Abstract
The field of pediatric and adult congenital cardiac catheterization has evolved rapidly in recent years. This review will focus on some of the newer endovascular technological and management strategies now being applied in the pediatric interventional laboratory. Emerging imaging techniques such as three-dimensional (3D) rotational angiography, multi-modal image fusion, 3D printing, and holographic imaging have the potential to enhance our understanding of complex congenital heart lesions for diagnostic or interventional purposes. While fluoroscopy and standard angiography remain procedural cornerstones, improved equipment design has allowed for effective radiation exposure reduction strategies. Innovations in device design and implantation techniques have enabled the application of percutaneous therapies in a wider range of patients, especially those with prohibitive surgical risk. For example, there is growing experience in transcatheter duct occlusion in symptomatic low-weight or premature infants and stent implantation into the right ventricular outflow tract or arterial duct in cyanotic neonates with duct-dependent pulmonary circulations. The application of percutaneous pulmonary valve implantation has been extended to a broader patient population with dysfunctional ‘native’ right ventricular outflow tracts and has spurred the development of novel techniques and devices to solve associated anatomic challenges. Finally, hybrid strategies, combining cardiosurgical and interventional approaches, have enhanced our capabilities to provide care for those with the most complex of lesions while optimizing efficacy and safety.
Keywords: cardiac catheterization, heart, angiography
Introduction
Over the last three decades, the pediatric cardiac catheterization laboratory has undergone a transformation from primarily a diagnostic tool to a modality for therapy. While this is an ongoing process, a variety of signature advances have taken place, altering management strategies. In the next few pages, we hope to highlight a few of those advances, which have improved the outcomes of children born with congenital lesions of the heart.
Advances in imaging
Three-dimensional rotational angiography and fusion imaging techniques
Three-dimensional rotational angiography (3DRA) is an emerging imaging modality that provides tableside, real-time acquisition of 3D volume rendered and cross-sectional images to aid the visualization of complex cardiac anatomy and navigation during diagnostic or interventional procedures 1– 4. Image acquisition is performed by rotation through an arc around the patient of the C-arm of the angiography system, equipped with flat-detector computer tomography (CT) 1, 4. The volume set is used to reconstruct the 3D structures of interest. These images can then be overlaid onto live fluoroscopy for road mapping during therapeutic procedures 2, 4. The so-registered 3D space can also be integrated with 3D datasets from magnetic resonance imaging (MRI) or CT studies.
Diagnostically, the advantage of 3DRA is its ability to profile the complexities of the cardiac anatomy from multiple projections, enhancing the appreciation of the spatial vascular relationships. To this end, several recent studies have reported the additive yield of using 3DRA compared to standard 2D biplane angiography 2, 3, 5. For example, in children with cavopulmonary connections, 3DRA can facilitate an understanding of the mechanisms underlying pulmonary artery (PA) stenosis and identify additional discrete proximal lesions at the anastomosis site 5. Additionally, the relationship of the trachea and bronchi to surrounding cardiovascular structures allows the assessment of airway anomalies and vascular compression frequently encountered in this population with significant implications for clinical management ( Figure 1) 6, 7.
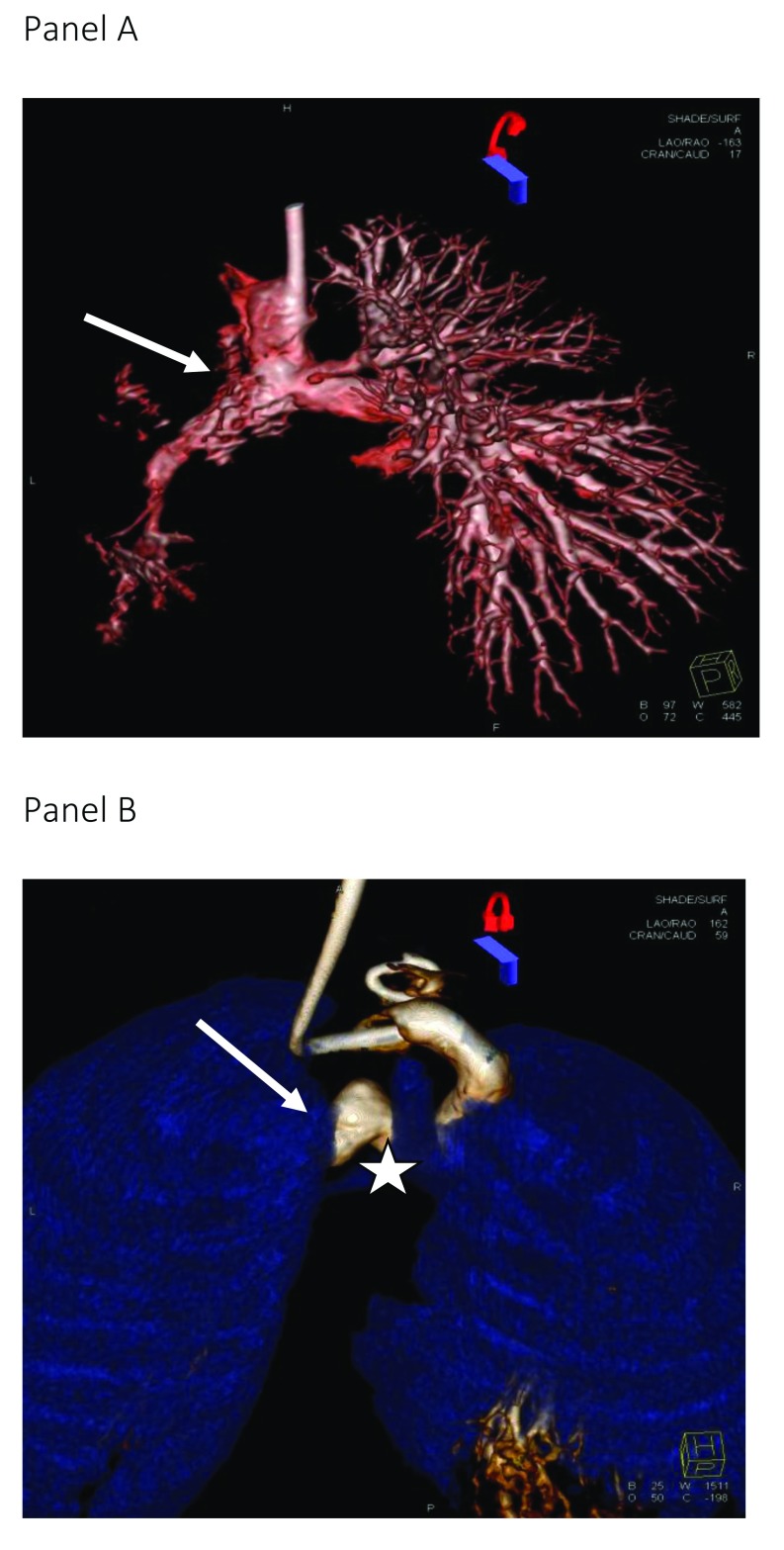
Figure 1. Rotational angiograms.
Panel A: a three-dimensional (3D)-rotational angiogram in a child with severe left pulmonary artery stenosis related to the retained ductal stent (arrow). The injection was in the bidirectional cavopulmonary connection. Panel B: this reconstruction from a 3D-rotational angiogram shows the relationships between vascular structures; in this case, left bronchial stenosis (star) is due to vascular compression (arrow) following previous arch reconstruction.
In the interventional setting, 3DRA is useful for planning and guiding stenting for aortic coarctation and complex PA anatomies, percutaneous pulmonary valve implantation, interventions in the Fontan circulation, or after an atrial switch repair 8– 12. Image fusion from disparate modalities and image overlay during fluoroscopic procedures can provide continuous visualization of the target lesion in any angulation and guidance for catheters, wires, and device placement, enhancing procedural efficiency 1. As such, shortened procedural time reduces radiation exposure not only to the child but also to laboratory personnel and total contrast dose 13, 14. Current challenges, however, include the inability to gate for cardiac and respiratory motion and the potential for misalignment of multi-modality image registration and distortion of anatomy by rigid interventional equipment. Innovations to address these concerns, including non-rigid registration techniques to compensate for translational motion, and the “triple overlay” technique, allow co-registration of pre-procedural CT or MR angiography and intra-procedural 3DRA and transesophageal echocardiography with live fluoroscopy 2, 11.
Three-dimensional printing, holography, and stereoscopic imaging for the interventional laboratory
Over the last two decades, catheter-directed interventions for congenital heart lesions have taken on a significant role in patient management. Indeed, many transcatheter interventions have become the standard of care for a number of abnormalities of heart valves, cardiac chambers, and proximal vessels 15. In this regard, children with complex congenital heart lesions, especially after complex operations, represent a challenge owing to their wide variation in complex morphology. One limiting factor in understanding and planning a percutaneous intervention is the limits of available 3D imaging modalities (MRI and CT) using images viewed on 2D screens. As such, these 2D representations make it inherently more difficult to appreciate the 3D relationships of cardiac structures unique to a particular intervention. Three-dimensional printing (also known as rapid prototyping, stereolithography, or additive manufacturing), while not a new technology, has only recently been used to fabricate physical models from 3D computerized imaging datasets 16. While the first medical applications were to produce surgical implants for oral and maxillofacial surgery and prosthetics for orthopedic surgery, the ability to generate a 3D model of complex cardiac anatomy has made this a tool for education, procedural planning, and device testing in both structural and congenital heart disease interventions ( Figure 2) 17, 18. The use of these constructed models representing true anatomical relationships has been a promising adjunct in planning a number of interventional procedures allowing for virtual device implantation 19– 27. However, expertise is required to generate these 3D models and the investment in resources is required to establish a 3D printing lab. As such, this technology has been limited thus far to teaching hospitals and research centers. In addition to the creation of physical models to view 3D anatomy, several augmented viewing modalities, using holography or stereoscopic imaging, are being evaluated.
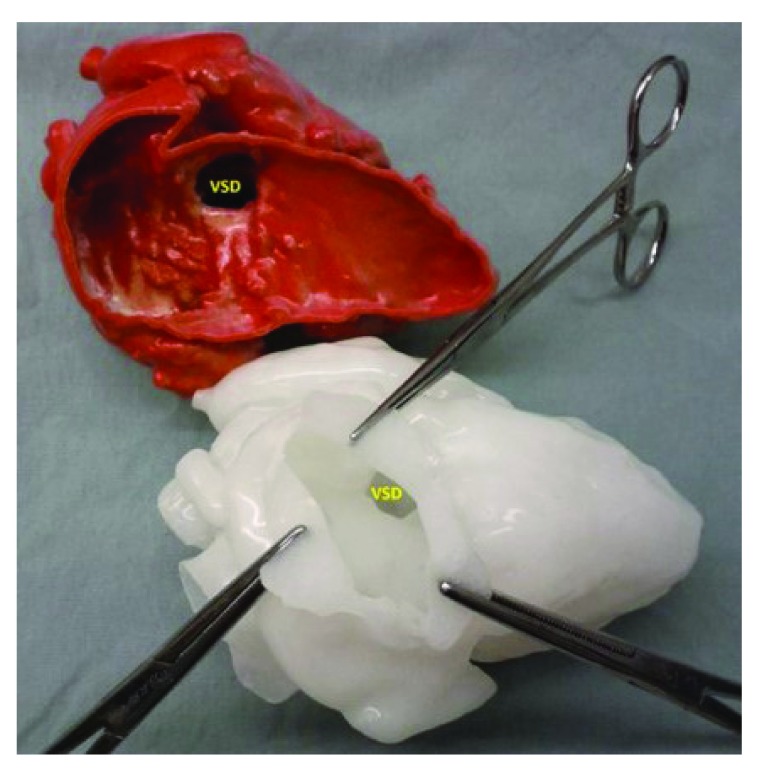
Figure 2. Three-dimensional modeling.
The upper image is a three-dimensional model obtained from a magnetic resonance angiogram with the free wall of the right ventricle cut away revealing the location of the ventricular septal defect (VSD); the lower model, made from a soft pliable material, shows the appearance of the VSD as seen through a virtual incision in the right atrium, as would be seen by the surgeon.
Radiation safety
Radiation safety awareness and techniques to reduce radiation exposure are essential for all procedures but have a special role in children with complex congenital cardiac lesions who often require long, and at times multiple, procedures during their lifetimes 28. A recent systematic review noted that radiation dose during pediatric cardiac catheterization remains varied and potentially substantial despite a downward trend in recent years 29. The observed decline in exposure estimates is attributed to improvement in physician awareness of dose optimization in tandem with technological advances 29– 31. However, the variation in radiation exposure amongst centers suggests that further initiatives towards minimizing radiation dose in children to “as low as reasonably achievable” (the ALARA principle) and standardization of practice are warranted 29. Comparisons between centers also highlight the inadequacy of fluoroscopy time alone as a metric of radiation dose estimate because of differences in programmed fluoroscopy modes, pulse rates, and cine acquisition frame rates 32, 33. Instead, recognition and comparison of actual radiation energy exposure such as dose area product and air kerma in relation to procedure types are more appropriate.
Several technical developments have impacted positively on the reduction of radiation exposure, notably the transition to flat panel detector (FPD) technology, which converts X-ray photon energy to digital signals more efficiently than image intensifiers 30, 34– 36. Innovation in FPD including increased pixel bit depth and usage of crystalline silicon rather than amorphous silicon combined with novel flat emitter X-ray tubes offers further reduction in radiation dose owing to better digitalization and lower detector noise 37. Additionally, alternative imaging techniques, such as transesophageal and/or intracardiac echocardiography or fusion of MRI/CT imaging with fluoroscopy, have been successfully used to reduce radiation exposure in various interventional procedures 32, 33.
Specific applications
Patent ductus arteriosus in preterm and low-birth-weight infants
A persistent patent ductus arteriosus (PDA) in the preterm newborn is often associated with important comorbidities (ventilator dependence, congestive heart failure, and failure to thrive) and increased mortality 38, 39. Definitive treatment strategies in this group remain debated, as both medical and surgical therapies have attendant risks 40, 41. Percutaneous ductal closure in these small newborns is generally limited by delivery sheath size, procedure-related hemodynamic instability, and the anchoring and retrievability characteristics of current devices 42. Although manufacturer recommendations for the most commonly used Amplatzer ductal occluders are a weight of 6 kg or more, there is no consensus on the minimum weight limit in practice.
Recently, there has been a growing body of evidence supporting the feasibility and efficacy of percutaneous PDA closure in the premature or small infant under 6 kg. The procedural success rates range from 88 to 94% in those with a median weight of between 2.5 and 6 kg 43– 47. Technical feasibility has also been demonstrated in extremely preterm (under 28 weeks) or very preterm (28 to under 32 weeks) infants under 2.5 kg, with the smallest noted in the literature weighing 755 g, having an uncomplicated closure with a 4 mm Amplatzer vascular plug II device 44, 48– 52. The adverse event rate was higher in the under 4 kg group, which was not unexpected given that premature infants are medically fragile 45, 53– 55, in keeping with the known inverse event rate related to patient weight at the time of catheterization 56. The increased risk of embolization is also noteworthy in low-weight and premature infants; hence, the retrievability of the occluder and duct morphology needs careful consideration 46, 47. There were no deaths attributable to the procedure reported in these studies.
Access-related complications, in particular acute arterial injury leading to vascular compromise of the extremity, is a significant concern when catheterizing these low-weight infants 57, 58. Some centers have avoided arterial access by adopting a transvenous-only approach guided by fluoroscopy and echocardiography 33. The risk of device-induced obstruction to the descending aorta or left PA (LPA) was low in these studies, and the majority tended to resolve over time with growth of the vessels 43– 45, 52. However, the severity of LPA stenosis may be underestimated because of outflow diversion to the right lung. Studies using scintigraphic perfusion imaging have shown evidence of decreased perfusion of the left lung after PDA occlusion, but the long-term clinical impact is unknown 59, 60. More recently, the introduction of a new microvascular plug (MVP™, Medtronic Inc.) for PDA closure in the extremely premature infant has shown promising results 61, 62. Advantages of the device include delivery through a microcatheter (<3 F), a flexible delivery cable, and diskless device design which minimizes the risk of device protrusion to the aorta or LPA.
The role of right ventricular flow tract stenting in symptomatic neonates with Fallot’s tetralogy
Primary repair in infants with tetralogy of Fallot (TOF) with good-sized confluent central PAs is the standard of care with excellent outcomes. However, the ideal management strategy for the symptomatic (cyanotic) neonate with TOF and one or more adverse risk factors, such as prematurity, low birth weight, unfavorable PA anatomy, or pulmonary atresia or non-cardiac co-morbidities, remains debated 63, 64. Conventional palliation with the Blalock–Taussig (BT) shunt in this high-risk group can result in complications such as shunt stenosis or occlusion, distortion and differential growth of the PAs, and pulmonary overcirculation 65– 67. Balloon dilation of the pulmonary valve alone has inconsistent benefit owing to frequently associated muscular obstruction in the infundibulum. Stent implantation to enlarge the right ventricular outflow tract (RVOT) is now increasingly used as a bridging procedure to palliate the cyanosis and promote PA growth ( Figure 3).
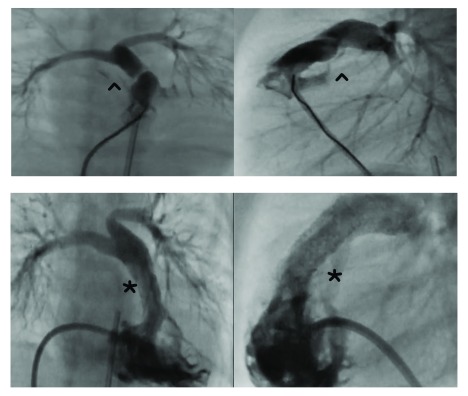
Figure 3. Hypoplastic pulmonary arteries and outflow stent.
Upper panels from right ventricular angiograms show the obstructive right ventricular outflow tract (^) in a newborn with Fallot’s tetralogy. The lower panels show stent implantation (*) to enlarge the outflow and improve pulmonary blood flow, relieving the cyanosis.
Several small studies have documented favorable outcomes of RVOT stents in isolation since 2008, focusing on technical aspects and acute outcomes 65, 67– 72. More recently, Sandoval and colleagues provided comparative data on 180 infants with TOF who underwent RVOT stenting, early primary repair, or standard repair over 3 months of age 63. While the RVOT stented group had worse PA anatomy and clinical factors compared to the early intervention group, final clinical outcomes were comparable to infants who underwent standard repair. RVOT stenting resulted in relief of cyanosis and PA growth, allowing time for somatic growth and resolution of non-cardiac comorbidities until definitive repair 63, 71. Quandt and colleagues compared outcomes of RVOT stenting and BT shunts, reporting better PA growth and hence shorter duration of palliation before complete repair after stenting. The RVOT stent group also had a shorter intensive care unit (ICU) and hospital stay, whilst mortality until surgical repair was similar in both groups 73, 74.
Several disadvantages of RVOT stenting exist, notably the technical complexity and risk of inadvertent perforation of the RVOT in low-weight infants with membranous pulmonary valve (PV) atresia, who undergo radiofrequency perforation to open the RVOT prior to stenting 63. A hybrid perventricular route may offer a more controlled approach to PV perforation and RVOT stent placement while avoiding cardiopulmonary bypass and femoral vessel complications and has been reported in a few case series, including a neonate as small as 1.3 kg 75, 76. There is a high re-intervention rate for additional stent implantation (if the initial implant did not cover the full extent of the outflow tract) and PA dilation, although in this subset of infants the PAs are hypoplastic and require repeat procedures to encourage growth 72.
Previous retrospective comparisons of early primary repair versus a staged repair with a BT shunt in symptomatic neonatal TOF showed equivalent mortality and outcomes, although shunted patients had a greater likelihood of avoiding a transannular patch at the time of repair 66. However, there are currently no published data comparing the outcomes of early primary repair, BT shunt, and RVOT stent. Ultimately, the preferred palliative option, whether surgical or percutaneous, is likely to depend on local expertise, patient factors, and clinical condition at the time of intervention.
The role of ductal stenting in the duct-dependent pulmonary circulation
While ductal stenting (DS) has been applied for well over two decades, the procedure has gained a wider acceptance as a palliative option for the cyanotic infant with a duct-dependent pulmonary circulation deemed unsuitable for primary repair ( Figure 4) 77, 78. The technique has been applied in various lesions, either leading to a biventricular repair or destined for univentricular palliation 77, 79, 80. Apart from avoidance of surgery and shunt-related adverse events, maintaining duct patency has been shown to promote significant and uniform PA growth compared to a BT shunt alone 77, 81, 82. The effective patency of the stented duct or PA growth potential was not significantly different between cases with a single source (ductal) of pulmonary blood flow or those with multiple sources of pulmonary blood flow 77, 81. In a few retrospective reports, DS has performed favorably and offered early survival advantage with improved hemodynamic stability compared to a BT shunt but with an increased likelihood of re-intervention prior to next-stage surgery 78– 80, 83.
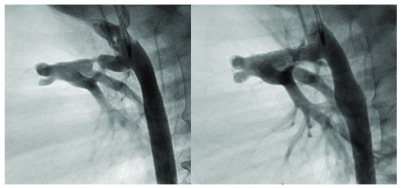
Figure 4. Ductal stent.
The left panel details a stenotic arterial duct as it connects with the main pulmonary artery in a child with ductal-dependent pulmonary circulation. The right panel shows the appearance of the duct after the placement of a ductal stent to support the pulmonary blood flow.
One major disadvantage of DS is progressive neointimal proliferation and consequent endoluminal narrowing with an unpredictable timecourse 82. The adequacy of palliation provided by DS, however, depends on the clinical setting. When DS is performed after RVOT intervention for critical PS or PA, short-term patency is usually adequate. In other conditions, such as univentricular palliation prior to a Glenn procedure or biventricular repair of complex anatomy, a longer lifespan of the stented duct from 6 to 12 months is desirable to allow somatic growth 82. The impact of drug-eluting stents used in adult coronary artery disease has been investigated in this setting. Sirolimus-eluting stents implanted in the porcine arterial duct had higher patency rates compared with bare-metal stents with anti-proliferative action on ductal smooth muscle 84. Early trials of drug-eluting stents in neonates showed no clinically significant adverse outcomes; however, their clinical efficacy has yet to be evaluated 85.
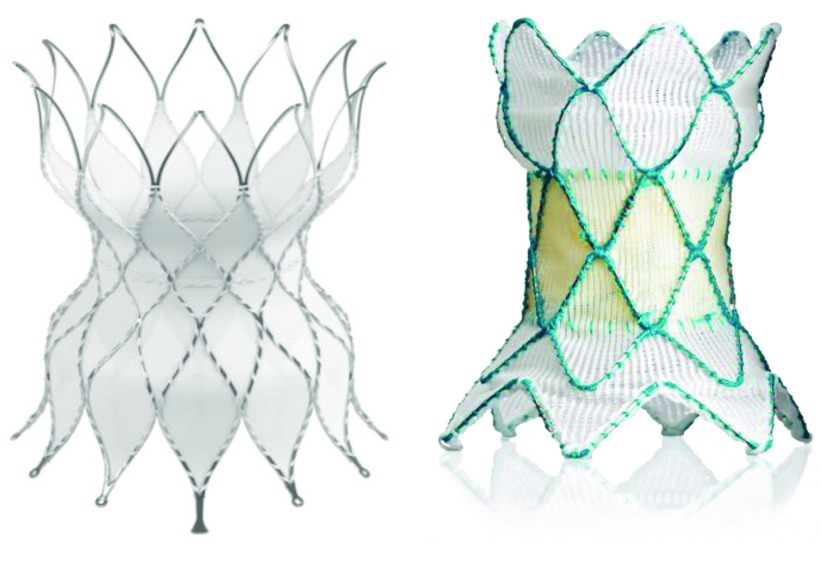
Percutaneous pulmonary valve implantation in the right ventricle to pulmonary artery conduit and native outflow tract
Percutaneous pulmonary valve implantation (PPVI) is a recognized alternative to surgical pulmonary valve replacement in selected patients with dysfunctional synthetic right ventricle-to-PA (RV-PA) conduits, bioprosthetic pulmonary valves, or homografts. Currently available percutaneous pulmonary valves include the Melody™ valve (Medtronic, Minneapolis, MN), which has a maximum outer diameter of 24 mm, and the Edwards Sapien™ valve system (Edwards Lifesciences Corp), with outer diameters of up to 29 mm.
Multiple studies have reported excellent procedural success and clinical efficacy of the Melody™ valve with thus far up to 7 years of follow up 86– 89. Published experience on the Sapien™ valve in the pulmonary position is comparatively less, although there are robust data supporting its use in the aortic position in high-risk or elderly patients with acquired aortic stenosis 90, 91. Early results of the COMPASSION (COngenital Multicenter Trial of Pulmonic VAlve Regurgitation Studying the SAPIEN Interventional THV) trial reported a good safety and efficacy profile, and several small studies have demonstrated comparable results to the Melody™ valve in the medium term 92– 95. With both the Melody™ and the Sapien™ valve, primary valve failure was rare and overall complication rates were low (0–5%) with reported mortality rates of up to 2% 86– 89, 92– 95. Coronary compression due to expansion of the implant occurred in less than 1% of cases and was significantly associated with an abnormal coronary artery course. Pre-implantation coronary artery compression testing is mandatory to avoid this potentially catastrophic complication 51, 96. Stent fractures which occurred with the Melody™ valve in the early experience have been addressed with routine RVOT pre-device implant stenting. The incidence of infective endocarditis after PPVI is estimated at approximately 3% per year with the Melody™ valve, whilst a lower incidence with the Sapien™ valve is suggested 97.
More recently, the application of both Melody™ and Sapien™ valves in the so-called “native” RVOT have been described in case reports and case series 98– 101. “Native” refers to the non-operated RVOT or one having a previous balloon pulmonary valvuloplasty/valvectomy for pulmonary stenosis or previous transannular patch repair for correction of TOF, which constitutes the vast majority of patients with a dysfunctional RVOT. The potentially distensible tissue in the contractile “native’” RVOT and the absence of a pre-existing “scaffold” such as in a conduit or bioprosthetic valve presents potential problems with implant valve stability, a paravalvular leak, or framework fracture. To overcome such issues, several techniques of pre-stenting the RVOT have been developed to create a “landing zone” for the selected transcatheter valve 11. Implantation of the Melody™ valve in the branch PAs has also been described 102.
For the dilated “native” RVOT often encountered after a transannular patch repair for TOF, hybrid approaches of RVOT plication through a limited sternotomy or thoracotomy and subsequent percutaneous or perventricular delivery of the Melody™ valve have been explored 103– 105. Paralleling the limited application of these first-generation PPVIs in the “native” RVOT is the development of self-expanding percutaneous valves such as the Harmony™ valve (Medtronic Inc, Minneapolis, USA), the Venus P™ valve (Medtech, Shenzhen, China), and a rendezvous Alterra™ stent (Edwards Lifesciences) as a landing zone for a Sapien™ valve 106– 111. The flared ends of these systems are designed to provide stability in the large pulsatile outflows, whilst the self-expanding nitinol frames adapt to various outflow anatomies ( Figure 5). Other concepts in development (in animal studies) include implantation of expandable conduits in the RVOT that can be dilated sequentially with growth or replaced with a percutaneous valve 112– 114.
Figure 5. Percutaneous pulmonary valves for the large outflow tract.
The left panel is a picture of the Edwards Alterra™ stent, designed to be placed in the right ventricular outflow tract in patients after transannular patch repair for Fallot’s tetralogy. The nitinol stent hosing (seen in the photo) provides a landing zone for a 29 mm Sapien™ valve. The right panel is a photo of Medtronic’s Harmony™ valve. This valve stent design has porcine pericardial valve leaflets sewn into the nitinol framework.
Transcatheter tricuspid and mitral valve replacement
The rapidly expanding transcatheter heart valve technology has been applied to the treatment of atrioventricular valve dysfunction with a growing experience in the tricuspid valve position. Implantation of percutaneous valves into degenerated bioprostheses (valve-in-valve, VIV) or into annuloplasty rings (valve-in-ring, VIR) is an attractive alternative to surgery in the high-risk and often-debilitated patient with acquired or congenital cardiac disease 115– 118. A large multicenter experience of tricuspid VIV implantation has recently been published, using both the Melody™ and the Sapien™ valves with favorable outcomes at more than a year follow up 117. The majority of patients were relatively young (median age 40 years), and over half had tricuspid valve disease associated with congenital heart disease including Ebstein’s anomaly, intrinsic tricuspid valve (TV) abnormalities, or TV injury related to previous surgery or catheter intervention. There was an excellent procedural success of 99%, with significant improvements in the degree of regurgitation or stenosis and symptomatic improvement. The 30-day mortality was 3.3%, and estimated freedom from tricuspid re-intervention at 1 year was 83%. The extension of the tricuspid VIV concept to VIR is considerably more challenging owing to larger diameters and geometric variability of surgically placed rings. Although this approach is technically feasible and clinically effective in reducing tricuspid regurgitation, paravalvular regurgitation has been common 119, 120.
Transcatheter mitral valve replacement remains in its early clinical stages 121. Mitral VIV implantation is more difficult to accomplish owing to difficulty in coaxial alignment of the percutaneous valve within the existing valve. The majority of implantations are performed using a direct transapical approach with the larger diameter Sapien™ valves 122– 124. A transvenous, transseptal technique utilizing an apical rail to facilitate the delivery of Melody™ valve into the dysfunctional mitral prosthesis has also been described 118. To date, the results of mitral VIV implantations within a high-pressure hemodynamic environment have shown good valve performance with low transvalvular gradients and low rates of paravalvular regurgitation in the short term 122– 125. Applying the Sapien™ valve to surgically implanted ring (VIR) is even less appealing because of incomplete sealing of the variable and D-shaped annuloplasty rings and the risk of causing left ventricular outflow tract obstruction. Recent successful case reports of the Melody™ VIR technique, however, offer significant promise 126, 127. Compared to Sapien™ valves, the Melody™ valve has a longer stent that is covered throughout its length, which provides good sealing at the subvalvar level 128. Transcatheter valve-in-native-ring for calcified native mitral stenosis has also been reported in high-risk adult patients 129– 131.
In infants and children with severe mitral stenosis or regurgitation associated with congenital heart disease, the Melody™ valve as a surgical implant has shown promising results after failed attempts at primary mitral valve repair 132, 133. The Melody™ valve has the potential for future expansions with child growth and is a viable option in the lack of appropriately sized mitral valve prostheses in these small children; however, further study is required to determine longer-term durability and safety.
Conclusion
Recent years have seen the rapid development of imaging and device technologies as well as percutaneous interventions in a variety of congenital cardiac lesions, with an increased application of percutaneous therapies to a broad range of patients. It is important, however, to remember that long-term outcomes for many such novel interventions are lacking, and rigorous prospective studies and data surveillance are required to determine safety and efficacy profiles before these become standard of care. Future innovations and growing experience in this field, in addition to increased collaboration between surgeons and interventionists, will undoubtedly continue to expand transcatheter options in the management of congenital heart disease, further improving the quality of life for the child and adult with congenital heart disease. This short review touches on some of the highlights that have been developed over the last decade. A number of percutaneous procedures (not mentioned) have become standard of care in many centers, and with continued diligence we can anticipate the continued application of such therapies.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Lourdes Prieto, Nicklaus Children’s Hospital, Miami, FL, USA
Shyam Sathanandam, Invasive Cardiac Imaging and Interventional Catheterization Laboratory, LeBonheur Children Hospital, University of Tennessee Health Science Center, Memphis, TN, USA
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 2 approved]
References
- 1. Glöckler M, Halbfaβ J, Koch A, et al. : Multimodality 3D-roadmap for cardiovascular interventions in congenital heart disease--a single-center, retrospective analysis of 78 cases. Catheter Cardiovasc Interv. 2013;82(3):436–42. 10.1002/ccd.24646 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 2. Fagan TE, Truong UT, Jone PN, et al. : Multimodality 3-dimensional image integration for congenital cardiac catheterization. Methodist Debakey Cardiovasc J. 2014;10(2):68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Glatz AC, Zhu X, Gillespie MJ, et al. : Use of angiographic CT imaging in the cardiac catheterization laboratory for congenital heart disease. JACC Cardiovasc Imaging. 2010;3(11):1149–57. 10.1016/j.jcmg.2010.09.011 [DOI] [PubMed] [Google Scholar]
- 4. Pedra CA, Fleishman C, Pedra SF, et al. : New imaging modalities in the catheterization laboratory. Curr Opin Cardiol. 2011;26(2):86–93. 10.1097/HCO.0b013e3283437fb4 [DOI] [PubMed] [Google Scholar]
- 5. Berman DP, Khan DM, Gutierrez Y, et al. : The use of three-dimensional rotational angiography to assess the pulmonary circulation following cavo-pulmonary connection in patients with single ventricle. Catheter Cardiovasc Interv. 2012;80(6):922–30. 10.1002/ccd.23461 [DOI] [PubMed] [Google Scholar]
- 6. Borik S, Volodina S, Chaturvedi R, et al. : Three-dimensional rotational angiography in the assessment of vascular and airway compression in children after a cavopulmonary anastomosis. Pediatr Cardiol. 2015;36(5):1083–9. 10.1007/s00246-015-1130-8 [DOI] [PubMed] [Google Scholar]
- 7. Truong UT, Fagan TE, Deterding R, et al. : Use of rotational angiography in assessing relationship of the airway to vasculature during cardiac catheterization. Catheter Cardiovasc Interv. 2015;86(6):1068–77. 10.1002/ccd.26004 [DOI] [PubMed] [Google Scholar]
- 8. Noble S, Miró J, Yong G, et al. : Rapid pacing rotational angiography with three-dimensional reconstruction: use and benefits in structural heart disease interventions. EuroIntervention. 2009;5(2):244–9. 10.4244/EIJV5I2A38 [DOI] [PubMed] [Google Scholar]
- 9. Fagan T, Kay J, Carroll J, et al. : 3-D guidance of complex pulmonary artery stent placement using reconstructed rotational angiography with live overlay. Catheter Cardiovasc Interv. 2012;79(3):414–21. 10.1002/ccd.23229 [DOI] [PubMed] [Google Scholar]
- 10. Stenger A, Dittrich S, Glöckler M: Three-Dimensional Rotational Angiography in the Pediatric Cath Lab: Optimizing Aortic Interventions. Pediatr Cardiol. 2016;37(3):528–36. 10.1007/s00246-015-1310-6 [DOI] [PubMed] [Google Scholar]
- 11. Wilson W, Osten M, Benson L, et al. : Evolving trends in interventional cardiology: endovascular options for congenital disease in adults. Can J Cardiol. 2014;30(1):75–86. 10.1016/j.cjca.2013.11.006 [DOI] [PubMed] [Google Scholar]
- 12. Goreczny S, Moszura T, Dryzek P, et al. : Three-dimensional image fusion guidance of percutaneous pulmonary valve implantation to reduce radiation exposure and contrast dose: A comparison with traditional two-dimensional and three-dimensional rotational angiographic guidance. Neth Heart J. 2017;25(2):91–9. 10.1007/s12471-016-0941-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Manica JL, Borges MS, Medeiros RF, et al. : A comparison of radiation dose between standard and 3D angiography in congenital heart disease. Arq Bras Cardiol. 2014;103(2):131–7. 10.5935/abc.20140118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peters M, Krings G, Koster M, et al. : Effective radiation dosage of three-dimensional rotational angiography in children. Europace. 2015;17(4):611–6. 10.1093/europace/euu207 [DOI] [PubMed] [Google Scholar]
- 15. Mullins CE: Cardiac Catheterization in Congenital Heart Disease: Pediatric and Adult. Blackwell Futura.2006. 10.1002/9780470986967 [DOI] [Google Scholar]
- 16. Noecker AM, Chen JF, Zhou Q, et al. : Development of patient-specific three-dimensional pediatric cardiac models. ASAIO J. 2006;52(3):349–53. 10.1097/01.mat.0000217962.98619.ab [DOI] [PubMed] [Google Scholar]
- 17. D'Urso PS, Barker TM, Earwaker WJ, et al. : Stereolithographic biomodelling in cranio-maxillofacial surgery: a prospective trial. J Craniomaxillofac Surg. 1999;27(1):30–7. 10.1016/S1010-5182(99)80007-9 [DOI] [PubMed] [Google Scholar]
- 18. Hananouchi T, Saito M, Koyama T, et al. : Tailor-made surgical guide based on rapid prototyping technique for cup insertion in total hip arthroplasty. Int J Med Robot. 2009;5(2):164–9. 10.1002/rcs.243 [DOI] [PubMed] [Google Scholar]
- 19. Ripley B, Kelil T, Cheezum MK, et al. : 3D printing based on cardiac CT assists anatomic visualization prior to transcatheter aortic valve replacement. J Cardiovasc Comput Tomogr. 2016;10(1):28–36. 10.1016/j.jcct.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Giannopoulos AA, Mitsouras D, Yoo SJ, et al. : Applications of 3D printing in cardiovascular diseases. Nat Rev Cardiol. 2016;13(12):701–18. 10.1038/nrcardio.2016.170 [DOI] [PubMed] [Google Scholar]
- 21. Valverde I, Gomez G, Coserria JF, et al. : 3D printed models for planning endovascular stenting in transverse aortic arch hypoplasia. Catheter Cardiovasc Interv. 2015;85(6):1006–12. 10.1002/ccd.25810 [DOI] [PubMed] [Google Scholar]
- 22. Olivieri L, Krieger A, Chen MY, et al. : 3D heart model guides complex stent angioplasty of pulmonary venous baffle obstruction in a Mustard repair of D-TGA. Int J Cardiol. 2014;172(2):e297–8. 10.1016/j.ijcard.2013.12.192 [DOI] [PubMed] [Google Scholar]
- 23. Cantinotti M, Valverde I, Kutty S: Three-dimensional printed models in congenital heart disease. Int J Cardiovasc Imaging. 2017;33(1):137–44. 10.1007/s10554-016-0981-2 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 24. Chaowu Y, Hua L, Xin S: Three-Dimensional Printing as an Aid in Transcatheter Closure of Secundum Atrial Septal Defect With Rim Deficiency: In Vitro Trial Occlusion Based on a Personalized Heart Model. Circulation. 2016;133(17):e608–10. 10.1161/CIRCULATIONAHA.115.020735 [DOI] [PubMed] [Google Scholar]
- 25. Kim MS, Hansgen AR, Wink O, et al. : Rapid prototyping: a new tool in understanding and treating structural heart disease. Circulation. 2008;117(18):2388–94. 10.1161/CIRCULATIONAHA.107.740977 [DOI] [PubMed] [Google Scholar]
- 26. Dankowski R, Baszko A, Sutherland M, et al. : 3D heart model printing for preparation of percutaneous structural interventions: description of the technology and case report. Kardiol Pol. 2014;72(6):546–51. 10.5603/KP.2014.0119 [DOI] [PubMed] [Google Scholar]
- 27. Lazkani M, Bashir F, Brady K, et al. : Postinfarct VSD management using 3D computer printing assisted percutaneous closure. Indian Heart J. 2015;67(6):581–5. 10.1016/j.ihj.2015.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Glatz AC, Purrington KS, Klinger A, et al. : Cumulative exposure to medical radiation for children requiring surgery for congenital heart disease. J Pediatr. 2014;164(4):789–794.e10. 10.1016/j.jpeds.2013.10.074 [DOI] [PubMed] [Google Scholar]
- 29. Gould R, McFadden SL, Hughes CM: Radiation dose in paediatric cardiac catheterisation: A systematic literature review. Radiography (Lond). 2017;23(4):358–64. 10.1016/j.radi.2017.02.001 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. Nicholson GT, Gao K, Kim SI, et al. : Direct physician reporting is associated with reductions in radiation exposure in pediatric cardiac catheterizations. Catheter Cardiovasc Interv. 2015;86(5):834–40. 10.1002/ccd.26098 [DOI] [PubMed] [Google Scholar]
- 31. Justino H: The ALARA concept in pediatric cardiac catheterization: techniques and tactics for managing radiation dose. Pediatr Radiol. 2006;36(Suppl 2):146–53. 10.1007/s00247-006-0194-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Glatz AC, Patel A, Zhu X, et al. : Patient radiation exposure in a modern, large-volume, pediatric cardiac catheterization laboratory. Pediatr Cardiol. 2014;35(5):870–8. 10.1007/s00246-014-0869-7 [DOI] [PubMed] [Google Scholar]
- 33. Ghelani SJ, Glatz AC, David S, et al. : Radiation dose benchmarks during cardiac catheterization for congenital heart disease in the United States. JACC Cardiovasc Interv. 2014;7(9):1060–9. 10.1016/j.jcin.2014.04.013 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Verghese GR, McElhinney DB, Strauss KJ, et al. : Characterization of radiation exposure and effect of a radiation monitoring policy in a large volume pediatric cardiac catheterization lab. Catheter Cardiovasc Interv. 2012;79(2):294–301. 10.1002/ccd.23118 [DOI] [PubMed] [Google Scholar]
- 35. Harbron RW, Pearce MS, Salotti JA, et al. : Radiation doses from fluoroscopically guided cardiac catheterization procedures in children and young adults in the United Kingdom: a multicentre study. Br J Radiol. 2015;88(1048): 20140852. 10.1259/bjr.20140852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smith BG, Tibby SM, Qureshi SA, et al. : Quantification of temporal, procedural, and hardware-related factors influencing radiation exposure during pediatric cardiac catheterization. Catheter Cardiovasc Interv. 2012;80(6):931–6. 10.1002/ccd.24359 [DOI] [PubMed] [Google Scholar]
- 37. Lamers LJ, Moran M, Torgeson JN, et al. : Radiation Reduction Capabilities of a Next-Generation Pediatric Imaging Platform. Pediatr Cardiol. 2016;37(1):24–9. 10.1007/s00246-015-1233-2 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Sellmer A, Bjerre JV, Schmidt MR, et al. : Morbidity and mortality in preterm neonates with patent ductus arteriosus on day 3. Arch Dis Child Fetal Neonatal Ed. 2013;98(6):F505–10. 10.1136/archdischild-2013-303816 [DOI] [PubMed] [Google Scholar]
- 39. Noori S, McCoy M, Friedlich P, et al. : Failure of ductus arteriosus closure is associated with increased mortality in preterm infants. Pediatrics. 2009;123(1):e138–44. 10.1542/peds.2008-2418 [DOI] [PubMed] [Google Scholar]
- 40. Heuchan AM, Clyman RI: Managing the patent ductus arteriosus: current treatment options. Arch Dis Child Fetal Neonatal Ed. 2014;99(5):F431–6. 10.1136/archdischild-2014-306176 [DOI] [PubMed] [Google Scholar]
- 41. Hamrick SE, Hansmann G: Patent ductus arteriosus of the preterm infant. Pediatrics. 2010;125(5):1020–30. 10.1542/peds.2009-3506 [DOI] [PubMed] [Google Scholar]
- 42. Abadir S, Boudjemline Y, Rey C, et al. : Significant persistent ductus arteriosus in infants less or equal to 6 kg: percutaneous closure or surgery? Arch Cardiovasc Dis. 2009;102(6–7):533–40. 10.1016/j.acvd.2009.04.004 [DOI] [PubMed] [Google Scholar]
- 43. Backes CH, Cheatham SL, Deyo GM, et al. : Percutaneous Patent Ductus Arteriosus (PDA) Closure in Very Preterm Infants: Feasibility and Complications. J Am Heart Assoc. 2016;5(2): pii: e002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Baspinar O, Sahin DA, Sulu A, et al. : Transcatheter closure of patent ductus arteriosus in under 6 kg and premature infants. J Interv Cardiol. 2015;28(2):180–9. 10.1111/joic.12196 [DOI] [PubMed] [Google Scholar]
- 45. Dimas VV, Takao C, Ing FF, et al. : Outcomes of transcatheter occlusion of patent ductus arteriosus in infants weighing ≤ 6 kg. JACC Cardiovasc Interv. 2010;3(12):1295–9. 10.1016/j.jcin.2010.08.022 [DOI] [PubMed] [Google Scholar]
- 46. Kang SL, Jivanji S, Mehta C, et al. : Outcome after transcatheter occlusion of patent ductus arteriosus in infants less than 6 kg: A national study from United Kingdom and Ireland. Catheter Cardiovasc Interv. 2017;90(7):1135–44. 10.1002/ccd.27212 [DOI] [PubMed] [Google Scholar]
- 47. Backes CH, Kennedy KF, Locke M, et al. : Transcatheter Occlusion of the Patent Ductus Arteriosus in 747 Infants <6 kg: Insights From the NCDR IMPACT Registry. JACC Cardiovasc Interv. 2017;10(17):1729–37. 10.1016/j.jcin.2017.05.018 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Zahn EM, Nevin P, Simmons C, et al. : A novel technique for transcatheter patent ductus arteriosus closure in extremely preterm infants using commercially available technology. Catheter Cardiovasc Interv. 2015;85(2):240–8. 10.1002/ccd.25534 [DOI] [PubMed] [Google Scholar]
- 49. Bentham J, Meur S, Hudsmith L, et al. : Echocardiographically guided catheter closure of arterial ducts in small preterm infants on the neonatal intensive care unit. Catheter Cardiovasc Interv. 2011;77(3):409–15. 10.1002/ccd.22637 [DOI] [PubMed] [Google Scholar]
- 50. Francis E, Singhi AK, Lakshmivenkateshaiah S, et al. : Transcatheter occlusion of patent ductus arteriosus in pre-term infants. JACC Cardiovasc Interv. 2010;3(5):550–5. 10.1016/j.jcin.2010.01.016 [DOI] [PubMed] [Google Scholar]
- 51. Roberts P, Adwani S, Archer N, et al. : Catheter closure of the arterial duct in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2007;92(4):F248–50. 10.1136/adc.2005.078600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zahn EM, Peck D, Phillips A, et al. : Transcatheter Closure of Patent Ductus Arteriosus in Extremely Premature Newborns: Early Results and Midterm Follow-Up. JACC Cardiovasc Interv. 2016;9(23):2429–37. 10.1016/j.jcin.2016.09.019 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. Pass RH, Hijazi Z, Hsu DT, et al. : Multicenter USA Amplatzer patent ductus arteriosus occlusion device trial: initial and one-year results. J Am Coll Cardiol. 2004;44(3):513–9. 10.1016/j.jacc.2004.03.074 [DOI] [PubMed] [Google Scholar]
- 54. Liddy S, Oslizlok P, Walsh KP: Comparison of the results of transcatheter closure of patent ductus arteriosus with newer Amplatzer devices. Catheter Cardiovasc Interv. 2013;82(2):253–9. 10.1002/ccd.24768 [DOI] [PubMed] [Google Scholar]
- 55. Gruenstein DH, Ebeid M, Radtke W, et al. : Transcatheter closure of patent ductus arteriosus using the AMPLATZER™ duct occluder II (ADO II). Catheter Cardiovasc Interv. 2017;89(6):1118–28. 10.1002/ccd.26968 [DOI] [PubMed] [Google Scholar]
- 56. Backes CH, Cua C, Kreutzer J, et al. : Low weight as an independent risk factor for adverse events during cardiac catheterization of infants. Catheter Cardiovasc Interv. 2013;82(5):786–94. 10.1002/ccd.24726 [DOI] [PubMed] [Google Scholar]
- 57. Glatz AC, Shah SS, McCarthy AL, et al. : Prevalence of and risk factors for acute occlusive arterial injury following pediatric cardiac catheterization: a large single-center cohort study. Catheter Cardiovasc Interv. 2013;82(3):454–62. 10.1002/ccd.24737 [DOI] [PubMed] [Google Scholar]
- 58. Brotschi B, Hug MI, Kretschmar O, et al. : Incidence and predictors of cardiac catheterisation-related arterial thrombosis in children. Heart. 2015;101(12):948–53. 10.1136/heartjnl-2014-306713 [DOI] [PubMed] [Google Scholar]
- 59. Kharouf R, Heitschmidt M, Hijazi ZM: Pulmonary perfusion scans following transcatheter patent ductus arteriosus closure using the Amplatzer devices. Catheter Cardiovasc Interv. 2011;77(5):664–70. 10.1002/ccd.22917 [DOI] [PubMed] [Google Scholar]
- 60. Sreeram N, Tofeig M, Walsh KP, et al. : Lung perfusion studies after detachable coil occlusion of persistent arterial duct. Heart. 1999;81(6):642–5. 10.1136/hrt.81.6.642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang-Giuffre EW, Breinholt JP: Novel use of the medtronic micro vascular plug for PDA closure in preterm infants. Catheter Cardiovasc Interv. 2017;89(6):1059–65. 10.1002/ccd.26855 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 62. Sathanandam S, Justino H, Waller BR, 3rd, et al. : Initial clinical experience with the Medtronic Micro Vascular Plug™ in transcatheter occlusion of PDAs in extremely premature infants. Catheter Cardiovasc Interv. 2017;89(6):1051–8. 10.1002/ccd.26878 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 63. Sandoval JP, Chaturvedi RR, Benson L, et al. : Right Ventricular Outflow Tract Stenting in Tetralogy of Fallot Infants With Risk Factors for Early Primary Repair. Circ Cardiovasc Interv. 2016;9(12): pii: e003979. 10.1161/CIRCINTERVENTIONS.116.003979 [DOI] [PubMed] [Google Scholar]
- 64. Stumper O, Ramchandani B, Noonan P, et al. : Stenting of the right ventricular outflow tract. Heart. 2013;99(21):1603–8. 10.1136/heartjnl-2013-304155 [DOI] [PubMed] [Google Scholar]
- 65. Dohlen G, Chaturvedi RR, Benson LN, et al. : Stenting of the right ventricular outflow tract in the symptomatic infant with tetralogy of Fallot. Heart. 2009;95(2):142–7. 10.1136/hrt.2007.135723 [DOI] [PubMed] [Google Scholar]
- 66. Kanter KR, Kogon BE, Kirshbom PM, et al. : Symptomatic neonatal tetralogy of Fallot: repair or shunt? Ann Thorac Surg. 2010;89(3):858–63. 10.1016/j.athoracsur.2009.12.060 [DOI] [PubMed] [Google Scholar]
- 67. Castleberry CD, Gudausky TM, Berger S, et al. : Stenting of the right ventricular outflow tract in the high-risk infant with cyanotic teratology of Fallot. Pediatr Cardiol. 2014;35(3):423–30. 10.1007/s00246-013-0796-z [DOI] [PubMed] [Google Scholar]
- 68. Quandt D, Ramchandani B, Bhole V, et al. : Initial experience with the cook formula balloon expandable stent in congenital heart disease. Catheter Cardiovasc Interv. 2015;85(2):259–66. 10.1002/ccd.25543 [DOI] [PubMed] [Google Scholar]
- 69. Haas NA, Laser TK, Moysich A, et al. : Stenting of the right ventricular outflow tract in symptomatic neonatal tetralogy of Fallot. Cardiol Young. 2014;24(2):369–73. 10.1017/S1047951113000279 [DOI] [PubMed] [Google Scholar]
- 70. Bertram H, Emmel M, Ewert P, et al. : Stenting of Native Right Ventricular Outflow Tract Obstructions in Symptomatic Infants. J Interv Cardiol. 2015;28(3):279–87. 10.1111/joic.12198 [DOI] [PubMed] [Google Scholar]
- 71. McGovern E, Morgan CT, Oslizlok P, et al. : Transcatheter stenting of the right ventricular outflow tract augments pulmonary arterial growth in symptomatic infants with right ventricular outflow tract obstruction and hypercyanotic spells. Cardiol Young. 2016;26(7):1260–5. 10.1017/S1047951115002231 [DOI] [PubMed] [Google Scholar]
- 72. Barron DJ, Ramchandani B, Murala J, et al. : Surgery following primary right ventricular outflow tract stenting for Fallot's tetralogy and variants: rehabilitation of small pulmonary arteries. Eur J Cardiothorac Surg. 2013;44(4):656–62. 10.1093/ejcts/ezt188 [DOI] [PubMed] [Google Scholar]
- 73. Quandt D, Ramchandani B, Penford G, et al. : Right ventricular outflow tract stent versus BT shunt palliation in Tetralogy of Fallot. Heart. 2017;103(24):1985–91. 10.1136/heartjnl-2016-310620 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 74. Quandt D, Ramchandani B, Stickley J, et al. : Stenting of the Right Ventricular Outflow Tract Promotes Better Pulmonary Arterial Growth Compared With Modified Blalock-Taussig Shunt Palliation in Tetralogy of Fallot-Type Lesions. JACC Cardiovasc Interv. 2017;10(17):1774–84. 10.1016/j.jcin.2017.06.023 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 75. Zampi JD, Armstrong AK, Hirsch-Romano JC: Hybrid perventricular pulmonary valve perforation and right ventricular outflow stent placement: a case report of a premature, 1.3-kg neonate with tetralogy of Fallot and pulmonary atresia. World J Pediatr Congenit Heart Surg. 2014;5(2):338–41. 10.1177/2150135113512136 [DOI] [PubMed] [Google Scholar]
- 76. Cools B, Boshoff D, Heying R, et al. : Transventricular balloon dilation and stenting of the RVOT in small infants with tetralogy of fallot with pulmonary atresia. Catheter Cardiovasc Interv. 2013;82(2):260–5. 10.1002/ccd.24548 [DOI] [PubMed] [Google Scholar]
- 77. Santoro G, Capozzi G, Capogrosso C, et al. : Pulmonary artery growth after arterial duct stenting in completely duct-dependent pulmonary circulation. Heart. 2016;102(6):459–64. 10.1136/heartjnl-2015-308493 [DOI] [PubMed] [Google Scholar]
- 78. Bentham JR, Zava NK, Harrison WJ, et al. : Duct Stenting Versus Modified Blalock-Taussig Shunt in Neonates With Duct-Dependent Pulmonary Blood Flow: Associations With Clinical Outcomes in a Multicenter National Study. Circulation. 2018;137(6):581–8. 10.1161/CIRCULATIONAHA.117.028972 [DOI] [PubMed] [Google Scholar]
- 79. McMullan DM, Permut LC, Jones TK, et al. : Modified Blalock-Taussig shunt versus ductal stenting for palliation of cardiac lesions with inadequate pulmonary blood flow. J Thorac Cardiovasc Surg. 2014;147(1):397–401. 10.1016/j.jtcvs.2013.07.052 [DOI] [PubMed] [Google Scholar]
- 80. Mallula K, Vaughn G, El-Said H, et al. : Comparison of ductal stenting versus surgical shunts for palliation of patients with pulmonary atresia and intact ventricular septum. Catheter Cardiovasc Interv. 2015;85(7):1196–202. 10.1002/ccd.25870 [DOI] [PubMed] [Google Scholar]
- 81. Santoro G, Gaio G, Capozzi G, et al. : Fate of Hypoplastic Pulmonary Arteries After Arterial Duct Stenting in Congenital Heart Disease With Duct-Dependent Pulmonary Circulation. JACC Cardiovasc Interv. 2015;8(12):1626–32. 10.1016/j.jcin.2015.05.027 [DOI] [PubMed] [Google Scholar]
- 82. Sivakumar K, Bhagyavathy A, Coelho R, et al. : Longevity of neonatal ductal stenting for congenital heart diseases with duct-dependent pulmonary circulation. Congenit Heart Dis. 2012;7(6):526–33. 10.1111/j.1747-0803.2012.00657.x [DOI] [PubMed] [Google Scholar]
- 83. Amoozgar H, Cheriki S, Borzoee M, et al. : Short-term result of ductus arteriosus stent implantation compared with surgically created shunts. Pediatr Cardiol. 2012;33(8):1288–94. 10.1007/s00246-012-0304-x [DOI] [PubMed] [Google Scholar]
- 84. Lee K, Hinek A, Chaturvedi RR, et al. : Rapamycin-eluting stents in the arterial duct: experimental observations in the pig model. Circulation. 2009;119(15):2078–85. 10.1161/CIRCULATIONAHA.107.737734 [DOI] [PubMed] [Google Scholar]
- 85. Lee K, Seto W, Benson L, et al. : Pharmacokinetics of sirolimus-eluting stents implanted in the neonatal arterial duct. Circ Cardiovasc Interv. 2015;8(5): pii: e002233. 10.1161/CIRCINTERVENTIONS.114.002233 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 86. Cheatham JP, Hellenbrand WE, Zahn EM, et al. : Clinical and hemodynamic outcomes up to 7 years after transcatheter pulmonary valve replacement in the US melody valve investigational device exemption trial. Circulation. 2015;131(22):1960–70. 10.1161/CIRCULATIONAHA.114.013588 [DOI] [PubMed] [Google Scholar]
- 87. Butera G, Milanesi O, Spadoni I, et al. : Melody transcatheter pulmonary valve implantation. Results from the registry of the Italian Society of Pediatric Cardiology. Catheter Cardiovasc Interv. 2013;81(2):310–6. 10.1002/ccd.24518 [DOI] [PubMed] [Google Scholar]
- 88. Eicken A, Ewert P, Hager A, et al. : Percutaneous pulmonary valve implantation: two-centre experience with more than 100 patients. Eur Heart J. 2011;32(10):1260–5. 10.1093/eurheartj/ehq520 [DOI] [PubMed] [Google Scholar]
- 89. McElhinney DB, Hellenbrand WE, Zahn EM, et al. : Short- and medium-term outcomes after transcatheter pulmonary valve placement in the expanded multicenter US melody valve trial. Circulation. 2010;122(5):507–16. 10.1161/CIRCULATIONAHA.109.921692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Leon MB, Smith CR, Mack M, et al. : Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597–607. 10.1056/NEJMoa1008232 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 91. Smith CR, Leon MB, Mack MJ, et al. : Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187–98. 10.1056/NEJMoa1103510 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 92. Kenny D, Hijazi ZM, Kar S, et al. : Percutaneous implantation of the Edwards SAPIEN transcatheter heart valve for conduit failure in the pulmonary position: early phase 1 results from an international multicenter clinical trial. J Am Coll Cardiol. 2011;58(21):2248–56. 10.1016/j.jacc.2011.07.040 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 93. Boone RH, Webb JG, Horlick E, et al. : Transcatheter pulmonary valve implantation using the Edwards SAPIEN™ transcatheter heart valve. Catheter Cardiovasc Interv. 2010;75(2):286–94. 10.1002/ccd.22250 [DOI] [PubMed] [Google Scholar]
- 94. Wilson WM, Benson LN, Osten MD, et al. : Transcatheter Pulmonary Valve Replacement With the Edwards Sapien System: The Toronto Experience. JACC Cardiovasc Interv. 2015;8(14):1819–27. 10.1016/j.jcin.2015.08.016 [DOI] [PubMed] [Google Scholar]
- 95. Faza N, Kenny D, Kavinsky C, et al. : Single-center comparative outcomes of the Edwards SAPIEN and Medtronic Melody transcatheter heart valves in the pulmonary position. Catheter Cardiovasc Interv. 2013;82(4):E535–41. 10.1002/ccd.24680 [DOI] [PubMed] [Google Scholar]
- 96. Morray BH, McElhinney DB, Cheatham JP, et al. : Risk of coronary artery compression among patients referred for transcatheter pulmonary valve implantation: a multicenter experience. Circ Cardiovasc Interv. 2013;6(5):535–42. 10.1161/CIRCINTERVENTIONS.113.000202 [DOI] [PubMed] [Google Scholar]
- 97. Hascoet S, Mauri L, Claude C, et al. : Infective Endocarditis Risk After Percutaneous Pulmonary Valve Implantation With the Melody and Sapien Valves. JACC Cardiovasc Interv. 2017;10(5):510–7. 10.1016/j.jcin.2016.12.012 [DOI] [PubMed] [Google Scholar]
- 98. Guccione P, Milanesi O, Hijazi ZM, et al. : Transcatheter pulmonary valve implantation in native pulmonary outflow tract using the Edwards SAPIEN™ transcatheter heart valve. Eur J Cardiothorac Surg. 2012;41(5):1192–4. 10.1093/ejcts/ezr130 [DOI] [PubMed] [Google Scholar]
- 99. Bertels RA, Blom NA, Schalij MJ: Edwards SAPIEN transcatheter heart valve in native pulmonary valve position. Heart. 2010;96(9):661. 10.1136/hrt.2009.189944 [DOI] [PubMed] [Google Scholar]
- 100. Levi DS, Sinha S, Salem MM, et al. : Transcatheter native pulmonary valve and tricuspid valve replacement with the sapien XT: Initial experience and development of a new delivery platform. Catheter Cardiovasc Interv. 2016;88(3):434–43. 10.1002/ccd.26398 [DOI] [PubMed] [Google Scholar]
- 101. Thanopoulos BV, Giannakoulas G, Arampatzis CA: Percutaneous pulmonary valve implantation in the native right ventricular outflow tract. Catheter Cardiovasc Interv. 2012;79(3):427–9. 10.1002/ccd.23095 [DOI] [PubMed] [Google Scholar]
- 102. Gillespie MJ, Dori Y, Harris MA, et al. : Bilateral branch pulmonary artery melody valve implantation for treatment of complex right ventricular outflow tract dysfunction in a high-risk patient. Circ Cardiovasc Interv. 2011;4(4):e21–3. 10.1161/CIRCINTERVENTIONS.111.962373 [DOI] [PubMed] [Google Scholar]
- 103. Carr JA: The results of catheter-based therapy compared with surgical repair of adult aortic coarctation. J Am Coll Cardiol. 2006;47(6):1101–7. 10.1016/j.jacc.2005.10.063 [DOI] [PubMed] [Google Scholar]
- 104. Travelli FC, Herrington CS, Ing FF: A novel hybrid technique for transcatheter pulmonary valve implantation within a dilated native right ventricular outflow tract. J Thorac Cardiovasc Surg. 2014;148(2):e145–6. 10.1016/j.jtcvs.2014.04.046 [DOI] [PubMed] [Google Scholar]
- 105. Boudjemline Y, Schievano S, Bonnet C, et al. : Off-pump replacement of the pulmonary valve in large right ventricular outflow tracts: a hybrid approach. J Thorac Cardiovasc Surg. 2005;129(4):831–7. 10.1016/j.jtcvs.2004.10.027 [DOI] [PubMed] [Google Scholar]
- 106. Promphan W, Prachasilchai P, Siripornpitak S, et al. : Percutaneous pulmonary valve implantation with the Venus P-valve: clinical experience and early results. Cardiol Young. 2016;26(4):698–710. 10.1017/S1047951115001067 [DOI] [PubMed] [Google Scholar]
- 107. Cao QL, Kenny D, Zhou D, et al. : Early clinical experience with a novel self-expanding percutaneous stent-valve in the native right ventricular outflow tract. Catheter Cardiovasc Interv. 2014;84(7):1131–7. 10.1002/ccd.25544 [DOI] [PubMed] [Google Scholar]
- 108. Husain J, Praichasilchai P, Gilbert Y, et al. : Early European experience with the Venus P-valve ®: filling the gap in percutaneous pulmonary valve implantation. EuroIntervention. 2016;12(5):e643–51. 10.4244/EIJV12I5A105 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 109. Jalal Z, Thambo J, Boudjemline Y: The future of transcatheter pulmonary valvulation. Arch Cardiovasc Dis. 2014;107(11):635–42. 10.1016/j.acvd.2014.07.046 [DOI] [PubMed] [Google Scholar]
- 110. Bergersen L, Benson LN, Gillespie MJ, et al. : Harmony Feasibility Trial: Acute and Short-Term Outcomes With a Self-Expanding Transcatheter Pulmonary Valve. JACC Cardiovasc Interv. 2017;10(17):1763–73. 10.1016/j.jcin.2017.05.034 [DOI] [PubMed] [Google Scholar]
- 111. Gillespie MJ, Benson LN, Bergersen L, et al. : Patient Selection Process for the Harmony Transcatheter Pulmonary Valve Early Feasibility Study. Am J Cardiol. 2017;120(8):1387–92. 10.1016/j.amjcard.2017.07.034 [DOI] [PubMed] [Google Scholar]
- 112. Amahzoune B, Szymansky C, Fabiani JN, et al. : A new endovascular size reducer for large pulmonary outflow tract. Eur J Cardiothorac Surg. 2010;37(3):730–2. 10.1016/j.ejcts.2009.08.034 [DOI] [PubMed] [Google Scholar]
- 113. Mollet A, Basquin A, Stos B, et al. : Off-pump replacement of the pulmonary valve in large right ventricular outflow tracts: a transcatheter approach using an intravascular infundibulum reducer. Pediatr Res. 2007;62(4):428–33. 10.1203/PDR.0b013e318142aa3e [DOI] [PubMed] [Google Scholar]
- 114. Boudjemline Y, Laborde F, Pineau E, et al. : Expandable right ventricular-to-pulmonary artery conduit: an animal study. Pediatr Res. 2006;59(6):773–7. 10.1203/01.pdr.0000219396.34610.4a [DOI] [PubMed] [Google Scholar]
- 115. Godart F, Baruteau AE, Petit J, et al. : Transcatheter tricuspid valve implantation: a multicentre French study. Arch Cardiovasc Dis. 2014;107(11):583–91. 10.1016/j.acvd.2014.07.051 [DOI] [PubMed] [Google Scholar]
- 116. Raval J, Nagaraja V, Eslick GD, et al. : Transcatheter valve-in-valve implantation: a systematic review of literature. Heart Lung Circ. 2014;23(11):1020–8. 10.1016/j.hlc.2014.06.001 [DOI] [PubMed] [Google Scholar]
- 117. McElhinney DB, Cabalka AK, Aboulhosn JA, et al. : Transcatheter Tricuspid Valve-in-Valve Implantation for the Treatment of Dysfunctional Surgical Bioprosthetic Valves: An International, Multicenter Registry Study. Circulation. 2016;133(16):1582–93. 10.1161/CIRCULATIONAHA.115.019353 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 118. Cullen MW, Cabalka AK, Alli OO, et al. : Transvenous, antegrade Melody valve-in-valve implantation for bioprosthetic mitral and tricuspid valve dysfunction: a case series in children and adults. JACC Cardiovasc Interv. 2013;6(6):598–605. 10.1016/j.jcin.2013.02.010 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 119. Aboulhosn J, Cabalka AK, Levi DS, et al. : Transcatheter Valve-in-Ring Implantation for the Treatment of Residual or Recurrent Tricuspid Valve Dysfunction After Prior Surgical Repair. JACC Cardiovasc Interv. 2017;10(1):53–63. 10.1016/j.jcin.2016.10.036 [DOI] [PubMed] [Google Scholar]
- 120. Bouleti C, Fassa AA, Himbert D, et al. : Transfemoral implantation of transcatheter heart valves after deterioration of mitral bioprosthesis or previous ring annuloplasty. JACC Cardiovasc Interv. 2015;8(1 Pt A):83–91. 10.1016/j.jcin.2014.07.026 [DOI] [PubMed] [Google Scholar]
- 121. Eleid MF, Whisenant BK, Cabalka AK, et al. : Early Outcomes of Percutaneous Transvenous Transseptal Transcatheter Valve Implantation in Failed Bioprosthetic Mitral Valves, Ring Annuloplasty, and Severe Mitral Annular Calcification. JACC Cardiovasc Interv. 2017;10(19):1932–42. 10.1016/j.jcin.2017.08.014 [DOI] [PubMed] [Google Scholar]
- 122. Cheung A, Webb JG, Barbanti M, et al. : 5-year experience with transcatheter transapical mitral valve-in-valve implantation for bioprosthetic valve dysfunction. J Am Coll Cardiol. 2013;61(17):1759–66. 10.1016/j.jacc.2013.01.058 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 123. Seiffert M, Conradi L, Baldus S, et al. : Transcatheter mitral valve-in-valve implantation in patients with degenerated bioprostheses. JACC Cardiovasc Interv. 2012;5(3):341–9. 10.1016/j.jcin.2011.12.008 [DOI] [PubMed] [Google Scholar]
- 124. Wilbring M, Alexiou K, Tugtekin SM, et al. : Pushing the limits-further evolutions of transcatheter valve procedures in the mitral position, including valve-in-valve, valve-in-ring, and valve-in-native-ring. J Thorac Cardiovasc Surg. 2014;147(1):210–9. 10.1016/j.jtcvs.2013.09.021 [DOI] [PubMed] [Google Scholar]
- 125. Hasan BS, McElhinney DB, Brown DW, et al. : Short-term performance of the transcatheter Melody valve in high-pressure hemodynamic environments in the pulmonary and systemic circulations. Circ Cardiovasc Interv. 2011;4(6):615–20. 10.1161/CIRCINTERVENTIONS.111.963389 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 126. Maisano F, Reser D, Pavicevic J, et al. : Successful first-in-man Melody transcatheter valve implant in a dehisced mitral annuloplasty ring transapical valve-in-ring implant. EuroIntervention. 2014;10(8):961–7. 10.4244/EIJV10I8A163 [DOI] [PubMed] [Google Scholar]
- 127. Kliger C, Al-Badri A, Wilson S, et al. : Successful first-in-man percutaneous transapical-transseptal Melody mitral valve-in-ring implantation after complicated closure of a para-annular ring leak. EuroIntervention. 2014;10(8):968–74. 10.4244/EIJV10I8A164 [DOI] [PubMed] [Google Scholar]
- 128. Borger MA, Kodali S: Transcatheter mitral valve-in-ring with the Melody prosthesis: a new therapeutic opportunity. EuroIntervention. 2014;10(8):903–5. 10.4244/EIJV10I8A156 [DOI] [PubMed] [Google Scholar]
- 129. Guerrero M, Greenbaum A, O'Neill W: First in human percutaneous implantation of a balloon expandable transcatheter heart valve in a severely stenosed native mitral valve. Catheter Cardiovasc Interv. 2014;83(7):E287–91. 10.1002/ccd.25441 [DOI] [PubMed] [Google Scholar]
- 130. Sinning J, Mellert F, Schiller W, et al. : Transcatheter mitral valve replacement using a balloon-expandable prosthesis in a patient with calcified native mitral valve stenosis. Eur Heart J. 2013;34(33):2609. 10.1093/eurheartj/eht254 [DOI] [PubMed] [Google Scholar]
- 131. Mellert F, Sinning JM, Werner N, et al. : First-in-man transapical mitral valve replacement using the Direct Flow Medical ® aortic valve prosthesis. Eur Heart J. 2015;36(31):2119. 10.1093/eurheartj/ehv167 [DOI] [PubMed] [Google Scholar]
- 132. Frigiola A, Pluchinotta FR, Saracino A, et al. : Surgical mitral valve replacement with the Melody valve in infants and children: the Italian experience. EuroIntervention. 2017;12(17):2104–9. 10.4244/EIJ-D-16-00853 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 133. Quiñonez LG, Breitbart R, Tworetsky W, et al. : Stented bovine jugular vein graft (Melody valve) for surgical mitral valve replacement in infants and children. J Thorac Cardiovasc Surg. 2014;148(4):1443–9. 10.1016/j.jtcvs.2013.10.059 [DOI] [PubMed] [Google Scholar]