Abstract
The endocannabinoid (eCB) system has emerged as a central integrator linking the perception of external and internal stimuli to distinct neurophysiological and behavioural outcomes (such as fear reaction, anxiety and stress-coping), thus allowing an organism to adapt to its changing environment. eCB signalling seems to determine the value of fear-evoking stimuli and to tune appropriate behavioural responses, which are essential for the organism’s long-term viability, homeostasis and stress resilience; and dysregulation of eCB signalling can lead to psychiatric disorders. An understanding of the underlying neural cell populations and cellular processes enables the development of therapeutic strategies to mitigate behavioural maladaptation.
If asked for the main reason why they use this largely illicit drug, the majority of cannabis users in the world would probably answer “it relaxes me” (REF. 1). This indicates that cannabinoid signalling in the brain and the body has a central role in the control of stress, fear and anxiety. Recently, the molecular, cellular and circuit mechanisms underlying these functions have started to be deciphered.
Appropriate behavioural responses to external (such as sensory inputs) and internal stimuli (such as endocrine, paracrine, metabolic and neuronal signals) are vital for an organism’s survival. Ideally, the consequent reactivity of the organism to stimuli is intrinsically regulated in an optimal manner, to avoid excessive or insufficient reactions, both of which can jeopardize the organism’s survival. A large body of data has emerged in recent years pointing to a crucial role of the endocannabinoid (eCB) system in the regulation of the behavioural domains of acquired fear, anxiety and stress-coping2–7. The eCB system modulates synaptic transmission processes8,9, thereby regulating behavioural outputs.
Despite the fact that the eCB system is widely distributed in the CNS9,10, its activity is highly specific and localized. To understand this specificity in the context of fear, anxiety and stress-coping, one needs an integrated view of the eCB-mediated control of relevant brain regions (mainly the hippocampus, prefrontal cortex (PFC), amygdala and hypothalamus) and their interregional connectivity, and of the communication of these brain regions with peripheral organs (via the hypothalamic–pituitary–adrenal (HPA) axis and sympathetic nervous system). Within distinct brain regions, eCB signalling can differentially modulate the activity of multiple cell types (neuronal subtypes9, astrocytes11 and microglia12), and in turn can execute context-related alterations in synaptic transmission, resulting in fine-tuned patterns of neuronal activity.
The eCB system classically includes cannabinoid receptor type 1 (CB1R) and CB2R, their endogenous lipid ligands (the eCBs; the most-studied of which are 2-arachidonoyl glycerol (2-AG) and N-arachidonoylethanolamine (AEA; also known as anandamide)), and eCB-synthesizing and -degrading enzymes9 (FIG. 1).
Figure 1. Architecture of eCB system components in neurons and glia.
In the CNS, endocannabinoid (eCB) system components show a distinct anatomical distribution. The Gi/o-coupled protein cannabinoid receptor type 1 (CB1R) is typically present at the presynaptic terminal. Its stimulation by 2-arachidonoyl glycerol (2-AG) or N-arachidonoylethanolamine (AEA) leads to the suppression of neurotransmitter release from the presynaptic terminal8,14. CB1R is also present in the outer mitochondrial membrane at both pre-and postsynaptic sites (mitochondrial CB1R (mtCB1R))146. Stimulation of the mtCB1R leads to inhibition of mitochondrial oxidative phosphorylation and ATP production in the mitochondria and can modulate neurotransmitter release through mechanisms that are still unknown (indicated by the question mark)146. AEA can also activate the postsynaptic non-selective cation channel transient receptor potential cation channel subfamily V member 1 (TRPV1)71,72,178,179, leading to an increase in postsynaptic current, whereas 2-AG can also stimulate postsynaptic GABAA receptors180. On depolarization of the postsynaptic terminal, for example, by activation of metabotropic receptors (metabotropic glutamate receptor 1 (mGluR1; also known as GRM1), mGluR5, muscarinic receptor type 1 (M1) or M2)8,14, 2-AG is postsynaptically synthesized ‘on-demand’ by diacylglycerol lipase-α (DAGLα) in dendritic spines of excitatory synapses181–183. 2-AG then travels to the presynaptic CB1R in a retrograde manner to inhibit neurotransmitter release184,185, thus acting as a negative-feedback mechanism to tune-down synaptic transmission, especially when the postsynaptic terminal is strongly activated. The major 2-AG degrading enzyme monoacylglycerol lipase (MAGL) is located at the presynaptic terminal186 or in astrocytes187, whereas α-β-hydrolase domain 6 (ABHD6), another 2-AG degrading enzyme, can limit 2-AG availability at the site of production188,189. Astrocytic MAGL seems to enable astrocyte–neuron transcellular shuttling and metabolism of 2-AG and arachidonic acid190. Several pathways are involved in AEA synthesis. One of the enzymes involved in AEA synthesis, N-acyl phosphatidyl ethanolamine-phospholipase D (NAPE-PLD), is predominantly expressed in the presynaptic terminal191,192, although it might also be synthesized postsynaptically41. Other AEA-synthesizing enzymes have been described but are not fully characterized193,194. The AEA-degrading enzyme fatty acid amide hydrolase (FAAH) is present at the postsynaptic terminal186. Thus, AEA can act in both an autocrine and a retrograde manner (an anterograde AEA-signalling mechanism awaits description). CB2R and possibly CB1R (indicated by a question mark) are also present on microglial cells and are involved in immune reactions12. Furthermore, whereas presynaptic CB1R is coupled to Go, CB1R on astrocytes is Gq-coupled11,37. Thus, agonist stimulation of the receptor leads to an increase in intracellular Ca2+ concentration, possibly with a concomitant release stimulation of ‘gliotransmitters’ (whose exact nature is not yet known, indicated by the question mark), finally modulating synaptic transmission11,37. eCB synthesis in microglia and astrocytes can be stimulated by the activation of P2X purinoreceptor 7 (P2X7) by ATP131,195.
Here, we discuss recent progress in understanding how the eCB system is an integral part of the interface between stimulus input and executive responses at the synaptic and behavioural levels, and how it is involved in feedback mechanisms leading to adapted neuronal and behavioural reactions. Our discussion also includes pathophysiological states, as observed in anxiety- and stress-related dysfunctions, such as anxiety disorders. We evaluate whether the activity of the eCB system is altered in these disease states and whether these observations might lead to possible approaches for therapeutic intervention.
Modulation of synaptic processes
The eCB system is widely distributed in the CNS10, constituting a complex signalling system9 that subserves multiple modes of synaptic transmission modulation8,13–15. The specific outcome of eCB-mediated modulation of synaptic transmission is dependent on the synapse-specific expression of the protein components of the eCB system (FIG. 1). Although the eCB system is highly abundant in the CNS10, not all synapses contain a functional eCB system. As CB1R is the major constituent of the eCB system, the expression of CB1R is highly indicative of the presence of eCB signalling at that particular synapse. The eCB system is expressed at some synapses in all brain regions that are important for the processing of anxiety, fear and stress, including the hippocampus16,17, the PFC18, the bed nucleus of the stria terminalis (BNST)19, the basolateral amygdala (BLA)16,20, the central amygdala (CeA)21,22 and various hypothalamic nuclei23. In cortical areas (including the cerebral cortex, hippocampus and cortical parts of the amygdala), CB1R is highly expressed in cholecystokinin (CCK)-positive GABAergic interneurons, whereas CB1R expression is largely absent from other interneuronal subtypes (for example, calretinin-and parvalbumin-positive interneurons)16,17. Much lower levels of CB1R expression are present in glutamatergic neurons of cortical regions9,10,16. However, cortical glutamatergic CB1R has been shown to have important functional roles, including the control of synaptic transmission and neuronal excitability15,24,25. CB1R is also present in the cholinergic, serotonergic and noradrenergic system, suggesting that the eCB system is involved in the suppression of the release of these neurotransmitters, although direct electrophysiological evidence is mostly lacking26–29. Furthermore, CB1R is present at very low levels in astrocytes30. CB2R is expressed in microglia, particularly in activated microglia, but the question of CB1R expression in these cells needs further investigation12.
At the synapse, eCBs function as retrograde messengers, binding to presynaptic CB1R, which in turn mediates the suppression of neurotransmitter release, leading to either transient eCB-mediated short-term depression (eCB-STD) or eCB-mediated long-term depression (eCB-LTD) of synaptic transmission (FIG. 2a). In addition, arachidonic acid (AA), which is both a precursor (in a lipid-esterified form) and a degradation product of eCBs, has recently been found to also act as a retrograde messenger, potentiating excitatory transmission in a process called depolarization-induced potentiation of excitation (DPE)31 (FIG. 2b). Interestingly, many years ago, it was reported that AA can modulate synaptic transmission by various mechanisms32,33. DPE has to be taken into consideration, as the genetic and pharmacological modulation of eCB-synthesizing and -degrading enzymes can lead to considerable changes in AA levels34–36, thereby presumably also influencing synaptic transmission. Owing to diffusion in the extracellular space of eCBs at the synapses, eCBs can also modulate neurotransmitter release at neighbouring synaptic terminals, leading to heterosynaptic suppression of neurotransmitter release14 (FIG. 3a). The eCB system and CB1R are also present and functional in astrocytes, thus eCB signalling is integrated into the concept of the ‘tripartite synapse’, including pre-and postsynaptic elements and surrounding astroglial processes11,37 (FIG. 3b).
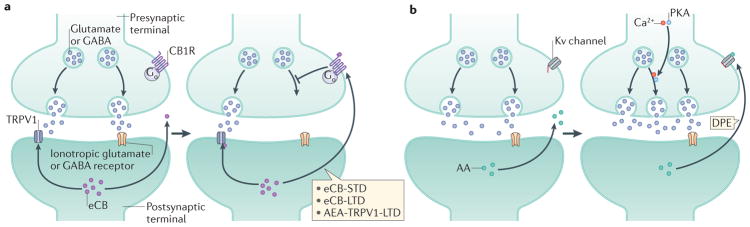
Figure 2. Regulation of synaptic excitatory and inhibitory transmission.
a | Schematic representation of endocannabinoid (eCB)-mediated suppression of synaptic transmission8,14; the mechanisms shown apply to both excitatory and inhibitory synapses. At excitatory synapses, afferent stimulation evokes increased glutamate release and subsequent activation of the postsynaptic terminal. This stimulates the synthesis of eCBs (such as N-arachidonoylethanolamine (AEA) and 2-arachidonoyl glycerol (2-AG)), which travel through the synaptic cleft to activate presynaptic cannabinoid receptor type 1 (CB1R), leading to the suppression of glutamate release. eCB-mediated short-term depression (eCB-STD, also termed depolarization-induced suppression of excitation (DSE)) or eCB-mediated long-term depression (eCB-LTD) can occur. A similar mechanism occurs at GABAergic synapses, in which postsynaptic activation by excitatory inputs can stimulate the production of eCBs, the inhibition of presynaptic CB1R and the retrograde suppression of GABA release. This form of eCB-STD is termed depolarization-induced suppression of inhibition (DSI). Both DSE and DSI require the synthesis of 2-AG by diacylglycerol lipase-α (DAGLα)184,185. AEA can also mediate LTD, although at a slower rate than 2-AG. AEA can act both through CB1R, to produce eCB-LTD, and through transient receptor potential cation channel subfamily V member 1 (TRPV1), to generate AEA-TRPV1-LTD (in an autocrine manner in which AEA activates postsynaptic TRPV1). AEA-TRPV1-LTD can occur at both glutamatergic and GABAergic synapses14,19,70–72,178,179. b | Schematic presentation of the modulation of excitatory transmission by the eCB precursor and degradation product arachidonic acid (AA). Postsynaptic AA acts in a retrograde manner via inhibition of presynaptic voltage-gated potassium (Kv) channels and potentiation of excitatory neurotransmission, a process called depolarization-induced potentiation of excitation (DPE)31. PKA, protein kinase A.
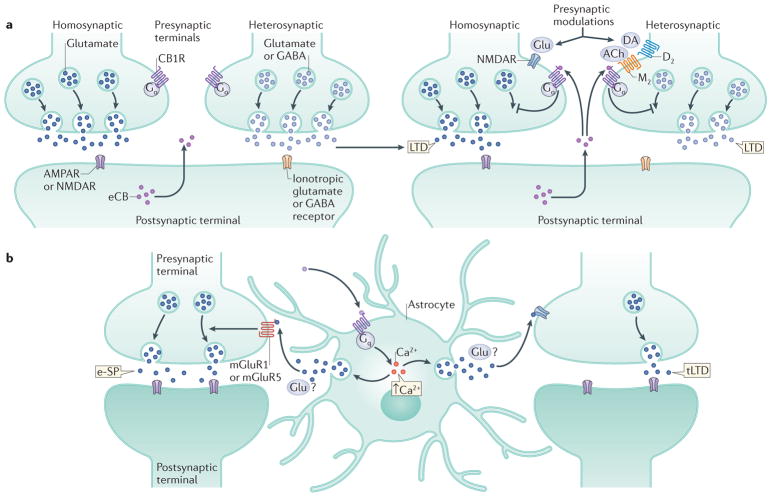
Figure 3. Heterosynaptic effects and eCB function in the tripartite synapse.
a | Schematic representation of homosynaptic and heterosynaptic effects of eCB signalling on neurotransmitter release. Typically, repetitive afferent stimulation causes glutamate (Glu) release from excitatory presynaptic sites, activating the postsynaptic terminal and inducing the generation and release of 2-arachidonoyl glycerol (2-AG), which then activates cannabinoid receptor type 1 (CB1R) on the same presynaptic terminal (a homosynaptic effect) and on the nearby synaptic terminal (a heterosynaptic effect). For long-term depression (LTD) to be produced, concomitant activation of other presynaptic receptors is required. For example, activation of NMDA receptor (NMDAR), dopamine (DA) receptor type 2 (D2) or muscarinic receptor type 2 (M2) by Glu, DA or acetycholine (ACh), respectively, is required. These associative mechanisms may ensure the selectivity of the synapses to be regulated by endocannabinoids (eCBs)14. b | Integration of the eCB system into the ‘tripartite synapse’ concept and modulation of synaptic transmission. Activation of CB1R on astrocytes leads to increased intracellular levels of Ca2+, promoting the release of ‘gliotransmitters’ (although this remains subject to debate, as indicated by the question mark), possibly including Glu. These gliotransmitters could then promote heterosynaptic excitatory potentiation (e-SP)133 or time-spiking-dependent LTD (tLTD) of glutamatergic transmission via presynaptic NMDAR134. AMPAR, AMPA receptor; mGluR, metabotropic glutamate receptor.
A central feature of the eCB system is that eCBs are synthesized on demand from cellular membrane lipids following various stimuli14. This is well documented for 2-AG, whose synthesis was shown to be stimulated after increased postsynaptic intracellular Ca2+ concentration or increased activity of phospholipase Cβ (PLCβ). The on-demand generation of AEA is not well characterized yet, owing to the lack of a detailed understanding of the mechanisms of AEA synthesis. The concept of on-demand eCB synthesis represents an attractive construct for understanding the roles of the eCB system in neuronal-network modulation and behaviours. In this construct, the eCB system is thought to be transiently activated at distinct synapses where the different cellular elements involved have been stimulated beyond a certain threshold. According to this view, the eCB system constitutes a brake mechanism used to fine-tune the network activity of specific brain circuits9,38. This mechanism, which seems to be mediated mainly by 2-AG, is an activity-driven process: eCB signalling is mostly silent when activity is low. Other molecules that induce eCB synthesis have also been identified, including corticosteroids39,40 and estradiol41, leading to the hypothesis that eCB signalling is the effector by which these hormones alter synaptic activity. Conversely, there are convincing data that AEA is involved in the tonic suppression of neurotransmitter release14.
Thus, the eCB system has emerged as a modulator of synaptic activity via a multitude of different mechanisms, resulting in either enhancement or suppression of general network activity. The eCB system is present throughout brain areas and neuronal circuits controlling anxiety4,42, fear4,42 and stress43, from sensory circuits to output nuclei.
Anxiety behaviour
Anxiety is an innate behavioural state associated with the anticipation of potential future threats that allows an organism to avoid potentially dangerous or harmful situations. Inputs from multiple senses are evaluated to assess these potential dangers and to initiate appropriate behavioural responses. The physiological and emotional state of the organism at the moment of this perception plays an important part in the evaluation of threat and determines the intensity of the autonomic, hormonal and behavioural outputs. Anxiety-like behaviours (for example, avoidance, decreased motion, increased heart rate and hypervigilance) occurring within the normal range of intensity are important for survival. However, when anxiety behaviours chronically exceed the normal range and become disproportionate to the actual level of danger, deleterious physical and psychological consequences ensue, eventually leading to anxiety-related neuropsychiatric disorders44,45. Increasing insights into the brain regions and neuronal circuits regulating anxiety have been gained during the last years42.
A large number of pharmacological and genetic studies support the role of the eCB system as an important regulator of anxiety-like behaviours4,46. Analysis of global CB1R-deficient mice revealed increased anxiety-like behaviour under highly aversive conditions but not under less aversive conditions47. This could occur because eCB signalling is mobilized only when the stimulus is very strong or because eCB synthesis has been sensitized by a previous negative event48. The conditional deletion of the gene encoding CB1R in cortical glutamatergic neurons, which interconnect several brain areas of the anxiety circuits, did not result in differences in behavioural responses in standard anxiety tests (for example, the elevated plus maze, which is a model of mild stress) under aversive47 or non-aversive conditions47,49. However, these mice exhibited decreased exploratory behaviour50,51 and increased thigmotaxis in the Morris water maze (a model of spatial learning that also places the animal under mild stress)52. These phenotypes might be seen as a neophobic behaviour, and thus as increased anxiety-like behaviour. Complementary to these loss-of-function studies, specific genetic-rescue experiments showed that re-expression of CB1R in cortical glutamatergic neurons is sufficient to almost completely restore wild-type anxiety-like behaviour in mice lacking CB1R expression in all cells of the body except cortical glutamatergic neurons53.
Conversely, the loss of CB1R in forebrain GABAergic neurons leads to increased exploratory behaviours in mildly aversive conditions50,51, which can be interpreted as reduced anxiety-like or neophobic behaviour. However, in the elevated plus maze, the exploratory behaviour of these conditional-knockout mice was the same as that of controls49. Thus, CB1Rs on cortical glutamatergic and GABAergic neurons exert opposing control on anxiety-like behaviours, but only when the environmental aversiveness exceeds a certain threshold. This is in agreement with the notion that the eCB system exerts a ‘buffering’ effect on neuronal activity in specific circuits. This function seems to operate within specific limits of neuronal activity, whereby a certain minimal strength of stimulus is needed to engage eCB signalling, and when this activity overcomes certain limits, the buffering capacity is exhausted9.
Numerous pharmacological studies support the notion of bidirectional regulation of anxiety circuits and behaviour by CB1R. It is well known that exogenous cannabinoids influence anxiety-like behaviour in a biphasic manner, with low and high doses exerting anxiolytic and anxiogenic states, respectively, in both animals and humans54. These pharmacological effects are mediated by CB1R. Cell-type-specific CB1R-deficient mice have enabled the identification of the underlying mechanisms of these biphasic effects49. The anxiolytic effect of cannabinoids at low doses depends on the presence of CB1R on cortical glutamatergic neurons, whereas the anxiogenic effect of higher doses is mediated by CB1R on forebrain GABAergic neurons. This observation is consistent with previous experiments in which the cannabinoid receptor agonist Δ9-tetrahydrocannabinol (THC) was locally injected into the ventral hippocampus or PFC: a low THC dose evoked an anxiolytic response, whereas a high THC dose led to an anxiogenic response55.
Taken together, these genetic and pharmacological experiments suggest a mechanism for the processing of anxiety-related stimuli in which CB1R on glutamatergic neurons and GABAergic neurons decreases excitatory and inhibitory drive, respectively, thereby explaining the opposing effects of manipulation of these two transmitter systems on anxiety49,51. Consistent with these behavioural data and the notion of opposing functions depending on cellular expression, mice with CB1R deficiency in cortical glutamatergic neurons were shown to exhibit overexcited hippocampal circuits (that is, increased long-term potentiation (LTP), spine density and dendritic branching in pyramidal neurons). By contrast, mice with CB1R deficiency in forebrain GABAergic neurons displayed decreased excitability of these circuits (that is, decreased LTP, spine density and dendritic branching in pyramidal neurons) (FIG. 4). These results indicate that the loss of CB1R in either neuronal population induced an allostatic shift and long-term dysregulation of the functions of hippocampal pyramidal neurons56. Interestingly, this bimodal control of excitability exerted by glutamatergic and GABAergic CB1R correlates well with the behavioural alterations observed.
Figure 4. Dichotomic CB1R function in glutamatergic and GABAergic neurons.
a | A prominent feature of the endocannabinoid (eCB) system in the forebrain is its distinct distribution in glutamatergic and GABAergic neurons, with low cannabinoid receptor type 1 (CB1R) expression in glutamatergic neurons and high CB1R expression in GABAergic neurons16,196. This is evident when immunostaining for CB1R of hippocampi in mice with CB1R deficiency in glutamatergic (Glu-CB1R-KO; left panel) and GABAergic (GABA-CB1R-KO; right panel) neurons; in comparison with wild-type controls (WT; middle panel). b | In principal neurons of the hippocampal CA1 formation, spine density and dendritic branching are increased in Glu-CB1R-KO mice (left panel) and decreased in GABA-CB1R-KO mice (right panel), as compared with these neurons in wild-type mice (middle panel)56. This coincides with increased and decreased hippocampal CA1 long-term potentiation (LTP) formation, respectively56. Moreover, the two mutant-mouse lines display opposing phenotypes in behaviours such as neophobia, exploration, fear relief and habituation. Thus, CB1R in cortical glutamatergic and forebrain GABAergic neurons calibrates excitatory synaptic balance and consequently regulates fear and anxiety-like behaviours. DSE, depolarization-induced suppression of excitation; DSI, depolarization-induced suppression of inhibition. Part a adapted with permission from REF. 196, Copyright ©1999–2015 John Wiley & Sons, Inc. All Rights Reserved. Part b adapted with permission from REF. 56, the Society for Neuroscience.
Genetic and pharmacological interference with eCB-synthesizing and -degrading enzymes, leading to alterations in eCB levels, has also revealed the importance of eCBs in the regulation of anxiety. Genetic deletion of fatty acid amide hydrolase (FAAH), the primary AEA-degrading enzyme in the CNS, leads to increased AEA levels in the brain and to decreased anxiety-like behaviour in the elevated plus maze and the light–dark test (animal models that measure anxiety-like behaviour)57. Similar effects occur with pharmacological blockade of FAAH under basal conditions57–59 and after chronic unpredictable stress (CUS)60. Currently, the analysis of conditional inactivation of FAAH is still pending but it should provide additional information to clarify the exact sites where this enzyme controls anxiety-related behaviours. Some years ago, a polymorphism in human FAAH was identified (rs324420; in which cysteine 385 is changed to alanine)61. This alteration leads to a destabilized FAAH enzyme and, consequently, to an increase in AEA signalling. Humans and mice homozygous in this allele (FAAHA/A) show decreased anxiety-like behaviour and increased fear-extinction learning62. Functional MRI (fMRI) investigations revealed increased functional connectivity between the ventromedial PFC (vmPFC) and amygdala in these people. Remarkably, the presence of this polymorphism results in comparable phenotypes in mice62.
With regard to 2-AG signalling, it has recently been reported that deficiency of the 2-AG-synthesizing enzyme, diacylglycerol lipase-α (DAGLα), leads to markedly decreased 2-AG brain levels and to increased anxiety-like behaviour34,36. The anxiety-like behaviour was rescued by pharmacological blockade of the 2-AG-degrading enzyme monoacylglycerol lipase (MAGL) with JZL184 (REF. 34). In agreement with these observations, impairment of 2-AG signalling in hippocampal glutamatergic neurons by viral overexpression of MAGL also led to decreased 2-AG levels and increased anxiety-like behaviour63. Conversely, pharmacological inhibition of MAGL in wild-type mice by JZL184 had anxiolytic effects under basal conditions60,64,65, under increased aversive conditions66 and after CUS60,67.
Non-CB1R actions in anxiety
The influence of eCBs on anxiety is made more complex by their activity at receptors other than CB1R. Postsynaptic transient receptor potential cation channel subfamily V member 1 (TRPV1) can be activated by AEA, thereby enhancing postsynaptic currents. Decreased anxiety-like behaviour has been observed in mice lacking TRPV1 (REF. 68). As TRPV1 is expressed and functional in both GABAergic and glutamatergic neurons23,69–72, the analysis of conditional TRPV1-deficient mice will be important and might reveal a behavioural dichotomy similar to that observed with CB1R. Furthermore, CB2R has been implicated in the eCB-dependent regulation of anxiety-like behaviours73,74. However, owing to the enigmatic expression of this receptor in neurons (BOX 1), the mechanistic basis of these observations is far from being understood.
Box 1. Enigmatic neuronal CB2R and its role in anxiety.
The presence of neuronal cannabinoid receptor type 2 (CB2R) was recently revealed in several brain structures156,157, although the low expression levels and technical aspects have continuously raised questions about the validity of these results157–159. Recent behavioural studies using genetically modified mice and pharmacological tools have provided evidence that CB2R is involved in several behavioural responses, including anxiety. Indeed, chronic pharmacological blockade of CB2R produced anxiolytic effects mediated by an alteration of GABAA receptor function160. Furthermore, transgenic mice overexpressing the CB2R in CNS neurons showed decreased anxiety-like behaviour and impaired anxiolytic effects of benzodiazepines161. CB2R was shown to be involved in the anxiolytic-like responses induced by 2-arachidonoyl glycerol (2-AG)64, and blockade of CB2R normalized the anxiety-like phenotype of fragile X mental retardation 1 (Fmr1)-knockout (Fmr1−/y) mice74. Despite these accumulating data, the molecular and cellular mechanisms by which the neuronal CB2R may influence behaviour have remained enigmatic. In this context, it is important to note that the CB2R is clearly expressed in brain immune cells (microglia). Thus, an immune cell–neuron interaction might be accountable for the behavioural phenotypes observed in CB2R-deficient mice, and the ‘enigmatic’ CB2R expression in neurons may not be functionally relevant in anxiety behaviours. To this end, further investigations using cell-type-specific deletions of the gene encoding CB2R are required.
The importance of CB1R-and TRPV1-mediated signalling in anxiety-like behaviour has also been investigated by using local applications of pharmacological agents that modulate eCB-system activity. These experiments revealed direct roles for CB1R-and TRPV1-mediated signalling within the ventral hippocampus55, PFC55,75, BLA55 and periaqueductal grey (PAG)76,77. Although they have a common ligand, AEA, the involvement of CB1R and TRPV1 in anxiety is opposite, constituting an intriguing antagonistic regulatory mode between both signalling systems78.
In conclusion, the eCB system controls anxiety-related brain regions at many different levels. Based on present knowledge, it seems that the general role of this system is to control excessive activation (thereby exerting anxiolytic functions). However, it is interesting to note that additional mechanisms (for example, CB1R signalling at GABAergic synapses) seem to mediate opposite (that is, anxiogenic) functions under certain conditions (FIG. 4). From an evolutionary point of view, the presence of CB1R and TRPV1, and the eCB signalling on both antagonizing neurotransmitter systems (that is, glutamatergic and GABAergic neurons), seems to be highly beneficial for the appropriate regulation of anxiety-like behaviour.
Fear behaviour
Anxiety is elicited by potentially dangerous but unspecified future threats, whereas fear is the response to specific and actual threatening stimuli. Thus, we will probably be anxious if we are walking in an area known to contain poisonous snakes (a potential but unspecific threat), but we feel fear when we encounter a poisonous snake directly (an actual and specific threat)79.
In a similar manner to anxiety, fear perception, elaboration and response involves neuronal, autonomic and hormonal responses. The behavioural reactions to specific threats can be passive in nature (that is, aimed at hiding from or passively avoiding the source of threat; for example, by freezing) or active in nature (that is, aimed at escaping and actively avoiding the danger). Fear can be innate (such as human fear of snakes and/or other animals) or acquired (when the individual learns that a certain stimulus represent a specific threat to well-being or life). All these modalities of fear and fear responses have been studied in experimental settings, and there is scientific literature linking these aspects to the eCB system2–5. Cued fear conditioning is the most widely used model to study fear circuits42,80. In this protocol, an animal learns to associate an initially neutral stimulus (called a conditioned stimulus (CS); for example, an acoustic, visual, tactile, gustatory or olfactory cue) with a simultaneous fear-inducing stimulus (known as an unconditioned stimulus (US); for example, a mild electric shock). After one or more pairings of the US with the CS, the subject associates the two stimuli, and the presentation of the CS alone is able to evoke a fear response79. This association of the two stimuli (the ‘fear learning’) is typically consolidated into long-term memory within 6–8 hours. In rodents, the strongest and most-immediate fear reaction is freezing79. Scoring of freezing behaviour during presentations of the CS alone is used to evaluate the strength of the fear elicited by the specific CS. However, the re-exposure of the subject to the fear stimulus triggers additional neuronal processes, aimed at adapting the behavioural response to changing environmental conditions81,82. Thus, short re-exposure to the CS triggers a second round of memory consolidation (called reconsolidation), whereby new information can be integrated into the original memory81. Conversely, prolonged or repeated exposure to the CS in the absence of the US triggers extinction, resulting in a decline of CS-evoked fear expression82,83. Clinically, extinction is thought to be impaired in patients suffering from specific fear-related disorders, such as phobias or post-traumatic stress disorder (PTSD). Thus, enhanced understanding of the mechanisms involved in fear extinction can lead to novel treatment options for these patients.
Brain regions and neural circuits regulating fear have been investigated intensively42. The eCB system is present in these fear-related brain areas and is centrally involved in the regulation of fear-memory processing3–5. Global genetic deletion and pharmacological blockade of CB1R consistently induces marked impairment in the decrease of fear responses (that is, freezing) after repeated or prolonged CS-alone presentations, but less in acquisition and consolidation of fear memory84,85. Subsequent experiments using mice lacking CB1R in cortical glutamatergic neurons revealed that CB1R in these cells is necessary for proper reduction of the fear response86. However, genetic-rescue experiments revealed that CB1R in cortical glutamatergic neurons is not sufficient to guarantee this behaviour53. This is in strong contrast to the CB1R-dependent control of anxiety behaviour, which was in large part rescued when CB1R was re-expressed (see above). CB1R deficiency in forebrain GABAergic neurons does not seem to have an essential role in the reduction of conditioned freezing responses86, although a recent study reported decreased freezing in GABAergic-specific CB1R mutants on the first re-exposure to the conditioned stimulus87. Further support for a role of CB1R in GABAergic interneurons comes from evidence that fear extinction can cause specific remodelling of perisomatic inhibitory synapses in the basal amygdala, including alterations in the localization of the CB1R on CCK-positive neurons in this region88. Furthermore, an interaction between the eCB system and CCK signalling has been demonstrated, as the decrease in fear extinction that is normally induced by CB1R antagonism was blunted in CCK-B receptor-deficient mice. This effect is possibly linked to CB1R expressed on GABAergic neurons in the amygdala89. CB1R can also control the expression of aversive memories in different brain regions. For instance, CB1R in the synaptic terminals of neurons of the medial habenula projecting to the interpeduncular nucleus was shown to promote the expression of aversive memories in fear conditioning and conditioned odour aversion experiments90. Interestingly, these recent results suggest that CB1R can increase or decrease aversive responses, depending on the specific brain circuits and cell types that are involved.
The role of 2-AG in fear extinction was shown in mice deficient in DAGLα, which have reduced 2-AG brain levels. These mutant mice exhibit no impairments in fear acquisition but show impaired fear extinction36. These data are in agreement with the requirement of the eCB system for proper fear extinction84. Interestingly, pharmacological enhancement of 2-AG with the MAGL inhibitor JZL184 promotes fear expression and impairs fear extinction, an effect that requires CB1R in forebrain GABAergic neurons87. In fact, genetic and pharmacological blockade of MAGL enhances hippocampal depolarization-induced suppression of inhibition (DSI; a form of eCB-STD at GABAergic synapses)91,92, leading to insufficient GABAergic transmission, which is consistent with increased fear expression. These results suggest that an optimal level of 2-AG is required for appropriate processing of fear responses and that having 2-AG levels that are too high or too low impairs the decrease in fear expression.
Pharmacological experiments that applied CB1R antagonists to specific brain regions revealed that eCB signalling in the BLA and CeA are important for different phases of fear extinction21. CB1R blockade in the BLA led to an impairment of long-term extinction, whereas CB1R antagonism in the CeA reduced within-session extinction21. In addition, it was reported that the magnitude of depolarization-induced suppression of excitation (DSE) and DSI in the CeA was increased on the day after fear conditioning, showing that CB1R-mediated synaptic plasticity in the CeA is a consequence of fear conditioning21. Pharmacological blockade of CB1R in the infralimbic subregion of the medial PFC also impairs fear extinction93.
Pharmacological enhancement of AEA signalling by inhibition of FAAH in the BLA facilitates fear extinction via activation of the CB1R94. In these pharmacological experiments, CB1R on both afferent synaptic terminals and local GABAergic interneurons is likely to be activated. Thus, although these experiments reveal the importance of CB1R signalling in the BLA, they do not give information on the specific neuronal circuits.
Reduction of fear responses
How does the eCB system modulate the fear reaction and the extinction process? Several theories have been proposed to explain the reduction of fear responses on repeated or prolonged exposure to a CS82, but the two primary theories refer to overlapping processes of ‘extinction’ and of ‘habituation’ (REFS 3,82). Both these processes are learning phenomena by which experience modifies future behavioural responses. Extinction is considered an active associative-learning process, in which a new association is formed, predicting the absence of the US after CS presentations82. Conversely, habituation is one of the simplest forms of memory and relies on the non-associative reduction of responses to repeated stimuli occurring even in very simple neuronal systems (for example, aplysia95). The eCB system has been proposed to participate in both of these processes. One possibility is that the local, CB1R-dependent control of neuronal transmission and plasticity might regulate associative properties of extinction, by favouring the activation of putative ‘no-fear’ neurons at the expense of ‘fear’ neurons in amygdalar circuits2. However, another hypothesis is that eCB signalling might potentiate or activate non-associative habituation processes to dampen the fear reaction after repeated CS-alone exposures96. The decrease of freezing response elicited by repeated tone presentations to mice previously exposed to a footshock not associated to the tone (non-associative sensitization) was shown to be strongly impaired in global CB1R-deficient mice and in conditional mutant mice lacking CB1R in cortical glutamatergic neurons3,96,97. Remarkably, this concept of habituation is congruent with the notion of how the eCB system works in stress-coping (see also below), in which it is activated by repeated exposure to a homotypic stress (that is, an identical repeated sensory input) and is required for habituation98. Therefore, the available data support the hypothesis that the eCB system is required for appropriate and efficient fear relief3, whereby the activity of the eCB system is increased with each re-exposure to a stimulus associated with a threat that is no longer present, and the fear response (for example, the freezing behaviour) is reduced in a manner inversely related to the elevation of eCB-mediated CB1R activation. This model still has to be verified experimentally by cell-type-specific analyses of eCB system activity under conditions of repeated exposures to the threatening stimuli.
The extinction of conditioned fear is inhibited by stress exposure, which can be problematic in the application of extinction-like procedures to treat PTSD in humans. In the inhibitory-avoidance paradigm, in which the animal (for example, a rat) learns to avoid places previously associated with punishments, exposure to stress enhances conditioning and reduces extinction. Both of these effects of stress are inhibited by CB1R agonist injection into the BLA99. Similarly, the effect of a single prolonged stress (SPS) to inhibit contextual fear extinction 1 week later is reduced by CB1R activation in BLA or hippocampus immediately after the SPS100. Furthermore, CB1R activation prevents SPS-induced upregulation of the glucocorticoid receptor in the BLA and hippocampus, suggesting that high CB1R activity at the time of trauma reduces the glucocorticoid receptor’s ability to hyperactivate the fear circuit. Chronic social-defeat stress also impairs contextual fear extinction, an effect that is alleviated by the treatment with the FAAH inhibitor URB597 (REF. 101). These data are consistent with the hypothesis that chronic stress creates a ‘hypocannabinergic state’ that results in impaired fear extinction and can be alleviated by CB1R agonists and indirect agonists (see below).
Fear-coping strategies
Several theories of fear refer to the coexistence of different coping strategies, or ‘styles’, which induce specific types of responses to threatening situations; these are usually classified as passive (or reactive) and active (or proactive)102,103. Another distinct feature of the eCB system is its role in the regulation of switching between these different strategies, that is, between a passive fear response (such as freezing) and active behaviours (such as escape attempts and risk assessment). In classical fear conditioning, after prolonged exposure to the threatening stimuli, a switch occurs from passive to active behaviour. Global CB1R deficiency disrupts this pattern and favours passive responses104. This phenotype seems to depend on CB1R expressed on cortical glutamatergic neurons, as mutants lacking CB1R in these neurons display longer freezing responses in fear conditioning and slower learning of avoidance behaviour in active-avoidance paradigms. Conversely, loss of CB1R on forebrain GABAergic neurons leads to the opposite phenotype, decreasing freezing and favouring active behaviours (in this case, digging and rearing) in fear conditioning, and promoting more efficient active avoidance. Interestingly, when all CS-induced responses (freezing, rearing and digging, which could all be considered rationally as ‘fear responses’) were summed over a sufficiently long period of CS presentation, there was no difference in the patterns displayed by either of the conditional mutants compared with wild-type controls; that is, there is no difference in total fear-related behaviours. These data provokingly suggest that the CB1R expressed on either cortical glutamatergic or forebrain GABAergic neurons does not strongly affect the ‘memory’ of the conditioning event and the consequent levels of perceived ‘fear’ by the individuals during CS exposition, but merely determines the individual coping style towards specific threats104.
Stress-coping
Stress can be defined as a reaction of the body to an internal or external challenge to prepare its response to possible dangers or injuries. Physical and psychological stress induces a pattern of responses that allow for coping with the immediate threat followed by recovery to homeostasis. The earliest responses to stress are neural and occur within seconds of the stress. Several neurotransmitters are involved in this process, including noradrenaline, serotonin, GABA, glutamate and the fast-reacting stress hormone adrenaline. Endocrine responses, mediated by activation of the HPA axis, with the ultimate release of adrenal glucocorticoids, occur minutes to hours after the stress. Preclinical data strongly support the hypothesis that eCB signalling is altered by stress (BOX 2) and represents a central mechanism by which stress alters synaptic plasticity in many brain regions.
Box 2. eCB-based synaptopathies.
A major function of the endocannabinoid (eCB) system is its ability to suppress neurotransmitter release in a retrograde manner (see the figure, part a). Many studies have investigated whether dysregulation of eCB signalling contributes to synaptopathies. Stress has a strong effect on eCB system functions. For example, acute stress results in increased activity of fatty acid amide hydrolase (FAAH) in the basolateral amygdala (BLA), via a corticotropin-releasing hormone (CRH) receptor type 1 (CRHR1)-mediated mechanism106 (see the figure, part b). Increased FAAH activity results in reduced concentrations of N-arachidonoylethanolamine (AEA) and thus in increased excitability of principal neurons in the BLA because AEA is not available for the retrograde suppression of glutamate release; eventually leading to increased anxiety-like behaviour. Chronic stress (see the figure, part c) has recently been shown to cause an impairment of 2-arachidonoyl glycerol (2-AG) synthesis, through collapse of a signalling cascade in glutamatergic neurons in the BLA, a process involving the activation of a metabotropic glucocorticoid receptor (mGR), leading to increased activity of protein tyrosine phosphatase 1B (PTP1B) via decreased palmitoylation and cytoplasmic activity of its inhibitor, LIM domain only 4 (LMO4; translocation out of dendrite). In consequence, PTP1B shows enhanced inhibition of metabotropic glutamate receptor 5 (mGluR5; also known as GRM5) phosphorylation, resulting in decreased diacylglycerol lipase-α (DAGLα) activity and 2-AG production162. Pharmacological inhibition of PTP1B rescues the insufficient 2-AG production and the anxiety-like phenotype after chronic stress162. Likewise, mutations in the gene fragile X mental retardation 1 (FMR1) in the fragile X syndrome (see the figure, part d) result in an uncoupling of DAGLα from the mGluR5–Homer complex163–165. This leads to impaired 2-AG production and decreased retrograde suppression of both GABAergic and glutamatergic transmission, and coincides with increased anxiety and cognitive impairments74. AMPAR, AMPA receptor; CB1R, cannabinoid receptor type 1; CORT, corticosterone; Gq, Gq family G protein; NMDAR, NMDA receptor.

Acute stress produces changes in the brain concentrations of the two major eCBs, AEA and 2-AG, and thereby alters CB1R signalling. Acute stress exposure reduces the concentration of AEA in the amygdala and PFC; these changes are accompanied by an increase in the activity of FAAH105 and are mediated by effects of corticotropin-releasing hormone (CRH) that alter FAAH activity106. In the amygdala, reduced AEA concentrations enable the activation of the HPA axis, and inhibition of FAAH reduces the glucocorticoid response105. Both stress and glucocorticoids increase the concentrations of 2-AG in the hypothalamus, hippocampus, PFC and raphe nuclei. In the hypothalamus, activation of a plasma membrane-associated glucocorticoid receptor rapidly increases levels of 2-AG, which acts to inhibit glutamate release107. In the PFC, the mechanism by which glucocorticoids elevate 2-AG levels is not clear, but this increase results in the inhibition of GABA release108. Both in the hypothalamus109 and in the PFC108, activation of CB1R signalling is required for glucocorticoid-mediated feedback inhibition of the HPA axis. Interestingly, recent data indicate that restraint stress is also able to switch eCB-dependent plasticity from LTD to LTP in the BNST, suggesting that the eCB system can regulate stress responses in several different brain regions110. Food deprivation was also shown to convert an eCB-dependent LTD of inhibitory transmission to a nitric oxide (NO)-dependent, CB1R-independent LTP of inhibitory transmission in the hypothalamus111, indicating that eCB signalling is centrally involved in plastic adaptations induced by different types of stress.
Chronic stress exposure also alters the eCB system throughout the brain. Exposure to non-habituating, chronic stress downregulates CB1R signalling in many brain regions involved in emotional processing, including the hippocampus112, striatum113, nucleus accumbens114, PFC115, dorsal raphe nucleus116, amygdala117 and hypothalamus118. In the hippocampus, hypothalamus and striatum, chronic stress reduces signalling by downregulating CB1R112,113,118. However, in the PFC, chronic stress increases CB1R mRNA expression but clearly reduces CB1R responsivity at GABAergic terminals115. Yet another mechanism is at play in the amygdala, where chronic stress increases FAAH activity and decreases AEA concentrations, which would be expected to decrease eCB signalling at the level of ligand availability117. Different neuronal types, such as cortical glutamatergic, forebrain GABAergic and serotonergic neurons, are differentially involved in the responses to acute and chronic stress in mice, again indicating multilevel control of stress responses by the eCB system28,86,119,120.
Repeated exposures to the same stress result in habituation of HPA axis activation and of the behavioural stress response. Repeated homotypic stress exposures sensitize the eCB system, which contributes to the habituation to stress98,121. The mechanism involves increased concentrations of 2-AG, possibly owing to reduced catabolism by MAGL48. The ability to habituate to repeated exposure to a non-threatening stimulus is protective, as it avoids the consequences of chronic stress. The ability of eCB-mediated synaptic plasticity to facilitate habituation could be one of the most critical roles of this process in the context of human psychopathology.
In summary, the brain’s eCB system links stress exposure to changes in synaptic plasticity, contributing to activation and feedback regulation of the HPA axis. More importantly for the understanding of human psychopathology, chronic stress can downregulate CB1R signalling in brain regions vital for the regulation of affective processes, whereas habituation to stress, which reduces its effect, is accompanied by enhanced eCB system activity. In more general terms, the hypothesis can be put forward that the eCB system facilitates the activation of resilience factors122,123 during and/or after stress exposure.
Future directions
Which specific neuronal circuits are regulated by the eCB system?
The eCB system is present in many brain circuits that are known to regulate anxiety, fear and stress-coping processes. Together with anatomical descriptions, substantial functional evidence corroborates the idea that eCB signalling modulates synaptic activity at many ‘nodes’ of these circuits. However, direct evidence of these functions of eCB signalling in freely behaving animals challenged with specific tasks to measure anxiety, fear and stress-coping behaviours is mostly missing and will require the manipulation of the activity of well-identified circuits in behaving animals. Therefore, most of the presently available evidence is correlative in nature and based on parallel mechanisms between in vitro, ex vivo and in vivo data, which are powerful and consistent but cannot be used to demonstrate causality. This limitation, which has negatively affected the progress of behavioural neurosciences in general, is being addressed by the advent of new technological approaches. For instance, experimental approaches such as optogenetics and pharmacogenetics124,125 will allow the examination of the direct causal relationship between the activity of specific circuits and behaviour in freely moving animals. The application of these techniques to the field of the eCB system, in combination with cell-type genetic manipulation of eCB system components using the Cre–loxP system and viral techniques, will allow the direct causal relationships between the function of, for example, CB1R in specific circuits and behavioural outputs to be uncovered126. Similarly, causal links between eCB system-meditated electrophysiological and/or synaptic modulations and behavioural outputs need to be established.
The eCB system and CNS–periphery crosstalk
The eCB system is also centrally involved in the crosstalk between central and peripheral processes regulating behaviour. This is well known in the control of energy balance and feeding, in which CB1R expression in the brain and in the periphery synergizes to regulate both metabolic activity and behavioural outputs127. This potential crosstalk has been extended to anxiety-and fear-related behaviours128. The anxiogenic effect in the elevated plus maze test and the freezing-promoting effect in fear-conditioning settings exerted by the CB1R antagonist rimonabant were blocked by the administration of peripherally restricted β-adrenergic receptor antagonists. Interestingly, this blockade also occurred when rimonabant was administered directly into the brain, suggesting that centrally mediated hyperactivation of the sympathetic nervous system is a primary consequence of CB1R blockade128. There is still much to be learned about eCB-mediated modulation of the crosstalk between the CNS and the periphery and how this can influence behavioural outputs (including in anxiety-and fear-related dimensions).
Astroglial CB1R in anxiety, fear and stress-coping
By secreting ‘gliotransmitters’ (for example, glutamate, GABA, ATP and d-serine)129 and providing energy supply and protection to neurons130, astrocytes can profoundly influence synaptic activity and brain function, including anxiety-and fear-related behaviours. Astrocytes and other glial cell types produce eCBs in response to activity-related ATP release131 and express low, but functionally important, levels of cannabinoid receptors11,132. Recent data indicate that physiological synaptic functions are regulated by astroglial cannabinoid receptors30,133–135. Interestingly, whereas the CB1R expressed at presynaptic terminals seems to reduce neurotransmitter release, the astroglial CB1R seems to potentiate synaptic glutamatergic signalling133,134. Considering that astroglial cells have been suggested to participate in anxiety, fear and stress-coping136,137, it will be interesting to assess whether similar astroglial CB1R-dependent mechanisms operate in the effect of cannabinoids and endocannabinoid signalling on these processes.
Brain bioenergetics in fear, anxiety and stress-coping: a role for CB1R?
The brain, with a weight of about 2% of the entire body, consumes up to 20% of the body’s energy138, presumably because bioenergetic processes in the brain are highly active and go beyond mere cell ‘housekeeping’ and survival. This has been demonstrated both biochemically139 and by fMRI140, and recent studies have revealed the profound effect of even limited alterations of energy supply (in the form of ATP) on synaptic functions141. Anxiety, fear and stress elicit high neuronal activity in distinct brain regions accompanied by high energy requirements, which mean a great demand for ATP. Mitochondria, which are the main cellular ‘power plants’ producing the large majority of ATP, are therefore crucial for efficient brain function, including the regulation of mood and anxiety142. The ability of cannabinoids to control mitochondrial activity was first reported in the 1970s143 and is now thought to be a potentially important way in which eCBs influence cellular and brain functions144,145. Recently, the presence of functional CB1R at mitochondrial membranes was demonstrated by different groups146,147 (see REFS 148,149 for methodological discussions). The brain mitochondrial CB1R (mtCB1R) directly regulates respiration and ATP production and, at the synaptic level, participates in eCB-dependent synaptic plasticity. The role of mtCB1R in anxiety-, fear-and stress-related circuits is not known yet, but this represents an interesting new field for future research.
Functions of peptide eCBs
The eCB family is typically represented by lipid fatty acid derivatives linked to glycerol (sn-2 acyl glycerol) or amines (N-acyl amides). However, evidence has recently accumulated that a family of peptide derivatives of α-haemoglobin, called peptide eCBs (pepcans)150, modulates CB1R activity. In particular, pepcan-12 acts as a negative allosteric modulator of CB1R150,151 and was identified in noradrenergic/adrenergic cells in the brain and in the adrenal glands152. Considering the importance of the regulation of the noradrenergic and adrenergic systems in stress regulation, further studies on pepcan-12 are keenly awaited.
Concluding remarks
The effects of phytocannabinoids on fear, anxiety and stress-coping have been appreciated for a long time, and the discovery of the active components of the plant Cannabis sativa — which is celebrated by this series of Review articles on endocannabinoid function in the brain15,153–155 — has fuelled the search for underlying mechanisms. Future studies will need to integrate new discoveries into the larger picture of the eCB-dependent regulation of anxiety, fear and stress responses. Based on its multiple and diverse mechanisms of action, the eCB system can also be considered as a suitable tool to shape and fine-tune diverse and complex behaviours. Indeed, in a bottom-up approach, starting from any of the elements of the eCB system (receptors, different types of ligands, triggers and enzymes involved in ligand synthesis and degradation), researchers are asked to follow several different pathways involving diverse elements of the mechanisms underlying complex behaviours, such as neuronal circuits, synaptic plasticity, astroglial functions, eCB biochemistry and bioenergetics. In the end, these studies raise strong hopes not only for a better understanding of basic behavioural processes but also for future therapeutic interventions to tackle their dysfunctions, which are particularly warranted in affective disorders (BOX 3). These reasons make the study of the eCB system a highly fascinating aspect of neuroscience, and the next decades of research will surely bring new and exciting discoveries and concepts.
Box 3. Therapeutic targeting.
Stimulation of cannabinoid receptors
Clinical findings suggest a negative correlation between endocannabinoid (eCB) system activity and anxiety166. Cannabinoid receptor type 1 (CB1R) agonists can have unacceptable side effects54, whereas increasing eCB levels by inhibiting eCB-degrading enzymes could be more selective. Enhancement of N-arachidonoylethanolamine (AEA) and 2-arachidonoyl glycerol (2-AG) by fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL) blockade, respectively, attenuates anxiety in rodents6,67,167. The anxiolytic effect of AEA depends on CB1R and is associated with cognitive impairments, whereas the anxiolytic effect of 2-AG is CB2R-dependent and not associated with cognitive disruption64. However, other studies reported a CB1R involvement in the 2-AG anxiolytic effect using different behavioural models65 and animal species66. As these indirect agonists have not yet been approved for use in humans, the option of direct CB1R stimulation has yet to be explored, whereby CB1R stimulation with Δ9-tetrahydrocannabinol (THC) enhances fear extinction in humans168, which warrants further investigation in post-traumatic stress disorders (PTSD)169.
Other strategies have been proposed to minimize cannabinoid side effects. Blockade of the mammalian target of rapamycin (mTOR) pathway prevents THC-induced cognitive impairment in mice, without modifying its anxiolytic effects170. COX2 (also known as PTGS2) inhibition increases eCB brain levels171, reduces anxiety in rodents172 and blocks THC-induced cognitive impairment173. Positive allosteric modulators regulating orthosteric ligand activity can open new perspectives for reducing side effects174,175. Alternatively, interruption of heterodimers between CB1R and serotonin 5-hydroxytryptamine receptor 2A (5-HT2AR) using cell-penetrable peptides selectively abrogates THC-induced memory impairments176.
Inhibition of cannabinoid receptors
An interesting therapeutic approach has recently been suggested for fragile X syndrome, which is caused by a mutation in the fragile X mental retardation 1 (FMR1) gene. An eCB system dysregulation seems to be responsible for the imbalance between excitatory and inhibitory inputs in the hippocampus, leading to the behavioural fragile X phenotype of Fmr1-knockout (Fmr1−/y) mice. CB1R blockade normalized the main cognitive and hippocampal neurological alterations in these mice, whereas CB2R blockade alleviated their anxiolytic phenotype74. The use of synthetic174,175 and endogenous150,177 negative allosteric modulators of cannabinoid receptors is another possible avenue to achieve better therapeutic effects with reduced side-effects.
Acknowledgments
B.L. was supported by the German Research Foundation (SFB TRR 58, CRC 1080 and FOR 926); G.M. by the Institut national de la santé et de la recherche médicale (INSERM), the European Commission Seventh Framework Programme (REPROBESITY, HEALTH-F2-2008-223713, PAINCAGE and HEALTH-2014-603191), the European Research Council (Endofood, ERC–2010–StG-260515, CannaPreg and ERC-2014-PoC-640923), the Fondation pour la Recherche Medicale (DRM20101220445), the Human Frontiers Science Program, Region Aquitaine, Agence Nationale de la Recherche (ANR Blanc NeuroNutriSens ANR-13-BSV4-0006 and BRAIN ANR-10-LABX-0043); R.M. by the grants SAF2014-59648P, RETICS-RTA#RD12/0028/0023, AGAUR#2014-SGR-1547 and Health-F2-2013-602891; and C.J.H. by the US National Institutes of Health grants DA038663, DA026996 and MH102838.
Glossary
- Endocannabinoid (eCB)
A type of lipid signalling molecule derived from arachidonic acid. The eCBs are the endogenous counterparts of the cannabinoids
- Microglia
Immune cells of the brain that are involved in defence
- Anxiety disorders
Mental disorders involving feelings of anxiety and fear, caused by physical or psychological harm. There are different forms, such as general anxiety disorders and specific phobias
- Thigmotaxis
Movement of an organism towards an object (for example, a wall), giving them a sense of increased safety
- Neophobic behaviour
Fear of anything new; unwillingness to try new things and break from routine
- Polymorphism
A genetic variant of a gene, with possible emergence of distinct phenotypes
- Habituation
A form of learning in which an organism reduces its response to a stimulus after repeated presentations of the stimulus
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- 1.Tart CT. Marijuana intoxication common experiences. Nature. 1970;226:701–704. doi: 10.1038/226701a0. [DOI] [PubMed] [Google Scholar]
- 2.Lafenetre P, Chaouloff F, Marsicano G. The endocannabinoid system in the processing of anxiety and fear and how CB1 receptors may modulate fear extinction. Pharmacol Res. 2007;56:367–381. doi: 10.1016/j.phrs.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Riebe CJ, Pamplona FA, Kamprath K, Wotjak CT. Fear relief—toward a new conceptual frame work and what endocannabinoids gotta do with it. Neuroscience. 2012;204:159–185. doi: 10.1016/j.neuroscience.2011.11.057. [DOI] [PubMed] [Google Scholar]
- 4.Ruehle S, Rey AA, Remmers F, Lutz B. The endocannabinoid system in anxiety, fear memory and habituation. J Psychopharmacol. 2012;26:23–39. doi: 10.1177/0269881111408958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akirav I. The role of cannabinoids in modulating emotional and non-emotional memory processes in the hippocampus. Front Behav Neurosci. 2011;5:34. doi: 10.3389/fnbeh.2011.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunduz-Cinar O, Hill MN, McEwen BS, Holmes A. Amygdala FAAH and anandamide: mediating protection and recovery from stress. Trends Pharmacol Sci. 2013;34:637–644. doi: 10.1016/j.tips.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLaughlin RJ, Hill MN, Gorzalka BB. A critical role for prefrontocortical endocannabinoid signaling in the regulation of stress and emotional behavior. Neurosci Biobehav Rev. 2014;42:116–131. doi: 10.1016/j.neubiorev.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Castillo PE, Younts TJ, Chavez AE, Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron. 2012;76:70–81. doi: 10.1016/j.neuron.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katona I, Freund TF. Multiple functions of endocannabinoid signaling in the brain. Annu Rev Neurosci. 2012;35:529–558. doi: 10.1146/annurev-neuro-062111-150420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marsicano G, Kuner R. In: Anatomical Distribution of Receptors, Ligands and Enzymes in the Brain and in the Spinal Cord: Circuitries and Neurochemistry in Cannabinoids in the Brain. Köfalvi A, editor. Springer; 2008. pp. 161–201. [Google Scholar]
- 11.Metna-Laurent M, Marsicano G. Rising stars: modulation of brain functions by astroglial type-1 cannabinoid receptors. Glia. 2015;63:353–364. doi: 10.1002/glia.22773. [DOI] [PubMed] [Google Scholar]
- 12.Stella N. Endocannabinoid signaling in microglial cells. Neuropharmacology. 2009;56:244–253. doi: 10.1016/j.neuropharm.2008.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alger BE, Kim J. Supply and demand for endocannabinoids. Trends Neurosci. 2011;34:304–315. doi: 10.1016/j.tins.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohno-Shosaku T, Kano M. Endocannabinoid-mediated retrograde modulation of synaptic transmission. Curr Opin Neurobiol. 2014;29:1–8. doi: 10.1016/j.conb.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 15.Soltesz I, et al. Weeding out bad waves: towards selective cannabinoid circuit control in epilepsy. Nat Rev Neurosci. 2015;16:264–277. doi: 10.1038/nrn3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- 17.Katona I, et al. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lafourcade M, et al. Molecular components and functions of the endocannabinoid system in mouse prefrontal cortex. PLoS ONE. 2007;2:e709. doi: 10.1371/journal.pone.0000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puente N, et al. Localization and function of the cannabinoid CB1 receptor in the anterolateral bed nucleus of the stria terminalis. PLoS ONE. 2010;5:e8869. doi: 10.1371/journal.pone.0008869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katona I, et al. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci. 2001;21:9506–9518. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamprath K, et al. Short-term adaptation of conditioned fear responses through endocannabinoid signaling in the central amygdala. Neuropsychopharmacology. 2011;36:652–663. doi: 10.1038/npp.2010.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramikie TS, et al. Multiple mechanistically distinct modes of endocannabinoid mobilization at central amygdala glutamatergic synapses. Neuron. 2014;81:1111–1125. doi: 10.1016/j.neuron.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cristino L, et al. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience. 2006;139:1405–1415. doi: 10.1016/j.neuroscience.2006.02.074. [DOI] [PubMed] [Google Scholar]
- 24.Marsicano G, et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- 25.Marinelli S, Pacioni S, Cannich A, Marsicano G, Bacci A. Self-modulation of neocortical pyramidal neurons by endocannabinoids. Nat Neurosci. 2009;12:1488–1490. doi: 10.1038/nn.2430. [DOI] [PubMed] [Google Scholar]
- 26.Kirilly E, Hunyady L, Bagdy G. Opposing local effects of endocannabinoids on the activity of noradrenergic neurons and release of noradrenaline: relevance for their role in depression and in the actions of CB1 receptor antagonists. J Neural Transm. 2013;120:177–186. doi: 10.1007/s00702-012-0900-1. [DOI] [PubMed] [Google Scholar]
- 27.Häring M, Marsicano G, Lutz B, Monory K. Identification of the cannabinoid receptor type 1 in serotonergic cells of raphe nuclei in mice. Neuroscience. 2007;146:1212–1219. doi: 10.1016/j.neuroscience.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 28.Häring M, et al. Cannabinoid type-1 receptor signaling in central serotonergic neurons regulates anxiety-like behavior and sociability. Front Behav Neurosci. 2015;9:235. doi: 10.3389/fnbeh.2015.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nyiri G, et al. GABAB and CB1 cannabinoid receptor expression identifies two types of septal cholinergic neurons. Eur J Neurosci. 2005;21:3034–3042. doi: 10.1111/j.1460-9568.2005.04146.x. [DOI] [PubMed] [Google Scholar]
- 30.Han J, et al. Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell. 2012;148:1039–1050. doi: 10.1016/j.cell.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 31.Carta M, et al. Membrane lipids tune synaptic transmission by direct modulation of presynaptic potassium channels. Neuron. 2014;81:787–799. doi: 10.1016/j.neuron.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 32.Piomelli D, et al. Dopamine activation of the arachidonic acid cascade as a basis for D1/D2 receptor synergism. Nature. 1991;353:164–167. doi: 10.1038/353164a0. [DOI] [PubMed] [Google Scholar]
- 33.Meves H. Arachidonic acid and ion channels: an update. Br J Pharmacol. 2008;155:4–16. doi: 10.1038/bjp.2008.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shonesy BC, et al. Genetic disruption of 2-arachidonoylglycerol synthesis reveals a key role for endocannabinoid signaling in anxiety modulation. Cell Rep. 2014;9:1644–1653. doi: 10.1016/j.celrep.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schlosburg JE, et al. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci. 2010;13:1113–1119. doi: 10.1038/nn.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jenniches I, et al. Anxiety, stress and fear response in mice with reduced endocannabinoid levels. Biol Psych. 2015 doi: 10.1016/j.biopsych.2015.03.033. http://dx.doi.org/10.1016/j.biopsych.2015.03.033. [DOI] [PubMed]
- 37.Navarrete M, Diez A, Araque A. Astrocytes in endocannabinoid signalling. Phil Trans R Soc B. 2014;369:20130599. doi: 10.1098/rstb.2013.0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lutz B. Endocannabinoid signals in the control of emotion. Curr Opin Pharmacol. 2009;9:46–52. doi: 10.1016/j.coph.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 39.Di S, et al. Activity-dependent release and actions of endocannabinoids in the rat hypothalamic supraoptic nucleus. J Physiol. 2005;569:751–760. doi: 10.1113/jphysiol.2005.097477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hill MN, Karatsoreos IN, Hillard CJ, McEwen BS. Rapid elevations in limbic endocannabinoid content by glucocorticoid hormones in vivo. Psychoneuroendocrinology. 2010;35:1333–1338. doi: 10.1016/j.psyneuen.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang GZ, Woolley CS. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron. 2012;74:801–808. doi: 10.1016/j.neuron.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tovote P, Fadok JP, Luthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci. 2015;16:317–331. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- 43.Bains JS, Cusulin JI, Inoue W. Stress-related synaptic plasticity in the hypothalamus. Nat Rev Neurosci. 2015;16:377–388. doi: 10.1038/nrn3881. [DOI] [PubMed] [Google Scholar]
- 44.Graham BM, Langton JM, Richardson R. Pharmacological enhancement of fear reduction: preclinical models. Br J Pharmacol. 2011;164:1230–1247. doi: 10.1111/j.1476-5381.2010.01175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sylvers P, Lilienfeld SO, LaPrairie JL. Differences between trait fear and trait anxiety: implications for psychopathology. Clin Psychol Rev. 2011;31:122–137. doi: 10.1016/j.cpr.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 46.Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annu Rev Psychol. 2013;64:21–47. doi: 10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- 47.Jacob W, et al. Endocannabinoids render exploratory behaviour largely independent of the test aversiveness: role of glutamatergic transmission. Genes Brain Behav. 2009;8:685–698. doi: 10.1111/j.1601-183X.2009.00512.x. [DOI] [PubMed] [Google Scholar]
- 48.Sumislawski JJ, Ramikie TS, Patel S. Reversible gating of endocannabinoid plasticity in the amygdala by chronic stress: a potential role for monoacylglycerol lipase inhibition in the prevention of stress-induced behavioral adaptation. Neuropsychopharmacology. 2011;36:2750–2761. doi: 10.1038/npp.2011.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rey AA, Purrio M, Viveros MP, Lutz B. Biphasic effects of cannabinoids in anxiety responses: CB1 and GABAB receptors in the balance of GABAergic and glutamatergic neurotransmission. Neuropsychopharmacology. 2012;37:2624–2634. doi: 10.1038/npp.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Häring M, Kaiser N, Monory K, Lutz B. Circuit specific functions of cannabinoid CB1 receptor in the balance of investigatory drive and exploration. PLoS ONE. 2011;6:e26617. doi: 10.1371/journal.pone.0026617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lafenetre P, Chaouloff F, Marsicano G. Bidirectional regulation of novelty-induced behavioral inhibition by the endocannabinoid system. Neuropharmacology. 2009;57:715–721. doi: 10.1016/j.neuropharm.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 52.Legler A, Monory K, Lutz B. Age differences in the role of the cannabinoid type 1 receptor on glutamatergic neurons in habituation and spatial memory acquisition. Life Sci. 2015;138:63. doi: 10.1016/j.lfs.2015.01.034. [DOI] [PubMed] [Google Scholar]
- 53.Ruehle S, et al. Cannabinoid CB1 receptor in dorsal telencephalic glutamatergic neurons: distinctive sufficiency for hippocampus-dependent and amygdala-dependent synaptic and behavioral functions. J Neurosci. 2013;33:10264–10277. doi: 10.1523/JNEUROSCI.4171-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moreira FA, Grieb M, Lutz B. Central side-effects of therapies based on CB1 cannabinoid receptor agonists and antagonists: focus on anxiety and depression. Best Pract Res Clin Endocrinol Metab. 2009;23:133–144. doi: 10.1016/j.beem.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 55.Rubino T, et al. CB1 receptor stimulation in specific brain areas differently modulate anxiety-related behaviour. Neuropharmacology. 2008;54:151–160. doi: 10.1016/j.neuropharm.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 56.Monory K, Polack M, Remus A, Lutz B, Korte M. Cannabinoid CB1 receptor calibrates excitatory synaptic balance in the mouse hippocampus. J Neurosci. 2015;35:3842–3850. doi: 10.1523/JNEUROSCI.3167-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moreira FA, Kaiser N, Monory K, Lutz B. Reduced anxiety-like behaviour induced by genetic and pharmacological inhibition of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) is mediated by CB1 receptors. Neuropharmacology. 2008;54:141–150. doi: 10.1016/j.neuropharm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 58.Kathuria S, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. This landmark publication shows the high efficacy of drugs that inhibit AEA hydrolysis and their potential use in the treatment of anxiety-related disorder. [DOI] [PubMed] [Google Scholar]
- 59.Patel S, Hillard CJ. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J Pharmacol Exp Ther. 2006;318:304–311. doi: 10.1124/jpet.106.101287. [DOI] [PubMed] [Google Scholar]
- 60.Lomazzo E, et al. Therapeutic potential of inhibitors of endocannabinoid degradation for the treatment of stress-related hyperalgesia in an animal model of chronic pain. Neuropsychopharmacology. 2015;40:488–501. doi: 10.1038/npp.2014.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sipe JC, Chiang K, Gerber AL, Beutler E, Cravatt BF. A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proc Natl Acad Sci USA. 2002;99:8394–8399. doi: 10.1073/pnas.082235799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dincheva I, et al. FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nat Commun. 2015;6:6395. doi: 10.1038/ncomms7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guggenhuber S, et al. Impaired 2-AG signaling in hippocampal glutamatergic neurons: aggravation of anxiety-like behavior and unaltered seizure susceptibility. Int J Neuropsychopharmacol. 2015 doi: 10.1093/ijnp/pyv091. http://dx.doi.org/10.1093/ijnp/pyv091. [DOI] [PMC free article] [PubMed]
- 64.Busquets-Garcia A, et al. Differential role of anandamide and 2-arachidonoylglycerol in memory and anxiety-like responses. Biol Psychiatry. 2011;70:479–486. doi: 10.1016/j.biopsych.2011.04.022. This study shows that anxiolytic-like effects of AEA are mediated by CB1R and associated with memory disruption, whereas 2-AG induces an anxiolytic effect via CB2R without affecting cognitive functions. [DOI] [PubMed] [Google Scholar]
- 65.Kinsey SG, O’Neal ST, Long JZ, Cravatt BF, Lichtman AH. Inhibition of endocannabinoid catabolic enzymes elicits anxiolytic-like effects in the marble burying assay. Pharmacol Biochem Behav. 2011;98:21–27. doi: 10.1016/j.pbb.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sciolino NR, Zhou W, Hohmann AG. Enhancement of endocannabinoid signaling with JZL184, an inhibitor of the 2-arachidonoylglycerol hydrolyzing enzyme monoacylglycerol lipase, produces anxiolytic effects under conditions of high environmental aversiveness in rats. Pharmacol Res. 2011;64:226–234. doi: 10.1016/j.phrs.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhong P, et al. Monoacylglycerol lipase inhibition blocks chronic stress-induced depressive-like behaviors via activation of mTOR signaling. Neuropsychopharmacology. 2014;39:1763–1776. doi: 10.1038/npp.2014.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marsch R, et al. Reduced anxiety, conditioned fear, and hippocampal long-term potentiation in transient receptor potential vanilloid type 1 receptor-deficient mice. J Neurosci. 2007;27:832–839. doi: 10.1523/JNEUROSCI.3303-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun FJ, et al. Increased expression of TRPV1 in the cortex and hippocampus from patients with mesial temporal lobe epilepsy. J Mol Neurosci. 2013;49:182–193. doi: 10.1007/s12031-012-9878-2. [DOI] [PubMed] [Google Scholar]
- 70.Puente N, et al. The transient receptor potential vanilloid-1 is localized at excitatory synapses in the mouse dentate gyrus. Brain Struct Funct. 2015;220:1187–1194. doi: 10.1007/s00429-014-0711-2. [DOI] [PubMed] [Google Scholar]
- 71.Chavez AE, Hernandez VM, Rodenas-Ruano A, Chan CS, Castillo PE. Compartment-specific modulation of GABAergic synaptic transmission by TRPV1 channels in the dentate gyrus. J Neurosci. 2014;34:16621–16629. doi: 10.1523/JNEUROSCI.3635-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chavez AE, Chiu CQ, Castillo PE. TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nat Neurosci. 2010;13:1511–1518. doi: 10.1038/nn.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bahi A, et al. β-caryophyllene, a CB2 receptor agonist produces multiple behavioral changes relevant to anxiety and depression in mice. Physiol Behav. 2014;135:119–124. doi: 10.1016/j.physbeh.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 74.Busquets-Garcia A, et al. Targeting the endocannabinoid system in the treatment of fragile X syndrome. Nat Med. 2013;19:603–607. doi: 10.1038/nm.3127. [DOI] [PubMed] [Google Scholar]
- 75.Aguiar DC, Terzian AL, Guimaraes FS, Moreira FA. Anxiolytic-like effects induced by blockade of transient receptor potential vanilloid type 1 (TRPV1) channels in the medial prefrontal cortex of rats. Psychopharmacology (Berl) 2009;205:217–225. doi: 10.1007/s00213-009-1532-5. [DOI] [PubMed] [Google Scholar]
- 76.Almeida-Santos AF, et al. Modulation of anxiety-like behavior by the endocannabinoid 2-arachidonoylglycerol (2-AG) in the dorsolateral periaqueductal gray. Behav Brain Res. 2013;252:10–17. doi: 10.1016/j.bbr.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 77.Terzian AL, Aguiar DC, Guimaraes FS, Moreira FA. Modulation of anxiety-like behaviour by transient receptor potential vanilloid type 1 (TRPV1) channels located in the dorsolateral periaqueductal gray. Eur Neuropsychopharmacol. 2009;19:188–195. doi: 10.1016/j.euroneuro.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 78.Moreira FA, Aguiar DC, Terzian AL, Guimaraes FS, Wotjak CT. Cannabinoid type 1 receptors and transient receptor potential vanilloid type 1 channels in fear and anxiety-two sides of one coin? Neuroscience. 2012;204:186–192. doi: 10.1016/j.neuroscience.2011.08.046. [DOI] [PubMed] [Google Scholar]
- 79.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 80.Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nader K, Hardt O. A single standard for memory: the case for reconsolidation. Nat Rev Neurosci. 2009;10:224–234. doi: 10.1038/nrn2590. [DOI] [PubMed] [Google Scholar]
- 82.Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- 83.Maren S. Seeking a spotless mind: extinction, deconsolidation, and erasure of fear memory. Neuron. 2011;70:830–845. doi: 10.1016/j.neuron.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marsicano G, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. This was the first study to demonstrate the effect of the eCB system on acquired fear response. [DOI] [PubMed] [Google Scholar]
- 85.Plendl W, Wotjak CT. Dissociation of within- and between-session extinction of conditioned fear. J Neurosci. 2010;30:4990–4998. doi: 10.1523/JNEUROSCI.6038-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dubreucq S, et al. Genetic dissection of the role of cannabinoid type-1 receptors in the emotional consequences of repeated social stress in mice. Neuropsychopharmacology. 2012;37:1885–1900. doi: 10.1038/npp.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Llorente-Berzal A, et al. 2-AG promotes the expression of conditioned fear via cannabinoid receptor type 1 on GABAergic neurons. Psychopharmacology (Berl) 2015;232:2811–2825. doi: 10.1007/s00213-015-3917-y. [DOI] [PubMed] [Google Scholar]
- 88.Trouche S, Sasaki JM, Tu T, Reijmers LG. Fear extinction causes target-specific remodeling of perisomatic inhibitory synapses. Neuron. 2013;80:1054–1065. doi: 10.1016/j.neuron.2013.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bowers ME, Ressler KJ. Interaction between the cholecystokinin and endogenous cannabinoid systems in cued fear expression and extinction retention. Neuropsychopharmacology. 2015;40:688–700. doi: 10.1038/npp.2014.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Soria-Gomez E, et al. Habenular CB receptors control the expression of aversive memories. Neuron. 2015;88:306–313. doi: 10.1016/j.neuron.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 91.Pan B, et al. Blockade of 2-arachidonoylglycerol hydrolysis by selective monoacylglycerol lipase inhibitor 4-nitrophenyl 4-(dibenzo[d][1,3] dioxol-5-yl(hydroxy)methyl)piperidine-1-carboxylate (JZL184) enhances retrograde endocannabinoid signaling. J Pharmacol Exp Ther. 2009;331:591–597. doi: 10.1124/jpet.109.158162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pan B, et al. Alterations of endocannabinoid signaling, synaptic plasticity, learning, and memory in monoacylglycerol lipase knock-out mice. J Neurosci. 2011;31:13420–13430. doi: 10.1523/JNEUROSCI.2075-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lin HC, Mao SC, Su CL, Gean PW. The role of prefrontal cortex CB1 receptors in the modulation of fear memory. Cereb Cortex. 2009;19:165–175. doi: 10.1093/cercor/bhn075. [DOI] [PubMed] [Google Scholar]
- 94.Gunduz-Cinar O, et al. Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Mol Psychiatry. 2013;18:813–823. doi: 10.1038/mp.2012.72. This study provides evidence for the conserved role of AEA in rodents and humans regarding amygdala function and threat processing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 96.Kamprath K, et al. Cannabinoid CB1 receptor mediates fear extinction via habituation-like processes. J Neurosci. 2006;26:6677–6686. doi: 10.1523/JNEUROSCI.0153-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kamprath K, et al. Endocannabinoids mediate acute fear adaptation via glutamatergic neurons independently of corticotropin-releasing hormone signaling. Genes Brain Behav. 2009;8:203–211. doi: 10.1111/j.1601-183X.2008.00463.x. [DOI] [PubMed] [Google Scholar]
- 98.Patel S, Roelke CT, Rademacher DJ, Hillard CJ. Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. Eur J Neurosci. 2005;21:1057–1069. doi: 10.1111/j.1460-9568.2005.03916.x. [DOI] [PubMed] [Google Scholar]
- 99.Ganon-Elazar E, Akirav I. Cannabinoid receptor activation in the basolateral amygdala blocks the effects of stress on the conditioning and extinction of inhibitory avoidance. J Neurosci. 2009;29:11078–11088. doi: 10.1523/JNEUROSCI.1223-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ganon-Elazar E, Akirav I. Cannabinoids and traumatic stress modulation of contextual fear extinction and GR expression in the amygdala-hippocampal-prefrontal circuit. Psychoneuroendocrinology. 2013;38:1675–1687. doi: 10.1016/j.psyneuen.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 101.Laricchiuta D, Centonze D, Petrosini L. Effects of endocannabinoid and endovanilloid systems on aversive memory extinction. Behav Brain Res. 2013;256:101–107. doi: 10.1016/j.bbr.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 102.Gozzi A, et al. A neural switch for active and passive fear. Neuron. 2010;67:656–666. doi: 10.1016/j.neuron.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 103.Koolhaas JM, de Boer SF, Coppens CM, Buwalda B. Neuroendocrinology of coping styles: towards understanding the biology of individual variation. Front Neuroendocrinol. 2010;31:307–321. doi: 10.1016/j.yfrne.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 104.Metna-Laurent M, et al. Bimodal control of fear-coping strategies by CB1 cannabinoid receptors. J Neurosci. 2012;32:7109–7118. doi: 10.1523/JNEUROSCI.1054-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hill MN, et al. Suppression of amygdalar endocannabinoid signaling by stress contributes to activation of the hypothalamic–pituitary–adrenal axis. Neuropsychopharmacology. 2009;34:2733–2745. doi: 10.1038/npp.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gray JM, et al. Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. J Neurosci. 2015;35:3879–3892. doi: 10.1523/JNEUROSCI.2737-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Di S, Malcher-Lopes R, Marcheselli VL, Bazan NG, Tasker JG. Rapid glucocorticoid-mediated endocannabinoid release and opposing regulation of glutamate and γ-aminobutyric acid inputs to hypothalamic magnocellular neurons. Endocrinology. 2005;146:4292–4301. doi: 10.1210/en.2005-0610. This study shows how hypothalamic eCBs and CB1R are involved in the negative feedback mechanism of the HPA axis. [DOI] [PubMed] [Google Scholar]
- 108.Hill MN, et al. Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J Neurosci. 2011;31:10506–10515. doi: 10.1523/JNEUROSCI.0496-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Evanson NK, Tasker JG, Hill MN, Hillard CJ, Herman JP. Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology. 2010;151:4811–4819. doi: 10.1210/en.2010-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Glangetas C, et al. Stress switches cannabinoid type-1 (CB1) receptor-dependent plasticity from LTD to LTP in the bed nucleus of the stria terminalis. J Neurosci. 2013;33:19657–19663. doi: 10.1523/JNEUROSCI.3175-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Crosby KM, Inoue W, Pittman QJ, Bains JS. Endocannabinoids gate state-dependent plasticity of synaptic inhibition in feeding circuits. Neuron. 2011;71:529–541. doi: 10.1016/j.neuron.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hill MN, et al. Downregulation of endocannabinoid signaling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacology. 2005;30:508–515. doi: 10.1038/sj.npp.1300601. This study provides strong evidence that the eCB system is dysregulated under chronic stress. [DOI] [PubMed] [Google Scholar]
- 113.Rossi S, et al. Chronic psychoemotional stress impairs cannabinoid-receptor-mediated control of GABA transmission in the striatum. J Neurosci. 2008;28:7284–7292. doi: 10.1523/JNEUROSCI.5346-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang W, et al. Deficiency in endocannabinoid signaling in the nucleus accumbens induced by chronic unpredictable stress. Neuropsychopharmacology. 2010;35:2249–2261. doi: 10.1038/npp.2010.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hillard C. In: Cannabinoids, Endocannabinoids and Stress in Cannabinoids. Di Marzo V, editor. Wiley-Blackwell; 2014. pp. 139–174. [Google Scholar]
- 116.Haj-Dahmane S, Shen RY. Chronic stress impairs alpha1-adrenoceptor-induced endocannabinoid-dependent synaptic plasticity in the dorsal raphe nucleus. J Neurosci. 2014;34:14560–14570. doi: 10.1523/JNEUROSCI.1310-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hill MN, et al. Disruption of fatty acid amide hydrolase activity prevents the effects of chronic stress on anxiety and amygdalar microstructure. Mol Psychiatry. 2013;18:1125–1135. doi: 10.1038/mp.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wamsteeker JI, Kuzmiski JB, Bains JS. Repeated stress impairs endocannabinoid signaling in the paraventricular nucleus of the hypothalamus. J Neurosci. 2010;30:11188–11196. doi: 10.1523/JNEUROSCI.1046-10.2010. This paper demonstrates the mechanistic underpinnings for how repeated stress interferes with eCB system activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Häring M, Grieb M, Monory K, Lutz B, Moreira FA. Cannabinoid CB1 receptor in the modulation of stress coping behavior in mice: the role of serotonin and different forebrain neuronal subpopulations. Neuropharmacology. 2013;65:83–89. doi: 10.1016/j.neuropharm.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 120.Steiner MA, Marsicano G, Wotjak CT, Lutz B. Conditional cannabinoid receptor type 1 mutants reveal neuron subpopulation-specific effects on behavioral and neuroendocrine stress responses. Psychoneuroendocrinology. 2008;33:1165–1170. doi: 10.1016/j.psyneuen.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 121.Patel S, Kingsley PJ, Mackie K, Marnett LJ, Winder DG. Repeated homotypic stress elevates 2-arachidonoylglycerol levels and enhances short-term endocannabinoid signaling at inhibitory synapses in basolateral amygdala. Neuropsychopharmacology. 2009;34:2699–2709. doi: 10.1038/npp.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ. Neurobiology of resilience. Nat Neurosci. 2012;15:1475–1484. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Franklin TB, Saab BJ, Mansuy IM. Neural mechanisms of stress resilience and vulnerability. Neuron. 2012;75:747–761. doi: 10.1016/j.neuron.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 124.Tye KM, Deisseroth K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat Rev Neurosci. 2012;13:251–266. doi: 10.1038/nrn3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Masseck OA, et al. Vertebrate cone opsins enable sustained and highly sensitive rapid control of Gi/o signaling in anxiety circuitry. Neuron. 2014;81:1263–1273. doi: 10.1016/j.neuron.2014.01.041. [DOI] [PubMed] [Google Scholar]
- 126.Soria-Gomez E, et al. The endocannabinoid system controls food intake via olfactory processes. Nat Neurosci. 2014;17:407–415. doi: 10.1038/nn.3647. [DOI] [PubMed] [Google Scholar]
- 127.Quarta C, et al. CB1 signaling in forebrain and sympathetic neurons is a key determinant of endocannabinoid actions on energy balance. Cell Metab. 2010;11:273–285. doi: 10.1016/j.cmet.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 128.Bellocchio L, et al. Activation of the sympathetic nervous system mediates hypophagic and anxiety-like effects of CB1 receptor blockade. Proc Natl Acad Sci USA. 2013;110:4786–4791. doi: 10.1073/pnas.1218573110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Araque A, et al. Gliotransmitters travel in time and space. Neuron. 2014;81:728–739. doi: 10.1016/j.neuron.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Belanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 131.Walter L, Dinh T, Stella N. ATP induces a rapid and pronounced increase in 2-arachidonoylglycerol production by astrocytes, a response limited by monoacylglycerol lipase. J Neurosci. 2004;24:8068–8074. doi: 10.1523/JNEUROSCI.2419-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stella N. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia. 2010;58:1017–1030. doi: 10.1002/glia.20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Navarrete M, Araque A. Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron. 2010;68:113–126. doi: 10.1016/j.neuron.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 134.Min R, Nevian T. Astrocyte signaling controls spike timing-dependent depression at neocortical synapses. Nat Neurosci. 2012;15:746–753. doi: 10.1038/nn.3075. [DOI] [PubMed] [Google Scholar]
- 135.Gomez-Gonzalo M, et al. Endocannabinoids induce lateral long-term potentiation of transmitter release by stimulation of gliotransmission. Cereb Cortex. 2015;25:3699–3712. doi: 10.1093/cercor/bhu231. [DOI] [PubMed] [Google Scholar]
- 136.Ostroff LE, Manzur MK, Cain CK, LeDoux JE. Synapses lacking astrocyte appear in the amygdala during consolidation of Pavlovian threat conditioning. J Comp Neurol. 2014;522:2152–2163. doi: 10.1002/cne.23523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hagemann TL, Paylor R, Messing A. Deficits in adult neurogenesis, contextual fear conditioning, and spatial learning in a Gfap mutant mouse model of Alexander disease. J Neurosci. 2013;33:18698–18706. doi: 10.1523/JNEUROSCI.3693-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Clark DD, Sokoloff L. In: Basic Neurochemistry: Molecular, Cellular and Medical Aspects. Siegel GJ, et al., editors. Lippincott; 1999. pp. 637–670. [Google Scholar]
- 139.Suzuki A, et al. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- 141.Rangaraju V, Calloway N, Ryan TA. Activity-driven local ATP synthesis is required for synaptic function. Cell. 2014;156:825–835. doi: 10.1016/j.cell.2013.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Marazziti D, et al. Mitochondrial alterations and neuropsychiatric disorders. Curr Med Chem. 2011;18:4715–4721. doi: 10.2174/092986711797379221. [DOI] [PubMed] [Google Scholar]
- 143.Chari-Bitron A, Bino T. Effect of 1-tetrahydrocannabinol on ATPase activity of rat liver mitochondria. Biochem Pharmacol. 1971;20:473–475. doi: 10.1016/0006-2952(71)90084-0. [DOI] [PubMed] [Google Scholar]
- 144.Nunn A, Guy G, Bell JD. Endocannabinoids in neuroendopsychology: multiphasic control of mitochondrial function. Phil Trans R Soc B. 2012;367:3342–3352. doi: 10.1098/rstb.2011.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zaccagnino P, et al. The endocannabinoid 2-arachidonoylglicerol decreases calcium induced cytochrome c release from liver mitochondria. J Bioenerg Biomembr. 2012;44:273–280. doi: 10.1007/s10863-012-9431-6. [DOI] [PubMed] [Google Scholar]
- 146.Benard G, et al. Mitochondrial CB1 receptors regulate neuronal energy metabolism. Nat Neurosci. 2012;15:558–564. doi: 10.1038/nn.3053. This study includes the first description of mitochondrial CB1R and the mechanisms by which it regulates energy metabolism. [DOI] [PubMed] [Google Scholar]
- 147.Koch M, et al. Hypothalamic POMC neurons promote cannabinoid-induced feeding. Nature. 2015;519:45–50. doi: 10.1038/nature14260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Morozov YM, et al. Antibodies to cannabinoid type 1 receptor co-react with stomatin-like protein 2 in mouse brain mitochondria. Eur J Neurosci. 2013;38:2341–2348. doi: 10.1111/ejn.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hebert-Chatelain E, et al. Cannabinoid control of brain bioenergetics: Exploring the subcellular localization of the CB1 receptor. Mol Metab. 2014;3:495–504. doi: 10.1016/j.molmet.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bauer M, et al. Identification and quantification of a new family of peptide endocannabinoids (Pepcans) showing negative allosteric modulation at CB1 receptors. J Biol Chem. 2012;287:36944–36967. doi: 10.1074/jbc.M112.382481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Straiker A, Mitjavila J, Yin D, Gibson A, Mackie K. Aiming for allosterism: evaluation of allosteric modulators of CB in a neuronal model. Pharmacol Res. 2015;99:370–376. doi: 10.1016/j.phrs.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hofer SC, et al. Localization and production of peptide endocannabinoids in the rodent CNS and adrenal medulla. Neuropharmacology. 2015 doi: 10.1016/j.neuropharm.2015.03.021. http://dx.doi.org/10.1016/j.neuropharm.2015.03.021. [DOI] [PubMed]
- 153.Mechoulam R, Hanus LO, Pertwee R, Howlett AC. Early phytocannabinoid chemistry to endocannabinoids and beyond. Nat Rev Neurosci. 2014;15:757–764. doi: 10.1038/nrn3811. [DOI] [PubMed] [Google Scholar]
- 154.Maccarrone M, Guzman M, Mackie K, Doherty P, Harkany T. Programming of neural cells by (endo)cannabinoids: from physiological rules to emerging therapies. Nat Rev Neurosci. 2014;15:786–801. doi: 10.1038/nrn3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Di Marzo V, Stella N, Zimmer A. Endocannabinoid signalling and the deteriorating brain. Nat Rev Neurosci. 2015;16:30–42. doi: 10.1038/nrn3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Van Sickle MD, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- 157.Zhang HY, et al. Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proc Natl Acad Sci USA. 2014;111:5007–5015. doi: 10.1073/pnas.1413210111. This study demonstrates the presence of CB2Rs in dopaminergic neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Brusco A, Tagliaferro PA, Saez T, Onaivi ES. Ultrastructural localization of neuronal brain CB2 cannabinoid receptors. Ann NY Acad Sci. 2008;1139:450–457. doi: 10.1196/annals.1432.037. [DOI] [PubMed] [Google Scholar]
- 159.Dhopeshwarkar A, Mackie K. CB2 cannabinoid receptors as a therapeutic target— What does the future hold? Mol Pharmacol. 2014;86:430–437. doi: 10.1124/mol.114.094649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Garcia-Gutierrez MS, Garcia-Bueno B, Zoppi S, Leza JC, Manzanares J. Chronic blockade of cannabinoid CB2 receptors induces anxiolytic-like actions associated with alterations in GABAA receptors. Br J Pharmacol. 2012;165:951–964. doi: 10.1111/j.1476-5381.2011.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Garcia-Gutierrez MS, Manzanares J. Overexpression of CB2 cannabinoid receptors decreased vulnerability to anxiety and impaired anxiolytic action of alprazolam in mice. J Psychopharmacol. 2011;25:111–120. doi: 10.1177/0269881110379507. [DOI] [PubMed] [Google Scholar]
- 162.Qin Z, et al. Chronic stress induces anxiety via an amygdalar intracellular cascade that impairs endocannabinoid signaling. Neuron. 2015;85:1319–1331. doi: 10.1016/j.neuron.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 163.Jung KM, et al. Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome. Nat Commun. 2012;3:1080. doi: 10.1038/ncomms2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhang L, Alger BE. Enhanced endocannabinoid signaling elevates neuronal excitability in fragile X syndrome. J Neurosci. 2010;30:5724–5729. doi: 10.1523/JNEUROSCI.0795-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tang AH, Alger BE. Homer protein-metabotropic glutamate receptor binding regulates endocannabinoid signaling and affects hyperexcitability in a mouse model of fragile X syndrome. J Neurosci. 2015;35:3938–3945. doi: 10.1523/JNEUROSCI.4499-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dlugos A, Childs E, Stuhr KL, Hillard CJ, de Wit H. Acute stress increases circulating anandamide and other N-acylethanolamines in healthy humans. Neuropsychopharmacology. 2012;37:2416–2427. doi: 10.1038/npp.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Blankman JL, Cravatt BF. Chemical probes of endocannabinoid metabolism. Pharmacol Rev. 2013;65:849–871. doi: 10.1124/pr.112.006387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rabinak CA, et al. Cannabinoid modulation of prefrontal-limbic activation during fear extinction learning and recall in humans. Neurobiol Learn Mem. 2014;113:125–134. doi: 10.1016/j.nlm.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Roitman P, Mechoulam R, Cooper-Kazaz R, Shalev A. Preliminary, open-label, pilot study of add-on oral Δ9-tetrahydrocannabinol in chronic post-traumatic stress disorder. Clin Drug Investig. 2014;34:587–591. doi: 10.1007/s40261-014-0212-3. [DOI] [PubMed] [Google Scholar]
- 170.Puighermanal E, et al. Dissociation of the pharmacological effects of THC by mTOR blockade. Neuropsychopharmacology. 2013;38:1334–1343. doi: 10.1038/npp.2013.31. This study identifies the mammalian target of rapamycin (mTOR) pathway as a novel strategy to dissociate beneficial effects and side effects of cannabinoid agonists. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hermanson DJ, et al. Substrate-selective COX-2 inhibition decreases anxiety via endocannabinoid activation. Nat Neurosci. 2013;16:1291–1298. doi: 10.1038/nn.3480. This study shows that the inhibition of eicosanoid synthesis by COX2 blockade could represent a novel strategy to tackle anxiety. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hermanson DJ, Gamble-George JC, Marnett LJ, Patel S. Substrate-selective COX-2 inhibition as a novel strategy for therapeutic endocannabinoid augmentation. Trends Pharmacol Sci. 2014;35:358–367. doi: 10.1016/j.tips.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chen R, et al. Δ9-THC-caused synaptic and memory impairments are mediated through COX-2 signaling. Cell. 2013;155:1154–1165. doi: 10.1016/j.cell.2013.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Shore DM, et al. Allosteric modulation of a cannabinoid G protein-coupled receptor: binding site elucidation and relationship to G protein signaling. J Biol Chem. 2014;289:5828–5845. doi: 10.1074/jbc.M113.478495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ross RA. Allosterism and cannabinoid CB1 receptors: the shape of things to come. Trends Pharmacol Sci. 2007;28:567–572. doi: 10.1016/j.tips.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 176.Vinals X, et al. Cognitive impairment induced by Δ-9-tetrahydrocannabinol occurs through heteromers between cannabinoid CB1 and serotonin 5-HT2A receptors. PLoS Biol. 2015;13:1002194. doi: 10.1371/journal.pbio.1002194. This paper demonstrates that the formation of a heteromer between cannabinoid CB1Rs and serotonin 5-hydroxytryptamine 2A (5-HT2A) receptors is responsible for the memory-impairment effects of cannabis, and that prevention of this interaction could allow selective harnessing of the beneficial effects of cannabinoids without the detrimental effects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Vallee M, et al. Pregnenolone can protect the brain from cannabis intoxication. Science. 2014;343:94–98. doi: 10.1126/science.1243985. This study includes a demonstration of pregnenolone as an endogenous allosteric and signal-specific inhibitor of CB1R that regulates the effect of (endo)cannabinoids on behaviour. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gibson HE, Edwards JG, Page RS, Van Hook MJ, Kauer JA. TRPV1 channels mediate long-term depression at synapses on hippocampal interneurons. Neuron. 2008;57:746–759. doi: 10.1016/j.neuron.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Puente N, et al. Polymodal activation of the endocannabinoid system in the extended amygdala. Nat Neurosci. 2011;14:1542–1547. doi: 10.1038/nn.2974. [DOI] [PubMed] [Google Scholar]
- 180.Sigel E, et al. The major central endocannabinoid directly acts at GABAA receptors. Proc Natl Acad Sci USA. 2011;108:18150–18155. doi: 10.1073/pnas.1113444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yoshida T, et al. Localization of diacylglycerol lipase-alpha around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J Neurosci. 2006;26:4740–4751. doi: 10.1523/JNEUROSCI.0054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Katona I, et al. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26:5628–5637. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Suarez J, et al. Distribution of diacylglycerol lipase alpha, an endocannabinoid synthesizing enzyme, in the rat forebrain. Neuroscience. 2011;192:112–131. doi: 10.1016/j.neuroscience.2011.06.062. [DOI] [PubMed] [Google Scholar]
- 184.Gao Y, et al. Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J Neurosci. 2010;30:2017–2024. doi: 10.1523/JNEUROSCI.5693-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tanimura A, et al. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron. 2010;65:320–327. doi: 10.1016/j.neuron.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 186.Gulyas AI, et al. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci. 2004;20:441–458. doi: 10.1111/j.1460-9568.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- 187.Uchigashima M, et al. Molecular and morphological configuration for 2-arachidonoylglycerol-mediated retrograde signaling at mossy cell-granule cell synapses in the dentate gyrus. J Neurosci. 2011;31:7700–7714. doi: 10.1523/JNEUROSCI.5665-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Marrs WR, et al. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat Neurosci. 2010;13:951–957. doi: 10.1038/nn.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Naydenov AV, et al. ABHD6 blockade exerts antiepileptic activity in PTZ-induced seizures and in spontaneous seizures in R6/2 mice. Neuron. 2014;83:361–371. doi: 10.1016/j.neuron.2014.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Viader A, et al. Metabolic interplay between astrocytes and neurons regulates endocannabinoid action. Cell Rep. 2015;12:808. doi: 10.1016/j.celrep.2015.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Nyilas R, et al. Enzymatic machinery for endocannabinoid biosynthesis associated with calcium stores in glutamatergic axon terminals. J Neurosci. 2008;28:1058–1063. doi: 10.1523/JNEUROSCI.5102-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Egertova M, Simon GM, Cravatt BF, Elphick MR. Localization of N-acyl phosphatidylethanolamine phospholipase D (NAPE-PLD) expression in mouse brain: A new perspective on N-acylethanolamines as neural signaling molecules. J Comp Neurol. 2008;506:604–615. doi: 10.1002/cne.21568. [DOI] [PubMed] [Google Scholar]
- 193.Tsuboi K, et al. Glycerophosphodiesterase GDE4 as a novel lysophospholipase D: a possible involvement in bioactive N-acylethanolamine biosynthesis. Biochim Biophys Acta. 2015;1851:537–548. doi: 10.1016/j.bbalip.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 194.Rahman IA, Tsuboi K, Uyama T, Ueda N. New players in the fatty acyl ethanolamide metabolism. Pharmacol Res. 2014;86:1–10. doi: 10.1016/j.phrs.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 195.Witting A, Walter L, Wacker J, Moller T, Stella N. P2X7 receptors control 2-arachidonoylglycerol production by microglial cells. Proc Natl Acad Sci USA. 2004;101:3214–3219. doi: 10.1073/pnas.0306707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Steindel F, et al. Neuron-type specific cannabinoid-mediated G protein signalling in mouse hippocampus. J Neurochem. 2013;124:795–807. doi: 10.1111/jnc.12137. [DOI] [PubMed] [Google Scholar]