clinical suspicion and diagnosis of bacterial infections is very difficult. To reduce in hospital-morbidity and mortality, early initiation of empiric antibiotherapy can be crucial, but bacterial cultures last long, and infection markers represented by CRP, leucocyte count, procalcitonin are of limited value in cirrhosis[11]. Therefore, in these specific clinical scenarios new biomarkers for diagnosis and prognosis are also needed.
Apart from acute decompensation, the prognosis of patients with cACLD is deeply influenced by hepatocellular carcinoma (HCC) occurrence[12]. The high mortality rate of HCC is owed partly to the absence of adequate monitoring in high-risk populations, and partly to insufficient diagnostic resources - especially for early tumor identification, which could still allow curative interventions. Serum alpha-fetoprotein (AFP) - which has been widely and commonly used as biomarker, either as a screening tool for early HCC detection or as a prognostic tool for tumor recurrence and patient survival, has a poor sensitivity since up to 40% of HCC and cirrhosis patients have normal AFP levels and only 10%-20% of patients with early-stage HCC have elevated AFP levels[13]. Therefore, more sensitive markers of disease are needed, particularly for the early detection of HCC disease and for HCC recurrence after curative treatment.
Given the reserved prognosis and the difficulty of management, this review proposes a synthesis of the existing evidences of metabolomics use in cACLD, decompensated liver cirrhosis, severe alcoholic hepatitis and HCC.
METABOLOMICS - NEW OPPORTUNITY FOR BIOMARKERS DISCOVERY
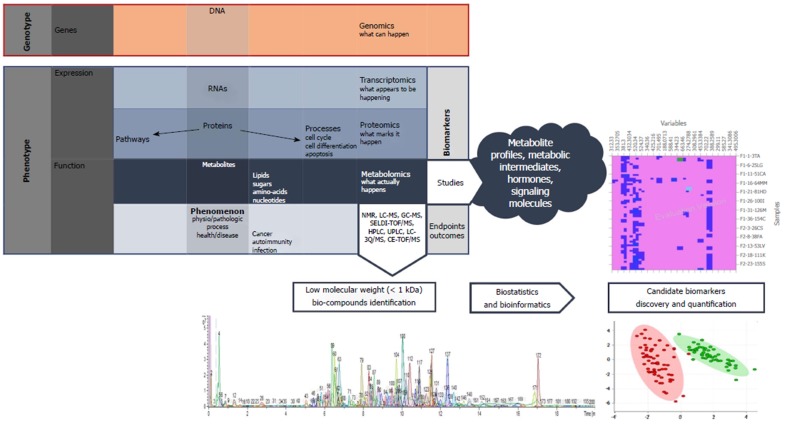
Although its recognition as a distinct scientific area is much more recent than the other “omics” such as genomics, transcriptomics, or proteomics, metabolomics provides a powerful platform for discovering novel biomarkers and biochemical pathways to improve diagnostic, prognostication, and therapy (Figure 1)[14,15]. It has the advantage of being much closer to the actual phenotype than the other omics, but the number of possible compounds is much higher. In contrast to genomics, transcriptomics, and proteomics, which address macromolecules with similar chemical properties, such as DNA, RNA, and proteins, metabolomics deals with diverse properties of low molecular weight bio-compounds[16].
Figure 1.
Integration of metabolites into the "omics" pathway and basic principles of metabolomics.
Metabolomics allows small metabolites, usually with a molecular weight under 1 kDa, and metabolic processes to be studied using nuclear magnetic resonance (NMR) spectroscopy, gas chromatography mass spectrometry (GC-MS), and liquid chromatography mass spectrometry (LC-MS)[17]. Given the nonvolatile character of biological materials (serum, urine, tissue or faeces) the most commonly used technique in clinical trials is LC-MS. The common pattern recognition methods of metabolomics include unsupervised and supervised ones: Principal component analysis (PCA) for the former and partial least squares-discriminant analysis (PLS-DA) for the latter group.
METABOLOMICS AND ADVANCED CHRONIC LIVER DISEASE
Once the cACLD patients develop decompensation the progression of the disease is very clear. The annual transition rate from compensated to decompensated stage is the highest in HBV cirrhosis, around 10% per year, being lower in HCV and alcoholic etiologies[18,19]. However, without HVPG measurement, the most difficult task is to identify the patients at risk of decompensation or to accurately identify the precipitating factor.
It seems that with the progression of chronic liver diseases the core metabolic phenotype is characterized by a decrease in phosphatidylcholines (PC) and increase in serum biliary acids[20]. This core metabolic phenotype appears early in the natural history of chronic liver diseases, regardless the etiology, and remains stable in the evolution, including different stages of cirrhosis or hepatic tumors, either cholangio or HCC.
When comparing the metabolic profile of patients infected with HCV without fibrosis with HCV cirrhosis, along with this core metabolic phenotype, there are other several disorders involving lipid, carbohydrate, protein, and energetic metabolism[21]. The HDL cholesterol and choline levels were lower in patients with cirrhosis compared to those without fibrosis. The perturbations of glucose metabolism are caused, firstly, by impaired tricarboxylic acid cycle activity due to mitochondrial dysfunction, and possibly on the second hand by insulin resistance that characterizes HCV infection, leading to increased serum glucose and citrate levels in the cirrhotic group. As a response to the relative carbohydrate deficiency, ketone bodies (hydroxybutyrate and acetoacetate) are used as preferential energy sources in the mitochondria, explaining their lower serum levels with the evolution of hepatic disease.
Regarding protein metabolism, there is an imbalance in the ratio of aromatic amino acids and branched chain amino acids in cirrhosis. In fact, only phenylalanine was founded elevated in serum of patients with cirrhosis, probably because disturbances of the gut microbiota in this situation[22].
Jimenez et al[23] has attempted to identify by NMR spectroscopy the metabolic profile of cirrhotic patients with minimal hepatic encephalopathy (MHE) vs cirrhotic patients without encephalopathy. MHE patients displayed increased serum concentrations of glucose, lactate, methionine and glycerol, as well as decreased levels of choline, branched chain amino acids, alanine, glycine, acetoacetate, and lipid moieties.
When serum metabolic profile by NMR spectroscopy of patients in different stages of chronic liver failure (CLF) according to the MELD score was analyzed, there is an evolutionary trend involving the representatives of the metabolism of lipids, carbohydrates and proteins[24]. Thus, there is a decrease in HDL cholesterol, choline and phosphatidylcholine, which are the more expressed in higher MELD patients. The glucose, lactic acid, butyrate, pyruvate and citrate levels increase in severe CLF and the protein metabolism is modified because increased skeletal muscle catabolism, expressed by increased levels of free amino acids (leucine, isoleucine, glutamine, methionine and valine) in parallel with the severity of liver disease.
Recently, it was proved that phosphatidylcholine and lysophosphatidylcholine may have also prognosis relevance in decompensated liver cirrhosis, serum levels of these compounds being negatively correlated with survival[25].
Therefore, there is no single biomarker for the different stages of advanced chronic liver disease, but a complex of biomarkers, a so-called metabolic fingerprint. This implies, regardless of the etiology of liver disease, progressive changes of the same classes of compounds in parallel with disease evolution.
METABOLOMICS AND ACUTE-ON-CHRONIC LIVER FAILURE
ACLF is a distinct syndrome that can occur in approximately one third of patients with decompensated liver cirrhosis[6]. The most common causes are bacterial infections, alcohol consumption and digestive bleeding, although in a large percentage of cases a precipitating factor cannot be identified[7].
Amathieu et al[26] compared the metabolic profile of patients with ACLF with the one of patients with decompensated liver cirrhosis who do not meet the criteria for ACLF. The patients with ACLF had decreased HDL cholesterol, increased lactic acid, pyruvate, and aromatic amino acids but these changes are rather the expression of the severity of liver disease.
Because indirect infection markers have limited value in cirrhotic patients and bacteriological studies last long, identifying bacterial infections in a patient with decompensated liver cirrhosis or ACLF could be difficult. Although there is a strong need for new biomarkers in infection, there are no publications regarding the metabolic profile of the infected cirrhotic patients.
In severe sepsis and septic shock in non-cirrhotic patients it was identified a urinary metabolic profile with prognostic value, characterized by higher levels of ethanol, glucose, hippurate, but lower levels of methionine, glutamine, arginine and phenylalanine in patients with lower survival[27]. A retrospective multicenter study in Greece and Germany, which enrolled a large number of patients proposed as a primary endpoint to differentiate the metabolic profile of patients with SIRS from patients with sepsis and as a secondary endpoint, to identify specific biomarkers for the different types of infections[28]. A regression model combining the sphingolipid SM C22:3 and the glycerophospholipid lysoPCaC24:0 was created for sepsis diagnosis with a sensitivity of 84.1% and specificity of 85.7%. The glycerophospholipid lysoPCaC26:1 was characteristic for patients with community-acquired pneumonia complicated with severe sepsis or septic shock. For the other types of infection, no biomarker or significant metabolic profile was found.
For diagnosis of sepsis in emergency department, one study identified a panel of 6 metabolites, represented by myristic acid, citric acid, isoleucine, norleucine, pyruvic acid and a phosphocholine like derivative, to have very good sensitivity (95%) and specificity (90%)[29].
It is to be demonstrated if all these metabolic markers of infection may be applied in the context of decompensated cirrhosis or ACLF.
METABOLOMICS AND ALCOHOLIC LIVER DISEASE
Alcohol liver disease encompasses a spectrum of injury ranging from simple steatosis to frank cirrhosis and alcohol consumption may represent a precipitating factor for decompensation or ACLF[8]. Because most cases of alcoholic hepatitis occur in patients with established cirrhosis, most of the times it is impossible to differentiate between severe alcoholic hepatitis and decompensated cirrhosis without liver biopsy[8]. Accordingly, new biomarkers capable to differentiate between severe alcoholic hepatitis and decompensated cirrhosis as well as markers capable to predict early the response to corticosteroid therapy, would be of great help in clinical practice.
Urinary indole-3-acetic acid has been identified as a potential biomarker for early alcoholic liver disease on animal model, by two studies performed by LC-MS[30,31]. Our group, in a pilot study, proved that lysophosphatidylcholine (LPC) 16:1 and 20:4 decease progressively with the severity of alcoholic liver disease and this is correlated with survival and the occurrence of liver related events[32]. However, if these metabolic changes are rather general in ACLD or specific to alcoholic liver disease remains to be proved.
There are only few small studies in the literature regarding the metabolic profile of the patient with severe alcoholic hepatitis. Enhanced adipose tissue lipolysis with increased fatty acid supply to the liver is a phenomenon observed in severe alcoholic hepatitis[33]. In severe alcoholic hepatitis, there is an impaired long-chain fatty acid beta-oxidation in the liver, first because of the oversaturation of hepatic metabolic capacity due to excessive fatty acid supply and second because of impaired mitochondrial function. Eicosapentaenoate (EPA; 20:5n3) and docosapentaenoate (DPA;22:5n6), 2 long chain essential fatty acids, have been identified as potential biomarkers for severe alcoholic hepatitis by Rachakonda et al[33,34], capable to differentiate severe alcoholic hepatitis from compensated alcoholic liver cirrhosis.
As a consequence of faulty beta-oxidation, the same study demonstrated a relative transition to lipid omega-oxidation in severe alcoholic hepatitis, with the increase of dicarboxylic acids such as tetradecanedioate, hexadecanedioate, octadecanedioate, which are endogenous ligands of the peroxisome proliferator activated receptor (PPAR) alpha, mechanism at least partially responsible for the developing of severe steatosis in alcoholic hepatitis[34]. Severe alcoholic hepatitis is characterized by intrahepatic cholestasis. An increase in sulphated bile acids has been demonstrated in the serum of patients with severe alcoholic hepatitis, with a decrease in bile acids from intestinal bacterial origin, reflecting probably gut microbiota disturbances[34]. Carbohydrate metabolism is also impaired and implicates mainly the dysfunction of the Krebs cycle activity, with an increase in serum concentrations of the intermediates of the cycle, like fumarate, succinate, malate, citrate. Glucose is poorly used in severe alcoholic hepatitis, and there is a shunting from glycolysis to the pentose phosphor pathway, the end product of this, xylonate, being an important biomarker for severe alcoholic hepatitis.
Branched chain amino acids originating in skeletal muscles appear to be an important energy substitute in severe alcoholic hepatitis, as their serum levels (valine and isoleucine) are reduced in parallel with the increase of their metabolites[34].
Ascha et al[35] identified two compounds, betaine and citrulline, as important biomarkers for the differentiation of severe alcoholic hepatitis from decompensated liver cirrhosis. Betaine is a methylating agent involved in preserving the integrity of the hepatic cell, while citrulline has intestinal origin and appears to be elevated alongside NO, secondary to an excessive nitric oxyde synthaseactivity in the context of significant portal hypertension in alcoholic hepatitis[35].
METABOLOMICS AND HCC
LPC is an important signaling molecule, involved in regulating cellular proliferation, cancer cell invasion, and inflammation[36] and it has been reported to be significantly decreased in the sera of HCC patients[37,38]. In a recent study, lower levels of LPC and PC, such as LPC (16:0), LPC (18:0), PC (16:0), and PC (18:0) were observed in HCC and liver cirrhosis samples compared with healthy controls[37]. Low levels of LPCs imply an anti-inflammatory status in HCC patients, and markedly low levels of LPCs represent a severe immune suppression status in cirrhotic patients. Similar LPC trends have also been found in other malignant diseases, such as renal cell carcinoma[39].
Other serum lipid compounds found to be discriminative between HCC and healthy controls are Free Fatty Acids (FFA). Amongst them, oleamide (cis-9, 10-octadecenoamide), the amide of FFA C18:1 (oleic acid), may represent a specific marker for HCC[36,40]. Gao et al[41] reported a gradual up-regulation of the ratio of FFA C16:1 to C16:0 and FFA C18:1 to C18:0 during hepatocarcinogenesis as a result of significantly increased level of stearoyl-CoA desaturase 1(SCD1), due to the increased demand for lipid synthesis in HCC.
Bile acids are synthesized in the liver and aid in fatty acid absorption and digestion and constitutes one of the most frequently reported compound classes suggested as discriminating between HCC patients and a control group. Cholic acid, chenodeoxycholic acid, lithocholic acid and deoxycholic acid had lower levels in HCC patients compared with cirrhosis[40,42]. Also, glycochenodeoxycholic acid 3-sulfate (3-sulfo- GCDCA), glycocholic acid (GCA), glycodeoxycholic acid (GDCA), taurocholic acid (TCA), and taurochenodeoxycholate (TCDCA) are down regulated HCC vs cirrhosis[37,42]. Bile acid downregulation in HCC may also reflect a metabolic shift away from β-oxidation and the reduced de novo bile acid production caused by the obliteration of healthy hepatocytes during chronic liver disease[43].
As the liver is the major organ of protein metabolism it is not surprising that a dysregulation of amino acids was found in several studies specifically a decrease in branched chain amino acids (BCAAs: leucine, isoleucine, and valine) and an increase in aromatic amino acids (AAAs: phenylalanine, tryptophan, tyrosine, and histidine) in HCC patients vs healthy controls, indicating enhanced BCAA catabolism and reduced AAA breakdown in the failing liver[44,45]. BCAAs have been reported to have a crucial role in cancer by regulating the anabolic process involving protein synthesis and degradation. Alteration of these metabolic pathways was observed after RFA intervention, indicating that application of electrical current during RFA treatment causes burns in the liver and produces coagulative necrosis which results in parenchymal and tumor cell death, enhancement of consumption of BCAA, such as isoleucine which may characterize the inflammatory response in liver[46].
Baniasadi et al[47] used a targeted approach based on liquid chromatography resolved tandem mass spectrometry (LC-MS/MS) on 73 metabolites out of which 16 were statistically different between the serum of HCC vs cirrhotic HCV patients. Among them, 4 metabolites (methionine, 5-hydroxymethyl-2′-deoxyuridine, N2,N2-dimethylguanosine and uric acid) showed an excellent separation between the two group patients with a sensitivity of 97% and specificity of 95% and an AUROC of 0.98.
Prognostication for HCC after curative treatment is difficult, in part due to the lack of useful biomarkers that would allow for the selection of patients at higher risk of tumor recurrence or enable accurate assessment of treatment response.
Goossens et al[46] evaluated through 1H-NMR analysis, preoperatively and at various time points post-RFA, the metabolic profile of serum samples from HCC patients in order to identify factors associated with treatment response and recurrence in viral and non-viral HCC patients. The analysis was able to discriminate in the serum of viral HCC between t0 (pre-ablation) and t2 ( at 1 to 4 mo post ablation), the t2 being mainly characterized by an increase of glucose, glycerol, methylhistidine , and a decrease of lipids, 3-hydroxybutyrate, and choline but it was not able to predict HCC recurrence.
Zhou et al[48] evaluated early postoperative recurrence metabolic disturbances in HCC patients and demonstrated that bile acids, steroids and fatty acids showed significant variation in the early recurrent HCC group compared to the late recurrence group. Moreover, with the combination of methionine, GCDCA and cholesterol sulfate, 80% of the early recurrent HCCs can be predicted correctly with the corresponding AUROC equal to 0.91.
As previously shown there are no specific metabolic changes during the carcinogenetic process and, therefore, by now there is no specific marker to be proposed for diagnostic and prognostic of patients with HCC. Although metabolomics is a powerful strategy for identifying a large panel of metabolites that exhibit promise in accurately diagnosing HCC, integrating two or more “omics” approaches can unveil the complex genomic-proteomic-metabolic network galvanizing cancer development. With this regard, Beyoğlu et al[49] performed a combined transcriptomics and metabolomics study and was able to evaluate the metabolic profile of G1 to G6 transcriptomics groups of HCC.
CONCLUSION
The main limitation to the generalization of the results of existing publication is that the methodology used by different groups is not uniform and, thus, the results are sometimes contradictory. Other possible explanation for this variability is the fact that the majority of the studies use non-targeted metabolic analysis and identification of different metabolites is based on molecular mass, which can be similar for different compounds. Despite the important progress that has been made in technology and in understanding the pathological processes, when talking about specific biomarkers for advanced chronic liver diseases we are still in an era of uncertainty and chiromancy.
However, what appears to be a fact, is that during the progression of liver diseases, regardless the etiology, there is a core represented by decrease in serum lysophosphatidylcholine and an increased in bile acids[20] (Table 1). These changes augment with the progression of the disease and that’s explains the prognostic relevance of these changes. Besides that, several candidate metabolomic biomarkers have been identified in these clinical scenarios. They reflect the changes that occur mainly in lipids, amino-acids and energetic metabolism. Nevertheless, none of them was widely and independently validated, or have been translated into clinical practice.
Table 1.
Principal metabolic changes in advanced liver diseases
| Condition | Lipids | Bile acids | Carbo-hydrates | Energy and oxidative stress | Proteins and Aminoacids |
| ACLD | ↓ HDL cholesterol | ↑ Glucose | ↓ OH-butyrate | ↑ Phe | |
| ↓ Choline | ↑ Glycerol | ↓ Aceto-acetate | ↓ Gli, Ala | ||
| ↓ Phosphatidylcholine | ↑ Lactate | ↓ Branched AA | |||
| ↓ Lipid moieties | ↑ Pyruvate | ↑ Leu, Iso-Leu, Val, Glu | |||
| ↑ Citrate | ↑ Methionine | ||||
| ACLF | ↓ HDL cholesterol | ↑ Lactate | ↑ Aromatic AA | ||
| ↑ Pyruvate | |||||
| ALD | ↓ Lyso-phosphatidilcholine | ↑ Sulphated bile acids | ↑ Fumarate, succinate, | ↑ Indole 3-acetic acid (u) | ↓ Val, Iso-Leu |
| ↑ Eico/doco -sapentaenoate | malate, citrate | ↑ Betaine | |||
| ↑ Tetra/hexa/octa -decanedioate | ↑ Xylonate | ↑ Citruline | |||
| HCC | ↓ Lysophosphatidilcholine | ↓ (Lito)cholic, (cheno)deoxy-cholic acids | ↑ Glucose, glycerol | ↓ OH-butyrate | ↓ BCAAs: Leu, Iso-Leu and Val |
| ↑ Oleamide | ↓ Xantine | ||||
| ↑ Stearoyl-coa desaturase | ↓ GCA, GDCA, GCDCA, TCA, TCDCA | ↑ Canavanino succinate | ↑ AAAs: Phe, Trp, Tyr, His | ||
| ↓ 3-Hydroxybutyrate | |||||
| ↓ Choline | ↑ Methionine, hydroxy-methyldeoxyuridine, dimethyl-guanosine, uric acid | ||||
| ↑ Methylhistidine |
ACLF: Acute on chronic liver failure; ACLD: Advanced chronic liver disease; ALD: Alcoholic liver disease; HCC: Hepatocellular carcinoma; AA: Aminoacids; AAA: Aromatic AA; BCAA: Branched chain AA; Ala: Alanine; Arg: Arginine; Gli: Glicine; Glu: Glutamate; His: Histidine; Phe: Phenylalanine; Leu: Leucine; Val: Valine; Trp: Triptofan; Tyr: Tyrosine; CA: Cholic acid; GCA: Glyco CA; GDCA: Glycodeoxy CA; GCDCA: Glycochenodeoxy CA; TCA: Tauro CA; TCDA: Tauro cheno deoxi CA; u: Urinary.
It is our strong belief that the diversity and quality of emerging data would allow the selection of the best method for metabolomics and further studies would validate new biomarkers for those scenarios where clinical needs are still unmet. Probably, the solution would be to interdisciplinary analyze the data through system’s biology, allowing the integration of clinical, biochemical, imaging and “omics” findings, so that we’ll be moving towards the era of personalized precision medicine in advanced chronic liver diseases.
Footnotes
Supported by Romanian Agency for Scientific Research, No. PN-II-RU-TE-2014-4-0356 (awarded to Stefanescu H), and No. PN-II-RU-TE-2014-4-0709 (awarded to Procopet B).
Conflict-of-interest statement: None.
Manuscript source: Invited manuscript
Peer-review started: October 29, 2017
First decision: November 28, 2017
Article in press: January 29, 2018
Specialty type: Gastroenterology and hepatology
Country of origin: Romania
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Chuang WL, Puoti C, Zheng YB, Zhu HF S- Editor: Song XX L- Editor: A E- Editor: Wang CH
Contributor Information
Bogdan Procopet, 3rd Medical Clinic, University of Medicine and Pharmacy, Cluj 400162, Romania; Hepatology Unit, Regional Institute of Gastroenterology and Hepatology, Cluj 400162, Romania.
Petra Fischer, 3rd Medical Clinic, University of Medicine and Pharmacy, Cluj 400162, Romania.
Oana Farcau, 3rd Medical Clinic, University of Medicine and Pharmacy, Cluj 400162, Romania.
Horia Stefanescu, Hepatology Unit, Regional Institute of Gastroenterology and Hepatology, Cluj 400162, Romania. horia.stefanescu@irgh.ro.
References
- 1.de Franchis R. Expanding consensus in portal hypertension Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol [Internet] 2015;63:543–545. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 2.Ripoll C, Groszmann R, Garcia-Tsao G, Grace N, Burroughs A, Planas R, Escorsell A, Garcia-Pagan JC, Makuch R, Patch D, et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481–488. doi: 10.1053/j.gastro.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 3.Procopeţ B, Tantau M, Bureau C. Are there any alternative methods to hepatic venous pressure gradient in portal hypertension assessment? J Gastrointestin Liver Dis. 2013;22:73–78. [PubMed] [Google Scholar]
- 4.Berzigotti A, Seijo S, Reverter E, Bosch J. Assessing portal hypertension in liver diseases. Expert Rev Gastroenterol Hepatol. 2013;7:141–155. doi: 10.1586/egh.12.83. [DOI] [PubMed] [Google Scholar]
- 5.Ripoll C, Bañares R, Rincón D, Catalina MV, Lo Iacono O, Salcedo M, Clemente G, Núñez O, Matilla A, Molinero LM. Influence of hepatic venous pressure gradient on the prediction of survival of patients with cirrhosis in the MELD Era. Hepatology. 2005;42:793–801. doi: 10.1002/hep.20871. [DOI] [PubMed] [Google Scholar]
- 6.Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–1437, 1437.e1-1437.e9. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 7.Arroyo V, Moreau R, Kamath PS, Jalan R, Ginès P, Nevens F, Fernández J, To U, García-Tsao G, Schnabl B. Acute-on-chronic liver failure in cirrhosis. Nat Rev Dis Primers. 2016;2:16041. doi: 10.1038/nrdp.2016.41. [DOI] [PubMed] [Google Scholar]
- 8.European Association for the Study of Liver. EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol. 2012;57:399–420. doi: 10.1016/j.jhep.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Fernández J, Acevedo J, Castro M, Garcia O, de Lope CR, Roca D, Pavesi M, Sola E, Moreira L, Silva A, et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology. 2012;55:1551–1561. doi: 10.1002/hep.25532. [DOI] [PubMed] [Google Scholar]
- 10.Fernández J, Acevedo J, Wiest R, Gustot T, Amoros A, Deulofeu C, Reverter E, Martínez J, Saliba F, Jalan R, et al. Bacterial and fungal infections in acute-on-chronic liver failure: prevalence, characteristics and impact on prognosis. Gut. 2017 doi: 10.1136/gutjnl-2017-314240. [DOI] [PubMed] [Google Scholar]
- 11.Alexopoulou A, Agiasotelli D, Vasilieva LE, Dourakis SP. Bacterial translocation markers in liver cirrhosis. Ann Gastroenterol. 2017;30:486–497. doi: 10.20524/aog.2017.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cabibbo G, Enea M, Attanasio M, Bruix J, Craxì A, Cammà C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010;51:1274–1283. doi: 10.1002/hep.23485. [DOI] [PubMed] [Google Scholar]
- 13.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Lindon JC, Holmes E, Nicholson JK. Metabonomics in pharmaceutical R&D. FEBS J. 2007;274:1140–1151. doi: 10.1111/j.1742-4658.2007.05673.x. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Zhang A, Sun H. Power of metabolomics in diagnosis and biomarker discovery of hepatocellular carcinoma. Hepatology. 2013;57:2072–2077. doi: 10.1002/hep.26130. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida M, Hatano N, Nishiumi S, Irino Y, Izumi Y, Takenawa T, Azuma T. Diagnosis of gastroenterological diseases by metabolome analysis using gas chromatography-mass spectrometry. J Gastroenterol. 2012;47:9–20. doi: 10.1007/s00535-011-0493-8. [DOI] [PubMed] [Google Scholar]
- 17.Patel NR, McPhail MJ, Shariff MI, Keun HC, Taylor-Robinson SD. Biofluid metabonomics using (1)H NMR spectroscopy: the road to biomarker discovery in gastroenterology and hepatology. Expert Rev Gastroenterol Hepatol. 2012;6:239–251. doi: 10.1586/egh.12.1. [DOI] [PubMed] [Google Scholar]
- 18.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838–851. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asrani SK, Kamath PS. Natural history of cirrhosis. Curr Gastroenterol Rep. 2013;15:308. doi: 10.1007/s11894-012-0308-y. [DOI] [PubMed] [Google Scholar]
- 20.Beyoğlu D, Idle JR. The metabolomic window into hepatobiliary disease. J Hepatol. 2013;59:842–858. doi: 10.1016/j.jhep.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Embade N, Mariño Z, Diercks T, Cano A, Lens S, Cabrera D, Navasa M, Falcón-Pérez JM, Caballería J, Castro A, et al. Metabolic Characterization of Advanced Liver Fibrosis in HCV Patients as Studied by Serum 1H-NMR Spectroscopy. PLoS One. 2016;11:e0155094. doi: 10.1371/journal.pone.0155094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vernocchi P, Del Chierico F, Putignani L. Gut Microbiota Profiling: Metabolomics Based Approach to Unravel Compounds Affecting Human Health. Front Microbiol. 2016;7:1144. doi: 10.3389/fmicb.2016.01144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiménez B, Montoliu C, MacIntyre DA, Serra MA, Wassel A, Jover M, Romero-Gomez M, Rodrigo JM, Pineda-Lucena A, Felipo V. Serum metabolic signature of minimal hepatic encephalopathy by (1)H-nuclear magnetic resonance. J Proteome Res. 2010;9:5180–5187. doi: 10.1021/pr100486e. [DOI] [PubMed] [Google Scholar]
- 24.Amathieu R, Nahon P, Triba M, Bouchemal N, Trinchet JC, Beaugrand M, Dhonneur G, Le Moyec L. Metabolomic approach by 1H NMR spectroscopy of serum for the assessment of chronic liver failure in patients with cirrhosis. J Proteome Res. 2011;10:3239–3245. doi: 10.1021/pr200265z. [DOI] [PubMed] [Google Scholar]
- 25.McPhail MJW, Shawcross DL, Lewis MR, Coltart I, Want EJ, Antoniades CG, Veselkov K, Triantafyllou E, Patel V, Pop O, et al. Multivariate metabotyping of plasma predicts survival in patients with decompensated cirrhosis. J Hepatol. 2016;64:1058–1067. doi: 10.1016/j.jhep.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amathieu R, Triba MN, Nahon P, Bouchemal N, Kamoun W, Haouache H, Trinchet JC, Savarin P, Le Moyec L, Dhonneur G. Serum 1H-NMR metabolomic fingerprints of acute-on-chronic liver failure in intensive care unit patients with alcoholic cirrhosis. PLoS One. 2014;9:e89230. doi: 10.1371/journal.pone.0089230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Simon M, Morales JM, Modesto-Alapont V, Gonzalez-Marrachelli V, Vento-Rehues R, Jorda-Miñana A, Blanquer-Olivas J, Monleon D. Prognosis Biomarkers of Severe Sepsis and Septic Shock by 1H NMR Urine Metabolomics in the Intensive Care Unit. PLoS One. 2015;10:e0140993. doi: 10.1371/journal.pone.0140993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neugebauer S, Giamarellos-Bourboulis EJ, Pelekanou A, Marioli A, Baziaka F, Tsangaris I, Bauer M, Kiehntopf M. Metabolite Profiles in Sepsis: Developing Prognostic Tools Based on the Type of Infection. Crit Care Med. 2016;44:1649–1662. doi: 10.1097/CCM.0000000000001740. [DOI] [PubMed] [Google Scholar]
- 29.Kauppi AM, Edin A, Ziegler I, Mölling P, Sjöstedt A, Gylfe Å, Strålin K, Johansson A. Metabolites in Blood for Prediction of Bacteremic Sepsis in the Emergency Room. PLoS One. 2016;11:e0147670. doi: 10.1371/journal.pone.0147670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manna SK, Patterson AD, Yang Q, Krausz KW, Idle JR, Fornace AJ, Gonzalez FJ. UPLC-MS-based urine metabolomics reveals indole-3-lactic acid and phenyllactic acid as conserved biomarkers for alcohol-induced liver disease in the Ppara-null mouse model. J Proteome Res. 2011;10:4120–4133. doi: 10.1021/pr200310s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manna SK, Thompson MD, Gonzalez FJ. Application of mass spectrometry-based metabolomics in identification of early noninvasive biomarkers of alcohol-induced liver disease using mouse model. Adv Exp Med Biol. 2015;815:217–238. doi: 10.1007/978-3-319-09614-8_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stefanescu H, Suciu A, Romanciuc F, Crisan D, Procopet B, Radu C, Tantau M, Socaciu C, Grigorescu M. Lyso-phosphatidylcholine: A potential metabolomic biomarker for alcoholic liver disease? Hepatology. 2016;64:678–679. doi: 10.1002/hep.28630. [DOI] [PubMed] [Google Scholar]
- 33.Rachakonda V, Gabbert C, Raina A, Li H, Malik S, DeLany JP, Behari J. Stratification of risk of death in severe acute alcoholic hepatitis using a panel of adipokines and cytokines. Alcohol Clin Exp Res. 2014;38:2712–2721. doi: 10.1111/acer.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rachakonda V, Gabbert C, Raina A, Bell LN, Cooper S, Malik S, Behari J. Serum metabolomic profiling in acute alcoholic hepatitis identifies multiple dysregulated pathways. PLoS One. 2014;9:e113860. doi: 10.1371/journal.pone.0113860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ascha M, Wang Z, Ascha MS, Dweik R, Zein NN, Grove D, Brown JM, Marshall S, Lopez R, Hanouneh IA. Metabolomics studies identify novel diagnostic and prognostic indicators in patients with alcoholic hepatitis. World J Hepatol. 2016;8:499–508. doi: 10.4254/wjh.v8.i10.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kimhofer T, Fye H, Taylor-Robinson S, Thursz M, Holmes E. Proteomic and metabonomic biomarkers for hepatocellular carcinoma: a comprehensive review. Br J Cancer. 2015;112:1141–1156. doi: 10.1038/bjc.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang B, Chen D, Chen Y, Hu Z, Cao M, Xie Q, Chen Y, Xu J, Zheng S, Li L. Metabonomic profiles discriminate hepatocellular carcinoma from liver cirrhosis by ultraperformance liquid chromatography-mass spectrometry. J Proteome Res. 2012;11:1217–1227. doi: 10.1021/pr2009252. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, Hong Z, Tan G, Dong X, Yang G, Zhao L, Chen X, Zhu Z, Lou Z, Qian B, et al. NMR and LC/MS-based global metabolomics to identify serum biomarkers differentiating hepatocellular carcinoma from liver cirrhosis. Int J Cancer. 2014;135:658–668. doi: 10.1002/ijc.28706. [DOI] [PubMed] [Google Scholar]
- 39.Lin L, Huang Z, Gao Y, Yan X, Xing J, Hang W. LC-MS based serum metabonomic analysis for renal cell carcinoma diagnosis, staging, and biomarker discovery. J Proteome Res. 2011;10:1396–1405. doi: 10.1021/pr101161u. [DOI] [PubMed] [Google Scholar]
- 40.Chen T, Xie G, Wang X, Fan J, Qiu Y, Zheng X, Qi X, Cao Y, Su M, Wang X, et al. Serum and urine metabolite profiling reveals potential biomarkers of human hepatocellular carcinoma. Mol Cell Proteomics. 2011;10:M110.004945. doi: 10.1074/mcp.M110.004945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao R, Cheng J, Fan C, Shi X, Cao Y, Sun B, Ding H, Hu C, Dong F, Yan X. Serum Metabolomics to Identify the Liver Disease-Specific Biomarkers for the Progression of Hepatitis to Hepatocellular Carcinoma. Sci Rep. 2015;5:18175. doi: 10.1038/srep18175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ressom HW, Xiao JF, Tuli L, Varghese RS, Zhou B, Tsai TH, Ranjbar MR, Zhao Y, Wang J, Di Poto C, et al. Utilization of metabolomics to identify serum biomarkers for hepatocellular carcinoma in patients with liver cirrhosis. Anal Chim Acta. 2012;743:90–100. doi: 10.1016/j.aca.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fitian AI, Cabrera R. Disease monitoring of hepatocellular carcinoma through metabolomics. World J Hepatol. 2017;9:1–17. doi: 10.4254/wjh.v9.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen S, Kong H, Lu X, Li Y, Yin P, Zeng Z, Xu G. Pseudotargeted metabolomics method and its application in serum biomarker discovery for hepatocellular carcinoma based on ultra high-performance liquid chromatography/triple quadrupole mass spectrometry. Anal Chem. 2013;85:8326–8333. doi: 10.1021/ac4016787. [DOI] [PubMed] [Google Scholar]
- 45.Fages A, Duarte-Salles T, Stepien M, Ferrari P, Fedirko V, Pontoizeau C, Trichopoulou A, Aleksandrova K, Tjønneland A, Olsen A, et al. Metabolomic profiles of hepatocellular carcinoma in a European prospective cohort. BMC Med. 2015;13:242. doi: 10.1186/s12916-015-0462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goossens C, Nahon P, Le Moyec L, Triba MN, Bouchemal N, Amathieu R, Ganne-Carrié N, Ziol M, Trinchet JC, Sellier N, et al. Sequential Serum Metabolomic Profiling after Radiofrequency Ablation of Hepatocellular Carcinoma Reveals Different Response Patterns According to Etiology. J Proteome Res. 2016;15:1446–1454. doi: 10.1021/acs.jproteome.5b01032. [DOI] [PubMed] [Google Scholar]
- 47.Baniasadi H, Gowda GA, Gu H, Zeng A, Zhuang S, Skill N, Maluccio M, Raftery D. Targeted metabolic profiling of hepatocellular carcinoma and hepatitis C using LC-MS/MS. Electrophoresis. 2013;34:2910–2917. doi: 10.1002/elps.201300029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou L, Liao Y, Yin P, Zeng Z, Li J, Lu X, Zheng L, Xu G. Metabolic profiling study of early and late recurrence of hepatocellular carcinoma based on liquid chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;966:163–170. doi: 10.1016/j.jchromb.2014.01.057. [DOI] [PubMed] [Google Scholar]
- 49.Beyoğlu D, Imbeaud S, Maurhofer O, Bioulac-Sage P, Zucman-Rossi J, Dufour JF, Idle JR. Tissue metabolomics of hepatocellular carcinoma: tumor energy metabolism and the role of transcriptomic classification. Hepatology. 2013;58:229–238. doi: 10.1002/hep.26350. [DOI] [PMC free article] [PubMed] [Google Scholar]