Abstract
Aggregated forms of α-synuclein play a crucial role in the pathogenesis of synucleinopathies such as Parkinson’s disease (PD). However, the molecular mechanisms underlying the pathogenic effects of α-synuclein are not completely understood. Here we show that asparagine endopeptidase (AEP) cleaves human α-synuclein, triggers its aggregation and escalates its neurotoxicity, thus leading to dopaminergic neuronal loss and motor impairments in a mouse model. AEP is activated and cleaves human α-synuclein at N103 in an age-dependent manner. AEP is highly activated in human brains with PD, and it fragments α-synuclein, which is found aggregated in Lewy bodies. Overexpression of the AEP-cleaved α-synuclein1–103 fragment in the substantia nigra induces both dopaminergic neuronal loss and movement defects in mice. In contrast, inhibition of AEP-mediated cleavage of α-synuclein (wild type and A53T mutant) diminishes α-synuclein’s pathologic effects. Together, these findings support AEP’s role as a key mediator of α-synuclein-related etiopathological effects in PD.
PD is characterized by the degeneration of dopaminergic neurons in the substantia nigra (SN) pars compacta. The etiology of PD appears to be multifactorial, involving both genetic and environmental components. To date, several cellular mechanisms, such as aberrant protein folding, oxidative stress and mitochondrial dysfunction have been implicated in the development and progression of this disease1. Most PD cases are sporadic, and their pathogenesis is unclear. However, several cases of familial PD have been described in which mutant genes have been identified as being causative in this disorder. These genes include the abundant presynaptic protein α-synuclein2,3 and two components of the proteasome pathway: L1 ubiquitin C-terminal hydrolase4 and parkin5. The misfolding of α-synuclein, the main component of Lewy pathology, plays a key role in the etiopathogenesis of PD, because the disorder is characterized by the accumulation of intraneuronal protein aggregates (Lewy bodies and Lewy neurites)6. Several mutations in the gene encoding α-synuclein, including A30P, A53T and E64K, have been identified in dominant forms of familial PD2,3. Moreover, duplication or triplication of the wild-type α-synuclein-encoding gene also causes PD, thus indicating that an increased α-synuclein protein level is sufficient to cause the disease. Furthermore, single-nucleotide polymorphisms in the α-synuclein-encoding gene are associated with an increased risk of developing sporadic PD7. The potential mechanisms of α-synuclein in PD have been explored by transgenic expression of human α-synuclein in various models8–10. Although these studies have demonstrated a pathological effect resulting from transgenic expression, including the formation of inclusion bodies, the molecular mechanisms involved in neurodegeneration remain obscure.
The physiological function of α-synuclein is poorly understood11. α-Synuclein is widely expressed in the nervous system, where it is found in presynaptic nerve terminals, closely associated with presynaptic vesicles11. The neurotoxicity of α-synuclein may be related to its oligomerization and fibrillization12 or may be due to a loss of functional protein, owing to the formation of insoluble aggregates. Mounting evidence indicates that C-terminal truncation promotes α-synuclein fibrillization13–16, in a process mediated by numerous proteases such as calpain17,18 and cathepsin D19,20. However, whether these proteases proteolytically cleave α-synuclein and mediate their pathological effects in an age-dependent manner in PD etiopathology remains unclear.
Mammalian AEP (also called legumain) is an endolysosomal cysteine protease that cleaves proteins after asparagine residues. AEP is synthesized as an inactive zymogen, and its activation requires sequential removal of C- and N-terminal propeptides at different pH thresholds21,22. AEP activity is controlled by the cysteine protease inhibitor cystatin C23. Recently, we have reported that neuronal AEP is activated by acidosis during excitotoxicity and contributes to neuronal apoptosis by degrading the DNase inhibitor SET24,25. Moreover, AEP-generated SET fragments inhibit protein phosphatase-2A and enhance tau hyperphosphorylation26. We have also shown that AEP cleaves TAR DNA-binding protein 43 in brain samples from humans with frontotemporal lobar degeneration27. Most recently, we have found that AEP cleaves tau at residues N255 and N368, thereby mediating formation of neurofibrillary tangles in human brains with Alzheimer’s disease (AD)28. Further, we have found that AEP acts as a δ-secretase that cleaves APP at residues N373 and N585, thereby regulating BACE1 fragmentation of the C-terminal fragment of APP and resulting in β-amyloid generation. Deletion of AEP from tau P301S or 5XFAD transgenic mice substantially alleviates the pathological and cognitive defects28,29.
PD is a chronic neurodegenerative disease, and aging is the major risk factor for PD development. We have recently reported that AEP protein levels are increased in the brain in an age-dependent manner28,29. Hence, it is possible that AEP might also process α-synuclein in the brains of people with PD during aging. In the current study, we tested this hypothesis and found that AEP cleaves α-synuclein at N103 in the human PD brain and promotes α-synuclein aggregation and neurotoxicity. Interestingly, AEP is activated in an age-dependent manner and contributes to and/or is concurrent with α-synuclein truncation in the SN. Overexpression of the α-synuclein1–103 fragment in the rodent SN induces dopaminergic neurodegeneration and a resultant motor phenotype, whereas blockade of cleavage of α-synuclein (either wild type or A53T mutant) by AEP ameliorates these α-synuclein-mediated pathological effects. Hence, our observations demonstrate that AEP contributes to the α-synuclein pathogenic activity in PD.
RESULTS
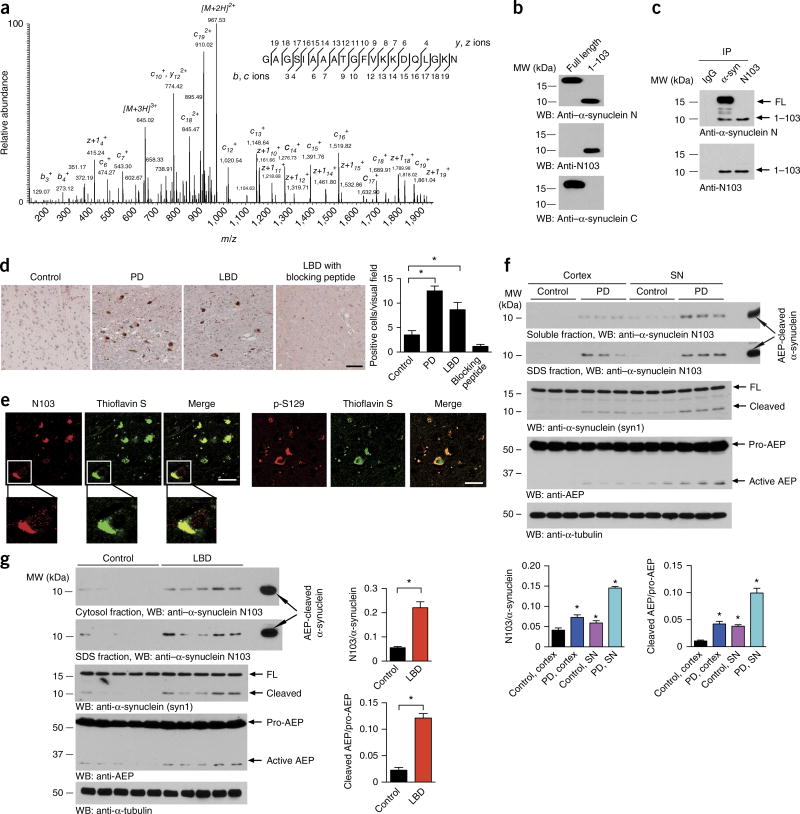
Cleavage of α-synuclein at N103 in PD and Lewy body dementia
To investigate the proteolytic processing of α-synuclein in PD, we conducted a proteomic analysis with immunoprecipitated α-synuclein from PD brains. Notably, LC–MS/MS analysis revealed an α-synuclein peptide fragment cleaved at N103 from the partial tryptic peptides (Fig. 1a). To detect the α-synuclein N103 fragment, we generated a neoepitope antibody (designated anti-N103) that specifically recognized the cleaved α-synuclein N103 fragment but not the full-length protein. ELISA measurements demonstrated that this antibody was highly selective for the α-synuclein1–103 fragment rather than intact α-synuclein (Supplementary Fig. 1a). Western blot analysis with recombinant full-length (FL) α-synuclein and its fragment comprising residues 1–103 confirmed that this antibody selectively recognized α-synuclein1–103 but not FL α-synuclein. The antibody also immunoprecipitated the cleaved N103 fragment from PD brains (Fig. 1b,c). To confirm that α-synuclein cleavage after N103 indeed occurs in people with synucleinopathy, we conducted immunohistochemistry (IHC) assays. IHC analysis of brain sections with anti–α-synuclein N103 clearly indicated that α-synuclein was cleaved after N103 in brains with PD or Lewy body dementia (LBD) but not in control brains without synucleinopathy. The antibody specificity was corroborated with a blocking peptide (Fig. 1d). Immunofluorescence (IF) staining confirmed that the α-synuclein N103 fragment was present as aggregates in Lewy bodies and synaptic structures (Fig. 1e and Supplementary Fig. 1b). Immunoblotting with the α-synuclein N103–specific antibody revealed that α-synuclein N103 was elevated specifically in both the soluble and insoluble fractions of SN tissues from PD brains, and its level was much higher than that observed in cortical tissues from the same subjects. By contrast, α-synuclein N103 was scarcely detectable in both brain regions in controls without synucleinopathy (Fig. 1f). Remarkably, α-synuclein N103 was readily detectable in brains with LBD compared with controls. These findings were further confirmed with anti-α-synuclein antibody. Interestingly, AEP was activated in the LBD brain samples, and this activation correlated with α-synuclein fragmentation patterns (Fig. 1g and Supplementary Fig. 1c–e). Thus, α-synuclein N103 fragmentation occurred predominantly in brains with PD or LBD but not in controls without synucleinopathy.
Figure 1.
α-synuclein is truncated after N103 in PD and LBD brains. (a) MS/MS spectrum showing the cleavage of α-synuclein after N103 in brain samples from subjects with PD. (b) Western blot (WB) with anti-α-synuclein antibodies. FL α-synuclein or the α-synuclein1–103 fragment were detected by immunoblotting with anti–α-synuclein N-terminus (N) antibody, anti–α-synuclein C-terminus (C) antibody and anti–α-synuclein N103 antibody. MW, molecular weight. (c) Immunoprecipitation (IP) of α-synuclein (α-syn) from human PD brain lysates with anti–α-synuclein N-terminus or anti–α-synuclein N103 antibodies. (d) Immunohistochemistry of the α-synuclein N103 fragment in SN sections from people with PD and cortex sections from people with LBD. Scale bar, 50 µm. Bar graph, quantification of positive cells in samples. Data are mean ± s.e.m.; n = 6 patients; *P < 0.05 by one-way ANOVA. (e) Brain sections were immunostained with anti–α-synuclein N103 or anti–phospho (p)-S129-α-synuclein (red) and then stained with thioflavin S (green), which stains Lewy bodies. Scale bars, 20 µm. Images are representative of nine sections from three subjects with LBD. (f,g) Western blots showing the presence of the α-synuclein N103 fragment in soluble and insoluble fractions of tissues from people with PD (f) or LBD (g), but not age-matched control tissues. α-Tubulin, loading control. Bar graphs show quantification as mean ± s.e.m.; n = 3 patients in f, n = 5 patients in g; *P < 0.05 compared with control, by one-way ANOVA in f and two-tailed Student’s t test in g. Results for an independent cohort of samples are shown in Supplementary Figure 1c,d. Blots shown are representative of three independent experiments; uncropped images are shown in Supplementary Data Set 1. Source data for graphs are available online.
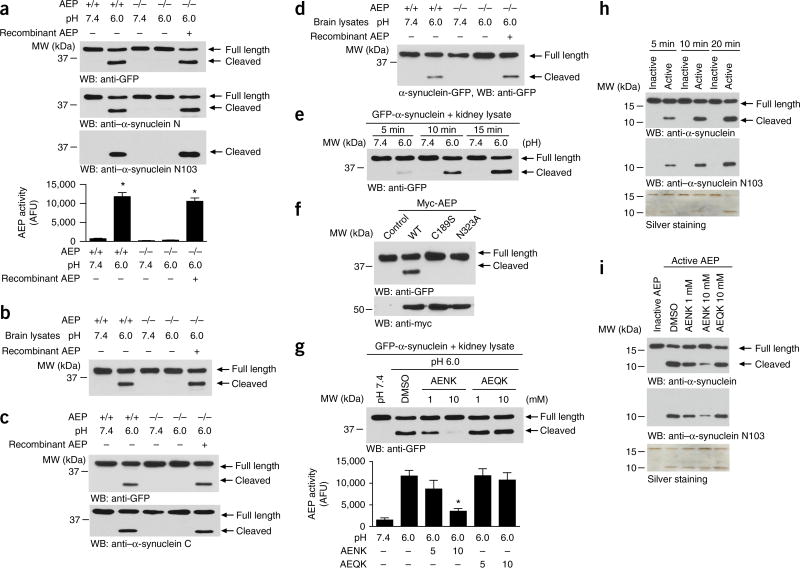
AEP cuts human α-synuclein at residue N103
AEP is the only reported mammalian enzyme that cleaves C terminal to asparagine. Hence, we hypothesized that AEP is responsible for the cleavage of α-synuclein after N103. To test this hypothesis, we transfected N-terminally GFP-tagged human α-synuclein into HEK293 cells and incubated cell lysates with kidney lysates (pH 7.4 or 6.0) prepared from wild-type or AEP-knockout mice. A truncated α-synuclein band was selectively observed in wild-type kidney lysates at pH 6.0 but was not observed in AEP-knockout kidney lysates or at pH 7.4. The cleavage of α-synuclein was reconstituted when exogenous active AEP was added to the AEP-knockout reactions (Fig. 2a). AEP enzymatic activity in the reactions was confirmed (Fig. 2a). GFP-tagged α-synuclein was also cleaved in mouse brain lysates in the same pattern as that in kidney lysates (Fig. 2b). We also tested the cleavage of C-terminally GFP-tagged α-synuclein in mouse kidney and brain lysates, and found the same results (Fig. 2c,d). When GFP-α-synuclein was incubated with wild-type kidney lysates for 5, 10 or 15 min, the intensity of the truncated α-synuclein fragment increased over time at pH 6.0 (Fig. 2e), thus indicating that AEP in the active kidney lysates truncates α-synuclein in a time-dependent manner. Cys189 is essential for the protease activity of AEP, and cleavage after N323 is obligatory for the maturation of the protease30. As expected, α-synuclein was cleaved by wild-type but not by mutated (C189S or N323A) AEP, thereby indicating that AEP’s enzymatic activity was responsible for the observed α-synuclein proteolytic cleavage (Fig. 2f). AEP is selectively inhibited by the small peptide AENK but not the control peptide AEQK24. Accordingly, AENK but not AEQK selectively antagonized GFP-α-synuclein cleavage by active AEP in a dose-dependent manner (Fig. 2g). Furthermore, recombinant active AEP directly cleaved recombinant α-synuclein in a time-dependent manner, and the resultant fragment was recognized by anti–α-synuclein N103 antibody. The cleavage was blocked by the AEP inhibitor AENK (Fig. 2h,i). Together, these results indicated that α-synuclein is a direct biological substrate of AEP.
Figure 2.
α-synuclein is a substrate of AEP. (a) α-synuclein cleavage assay in kidney lysates. N-terminal GFP-tagged α-synuclein was incubated with kidney lysates from wild-type (+/+) or AEP-knockout (−/−) mice at pH 7.4 or pH 6.0 at 37 °C for 15 min. Western blot (WB) showing α-synuclein cleavage at pH 6.0 (top) when AEP was activated (bottom). Bar graph shows data as mean ± s.e.m.; n = 3 independent experiments; *P < 0.05 compared with the pH 7.4 group by one-way ANOVA. AFU, arbitrary fluorescence units. (b) α-synuclein cleavage assay in brain lysates. N-terminally GFP-tagged α-synuclein was incubated with brain lysates from wild-type or AEP-knockout mice at pH 7.4 or pH 6.0 at 37 °C for 15 min. (c,d) Processing of C-terminally GFP-tagged α-synuclein in mouse kidney lysates (c) and brain lysates (d). (e) Time-dependent cleavage of α-synuclein by AEP. (f) α-Synuclein cleavage by wild-type and mutant AEP. (g) The proteolysis of α-synuclein is blocked by AENK peptide but not AEQK peptide (top). The effect of AENK on AEP was confirmed by an enzymatic activity assay (bottom). Graph shows mean ± s.e.m.; n = 3 independent experiments; *P < 0.05 compared with wild type, by one-way ANOVA. (h) Recombinant AEP has high cleavage activity toward recombinant α-synuclein. The AEP-generated α-synuclein fragment was recognized by anti–α-synuclein N103 antibody. Silver-stained gel shows all the fragments. (i) AEP inhibitor blocks the cleavage of recombinant α-synuclein by AEP. Silver-stained gel shows all the fragments. Blots shown are representative of three independent experiments; uncropped images are shown in Supplementary Data Set 1. Source data for graphs are available online.
To determine whether there are multiple AEP-cleavage sites in α-synuclein, we conducted a mapping assay with a variety of truncated α-synuclein recombinant proteins. FL α-synuclein produced a fragment of the same size as the 1–103 fragment, thus indicating that α-synuclein was cut near residue 103 (Supplementary Fig. 2a). MS analysis identified the α-synuclein N103 fragment from purified mammalian GST-α-synuclein recombinant proteins cleaved by active AEP in vitro (Supplementary Fig. 2b). Because AEP selectively cleaves its substrates after asparagine residues, and human α-synuclein contains three asparagine residues (N65, N103 and N122), we prepared three α-synuclein point mutants with asparagine changed to alanine. Both N-terminal and C-terminal GFP-tagged α-synuclein N65A and N122A mutants were cleaved as efficiently as wild-type α-synuclein by AEP, whereas the N103A mutation completely abolished cleavage (Supplementary Fig. 2c,d). Interestingly, mouse α-synuclein does not contain residue N103, but human α-synuclein does. Accordingly, human but not mouse α-synuclein was selectively fragmented by AEP (Supplementary Fig. 2e,f).
To confirm the interaction between AEP and α-synuclein, we performed coimmunoprecipitation and found that α-synuclein interacted with AEP in human PD brains (Supplementary Fig. 3a), where it may cleave α-synuclein. The proteolytic actions in human PD brain lysates were selectively blocked by anti-AEP but not control IgG, thus supporting the specific processing of α-synuclein N103 by AEP (Supplementary Fig. 3b). Immunofluorescence staining with human brain slides revealed that AEP strictly colocalized with LAMP1, the specific marker for lysosomes in control brains, whereas it leaked out into the cytoplasm and was not tightly colocalized with LAMP1 in PD brains (Supplementary Fig. 3c). Indeed, our subcellular fractionation assay also demonstrated that the activity of AEP was enhanced in the cytoplasm in PD brains compared with controls (Supplementary Fig. 3d). Cleavage assays with exogenous α-synuclein recombinant proteins demonstrated that PD and LBD brain cytosolic fractions displayed much stronger N103 cleavage activities than those of control cytosolic fractions, whereas the lysosomal fractions exhibited similar effects among control, PD and LBD brains (Supplementary Fig. 3e). These results suggested that AEP may leak from the lysosomes into the cytoplasm, where it cleaves α-synuclein in synucleinopathies.
AEP cleaves α-synuclein independently of post-translational modifications
Phosphorylation of α-synuclein at S129 and Y125 regulates α-synuclein aggregation and toxicity31. To test whether S129 phosphorylation influences α-synuclein cleavage by AEP, we conducted an in vitro cleavage assay using recombinant α-synuclein and S129-phosphorylated α-synuclein. The S129-phosphorylated and nonphosphorylated α-synuclein were cleaved in a time-dependent manner with similar rates, thus indicating that phosphorylation of α-synuclein at S129 does not interfere with AEP cleavage (Supplementary Fig. 4a). α-Synuclein is also phosphorylated at Y125 by the Src family protein tyrosine kinase Fyn32. Nonetheless, both wild-type human α-synuclein and the Y125F mutant exhibited comparable cleavage rates by AEP (Supplementary Fig. 4b). Moreover, phosphorylation of α-synuclein at Y125 by overexpression of constitutively active or kinase-dead Fyn mutants did not affect its cleavage rate, thus further confirming that Y125 phosphorylation does not affect human α-synuclein cleavage (Supplementary Fig. 4c,d). We also tested the cleavage rate of the familial PD-associated A53T and A30P missense mutations and found that these mutants were also cleaved at the same rate as wild-type α-synuclein (Supplementary Fig. 4e). A number of proteinases have been implicated in α-synuclein cleavage, including calpain, cathepsin D and the proteasome33. To explore their potential effects on N103 fragmentation, we used specific inhibitors or a protease-inhibitor cocktail inhibiting serine proteases, aminpeptidases, cysteine proteases and acid proteases. Notably, α-synuclein cleavage was inhibited by only the peptide inhibitor AENK but not by any of the other protease inhibitors or the protease-inhibitor cocktail (Supplementary Fig. 4f). Thus, these results indicated that AEP is the major protease cleaving human α-synuclein at N103, and its proteolytic activity is independent of other post-translational modifications.
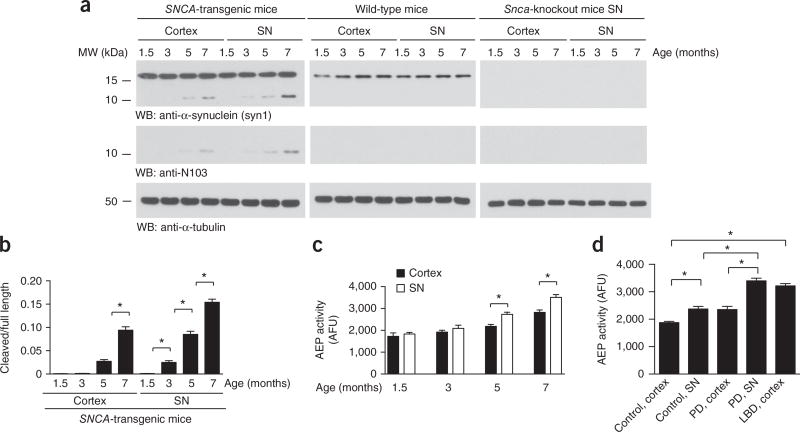
AEP is activated and cleaves α-synuclein in an age-dependent manner
We have recently reported that AEP is selectively upregulated and activated in the brain in an age-dependent manner28,29. To explore whether this enzyme truncates human α-synuclein in a similar temporal pattern, we used a mouse transgenic model with the mouse α-synuclein-encoding gene (Snca) replaced with a human version, and we performed immunoblotting on SN and cortex-tissue samples from mice at 1.5, 3, 5 and 7 months of age. We found that α-synuclein was cleaved at N103 in an age-dependent manner. Fragmentation was clearly more prominent in the SN than the cortex, in a manner tightly coupled to the spatial and temporal patterns of AEP activity. In contrast, no cleavage signals were detected in wild-type or Snca-knockout SN tissues (Fig. 3a–c). Therefore, these findings indicated that AEP specifically cleaves human α-synuclein in an age-dependent manner, more prominently in the SN region than in the cortex. We also extended these studies to wild-type mice and found that AEP activity increased in an age-dependent manner, and the SN activity was much higher than that in the cortex (Supplementary Fig. 5). Interestingly, we observed the same result in SN and cortex samples from human PD brains (Fig. 3d). Hence, AEP is prone to being activated in the SN versus the cortex.
Figure 3.
AEP is activated and cleaves α-synuclein in an age-dependent manner. (a) Western blot (WB) showing the processing of α-synuclein during aging in human SNCA-transgenic mice. α-Tubulin, loading control. (b) Quantification of AEP-cleaved α-synuclein in the cortex and SN of SNCA-transgenic mice at different ages. Data are mean ± s.e.m.; n = 3 mice per group; *P < 0.05 by one-way ANOVA. (c) AEP activity in the cortex and SN in SNCA-transgenic mice at different ages. Data are mean ± s.e.m.; n = 5 mice per group; *P < 0.05 by one-way ANOVA. AFU, arbitrary fluorescence units. (d) AEP activity in the cortex and SN from PD, LBD and age-matched control tissues. Data are mean ± s.e.m.; n = 4 different subjects for control and LBD samples; n = 3 different subjects for PD samples; *P < 0.05 by one-way ANOVA. Blots shown are representative of three independent experiments; uncropped images are shown in Supplementary Data Set 1. Source data for graphs are available online.
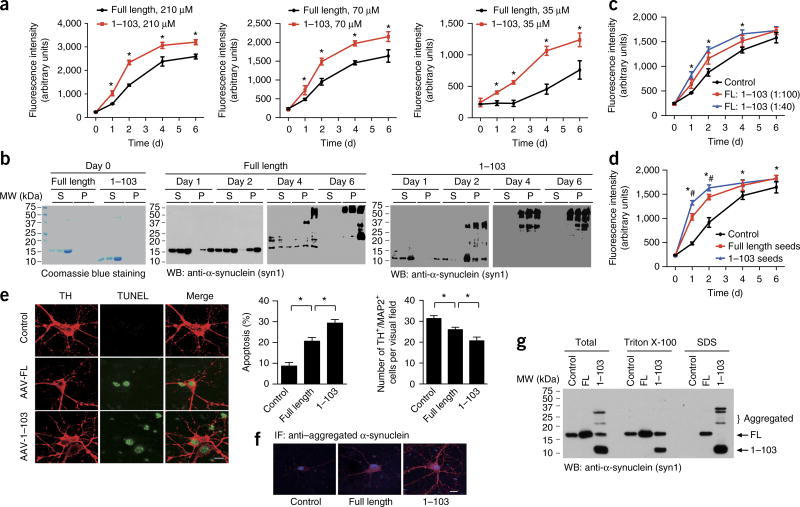
Cleavage of α-synuclein by AEP promotes its aggregation and toxicity
To determine the effect of AEP cleavage of α-synuclein on fibrillization, we monitored the kinetics of filament formation by FL α-synuclein or the AEP-derived α-synuclein1–103 fragment at different concentrations, by using thioflavin T staining. We found that the α-synuclein1–103 fragment aggregated much faster and to a greater extent than did FL α-synuclein (Fig. 4a). Ultracentrifugation assays revealed that more aggregated α-synuclein1–103 than FL α-synuclein was present in the pellet (Fig. 4b). Electron microscopy confirmed the aggregation of α-synuclein into fibrils (Supplementary Fig. 6a). Furthermore, the α-synuclein1–103 fragment significantly promoted the aggregation of FL α-synuclein even at very low concentrations (Fig. 4c). We also tested the seeding capacity of fibrils from the α-synuclein1–103 fragment and that from the full-length protein. We found that α-synuclein1–103 fibrils, compared with FL α-synuclein fibrils, seeded the α-synuclein with a much higher capacity (Fig. 4d). Thus, these findings suggested that AEP cleavage of α-synuclein may promote α-synuclein aggregation.
Figure 4.
AEP cleavage of α-synuclein promotes α-synuclein aggregation and toxic effects. (a) Thioflavin T assay showing the kinetics of aggregation of purified recombinant FL α-synuclein and the α-synuclein1–103 fragment at different concentrations. Data are mean ± s.e.m.; n = 3 independent experiments; *P < 0.05 by two-tailed Student’s t test. (b) Ultracentrifugation assay. The soluble and aggregated α-synuclein was analyzed by western blotting (S, supernatant; P, pellet). The blots shown are representative of three independent experiments. (c) The α-synuclein1–103 fragment promotes the aggregation of FL α-synuclein. 70 µM α-synuclein aggregation was assessed with thioflavin T assays in the presence of 1:100 or 1:40 α-synuclein1–103 fragment. Data are mean ± s.e.m.; n = 3 independent experiments; *P < 0.05 compared with control, by one-way ANOVA. (d) Seeding of full-length α-synuclein by fibrils of the α-synuclein1–103 fragment (blue) or fibrils of FL α-synuclein (red). Data are mean ± s.e.m.; n = 3 independent experiments; *P < 0.05 compared with control; #P < 0.05 compared with fibrils from FL α-synuclein, by one-way ANOVA. (e) The neurotoxicity of FL α-synuclein and the α-synuclein1–103 fragment. α-Synuclein (without tag) was expressed in primary cultured SN neurons. Cell death was determined by TUNEL staining and TH+/MAP2+ cell counts. Scale bar for micrographs, 20 µm. Data shown in graphs are mean ± s.e.m.; n = 3 independent experiments; *P < 0.05 by one-way ANOVA. (f) Immunostaining showing the aggregation of α-synuclein. Scale bar, 20 µm. (g) Western blot (WB) showing the aggregation of α-synuclein1–103 fragments into higher-molecular-weight species. Blots shown are representative of three independent experiments; uncropped images are shown in Supplementary Data Set 1. Source data for graphs are available online.
α-Synuclein truncation and oligomerization are tightly associated with its neurotoxicity12; therefore, we conducted dopaminergic neuronal cell death assays. As expected, overexpression of α-synuclein induced apoptosis of dopaminergic neurons, and this effect was more pronounced with the α-synuclein1–103 fragment (Fig. 4e). In addition, the α-synuclein1–103 fragment formed more aggregates, as indicated by IF staining with an antibody specific to aggregated α-synuclein (Fig. 4f). Furthermore, the α-synuclein1–103 fragment formed more abundant higher-molecular-weight species than did FL α-synuclein (Fig. 4g). Hence, the AEP-derived α-synuclein N103 fragment is more prone to aggregation and exhibits stronger neurotoxicity. IF staining and subcellular fractionation assays indicated that most FL and truncated α-synuclein localized in the cytosolic fraction (Supplementary Fig. 6b,c).
AEP-cleaved α-synuclein induces dopamine neuronal loss and motor deficits in vivo
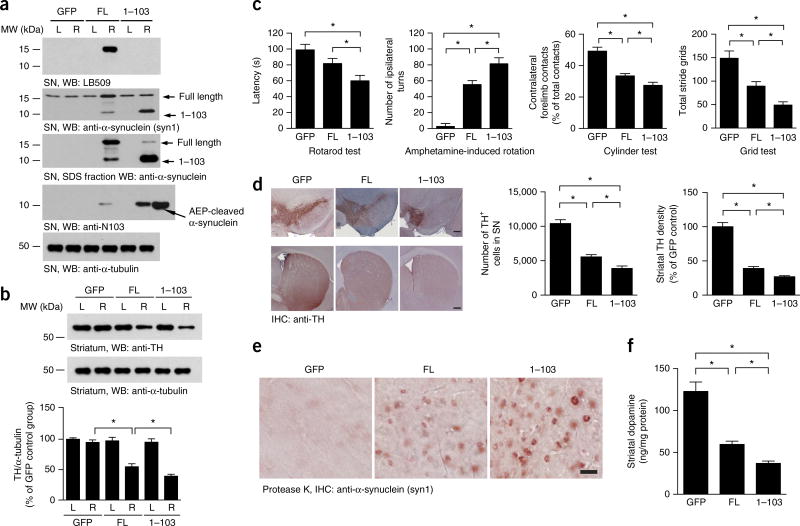
To assess the pathological roles of the α-synuclein1–103 fragment in vivo, we used adenoassociated viruses (AAVs) expressing either FL wild-type α-synuclein or the 1–103 fragment, which were delivered by unilateral injection into the mouse SN. Four months after the vector delivery, we performed IHC and IF staining on the brain sections to validate transduction. IHC analysis with LB509, an antibody recognizing residues 115–122 of human α-synuclein, revealed that FL α-synuclein was strongly expressed, whereas the α-synuclein1–103 fragment was not detected (Supplementary Fig. 7a). By contrast, IF with the specific anti–α-synuclein N103 antibody demonstrated robust immunoreactivity toward α-synuclein1–103 in brains injected with AAVs expressing the α-synuclein1–103 fragment. In addition, some α-synuclein N103 signal was detectable in brains injected with FL human α-synuclein (Supplementary Fig. 7b), thus indicating that the FL human α-synuclein had been truncated at N103 in the SN. Immunoblotting with the same specific antibodies selectively recognizing FL human α-synuclein or the N103 truncation also confirmed these observations (Fig. 5a). As expected, overexpression of the α-synuclein1–103 fragment resulted in a significant loss of tyrosine hydroxylase (TH) expression in the striatum. In contrast, the effect of FL α-synuclein expression was much less pronounced (Fig. 5b). To investigate the effect of the α-synuclein1–103 fragment on motor function, we performed a panel of behavioral tests including rotarod tests, amphetamine-induced rotational behavior tests, cylinder tests and grid assays. The rotarod test showed decreased latency in mice injected with AAV-α-synuclein1–103 compared with FL α-synuclein (Fig. 5c), thus indicating that the α-synuclein1–103 fragment triggers much more severe dopaminergic neuronal loss than does FL. In agreement with this finding, overexpression of the α-synuclein1–103 fragment, as compared with FL overexpression, produced increased amphetamine-induced rotational asymmetry and increased forepaw impairment (cylinder test) (Fig. 5c). The grid test also demonstrated an evident motor deficit in AAV-α-synuclein1–103 mice compared with AAV-FL mice (Fig. 5c). Together, these behavioral tests indicated that the human α-synuclein1–103 fragment induces more severe motor impairment than does the FL counterpart. Quantification of TH immunoreactivity (stereological cell counts of TH-positive neurons and densitometry of TH-positive striatal terminals) supported these findings: the SN and striatum in mice injected with AAV-α-synuclein1–103, compared with AAV-α-synuclein FL, contained significantly fewer TH-positive neurons and termini (Fig. 5d). Immunostaining of the proteinase K–treated slides indicated more aggregates in mice injected in the SN with AAV-α-synuclein1–103 compared with AAV-α-synuclein FL (Fig. 5e). Finally, HPLC analysis of striatal tissues showed significantly decreased dopamine concentrations in mice injected with the AAV-α-synuclein1–103 fragment compared with AAV-α-synuclein FL (Fig. 5f). Hence, these findings demonstrated that the AEP-generated α-synuclein1–103 fragment is sufficient to induce a PD-like pattern of neurodegeneration.
Figure 5.
The α-synuclein1–103 fragment induces dopaminergic cell death. (a) Western blots (WB) showing the expression of FL α-synuclein and the α-synuclein1–103 fragment in SN. α-Tubulin, loading control. L, left; R, right. (b) Western blots showing the expression of TH in the striatum, Bar graph, quantification; data are mean ± s.e.m.; n = 3 mice; *P < 0.05 by one-way ANOVA. Blots shown are representative of three independent experiments; uncropped images are shown in Supplementary Data Set 1. (c) The α-synuclein1–103 fragment induces behavioral impairment, as demonstrated by rotarod tests, amphetamine-induced rotation tests, cylinder tests and grid tests. Data are mean ± s.e.m.; n = 9 mice per group; *P < 0.05 by one-way ANOVA. Source data are available online. (d) TH immunostaining of the striatum and SN. Right, unbiased stereological cell counts in the SN and total density of striatal dopaminergic terminals. Scale bars, 200 µm. Bar graph, quantification; data are mean ± s.e.m.; n = 5 mice per group; *P < 0.05 by one-way ANOVA. (e) Immunostaining showing the aggregation of α-synuclein in SN slides after incubation with 10 µg/ml proteinase K. (f) Concentrations of dopamine in the striatal tissues, as determined by HPLC. Data are mean ± s.e.m.; n = 4 mice per group; *P < 0.05 by one-way ANOVA. Source data for graphs are available online.
Knockout of AEP ameliorates α-synuclein’s pathological effects
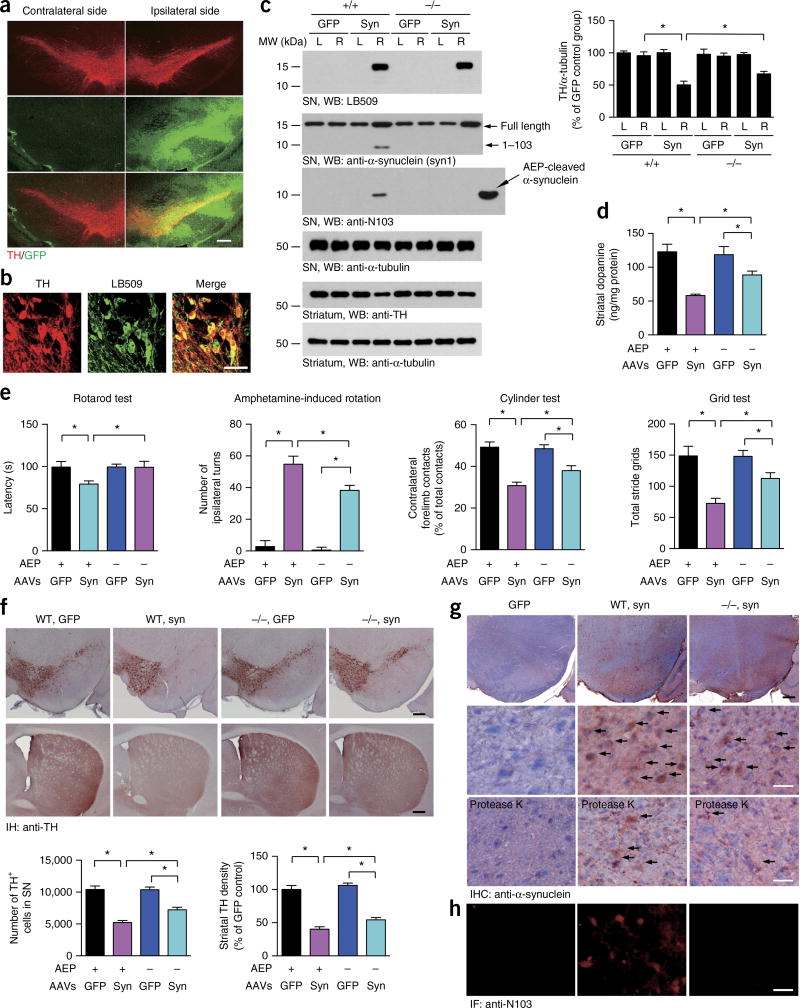
We then sought to further confirm the role of AEP in the cleavage of α-synuclein and the subsequent dopaminergic neuronal loss and motor deficits. To do so, we used AAVs to overexpress human α-synuclein in wild-type and AEP-knockout mice. Animals were euthanized 4 months after the injection, and IF analysis confirmed that TH-positive dopaminergic neurons in the injected SN expressed GFP or human α-synuclein (Fig. 6a,b). Immunoblotting confirmed these observations. As predicted, FL α-synuclein was selectively cleaved at N103 by AEP in wild-type but not knockout mice. Injection of α-synuclein virus induced a significant striatal TH-immunoreactivity loss. Deletion of AEP partially reversed the loss of striatal TH expression (Fig. 6c). Similarly, the striatal dopamine content was significantly decreased in wild-type mice compared with AEP-knockout mice (Fig. 6d). In agreement with these observations, overexpression of human α-synuclein induced more severe motor deficits in wild-type mice than GFP control mice, as detected through the motor tests described above. Again, this effect was significantly alleviated in AEP-knockout mice (Fig. 6e). A quantitative analysis of TH-positive neurons demonstrated that dopaminergic neurons were drastically lost in the SN, with concomitant denervation of the striatum, when human α-synuclein was overexpressed in wild-type mice, as compared with GFP control mice. This neurotoxic effect was reversed when AEP was depleted (Fig. 6f). IHC with anti–human α-synuclein and IF analysis with anti–α-synuclein N103 showed that α-synuclein was successfully overexpressed in the SN regions and that α-synuclein was truncated at N103 in wild-type but not knockout mice (Fig. 6g,h). The intraneuronal aggregation of α-synuclein was more pronounced in wild-type mice than knockout mice, and the aggregates were partially resistant to proteinase K digestion (Fig. 6g). Furthermore, the α-synuclein aggregates exhibited coimmunostaining with 14-3-3 and ubiquitin, thus suggesting that these aggregates were Lewy body–like inclusions (Supplementary Fig. 7c–f). Therefore, these data indicated that the deletion of AEP ameliorates α-synuclein fragmentation, attenuates its aggregation and alleviates its neurotoxicity.
Figure 6.
Deletion of AEP attenuates α-synuclein aggregation and dopaminergic cell death in mice overexpressing human α-synuclein. (a) Immunofluorescence showing the expression of AAV-GFP (green) in TH-positive neurons (red). Scale bar, 200 µm. (b) Double-label immunofluorescence images showing the AAV-mediated expression of human α-synuclein (green) in TH-positive neurons (red). Scale bar, 50 µm. (c) Western blots (WB) showing the expression and truncation of human α-synuclein (syn) in the SN and striatum of wild-type (+/+) and AEP knockout (−/−) mice. L, left; R, right. α-Tubulin, loading control. Bar graph, quantification; data are mean ± s.e.m.; n = 3 mice per group; *P < 0.05 by one-way ANOVA. Blots shown are representative of three independent experiments; uncropped images are shown in Supplementary Data Set 1. (d) Concentrations of dopamine in the striatal tissues, as determined by HPLC. Data are mean ± s.e.m.; n = 4 mice per group; *P < 0.05 by one-way ANOVA. (e) Effect of AEP deletion on behavioral impairments induced by overexpression of α-synuclein, as shown by rotarod tests, amphetamine-induced rotation tests, cylinder tests and grid tests. Data are mean ± s.e.m.; n = 9 AAV-GFP-injected WT mice; n = 8 mice in the other three groups; *P < 0.05 by one-way ANOVA. (f) TH immunostaining of the striatum and SN. Bottom, unbiased stereological cell counts in the SN and the total density of striatal dopaminergic terminals. Scale bars, 200 µm. Bar graphs, quantification; data are mean ± s.e.m.; n = 5; *P < 0.05 by one-way ANOVA. (g) α-synuclein aggregation in the SN in AAV-injected mice. The brain slides were stained with anti–human α-synuclein with or without preincubation with proteinase K. The number of proteinase K–resistant α-synuclein inclusions in AEP knockout mice was only half of that in wild-type mice. Black scale bar, 200 µm. White scale bars, 50 µm. (h) IHC with anti–α-synuclein N103 antibody, showing the cleavage of α-synuclein in wild-type but not AEP-knockout mice. Scale bar, 50 µm. Source data for graphs are available online.
Blockade of α-synuclein (A53T) cleavage by AEP prevents its pathological activities
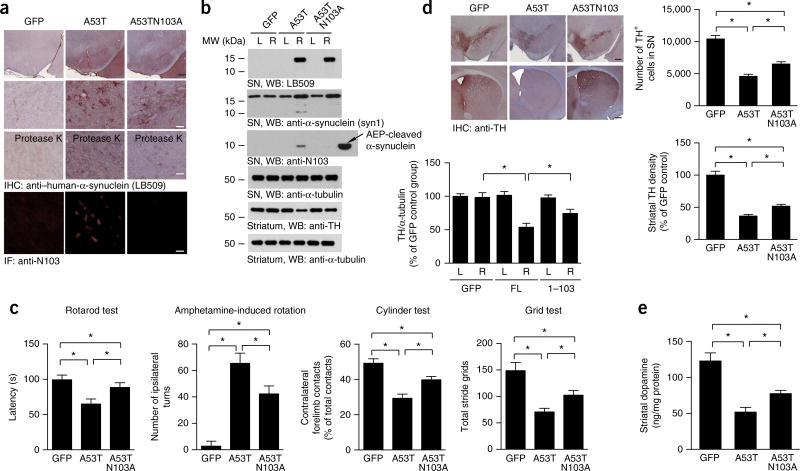
The A53T mutant of α-synuclein is linked to autosomal-dominant early-onset PD. Transgenic mice expressing mutant A53T show age-dependent formation of intraneuronal α-synuclein inclusions that recapitulate the features in humans with PD, and they also develop severe and complex motor impairment34,35. To assess whether these pathological effects caused by the A53T mutant are related to N103 cleavage by AEP, we generated an uncleavable mutant (A53TN103A) that was overexpressed through AAVs in the SN in wild-type mice. Four months after the AAV injection, expression of human α-synuclein (A53T) or its uncleavable mutant was confirmed with IHC. Proteinase K–resistant α-synuclein aggregates were more abundant after expression of A53T as compared with the uncleavable mutant (Fig. 7a). IF analysis with anti-N103 antibody showed that A53T proteins were clearly fragmented at N103; in contrast, no cleavage of A53TN103A proteins was observed (Fig. 7a). AEP activity assays indicated that the ratio between cytosolic and lysosomal AEP activities increased in mice injected with AAV-α-synuclein wild type and AAV-α-synuclein A53T, as compared with mice injected with AAV-GFP, thus indicating lysosomal leakage (Supplementary Fig. 7g). Immunoblotting verified that both mutants were highly expressed and that the A53T protein was truncated at N103, whereas the uncleavable mutant remained intact. In agreement with our findings above, mice injected with A53TN103A-mutant α-synuclein virus, as compared with A53T mice, were partially spared from motor deficits, thus implicating AEP cleavage of α-synuclein in movement disorders elicited by the A53T mutant (Fig. 7c). Accordingly, TH immunoreactivity in both the SN and striatum was significantly lower in A53T-expressing brains than in brains expressing the uncleavable mutant (Fig. 7b,d). Finally, striatal dopamine concentrations in mice injected with the uncleavable mutant were significantly higher than those in mice injected with the A53T mutant (Fig. 7e). Collectively, these data suggested that blockade of α-synuclein (A53T) cleavage by AEP antagonizes its deleterious pathological activities, thus again supporting PD-relevant AEP-mediated truncation of α-synuclein.
Figure 7.
Preservation of dopaminergic neurons in mice expressing uncleavable α-synuclein A53TN103A. (a) Immunostaining of FL α-synuclein and the N103 fragment in the SN in mice. The aggregation of α-synuclein was detected after the slides were preincubated with 10 µg/ml proteinase K. Black scale bar, 200 µm. White scale bars, 50 µm. (b) Western blots (WB) showing the expression and truncation of α-synuclein A53T and A53TN103A in the SN and striatum. L, left; R, right. α-Tubulin, loading control. Bar graph, quantification; data are mean ± s.e.m.; n = 3; *P < 0.05 by one-way ANOVA. Blots shown are representative of three independent experiments; uncropped images are shown in Supplementary Data Set 1. (c) The N103A mutation attenuates behavioral impairment induced by α-synuclein A53T. The mice underwent rotarod tests, amphetamine-induced rotation tests, cylinder tests and grid tests. Data are mean ± s.e.m.; n = 10 for the A53TN103A group; n = 9 for the GFP and A53T groups; *P < 0.05 by one-way ANOVA. (d) TH immunostaining of the striatum and SN. Right, unbiased stereological cell counts in the SN and total density of striatal dopaminergic terminals. Data are mean ± s.e.m.; n = 5; *P < 0.05 by one-way ANOVA. Scale bar, 200 µm. (e) Concentrations of dopamine in the striatal tissues, as determined by HPLC. Data are mean ± s.e.m.; n = 4; *P < 0.05 by one-way ANOVA. Source data for graphs are available online.
DISCUSSION
AEP is a lysosomal protease that leaks into the cytoplasm in neurodegenerative diseases26. Here we report that AEP cleaves human α-synuclein at N103 in an age-dependent manner and triggers α-synuclein aggregation and neurotoxicity, thus leading to dopaminergic neuronal loss and motor impairment. Notably, we found that AEP is highly activated in human PD and LBD brains compared with age-matched healthy controls, especially in the SN. As a consequence, human α-synuclein is cleaved at N103, and this fragment is present in Lewy bodies. In agreement with these observations, we showed that human α-synuclein was cleaved at N103 in human α-synuclein–transgenic mice in an age-dependent manner, and this activity was tightly coupled with the temporal pattern of AEP activity escalation. The age-dependent activation of AEP indicates that AEP expression is gradually increased, and/or endogenous AEP inhibitors, such as cystatin C, are decreased during aging. Furthermore, the pH in the brain gradually decreases during aging36, and this decrease may elicit AEP activation. Thus, AEP processes α-synuclein in an age-dependent manner. Next, we provided extensive evidence demonstrating that AEP directly cleaves human but not mouse α-synuclein at the N103 residue. Interestingly, although human α-synuclein shares 95% identity with its mouse counterpart, mouse α-synuclein does not have an N103 residue. Notably, the A53T mutation in human α-synuclein causes dominant forms of familial PD, whereas the fifty-third position in wild-type mouse α-synuclein is T (Supplementary Fig. 2e). Hence, mouse α-synuclein possesses different key residues from the human version, and these residues may exhibit distinct biological activities.
Cystatin C is the endogenous inhibitor of AEP. Mounting evidence implicates cystatin C in the etiology of neurodegenerative diseases including AD, stroke and epilepsy37. During aging, an elevated AEP level and/or decreased cystatin C level may mediate the gradual cleavage of α-synuclein and promote its aggregation during the pathogenesis of synucleinopathies. Here, we also provided evidence that AEP is secreted from lysosomes into the cytoplasm in human PD brains, where it may cleave α-synuclein (Supplementary Fig. 3). These observations are consistent with findings from previous reports indicating that the lysosomal AEP penetrates into the cytoplasm in human AD brains and cleaves both tau and SET, a PP2A inhibitor, thereby mediating AD pathogenesis26. In fact, AEP has been found to be active in the near-neutral pH milieus of the cytosol, nucleus and cell surface38.
Post-translational modifications including phosphorylation, truncation and ubiquitination can affect the structure and aggregation properties of α-synuclein39. C-terminal truncation promotes α-synuclein fibrillization, in a process mediated by numerous proteases including neurosin, calpain, cathepsin D and metalloproteinase12. Nevertheless, the roles of these proteases in the pathogenesis of PD remain elusive. Here we provided extensive biochemical evidence demonstrating that AEP cleaves α-synuclein at N103 in an age-dependent manner. Notably, the cleaved human α-synuclein1–103 fragment is more prone to aggregation than the full-length protein (Fig. 4) and forms Lewy body-like aggregations in the SN (Fig. 6, Supplementary Fig. 7), thus providing further evidence of the role of C-terminally truncated α-synuclein in the pathogenesis of neurodegenerative diseases including PD. We observed some minor truncated α-synuclein bands at ~12 kDa in human and mouse brains (Figs. 1, 5 and 7). This result is consistent with previous findings of distinct α-synuclein truncations at sites downstream of N103, including at positions 119 (ref. 14), 121 (ref. 40) and 122 (ref. 18), in synucleinopathies and in vivo models. Under different stresses or animal models, a variety of proteases including caspases or calpains may be activated and mediate α-synuclein proteolytic cleavage at various sites, thus contributing to α-synuclein aggregation and neurotoxicity. Nonetheless, AEP cleavage of α-synuclein at N103 during the aging process provides an additional mechanism accounting for AEP’s detrimental effect.
To test the hypothesis that AEP cleavage of human α-synuclein contributes to PD pathogenesis, we provided gain-of-function and loss-of-function evidence (Figs. 5–7). As expected, the human α-synuclein1–103 fragment was more potent than FL α-synuclein in provoking dopaminergic neuronal loss and motor deficits (Fig. 5). However, although overexpression of FL human α-synuclein in wild-type mice notably produced clear dopaminergic neurodegeneration, as compared with that in GFP controls, far fewer pathological hallmarks were observed in AEP-null mice (Fig. 6). This result suggested that AEP promotes the neurotoxic effect of α-synuclein. The same scenario applied to the A53T mutant, which was even more toxic. We demonstrated that blockade of A53T α-synuclein cleavage by AEP greatly suppressed its pathologic activities in PD (Fig. 7). Furthermore, the amount of α-synuclein1–103 fragments in human PD SN tissue and mouse SN injected with α-synuclein virus was comparable (Supplementary Fig. 8), thus suggesting that the amount in the human PD SN was sufficient to induce neurotoxicity. Together, our in vivo data support the hypothesis that AEP cleavage of human α-synuclein promotes the onset of PD pathologies. These findings notably indicate that the α-synuclein1–103 fragment might act as the major pathological trigger of dopaminergic neuronal loss in PD. Therefore, these findings suggest that AEP may be a novel drug target and that the inhibition of AEP may hold promise as a useful strategy for treating PD.
ONLINE METHODS
Mice
Human SNCA–transgenic mice carrying a transgene containing the human SNCA gene and a knockout allele of the mouse Snca gene were from Jackson Laboratory (stock no. 023837). The AEP-knockout mice on a mixed 129/Ola and C57BL/6 background were generated as previously reported41. Only male mice were used in the experiments. Animal care and handling was performed according to Emory Medical School guidelines. Sample size was determined in Power and Precision software (Biostat). The mice were randomized into different groups by using a random number table. Investigators were blinded to the group allocation during the animal experiments. The protocol was reviewed and approved by the Emory Institutional Animal Care and Use Committee.
Human tissue samples
Postmortem brain samples were dissected from the frozen brains of five control cases, LBD cases, and PD cases obtained from the Emory Alzheimer’s Disease Research Center. The study was approved by the biospecimen committee at Emory University. PD and LBD cases were clinically diagnosed and neuropathologically confirmed. Informed consent was obtained from all subjects.
Cell lines, transfection and infection
HEK293 cells were obtained from the ATCC and tested for mycoplasma contamination before use. Cells were transfected with plasmids encoding wild-type or point-mutant GFP-α-synuclein, myc-AEP, myc–AEP C189S, or myc–AEP N323A by the calcium phosphate precipitation method. AAVs were used to express wild-type, mutant and truncated α-synuclein in primary neurons and SH-SY5Y cells. The neurotoxicity was analyzed through TUNEL analysis or LDH assays.
In vitro α-synuclein cleavage assay
To assess the cleavage of α-synuclein by AEP in vitro, HEK293 cells were transfected with GST- or GFP-tagged α-synuclein DNA through the calcium phosphate precipitation method. 48 h after transfection, cells were collected, washed once in PBS, lysed in buffer (50 mM sodium citrate, 5 mM DTT, 0.1% CHAPS, pH 5.5, and 0.5% Triton X-100), and centrifuged for 10 min at 14,000g at 4 °C. The supernatant was then incubated with mouse kidney lysate or recombinant AEP at pH 7.4 or 6.0 at 37 °C for different time points. To test the effects of cathepsin, calpain and AEP inhibitors on the cleavage of α-synuclein by AEP, inhibitors were used against cathepsin (E64, Sigma-Aldrich), calpains (ALLN, Sigma-Aldrich) and AEP (AENK peptide inhibitor and inactive control AEQK). To measure the cleavage of purified α-synuclein fragments by AEP, GST-tagged α-synuclein was purified with glutathione beads. The purified α-synuclein protein was incubated with recombinant AEP protein (Novoprotein, 5 µg ml−1) in buffer (50 mM sodium citrate, 5 mM DTT, 0.1% CHAPS and 0.5% Triton X-100, pH 6.0). The samples were then boiled in 1× SDS loading buffer and analyzed by immunoblotting.
AEP activity assay
Tissue homogenates or cell lysates (10 µg) were incubated in 200 µl assay buffer (20 mM citric acid, 60 mM Na2HPO4, 1 mM EDTA, 0.1% CHAPS and 1 mM DTT, pH 6.0) containing 20 µM AEP substrate Z-Ala-Ala-Asn-AMC (Bachem). AMC released by substrate cleavage was quantified by measuring at 460 nm in a fluorescence plate reader at 37 °C for 1 h in kinetic mode for 5 min.
Mass spectrometry analysis
Protein samples were in-gel-digested with 10 ng/µl Glu-C. Peptide samples were resuspended in loading buffer (0.1% formic acid, 0.03% trifluoroacetic acid and 1% acetonitrile) and loaded onto a 20-cm nano-HPLC column (internal diameter, 75 µm) packed with Reprosil-Pur 120 C18-AQ 1.9-µm beads (Dr. Maisch) and eluted over a 2-h 1–50% buffer B reverse-phase gradient (buffer A, 0.1% formic acid and 1% acetonitrile in water; buffer B, 0.1% formic acid in acetonitrile) generated by a Dionex RSLCnano UPLC system (Thermo). Peptides were ionized with 2.0-kV electrospray ionization voltage from a nano-ESI source on an Orbitrap Fusion mass spectrometer (Thermo). Data-dependent acquisition of MS spectra at 120,000 resolution (FWHM), and MS/MS spectra were obtained in the Orbitrap after electron-transfer dissociation (ETD) with supplemental activation with high energy (EThcD) for peptide masses corresponding to the 3+ and 4+ charge states (645.3536 and 484.2669, respectively) of the peptide GAGSIAAATGFVKKDQLGKN. To identify AEP-cleavage sites in human α-synuclein, Proteome Discoverer 2.0 (PD) was used to search and match MS/MS spectra to a complete human proteome database (NCBI reference sequence revision 62, with 68746 entries) with a ±10-p.p.m. mass-accuracy threshold and allowable cleavages at glutamates and asparagines. A percolator was used to filter the peptide spectral matches (PSM) to a false discovery rate (FDR) of <1%. All MS/MS spectra for putative AEP-generated α-synuclein cleavage sites were manually inspected.
Generation of antibodies specific to AEP-generated α-synuclein (anti–α-synuclein N103)
Two rabbits were immunized with the peptide Ac-CVKKDQLGKN-OH, which included the nine amino acids in α-synuclein that precede the AEP-cleavage site at N103 as well as an N-terminal cysteine residue to allow for coupling to KLH. The rabbits were received booster injections four times with the immunizing peptide with 3-week intervals between injections. The antiserum was affinity purified by chromatography with the immunizing peptide and was then absorbed to a spanning peptide spanning the N103 cleavage site (Ac-CKDQLGKNEEGAPQE-amide). The titers against the immunizing peptide were determined by ELISA. The maximal dilution giving a positive response with chromogenic substrate for horseradish peroxidase was 1:30,000. The immunoactivity of the antiserum was further confirmed by western blotting and immunohistochemistry.
Western blot analysis
The mouse brain tissue or human tissue samples were lysed in lysis buffer (50 mM Tris, pH 7.4, 40 mM NaCl, 1 mM EDTA, 0.5% Triton X-100, 1.5 mM Na3VO4, 50 mM NaF, 10 mM sodium pyrophosphate and 10 mM sodium β-glycerophosphate, supplemented with a cocktail of protease inhibitors), and centrifuged for 15 min at 16,000g. The supernatant was boiled in SDS loading buffer. After SDS–PAGE, the samples were transferred to a nitrocellulose membrane. Primary antibodies to the following targets were used: GST-HRP (Sigma-Aldrich, GERPN1236), α-tubulin (Sigma-Aldrich, T9026), HA (Santa Cruz, H3663), myc (Santa Cruz, M4439), α-synuclein N terminus (Santa Cruz, sc-514908); anti-α-synuclein (syn1, clone 42, BD Bioscience, 610787), AEP (clone 6E3, gift from C. Watts), TH (Cell Signaling Technology, 2792), α-synuclein C terminus (Cell Signaling Technology, 2642) and α-synuclein LB509 (Thermo Fisher Scientific, 180215).
Immunostaining
Paraffin-embedded human brain sections or free-floating mouse brain sections were treated with 0.3% H2O2 for 10 min. Sections were thereafter washed three times in PBS, blocked in 1% BSA and 0.3% Triton X-100 for 30 min and incubated overnight with anti-TH (1:1,000) antibody, anti–α-synuclein N103 antibody (1:1,000) or α-synuclein LB509 antibody (1:500) at 4 °C. The signal was developed with a Histostain-SP kit (Invitrogen). To detect the localization of the α-synuclein1–103 fragment in Lewy bodies in human PD brain sections, the sections were incubated with rabbit anti–α-synuclein N103 primary antibody and mouse anti-α-synuclein antibody overnight at 4 °C. The slides were washed three times in PBS and incubated with Texas red–conjugated anti-mouse IgG and FITC-conjugated anti-rabbit IgG for 1 h at room temperature. The slides were washed three times in PBS, then covered with a glass cover with mounting solution and examined under a fluorescence microscope (Olympus). The primary cultured neurons were infected with AAVs encoding full-length α-synuclein or the α-synuclein1–103 fragment, and were double-stained for α-synuclein/TH, TUNEL/TH or TH/N103. The percentages of TUNEL+/TH+ cells over TH+ cells were calculated. The researchers who performed immunostaining and cell counting were blinded to the group allocations.
Coimmunoprecipitation
The mouse brain tissue samples were lysed in lysis buffer (50 mM Tris, pH 7.4, 40 mM NaCl, 1 mM EDTA, 0.5% Triton X-100, 1.5 mM Na3VO4, 50 mM NaF, 10 mM sodium pyrophosphate and 10 mM sodium β-glycerophosphate, supplemented with a cocktail of protease inhibitors) and centrifuged for 15 min at 16,000g. The supernatant was incubated with anti-α-synuclein antibody and Protein A/G–agarose overnight at 4 °C. After extensive washing with lysis buffer, the bound proteins were eluted from the beads by boiling in Laemmli sample buffer and were subjected to western blot analyses.
In vitro aggregation of α-synuclein
The aggregation of α-synuclein was induced as described previously42. Briefly, purified α-synuclein protein (rPeptide, 5 mg ml−1) was first incubated with vehicle or AEP in for 1 h and then dialyzed against PBS, pH 7.0. The samples were incubated at 37 °C with continuous shaking for 3 d. The aggregation kinetics of α-synuclein was measured with thioflavin T staining. 100 µl of 20 µM thioflavin T was mixed with 2 µl α-synuclein protein and incubated for 5–10 min at room temperature. Fluorometric readings were taken at 450-nm excitation and 510-nm emission. The remaining solutions of aggregated α-synuclein were centrifuged at 100,000g for 30 min to separate the aggregated α-synuclein pellet and the nonaggregated α-synuclein supernatant, and analyzed by western blotting.
AAV vector packaging and stereotaxic injection
293T cells were transfected with a plasmid encoding the viral genome together with a plasmid encoding AAV2 rep, AAV5 cap, as well as helper functions. 72 h post-transduction, virus particles were harvested by repeated freeze–thaw cycles, purified with an iodixanol gradient and subsequent column chromatography, dialyzed against modified (enhanced Mg2+/Ca2+) PBS and concentrated to the desired titer with an Apollo concentrator (Orbital Biosciences). Three-month-old wild-type C57BL/6J mice were anesthetized with phenobarbital (75 mg kg−1). Unilateral intracerebral injection of AAVs was performed stereotaxically at coordinates anteroposterior (AP) −3.1 mm and mediolateral (ML) −1.2 mm relative to the bregma, and dorsoventral (DV) −4.0 mm from the dural surface. 2 µl of viral suspension containing 3 × 1013 vector genomes (vg) ml−1 was injected into each site with a 10-µl glass syringe with a fixed needle at a rate of 0.25 µl min−1. The needle remained in place for 5 min before it was removed slowly (over 2 min). The mice were placed on a heating pad until they began to recover from the surgery.
Primary neuron cultures
Primary rat ventral midbrain culture neurons were cultured as previously described43. To measure the effects of α-synuclein fragments on neurons, neurons cultured 7 d in vitro (DIV 7) were infected with AAVs encoding FL α-synuclein or α-synuclein1–103. 5 d later, the neurons were fixed in 4% formaldehyde, permeabilized and immunostained with anti-TH antibody. The toxic effect of α-synuclein fragments was detected with an In situ cell death detection kit (Roche). The apoptotic index was expressed as a percentage of TUNEL-positive neurons out of the total number of TH-positive neurons.
Behavioral tests
Motor impairments were tested 15 weeks after the virus injection with rotarod tests, cylinder tests, amphetamine-induced rotation tests, and grid tests. In the rotarod tests, animals were trained for 2 min at a speed of 4 r.p.m. After this initial training, mice performed eight trials for a maximum of 5 min with increasing speed starting from 4 r.p.m. and increasing to 40 r.p.m. The fall-off time was recorded. In the cylinder tests, mice were placed individually inside a glass cylinder (12-cm diameter, 22 cm-height). Video recordings were examined by an observer blinded to the animal’s identity. Between 20 and 30 wall touches per animal (contacts with fully extended digits executed with the forelimb ipsilateral and contralateral to the lesion) were counted. In the amphetamine-induced rotation tests, animals that had been unilaterally injected with AAV vectors were challenged with d-amphetamine (free base, 2 mg/kg in saline, intramuscular, Sigma). The number of completed circles either to the left or to the right was counted.
Stereological quantification of TH-positive cells
The number of TH-positive cells in the SN was estimated with a random-sampling stereological counting method. For each animal, every fourth section throughout the rostrocaudal extent of the SN and every fourth section covering the entire extent of the STR were incorporated into the counting procedure. The investigator was blinded to the conditions of the experiment.
HPLC analysis of dopamine
Dopamine levels were determined by HPLC with coulometric detection through established methods44. Each brain sample was processed individually. Samples were homogenized in 0.1 N HClO4 solution (containing 0.01% sodium metabisulfite and 25 ng/ml internal standard 3, 4-dihydroxybenzylamine HBr) and centrifuged at 13,000g for 15 min at 4 °C. Aliquots of supernatant fractions were filtered with a 0.2-µm HT Tuffryn membrane (Pall), then injected into an Ultrasphere 5 µm ODS column, 250 × 4.6-mm (Hichrom Limited) and separated with a mobile phase containing 0.1 M sodium phosphate, 0.1 mM EDTA, 0.30 mM sodium octyl sulfate and 5% (v/v) acetonitrile, pH 3.2. The dopamine amount (ng/sample) was then quantified by comparison to internal standards, with a standard curve generated with 0.1–5 ng of dopamine standard. The protein level (mg/sample) was determined with Lowry protein assays with a standard curve generated with 0–95 µg bovine serum albumin45.
Statistical analysis
Statistical analysis was performed with either Student’s t test (two-group comparison) or one-way ANOVA followed by LSD post hoc test (more than two groups), and differences with P values <0.05 were considered significant. Detailed information on the experimental design and reagents can be found in the Life Sciences Reporting Summary.
Data availability
Source data files for Figures 1–7 are available online. All other data are available from the corresponding author upon reasonable request.
Supplementary Material
Acknowledgments
This work was supported by grants from the Michael J. Fox Foundation (grant ID 11137) to K.Y.; a grant from the National Natural Science Foundation (NSFC) of China (no. 81571249) to Zhentao Zhang; NSFC grant (no. 81528007) to K.Y. and J.-Z.W.; a National Key Basic Research Program of China grant (2010CB945202) to Y.E.S.; an NSFC grant (81330030) to Y.E.S.; and grants from the US Public Health Service (P30EY006360 and R01EY004864) to P.M.I. We thank the ADRC at Emory University for providing human PD, LBD and healthy-control samples, and C. Watts (University of Cambridge) for providing anti-AEP.
Footnotes
Note: Any Supplementary Information and Source Data files are available in the online version of the paper.
AUTHOR CONTRIBUTIONS
K.Y. conceived the project, designed the experiments and wrote the manuscript. Zhentao Zhang designed and performed most of the experiments. S.S.K. and X.L. prepared primary neurons and assisted with animal experiments. M.J.B. and F.P.M. provided clones and packaged viral vectors. D.M.D. and N.T.S. performed the mass spectrometry analysis. L.H. and P.M.I. performed the HPLC experiments and critically read and edited the manuscript. Zhaohui Zhang, E.H.A., L.J., Y.E.S., F.P.M. and J.-Z.W. designed the experiments, assisted with data analysis and interpretation and critically read the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Maries E, Dass B, Collier TJ, Kordower JH, Steece-Collier K. The role of alpha-synuclein in Parkinson’s disease: insights from animal models. Nat. Rev. Neurosci. 2003;4:727–738. doi: 10.1038/nrn1199. [DOI] [PubMed] [Google Scholar]
- 2.Polymeropoulos MH, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 3.Krüger R, et al. Ala30Pro mutation in the gene encoding α-synuclein in Parkinson’s disease. Nat. Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 4.Leroy E, Boyer R, Polymeropoulos MH. Intron-exon structure of ubiquitin c-terminal hydrolase-L1. DNA Res. 1998;5:397–400. doi: 10.1093/dnares/5.6.397. [DOI] [PubMed] [Google Scholar]
- 5.Kitada T, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 6.Spillantini MG, et al. α-Synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 7.Olanow CW, Brundin P. Parkinson’s disease and alpha synuclein: is Parkinson’s disease a prion-like disorder? Mov. Disord. 2013;28:31–40. doi: 10.1002/mds.25373. [DOI] [PubMed] [Google Scholar]
- 8.Masliah E, et al. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287:1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- 9.Kahle PJ, Neumann M, Ozmen L, Haass C. Physiology and pathophysiology of alpha-synuclein: cell culture and transgenic animal models based on a Parkinson’s disease-associated protein. Ann. NY Acad. Sci. 2000;920:33–41. doi: 10.1111/j.1749-6632.2000.tb06902.x. [DOI] [PubMed] [Google Scholar]
- 10.Feany MB, Bender WWA. Drosophila model of Parkinson’s disease. Nature. 2000;404:394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- 11.Burré J, et al. Properties of native brain α-synuclein. Nature. 2013;498:E4–E6. doi: 10.1038/nature12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of α-synuclein: from structure and toxicity to therapeutic target. Nat. Rev. Neurosci. 2013;14:38–48. doi: 10.1038/nrn3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W, et al. Aggregation promoting C-terminal truncation of alpha-synuclein is a normal cellular process and is enhanced by the familial Parkinson’s disease-linked mutations. Proc. Natl. Acad. Sci. USA. 2005;102:2162–2167. doi: 10.1073/pnas.0406976102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu CW, et al. A precipitating role for truncated alpha-synuclein and the proteasome in alpha-synuclein aggregation: implications for pathogenesis of Parkinson disease. J. Biol. Chem. 2005;280:22670–22678. doi: 10.1074/jbc.M501508200. [DOI] [PubMed] [Google Scholar]
- 15.Hoyer W, Cherny D, Subramaniam V, Jovin TM. Impact of the acidic C-terminal region comprising amino acids 109–140 on alpha-synuclein aggregation in vitro. Biochemistry. 2004;43:16233–16242. doi: 10.1021/bi048453u. [DOI] [PubMed] [Google Scholar]
- 16.Murray IV, et al. Role of alpha-synuclein carboxy-terminus on fibril formation in vitro. Biochemistry. 2003;42:8530–8540. doi: 10.1021/bi027363r. [DOI] [PubMed] [Google Scholar]
- 17.Mishizen-Eberz AJ, et al. Distinct cleavage patterns of normal and pathologic forms of alpha-synuclein by calpain I in vitro. J. Neurochem. 2003;86:836–847. doi: 10.1046/j.1471-4159.2003.01878.x. [DOI] [PubMed] [Google Scholar]
- 18.Mishizen-Eberz AJ, et al. Cleavage of alpha-synuclein by calpain: potential role in degradation of fibrillized and nitrated species of alpha-synuclein. Biochemistry. 2005;44:7818–7829. doi: 10.1021/bi047846q. [DOI] [PubMed] [Google Scholar]
- 19.Sevlever D, Jiang P, Yen SH. Cathepsin D is the main lysosomal enzyme involved in the degradation of alpha-synuclein and generation of its carboxy-terminally truncated species. Biochemistry. 2008;47:9678–9687. doi: 10.1021/bi800699v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi M, et al. Oxidative stress-induced phosphorylation, degradation and aggregation of alpha-synuclein are linked to upregulated CK2 and cathepsin D. Eur. J. Neurosci. 2007;26:863–874. doi: 10.1111/j.1460-9568.2007.05736.x. [DOI] [PubMed] [Google Scholar]
- 21.Halfon S, Patel S, Vega F, Zurawski S, Zurawski G. Autocatalytic activation of human legumain at aspartic acid residues. FEBS Lett. 1998;438:114–118. doi: 10.1016/s0014-5793(98)01281-2. [DOI] [PubMed] [Google Scholar]
- 22.Gamblin TC, et al. Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2003;100:10032–10037. doi: 10.1073/pnas.1630428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alvarez-Fernandez M, et al. Inhibition of mammalian legumain by some cystatins is due to a novel second reactive site. J. Biol. Chem. 1999;274:19195–19203. doi: 10.1074/jbc.274.27.19195. [DOI] [PubMed] [Google Scholar]
- 24.Liu Z, et al. Neuroprotective actions of PIKE-L by inhibition of SET proteolytic degradation by asparagine endopeptidase. Mol. Cell. 2008;29:665–678. doi: 10.1016/j.molcel.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madeira A, Pommet JM, Prochiantz A, Allinquant B. SET protein (TAF1beta, I2PP2A) is involved in neuronal apoptosis induced by an amyloid precursor protein cytoplasmic subdomain. FASEB J. 2005;19:1905–1907. doi: 10.1096/fj.05-3839fje. [DOI] [PubMed] [Google Scholar]
- 26.Basurto-Islas G, Grundke-Iqbal I, Tung YC, Liu F, Iqbal K. Activation of asparaginyl endopeptidase leads to Tau hyperphosphorylation in Alzheimer disease. J. Biol. Chem. 2013;288:17495–17507. doi: 10.1074/jbc.M112.446070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herskowitz JH, et al. Asparaginyl endopeptidase cleaves TDP-43 in brain. Proteomics. 2012;12:2455–2463. doi: 10.1002/pmic.201200006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Z, et al. Cleavage of tau by asparagine endopeptidase mediates the neurofibrillary pathology in Alzheimer’s disease. Nat. Med. 2014;20:1254–1262. doi: 10.1038/nm.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Z, et al. Delta-secretase cleaves amyloid precursor protein and regulates the pathogenesis in Alzheimer’s disease. Nat. Commun. 2015;6:8762. doi: 10.1038/ncomms9762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li DN, Matthews SP, Antoniou AN, Mazzeo D, Watts C. Multistep autoactivation of asparaginyl endopeptidase in vitro and in vivo. J. Biol. Chem. 2003;278:38980–38990. doi: 10.1074/jbc.M305930200. [DOI] [PubMed] [Google Scholar]
- 31.Chen L, et al. Tyrosine and serine phosphorylation of alpha-synuclein have opposing effects on neurotoxicity and soluble oligomer formation. J. Clin. Invest. 2009;119:3257–3265. doi: 10.1172/JCI39088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellis CE, Schwartzberg PL, Grider TL, Fink DW, Nussbaum RL. α-Synuclein is phosphorylated by members of the Src family of protein-tyrosine kinases. J. Biol. Chem. 2001;276:3879–3884. doi: 10.1074/jbc.M010316200. [DOI] [PubMed] [Google Scholar]
- 33.Beyer K, Ariza A. α-Synuclein posttranslational modification and alternative splicing as a trigger for neurodegeneration. Mol. Neurobiol. 2013;47:509–524. doi: 10.1007/s12035-012-8330-5. [DOI] [PubMed] [Google Scholar]
- 34.Giasson BI, et al. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron. 2002;34:521–533. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- 35.Lee MK, et al. Human alpha-synuclein-harboring familial Parkinson’s disease-linked Ala-53 → Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc. Natl. Acad. Sci. USA. 2002;99:8968–8973. doi: 10.1073/pnas.132197599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forester BP, et al. Age-related changes in brain energetics and phospholipid metabolism. NMR Biomed. 2010;23:242–250. doi: 10.1002/nbm.1444. [DOI] [PubMed] [Google Scholar]
- 37.Gauthier S, Kaur G, Mi W, Tizon B, Levy E. Protective mechanisms by cystatin C in neurodegenerative diseases. Front. Biosci. (Schol. Ed.) 2011;3:541–554. doi: 10.2741/s170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dall E, Brandstetter H. Structure and function of legumain in health and disease. Biochimie. 2016;122:126–150. doi: 10.1016/j.biochi.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 39.Oueslati A, Fournier M, Lashuel HA. Role of post-translational modifications in modulating the structure, function and toxicity of alpha-synuclein: implications for Parkinson’s disease pathogenesis and therapies. Prog. Brain Res. 2010;183:115–145. doi: 10.1016/S0079-6123(10)83007-9. [DOI] [PubMed] [Google Scholar]
- 40.Wang W, et al. Caspase-1 causes truncation and aggregation of the Parkinson’s disease-associated protein α-synuclein. Proc. Natl. Acad. Sci. USA. 2016;113:9587–9592. doi: 10.1073/pnas.1610099113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shirahama-Noda K, et al. Biosynthetic processing of cathepsins and lysosomal degradation are abolished in asparaginyl endopeptidase-deficient mice. J. Biol. Chem. 2003;278:33194–33199. doi: 10.1074/jbc.M302742200. [DOI] [PubMed] [Google Scholar]
- 42.Volpicelli-Daley LA, Luk KC, Lee VM. Addition of exogenous α-synuclein preformed fibrils to primary neuronal cultures to seed recruitment of endogenous α-synuclein to Lewy body and Lewy neurite-like aggregates. Nat. Protoc. 2014;9:2135–2146. doi: 10.1038/nprot.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Z, et al. 7,8-dihydroxyflavone prevents synaptic loss and memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2014;39:638–650. doi: 10.1038/npp.2013.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pozdeyev N, et al. Dopamine modulates diurnal and circadian rhythms of protein phosphorylation in photoreceptor cells of mouse retina. Eur. J. Neurosci. 2008;27:2691–2700. doi: 10.1111/j.1460-9568.2008.06224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Source data files for Figures 1–7 are available online. All other data are available from the corresponding author upon reasonable request.